Abstract
Little is known about transient effects of foods and nutrients on reactivity to mental stress. In a randomized crossover study of healthy adults (n = 20), we measured heart rate variability (respiratory sinus arrhythmia), blood pressure, and other hemodynamic variables after three test meals varying in type and amount of fat. Measurements were collected at rest and during speech and cold pressor tasks. There were significant post-meal changes in resting diastolic blood pressure (−4%), cardiac output (+18%), total peripheral resistance (−17%), and interleukin-6 (−27%). Heart rate variability and hemodynamic reactivity to stress was not affected by meal content. We recommend that future studies control for time since last meal and continue to examine effects of meal content on heart rate variability.
Keywords: Cardiovascular reactivity, heart rate variability, polyunsaturated fatty acids, psychological stress, flax, nutrition
Globally, hypertension is the leading risk factor for mortality (World Health Organization, 2009), and in the United States, it affects approximately 1 in 3 adults (Roger et al., 2011). The reactivity hypothesis posits that individuals who exhibit exaggerated reactivity to stress are at greater risk of developing hypertension. A recent meta-analysis of 36 studies concluded that greater stress reactivity consistently predicts higher blood pressure and incident hypertension up to 36 years later, independent of traditional risk factors (Chida & Steptoe, 2010). Sustained elevations in blood pressure are likely due to chronic elevation of cardiac output and total peripheral resistance (Brownley, Hurwitz, & Schneiderman, 2000).
Diet is also implicated in the development of hypertension. The well-known Dietary Approaches to Stop Hypertension (DASH) diet studies have shown that a diet rich in fruits, vegetables, and low-fat dairy and low in saturated fat can reduce blood pressure by 11.4/5.5 mmHg (systolic/diastolic) in as little as eight weeks (Appel et al., 1997). Consumption of polyunsaturated fats, such as those found in nuts, seeds, and fish, is also associated with lower blood pressure (Morris & Sacks, 1994; Puska et al., 1983). Polyunsaturated fats may benefit cardiovascular health via decreases in peripheral vascular resistance and blood pressure (West et al., 2010), antiarrhythmic effects (Albert et al., 2005; Burr et al., 1989; GISSI-Prevenzione, 1999), enhanced heart rate variability and vagal tone (Mozaffarian et al., 2005), or improved vascular function (Ros et al., 2004; West, et al., 2010). In contrast, the atherosclerotic properties of saturated fat and cholesterol may elevate blood pressure by decreasing vessel size and elasticity (Tortora & Grabowski, 2000). There is additional evidence from animal studies that high saturated fat diets are associated with increases in sympathetic nervous system activity (Kaufman, Peterson, & Smith, 1991; Prior et al., 2010; Schwartz, Young, & Landsberg, 1983). For example, rats fed a high-fat diet for 12 weeks had significantly greater renal sympathetic nerve activity compared to regular chow-fed rats (Barnes et al., 2003).
Despite the independent associations of poor diet and exaggerated reactivity with hypertension, the potential effect of recent meals or dietary patterns on reactivity has been less well studied. Of the 36 studies analyzed by Chida & Steptoe (2010), only one considered background diet in the analyses and only three imposed fasting periods prior to testing to control for acute effects of eating. Dietary patterns over relatively short-term periods have been associated with changes in reactivity. For example, blood pressure and total peripheral resistance during stress were reduced after six weeks of a diet high in polyunsaturated fat compared to a diet high in saturated fat (West, et al., 2010). Straznicky and colleagues reported that stress reactivity was significantly lower after two weeks on a low fat diet compared to a high saturated fat diet (Straznicky, Louis, McGrade, & Howes, 1993).
In addition to habitual diet, hemodynamics and reactivity can be affected by a single meal. Studies have demonstrated postprandial increases in resting heart rate, cardiac output, and stroke volume, and decreases in pre-ejection period, total peripheral resistance, and heart rate variability (Chang, Ko, Lien, & Chou, 2010; Cozzolino et al., 2010; DeMey, Hansenschmidt, Enterling, & Meineke, 1989; Host et al., 1996; Kearney, Cowley, Stubbs, & Macdonald, 1996; Sidery, Cowley, & Macdonald, 1993; Sidery, Macdonald, Cowley, & Fullwood, 1991; Tentolouris et al., 2003; Uijtdehaage, Shapiro, & Jaquet, 1994). These postprandial shifts have minimal effect on the magnitude of stress responses when compared to fasting conditions (Uijtdehaage, et al., 1994), but meal content (relative amounts of fat, protein, and carbohydrates) may moderate postprandial stress reactivity. A high carbohydrate meal was reported to augment reactivity compared to a high protein meal (Uijtdehaage, et al., 1994), and greater reactivity was observed after a meal high in saturated fat compared to a meal with minimal saturated fat (Faulk & Bartholomew, 2011; Jakulj et al., 2007). However, no previous studies have compared the relative effect of a single meal containing saturated vs unsaturated fat on postprandial stress reactivity.
