Abstract
In the Women’s Health Initiative (WHI) trial of calcium plus vitamin D (CaD), we examined the treatment effect on incidence and mortality for all invasive cancers. Postmenopausal women (N = 36,282) were randomized to 1,000 mg of elemental calcium with 400 IU vitamin D3 or placebo. Cox models estimated risk of cancer incidence and mortality. After 7.0 yr, 1,306 invasive cancers were diagnosed in the supplement and 1,333 in the placebo group [hazard ratio (HR) = 0.98; CI = 0.90, 1.05, unweighted P = 0.54]. Mortality did not differ between supplement (315, annualized% = .26) and placebo [(347, 0.28%; P = 0.17; HR = 0.90 (0.77, 1.05)]. Significant treatment interactions on incident cancer were found for family history of cancer, personal total intake of vitamin D, smoking, and WHI dietary trial randomized group. Calcium/vitamin D supplementation did not reduce invasive cancer incidence or mortality. Supplementation lowered cancer risk in the WHI healthy diet trial arm and in women without a first-degree relative with cancer. The interactions are only suggestive given multiple testing considerations. The low vitamin D dose provided, limited adherence, and lack of serum 25(OH)D values should be considered when interpreting these findings.
INTRODUCTION
The majority of work concerned with cancer prevention and calcium/vitamin D has focused on breast and colorectal cancer (1). The Women’s Health Initiative (WHI) randomized clinical trial of calcium plus vitamin D (CaD) supplementation compared to placebo did not find a significant effect of active treatment on colorectal cancer incidence (2) or breast cancer incidence or mortality (3) over a 7-yr period. These findings failed to support certain previous observational (colon cancer) and polyp prevention studies that had suggested an association between higher calcium/vitamin D intakes and lower risk of colon and breast cancer risk (4-7). However not all observational studies to date support an association between calcium and vitamin D intake from foods or supplements and colorectal cancer (8,9). It is similarly unclear, from epidemiological studies whether calcium or vitamin D intake reduces breast cancer risk. Two prospective cohort studies found no association between calcium levels and breast cancer risk (10,11). While most prospective studies have found that vitamin D reduces the risk of breast cancer, the effects may be modified by timing and by tumor type (12). In addition to colon and breast cancer risk, vitamin D and calcium intake effects on other cancers have been studied, but results are also not conclusive (1).
Despite negative findings for the primary endpoints in WHI, the CaD trial did report fewer deaths in the calcium and vitamin D group compared to placebo but the difference was not statistically significant [744 vs. 807 deaths, respectively; hazard ratio (HR) 0.9195%, CI 0.83–1.06] (13). However, their analyses were not specific to cause of death. With the current recommendations to stay out of the sun to prevent skin cancer, a significant source of vitamin D may be lost. It is essential to better understand the influence of vitamin D supplementation on cancer risk. Most of the published literature has correlated total vitamin D and calcium exposure (foods and supplements combined) using observational study designs (9,14). We examine the potential effects of CaD assignment on total invasive cancer incidence and mortality in the context of a randomized and blinded clinical trial and supplementation of 400 IU of vitamin D3 and 1,000 mg of elemental calcium.
MATERIALS AND METHODS
Study Population, Eligibility, and Consent
The WHI study was designed to determine factors associated with morbidity and mortality in postmenopausal women. Between 1993 and 1998, postmenopausal women 50 to 79 yr of age were enrolled in the WHI randomized trials assessing the risks and benefits of hormone therapy (HT) and dietary modification (DM) (15-18). Exclusion criteria for entry into the trials were related to competing risks, safety, adherence, and retention. One yr later, participants in the HT and DM trials were invited to enroll in the randomized trial of calcium plus vitamin D compared to placebo. The trial was designed to determine whether calcium plus vitamin D supplementation would prevent hip fracture, which was the primary outcome, and colorectal cancer, a designated secondary outcome (19). Results of those outcomes, as well as of breast cancer, have been reported (2,3,19). Exclusion criteria for the CaD trial included a predicted survival of less than 3 yr, a history of renal calculi or hypercalcemia, current use of oral corticosteroids, and current daily use of at least 600 IU of supplemental vitamin D (single supplement and multivitamin combined) or calcitriol (19). The majority of study women (91%) joined the CaD trial during their first annual clinic visit with 9% the following year. Fifty-four percent of CaD trial participants had been enrolled in one of the trials assessing hormone therapy, 69% had been enrolled in the trial assessing dietary modification, and 14% were in both trials. The protocol and consent forms were approved by the institutional review board at each participating institution and all women provided written informed consent.
Randomization, Blinding, and Intervention
Randomization was done using a permuted-block algorithm with participants stratified according to clinical center and age. Of the 36,282 participants, 18,176 were randomized to receive 1,000 mg of elemental calcium as calcium carbonate combined with 400 IU of vitamin D3 (GlaxoSmithKline, Brentford, UK). Each pill contained half the dose and 2 pills were taken daily. The 18,106 women randomized to placebo received identical-appearing tablets each also taken twice daily. Blinding of the study was achieved by identical-looking pills and bottles. The participants were given chewable tablets until 1997, when both chewable and made-to-swallow options were offered. Although 61% started with a chewable form, by the end of the study 70% chose to take the made-to-swallow form.
Follow-Up Procedures and Outcome Ascertainment
Four weeks after randomization, study personnel telephoned participants to reinforce adherence to study medications and assess symptoms related to medication intake. Thereafter, participants came to annual clinic visits and were contacted semiannually by telephone or mail to obtain self-reported updates on medical history. Medical records were obtained for any self-reported cancers and were reviewed and verified by both local and central physician adjudicators who were blinded to randomization status. Cancers were coded according to the Surveillance, Epidemiology, and End Results system (20) and 99.4% of reported cancers were centrally confirmed. Adherence was assessed by weighing returned pill bottles. Regardless of their adherence, participants were followed up until they died, were lost to follow-up, requested no further contact, or until their close-out visit at the end of the main study when they stopped taking all study medications. At that visit they had a medical history update and were subsequently unblinded. Protocol-specified cancer screenings included mammograms and pelvic/Pap exams taken every 1–2 yr, with other health screening decided by each participant and their personal physician. Reported symptoms or concerns related to study medications, as a rule, were managed by temporary reduction in the number of pills taken. Study pills were discontinued if kidney stones, hypercalcemia, dialysis, or the use of calcitriol or of daily supplements of more than 1,000 IU of vitamin D was reported. As previously reported, during Year 1, 60% of the participants took at least 80% of their study medications, and this percentage remained stable through Year 6, with negligible differences between placebo and active groups. At least 70% took 50% or more of their study medication through Year 6 (2). An independent data and safety monitoring board reviewed the trial data semiannually. Closeout visits occurred as planned between October 1, 2004 and March 31, 2005.
Additional Study Variables
Other factors that were assessed for their potential interaction with treatment assignment included age, race/ethnicity, education, first-degree relative with cancer, body mass index (BMI), physical activity, kcal intake, total calcium, and vitamin D intakes (diet plus supplements from the food frequency questionnaire) (21), geographic latitude, regional solar irradiance (in Langleys), alcohol intake and smoking at baseline, prior hormone therapy use, and assignment in the DM and HT trials.
STATISTICAL ANALYSIS
Primary analyses used time-to-event methods, according to the intention-to-treat principle. The incidence of cancer was compared in the 2 groups with the use of hazard ratios (with % CI) and Wald statistic P values from Cox proportional-hazards models (22), stratified according to age, and treatment assignment in the HT and DM trials. Comparisons of treatment assignments were made by use of a 2-sided, weighted log-rank test as specified in the protocol, with weight increasing linearly from 0 at randomization to a maximum of one at 10 yr, to enhance the statistical power of the study according to the design assumptions. Both Bonferroni’s adjusted and unadjusted tests of significance are given for the weighted log-rank test. Kaplan–Meier estimates were used to describe event rates over time. Potential differential effects across subgroups of important risk factors for cancer were tested individually with the use of a likelihood ratio test for interaction between the risk factor and treatment assignment after including both as main effects. A total of 18 subgroup comparisons were tested, accordingly, the results of one test would be expected to be significant at the 0.05 level by chance alone. Participants with missing values were excluded from analyses requiring that value. All reported P values are 2-sided and, along with the confidence intervals, were not adjusted for multiplicity, unless noted. In addition, sensitivity analyses censoring women 6 mo after they became less than 80% adherent were done.
