Abstract
A prospective, behavioral high-risk design provided a theoretically guided examination of vulnerability to first onset of bipolar spectrum disorder based on the Behavioral Approach System (BAS) model. Adolescents (ages 14–19) at an “age of risk” for bipolar disorder onset were screened on BAS sensitivity by interviewers blind to current symptoms, lifetime history, and family history of psychopathology. Participants were selected with high versus moderate levels of BAS sensitivity and administered a lifetime diagnostic interview. Those with a bipolar spectrum disorder, psychosis, or hypomanic episode with onset prior to the BAS sensitivity assessment were excluded. High BAS (n = 171) and Moderate BAS (n = 119) sensitivity participants in the final sample completed baseline measures of symptoms, goal-setting, and reward responsiveness and were followed prospectively with semistructured diagnostic interviews every 6 months. Consistent with the vulnerability hypothesis of the BAS model of bipolar disorder, high BAS participants had a greater likelihood, and shorter time to onset, of bipolar spectrum disorder than moderate BAS participants across an average of 12.8 months of follow-up (12.9% vs. 4.2%), controlling for baseline depressive and hypomanic symptoms, and family history of bipolar disorder. High reward responsiveness on a behavioral task and ambitious goal-striving for popular fame and financial success (but not impulsivity) also predicted first onset of bipolar spectrum disorder controlling for the covariates and BAS risk group, and ambitious goal-striving partially mediated the BAS risk group effect. We discuss implications of the findings for the BAS model of bipolar disorder and early intervention efforts.
Keywords: bipolar spectrum disorder, first onset, Behavioral Approach System (BAS), reward responsiveness, goal-striving
Bipolar spectrum disorders (BSDs) occur in 4.4% of the US population (Merikangas et al., 2007) and can be associated with severe personal, social, and economic costs. Within the bipolar category, a group of disorders appears to form a spectrum of severity from the milder bipolar disorder not otherwise specified (BiNOS) and cyclothymia, to bipolar II disorder, to full-blown bipolar I disorder at the most severe end of the continuum (e.g., Akiskal, Djenderedjian, Rosenthal, & Khani, 1977; Alloy et al., in press; Birmaher et al., 2009; Cassano et al., 1999; Goodwin & Jamison, 2007). Moreover, milder forms of bipolar disorder sometimes progress to more severe forms (e.g., Akiskal et al., 1977; Alloy et al., in press; Birmaher et al., 2009; Kochman et al., 2005). BSDs are often associated with divorce, substance abuse, suicide, and significant impairment in work and academic functioning (e.g., Angst, Stassen, Clayton & Angst, 2002; Conway, Compton, Stinson, & Grant, 2006; Judd et al., 2008; Nusslock, Alloy, Abramson, Harmon-Jones, & Hogan, 2008).
Adolescence may be a critical developmental period constituting an “age of risk” (Weissman et al., 1996) for bipolar conditions. Although there has been increased recognition and diagnosis of BSDs in prepubertal children (see Youngstrom, Birmaher, & Findling, 2008, for a review), the first peak in onset of the adult form of bipolar disorder usually occurs between ages 15–19 (Alloy, Abramson, Walshaw, Keyser, & Gerstein, 2006a; Burke, Burke, Regier, & Rao, 1990; Kennedy et al., 2005; Kessler et al., 1997; Oedegaard et al., 2009; Weissman et al., 1996). Admixture analyses indicate that the earliest high-risk period for onset of adult bipolar disorder is around age 17 (Bellivier, Golmard, Henry, Leboyer, & Schurhoff, 2001; Bellivier et al., 2003), and longitudinal follow-ups of a community sample (Beesdo et al., 2009) and an offspring of bipolar parents sample (Hillegers, Reichart, Wals, Verhulst, Ormel, & Nolen, 2005) also report initial onset of bipolar disorder in adolescence.
Inasmuch as BSDs frequently emerge during adolescence, the ability to identify at-risk adolescents before the initial onset of a BSD may facilitate early and targeted intervention. In turn, early intervention may prevent disorder expression or improve long-term prognosis. Given evidence that bipolar disorder has a strong genetic predisposition (e.g., Merikangas et al., 2002; McGuffin et al., 2003), at present, the best way to identify adolescents at risk for future BSD is to find those with a first-degree relative with the disorder. However, some individuals who develop a BSD either do not have, or are unaware that they have, relatives with the disorder. Thus, alternative approaches for identifying at-risk adolescents that do not rely on a genetic risk paradigm would be extremely valuable. Consequently, we use a behavioral high-risk design to identify adolescents hypothetically vulnerable to BSDs, with the possibility open that the behavioral risk factor itself could be related to genetic predisposition.
In this article, we present findings from the first prospective study of initial onset of BSD among adolescents at an “age of risk” for onset of the disorder selected on indicators of biobehavioral vulnerability. The Teen Emotion and Motivation (TEAM) Project uses a prospective, behavioral high-risk design to provide a theoretically guided examination of vulnerability to initial onset of BSD based on the Behavioral Approach System (BAS) model.
Behavioral Approach System (BAS) Model: Theory and Evidence
The BAS is a biobehavioral system that regulates approach motivation and goal-directed behavior to attain rewards (e.g., Gray, 1994). It is activated by goal- or reward-relevant stimuli, which can be either internal (expectancies of goal attainment) or external (presence of a desired goal). BAS activation has been associated with increased motor behavior, incentive-reward motivation, and positive goal-striving emotions such as hope and happiness (e.g., Depue & Collins, 1999; Gray, 1994), as well as with anger when goal striving is frustrated or blocked (Carver, 2004; Harmon-Jones & Allen, 1998; Harmon-Jones & Sigelman, 2001). The BAS has also been linked with a reward-sensitive neural network, involving dopaminergic projections from the limbic system to the frontal cortex (Depue & Iacono, 1989). Although Gray’s general Reinforcement Sensitivity Theory has been revised more recently (e.g., Gray & McNaughton, 2000), the present study is designed to test aspects of Depue and colleagues’ (Depue & Iacono, 1989; Depue, Krauss, & Spoont, 1987) earlier BAS dysregulation model of BSDs and recent expansions of the Depue model.
The BAS model of bipolar disorder (Depue & Iacono, 1989; Depue et al., 1987) has been expanded recently (Alloy & Abramson, 2010; Alloy, Abramson, Urosevic, Bender, & Wagner, 2009a; Johnson, 2005; Urosevic, Abramson, Harmon-Jones, & Alloy, 2008) and provides a single theme – approach motivation/reward sensitivity – to organize a diverse array of symptoms and account for both poles of bipolar disorder. According to this model, vulnerability to BSDs is reflected in an overly sensitive BAS that is hyper-reactive to goal- and reward-relevant cues. This hypersensitivity can lead to excessive BAS activation in response to events involving rewards or goal striving and attainment, and to excessive BAS deactivation or shutdown of behavioral approach/engagement in response to events involving definite failures, losses, or nonattainment of goals. In turn, excessive BAS activation is hypothesized to lead to (hypo)manic symptoms, such as excessive goal-directed behavior, increased energy, decreased need for sleep, optimism, grandiosity, and euphoria (or irritability if the BAS activation-triggering event involves goal frustrations or obstacles). In contrast, excessive BAS deactivation may lead to depressive symptoms, such as decreased goal-directed activity, decreased energy, loss of interest and anhedonia, hopelessness, and sadness. Thus, according to the BAS model, individuals with or vulnerable to BSDs have a single vulnerability, a hypersensitive BAS, but polarity-specific BAS-relevant triggers for (hypo)manic and bipolar depressive episodes (e.g., Alloy et al., 2009a; Urosevic et al., 2008). It is important to emphasize that the hypothesized vulnerability to BSDs in this model is a tendency (i.e., a propensity) toward excessive BAS activation and deactivation, not the actual activation or deactivation (dysregulation) itself, which is considered the more proximal precursor of mood symptoms/episodes.
Considerable evidence supports the BAS model of BSD. Individuals with BSDs or vulnerable to bipolar disorder because they exhibit a hypomanic personality have significantly higher levels of self-reported BAS sensitivity than do individuals without mood disorders or hypomanic personality (Alloy et al., 2008; Meyer, Johnson, & Carver, 1999; Meyer, Johnson, & Winters, 2001; Salavert et al., 2007; Van der Gucht, Morriss, Lancaster, Kinderman, & Bentall, 2009). The cognitive styles of individuals with BSDs are characterized by BAS-relevant themes of goal-striving, perfectionism, and self-criticism (e.g., Alloy et al., 2009b; Lam, Wright, & Smith, 2004: Scott, Stanton, Garland, & Ferrier, 2000). Also, compared to healthy controls, individuals with BSDs exhibit increased relative left frontal cortical activity as assessed by electroencephalography (EEG), a neurobiological indicator of BAS sensitivity and activation, both in the resting state and in response to anticipation of rewards (Harmon-Jones et al., 2008; Kano, Nakamura, Matsuoka, Iida, & Nakajima, 1992) and frustrations (Harmon-Jones et al., 2002). Moreover, on functional magnetic resonance imaging (fMRI), compared to healthy controls, both euthymic and manic bipolar individuals display increased activation of the ventral striatum and lateral orbitofrontal cortex, which are part of the corticolimbic circuit subserving reward-related processing (e.g., Bermpohl et al., 2010; Nusslock et al., 2011).
Individuals with BSDs also exhibit excessive goal-striving and greater responsiveness to rewards. Consistent with both the BAS model and a related goal dysregulation account of bipolar disorder (Johnson, 2005), BSD individuals or those vulnerable to mania exhibit ambitious goal-setting compared with controls. This is particularly true for goals involving popular fame and financial success (Carver & Johnson, 2009; Gruber & Johnson, 2009; Johnson & Carver, 2006; Johnson, Eisner & Carver, 2009). Individuals with bipolar disorders are also less likely than controls to decrease their goal-striving efforts after unexpectedly high progress toward a goal (Fulford, Johnson, Llabre & Carver, 2010) and they continue to exhibit greater left frontal cortical activity in response to challenging tasks when striving for rewards than do controls (Harmon-Jones et al., 2008). In addition, individuals with BSDs exhibit greater behavioral, emotional, and cognitive responsiveness to rewards and an inability to delay rewards (Eisner, Johnson, & Carver, 2008; Hayden et al., 2008; Johnson, Ruggero, & Carver, 2005; Swann, Lijffijt, Lane, Steinberg, & Moeller, 2009). Thus, ambitious goal-striving and excessive reward responsiveness, hypothesized to be part of the nomological network of the BAS sensitivity construct, appear to be important features of bipolar disorder.
