Abstract
BACKGROUND
Many observational studies indicate higher oral contraceptive failure among obese women, but most clinical trials and physiological studies do not support these differences. Limited data indicate higher failure rates among obese contraceptive patch users. Data regarding contraceptive vaginal ring (CVR) performance in obese women are needed.
METHODS
20 normal weight (BMI 19.0–24.9, median 21.65) and 20 obese (BMI 30.0–39.9, median 33.7) women enrolled in a prospective study of ethinyl estradiol (EE) and etonorgestrel (ENG) pharmacokinetics and of ovarian follicle development, endometrial thickness, and bleeding patterns, all measured biweekly during the second cycle of CVR use.
RESULTS
Thirty-seven women completed follow-up. Mean day 0–21 EE concentrations were lower among obese versus normal weight women (15.0 versus 22.0 pg/mL, respectively. p = 0.004), while ENG concentrations were similar (1138 versus 1256 pg/mL, respectively. p = 0.39). Follicular development was minimal in both groups, with only five women achieving a maximum follicle diameter > 13mm at any time during 3 weeks follow-up (3 normal weight and 2 obese women); these women had serum progesterone levels < 1.0. Obese women reported more bleeding or spotting than normal weight women (3.6 versus 1.4 days, respectively. p = 0.01).
CONCLUSIONS
While obese women had lower EE levels during CVR use, they had excellent suppression of ovarian follicle development, similar to normal weight women. This predicts that CVR effectiveness will be similar in women with a BMI up to 39.9. The lower serum EE levels in the obese women may explain the greater reported bleeding or spotting days.
Keywords: contraceptive vaginal ring, pharmacokinetics, pharmacodynamics, obesity
INTRODUCTION
Several observational studies have reported that heavier women had higher failure rates during use of combination oral contraceptives (OC)1–2, and one clinical trial identified higher failure rates for heavier women using the contraceptive patch3. In contrast, several more recent prospective studies found little or no effect of weight on OC failure4–6. Only one analysis has evaluated the relationship between contraceptive vaginal ring (CVR) failure rates and weight; that study found no effect of weight on CVR failure rates, but it included too few obese women to provide a precise answer7. A recent physiological study of compliant OC users identified some differences in the pharmacokinetics of the contraceptive hormones between the normal weight and obese women8; however, normal weight and obese women experienced no differences in ovarian suppression or ovulation during OC use9. The goal of the present study was to compare pharmacokinetics and ovarian suppression during CVR use in normal weight and obese women.
MATERIALS AND METHODS
This clinical trial compared ovarian follicle development and serum ethinyl estradiol and etonogestrel levels between normal weight and obese women using the CVR (daily release of 15 mcg ethinyl estradiol (EE2) and 120 mcg etonogestrel (ENG)). Participant related activities were conducted between July and December 2008. The Columbia University Institutional Review Board approved the study, and all participants gave informed consent. We recruited participants from a cohort of women who had participated in a similar previous study of oral contraceptives several months prior to enrollment in this study. The women were initially recruited via advertisements online, in newspapers, and fliers9.
Eligible women were aged 18–35 years with a recent history of regular, spontaneous menstrual cycles, were willing to use the CVR and commit to at least eight bi-weekly study visits during the second ring cycle. We excluded women with any medical contraindications to the use of combined hormonal contraception based on the WHO Medical Eligibility Criteria and only enrolled women in WHO Category 110. Women using medications known to affect the CYPp450 system were ineligible. All participants had normal-appearing ovaries on a baseline sonogram using a TITAN (SonoSite, Inc., Bothell, WA) with a 7.5 MHz transvaginal probe, and were either normal weight [body mass index (BMI; kg/m2) 19.0–24.9] or obese (BMI 30.0–39.9) based on standardized height and body weight measurements on the day of enrollment. We measured body weight (kg) using the BC-418 (Tanita Corp., Tokyo, Japan), body composition analyzer and used the same machine for all measurements.