Any women with prior history of invasive cancer at baseline (enrollment) or a diagnosis of invasive cancer in the interval between HT/DM randomization and CaD randomization were excluded from these analyses. Analyses included all invasive cancers reported from the date of CaD randomization until the date of diagnosis of first invasive cancer, loss to follow-up, death, or date of close-out. Only invasive and centrally adjudicated cancers are included in these analyses. This made available 2,639 total cancers for analysis. Of these 1,028 breast cancers, 305 colorectal cancers, 101 ovarian cancers, 173 endometrial cancers, and 1,246 other cancers (than breast, colorectal, ovarian and endometrial) were reported. A total of 662 cancer deaths occurred.
RESULTS
Participant Characteristics and Personal Supplement Use
Demographic characteristics, health behaviors, and medical history were balanced between randomization groups (17,343 women in the supplement group and 17,327 in the placebo group). Self-reported baseline total calcium and vitamin D intakes from diet were similar in the randomization groups (Table 1), with personal (not part of study treatment) vitamin D supplement use of 400 IU/day or greater reported by 37.7% of women in the placebo group and 37.1% of women in the active treatment group.
TABLE 1.
Characteristics of participants in Women’s Health Initiative CaD trial enrollment group assignment excluding those with cancer prior to the start of the trial
| Characteristic | CaD N (%) | Placebo N (%) | P Value |
|---|---|---|---|
| Age at screening | 0.99 | ||
| 50–59 | 6,441 (37.1) | 6,444 (37.2) | |
| 60–69 | 7,913 (45.6) | 7,892 (45.5) | |
| 70–79 | 2,989 (17.2) | 2,991 (17.3) | |
| Ethnicity | 0.47 | ||
| White | 14,335 (82.7) | 14,435 (83.3) | |
| Black | 1,614 (9.3) | 1,570 (9.1) | |
| Hispanic | 760 (4.4) | 700 (4.0) | |
| American Indian | 75 (0.4) | 69 (0.4) | |
| Asian/Pacific Islander | 356 (2.1) | 337 (1.9) | |
| Unknown | 203 (1.2) | 216 (1.2) | |
| Education | 0.71 | ||
| ≤8 yr | 271 (1.6) | 239 (1.4) | |
| Some high school | 665 (3.8) | 647 (3.7) | |
| High school diploma/GED | 3,175 (18.3) | 3,236 (18.7) | |
| School after high school | 6,848 (39.5) | 6,817 (39.3) | |
| College degree or higher | 6,273 (36.2) | 6,277 (36.2) | |
| First-degree relative with cancer | 10,990 (63.4) | 10,931 (63.1) | 0.79 |
| Body mass index (baseline) | 0.27 | ||
| <25 | 4,556 (26.3) | 4,642 (26.8) | |
| 25–<30 | 6,154 (35.5) | 6,229 (35.9) | |
| ≥30 | 6,549 (37.8) | 6,367 (36.7) | |
| Physical activity (METs/wk) | 0.57 | ||
| <1.5 | 3,830 (22.1) | 3,751 (21.6) | |
| 1.5–6.7 | 4,117 (23.7) | 4,170 (24.1) | |
| 6.8–15.5 | 3,917 (22.6) | 3,832 (22.1) | |
| ≥15.6 | 3,906 (22.5) | 3,969 (22.9) | |
| Total dietary calorie intake | 0.11 | ||
| <1,171 kcal | 3,395 (19.6) | 3,389 (19.6) | |
| 1,171–1,483 kcal | 3,404 (19.6) | 3,399 (19.6) | |
| 1,484–1,790 kcal | 3,368 (19.4) | 3,432 (19.8) | |
| 1,791–2,232 kcal | 3,503 (20.2) | 3,301 (19.1) | |
| ≥2,233 kcal | 3,336 (19.2) | 3,465 (20.0) | |
| Percent energy from fat | 0.06 | ||
| <31.4 | 3,433 (19.8) | 3,302 (19.1) | |
| 31.4–34.2 | 3,356 (19.4) | 3,412 (19.7) | |
| 34.3–37.2 | 3,309 (19.1) | 3,504 (20.2) | |
| 37.3–41.1 | 3,488 (20.1) | 3,369 (19.4) | |
| ≥41.2 | 3,420 (19.7) | 3,399 (19.6) | |
| Multivitamin use (current, daily) | 6,139 (35.4) | 6,238 (36.0) | 0.31 |
| Calcium single supplement (personal use) | 0.54 | ||
| Yes | 3,372 (19.4) | 3,333 (19.2) | |
| Mg, mean (SD) | 893.6 (686.4) | 884.5 (647.4) | |
| Calcium intake (mg) diet | 0.93 | ||
| <400 mg | 2,289 (13.2) | 2,273 (13.1) | |
| 400–800 mg | 7,263 (41.9) | 7,265 (41.9) | |
| 800–1,200 mg | 4,579 (26.4) | 4,523 (26.1) | |
| >1,200 mg | 2,875 (16.6) | 2,925 (16.9) | |
| Calcium intake (mg) total | 0.62 | ||
| <400 mg | 1,240 (7.1) | 1,189 (6.9) | |
| 400–800 mg | 4,515 (26.0) | 4,465 (25.8) | |
| 800–1,200 mg | 4,482 (25.8) | 4,442 (25.6) | |
| >1,200 mg | 6,769 (39.0) | 6,890 (39.8) | |
| Vitamin D single supplement (personal use) | 0.57 | ||
| Yes | 560 (3.2) | 571 (3.3) | |
| Mg, mean (SD) | 492.9 (349.4) | 508.4 (377.9) | |
| Vitamin D intake diet (IU) | 0.97 | ||
| <200 IU | 11,759 (67.8) | 11,699 (67.5) | |
| 200–<400 IU | 4,448 (25.6) | 4,468 (25.8) | |
| 400–<600 IU | 658 (3.8) | 677 (3.9) | |
| ≥600 IU | 141 (0.8) | 142 (0.8) | |
| Vitamin D intake total (IU) | 0.21 | ||
| <200 IU | 6,537 (37.7) | 6,368 (36.8) | |
| 200–<400 IU | 3,230 (18.6) | 3,279 (18.9) | |
| 400–<600 IU | 3,973 (22.9) | 4,125 (23.8) | |
| ≥600 IU | 3,266 (18.8) | 3,214 (18.5) | |
| Latitude | 0.96 | ||
| South <35°N | 5,186 (29.9) | 5,205 (30.0) | |
| Middle 35–40°N | 4,787 (27.6) | 4,771 (27.5) | |
| North >40°N | 7,370 (42.5) | 7,351 (42.4) | |
| Region by solar irradiance in Langley categories | 0.99 | ||
| 475–500 | 3,646 (21.0) | 3,669 (21.2) | |
| 400–430 | 2,889 (16.7) | 2,872 (16.6) | |
| 375–380 | 1,918 (11.1) | 1,920 (11.1) | |
| 350 | 3,746 (21.6) | 3,710 (21.4) | |
| 300–325 | 5,144 (29.7) | 5,156 (29.8) | |
| Alcohol intake | 0.88 | ||
| Nondrinker | 1,799 (10.4) | 1,821 (10.5) | |
| Past drinker | 3,026 (17.4) | 3,042 (17.6) | |
| <1 drink per mo | 2,420 (14.0) | 2,410 (13.9) | |
| <1 drink per wk | 3,683 (21.2) | 3,603 (20.8) | |
| 1−<7 drinks per wk | 4,469 (25.8) | 4,508 (26.0) | |
| 7+ drinks per wk | 1,804 (10.4) | 1,818 (10.5) | |
| Smoking | 0.51 | ||
| Never smoked | 8,935 (51.5) | 9,067 (52.3) | |
| Past smoked | 6,902 (39.8) | 6,791 (39.2) | |
| Current smoker | 1,324 (7.6) | 1,290 (7.4) | |
| Prior hormone use | 0.52 | ||
| Never user | 2,830 (16.3) | 2,791 (16.1) | |
| Former | 5,540 (31.9) | 5,441 (31.4) | |
| Current E alone (personal and HT trial) | 4,247 (24.5) | 4,340 (25.0) | |
| Current E + P (personal and HT trial) | 4,726 (27.3) | 4,755 (27.4) | |
| HT duration use | 0.55 | ||
| Nonuser | 8,416 (48.5) | 8,276 (47.8) | |
| <5 yr | 3,886 (22.4) | 3,931 (22.7) | |
| 5−<10 yr | 2,032 (11.7) | 2,049 (11.8) | |
| 10+ yr | 3,009 (17.3) | 3,071 (17.7) | |
| Dietary modification (DM) trial enrollment | 0.40 | ||
| Not randomized | 5,382 (31.0) | 5,308 (30.6) | |
| DM intervention | 4,542 (26.2) | 4,647 (26.8) | |
| DM usual diet | 7,419 (42.8) | 7,372 (42.5) | |
| HT trial enrollment | 0.73 | ||
| No | 9,566 (55.2) | 9,566 (55.2) | |
| E + P active | 2,450 (14.1) | 2,471 (14.3) | |
| E + P placebo | 2,427 (14.0) | 2,341 (13.5) | |
| E alone active | 1,452 (8.4) | 1,475 (8.5) | |
| E alone placebo | 1,448 (8.3) | 1,474 (8.5) |
The Langley is a unit of solar radiance and relates to the amount that reaches a given area of the earth’s surface. The information is from national weather data on total solar irradiance in the United States and is adapted from Garland CF and Garland FC: Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol 9, 227–231, 1980.