The mechanisms responsible for postprandial differences in reactivity are not clear, but may be attributed to the body’s response to glucose (found in carbohydrates) and saturated fat. Carbohydrate consumption may influence hemodynamics through the action of insulin, which is released in response to the glucose in carbohydrates. Insulin has vasodilatory properties (Baron, 1994), and also appears to induce sympathetic nervous system activity. Plasma norepinephrine and muscle sympathetic nerve activity reportedly increase following meals high in carbohydrates compared to high fat or high protein meals (Baliga, Burden, Sidhu, Rampling, & Kooner, 1997; Berne, Fagius, Pollare, & Hjemdahl, 1992; Fagius & Berne, 1994; Heseltine, Potter, Hartley, Macdonald, & James, 1990; Potter et al., 1989; Sidery, et al., 1991).
Dietary fat may influence hemodynamics via changes in vascular structure and function. Saturated fat and triglycerides substantially impair vascular endothelial function (Giannattasio et al., 2005; Vogel, Corretti, & Plotnick, 1997; Williams et al., 1999), a cardiovascular risk factor that may contribute to the development and progression of hypertension (Beevers, Lip, & O’Brien, 2001; Celermajer, 1997). In contrast, polyunsaturated fat appears to improve vascular endothelial function up to four hours after a single meal (Cortes et al., 2006; Ros, et al., 2004; West et al., 2005). Polyunsaturated fats may also reduce blood pressure by attenuating total peripheral resistance (West, et al., 2010). No previous studies have examined whether the opposing effects of saturated and polyunsaturated fat on vascular function translate into different hemodynamic responses to stress following a single meal, and no studies have determined whether changes in insulin or lipids mediate the acute effects of eating different meals onreactivity.
We designed a randomized crossover study to examine the acute effects of standardized meals on cardiovascular reactivity. Resting hemodynamics, heart rate variability, and metabolic parameters were assessed in the fasting state, two hours after consumption of test meals, and during exposure to standard laboratory speech and cold pressor tasks. Similar to our previous study, we compared a high saturated fat/low carbohydrate meal to a low saturated fat/high carbohydrate meal (Jakulj, et al., 2007). We hypothesized that reactivity would be greater after the saturated fat meal compared to the carbohydrate meal, and that differences in insulin and lipids would mediate these effects. Due to the significant improvements in vascular function observed after meals high in polyunsaturated fats (Cortes, et al., 2006; Ros, et al., 2004; West, et al., 2010), we also included a high polyunsaturated fat/low carbohydrate meal containing fatty acids from flax seed and flax oil. We hypothesized that reactivity following the polyunsaturated fat meal would be attenuated compared to the saturated fat meal due to vascular changes, and augmented compared to the carbohydrate meal due to differences in insulin.
Methods
Participants
Twenty healthy young adults (7 females) ages 20–31 years with a body mass index (BMI; kg/m2) between 19 and 29 participated in the study. Sixty percent were White, 30% Asian, and 10% African-American. Prior to study enrollment, potential participants completed a phone interview in which a research assistant explained the study and reviewed the individual’s medical history. Individuals who self-reported having a history of hypertension, cardiovascular disease, diabetes, sleep disorders, anxiety, depression, peripheral artery disease, hemophilia, celiac disease, or allergies to food, latex, or adhesive tape were excluded. Self-identified current smokers, pregnant women, or individuals unable to follow study procedures were also excluded. Due to blood draw difficulties and technical problems, blood-derived data were available from 19 participants and hemodynamic and heart rate variability data were available from 18 participants. Written informed consent was obtained from all participants, and approval for the study was granted by the Pennsylvania State University Institutional Review Board.
Experimental Design
The study was a randomized, 3-period, crossover design. Participants were instructed to maintain their normal diet in the week prior to each visit. Visits were scheduled for the afternoon and time of day was held constant for each participant. Instructions were given for a low fat meal to be consumed 6 hours prior to the testing session and standardized instructions were provided. Participants were asked to fast and abstain from alcohol and caffeine for 6 hours prior to each visit. Cardiovascular parameters were measured during 20 min of rest, immediately followed by collection of a blood sample and consumption of one of three test meals. Two hours later, an additional blood sample was collected and cardiovascular parameters were measured during a 20 min rest period, two stress tasks (described below), and two recovery periods. Identical procedures were followed on the second and third testing days, which took place a minimum of 24 hours apart. The order of meal presentation was randomized and counterbalanced. All women were tested during the early follicular phase of their menstrual cycle via self-report.
Macronutrient Profiles of the Test Meals
Three standardized meals were prepared in a metabolic kitchen: 1) a high saturated fat meal (SatFat), 2) a low fat meal (Control), and 3) a meal high in polyunsaturated fat from flax oil and flax seed (Flax). The meals were equivalent with regard to total calories, protein, cholesterol, and sodium (Table 1). The Control meal contained little fat, the Flax meal was rich in mono-and polyunsaturated fat and contained relatively little saturated fat, and the SatFat meal was rich in saturated fat and contained relatively little mono- and polyunsaturated fat. The SatFat and Flax meals were relatively low in carbohydrates, and the Control meal was rich in carbohydrates.