Participants received two CVRs and completed one ring cycle (21 days with continuous ring use, followed by seven ring-free days) prior to the planned study cycle. They inserted the second ring on day 1 of the study cycle. Participants then underwent bi-weekly visits for venipuncture to obtain samples to measure ethinyl estradiol (EE2) and etonogestrel (ENG); we collected these samples at approximately days 3, 6, 9, 12, 15, 18, and 21 +/− one day. Specimens were allowed to clot for at least 10 minutes at room temperature, and then separated at 3400 RPM by centrifuge. The serum was transferred to clean tubes and stored in aliquots at −80°C until analysis. For this study the Biomarkers Core Laboratory of the Irving Institute of Clinical and Translational Research at Columbia University Medical Center developed ENG and EE2 assays using the tandem mass spectrometry11. In short, ENG and EE2 were measured in serum by UPLC/MS/MS after liquid/liquid extraction using D8-Progesterone or D4-EE2 as the internal standards (I.S.) for ENG and EE2 respectively. EE2 was derivatized with dansyl chloride prior to analysis. The steroids were quantified by positive electrospray ionization in multiple reaction monitoring mode using the Waters Xevo TQ-S system (Waters, Milford, MA). The method was linear between 50 to 2000 pg/mL and 1 to 100 pg/ml for ENG and EE2 respectively (LOQ: 50pg/ml and 1pg/ml). The intra- and inter-assay coefficient of variation were <6% and <13% respectively for ENG; <3.9% and <4.4% respectively for EE2.
Participants also underwent biweekly vaginal sonograms during use of the second ring to measure ovarian follicle-like structures using the same Sonosite TITAN with a 7.5 MHz transvaginal probe; we measured follicle diameter (FD) in two perpendicular diameters and recorded the dimensions of all follicles with a mean diameter of at least 8 mm. We also noted a corpus luteum or ruptured follicle, if present, and measured anterior-posterior endometrial thickness. Each sonogram took place on the same day as the venipunctures. We measured serum progesterone for all participants who had any ovarian follicle with a diameter of 13 mm or greater. At all visits the study physician confirmed that the CVR was present in the vagina. Participants were compensated for their time and travel costs.
Participants recorded daily bleeding and spotting throughout the study using paper diaries. The loss of blood requiring the use of a sanitary pad or tampon was noted as “bleeding” and the loss of blood requiring a panty liner or no protection as “spotting”12–14. Participants reported the total number of bleeding and spotting days, both scheduled and unscheduled, and the investigators did not impose a definition of which days were schedule and which were unscheduled.
We compared serum ENG and EE2 levels using the area under the concentration-time curve (AUC) from days 0 to day 21 using the linear trapezoidal approximation, assuming that the day 0 level was 0 for both hormones (as by day zero 97% of EE2 has been eliminated and over 99% of ENG). We also compared the geometric mean serum levels for both hormones in normal weight versus obese women at the end of weeks 1, 2, and 3 using the log-rank test. We assessed ovarian suppression in this study as the proportion of participants in each BMI group who had a maximum FD exceeding pre-specified thresholds, 8mm or 13 mm15–22. We used chi-square tests and Fisher’s Exact Test as appropriate to assess the association between obesity and maximum FD. For each participant, we calculated mean endometrial thickness and number of bleeding and spotting days during the study cycle, and used a t-test to compare these variables between the BMI groups.
A final sample size of 17 participants in each group was planned a priori in order to have 80% power to identify a one standard deviation difference in the mean serum levels of the contraceptive hormones, based on expected values for normal weight women23–24.
RESULTS
We enrolled 40 women into the study. Prior to the study cycle one woman fractured her pelvis in a motor vehicle accident and another experienced a venous thromboembolism following a long-haul flight (flight duration greater than 4 hours). We excluded both of these women from continuation in the study. We also excluded one woman due to removal of the CVR during the study cycle, leaving 18 normal weight and 19 obese women for analysis. Table 1 presents the baseline characteristics of the participants, whose BMI differed by design, and who differed somewhat by ethnicity.
Table 1.