CaD = calcium plus vitamin D; MET = metabolic equivalent tasks; HT = hormone therapy.
During the trial, personal calcium and vitamin D supplement use was similar in the 2 randomization groups. At Year 6, personal vitamin D supplement use, mostly in multivitamin preparations, was reported by 52.8% of women in the placebo group and 52.0% of women in the supplement group. During the course of the trial, personal calcium intake increased by approximately 100 mg daily in both randomization groups.
Primary Analyses
After a mean follow-up of 7 yr, a total of 1,306 invasive cancers were diagnosed in the supplement group and 1,333 in the placebo group [HR = 0.98 (0.90, 1.05), P = 0.54] (Table 2). None of the specific primary sites of invasive cancers differed significantly between the treatment and control groups. A sensitivity analysis censoring follow-up of 6 mo after nonadherence found 696 invasive cancers in the treatment and 768 in the placebo groups [HR = 0.94 (0.85, 1.05), P = 0.27]. These results are shown in Table 3.
TABLE 2.
Incidence (annualized rate) of invasive cancer by site
| Site Grouping/Site | Number of Cases (Annualized %)
|
HR (95% CI)* |
P Value
|
||
|---|---|---|---|---|---|
| CaD | Placebo | Unweighted | Weighted | ||
| Total cancer | 1,306 (1.10) | 1,333 (1.13) | 0.98 (0.90, 1.05) | 0.54 | 0.78 |
| Bone, connective tissue, and skin (overall) | 563 (0.47) | 589 (0.49) | 0.96 (0.85, 1.07) | 0.44 | 0.12 |
| Bones/joints/articular cartilage (limbs) | 1 (<0.01) | 0 (<0.01) | |||
| Bones/joints/articular cartilage (other) | 2 (<0.01) | 1 (<0.01) | 1.94 (0.18, 21.41) | 0.59 | 0.95 |
| Breast | 505 (0.42) | 523 (0.44) | 0.96 (0.85, 1.09) | 0.55 | 0.26 |
| Connective/subcutaneous/soft tissues | 4 (<0.01) | 5 (<0.01) | 0.81 (0.22, 3.01) | 0.75 | 0.54 |
| Heart | 0 (0.00) | 1 (<0.01) | |||
| Melanoma of the skin | 54 (0.04) | 60 (0.05) | 0.91 (0.63, 1.32) | 0.62 | 0.27 |
| Digestive organs and peritoneum (overall) | 227 (0.19) | 240 (0.20) | 0.94 (0.78, 1.13) | 0.50 | 0.69 |
| Anus | 1 (<0.01) | 5 (<0.01) | 0.20 (0.02, 1.71) | 0.14 | 0.29 |
| Appendix | 0 (0.00) | 2 (<0.01) | |||
| Biliary tract, parts of | 13 (0.01) | 9 (<0.01) | 1.43 (0.61, 3.35) | 0.41 | 0.88 |
| Colon | 117 (0.10) | 118 (0.10) | 0.98 (0.76, 1.27) | 0.90 | 0.72 |
| Esophagus | 6 (<0.01) | 12 (<0.01) | 0.50 (0.19, 1.32) | 0.16 | 0.09 |
| Gallbladder | 2 (<0.01) | 2 (<0.01) | 1.04 (0.15, 7.38) | 0.97 | 0.95 |
| Liver | 4 (<0.01) | 9 (<0.01) | 0.45 (0.14, 1.47) | 0.19 | 0.36 |
| Pancreas | 32 (0.03) | 36 (0.03) | 0.88 (0.55, 1.41) | 0.59 | 0.46 |
| Rectal | 41 (0.03) | 29 (0.02) | 1.42 (0.88, 2.28) | 0.15 | 0.16 |
| Retroperitoneum | 5 (<0.01) | 6 (<0.01) | 0.84 (0.26, 2.77) | 0.78 | 0.89 |
| Small intestine | 4 (<0.01) | 2 (<0.01) | 1.88 (0.34, 10.28) | 0.47 | 0.51 |
| Stomach | 9 (<0.01) | 12 (<0.01) | 0.73 (0.31, 1.73) | 0.47 | 0.89 |
| Other digestive organs | 0 (0.00) | 2 (<0.01) | — | - | - |
| Genital (overall) | 155 (0.13) | 144 (0.12) | 1.07 (0.85, 1.35) | 0.55 | 0.78 |
| Cervix | 6 (<0.01) | 0 (0.00) | |||
| Endometrium | 85 (0.12) | 88 (0.12) | 0.95 (0.71, 1.28) | 0.75 | 0.56 |
| Genital organs | 8 (<0.01) | 3 (<0.01) | 2.56 (0.68, 9.65) | 0.17 | 0.50 |
| Ovary | 50 (0.05) | 51 (0.05) | 0.98 (0.66, 1.44) | 0.90 | 0.98 |
| Uterus, not otherwise specified | 10 (<0.01) | 8 (<0.01) | 1.25 (0.49, 3.17) | 0.64 | 0.70 |
| Vulva | 3 (<0.01) | 3 (<0.01) | 0.99 (0.20, 4.91) | 0.99 | 0.97 |
| Lip, oral cavity, and pharynx (overall) | 15 (0.01) | 11 (<0.01) | 1.33 (0.61, 2.89) | 0.48 | 0.34 |
| Oral (mouth) | 9 (<0.01) | 6 (<0.01) | 1.43 (0.51, 4.02) | 0.50 | 0.64 |
| Oropharaynx | 0 (0.00) | 1 (<0.01) | |||
| Parotoid gland (Stensen’s duct) | 3 (<0.01) | 0 (0.00) | |||
| Salivary glands, major (other/unspecified) | 2 (<0.01) | 0 (0.00) | |||
| Tongue, part of (other/unspecified) | 1 (<0.01) | 4 (<0.01) | 0.25 (0.03, 2.20) | 0.21 | 0.34 |
| Malignant neoplasm of lymphatic and hematopoietic tissue (overall) | 97 (0.08) | 122 (0.10) | 0.80 (0.61, 1.04) | 0.10 | 0.16 |
| Leukemia | 28 (0.02) | 29 (0.02) | 0.97 (0.58, 1.63) | 0.91 | 0.92 |
| Lymphoma, Hodgkin’s | 2 (<0.01) | 8 (<0.01) | 0.26 (0.06, 1.23) | 0.09 | 0.14 |
| Lymphoma, non-Hodgkin’s | 47 (0.04) | 65 (0.05) | 0.72 (0.49, 1.05) | 0.09 | 0.20 |
| Multiple myeloma | 21 (0.02) | 20 (0.02) | 1.09 (0.59, 2.01) | 0.79 | 0.59 |
| Respiratory and intrathoracic organs (overall) | 114 (0.09) | 130 (0.11) | 0.87 (0.68, 1.12) | 0.29 | 0.29 |
| Larynx | 3 (<0.01) | 2 (<0.01) | 1.45 (0.24, 8.69) | 0.68 | 0.77 |
| Lung | 109 (0.09) | 126 (0.10) | 0.86 (0.67, 1.12) | 0.26 | 0.28 |
| Respiratory system | 2 (<0.01) | 1 (<0.01) | 1.92 (0.17, 21.24) | 0.59 | 0.71 |
| Thymus | 0 (0.00) | 1 (<0.01) | 0.99 | 0.99 | |
| Urinary (overall) | 66 (0.05) | 53 (0.04) | 1.24 (0.86, 1.78) | 0.25 | 0.04 |
| Bladder | 34 (0.03) | 23 (0.02) | 1.49 (0.88, 2.53) | 0.14 | 0.12 |
| Kidney | 28 (0.02) | 27 (0.02) | 1.02 (0.60, 1.74) | 0.94 | 0.26 |
| Ureter | 2 (<0.01) | 0 (0.00) | |||
| Urinary organs (other/unspecified) | 2 (<0.01) | 4 (<0.01) | 0.48 (0.09, 2.62) | 0.40 | 0.72 |
| Other and unspecified (overall) | 45 (0.04) | 40 (0.03) | 1.11 (0.72, 1.70) | 0.63 | 0.27 |