Table 1.
Nutrient composition of test meals
| Nutrient | Unsaturated Fat Flax Meal | High Saturated Fat Meal | Low Fat Control Meal |
|---|---|---|---|
| Calories (kcal) | 855 | 826 | 840 |
| Total fat (g) | 45.3 | 45.4 | 3.4 |
| Saturated fat (g) | 5.8 | 14.5 | 1.1 |
| MUFA (g) | 10.2 | 16.2 | 0.8 |
| PUFA (g) | 26.4 | 11.2 | 1.3 |
| Omega-3 (g) | 20.3 | 1.0 | 0.1 |
| Omega-6 (g) | 6.0 | 9.9 | 1.1 |
| Carbohydrates (g) | 79.0 | 71.6 | 183.1 |
| Protein (g) | 31.7 | 32.5 | 23.5 |
| Cholesterol (mg) | 269.9 | 271.2 | 8.3 |
| Fiber (g) | 8.4 | 5.9 | 6.1 |
| Sodium (mg) | 1541.0 | 1497.0 | 1504.0 |
Note. Values determined with Nutrition Data System for Research Software, 2006. MUFA = monounsaturated fatty acids; PUFA = polyunsaturated fatty acids.
The SatFat meal was modeled on the fast food meal used in our previous study. It consisted of two breakfast sandwiches (English muffin with egg and Canadian bacon or pork sausage), liquid margarine, and hashed brown potatoes. The Control meal included frosted corn flakes, bran buds, skim milk, light fat-free yogurt, bread, fat-free margarine spread, jelly, and an orange drink. The Flax meal consisted of banana flax muffins (containing a total of 10.5g of flaxseed and 32g of flax oil), one hard-boiled egg, fat-free margarine spread, scrambled egg substitute, salt and pepper. Participants drank water or a decaffeinated beverage. Meals were consumed within 20 min.
Physiological Measurements
Systolic and diastolic blood pressures were obtained at 1–4-min intervals (depending on task) with an automatic oscillometric blood pressure monitor attached to the left arm (Dinamap, Critikon Pro 100, GE Medical Systems). Impedance cardiography utilizing a tetrapolar band configuration with spot electrocardiographic (ECG) electrodes was used to estimate heart rate, stroke volume, and pre-ejection period (Hutcheson Impedance Cardiograph and the Cardiac Output Program, Bio-Impedance Technology, Inc., Chapel Hill, NC). Cardiac output and total peripheral resistance were calculated according to standard formulae (Sherwood et al., 1990).
Heart rate variability is a noninvasive method used to index autonomic influence on cardiac function by analyzing the beat-to-beat fluctuations in R-R interval (Task Force, 1996). Spectral analysis of the interbeat fluctuations allows identification of frequency bands with their own physiological determinants (Akselrod et al., 1981). Oscillations in the high frequency (HF) band (0.15 Hz to 0.4 Hz) are believed to reflect vagal modulation of heart rate. We assessed heart rate variability by sampling raw interbeat intervals (R-R) at 1,000 Hz from the ECG. The R-R interval sequences were visually inspected, and the data considered artifactual were manually replaced by interpolated or extrapolated data. HF heart rate variability was calculated with autoregressive spectra using commercial software (Nevrokard, Medistar Inc.) and standard methods (Boardman, Schlindwein, Rocha, & Leite, 2002). Values were normalized for total power excluding very low frequency power (e.g., HF/[total power very − low frequency]*100), and did not require further transformation for statistical normality (skew = −0.115).
Venous blood samples were collected during each visit in the fasting state and two hours following the test meals. Participants were given the option of intravenous catheterization or separate venipunctures for the two blood draws; one participant chose catheterization and this was held constant across visits. Blood was analyzed at a local clinical laboratory for insulin, glucose, total cholesterol, high density lipoprotein (HDL) cholesterol, and triglycerides. Low density lipoprotein (LDL) cholesterol was not examined because of the substantial postmeal increases in triglycerides, which render equations for estimating LDL cholesterol unreliable. Serum concentrations of the inflammatory cytokines interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-α (TNFα) were measured via high sensitivity enzyme-linked immunosorbent assays (ELISA) kits from R&D Systems (Minneapolis, MN) in duplicate (assay CV < 11% for all). High sensitivity C-reactive protein (CRP) was measured by latex-enhanced immunonephelometry (Quest Diagnostics, Pittsburgh, PA; assay CV < 8%).
Stress Tasks
In keeping with several previous studies (West, et al., 2010; West, Likos-Krick, Brown, & Mariotti, 2005), participants were given 2 min to prepare for a 3 min speech on one of three hypothetical situations (being falsely accused of shoplifting, being prevented from boarding a flight, and receiving a traffic ticket). Participants were told that the speech would be videotaped and evaluated by the researchers for content and clarity. During the cold pressor task, participants immersed one foot up to the ankle into 4°C water for 2.5 min. Each task was followed by a 10 min recovery period. Means were calculated for each of the seven conditions (fasting baseline, post-meal rest, speech prep, speech task, recovery 1, cold pressor, recovery 2).