The BAS model also has predictive validity with respect to the course of BSDs. BAS-relevant life events involving goal-striving or goal-attainment, but not positive events in general, predict onsets of hypomanic or manic episodes among BSD individuals (Johnson et al., 2000; 2008; Nusslock, Abramson, Harmon-Jones, Alloy, & Hogan, 2007). In addition, high self-reported BAS sensitivity predicts prospective increases in manic symptoms among recovered bipolar I patients (Meyer et al., 2001), greater likelihood and faster onset of hypomanic and manic episodes (Alloy et al., 2008; Salavert et al., 2007), and a greater likelihood of progressing to a more severe bipolar diagnosis (e.g., bipolar I disorder) over follow-up among individuals with milder bipolar conditions (Alloy et al., in press).
BAS Hypersensitivity as a Vulnerability for First Onset of Bipolar Spectrum Disorder
The research reviewed above provides considerable evidence supporting the BAS model of BSD. Prior studies demonstrate that high BAS sensitivity confers risk for recurrence of mood episodes/symptoms and for a more severe course of bipolar disorder. However, as yet, no study has examined whether high BAS sensitivity, reward responsiveness, or ambitious goal-striving actually provides vulnerability to first onset of BSDs. The “behavioral high-risk design” (e.g., Alloy et al., 2000; 2006b; Kwapil et al., 2000) is a powerful strategy for testing whether BAS hypersensitivity indeed confers vulnerability for onset of BSD, rather than just modulating its course. In this design, one selects individuals at high versus low risk for BSD based on the presence versus absence of the hypothesized vulnerability, in this case, BAS hypersensitivity. One then compares these groups on their likelihood of developing BSD in the future.
Demonstrating the potential fruitfulness of testing the BAS vulnerability hypothesis with a behavioral high-risk design, Alloy and colleagues (2006c) selected 18–24 year-olds with high versus moderate BAS sensitivity and compared their lifetime histories of mood disorders, current symptoms, and personality, blind to their BAS scores. High BAS sensitivity participants were more likely to receive a lifetime BSD diagnosis (50%) than were moderate BAS sensitivity participants (8.3%) and also scored higher on hypomanic symptoms and personality. These findings suggest that high BAS sensitivity may indeed confer risk for BSDs. However, the causal direction of the association between high BAS sensitivity and increased BSDs is unclear in this retrospective study.
The Present Study
Consequently, the present study employed a theoretically guided, prospective behavioral high-risk design to test the BAS vulnerability hypothesis for predicting first onset of BSD among adolescents at an “age of risk” for onset of bipolar disorder. Given that the crucial indicator of BSD onset is occurrence of hypomania or mania, first onset of BSD in this study was operationalized by onset of at least one hypomanic episode either alone or as part of a Bipolar II, Cyclothymia, or BiNOS diagnosis. Multiple investigators (e.g., Akiskal et al., 2000; Angst et al., 2003; 2010; Benazzi, 2007; Cassano et al., 1999; Keck et al., 2008; Merikangas et al., 2007; Zimmerman et al., 2009) have argued for broadening the diagnostic criteria for BSDs in DSM-5 to better account for subsyndromal hypomanic presentations based on evidence that subsyndromal hypomania (that falls short of DSM-IV criteria) is clinically significant and associated with role impairment (e.g., Merikangas et al., 2007), occurrence of criterial bipolar mood episodes (Angst et al., 2010), conversion to bipolar I or II diagnosis (e.g., Zimmerman et al., 2009), bipolar family history (e.g., Akiskal et al., 2000; Angst et al., 2003; Cassano et al., 1992; Zimmerman et al., 2009) and psychiatric comorbidity (e.g., Angst et al., 2003; 2010; Merikangas et al., 2007; Zimmerman et al., 2009). Thus, in line with this increased recognition of subsyndromal bipolar presentations, and because our goal was to predict initial onset of bipolarity, we considered onset of a hypomanic episode to indicate onset of a bipolar spectrum condition. Moreover, Alloy et al. (in press) validated the criteria for the ‘soft’ BiNOS conditions included as onsets in the present study by demonstrating that individuals who met these criteria were likely to progress to DSM-IV bipolar I or II diagnoses over follow-up.
Adolescents (ages 14–19) were screened for BAS sensitivity by interviewers blind to their current symptoms, lifetime history, and family history of psychopathology, using the two BAS sensitivity measures previously validated as predictors of lifetime history of BSD by Alloy et al. (2006c) in their retrospective high-risk design – Carver and White’s (1994) Behavioral Inhibition System/Behavioral Activation System (BIS/BAS) Scales and Torrubia, Avila, Molto, and Caseras’ (2001) Sensitivity to Punishment/Sensitivity to Reward Questionnaire (SPSRQ). Although the BAS subscale of the BIS/BAS and the Sensitivity to Reward (SR) subscale of the SPSRQ are designed to assess BAS sensitivity and lability in the upward (activated) direction and thus, risk for (hypo)mania, it is an empirical question as to whether these two measures also are relevant to BAS lability in the downward (deactivated) direction, and risk for depression within the context of BSD. Individuals prone to lability in the upward direction may also be prone to lability in the downward direction. Prior evidence suggests that high BAS sensitivity on the Carver and White (1994) BAS scale does, in fact, predict sadness following frustrative non-reward (Carver, 2004) and major depressive episode onset among individuals with Cyclothymia or BiNOS (Alloy et al., in press).
Adolescents who exhibited high versus moderate levels of BAS sensitivity on both the BAS and SR were invited for further screening with a lifetime psychiatric diagnostic interview. We used a moderate BAS sensitivity, rather than a low BAS sensitivity, group as the low-risk comparison group for three reasons (Alloy et al., 2006c). First, interpretation of differences in BSD onset rates between high and low BAS groups would be unclear, because such differences could be due to increased vulnerability in the high BAS group or decreased vulnerability in the low BAS group. Comparison of a high BAS group to a moderate BAS group resolves this ambiguity. Second, a moderate BAS group is closer to the mean on the BAS sensitivity dimension and, thus, more normal from a statistical perspective. Third, low BAS sensitivity has been associated with vulnerability to unipolar depression (e.g., Depue & Iacono, 1989; Depue et al., 1987; Fowles, 1993; Kasch, Rottenberg, Arnow & Gotlib, 2002). We excluded adolescents who met Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition (DSM-IV-TR; American Psychiatric Association, 2000) or Research Diagnostic Criteria (RDC; Spitzer, Endicott & Robins, 1978) for any BSD, hypomanic episode, or psychosis with onset prior to the date of BAS screening.
High and Moderate BAS sensitivity participants in the final sample completed baseline measures of symptoms, goal-setting, and reward responsiveness at Time 1 and then were followed prospectively with structured diagnostic interviews every 6 months. To investigate further components of the nomological network of the BAS construct, we included measures of ambitious goal-setting and reward responsiveness as additional predictors of first onset of BSD, given that both goal-setting and reward responsiveness are related to self-reported vulnerability to mania and past history of (hypo)mania. Measures of self-reported BAS sensitivity (BAS and SR), goal-setting, and reward responsiveness may each assess a different component of BAS that is imperfectly correlated with the other measures. Indeed, Carver and White’s BAS scale may be more strongly associated with pre-goal attainment motivational states (ambitious goal-setting) than with post-goal reward responsiveness (Alloy et al., 2006c; Davidson, 1994).
We hypothesized that the High BAS group would be more likely than the Moderate BAS group to develop a first onset of a BSD. Second, we hypothesized that high responsiveness to rewards and ambitious goal-striving for popular fame and financial success also would predict onset of BSD, and that reward responsiveness or ambitious goal-striving would mediate any obtained association between BAS risk group and prospective onset of BSD. Finally, we examined the specificity of our findings to BAS sensitivity. We hypothesized that the individual difference trait of impulsivity, found to be elevated in individuals with BSDs (e.g., Swann, Anderson, Dougherty, & Moeller, 2001; Swann, Dougherty, Pazzaglia, Pham, & Moeller, 2004), would not predict first onset of BSD as well as would high BAS risk group status. Although impulsivity and BAS sensitivity are correlated, measures of these constructs load on separate factors in factor analyses (see Dawe, Gullo, & Loxton, 2004 for review), and thus, they may have differential ability to predict onset of BSD.
Methods
Participants
Sample selection
Adolescents (ages 14–19) were selected for Project TEAM based on a two-phase screening procedure. In Phase I, we screened students (grades 9–12, ages 14–18) from 13 Philadelphia public high schools during homeroom advisory periods, as well as college students (ages 17–19) from two universities through the dorms and the universities’ online screening systems. Following procedures in Alloy et al. (2006c), students were screened on demographics and two self-report BAS sensitivity measures: the BIS/BAS Scales (Carver & White, 1994) and SPSRQ (Torrubia et al., 2001). Students scoring in the highest 15th percentile on both the BAS-Total (high BAS-T score cutpoint ≥ 43) and SR scale (high SR cutpoint ≥ 16) were categorized as High BAS (HBAS). Participants were classified as Moderate BAS (MBAS) if they scored between the 40th and 60th percentiles on both the BAS-T (cutpoints ≥ 37 and ≤ 39) and SR (cutpoints ≥ 10.4 and ≤ 12.6) subscales. Of 9991 Phase I students, 7.77% (n = 776) qualified for HBAS and 4.04% (n = 404) for MBAS status.
We attempted to invite students who met the Phase I criteria for the HBAS or MBAS groups for Phase II screening1; 244 HBAS (31.4%) and 146 MBAS (36.1%) students participated in Phase II. In Phase II, participants were administered the mood and psychosis disorder sections of an expanded Schedule for Affective Disorders and Schizophrenia – Lifetime (exp-SADS-L) interview, as well as the Beck Depression Inventory (BDI; Beck, Rush, Shaw & Emery, 1979) to assess depressive symptoms and the Altman Self-Rating Mania Scale (ASRM; Altman, Hedeker, Peterson, & Davis, 1997) to assess (hypo)manic symptoms. The remainder of the exp-SADS-L interview was administered to participants in the final sample at a baseline session. Exp-SADS-L interviewers were blind to participants’ BAS risk group. At Phase II, parental consent and adolescent assent were obtained for participants aged < 18, whereas participants ≥ 18 provided their own written consent.