Characteristics of Participants
| Variable | Overall (n=37) | Normal Weight (n=18) | Obese (n=19) | p-value |
|---|---|---|---|---|
|
| ||||
| Age | 26.6 ± 4.5 | 27.1 ± 4.7 | 26.2 ± 4.3 | 0.6 |
|
| ||||
| Hispanic | 14 (38) | 4 (22) | 10 (53) | |
| Non-Hispanic Black | 12 (32) | 6 (33.5) | 6 (31) | |
| Non-Hispanic White | 9 (24.5) | 6 (33.5) | 3 (16) | 0.15 |
| Asian | 2 (5.5) | 2 (11) | 0 (0) | |
|
| ||||
| Age Menarche | 12.0 ± 1.9 | 12.4 ± 1.8 | 11.6 ± 1.9 | 0.3 |
|
| ||||
| Ever Pregnant | 18 (49) | 9 (50) | 9 (47) | |
| Never Pregnant | 19 (51) | 9 (50) | 10 (53) | 1.0 |
|
| ||||
| Current Smoker | 5 (14) | 2 (11) | 3 (16) | |
| Past or Never Smoker | 32 (86) | 16 (89) | 16 (84) | 0.63 |
|
| ||||
| Height (cm) | 164.4 ± 5.7 | 162.6 ± 5.2 | 166.1 ± 5.7 | 0.1 |
|
| ||||
| Weight (kg) | -- | 56.9 ± 5.5 | 94.7 ± 10.4 | <0.0001 |
|
| ||||
| BMI | -- | 21.5 ± 1.3 | 34.3 ± 3.0 | <0.0001 |
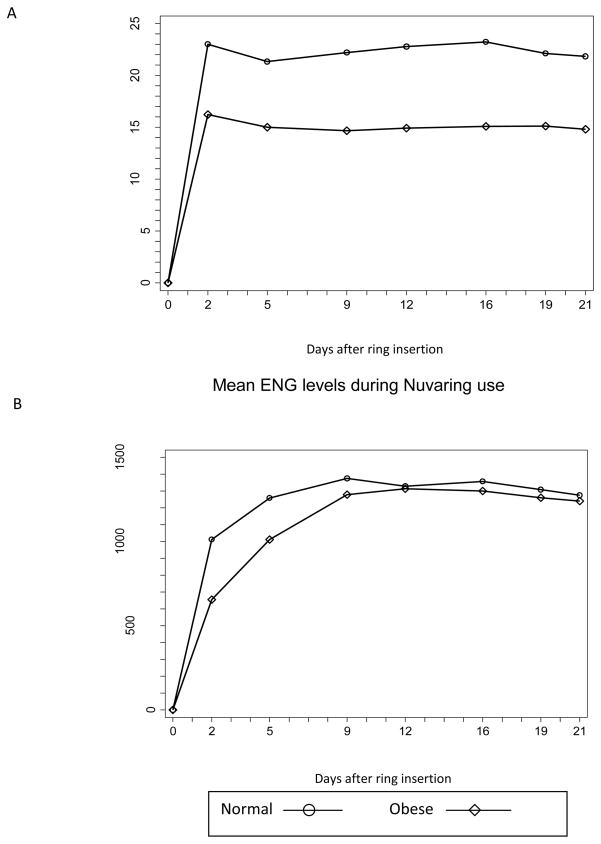
Figure 1 shows the mean EE2 and ENG levels during CVR use. We did not measure the serum levels immediately prior to ring insertion, and assume these day 0 levels to be 0 for both hormones. The day 0–21 average concentration for EE2 was 22.0 for normal weight women and 15.0 for obese women (p = 0.004). The day 0–21 average concentration for ENG was 1256 for normal weight women and 1138 for obese women (p = 0.39). The EE2 levels were lower in the obese participants during all three weeks of CVR use; however, the ENG levels were similar between the normal weight and obese participants (table 2).
Fig 1.
Serum concentrations of EE2 and ENG in 18 normal-weight and 19 obese contraceptive vaginal ring users.
Table 2.