| Adrenal gland | 1 (<0.01) | 0 (0.00) | |||
| Brain | 16 (0.01) | 10 (<0.01) | 1.58 (0.72, 3.49) | 0.26 | 0.13 |
| Endocrine glands | 0 (0.00) | 1 (<0.01) | |||
| Eye and adnexa | 4 (<0.01) | 4 (<0.01) | 0.99 (0.25, 3.94) | 0.98 | 0.93 |
| Thyroid | 20 (0.02) | 22 (0.02) | 0.90 (0.49, 1.65) | 0.74 | 0.97 |
| Other/unknown | 4 (<0.01) | 2 (<0.01) | 1.86 (0.34, 10.17) | 0.48 | 0.48 |
| Other ill defined sites | 0 (0.00) | 1 (<0.01) | |||
| Unknown primary site | 21 (0.02) | 15 (0.01) | 1.37 (0.71, 2.66) | 0.35 | 0.07 |
CaD = calcium plus vitamin D.
TABLE 3.
Incidence (annualized rate) of invasive cancer by site (adherent women)
| Site Grouping/Site | Number of Cases (Annualized %)
|
HR (95% CI)* |
P Value
|
||
|---|---|---|---|---|---|
| CaD | Placebo | Unweighted | Weighted | ||
| Total cancer | 696 (1.08) | 768 (1.15) | 0.94 (0.85, 1.05) | 0.27 | 0.42 |
| Bone, connective tissue, and skin (overall) | 302 (0.47) | 339 (0.50) | 0.93 (0.80, 1.09) | 0.36 | 0.22 |
| Bones/joints/articular cartilage (limbs) | 1 (<0.01) | 0 (0.00) | — | — | — |
| Bones/joints/articular cartilage (other) | 1 (<0.01) | 1 (<0.01) | 1.07 (0.07, 17.26) | 0.96 | 0.69 |
| Breast | 263 (0.40) | 301 (0.45) | 0.91 (0.77, 1.07) | 0.26 | 0.18 |
| Connective/subcutaneous/soft tissues | 4 (<0.01) | 3 (<0.01) | 1.48 (0.33, 6.63) | 0.61 | 0.83 |
| Heart | 0 (0.00) | 0 (0.00) | — | — | — |
| Melanoma of the skin | 36 (0.05) | 35 (0.05) | 1.09 (0.68, 1.73) | 0.73 | 0.73 |
| Digestive organs and peritoneum (overall) | 122 (0.19) | 140 (0.21) | 0.90 (0.70, 1.14) | 0.38 | 0.37 |
| Anus | 1 (<0.01) | 2 (<0.01) | 0.54 (0.05, 5.96) | 0.62 | 0.70 |
| Appendix | 0 (0.00) | 1 (<0.01) | — | — | — |
| Biliary tract, parts of | 8 (0.01) | 5 (<0.01) | 1.53 (0.50, 4.69) | 0.45 | 0.65 |
| Colon | 60 (0.09) | 69 (0.10) | 0.89 (0.63, 1.26) | 0.50 | 0.67 |
| Esophagus | 6 (<0.01) | 7 (0.01) | 0.93 (0.31, 2.78) | 0.89 | 0.57 |
| Gallbladder | 2 (<0.01) | 1 (<0.01) | 2.14 (0.19, 23.56) | 0.54 | 0.37 |
| Liver | 4 (<0.01) | 5 (<0.01) | 0.87 (0.23, 3.27) | 0.84 | 0.83 |
| Pancreas | 16 (0.02) | 24 (0.04) | 0.68 (0.36, 1.28) | 0.23 | 0.13 |
| Rectal | 22 (0.03) | 18 (0.03) | 1.27 (0.68, 2.37) | 0.46 | 0.29 |
| Retroperitoneum | 1 (<0.01) | 4 (<0.01) | 0.26 (0.03, 2.37) | 0.23 | 0.54 |
| Small intestine | 2 (<0.01) | 1 (<0.01) | 2.02 (0.18, 22.30) | 0.57 | 0.83 |
| Stomach | 3 (<0.01) | 4 (<0.01) | 0.78 (0.17, 3.48) | 0.74 | 0.78 |
| Other digestive organs | 0 (0.00) | 1 (<0.01) | |||
| Genital (overall) | 85 (0.13) | 78 (0.11) | 1.14 (0.84, 1.55) | 0.41 | 0.72 |
| Cervix | 3 (<0.01) | 0 (0.00) | |||
| Endometrium | 53 (0.13) | 48 (0.12) | 1.14 (0.77, 1.68) | 0.52 | 0.56 |
| Genital organs | 5 (<0.01) | 0 (0.00) | |||
| Ovary | 22 (0.04) | 28 (0.05) | 0.81 (0.47, 1.43) | 0.47 | 0.16 |
| Uterus, not otherwise specified | 6 (<0.01) | 4 (<0.01) | 1.51 (0.43, 5.35) | 0.52 | 0.86 |
| Vulva | 1 (<0.01) | 1 (<0.01) | 1.10 (0.07, 17.66) | 0.95 | 0.85 |
| Lip, oral cavity, and pharynx (overall) | 8 (0.01) | 9 (0.01) | 0.94 (0.36, 2.44) | 0.90 | 0.99 |
| Oral (mouth) | 5 (<0.01) | 5 (<0.01) | 1.06 (0.31, 3.67) | 0.92 | 0.73 |
| Oropharaynx | 0 (0.00) | 1 (<0.01) | |||
| Parotoid gland (Stensen’s duct) | 2 (<0.01) | 0 (0.00) | |||
| Salivary glands, major (other/unspecified) | 1 (<0.01) | 0 (0.00) | |||
| Tongue, part of (other/unspecified) | 0 (0.00) | 3 (<0.01) | |||
| Malignant neoplasm of lymphatic and hematopoietic tissue (overall) | 61 (0.09) | 75 (0.11) | 0.85 (0.61, 1.19) | 0.35 | 0.49 |
| Leukemia | 16 (0.02) | 19 (0.03) | 0.89 (0.46, 1.74) | 0.74 | 0.87 |
| Lymphoma, Hodgkin’s | 2 (<0.01) | 5 (<0.01) | 0.40 (0.08, 2.07) | 0.28 | 0.24 |
| Lymphoma, non-Hodgkin’s | 31 (0.05) | 39 (0.06) | 0.83 (0.52, 1.33) | 0.44 | 0.84 |
| Multiple myeloma | 12 (0.02) | 12 (0.02) | 1.10 (0.60, 2.04) | 0.75 | 0.89 |
| Respiratory and intrathoracic organs (overall) | 62 (0.09) | 73 (0.11) | 0.88 (0.63, 1.24) | 0.47 | 0.78 |
| Larynx | 1 (<0.01) | 1 (<0.01) | 1.04 (0.07, 16.69) | 0.98 | 0.62 |
| Lung | 60 (0.09) | 71 (0.10) | 0.88 (0.62, 1.24) | 0.46 | 0.80 |
| Respiratory system | 1 (<0.01) | 1 (<0.01) | 1.00 (0.06, 15.94) | 0.99 | 0.99 |
| Thymus | 0 (0.00) | 0 (0.00) | |||
| Urinary (overall) | 26 (0.04) | 35 (0.05) | 0.77 (0.46, 1.27) | 0.30 | 0.38 |
| Bladder | 12 (0.02) | 15 (0.02) | 0.84 (0.39, 1.79) | 0.65 | 0.35 |
| Kidney | 14 (0.02) | 18 (0.03) | 0.79 (0.39, 1.59) | 0.52 | 0.89 |
| Ureter | 0 (0.00) | 0 (0.00) | |||
| Urinary organs (other/unspecified) | 0 (0.00) | 2 (<0.01) | |||
| Other and unspecified (overall) | 18 (0.03) | 15 (0.02) | 1.28 (0.65, 2.55) | 0.48 | 0.27 |
| Adrenal gland | 1 (<0.01) | 0 (0.00) | |||
| Brain | 5 (<0.01) | 5 (<0.01) | 1.05 (0.30, 3.63) | 0.94 | 0.86 |
| Endocrine glands | 0 (0.00) | 0 (0.00) | |||
| Eye and adnexa | 3 (<0.01) | 3 (<0.01) | 1.09 (0.22, 5.38) | 0.92 | 0.94 |
| Thyroid | 7 (0.01) | 7 (0.01) | 1.08 (0.38, 3.08) | 0.89 | 0.46 |
| Other/unknown | 2 (<0.01) | 0 (0.00) | |||
| Other ill defined sites | 0 (0.00) | 0 (0.00) | |||
| Unknown primary site | 9 (0.01) | 6 (<0.01) | 1.53 (0.54, 4.29) | 0.42 | 0.24 |
CaD = calcium plus vitamin D.