Participants were excluded from the final sample if they met DSM-IV-TR (American Psychiatric Association, 2000) or RDC (Spitzer et al., 1978) criteria for: 1) any BSD (Bipolar I, Bipolar II, Cyclothymia, BiNOS) or a hypomanic (Hyp) episode with onset prior to the participant’s Phase I screening date, and 2) any lifetime psychotic disorder (Schizophrenia, Schizoaffective Disorder, Major Depressive Disorder with psychosis). They also were excluded if they lacked fluency in English. Participants were not excluded if they met criteria for a non-psychotic DSM-IV-TR or RDC major depressive (MD) or RDC minor depressive (MiD) episode with onset prior to Phase I screening, as prior depressive episodes without mania (Ma) or hypomania (Hyp) may reflect unipolar depression rather than bipolar disorder.
Of 390 participants interviewed in Phase II, 22 were excluded because they met criteria for a BSD or Hyp episode with onset prior to their Phase I BAS screening, 7 were excluded because they met criteria for a psychotic disorder or exhibited psychotic symptoms, and another 5 were excluded for poor English fluency. Another 66 eligible Phase II participants have not yet completed their Time 1 (baseline) assessment and, thus, were missing data needed for the present study. Notably, of the 22 participants excluded because they already had met criteria for BSD or Hyp episode, 17 were HBAS and 5 were MBAS, Χ2 = 3.84, p = .05. This replicates Alloy et al.’s (2006c) prior findings in a younger sample.
The final sample for the current analyses had 171 HBAS and 119 MBAS participants (mean age = 17.44; SD = 1.56), with 53.1% Caucasian, 26.2% African American, 7.2% Hispanic/Latino, 7.0% Asian or Pacific Islander, 3.1% Biracial, and 3.4% Other ethnicity participants. Table 1 presents the demographic characteristics and means and SDs of the BIS/BAS, SPSRQ, BDI, and ASRM scores for each group. The risk groups didn’t differ from each other on age (t(288) = 1.32, ns), gender (Χ2(1) = 1.73, ns), or race/ethnicity (Χ2(6)= 10.62, ns). In addition, the two groups didn’t differ on BIS (t(288) = 0.24, ns) or SP (t(288) = 0.58, ns) scores, but obviously, the HBAS group had significantly higher BAS-T (t(288) = 28.53, p < .001) and SR (t(288) = 28.90, p < .001) scores than the MBAS group. Finally, the two groups didn’t differ on initial BDI (t(277) = 1.23, ns) or ASRM (t(277) = 1.47, ns) symptom scores.
Table 1.
Demographic Characteristics of the Study Sample
| High BAS (N = 171) |
Moderate BAS (N = 119) |
|
|---|---|---|
| Age | 17.60 (1.45) | 17.20 (1.69) |
| Sex | 63.0% Female | 71.0% Female |
| Race/Ethnicity | 55.0% Caucasian | 55.0% Caucasian |
| 30.0% African Amer. | 25.0% African Amer. | |
| 3.8% Hispanic/Latino | 6.3% Hispanic/Latino | |
| 9.4% Asian/Pacific Isl. | 4.4% Asian/Pacific Isl. | |
| 0.9% Biracial | 4.8% Biracial | |
| 0.9% Other | 4.5% Other | |
| BIS | 19.83 (4.07) | 19.72 (3.34) |
| BAS-T | 46.08 (2.76) | 38.07 (1.59) |
| SP | 10.50 (5.29) | 10.86 (5.18) |
| SR | 18.06 (1.89) | 11.43 (1.96) |
| BDI | 7.08 (6.44) | 6.13 (6.23) |
| ASRM | 6.23 (3.98) | 5.55 (3.67) |
Note. Standard deviations are in parentheses. BIS = Behavioral Inhibition System scores from the BIS/BAS Scales; BAS-T = Behavioral Approach System – Total scores from the BIS/BAS Scales; SP = Sensitivity to Punishment scores from the SPSRQ; SR = Sensitivity to Reward from the SPSRQ; BDI = Beck Depression Inventory; ASRM = Altman Self-Rating Mania Scale.
Sample representativeness
Participants in the final sample didn’t differ from the Phase I screening sample on age (t(9881) = 1.225, ns), gender (Χ2(1) = 0.82, ns), or race/ethnicity (Χ2(6) = 10.54, ns). The final sample didn’t differ from Phase II eligible participants not included in the present analyses on age (t(388) = −0.70, ns), gender (Χ2(1) = 2.20, ns), race/ethnicity (Χ2(6) = 5.77, ns), BAS-T (t(388) = 0.99, ns), or SR (t(388) = 0.097, ns) scores.
Measures
BAS sensitivity measures
The BIS/BAS Scales (Carver & White, 1994) and SPSRQ (Torrubia et al., 2001) were used to select the risk groups. The BIS/BAS is the most frequently used self-report measure to assess individual differences in BIS and BAS sensitivity. It consists of 20 items on 4-point Likert scales (1 = strongly disagree, 4 = strongly agree) and is comprised of three BAS subscales and one BIS subscale. We used the BAS-Total (BAS-T) score, calculated as the sum of all BAS items, as one of our screening measures. The BIS/BAS has demonstrated internal consistency and retest reliability (Carver & White, 1994), as well as construct validity, exhibiting expected associations with prefrontal cortical activity, affect, personality traits, and performance on reaction-time and learning tasks involving incentives (Colder & O'Conner, 2004; Harmon-Jones & Allen, 1997; Kambouroupolis & Staiger, 2004; Sutton & Davidson, 1997; Zinbarg & Mohlman, 1998). Internal consistencies of the BAS-T and BIS scales in the Phase I screening sample were α’s = .80 and .72, respectively.
The SPSRQ was designed to improve on the BIS/BAS by including items that focus on sensitivity to specific types of rewards and punishments, whereas the BIS/BAS items focus on generalized sensitivity to punishment and reward. The SPSRQ has 24 SR (e.g., “Does the good prospect of obtaining money motivate you strongly to do some things?”; “Do you often do things to be praised?”) and 24 SP (“Do you often refrain from doing something because you are afraid of it being illegal?”; “Is it difficult for you to telephone someone you do not know?”) “yes” or “no” items designed to assess BAS and BIS sensitivity, respectively. Both subscales have acceptable internal consistency, with α’s = .75–.83 (Torrubia et al., 2001). In Phase I of this study, α’s for the SR and SP scales were .76 and .84, respectively. Three-month retest reliabilities are .87 for the SR and .89 for the SP scale (Torrubia et al., 2001). Findings also support the construct validity of the SPSRQ in terms of expected correlations with extraversion, impulsivity, sensation seeking, and neuroticism, and associations with proneness to various personality disorders (e.g., Alloy et al., 2006b; Caseras, Torrubia & Farre, 2001; Torrubia et al., 2001). BAS-T and SR scores correlated r = .40 in our Phase I sample.
Impulsivity
At baseline (Time 1), participants completed the Barratt Impulsiveness Scale – Version 11 (Patton, Stanford, & Barratt, 1995). The Barratt is a widely used self-report questionnaire for assessing impulsivity that includes three factors: Attentional Impulsivity, Motor Impulsivity, and Nonplanning. The three factors intercorrelate significantly, with r’s = .46 – .53 and internal consistency in an undergraduate sample was good (α = .82). The Barratt predicts risk-taking behaviors in high school and college students (Stanford, Greve, Boudreaux, Mathias, & Brumbelow, 1996) and has been validated in bipolar samples (e.g., Swann et al., 2001). Internal consistency in this study was α = .79.
Symptom measures
During Phase II screening, participants also completed self-report measures of depressive and hypomanic/manic symptoms. The BDI (Beck et al., 1979) has 21 items assessing the severity of affective, cognitive, motivational, and somatic symptoms of depression. It has good internal (α’s = .81–.86) and retest reliability (r’s = .48–.86) and validity in nonclinical samples (Beck, Steer, & Garbin, 1988). In our Phase II sample, α = .87.
Initial symptoms of (hypo)mania at Phase II were assessed with the ASRM (Altman et al., 1997). The ASRM has 5 items rated on 5-point Likert scales that assess inflated self-confidence, talkativeness, elation, reduced need for sleep, and excessive activity. ASRM items load on a single factor and ASRM scores are highly correlated with both clinical interview and other self-report measures of mania (Altman, Hedeker, Peterson, & Davis, 2001). In our Phase II sample, α = .75.
Reward responsiveness and goal-setting measures
Participants completed measures of reward responsiveness and goal-setting at baseline (Time 1). The original Card Arranging Reward Responsivity Objective Test (CARROT; Al-Adawi, Powell, & Greenwood, 1998; Powell, Al-Adawi, Morgan, & Greenwood, 1996) is a 3-trial card-sorting task used frequently to measure reward responsiveness. It assesses the extent to which participants increase their speed on a psychomotor task when offered small financial incentives. Participants receive 60 cards, each with 5 digits on it, with one of the digits a 1, 2, or 3. Participants sort the cards into 3 correspondingly numbered trays. Trial 1 establishes baseline sorting speed. In Trial 2, the cards are reshuffled and participants are given 75% of their Trial 1 sorting time to resort them. Trial 3 is identical to Trial 2 except participants receive 25 cents for every 5 cards sorted (quarters are placed in view of the participant after every 5th card sorted). The dependent measure of reward responsiveness is the difference between the number of cards sorted in Trial 3 with reward incentives minus the number sorted in the non-rewarded Trial 2. The CARROT has been validated in normal, addicted, and bipolar disorder samples (e.g., Al-Adawi et al., 1998; Dawkins, Powell, West, Powell, & Pickering, 2006; Hayden et al., 2008; Kambouropoulos & Staiger, 2002; Powell et al., 1996) and correlates with BAS sensitivity on the SR scale (Kambouropoulos & Staiger, 2004), but not the BAS scale (Hayden et al., 2008). CARROT reward responsiveness is also associated with a polymorphism of the D2 dopamine receptor (DRD2) gene (White, Morris, Lawford, & Young, 2008).