Statistical Analysis
The study was designed with 80% power and alpha set at 0.05 to detect significant between-treatment differences in diastolic blood pressure of 4.5 mmHg and total peripheral resistance of 47.8 dyne sec-cm5, based on our previous study (Jakulj, et al., 2007). Variables were tested for normality; insulin, triglycerides, IL-1β, and CRP were log-transformed. The mixed models procedure in SAS (v.9.2, Cary, NC) was used to test the effects of treatment, condition, visit, and their interactions. Following the published guidelines for use of mixed models in psychophysiological research (Bagiella, Sloan, & Heitjan, 2000), model fit was evaluated using several covariance structures. Compound symmetry was selected for the final model. The first set of analyses examined changes in resting values from pre-meal to post-meal. Preliminary models included treatment, condition (fasting or post-meal), visit, their interactions, age, sex, and BMI. The treatment x visit interaction, age, sex, and BMI did not significantly change the results and were removed from the final model. Next, we examined the effects of meal composition on heart rate variability and hemodynamic reactivity to stress assessed two hours after the meals. Stress data were analyzed as reactivity change scores (calculated as task level minus postmeal rest). Preliminary models included treatment, condition (speech prep, speech task, recovery 1, cold pressor, and recovery 2), visit, their interactions, age, sex, and BMI. Treatment x condition, treatment x visit, age, sex and BMI did not significantly change the results and were removed from the final model. Significant effects (p ≤ 0.05) were further examined with Tukey’s post-hoc test. Effect sizes reflect Cohen’s d (Cohen, 1988). Tables and the figure depict unadjusted means ± SE. Effect sizes were calculated using adjusted means from the mixed models procedure, which are within-group means adjusted for random effects and unbalanced groups.
Results
Effects of Eating on Resting Measures: Pre- to Post-meal Comparisons
Eating a meal, regardless of its macronutrient composition, was associated with significant shifts in many of the hemodynamic and autonomic variables. As shown in Table 2, resting diastolic blood pressure, F(1,84) = 14.17, p < .001, Cohen’s d = −0.51, pre-ejection period, F(1,81) = 280.42, p < .001, Cohen’s d = −1.33, total peripheral resistance, F(1,83) = 148.13, p < .001, Cohen’s d = −1.27, and HF heart rate variability, F(1,82) = 5.45, p < .05, Cohen’s d = −0.28, were significantly lower two hours after the meals compared to the fasting state. We observed significant post-meal increases in heart rate, F(1,84) = 22.11, p < .001, Cohen’s d = 0.52, stroke volume, F(1,83) = 38.52, p < .001, Cohen’s d = 0.51, and cardiac output, F(1,83) = 122.23, p < .001, Cohen’s d = 1.44. Meal content had no effect on hemodynamic or heart rate variability measurements under resting conditions, Fs(2,81–84) = 0.16–1.54, ps > .10.
Table 2.
Effects of a meal on systemic hemodynamics and heart rate variability
| Fasting | Post-Meal | Cohen’s d | |
|---|---|---|---|
| Systolic Blood Pressure (mmHg) | 108.5 ± 1.2 | 108.7 ± 1.2 | 0.02 |
| Diastolic Blood Pressure (mmHg)*** | 63.2 ± 0.8 | 60.7 ± 0.8 | −0.51 |
| Heart Rate (bpm)*** | 62.7 ± 1.2 | 67.1 ± 1.3 | 0.52 |
| Stroke Volume (ml/beat)*** | 99.7 ± 2.8 | 109.7 ± 3.0 | 0.51 |
| Cardiac Output (l/min)*** | 6.1 ± 0.1 | 7.2 ± 0.1 | 1.44 |
| Pre-Ejection Period (ms)*** | 123.2 ± 1.4 | 110.5 ± 1.4 | −1.33 |
| Total Peripheral Resistance (dyne·sec-cm5)*** | 1043.9 + 23.7 | 867.7 + 17.7 | −1.27 |
| HF Heart Rate Variability (nu)* | 62.3 ± 2.6 | 57.5 ± 2.5 | −0.28 |
Note. Data are unadjusted means ± SE of within-subject contrasts, n=18. Cohen’s d was calculated using adjusted means from the mixed models procedure. HF = high frequency.