Mean EthinylEstradiol and Etonorgestrel Levels During Contractive Vaginal Ring Use
| Normal Weight Mean (ng/L ± SD1) |
Obese Mean (ng/L ± SD) |
p-value | |
|---|---|---|---|
| EE | |||
| Week 1 | 21.8 (17.5, 27.1) | 14.8 (12.5, 17.5) | 0.008 |
| Week 2 | 23.5 (19.6, 28.2) | 14.9 (12.5, 17.8) | 0.001 |
| Week 3 | 21.9 (17.9, 26.6) | 14.8 (12.7, 17.3) | 0.004 |
| ENG | |||
| Week 1 | 1349 (1179, 1542) | 1190 (968, 1464) | 0.35 |
| Week 2 | 1360 (1196, 1547) | 1311 (1106, 1553) | 0.75 |
| Week 3 | 1275 (1116, 1457) | 1240 (1041, 1477) | 0.39 |
All participants underwent biweekly transvaginal sonograms during CVR use. As shown in Table 3, during week 1 (which immediately followed the ring-free week), 30% of the women had a largest follicle with a mean follicle diameter > 8mm; during weeks 2 and 3, respectively, only 14% and 11% of participants had follicles > 8 mm. During all 3 weeks of CVR use, only five participants (11%) had a largest follicle with a mean FD > 13mm and no corpora lutea were observed. All serum progesterone levels in these four participants were less than 1.0 ng/mL. Maximum FD’s were similar between the normal weight and obese participants. During follow-up, the mean endometrial thickness was 3.9mm (sd 1.6) in the normal weight participants, and 4.7mm (sd 1.8) in the obese participants (p = 0.19).
Table 3.
Maximum Follicular Diameter during contraceptive vaginal ring use
| Maximum Follicular Diameter | Overall n=37 (%) |
Normal Weight n=18 (%) |
Obese n=19 (%) |
p-value |
|---|---|---|---|---|
|
| ||||
| Week 1 | ||||
| ≥ 8 mm | 11 (30) | 5 (28) | 6 (32) | 0.80 |
| ≥ 13 mm | 4 (11) | 3 (17) | 1 (5) | 0.26 |
|
| ||||
| Week 2 | ||||
| ≥ 8 mm | 5 (14) | 4 (22) | 1 (5) | 0.13 |
| ≥ 13 mm | 4 (11) | 3 (17) | 1 (5) | 0.26 |
|
| ||||
| Week 3 | ||||
| ≥ 8 mm | 4 (11) | 2 (11) | 2 (11) | 0.95 |
| ≥13 mm | 4 (11) | 2 (11) | 2 (11) | 0.95 |
Categorical Variables given as n(%)
Total bleeding and spotting days were more frequent among the obese participants, particularly during week 1 (Table 4). During week 1, 10/18 normal weight women and 16/19 obese women reported bleeding or spotting (p = 0.1). In all of these women the week 1 bleeding took place at the end of withdrawal bleeding and was not a separate episode of unscheduled bleeding.
Table 4.
Mean Total Bleeding Days Weeks 1–3
| Week | Type of Bleeding | Overall (n=37) | Normal Weight (n=18) | Obese (n=19) | P-Value |
|---|---|---|---|---|---|
|
| |||||
| Week 1 | Bleeding | 0.8 ± 1.3 | 0.3 ± 0.8 | 1.2 ± 1.6 | 0.06 |
| Spotting | 1.1 ± 1.1 | 0.7 ±0.8 | 1.6 ± 1.3 | 0.02 | |
| Bleeding or Spotting | 1.9 ± 1.8 | 1.0 ± 1.0 | 2.7 ± 2.0 | 0.002 | |
|
| |||||
| Week 2 | Bleeding | 0.3 ± 1.0 | 0.1± 0.5 | 0.4 ± 1.3 | 0.4 |
| Spotting | 0.1 ± 0.4 | 0.1 ± 0.2 | 0.2 ± 0.5 | 0.3 | |
| Bleeding or Spotting | 0.4 ± 1.3 | 0.2 ± 0.7 | 0.6 ± 1.7 | 0.3 | |
|
| |||||
| Week 3 | Bleeding | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.1 ± 0.2 | 0.5 |
| Spotting | 0.2 ± 0.6 | 0.1 ± 0.3 | 0.3 ± 0.7 | 0.5 | |
| Bleeding or Spotting | 0.3 ± 0.7 | 0.2 ± 0.7 | 0.3 ± 0.8 | 0.7 | |
|
| |||||
| Weeks 1–3 Overall | Bleeding | 1.1 ± 1.6 | 0.6 ± 1.0 | 1.6 ± 1.9 | 0.04 |
| Spotting | 1.5 ± 1.7 | 0.8 ± 1.0 | 2.0 ± 1.9 | 0.03 | |
| Bleeding or Spotting | 2.6 ± 2.7 | 1.4 ± 1.7 | 3.6 ± 3.0 | 0.01 | |
Mean EE2 levels during contraceptive vaginal ring use
DISCUSSION
Obese women using the CVR achieve lower serum EE2 levels than normal weight women, but ENG levels are similar. The hormone levels found here among normal weight women are nearly identical to those found among normal weight Dutch women supporting the validity of these results23. Women in both groups had substantial suppression of ovarian follicular development, and we observed no ovulations during the study cycle. To compare follicular development between obese and normal weight women, we would have needed a much larger sample size. Obese women reported more bleeding and spotting days during CVR use, which might be related to their lower EE2 levels. All women had previously used an OC and had one cycle of ring use prior to the study cycle. We do not know if our observed results and differences between groups would be different among women during first-ever use of hormonal contraceptives.