The Willingly Approached Set of Statistically Unlikely Pursuits (WASSUP; Johnson & Carver, 2006) was used to measure the tendency toward overly ambitious goal-setting at Time 1. Participants rate the likelihood that they will set each of 30 goals for themselves, from 1 (“no chance I will set this goal for myself”) to 5 (“definitely WILL set this goal for myself”). Factor analyses of the WASSUP yielded 7 subscales tapping different types of aspirations: Popular Fame, Financial Success, Political Influence, idealized relations with Friends, idealized relations with Family, impact on World Well-being, and Fulfillment. Given that prior work (Carver & Johnson, 2009; Gruber & Johnson, 2009; Johnson & Carver, 2006; Johnson et al., 2009) found that it is the WASSUP Popular Fame (e.g., “you will have a major role in a movie”) and Financial Success (e.g., “you will have 20 million dollars or more”) subscales that distinguish manic individuals and individuals at risk for mania from various control groups, we used these two subscales in this study. Internal reliabilities for these two subscales are acceptable (α = .88 for Popular Fame and .73 for Financial Success; Johnson & Carver, 2006, Study 2), and, in the present sample, α’s = .90 and .75 for Popular Fame and Financial Success.
Phase II diagnostic interview
The SADS-L (Endicott & Spitzer, 1978) is a semi-structured diagnostic interview assessing current and lifetime history of Axis I disorders. The mood disorders and psychosis sections of an expanded SADS-L (exp-SADS-L) described in detail by Alloy et al. (2008) and Nusslock et al. (2007) was given during Phase II to determine eligibility for the final study sample, with the remainder administered at baseline (Time 1) to participants in the final sample. Expansions of the original SADS-L included: 1) additional probes to allow for DSM-IV-TR as well as RDC diagnoses; 2) more items and improvements in the probes in the Depression, Mania/Hypomania, and Cyclothymia sections; 3) additional probes to assess the precise number of days and % of waking hours on each day that participants felt depressed or euphoric/irritable in the Depression and Mania/Hypomania sections, respectively; 4) improvements of probes in the mood disorders sections based on Depue’s Behavioral Variability Interview (1985); 5) addition of items that assess frequency, duration, and switch rapidity of depressive and hypomanic periods in the Cyclothymia section; 6) additional probes to examine whether changes in participants’ behavior were observable by others; 7) use of 5-point scales (0–4) to make symptom ratings, with 3 as the cutoff for presence of the symptom; 8) sections about past disorders were placed immediately after the corresponding current sections to increase understanding; and 9) sections were added to assess eating disorders, ADHD, and acute stress disorder; additional probes were added in the anxiety disorders section; and an organic rule-out module, medical history, and family history sections were appended. The exp-SADS-L has demonstrated excellent inter-rater reliability, with Κ > .90 for unipolar depression diagnoses based on 80 jointly rated interviews (Alloy et al., 2000) and Κ > .96 for BSDs based on 105 jointly rated interviews (Alloy et al., 2008).
In Project TEAM, the exp-SADS-L (and exp-SADS-C described below) interviews were administered by Clinical Psychology postdoctoral fellows, Ph.D. and masters students, and post-BA psychology majors blinded to participants’ BAS risk group and Phase I BIS/BAS and SPSRQ scores. Interviewers completed an intensive training program conducted by a senior diagnostician with 20 years of experience, involving about 200 hours of reading, didactic instruction, watching videotaped interviews, role-playing, discussing case vignettes, observation of live interviews by experienced interviewers, and extensive practice conducting live interviews with supervision and feedback from the trainer and the principal investigators (PIs). For both the exp-SADS-L and exp-SADS-C, consensus DSM-IV-TR and RDC diagnoses were determined by a 3-tiered consensual standardized review procedure involving project interviewers, the senior trainer, and the PIs. To gain further specificity, diagnosis of cyclothymia was operationalized as fulfilling all DSM-IV-TR or RDC criteria plus: 1) at least two ≥ 2-day episodes of Hyp mood with at least two additional Hyp symptoms per year; 2) at least two ≥ 2-day episodes of depressed mood with at least two additional depressive symptoms per year; 3) presence of Hyp and depressed mood for at least 50% of the day during the respective mood episodes; and 4) presence of this pattern for at least two years if ≥ age 18 or one year if < age 18. A diagnosis of BiNOS was given if individuals exhibited: 1) diagnosable Hyp episodes without diagnosable MD or MiD episodes; 2) a cyclothymic pattern but with Hyp and depressive periods that did not meet minimum 2-day duration criteria for mood episodes in cyclothymia (i.e., they exhibited 1-day Hyp and depressive periods); and 3) Hyp and depressive periods too infrequent to qualify for cyclothymia (i.e., one Hyp and depressive period per year).
Information regarding age of onset was obtained from the exp-SADS-L or exp-SADS-C and was operationalized as the earliest age at which the participant met criteria for either a MD or Hyp or Ma episode (for those with bipolar I or II diagnoses), the earliest age at which the participant exhibited at least one depressive period and at least one Hyp period within a 1-year interval (for those with BiNOS or cyclothymia diagnoses), or the earliest age at which the participant met criteria for a Hyp episode. Information regarding family history of BSDs in first-degree relatives was also obtained from the exp-SADS-L using the family history method and the Family History – Research Diagnostic Criteria (FH-RDC; Andreason, Endicott, Spitzer, & Winokur, 1977). Inasmuch as family history of bipolar disorder is a known risk factor for BSD in first-degree relatives (McGuffin et al., 2003; Merikangas et al., 2002), we controlled for family history of BSDs in our analyses predicting first onset of BSDs.
Prospective diagnostic interview
Prospective onsets of mood episodes and diagnoses were assessed with an expanded SADS-Change (exp-SADS-C) interview (Spitzer & Endicott, 1978) described in detail in Alloy et al. (2008) and Nusslock et al. (2007). It was administered approximately every 6 months during the prospective follow-up by interviewers blinded to participants’ BAS risk group, Phase I BIS/BAS and SPSRQ scores, and Phase II diagnostic information and family history. The exp-SADS-C was expanded identically to the exp-SADS-L and features of the Longitudinal Interval Follow-up Evaluation (LIFE II; Shapiro & Keller, 1979) were added to track the course of symptoms and episodes during follow-up. However, the exp-SADS-C inquired about the presence of each symptom of depression and (hypo)mania more frequently (daily) than does the LIFE II (weekly) during the 6-month interval. Consensus diagnoses were made with the same 3-tiered diagnostic review procedure as for the exp-SADS-L. Inter-rater reliability for the exp-SADS-C in joint ratings of 60 interviews was good (Κ > .80; Alloy et al., 2008). Also, a validity study showed that participants dated symptoms on the exp-SADS-C with at least 70% accuracy compared to daily symptom ratings obtained prospectively over 4 months (Alloy et al., 2008). In the current study, inter-rater reliability for any BSD on the exp-SADS-L or exp-SADS-C interviews was Κ = 0.90 based on 100 jointly rated interviews. For Bipolar I, Κ = 1.0, for Bipolar II, Κ = 0.92, and for BiNOS, Κ = 0.88.
Procedure
Participants eligible for the final sample were invited for a baseline (Time 1) assessment and prospective study, and additional informed consent and assent were obtained at this time. At Time 1, they completed the remainder of the exp-SADS-L interview and the CARROT, WASSUP, and Barratt. Then they were followed approximately every 6 months with the exp-SADS-C. This study is based on an average of 12.8 months (SD = 9.7 months) of follow-up. Follow-up time did not differ by group (HBAS M = 12.12 mo., SD = 9.47 mo.; MBAS M = 13.50 mo., SD = 9.95 mo.); t(288) = 1.65, ns.
Results
Twenty-six participants had a first onset of BSD during the follow-up, with HBAS (n = 21; 12.3%) more likely than MBAS (n = 5; 4.2%) individuals to experience a first onset, Χ2 (1) = 9.846 p < .002. Of these 26 bipolar onset cases, 16 had Bipolar II (onset of at least one Hyp and one MD episode or onset of Hyp episode in a participant with a previous MD), 1 had Cyclothymia, and 9 had BiNOS (onset of at least one Hyp episode). Of the 9 BiNOS cases, 7 had an onset of at least one Hyp episode that fully met DSM-IV-TR or RDC criteria. The mean age of first onset of BSD was 18.70 years (SD = 2.09 years). Gender and race/ethnicity did not predict BSD onset; thus, these demographic characteristics were not included as covariates in the main hypothesis-testing analyses. Length of follow-up also was unrelated to demographic characteristics and BAS risk group status. Table 2 presents the correlations between the measures used in this study. BAS risk group correlated significantly with ambitious goal-setting (WASSUP), but not with CARROT reward responsiveness. The CARROT also did not correlate with the WASSUP.
Table 2.
Correlations among Study Variables
| Risk | BAS-T | SR | Barratt | CARROT | WASS P | WASS F | ASRM | BDI | ProBSD | |
|---|---|---|---|---|---|---|---|---|---|---|
| Risk | ----- | .872*** | .855*** | .214*** | −.076 | .210*** | .186** | .089 | .075 | .139* |
| BAS-T | ----- | .786*** | .230*** | −.068 | .187** | .166** | .052 | −.011 | .180** | |
| SR | ----- | .230*** | −.087 | .143* | .151* | .082 | .054 | .134* | ||
| Barratt | ----- | .054 | .012 | −.017 | .043 | .238*** | .049 | |||
| CARROT | ----- | −.088 | −.053 | .042 | −.048 | .158** | ||||
| WASS P | ----- | .600*** | .167** | .053 | .168** | |||||
| WASS F | ----- | .159** | .060 | .120* | ||||||
| ASRM | ----- | .009 | .124* | |||||||
| BDI | ----- | .090 | ||||||||
| ProBSD | ----- |
Note.
p < .05;
p < .01;
p < .001
Risk = BAS risk group; BAS-T = BAS-Total from BIS/BAS Scales; SR = Sensitivity to Reward scale from SPSRQ; Barratt = Barratt Impulsiveness Scale; CARROT = Card Arranging Reward Responsivity Objective Test; WASS P = Willingly Assumed Set of Statistically Unlikely Pursuits (WASSUP) Popular Fame subscale; WASS F = WASSUP Financial Success subscale; ASRM = Altman Self-Rating Mania Scale; BDI = Beck Depression Inventory; ProBSD = Prospective first onset of bipolar spectrum disorder.