Subgroup Analyses
Analyses assessing the interaction of 18 subgroup categories and risk of incident cancer according to treatment assignment are shown in Fig. 1. Among women without a first-degree relative with cancer, fewer total invasive cancers were seen in the supplement group than in the placebo group [HR = 0.84 (0.72, 0.97), P < .02]. In the highest quartile (>600 units) of reported total vitamin D intake (diet plus personal supplement) at the time of WHI enrollment, more invasive cancers were seen in the treatment group than in the placebo group [HR = 1.22 (1.02, 1.45), P < .03]. Smoking status interacted (P < .04) with treatment assignment with past smokers (but not never or current smokers) having lower invasive cancer rates on CaD compared to placebo [HR = 0.87 (0.78, 0.98), P < .04]. We also assessed the interaction of CaD assignment according to hormone treatment trial (HT) assignment, the lowfat dietary trial (DM) assignment or both. There were significantly fewer invasive cancers among participants who were in the intervention arm of the dietary modification (lowfat, high fruits/vegetables) trial if they were randomized to CaD compared to placebo [HR = 0.83 (0.72, 0.96), P < .02]. No such effect was seen for assignment to either treatment or place in the HT trials.
FIG. 1.
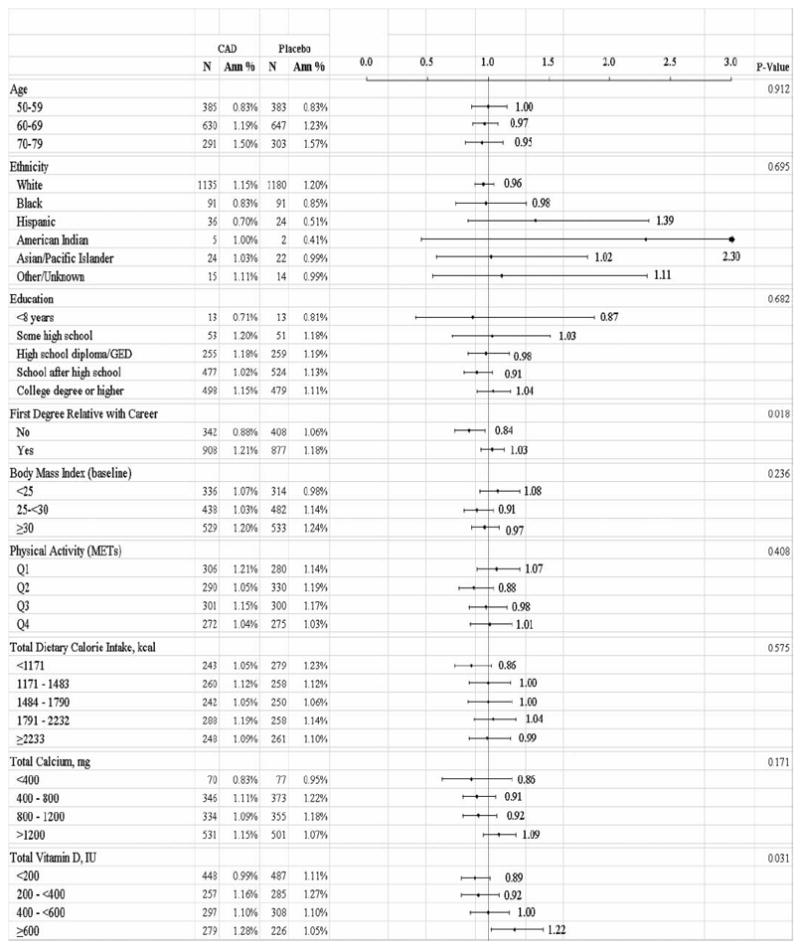
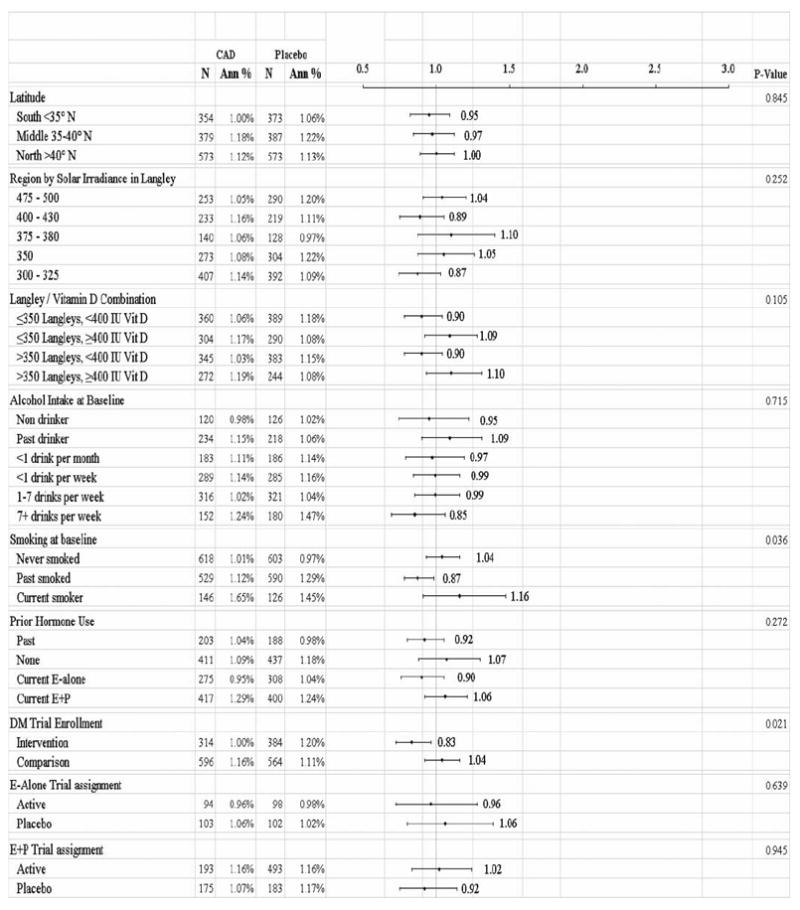
Annualized overall rate of invasive cancer, according to calcium plus vitamin D (CaD) and/or baseline characteristics by treatment group.