In our previous study of normal weight and obese oral contraceptive (OC) users, we also found lower EE levels, similar progestin levels (in that study, the OC progestin was levonorgestrel), and no difference in follicular development in obese compared to normal weight OC users8–9. Why might obesity decrease the EE2 levels substantially and have so little effect on ENG levels? ENG is bound to both SHBG and albumin, while ethinyl estradiol is 98% bound to albumin25–26. Human adipose tissue has a network of pre-receptor steroid-converting enzymes that metabolize androgens and other hormones27. The low affinity albumin binding of EE2 makes the circulating EE2 vulnerable to greater metabolism by steroid-converting enzymes present in adipocytes. Whether adipocytes may also metabolize contraceptive progestins is not yet described. Pending further studies, enhanced metabolism of the EE2 by adipocytes could account for the observed differences in the serum levels between the normal weight and obese participants.
The present study was far too small to assess contraceptive failure as an outcome; however, these physiological results provide reassurance that the CVR will be effective among obese women. Levels of ENG were the same in normal weight and obese women and this ideally should correlate with efficacy; this result supports the vaginal ring having similar efficacy in normal weight and obese women. A caveat is that this study excluded women with a BMI > 40, and thus this reassurance cannot extend to women who are morbidly obese. Because concerns about increased risk of venous thromboembolism may limit the use of the CVR or other estrogen-containing contraceptives among the morbidly obese women28–29, the question of effectiveness in such women is somewhat less important than effectiveness among the increasingly prevalent group of women with BMI from 30–39.9.
Acknowledgments
FINANCIAL SUPPORT FOR THE STUDY:
NIH Grant RO1 HD04578
NIH CTSA Grant UL1 RR024156
Anonymous Foundation
Merck donation of Nuvarings and Laboratory assays
Footnotes
These standard deviations are not symmetrical because calculations were done using log transformed values. The standard deviations above are presented in the original units for ease of interpretation
CONFLICT OF INTEREST: C.L.W. is an advisory board member of Teva and Agile and a consultant for Merck and Bayer. The remaining authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holt VL, Cushing-Haugen KL, Daling JR. Body weight and risk of oral contraceptive failure. Obstet Gynecol. 2002;99:820–7. doi: 10.1016/s0029-7844(02)01939-7. [DOI] [PubMed] [Google Scholar]
- 2.Holt VL, Scholes D, Wicklund KG, Cushing-Haugen KL, Darling JR. Body mass index, weight, and oral contraceptive failure risk. Obstet Gynecol. 2005;105:46–52. doi: 10.1097/01.AOG.0000149155.11912.52. [DOI] [PubMed] [Google Scholar]
- 3.Zieman M, Guillebaud J, Weisberg E, Shangold GA, Fisher AC, Creasy GW. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system: the analysis of pooled data. Fertil Steril. 2002 Feb;77(2 Suppl 2):S13–8. doi: 10.1016/s0015-0282(01)03275-7. [DOI] [PubMed] [Google Scholar]