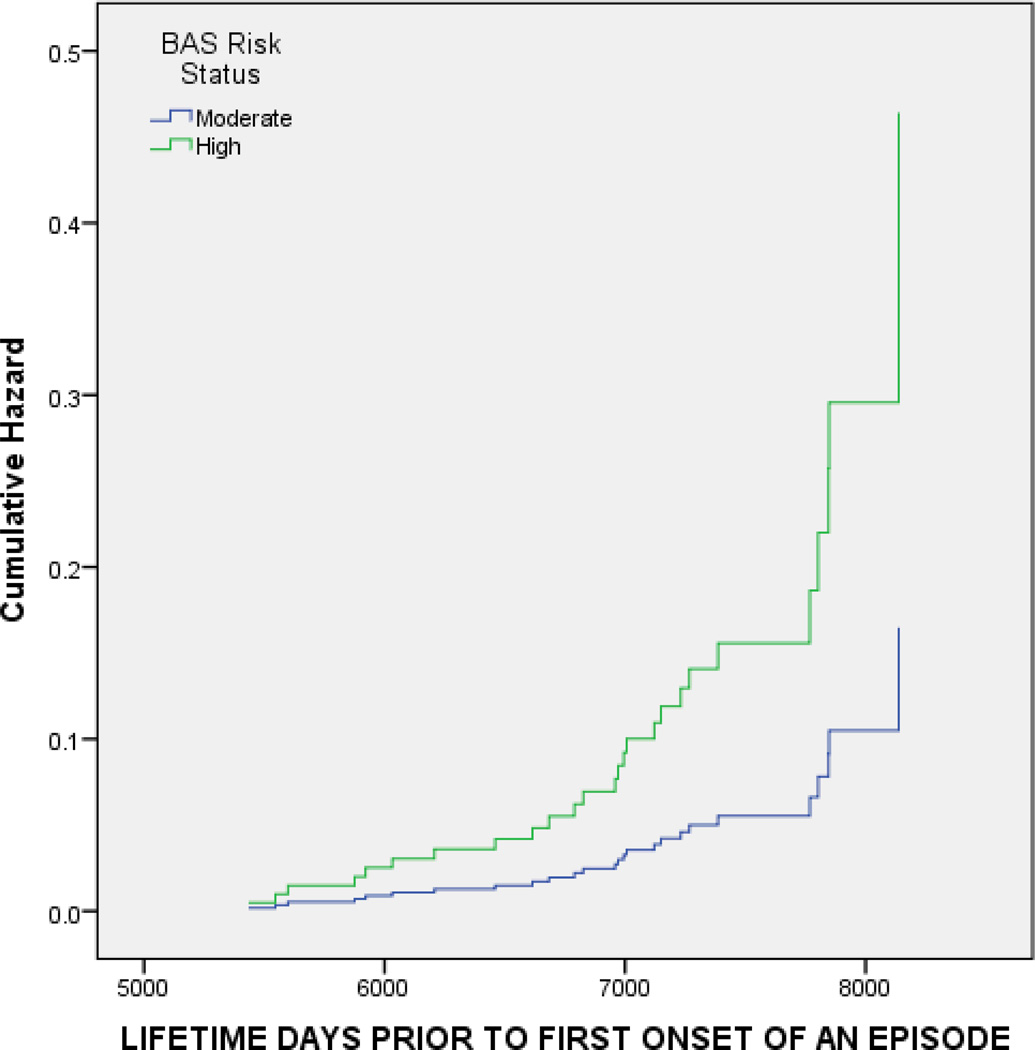
To test Hypothesis 1 that BAS risk group would predict first onset of BSD, we conducted Cox proportional hazard regression (survival) analysis with time (days since participants’ birth) to first onset of a BSD as the outcome. We used survival analysis because it allows for varying length of follow-up in longitudinal studies and, thus, minimizes bias due to attrition, takes into account participants’ ages, and accounts for censored, or missing, data (Cox, 1972; Willett & Singer, 1993). Initial (Phase II) depressive (BDI) and (hypo)manic (ASRM) symptoms and family history of BSD were entered in Step 1 to account for any effects of baseline symptoms and family history on the prospective occurrence of a BSD. BAS risk group was entered in Step 2. Of the 3 covariates included in the model, only ASRM scores (p = .023) significantly predicted onset of BSD. Participants with higher baseline (hypo)manic symptoms experienced a shorter time to develop a BSD. Controlling for these 3 covariates, BAS risk group also significantly predicted first onset of BSD (Wald = 4.240, p = .039, OR = 2.817, 95% CI = 1.051 – 7.547).2 HBAS participants had a significantly shorter time to develop a first onset of BSD than did MBAS participants. Table 3 displays the results of this analysis and Figure 1 displays the survival curves for BSD onset as a function of high vs. moderate BAS risk group.
Table 3.
Cox Proportional Hazard Regression Models Predicting Time to First Onset of Bipolar Spectrum Disorder Controlling for Initial Depressive (BDI) and Hypomanic/Manic (ASRM) Symptoms and Family History of Bipolar Disorder
| Predictor | Wald | p | OR | 95% CI |
|---|---|---|---|---|
| Step 1 | ||||
| BDI | 2.353 | .125 | 1.044 | 0.988 – 1.103 |
| ASRM | 5.408 | .020 | 1.122 | 1.018 – 1.237 |
| Fam Hx of BD | 0.251 | .616 | 1.381 | 0.391 – 4.879 |
| Step 2 | ||||
| BAS Risk Group | 4.240 | .039 | 2.817 | 1.051 – 7.547 |
| Step 3 | ||||
| CARROT | 5.347 | .021 | 1.061 | 1.009 – 1.116 |
| BAS Risk Group | 5.074 | .024 | 3.338 | 1.169 – 9.528 |
| (controlling for CARROT) | ||||
| Step 3 | ||||
| WASSUP Pop Fame | 10.302 | .001 | 1.096 | 1.036 – 1.159 |
| BAS Risk Group | 2.524 | .112 | 2.252 | 0.827 – 6.133 |
| (controlling for WASSUP Pop Fame) | ||||
| Step 3 | ||||
| WASSUP Fin Succ | 5.218 | .022 | 1.131 | 1.018 – 1.257 |
| BAS Risk Group | 2.932 | .087 | 2.417 | 0.880 – 6.635 |
| (controlling for WASSUP Fin Succ) | ||||
Note. BDI = Beck Depression Inventory; ASRM = Altman Self-Rating Mania Scale; Fam Hx of BD = Family history of bipolar disorder; BAS = Behavioral Approach System; CARROT = Card Arranging Reward Responsivity Objective Test; WASSUP – Willingly Assumed Set of Statistically Unlikely Pursuits; Pop Fame = Popular Fame subscale; Fin Succ = Financial Success subscale
Statistics are shown for each step of the model as the predictors on that step are entered.
Figure 1.
Time to first onset of bipolar spectrum disorder (in days since participants’ birth) as a function of Behavioral Approach System (BAS) risk group status.
To examine whether reward responsiveness (CARROT) and/or ambitious goal-striving (WASSUP) also predicted onset of BSD (Hypothesis 2), either CARROT, WASSUP Popular Fame, or WASSUP Financial Success scores were added on Step 3 of this survival analysis (see Table 3). Controlling for BDI, ASRM, family history, and BAS risk group, CARROT scores significantly predicted time to onset of BSD (Wald = 5.347, p = .021, OR = 1.061, 95% CI = 1.009 – 1.116), as did WASSUP Popular Fame (Wald = 10.302, p = .001, OR = 1.096, 95% CI = 1.036 – 1.159) and WASSUP Financial Success scores (Wald = 5.218, p = .022, OR = 1.131, 95% CI = 1.018 – 1.257).3 Greater reward responsiveness on the CARROT and more ambitious goal-striving on the WASSUP Popular Fame and Financial Success scales predicted shorter time to first onset of BSD over and beyond BAS risk group. As shown in Table 3, although BAS risk group remained predictive of onset of BSD with CARROT added to the survival analysis (Wald = 5.074, p = .024; OR = 3.338), BAS risk group was no longer a significant predictor with WASSUP Popular Fame (Wald = 2.524, p = .112, OR = 2.252) or Financial Success (Wald = 2.932, p = .087, OR = 2.417) added to the analysis. This indicates that WASSUP ambitious goal-striving partially mediated the association between BAS risk group and BSD onset, whereas CARROT reward responsiveness was not a mediator.
To examine whether trait impulsivity (Barratt) predicted first onset of BSD (Hypothesis 3), we conducted survival analyses with time to onset of BSD as the outcome and BDI, ASRM, and family history of BSD as covariates on Step 1. Barratt scores were entered on Step 2. Barratt scores did not predict time to onset of BSD (Wald = .020, p = .887, OR = 0.997, 95% CI = 0.955 – 1.041).
Discussion
This study employed a theoretically guided, prospective behavioral high-risk design to examine first onset of BSDs in adolescents with no prior history of bipolar disorder, but selected to be at high versus low risk for these disorders based on exhibiting high versus moderate BAS sensitivity. We also examined reward responsiveness and ambitious goal-setting, constructs related to bipolar disorder in prior studies and part of the nomological network of the BAS construct, as additional predictors of first onset of BSDs.
Consistent with the vulnerability hypothesis of the BAS model of bipolar disorders (Alloy & Abramson, 2010; Alloy et al., 2009a; Depue & Iacono, 1989; Depue et al., 2007; Urosevic et al., 2008), we found that high BAS sensitivity participants were more likely and exhibited a shorter time to develop a BSD across an average of 12.8 months of follow-up than were moderate BAS sensitivity participants, controlling for baseline mood symptoms and family history. Thus, any initial depressive or hypomanic symptoms associated with high BAS sensitivity status are unlikely to be plausible explanations for the BAS sensitivity risk effect. Moreover, controlling for family history of BSD in addition to baseline mood symptoms is a very conservative test of the BAS vulnerability hypothesis. This is because any variance in prospective BSD onset shared between BAS sensitivity and baseline mood symptoms and family history is allocated to the initial symptoms and family history. That high BAS sensitivity predicted onset of BSD in adolescents with no prior history of bipolar disorder and despite controlling for initial mood symptoms provides an important test of the BAS vulnerability hypothesis, because the test is truly prospective.