Cancer Mortality
Cancer mortality was not significantly different in the treatment and placebo groups. There were 315 (annualized percent, 0.26%) deaths in the treated group and 347 (0.28%) in the placebo group [HR = 0.90 (0.77, 1.05), unweighted P = 0.17]; and weighted P = 0.25.
DISCUSSION
In this randomized, double-blind, placebo-controlled trial, daily supplementation with 1,000 mg of elemental calcium combined with 400 IU of vitamin D3 did not have an effect on total invasive cancer incidence and cancer mortality. None of the specific primary sites of invasive cancers differed significantly between the treatment and control groups. A sensitivity analysis censoring follow-up at 6 mo after non-adherence was not significant as well, with 696 invasive cancers in the treatment and 768 in the placebo groups [0.94 (0.85, 1.05), P = 0.27].
The findings of the present study are not inconsistent with previous work. Few other studies have prospectively examined the association of calcium and vitamin D intake to the multiplicity of specific cancers or overall cancer risk. While colorectal cancer has been the most intensively investigated cancer in this context, there is not complete agreement as to what dietary factors protect against or promote its development (23). More specifically, the results of randomized trials have been inconsistent in linking calcium and vitamin D intake to incident risk or mortality (1). One recent meta-analysis of serum vitamin D levels from observational studies concluded that there was a consistent inverse relationship between serum 25-hydroxyvitamin D levels and colorectal cancer, but not for breast or prostate cancer (24). They note however that clinical trials have failed to demonstrate protection. The authors suggest an alternative hypothesis that vitamin D status may reflect an individual’s propensity to develop colorectal cancer rather than be the cause of that cancer. Many observers agree that higher intake levels of vitamin D (and/or more sun exposure) than represented in these earlier studies may be required to increase serum levels sufficiently for cancer protection (25,26). Additional clinical trials of calcium and vitamin D supplementation and disease outcomes are needed to adequately establish the Institute of Medicine’s Dietary Reference Intakes (DRIs) that were in place during the conduct of this trial (27) or the recently updated DRIs’ increasing the recommended intakes (28). The total dosage estimated to be required to achieve the proposed high levels of vitamin D are well beyond those used in WHI and some other randomized trials (29). Several reviews have concluded that evidence points to a protective effect of vitamin D for a range of cancer types (e.g., 30,31) but the source (sun, diet, supplements), parameters, and conditions for protection vary across studies and remain poorly understood.
The subgroup analyses indicated 4 significant interactions from among 18 tests performed and should not be viewed as conclusive given the possibility of chance effects with multiple testing. First, in the active treatment group compared to placebo there was a significant 14% lower incidence of invasive cancer among women without a first-degree relative with cancer. Similar or related findings have been reported in some studies (e.g., 32-34) but not others (e.g., 35). Studies have explored vitamin D receptor genes as an inherited mechanism that potentially could underlie such family history interaction effects (36). In the case of colon cancer, which has been studied the most intensively, thus far, variants in vitamin D-related genes that might modify the association between vitamin D levels and colon cancer risk have not supported a straightforward mechanism (37). Recently, an examination of the retinoid-X-receptor gene, which is a link in possible vitamin D anti-carcinogenic activity, was found to interact with vitamin D and calcium-rich food consumption to affect risk of renal cell carcinoma (36). Information on these genetic variants is not currently available in the WHI CaD cohort.
A second significant interaction suggested that in the highest quartile (>600 units) of self-reported total vitamin D intake (diet plus supplement), more invasive cancers occurred in the supplement group than in the placebo group. There are few prospective studies to draw on to support or refute this finding. Fedirko et al. (23) found the estimated treatment effects of vitamin D or calcium in reducing DNA damage were strongest among participants with higher baseline colon crypt vitamin D receptor expression. This appears counter to the present finding. Others have also demonstrated interactions between vitamin D levels and calcium on cancer risk (38). It is difficult to find relevant comparisons with earlier studies since most randomized trials have been of older women with deficient baseline levels of vitamin D (39). Since there are not serum 25-hydroxyvitamin D 25(OH)D levels available on all women in the highest quartile, we cannot assess whether 25(OH)D status, inclusive of sun exposure, is related to increased risk of disease. Previous research shows that high intake and supplementation levels do not necessarily agree with overall systemic vitamin D status as assessed by serum 25(OH)D(40).
A third significant interaction found that higher cancer rates were seen in smokers who were in the active arm of the CaD trial. However, previous smokers in the active arm had somewhat lower cancer rates than never smokers making interpretation of this finding unclear. Most studies adjust for smoking rather than examining possible interactions with calcium and/or vitamin D.
The fourth interaction showed that enrolment in the active dietary intervention (lowfat, high fruits and vegetables) trial arm together with the active CaD arm was associated with lower cancer rates than for women receiving usual diet and placebo. The dietary intervention alone produced a reduction that did not reach significance in breast cancer cases (41). This interaction suggests that a combination of positive interventions has achieved a threshold for protection. A recent meta-analysis indicated that the highest versus lowest categories of the “unhealthy” Western diet produced a small effect on breast cancer that was not significant (42), which was similar to the WHI dietary modification trial result. The possibility of a positive outcome by combining 2 weaker interventions (CaD supplementation and a healthy diet) is intriguing and warrants further exploration.
In WHI, it was shown that breast cancer was increased at the time of estrogen plus progesterone trial closure (43) and there was a greater risk of fatal and nonfatal malignancies 3 yr after stopping this randomized treatment compared with placebo (44). Estrogen alone, which was restricted to women with a hysterectomy did not similarly affect cancer incidence. Treatment with calcium and vitamin D did not produce significant interactions with hormone trial assignments.
Earlier, WHI investigators reported that calcium and vitamin D supplementation in the dosage provided in this trial did not reduce the incidence of invasive colorectal cancer or breast cancer in this cohort of postmenopausal women (2,3). In addition, in a subset of women with serum measures of 25-hydroxyvitamin D, levels were not found to be associated with subsequent breast cancer risk (3). Some meta-analyses of observational studies have recently supported a role of both calcium and vitamin D in lower breast cancer rates (45,46) although not all studies support the association (11,47,48). A recent meta analysis of breast cancer and serum 25 (OH)D levels observed that samples taken prior to diagnosis in nested designs were generally not found to be associated with protection, whereas serum D levels taken after diagnosis in case-control studies were associated with protection, suggesting timing of sample collection in relation to the steps in the carcinogenesis process may be important (49). In addition, several factors suggested by Chlebowski et al. (3) may explain conflicting results, including inadequate adjustment for important covariates (e.g., BMI and physical activity) and the level of vitamin D intake. In addition, genetic and environmental influences may independently affect vitamin D levels and breast cancer risk. Similar considerations may also be important in other cancers, including colorectal cancer.
There are studies providing evidence of a reduction of cancer risk related to calcium (and/or dairy intake) and vitamin D intake. In a randomized, placebo-controlled design, Lappe et al. (25) found a remarkable 60% to 77% reduction in relative risk of cancer in a population-based study of women who were not deficient serum levels of vitamin D and who increased their vitamin D intake by 1,100 IU. The NIH-AARP observational study found that calcium intake was associated with reduced total cancer in women but not men (50). Risk decreased with increases in calcium until about 1,300 mg/d, after which there was no additional benefit. Dairy food and calcium intakes were inversely associated with cancers of the digestive system [multivariate relative risk for the highest quintile of total calcium versus the lowest, HR = 0.84; (0.77–0.92) in men and HR = 0.77; (0.69–0.91) in women]. Decreased risk was particularly pronounced for colorectal cancer in this study. A recent case-control study also found a significant reduction in colon cancer (not rectal) after the fifth decile of vitamin D intake (51). Disparate results were found in the large Japan Public Health Center-based Prospective Study, which found a reduction in colon cancer in the highest quintile of dietary calcium intake compared with the lowest among men but not women and without a dose-response relation (52). No statistically significant association with dietary vitamin D intake was seen in either men or women. Again it appears that calcium/vitamin D is inconsistently associated with those cancers believed to be the most sensitive to their effects. However, since the WHI CaD trial was designed as a combined intervention, the comparison with studies that focus on the individual effects of either calcium or vitamin D intake is not straightforward.