- 4.Dinger JC, Cronin M, Möhner S, Schellschmidt I, Minh TD, Westhoff C. Oral contraceptive effectiveness according to body mass index, weight, age, and other factors. Am J Obstet Gynecol. 2009 Sep;201(3):263. e1–9. doi: 10.1016/j.ajog.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Dinger J, Minh TD, Buttmann N, Bardenheuer K. Effectiveness of oral contraceptive pills in a large U.S. cohort comparing progestogen and regimen. Obstet Gynecol. 2011 Jan;117(1):33–40. doi: 10.1097/AOG.0b013e31820095a2. [DOI] [PubMed] [Google Scholar]
- 6.Westhoff CL, Hait HI, Reape KZ. Body weight does not impact pregnancy rates during use of a low-dose extended-regimen 91-day oral contraceptive. Contraception. 2012 Mar;85(3):235–9. doi: 10.1016/j.contraception.2011.08.001. Epub 2011 Sep 19. [DOI] [PubMed] [Google Scholar]
- 7.Westhoff C. Higher body weight does not affect NuvaRing’s efficacy [abstract] Obstet Gynecol. 2005;105(Suppl 4):56S. [Google Scholar]
- 8.Westhoff CL, Torgal AH, Mayeda ER, Stanczyk FZ, Lerner JP, Benn EK, Paik M. Ovarian suppression in normal-weight and obese women during oral contraceptive use: a randomized controlled trial. Obstet Gynecol. 2010 Aug;116(2 Pt 1):275–83. doi: 10.1097/AOG.0b013e3181e79440. [DOI] [PubMed] [Google Scholar]
- 9.Westhoff CL, Torgal AH, Mayeda ER, Pike MC, Stanczyk FZ. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010 Jun;81(6):474–80. doi: 10.1016/j.contraception.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization; Organization WH. Reproductive Health and Research. Geneva: World Health Organization; 2004. Medical eligibility criteria for contraceptive use. [Google Scholar]
- 11.Anari MR, Bakhtiar R, Zhu B, Huskey S, Franklin RB, Evans DC. Derivatization of ethinylestradiol with dansyl chloride to enhance electrospray ionization: application in trace analysis of ethinylestradiol in rhesus monkey plasma. Anal Chem. 2002 Aug 15;74(16):4136–44. doi: 10.1021/ac025712h. [DOI] [PubMed] [Google Scholar]
- 12.Belsey EM, Machin D, d’Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. World Health Organization Special Programme of Research, Development and Research Training in Human Reproduction. Contraception. 1986 Sep;34(3):253–60. doi: 10.1016/0010-7824(86)90006-5. [DOI] [PubMed] [Google Scholar]
- 13.Mishell DR, Jr, Guillebaud J, Westhoff C, Nelson AL, Kaunitz AM, Trussell J, Davis AJ. Combined hormonal contraceptive trials: variable data collection and bleeding assessment methodologies influence study outcomes and physician perception. Contraception. 2007 Jan;75(1):4–10. doi: 10.1016/j.contraception.2006.08.008. Epub 2006 Oct 13. [DOI] [PubMed] [Google Scholar]
- 14.Mishell DR, Jr, Guillebaud J, Westhoff C, Nelson AL, Kaunitz AM, Trussell J, Davis AJ. Recommendations for standardization of data collection and analysis of bleeding in combined hormone contraceptive trials. Contraception. 2007 Jan;75(1):11–5. doi: 10.1016/j.contraception.2006.08.012. Epub 2006 Oct 11. [DOI] [PubMed] [Google Scholar]
- 15.Broome M, Clayton J, Fotherby K. Enlarged follicles in women using oral contraceptives. Contraception. 1995;52:13–6. doi: 10.1016/0010-7824(95)00127-v. [DOI] [PubMed] [Google Scholar]
- 16.Schlaff WD, Lynch AM, Hughes HD, Cedars MI, Smith DL. Manipulation of the pill-free interval in oral contraceptive pill users: the effect on follicular suppression. Am J Obstet Gynecol. 2004;190:943–51. doi: 10.1016/j.ajog.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Hoogland HJ, Skouby SO. Ultrasound evaluation of ovarian activity under oral contraceptives. Contraception. 1993 Jun;47(6):583–90. doi: 10.1016/0010-7824(93)90025-3. [DOI] [PubMed] [Google Scholar]