Our results extend prior work demonstrating that high BAS sensitivity is associated with greater likelihood of a past BSD (Alloy et al., 2006c), as well as prior studies demonstrating an association between high BAS sensitivity and prospective recurrences of mood episodes in samples with BSDs (Alloy et al., 2008, Meyer et al., 2001; Salavert et al., 2007) and progression to more severe diagnoses along the bipolar spectrum (Alloy et al., in press). As such, they are consistent with the hypothesis derived from the BAS model that BAS hypersensitivity provides vulnerability for both initial onset and a more severe course of BSD.
Our findings also demonstrated the specificity of the high BAS sensitivity – onset of BSD association. Our findings suggest that another personality trait that is also elevated in individuals with BSDs, impulsivity, does not increase vulnerability to first onset of BSD, as does BAS hypersensitivity. Given that impulsivity predicted conversion to bipolar I disorder (onset of full-blown mania) in a prior study (Alloy et al., in press), it may be that impulsivity is especially relevant to predicting risky, impairing behaviors seen in mania, but not initial onset of less severe bipolar spectrum conditions.
High reward responsiveness as assessed by the CARROT also predicted first onset of BSD above and beyond BAS risk group. Although prior research found that individuals with BSDs exhibit increased behavioral, cognitive, emotional, and neurobiological responses to rewards or their anticipation (Eisner et al., 2008; Harmon-Jones et al., 2008; Hayden et al., 2008; Johnson et al., 2005; Swann et al., 2009), the present findings indicate that high levels of reward responsiveness actually predict initial onset of BSDs and may be an additional vulnerability for these disorders. In addition, these findings provide a complement to the predictive effect of BAS risk group, which was based on two self-report measures of BAS sensitivity, with a behavioral task of responsiveness to financial incentives.
Finally, we also found that highly ambitious goal-striving for popular fame and financial success on the WASSUP predicted shorter time to first onset of BSD and partially mediated the effect of BAS risk group in predicting onset of BSD. This finding extends prior research showing that individuals with BSDs or vulnerable to mania based on exhibiting hypomanic personality set extremely high goals for themselves, especially with regard to achieving popular fame and financial success (Carver & Johnson, 2009; Gruber & Johnson, 2009; Johnson & Carver, 2006; Johnson et al., 2009), and provides further support for Johnson’s (2005) goal dysregulation model of bipolar disorder. Setting ambitious goals may be especially related to the grandiosity seen in hypomania/mania. The fact that ambitious goal-striving, but not behavioral reward responsiveness, partially mediated the effect of BAS sensitivity measured by the BIS/BAS and SPSRQ questionnaires in predicting first onset of BSD suggests that high self-reported BAS sensitivity may be more strongly related to pre-goal than post-goal attainment motivational states (Alloy et al., 2006c, Davidson, 1994). Consistent with this line of reasoning, some studies have found that the Drive and Fun Seeking subscales (assessing vigor in pursuing rewards and willingness to approach rewards, respectively) of the BIS/BAS are more strongly associated with hypomania and BSD than the Reward Responsiveness subscale (Carver & White, 1994; Meyer et al., 2001). Moreover, our findings indicate that the multi-method assessment of propensity to approach and respond strongly to incentives may be useful in predicting risk for bipolar disorders.
Our findings have important clinical implications. They suggest that it may be possible to identify adolescents at risk for initial onset of BSD based on various measures of BAS sensitivity, goal-striving, and reward responsiveness before onset occurs. It then may be possible to intervene early with these at-risk adolescents to prevent onset of bipolar disorder, or if that is not possible, to at least lessen the severity of the disorder’s course.
Study Strengths and Limitations
A major strength of the present study is the truly prospective design with a theory-based assessment of vulnerability to BSDs. Additional strengths include examination of specificity of the BAS hypersensitivity – BSD onset association, standardized diagnostic interviews, interviewers who were blinded to BAS risk status and the other predictors, frequent assessment intervals providing sufficient sensitivity for assessing mood symptoms, behavioral as well as self-report measures of vulnerability, inclusion of a large, ethnically diverse, community sample, increasing generalizability of study findings, and highly conservative statistical tests of the study hypotheses. Moreover, this is the first study of first onset of BSDs based on the BAS model.
However, it is important to acknowledge this study’s limitations as well. Although our sample was ethnically diverse and representative of the larger adolescent community population from which it was drawn on demographics, our results may not generalize to clinical adolescent samples or to other community samples. Second, family history of BSD was assessed via the family history method rather than the more accurate family study method involving direct interviews with relatives. Third, although we included the CARROT behavioral measure of reward responsiveness, most of our vulnerability measures were self-report questionnaires. These self-report questionnaires are reliable and valid assessments of BAS sensitivity and goal-striving; however, future studies may benefit from additional behavioral and neurobiological (e.g., EEG, imaging) assessments of BAS sensitivity and activation levels. Fourth, the self-report measures of BAS sensitivity (BAS, SR) used here most likely assess lability or dysregulation of the BAS in the upward direction (activation) better than BAS lability or dysregulation in the downward (deactivation) direction. Further instrument development is needed to more powerfully test the BAS hypersensitivity model of BSD. Finally, this study examined the propensity of the BAS to become dysregulated (overactivated) as a vulnerability to first onset of BSD, but not BAS dysregulation itself. Future research will need to investigate the nature of BAS dysregulation processes themselves as vulnerabilities for or mechanisms involved in bipolar disorders.
Conclusion
In summary, this is the first prospective study of first onset of bipolar spectrum disorders in adolescents with no prior history of bipolar disorder selected to be at risk on the basis of high BAS sensitivity. Our findings support the vulnerability hypothesis of the BAS model and indicate that high BAS sensitivity, ambitious goal-striving, and high reward responsiveness are vulnerabilities for first onset of bipolar spectrum disorders.
Acknowledgements
This research was supported by National Institute of Mental health Grant MH 77908 to Lauren B. Alloy.
Footnotes
Our target was to obtain 200 HBAS and 150 MBAS participants. Some Phase I eligible participants refused participation in Phase II, many agreed to participate in Phase II but then cancelled or no-showed for their appointments 4–5 times (at which point, we stopped trying to reschedule them), and we were unable to contact some participants despite repeated attempts.
BAS risk group still predicted time to first onset of BSD significantly controlling for BDI scores, ASRM scores, and family history of BSD, even with the exclusion of the 2 BiNOS cases who had an onset of a Hyp episode that did not meet either DSM-IV-TR or RDC criteria (Wald = 3.953, p == .047, OR = .2.748, 95% CI = 1.014 – 7.443).
WASSUP total scores also predicted time to first onset of BSD, controlling for depressive and hypomanic symptoms and family history of bipolar disorder (Wald = 5.646, p = .017, OR = 1.029, 95% CI = 1.005 – 1.054), although they did not predict as well as either WASSUP Popular Fame or Financial Success subscales.
Contributor Information
Lauren B. Alloy, Temple University
Rachel E. Bender, Temple University
Wayne G. Whitehouse, Temple University
Clara A. Wagner, Temple University
Richard T. Liu, Temple University
David A. Grant, Temple University
Shari Jager-Hyman, Temple University.
Ashleigh Molz, Temple University.
James Y. Choi, Temple University
Eddie Harmon-Jones, Texas A & M University.