p < .05,
p < .01,
p < .001
Fasting and postprandial means of metabolic variables are displayed in Table 3. Treatment x condition interactions emerged for triglycerides, F(2,85) = 9.75, p < .001, and insulin, F(2,87) = 13.41, p < .001. Postprandial triglycerides were significantly greater following the Flax (M = 172.9, SE = 22.1, Tukey p < .001, Cohen’s d = 0.69) and SatFat meals (M = 176.4, SE = 31.1, Tukey p < .001, Cohen’s d = 0.77) than Control meal (M = 111.0, SE = 18.0). Conversely, postprandial insulin was significantly lower following the Flax (M = 37.3, SE = 8.0, Tukey p < .001, Cohen’s d = −0.88) and SatFat meals (M = 27.1, SE = 6.8, Tukey p < .001, Cohen’s d = −1.17) than Control meal (M = 73.6, SE = 19.6). The Flax and SatFat meals did not differ from each other with regard to postprandial triglycerides or insulin. At two hours post-meal, glucose, F(1,87) = 9.85, p < .01, Cohen’s d = −0.73, HDL cholesterol, F(1,85) = 9.21, p < .01, Cohen’s d = −0.21, and IL-6, F(1,85) = 26.27, p < .001, Cohen’s d = −0.69, were significantly decreased relative to the fasting baseline. There were no significant differences in fasting vs post-meal levels of total cholesterol, F(1,85) = 1.72, p > .10, Cohen’s d = −0.06, CRP, F(1,71) = 0.48, p > .10, Cohen’s d = −0.06, TNFα, F(1,84) = 3.16, p > .05, Cohen’s d = −0.25, and IL-1β, F(1,85) = 0.71, p > .10, Cohen’s d = −0.11.
Table 3.
Effects of a meal on metabolic and inflammatory parameters
| Fasting | Post-Meal | Cohen’s d | |
|---|---|---|---|
| Total Cholesterol (mg/dl) | 166.7 ± 4.7 | 164.7 ± 4.4 | −0.06 |
| HDL Cholesterol (mg/dl)** | 52.8 ± 1.8 | 50.1 ± 1.8 | −0.21 |
| Triglycerides (mg/dl)***§ | 101.5 ± 8.3 | 152.7 ± 14.3 | 0.72 |
| Glucose (mg/dl)** | 87.3 ± 0.9 | 79.9 ± 2.5 | −0.73 |
| Insulin (μg/ml)***§ | 9.3 ± 0.4 | 46.1 ± 7.9 | 2.46 |
| IL-1β (pg/ml) | 0.30 ± 0.1 | 0.25 + 0.1 | −0.11 |
| IL-6 (pg/ml)*** | 0.82 ± 0.1 | 0.60 ± 0.0 | −0.69 |
| TNFα (pg/ml) | 2.95 ± 0.1 | 2.75 ± 0.1 | −0.25 |
| CRP (mg/l) | 1.06 ± 0.2 | 0.98 ± 0.2 | −0.06 |
Note. Data are unadjusted means ± SE of within-subject contrasts, n = 19. Cohen’s d was calculated using adjusted means from the mixed models procedure. HDL = high density lipoprotein; IL-1β = interleukin-1β IL-6 = interleukin-6; TNFα = tumor necrosis factor-α; CRP = C-reactive protein.
p < .05,
p < .01,
p < .001
Significant treatment x condition interaction; triglycerides and insulin following the Control meal significantly different from the Flax and SatFat meals.
Effects of Meal Composition on Cardiovascular and Autonomic Reactivity to Acute Stress
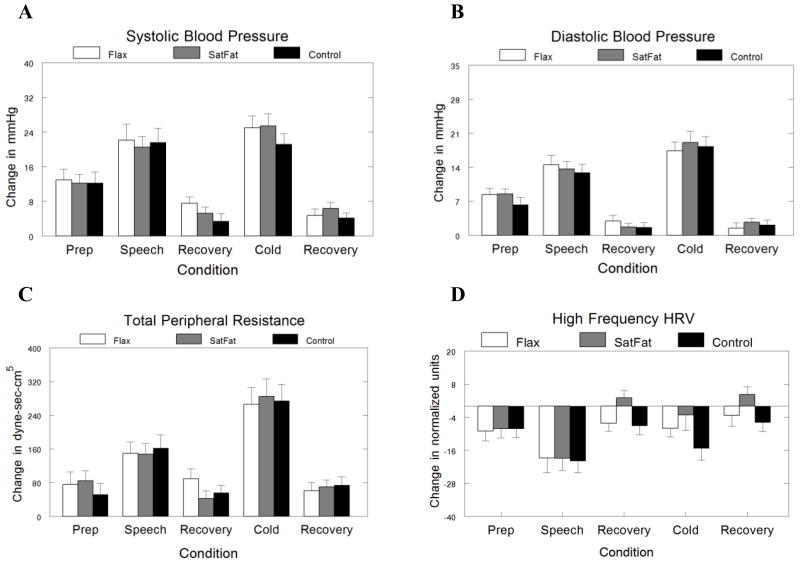
Contrary to our hypotheses, meal composition had no effect on hemodynamic or heart rate variability reactivity, Fs(2,228–250) = 0.06−1.76, ps > .10. As expected, the stressors produced significant changes in hemodynamics and heart rate variability (see Figure). Relative to the post-meal baseline, the speech task increased systolic blood pressure, F(4, 236) = 68.50, Tukey p < .001, Cohen’s d = 2.94, diastolic blood pressure, F(4,238) = 91.13, Tukey p < .001, Cohen’s d = 3.24, heart rate, F(4,233) = 34.46, Tukey p < .001, Cohen’s d = 2.11, total peripheral resistance, F(4,234) = 43.53, Tukey p < .001, Cohen’s d = 1.96, and decreased stroke volume, F(4,237) = 10.80, Tukey p < .001, Cohen’s d = −2.15, pre-ejection period, F(4,225) = 21.60, Tukey p < .001, Cohen’s d = −0.60, and HF heart rate variability, F(4,212) = 14.17, Tukey p < .001, Cohen’s d = −2.23. The cold pressor increased systolic blood pressure, F(4, 236) = 68.50, Tukey p < .001, Cohen’s d = 3.26, diastolic blood pressure, F(4,238) = 91.13, Tukey p < .001, Cohen’s d = 4.32, heart rate, F(4,233) = 34.46, Tukey p < .001, Cohen’s d = 1.27, total peripheral resistance, F(4,234) = 43.53, Tukey p < .001, Cohen’s d = 3.44, pre-ejection period, F(4,225) = 21.60, Tukey p < .001, Cohen’s d = 0.52, and decreased stroke volume, F(4,237) = 10.80, Tukey p < .001, Cohen’s d = −2.14, and HF heart rate variability, F(4,212) = 14.17, Tukey p < .001, Cohen’s d = −1.03.