The limitations of the WHI randomized trial of calcium and vitamin D have been described in earlier publications (2,3,19). To summarize, about one-quarter of the participants had stopped taking study pills by the end of the study, possibly compromising statistical power. A 7-yr long intervention trial may not be sufficiently long to see treatment effects. Any separate effects of calcium or vitamin D could not be determined as they were combined in the randomized treatment. Also, if carcinogenesis is a multistep process, the timing of the treatment may not have coincided with a critical process (53). Since the protocol did not restrict participant’s use of personal supplements, the possibility of seeing an effect if one had been present may have been diminished. The study dosage was similar to that recommended by the Institute of Medicine (54) but design of the trial did not have the benefit of prior clinical trials to inform dose selection. A recent review of clinical trials of vitamin D would classify the WHI as a “low-dose” study (55), with current trials of vitamin D including doses double or more than that in the WHI CaD trial. Whether higher doses of vitamin D may have beneficial effects remains to be proven. One small clinical trial of larger dose vitamin D with calcium supported a potential effect of 1,100 IU on total cancer (25).
In summary, calcium and vitamin D supplementation in the dosage provided in this trial did not reduce the incidence of invasive cancers or cancer mortality in postmenopausal women. However, women who received calcium and vitamin D and were in the active arm of the dietary modification trial had a significantly lower risk of developing cancer. Further exploration of this result and whether and how other factors, especially genomics, may influence this association is warranted.
Acknowledgments
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100–2, 32105–6, 32108–9, 32111–13, 32115, 32118–19, 32122, 42107–26, 42129–32, and 44221. The funding organization had representation on the steering committee, which governed the design and conduct of the study, the interpretation of the data, and approval of the article but did not participate in the preparation of the article. The corresponding author has full access to the data and made the final decision when and where to submit the article for publication.
R. T. Chlebowski has received a speaker’s fee and honorarium for advisory boards and consulting from AstraZeneca and Novartis; honorarium for advisory boards and consulting for Lilly, Amgen, and Pfizer; and grant support from Amgen. All of the authors have received grant support from National Institutes of Health; Robert L. Brunner and R. T. Chlebowski additionally have received grant support from the National Cancer Institute of Canada. M. L. S. Gass has received grant support from Wyeth.
We gratefully acknowledge the dedicated efforts of investigators and staff at the 40 WHI clinical centers, the WHI Clinical Coordinating Center, and the National Heart, Lung, and Blood Institute program office (listing available at http://www.whi.org). Most importantly, we recognize the WHI participants for their extraordinary commitment to the WHI program.
Footnotes
The remaining authors do not report conflicts of interest.
Publisher's Disclaimer: Full terms and conditions of use: http://www.tandfonline.com/page/terms-and-conditions
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan, sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Contributor Information
Robert L. Brunner, Department of Family and Community Medicine, University of Nevada School of Medicine, Reno, Nevada, USA
Jean Wactawski-Wende, Department of Social and Preventive Medicine, University of Buffalo, Buffalo, New York, USA.
Bette J. Caan, Division of Research, Kaiser Permanente, Oakland, California, USA
Barbara B. Cochrane, Family and Child Nursing, University of Washington, Seattle, Washington, USA
Rowan T. Chlebowski, Division of Medicine Oncology/Hematology, University of California–Torrance, LA BioMed at Harbor–UCLA Medical Center, Torrance, California, USA
Margery L. S. Gass, The North American Menopause Society, Mayfield Heights, Ohio, USA
Elizabeth T. Jacobs, Mel and Enid Zuckerman College of Public Health, Tucson, Arizona, USA
Andrea Z. LaCroix, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA
Dorothy Lane, Department of Preventive Medicine, School of Medicine, State University of New York at Stony Brook, Stony Brook, New York, USA.
Joseph Larson, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA.
Karen L. Margolis, HealthPartners Research Foundation, Minneapolis, Minnesota, USA
Amy E. Millen, Department of Social and Preventive Medicine, University of Buffalo, Buffalo, New York, USA
Gloria E. Sarto, University of Wisconsin Center for Women’s Health Research, Madison, Wisconsin, USA
Mara Z. Vitolins, PHS/Department of Epidemiology and Prevention, Health Sciences, Winston-Salem, North Carolina, USA
Robert B. Wallace, Department of Epidemiology, University of Iowa, Iowa City, Iowa, USA
References
- 1.International Agency for Research on Cancer: Vitamin D and Cancer. IARC Working Group Reports. Vol. 5. International Agency for Research on Cancer; Lyon, France: 2008. [Google Scholar]
- 2.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 3.Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski-Wende J, et al. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. 2008;100:1581–1591. doi: 10.1093/jnci/djn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters U, Chatterjee N, McGlynn KA, Schoen RE, Church TR, et al. Calcium intake and colorectal adenoma in a U.S. colorectal cancer early detection program. Am J Clin Nutr. 2004;80:1358–65. doi: 10.1093/ajcn/80.5.1358. [DOI] [PubMed] [Google Scholar]
- 5.Abbas S, Linseisen J, Slanger T, Kropp S, Mutschelknauss E, et al. Serum 25-hydroxyvitamin D and risk of postmenopausal breast cancer—results of a large case-control study. Carcinogenesis. 2008;29:93–99. doi: 10.1093/carcin/bgm240. [DOI] [PubMed] [Google Scholar]
- 6.Cui Y, Rohan TE. Vitamin D, calcium, and breast cancer risk: a review. Cancer Epidemiol Biomarkers Prev. 2006;15:1427–1437. doi: 10.1158/1055-9965.EPI-06-0075. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman DA, Prindiville S, Weiss DG, Willett W. VA Cooperative Study Group 380. risk factors for advanced colonic neoplasia and hyper-plastic polyps in asymptomatic individuals. JAMA. 2003;290:59–67. doi: 10.1001/jama.290.22.2959. [DOI] [PubMed] [Google Scholar]
- 8.Kampman E, Slattery ML, Caan B, Potter JD. Calcium, vitamin D, sunshine exposure, dairy products and colon cancer risk (United States) Cancer Causes Control. 2000;11:459–466. doi: 10.1023/a:1008914108739. [DOI] [PubMed] [Google Scholar]
- 9.Huncharek M, Muscat J, Kupelnick B. Colorectal cancer risk and dietary intake of calcium, vitamin D, and dairy products: a metaanalysis of 26,335 cases from 60 observational studies. Nutr Cancer. 2009;61:47–69. doi: 10.1080/01635580802395733. [DOI] [PubMed] [Google Scholar]
- 10.Sprague BL, Skinner HG, Trentham-Dietz A, Lee KE, Klein BE, et al. Serum calcium and breast cancer risk in a prospective cohort study. Ann Epidemiol. 2010;20:82–85. doi: 10.1016/j.annepidem.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsson SC, Bergkvist L, Wolk A. Long-term dietary calcium intake and breast cancer risk in a prospective cohort of women. Am J Clin Nut. 2009;89:277–282. doi: 10.3945/ajcn.2008.26704. [DOI] [PubMed] [Google Scholar]