Lyn Y. Abramson, University of Wisconsin-Madison
References
- Akiskal HS, Bourgeois ML, Angst J, Post R, Moller H, Hirschfeld R. Re-evaluation the prevalence of and diagnostic composition within the broad clinical spectrum of bipolar disorders. Journal of Affective Disorders. 2000;59(Suppl 1):S5–S30. doi: 10.1016/s0165-0327(00)00203-2. [DOI] [PubMed] [Google Scholar]
- Akiskal HS, Djenderedjian AH, Rosenthal RH, Khani MK. Cyclothymic disorder: Validating criteria for inclusion in the bipolar affective group. American Journal of Psychiatry. 1977;134:1227–1233. doi: 10.1176/ajp.134.11.1227. [DOI] [PubMed] [Google Scholar]
- Al-Adawi S, Powell JH. The influence of smoking on reward responsiveness and cognitive functions: A natural experiment. Addiction. 1997;92:1773–1782. [PubMed] [Google Scholar]
- Al-Adawi S, Powell JH, Greenwood RJ. Motivational deficits after brain injury: A neuropsychological approach using new assessment techniques. Neuropsychology. 1998;12:115–124. doi: 10.1037//0894-4105.12.1.115. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY. The role of the Behavioral Approach System (BAS) in Bipolar spectrum disorders. Current Directions in Psychological Science. 2010;19:189–194. doi: 10.1177/0963721410370292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Hogan ME, Whitehouse WG, Rose DT, Robinson MS, et al. The Temple – Wisconsin Cognitive Vulnerability to Depression (CVD) Project: Lifetime history of Axis I psychopathology in individuals at high and low cognitive risk for depression. Journal of Abnormal Psychology. 2000;109:403–418. [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Urosevic S, Bender RE, Wagner CA. Longitudinal predictors of bipolar spectrum disorders: A Behavioral Approach System (BAS) perspective. Clinical Psychology: Science and Practice. 2009a;16:206–226. doi: 10.1111/j.1468-2850.2009.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, Hughes ME, et al. Behavioral Approach System and Behavioral Inhibition System sensitivities: Prospective prediction of bipolar mood episodes. Bipolar Disorders. 2008;10:310–322. doi: 10.1111/j.1399-5618.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Smith J, Hughes M, et al. Behavioral Approach System (BAS) sensitivity and bipolar spectrum disorders: A retrospective and concurrent behavioral high-risk design. Motivation and Emotion. 2006c;30:143–155. [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Gerstein RK, Keyser JD, Whitehouse WG, et al. Behavioral Approach System (BAS) – relevant cognitive styles and bipolar spectrum disorders: Concurrent and prospective associations. Journal of Abnormal Psychology. 2009b;118:459–471. doi: 10.1037/a0016604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Keyser J, Gerstein RK. A cognitive vulnerability-stress perspective on bipolar spectrum disroders in a normative adolescent brain, cognitive, and emotional development context. Development and Psychopathology. 2006a;18:1055–1103. doi: 10.1017/S0954579406060524. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Whitehouse WG, Hogan ME, Panzarella C, Rose DT. Prospective incidence of first onsets and recurrences of depression in individuals at high and low cognitive risk for depression. Journal of Abnormal Psychology. 2006b;115:145–156. doi: 10.1037/0021-843X.115.1.145. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Urosevic S, Abramson LY, Jager-Hyman S, Nusslock R, Whitehouse WG, Hogan ME. Progression along the bipolar spectrum: A longitudinal study of predictors of conversion from bipolar spectrum conditions to bipolar I and II disorders. Journal of Abnormal Psychology. doi: 10.1037/a0023973. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman EG, Hedeker D, Peterson JL, Davis JM. The Altman self-rating mania scale. Biological Psychiatry. 1997;42:948–955. doi: 10.1016/S0006-3223(96)00548-3. [DOI] [PubMed] [Google Scholar]
- Altman EG, Hedeker D, Peterson JL, Davis JM. A comparative evaluation of three self-rating scales for acute mania. Biological Psychiatry. 2001;50:468–471. doi: 10.1016/s0006-3223(01)01065-4. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual. 4th Edition. Washington, D.C: Author; 2000. Text Revision. [Google Scholar]
- Andreason N, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria: Reliability and validity. Archives of General Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: Follow-up over 34–38 years. Journal of Affective Disorders. 2002;68:167–181. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- Angst J, Cui L, Swendsen J, Rothen S, Cravchik A, Kessler RC, et al. Major depressive disorder with subthreshold bipolarity in the National Comorbidity Survey Replication. American Journal of Psychiatry. 2010;167:1194–1201. doi: 10.1176/appi.ajp.2010.09071011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst J, Gamma A, Benazzi F, Ajdacic V, Eich D, Rossler W. Toward a redefinition of subthreshold bipolarity: Epidemiology and proposed criteria for bipolar-II, minor bipolar disorders and hypomania. Journal of Affective Disorders. 2003;73:133–146. doi: 10.1016/s0165-0327(02)00322-1. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guilford Press; 1979. [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Beesdo K, Hofler M, Leibenluft E, Lieb R, Bauer M, Pfenning A. Mood episodes and mood disorders: Patterns of incidence and conversion in the three decades of life. Bipolar Disorders. 2009;11:637–649. doi: 10.1111/j.1399-5618.2009.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellivier F, Golmard J, Henry C, Leboyer M, Schurhoff F. Admixture analysis of age at onset in bipolar I affective disorder. Archives of General Psychiatry. 2001;58:510–512. doi: 10.1001/archpsyc.58.5.510. [DOI] [PubMed] [Google Scholar]
- Bellivier F, Golmard J, Rietschel M, Schulze TG, Malafosse A, Preisig M, et al. Age of onset in bipolar I affective disorder: Further evidence for three subgroups. American Journal of Psychiatry. 2003;160:999–1001. doi: 10.1176/appi.ajp.160.5.999. [DOI] [PubMed] [Google Scholar]
- Benazzi F. Testing new diagnostic criteria for hypomania. Annals of Clinical Psychiatry. 2007;19:99–104. doi: 10.1080/10401230701338219. [DOI] [PubMed] [Google Scholar]
- Bermpohl F, Kahnt T, Dalanay U, Hagele C, Sajonz B, Wegner T, et al. Altered representation of expected value in the orbitofrontal cortex in mania. Human Brain Mapping. 2010;31:958–969. doi: 10.1002/hbm.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Goldstein B, Strober M, Gill MK, Hunt J, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: The Course and Outcome of Bipolar Youth (COBY) study. American Journal of Psychiatry. 2009;166:795–804. doi: 10.1176/appi.ajp.2009.08101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke KC, Burke JD, Regier DA, Rae DS. Age at onset of selected mental disorders in five community populations. Archives of General Psychiatry. 1990;47:511–518. doi: 10.1001/archpsyc.1990.01810180011002. [DOI] [PubMed] [Google Scholar]
- Carver CS. Negative affect deriving from the Behavioral Approach System. Emotion. 2004;4:3–22. doi: 10.1037/1528-3542.4.1.3. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL. Tendencies toward mania and tendencies toward depression have distinct motivational, affective, and cognitive correlates. Cognitive Therapy and Research. 2009;33:552–569. doi: 10.1007/s10608-008-9213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Cassano GB, Akiskal HS, Savino M, Musetti L, Perugi G. Proposed subtypes of bipolar II and related disorders: with hypomanic episodes (or cyclothymia) and with hyperthymic temperament. Journal of Affective Disorders. 1992;26:127–140. doi: 10.1016/0165-0327(92)90044-7. [DOI] [PubMed] [Google Scholar]
- Cassano GB, Dell’Osso L, Frank E, Miniati M, Fagiolini A, Shear K, Pini S, Maser J. The bipolar spectrum: A clinical reality in search of diagnostic criteria and an assessment methodology. Journal of Affective Disorders. 1999;54:319–328. doi: 10.1016/s0165-0327(98)00158-x. [DOI] [PubMed] [Google Scholar]
- Colder CR, O'Connor RM. Gray’s reinforcement sensitivity model and child psychopathology: Laboratory and questionnaire assessment of the BAS and BIS. Journal of Abnormal Child Psychology. 2004;32:435–451. doi: 10.1023/b:jacp.0000030296.54122.b6. [DOI] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: Results from the National Epidemiologic Survey on Alcohol and related conditions. Journal of Clinical Psychiatry. 2006;67:247–257. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life tables. Journal of the Royal Statistical Society. 1972;34:187–220. [Google Scholar]
- Davidson RJ. Asymmetric brain function, affective style, and psychopathology: The role of early experience and plasticity. Development and Psychopathology. 1994;6:741–758. [Google Scholar]
- Dawe S, Gullo MJ, Loxton NJ. Reward drive and rash impulsiveness as dimensions of impulsivity: Implications for substance misuse. Addictive Behaviors. 2004;29:1389–1405. doi: 10.1016/j.addbeh.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo controlled experimental study of nicotine: I-effects on incentive motivation. Psychopharmacology. 2006;189:355–367. doi: 10.1007/s00213-006-0588-8. [DOI] [PubMed] [Google Scholar]
- Depue RA. Behavioral Variability Interview. Minneapolis, MN: University of Minnesota; 1985. [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22:491–517. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Depue RA, Iacono WG. Neurobehavioral aspects of affective disorders. Annual Reviews in Psychology. 1989;40:457–492. doi: 10.1146/annurev.ps.40.020189.002325. [DOI] [PubMed] [Google Scholar]
- Depue RA, Krauss S, Spoont MR. A two-dimensional threshold model of seasonal bipolar affective disorder. In: Magnusson D, Ohman A, editors. Psychopathology: An interactional perspective. New York: Academic Press; 1987. pp. 95–123. [Google Scholar]
- Eisner L, Johnson SL, Carver CS. Cognitive responses to failure and success relate uniquely to bipolar depression versus mania. Journal of Abnormal Psychology. 2008;117:154–163. doi: 10.1037/0021-843X.117.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL. A diagnostic interview: The schedule for Affective Disorders and Schizophrenia. Archives of General Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- Fowles DC. Biological variables in psychopathology: A psychobiological perspective. In: Sutker PB, Adams HE, editors. Comprehensive handbook of psychopathology. 2nd Edition. New York: Plenum Press; 1993. pp. 57–82. [Google Scholar]
- Fulford D, Johnson SL, Llabre MM, Carver CS. Pushing and coasting in dynamic goal pursuit: Coasting is attenuated in bipolar disorder. Psychological Science. 2010;21:1021–1027. doi: 10.1177/0956797610373372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. Manic-depressive illness. 2nd edition. New York: Oxford University Press; 2007. [Google Scholar]
- Gray JA. Three fundamental emotion systems. In: Eckman P, Davidson RJ, editors. The nature of emotion: Fundamental questions. New York: Oxford University Press; 1994. pp. 243–247. [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety. Oxford, England: Oxford University Press; 2000. [Google Scholar]