Discussion
This study examined the effect of consuming meals differing in macronutrient content on heart rate variability and systemic hemodynamics at rest and during mental stress in healthy young adults. Regardless of meal content, there were significant postprandial changes in resting measures of hemodynamics and autonomic function. On average, resting diastolic blood pressure decreased by 2.5 mmHg and cardiac output was reduced by 1.1 l/min two hours postprandially. Similarly, HF heart rate variability decreased by 4.8 nu, indicating a postprandial decrease in cardiac parasympathetic tone (Berntson et al., 1997; Task Force, 1996). While the observed changes may seem modest, even small differences in hemodynamics have been associated with reductions in cardiovascular risk (Liao et al., 1997; MacMahon et al., 1990).
As expected, significantly larger increases in triglycerides and smaller increases in insulin were observed two hours after the SatFat and Flax meals compared to the Control meal. Cholesterol and glucose were significantly lower two hours after the meals compared to fasting values, which corresponds to the known time course of nutrient metabolism in non-diabetic individuals (Ginsberg et al., 1994; Matsuda & DeFronzo, 1999). In contrast to previous reports of more favorable inflammatory profiles following meals low in saturated fat compared to meals high in saturated fat (Blum, Aviram, Ben-Amotz, & Levy, 2006; Ceriello et al., 2005), we did not observe differential effects of meal content on postprandial inflammation. Only IL-6 changed postprandially, and it decreased uniformly after all three meals. It is possible that the low baseline levels of inflammation in these healthy participants prevented detection of meal-induced differences in inflammation, or that our SatFat meal was not optimally designed to induce inflammation. Given the recent interest in IL-6 reactivity to stress (Chida & Steptoe, 2010), it is critical that future studies account for the postprandial decline in IL-6.
In our previous study, we demonstrated that commercially available breakfast foods high in saturated fat (similar to the meal prepared for this study) significantly augmented blood pressure and vascular resistance responses to mental stress presented two hours later (Jakulj, et al., 2007). We failed to replicate that finding in the present study. Although our high saturated fat meal was designed to be as similar as possible to the previously used fast food breakfast, the food for the present study was freshly prepared in the research kitchen. It is possible that differences in cooking methods or types of oil used may have affected our results. Furthermore, we intentionally designed the meals to minimize differences in fiber, sodium, and protein, and controlling these nutrients in the current study may have prevented us from replicating our earlier results.
We did observe the expected differences in postprandial insulin and triglyceride responses to the high saturated and polyunsaturated meals compared to the low fat (high carbohydrate) Control meal. However, these varying metabolic responses did not appear to moderate stress reactivity. As insulin is associated with both vasodilation (Baron, 1994) and enhanced sympathetic activity (Baliga, et al., 1997; Berne, et al., 1992; Fagius & Berne, 1994; Heseltine, et al., 1990; Potter, et al., 1989; Sidery, et al., 1991), it is possible that these opposing properties combined to create a null effect of insulin on hemodynamics in the present study. Elevated triglyceride levels have been inversely associated with impaired vascular endothelial function in acute settings (Vogel, et al., 1997; West, Hecker, et al., 2005); however, the triglyceride increase following the two high fat meals did not appear to affect vascular reactivity assessed by total peripheral resistance. Our results provide evidence that unique metabolic profiles induced by single meals differing in macronutrient content have no acute effect on hemodynamic responses to stress.