- 12.Bertone-Johnson ER. Prospective studies of dietary vitamin D and breast cancer: more questions raised than answered. Nutr Rev. 2007;65:459–466. doi: 10.1111/j.1753-4887.2007.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 13.LaCroix AZ, Kotchen J, Anderson G, Brzyski R, Cauley JA, et al. Calcium plus vitamin D supplementation and mortality in postmenopausal women: the Women’s Health Initiative calcium-vitamin D randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2009;64:559–567. doi: 10.1093/gerona/glp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garland CF, Gorham ED, Mohr SB, Garland FC. Vitamin D for cancer prevention: global perspective. Ann Epidemiol. 2009;19:468–483. doi: 10.1016/j.annepidem.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Anderson GL, Manson J, Wallace R, Lund B, Hall D, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(Suppl):S5–S17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 16.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13(Suppl):S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 17.Ritenbaugh C, Patterson RE, Chlebowski RT, Caan B, Fels-Tinker L, et al. The Women’s Health Initiative Dietary Modification trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13(Suppl):S87–S97. doi: 10.1016/s1047-2797(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 18.Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, et al. The Women’s Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13(Suppl):S78–S86. doi: 10.1016/s1047-2797(03)00045-0. [DOI] [PubMed] [Google Scholar]
- 19.Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 20.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13(Suppl):S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 21.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, et al. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 22.Cox DR. Regression models and lifetables. J R Stat Soc [B] 1972;34:187–220. [Google Scholar]
- 23.Fedirko V, Bostick RM, Long Q, Flanders WD, McCullough ML, et al. Effects of supplemental vitamin D and calcium on oxidative DNA damage marker in normal colorectal mucosa: a randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2010;19:280–291. doi: 10.1158/1055-9965.EPI-09-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128:1414–1424. doi: 10.1002/ijc.25439. [DOI] [PubMed] [Google Scholar]
- 25.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 26.Vieth R. Vitamin D and cancer mini-symposium: the risk of additional vitamin D. Ann Epidemiol. 2009;19:441–445. doi: 10.1016/j.annepidem.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine: Vitamin D. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academy Press; 1997. pp. 250–287. [Google Scholar]
- 28.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garland CF, Gorham ED, Mohr SB, Grant WB, Giovannucci EL, et al. Vitamin D and prevention of breast cancer: pooled analysis. J Steroid Biochem Mol Biol. 2007;103:708–711. doi: 10.1016/j.jsbmb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Ingraham BA, Bragdon B, Nohe A. Molecular basis of the potential of vitamin D to prevent cancer. Curr Med Res Opin. 2008;24:139–149. doi: 10.1185/030079908x253519. [DOI] [PubMed] [Google Scholar]
- 31.Grant WB. How strong is the evidence that solar ultraviolet B and vitamin D reduce the risk of cancer? An examination using Hill’s criteria for causality. Dermato-Endocrinology. 2009;1:17–24. doi: 10.4161/derm.1.1.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sellers TA, Bazyk AE, Bostick RM, Kushi LH, Olson JE, et al. Diet and risk of colon cancer in a large prospective study of old: an analysis stratified on family history (Iowa, United States) Cancer Causes Control. 1998;9:357–367. doi: 10.1023/a:1008886715597. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez E, Gallus S, LaVecchia C, Talamini R, Negri E, et al. Family history and environmental risk factors for colon cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:658–661. [PubMed] [Google Scholar]
- 34.Ochs-Balcom HM, Cicek MS, Thompson CL, Tucker TC, Elston RC, et al. Association of vitamin D receptor gene variants, adiposity and colon cancer. Carcinogenesis. 2008;29:1788–1793. doi: 10.1093/carcin/bgn166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghadirian P, Boyle P, Simard A, Baillargeon J, Maisonneuve P, et al. Reported family aggregation of pancreatic cancer within a population-based case-control study in the Francophone community in Montreal, Canada. Int J Pancreato. 1991;10:183–196. doi: 10.1007/BF02924156. [DOI] [PubMed] [Google Scholar]
- 36.Karami S, Brennan P, Navratilova M, Mates D, Zaridze D, et al. Vitamin D pathway genes, diet, and risk of renal cell carcinoma. [November 5, 2009];Int J Endocrinol. 2010 :879362. doi: 10.1155/2010/879362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poynter JN, Jacobs ET, Figueiredo JC, Lee WH, Conti DV, et al. Genetic variation in the vitamin D receptor (VDR) and the vitamin D-binding protein (GC) and risk for colorectal cancer: results from the Colon Cancer Family Registry. Cancer Epidemiol Biomarkers Prev. 2010;19:525–536. doi: 10.1158/1055-9965.EPI-09-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahn J, Peters U, Albanes D, Purdue MP, Abnet CC, et al. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;100:796–804. doi: 10.1093/jnci/djn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 40.Millen AE, Wactawski-Wende J, Pettinger M, Melamed ML, Tylavsky FA, et al. Predictors of serum 25-hydroxyvitamin D concentrations among post-menopausal women: the Women’s Health Initiative Calcium plus Vitamin D Clinical Trial. Am J Clin Nutr. 2010;9:1324–1335. doi: 10.3945/ajcn.2009.28908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, et al. Lowfat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2009;295:629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 42.Brennan SF, Cantwell MM, Cardwell CR, Velentzis LS, Woodside JV. Dietary patterns and breast cancer risk: a systematic review and meta-analysis. Am J Clin Nutr. 2010;91:1294–1302. doi: 10.3945/ajcn.2009.28796. [DOI] [PubMed] [Google Scholar]
- 43.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 44.Heiss G, Wallace R, Anderson GL, Aragaki A, Beresford SA, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299:1036–1045. doi: 10.1001/jama.299.9.1036. [DOI] [PubMed] [Google Scholar]
- 45.Chen P, Hu P, Xie D, Qin Y, Wang F, et al. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2009;121:469–477. doi: 10.1007/s10549-009-0593-9. [DOI] [PubMed] [Google Scholar]
- 46.Gissel T, Rejnmark L, Mosekilde L, Vestergaard P. Intake of vitamin D and risk of breast cancer—a meta-analysis. J Steroid Biochem Mol Biol. 2008;111:195–199. doi: 10.1016/j.jsbmb.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Lin J, Manson JE, Lee IM, Cook NR, Buring JE, et al. Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med. 2007;267:1050–1059. doi: 10.1001/archinte.167.10.1050. [DOI] [PubMed] [Google Scholar]
- 48.Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, et al. Intake of dairy products, calcium, and vitamin D and risk of breast cancer. J Natl Cancer Inst. 2002;94:1301–1311. doi: 10.1093/jnci/94.17.1301. [DOI] [PubMed] [Google Scholar]
- 49.Yin L, Grandi N, Raum E, Haug U, Arndt V, et al. Meta-analysis: serum vitamin D and breast cancer risk. Eur J Cancer. 2010;46:2196–2205. doi: 10.1016/j.ejca.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 50.Park Y, Leitzmann MF, Subar AF, Hollenbeck A, Schatzkin A. Dairy food, calcium, and risk of cancer in the NIH-AARP Diet and Health Study. Arch Intern Med. 2009;169:391–401. doi: 10.1001/archinternmed.2008.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lipworth L, Rossi M, McLaughlin JK, Negri E, Talamini R, et al. Dietary vitamin D and cancers of the oral cavity and esophagus. Ann Oncol. 2009;20:1576–1581. doi: 10.1093/annonc/mdp036. [DOI] [PubMed] [Google Scholar]
- 52.Ishihara J, Inoue M, Iwasaki M, Sasazuki S, Tsugane S. Dietary calcium, vitamin D, and the risk of colorectal cancer. Am J Clin Nutr. 2008;88:1576–1583. doi: 10.3945/ajcn.2008.26195. [DOI] [PubMed] [Google Scholar]
- 53.Bresalier RS. Chemoprevention of colorectal cancer: why all the confusion? Curr Opin Gastroenterol. 2008;24:48–50. doi: 10.1097/MOG.0b013e3282f31d36. [DOI] [PubMed] [Google Scholar]
- 54.Institute of Medicine Food and Nutrition Board. Dietary Reference Intakes: A Risk Assessment Model for Establishing Upper Intake Levels for Nutrients. National Academy Press; Washington, DC: 1998. [PubMed] [Google Scholar]
- 55.Bischoff-Ferrari HA, Shao A, Dawson-Hughes B, Hathcock J, Giovannucci E, et al. Benefit-risk assessment of vitamin D supplementation. Osteoporos Int. 2009;2:1121–1132. doi: 10.1007/s00198-009-1119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