- 18.Grimes DA, Godwin AJ, Rubin A, Smith JA, Lacarra M. Ovulation and follicular development associated with three low-dose oral contraceptives: a randomized controlled trial. Obstet Gynecol. 1994 Jan;83(1):29–34. [PubMed] [Google Scholar]
- 19.Klipping C, Duijkers I, Trummer D, Marr J. Suppression of ovarian activity with a drospirenone-containing oral contraceptive in a 24/4 regimen. Contraception. 2008 Jul;78(1):16–25. doi: 10.1016/j.contraception.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Endrikat J, Parke S, Trummer D, Schmidt W, Duijkers I, Klipping C. Ovulation inhibition with four variations of a four-phasic estradiol valerate/dienogest combined oral contraceptive: results of two prospective, randomized, open-label studies. Contraception. 2008 Sep;78(3):218–25. doi: 10.1016/j.contraception.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Rible RD, Taylor D, Wilson ML, Stanczyk FZ, Mishell DR., Jr Follicular development in a 7-day versus 4-day hormone-free interval with an oral contraceptive containing 20 mcg ethinyl estradiol and 1 mg norethindrone acetate. Contraception. 2009 Mar;79(3):182–8. doi: 10.1016/j.contraception.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Spona J, Binder N, Höschen K, Feichtinger W. Suppression of ovarian function by a combined oral contraceptive containing 0.02 mg ethinyl estradiol and 2 mg chlormadinone acetate given in a 24/4-day intake regimen over three cycles. Fertil Steril. 2010 Sep;94(4):1195–201. doi: 10.1016/j.fertnstert.2009.06.057. [DOI] [PubMed] [Google Scholar]
- 23.Timmer CJ, Mulders TM. Pharmacokinetics of etonogestrel and ethinylestradiol released from a combined contraceptive vaginal ring. Clin Pharmacokinet. 2000;39:233–42. doi: 10.2165/00003088-200039030-00005. [DOI] [PubMed] [Google Scholar]
- 24.Mulders TM, Dieben TO. Use of the novel combined contraceptive vaginal ring NuvaRing for ovulation inhibition. Fertility and Sterility. 2001;75:865–870. doi: 10.1016/s0015-0282(01)01689-2. [DOI] [PubMed] [Google Scholar]
- 25.Hammond GL, Langley MS, Robinson PA, Nummi S, Lund L. Serum steroid binding protein concentrations, distribution of progestogens, and bioavailability of testosterone during treatment with contraceptives containing desogestrel or levonorgestrel. Fertil Steril. 1984 Jul;42(1):44–51. doi: 10.1016/s0015-0282(16)47956-2. [DOI] [PubMed] [Google Scholar]
- 26.Kuhnz W, Pfeffer M, al-Yacoub G. Protein binding of the contraceptive steroids gestodene, 3-keto-desogestrel and ethinylestradiol in human serum. J Steroid Biochem. 1990 Feb;35(2):313–8. doi: 10.1016/0022-4731(90)90290-9. [DOI] [PubMed] [Google Scholar]
- 27.Blouin K, Veilleux A, Luu-The V, Tchernof A. Androgen metabolism in adipose tissue: recent advances. Mol Cell Endocrinol. 2009;301:97–103. doi: 10.1016/j.mce.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 28.Cushman M, Callas PW, Denenberg JO, Bovill EG, Criqui MH. Risk factors for peripheral venous disease resemble those for venous thrombosis: the San Diego Population Study. J Thromb Haemost. 2010 Aug;8(8):1730–5. doi: 10.1111/j.1538-7836.2010.03924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Royal College of Obstetricians & Gynaecologists, Faculty of family planning and reproductive health care. UK medical eligibility criteria for contraceptive use (UKMEC 2005/2006) 2006 Jul; Retrieved April 4, 2012 from: http://www.fsrh.org/pdfs/archive/UKMEC2005_06.pdf.