To our knowledge, this is the first study that has examined the effects of meal content on autonomic reactivity to stress. Larger reductions in vagally-mediated heart rate variability during acute stress have been prospectively associated with increases in diastolic blood pressure three years later (Matthews, Salomon, Brady, & Allen, 2003; Steptoe & Marmot, 2005). In the present study, HF heart rate variability reactivity was not differentially affected by meals eaten two hours prior to testing. However, resting HF heart rate variability was significantly lower following the meal compared to fasting values. Future studies should assess whether this postprandial shift in HF heart rate variability affects reactivity magnitude compared to a fasting state.
Our study has several limitations. First, our sample consisted of healthy, young adults, and therefore these results cannot be generalized to individuals with medical conditions affecting metabolism. Diet-related therapies have a greater effect on blood pressure in hypertensives than normotensives (Appel, et al., 1997), and it is possible that different results would have been obtained if we had enrolled individuals with elevated blood pressure. We acknowledge that the sample size is modest. However, our use of a repeated measures design increases statistical power. It is important to note that our sample size is comparable to the work by Uijtdehaage and colleagues (1994). Age may also be an important consideration because symptomatic postprandial hypotension is known to be more prevalent in older adults, and the underlying hemodynamic shifts may differ in important ways from those observed in younger participants (Luciano, Brennan, & Rothberg, 2010).
A potential confound present in our study was that we did not measure respiration for use in HF analysis. Heart rate variability is sensitive to changes in respiration, which may have varied across the resting and stressor conditions and could have adversely affected the results (Berntson, et al., 1997; Grossman & Taylor, 2007). This may be particularly important when measuring HRV during stressors that affect respiration rate, such as the speech task. However, as respiration is not expected to differ across multiple exposures to the speech task within the same individual, we believe the crossover within-subjects design of this study minimizes the potential confounding effect of respiration on our measure of HF heart rate variability. Furthermore, the studies using HF heart rate variability to predict future CV risk typically assess heart rate variability under ambulatory conditions and do not record or adjust for respiration (Singh et al., 1998; Tsuji et al., 1996). As we are interested in the significance of heart rate variability with regard to future risk, we attempted to assess heart rate variability using similar methods to the prognostic studies. However, this should be noted as a limitation of the present study.
We did not include a “no meal” condition, which prevented us from confirming earlier findings that postprandial hemodynamic shifts generally do not affect reactivity (Uijtdehaage, et al., 1994). We tested the acute effect of eating a single meal, thus we also cannot draw conclusions about the effect of habitual diet on reactivity. Our postprandial measurements began two hours after the meal in accord with previous studies (Jakulj, et al., 2007; Uijtdehaage, et al., 1994). Changes in cardiovascular parameters typically peak around 30 minutes after eating (DeMey, et al., 1989), and the vascular benefits of polyunsaturated fats are typically observed 3–4 hours after eating coinciding with the peak triglyceride concentration (West, Hecker, et al., 2005). Future studies should include a longer monitoring period beginning closer to the end of the meal to determine the time course of these responses.
Previous studies that found meal-related differences in stress reactivity did not collect blood samples, and it is possible that existing treatment effects were masked by venipuncture-induced changes in sympathetic outflow. Venipuncture is a known stressor that may cause significant changes in neuroendocrine activity and cardiovascular parameters (Turner, Sherwood, & Light, 1992). Only one participant chose the option of intravenous catheterization in place of two separate venipunctures, and the results did not substantively change when this participant was removed from the analysis. We attempted to further minimize this potential confound by keeping sampling method the same across visits and discarding the cardiovascular data collected during the first 17 minutes of the post-meal resting period to allow for normalization of cardiovascular parameters.
In conclusion, consuming a meal causes significant changes in resting heart rate variability, systemic hemodynamics, and IL-6 concentrations. These findings highlight the need for researchers to standardize meal consumption (or institute a longer fasting period) prior to assessing autonomic function and systemic hemodynamics to minimize the confounding effects of food.
Figure.
Hemodynamic reactivity to acute stress as a function of meal type. Values are unadjusted means ± SE for within-subjects comparisons, n=18. A) Systolic blood pressure. B) Diastolic blood pressure. C) Total peripheral resistance. D) High frequency (HF) heart rate variability.
Acknowledgments
This research was supported by the Flax Council of Canada and NIH Grant M01 RR 10732. Dr. West has worked as a consultant for the Flax Council of Canada.
The services provided by the General Clinical Research Center of The Pennsylvania State University are appreciated. The authors thank Julian F. Thayer for his expert contribution to this project.