- Gruber J, Johnson SL. Positive emotional traits and ambitious goals among people at risk for mania: The need for specificity. International Journal of Cognitive Therapy. 2009;2:176–187. doi: 10.1521/ijct.2009.2.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, Abramson LY, Nusslock R, Sigelman JD, Urosevic S, Turonie LD, et al. Effect of bipolar disorder on left frontal cortical responses to goals differing in valence and task difficulty. Biological Psychiatry. 2008;63:693–698. doi: 10.1016/j.biopsych.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJB. Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology. 1997;106:159–163. doi: 10.1037//0021-843x.106.1.159. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJB. Anger and prefrontal brain activity: EEG asymmetry consistent with approach motivation despite negative affect valence. Journal of Personality and Social Psychology. 1998;74:1310–1316. doi: 10.1037//0022-3514.74.5.1310. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Sigelman JD. State anger and prefrontal brain activity: Evidence that insult-related relative left prefrontal activity is associated with experienced anger and aggression. Journal of Personality and Social Psychology. 2001;80:797–803. [PubMed] [Google Scholar]
- Hayden EP, Bodkins M, Brenner C, Shekhar A, Nurnberger JI, O’Donnell BF, et al. A multimethod investigation of the Behavioral Activation System in bipolar disorder. Journal of Abnormal Psychology. 2008;117:164–170. doi: 10.1037/0021-843X.117.1.164. [DOI] [PubMed] [Google Scholar]
- Hillegers MHJ, Reichart CG, Wals M, Verhulst FC, Ormel J, Nolen WA. Five-year prospective outcome of psychopathology in the adolescent offspring of bipolar parents. Bipolar Disorders. 2005;7:344–350. doi: 10.1111/j.1399-5618.2005.00215.x. [DOI] [PubMed] [Google Scholar]
- Johnson SL. Mania and dysregulation in goal pursuit: A review. Clinical Psychology Review. 2005;25:241–262. doi: 10.1016/j.cpr.2004.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Carver CS. Extreme goal setting and vulnerability to mania among undiagnosed young adults. Cognitive Therapy and Research. 2006;30:377–395. doi: 10.1007/s10608-006-9044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Cueller AK, Ruggero C, Winett-Perlman C, Goodnick P, White R, et al. Life events as predictors of mania and depression in bipolar I disorder. Journal of Abnormal Psychology. 2008;117:268–277. doi: 10.1037/0021-843X.117.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Eisner LR, Carver CS. Elevated expectancies among persons diagnosed with bipolar disorder. British Journal of Clinical Psychology. 2009;48:217–222. doi: 10.1348/014466509X414655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Ruggero C, Carver CS. Cognitive, behavioral and affective responses to reward: Links with hypomanic vulnerability. Journal of Social and Clinical Psychology. 2005;24:894–906. [Google Scholar]
- Johnson SL, Sandrow D, Meyer B, Winters R, Miller I, Solomon D, et al. Increases in manic symptoms after life events involving goal attainment. Journal of Abnormal Psychology. 2000;109:721–727. doi: 10.1037//0021-843x.109.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd LL, Schettler PJ, Solomon DA, Maser JD, Coryell W, Endicott J, Akiskal HS. Psychosocial disability and work role function compared across the long-term course of bipolar I, bipolar II, and unipolar major depressive disorders. Journal of Affective Disorders. 2008;108:49–58. doi: 10.1016/j.jad.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Kambouropoulos N, Staiger PK. The influence of sensitivity to reward on reactivity to alcohol-related cues. Addiction. 2002;96:1175–1185. doi: 10.1046/j.1360-0443.2001.968117510.x. [DOI] [PubMed] [Google Scholar]
- Kambouropoulos N, Staiger PK. Reactivity to alcohol-related cues: Relationship among cue type, motivational processes, and personality. Psychology of Addictive Behaviors. 2004;18:275–283. doi: 10.1037/0893-164X.18.3.275. [DOI] [PubMed] [Google Scholar]
- Kano K, Nakamura M, Matsuoka T, Iida H, Nakajima T. The topographical features of EEGs in patients with affective disorders. Electroencephalography and Clinical Neurophysiology. 1992;83:124–129. doi: 10.1016/0013-4694(92)90025-d. [DOI] [PubMed] [Google Scholar]
- Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. Journal of Abnormal Psychology. 2002;111:589–597. doi: 10.1037//0021-843x.111.4.589. [DOI] [PubMed] [Google Scholar]
- Kennedy N, Boydell J, Kalidindi S, Fearon P, Jones PB, van Os J, et al. Gender differences in incidence and age at onset of mania and bipolar disorder over a 35-year period in Camberwell, England. American Journal of Psychiatry. 2005;162:257–262. doi: 10.1176/appi.ajp.162.2.257. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Rubinow DR, Holmes C, Abelson JM, Zhao S. The epidemiology of DSM-III-R bipolar I disorder in a general population survey. Psychological Medicine. 1997;27:1079–1089. doi: 10.1017/s0033291797005333. [DOI] [PubMed] [Google Scholar]
- Kochman FJ, Hantouche EG, Ferrari P, Lancrenon S, Bayart D, Akiskal HS. Cyclothymic temperament as a prospective predictor of bipolarity and suicidality in children and adolescents with major depressive disorder. Journal of Affective Disorders. 2005;85:181–189. doi: 10.1016/j.jad.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Kwapil TR, Miller MB, Zinser MC, Chapman LJ, Chapman J, Eckblad M. A longitudinal study of high scorers on the Hypomanic Personality Scale. Journal of Abnormal Psychology. 2000;109:222–226. [PubMed] [Google Scholar]
- Lam D, Wright K, Smith N. Dysfunctional assumptions in bipolar disorder. Journal of Affective Disorders. 2004;79:193–199. doi: 10.1016/S0165-0327(02)00462-7. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Archives of General Psychiatry. 2003;60:497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RMA, Petukhova M, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Chakravarti A, Moldin SO, Araj H, Blangero JC, Burmeister M, et al. Future of genetics of mood disorders research. Biological Psychiatry. 2002;52:457–477. doi: 10.1016/s0006-3223(02)01471-3. [DOI] [PubMed] [Google Scholar]
- Meyer B, Johnson SL, Carver CS. Exploring behavioral activation and inhibition sensitivities among college students at risk for bipolar spectrum symptomatology. Journal of Psychopathology and Behavioral Assessment. 1999;21:275–292. doi: 10.1023/A:1022119414440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B, Johnson SL, Winters R. Responsiveness to threat and incentive in bipolar disorder: Relations of the BIS/BAS scales with symptoms. Journal of Psychopathology and Behavioral Assessment. 2001;23:133–143. doi: 10.1023/A:1010929402770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Abramson LY, Harmon-Jones E, Alloy LB, Hogan ME. A goal-striving life event and the onset of bipolar episodes: Perspective from the Behavioral Approach System (BAS) dysregulation theory. Journal of Abnormal Psychology. 2007;116:105–115. doi: 10.1037/0021-843X.116.1.105. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Alloy LB, Abramson LY, Harmon-Jones E, Hogan ME. Impairment in the achievement domain in bipolar spectrum disorders: Role of Behavioral Approach System (BAS) hypersensitivity and impulsivity. Minerva Pediatrica. 2008;60:41–50. [PubMed] [Google Scholar]
- Nusslock R, Almeida JRC, Forbes EE, Versace A, LaBarbara EJ, Klein CR, Phillips ML. Waiting to win: Elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar adults. 2011 doi: 10.1111/j.1399-5618.2012.01012.x. Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oedegarrd KJ, Syrstad VEG, Morken G, Akiskal HS, Fasmer OB. A study of age at onset of affective temperaments in a Norwegian sample of patients with mood disorders. Journal of Affective Disorders. 2009;118:229–233. doi: 10.1016/j.jad.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Powell JH, Al-Adawi S, Morgan J, Greenwood RJ. Motivational deficits after brain injury: Effects of bromocriptine in 11 patients. Journal of Neurology, Neurosurgery, and Psychiatry. 1996;60:416–421. doi: 10.1136/jnnp.60.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salavert J, Caseras X, Torrubia R, Furest S, Arranz B, Duenas R, et al. The functioning of the Behavioral Activation and Inhibition Systems in bipolar I euthymic patients and its influence in subsequent episodes over an 18-month period. Personality and Individual Differences. 2007;42:1323–1331. [Google Scholar]
- Scott J, Stanton B, Garland A, Ferrier IN. Cognitive vulnerability in patients with bipolar disorder. Psychological Medicine. 2000;30:467–472. doi: 10.1017/s0033291799008879. [DOI] [PubMed] [Google Scholar]
- Shapiro RW, Keller MB. Longitudinal Interval Follow-up Evaluation (LIFE) Boston, MA: Massachusetts General Hospital; 1979. [Google Scholar]
- Spitzer RL, Endicott J. Schedule for Affective Disorders and Schizophrenia – Change Version. Biometrics Research, New York State Psychiatric Institute; 1978. [Google Scholar]
- Spitzer RL, Endicott J, Robins E. Research diagnostic criteria (RDC) for a selected group of functional disorders. 3rd ed. New York: New York Psychiatric Institute; 1978. [Google Scholar]
- Stanford MS, Greve KW, Boudreauz JK, Mathias CW, Brumbelow JL. Impulsiveness and risk-taking behavior: Comparison of high-school and college students using the Barratt Impulsiveness Scale. Personality and Individual Differences. 1996;21:1073–1075. [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science. 1997;8:204–210. [Google Scholar]
- Swann AC, Anderson JC, Dougherty DM, Moeller FG. Measurement of inter-episode impulsivity in bipolar disorder. Psychiatry Research. 2001;101:95–97. doi: 10.1016/s0165-1781(00)00249-3. [DOI] [PubMed] [Google Scholar]
- Swann AC, Dougherty DM, Pazzaglia P, Pham M, Moeller FG. Impulsivity: A link between bipolar disorder and substance abuse. Bipolar Disorders. 2004;6:204–212. doi: 10.1111/j.1399-5618.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Severity of bipolar disorder is associated with impairment of response inhibition. Journal of Affective Disorders. 2009;116:30–36. doi: 10.1016/j.jad.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrubia R, Avila C, Molto J, Caseras X. The Sensitivity to Punishment and Sensitivty to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Personality and Individual Differences. 2001;31:837–862. [Google Scholar]
- Urošević S, Abramson LY, Harmon-Jones E, Alloy LB. Dysregulation of the behavioral approach system (BAS) in bipolar spectrum disorders: Review of theory and evidence. Clinical Psychology Review. 2008;28:1188–1205. doi: 10.1016/j.cpr.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Gucht E, Morriss R, Lancaster G, Kinderman P, Bentall RP. Psychological processes in bipolar affective disorder: Negative cognitive style and reward processing. British Journal of Psychiatry. 2009;194:146–151. doi: 10.1192/bjp.bp.107.047894. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–299. [PubMed] [Google Scholar]
- White MJ, Morris CP, Lawford BR, Young RM. Behavioral phenotypes of impulsivity related to the ANKKI gene are independent of an acute stressor. Behavioral and Brian Functions. 2008;4:1–9. doi: 10.1186/1744-9081-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett JB, Singer JD. Investigating onset, cessation, relapse, and recovery: Why and how you can use discrete-time survival analysis to examine event occurrence. Journal of Consulting and Clinical Psychology. 1993;61:952–965. doi: 10.1037//0022-006x.61.6.952. [DOI] [PubMed] [Google Scholar]
- Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: Validity, phenomenology, and recommendations for diagnosis. Bipolar Disorders. 2008;10:194–214. doi: 10.1111/j.1399-5618.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman P, Bruckl t, Nocon A, Pfister H, Lieb R, Wittchen HU, et al. Heterogeneity of DSM-IV major depressive disorder as a consequence of subthreshold bipolarity. Archives of General Psychiatry. 2009;66:1341–1352. doi: 10.1001/archgenpsychiatry.2009.158. [DOI] [PubMed] [Google Scholar]
- Zinbarg RE, Mohlman J. Individual differences in the acquisition of affectively valenced associations. Journal of Personality and Social Psychology. 1998;74:1024–1040. doi: 10.1037//0022-3514.74.4.1024. [DOI] [PubMed] [Google Scholar]