S. West is a co-investigator on a grant that is partially funded by the Flax Council of Canada.
Footnotes
K. Sauder, E. Johnston, A. Skulas-Ray, and T. Campbell have no conflicts of interest.
Contributor Information
Katherine A. Sauder, Department of Biobehavioral Health, Pennsylvania State University
Elyse R. Johnston, Department of Biobehavioral Health, Pennsylvania State University
Ann C. Skulas-Ray, Department of Nutritional Sciences, Pennsylvania State University
Tavis S. Campbell, Department of Psychology, University of Calgary
Sheila G. West, Departments of Biobehavioral Health and Nutritional Sciences, Pennsylvania State University
References
- Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213(4504):220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- Albert CM, Oh K, Whang W, Manson JE, Chae CU, Stampfer MJ, Hu FB. Dietary alpha-linolenic acid intake and risk of sudden cardiac death and coronary heart disease. Circulation. 2005;112(21):3232–3238. doi: 10.1161/CIRCULATIONAHA.105.572008. [DOI] [PubMed] [Google Scholar]
- Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASHCollaborative Research Group. New England Journal of Medicine. 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- Bagiella E, Sloan RP, Heitjan DF. Mixed-effects models in psychophysiology. Psychophysiology. 2000;37(1):13–20. doi: 10.1111/1469-8986.3710013. [DOI] [PubMed] [Google Scholar]
- Baliga RR, Burden L, Sidhu MK, Rampling MW, Kooner JS. Effects of components of meals (carbohydrate, fat, protein) in causing postprandial exertional angina pectoris. American Journal of Cardiology. 1997;79(10):1397–1400. doi: 10.1016/S0002-9149(97)00149-5. [DOI] [PubMed] [Google Scholar]
- Barnes MJ, Lapanowski K, Conley A, Rafols JA, Jen KL, Dunbar JC. High fat feeding is associated with increased blood pressure, sympathetic nerve activity and hypothalamic mu opioid receptors. Brain Research Bulletin. 2003;61(5):511–519. doi: 10.1016/S0361-9230(03)00188-6. [DOI] [PubMed] [Google Scholar]
- Baron AD. Hemodynamic actions of insulin. American Journal of Physiology. 1994;267(2 Pt 1):E187–202. doi: 10.1152/ajpendo.1994.267.2.E187. [DOI] [PubMed] [Google Scholar]
- Beevers G, Lip GY, O’Brien E. ABC of hypertension: The pathophysiology of hypertension. British Medical Journal. 2001;322(7291):912–916. doi: 10.1136/bmj.322.7291.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne C, Fagius J, Pollare T, Hjemdahl P. The sympathetic response to euglycaemic hyperinsulinaemia. Evidence from microelectrode nerve recordings in healthy subjects. Diabetologia. 1992;35(9):873–879. doi: 10.1007/BF00399935. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Blum S, Aviram M, Ben-Amotz A, Levy Y. Effect of a Mediterranean meal on postprandial carotenoids, paraoxonase activity and C-reactive protein levels. Annals of Nutrition and Metabolism. 2006;50(1):20–24. doi: 10.1159/000089560. [DOI] [PubMed] [Google Scholar]
- Boardman A, Schlindwein FS, Rocha AP, Leite A. A study on the optimum order of autoregressive models for heart rate variability. Physiological Measurement. 2002;23(2):325–336. doi: 10.1088/0967-3334/23/2/308. [DOI] [PubMed] [Google Scholar]
- Brownley KA, Hurwitz BE, Schneiderman N. Cardiovascular Psychophysiology. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 2. New York: Cambridge University Press; 2000. [Google Scholar]
- Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Deadman NM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2(8666):757–761. doi: 10.1016/S0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- Celermajer DS. Endothelial dysfunction: Does it matter? Is it reversible? Journal of the American College of Cardiology. 1997;30(2):325–333. doi: 10.1016/s0735-1097(97)00189-7. S0735-1097(97)00189-7 [pii] [DOI] [PubMed] [Google Scholar]
- Ceriello A, Assaloni R, Da Ros R, Maier A, Piconi L, Quagliaro L, Giugliano D. Effect of atorvastatin and irbesartan, alone and in combination, on postprandial endothelial dysfunction, oxidative stress, and inflammation in type 2 diabetic patients. Circulation. 2005;111(19):2518–2524. doi: 10.1161/01.CIR.0000165070.46111.9F. [DOI] [PubMed] [Google Scholar]
- Chang CS, Ko CW, Lien HC, Chou MC. Varying postprandial abdominovagal and cardiovagal activity in normal subjects. Neurogastroenterology and Motility. 2010;22(5):546–551. doi: 10.1111/j.1365-2982.2009.01455.x. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: A meta-analysis of prospective evidence. Hypertension. 2010;55(4):1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1988. [Google Scholar]
- Cortes B, Nunez I, Cofan M, Gilabert R, Perez-Heras A, Casals E, Ros E. Acute effects of high-fat meals enriched with walnuts or olive oil on postprandial endothelial function. Journal of the American College of Cardiology. 2006;48(8):1666–1671. doi: 10.1016/j.jacc.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Cozzolino D, Furlan R, Gruosso D, Di Maggio C, Miraglia Del Giudice E, Torella R, Giugliano D. Effects of a mixed meal on hemodynamics and autonomic control of the heart in patients with type 1 diabetes. Journal of Clinical Endocrinology and Metabolism. 2010;95(1):194–200. doi: 10.1210/jc.2009-1663. [DOI] [PubMed] [Google Scholar]
- DeMey C, Hansenschmidt S, Enterling D, Meineke I. Time course and nature of postprandial hemodynamic changes in normal man. Clinical Physiology. 1989;9(1):77–87. doi: 10.1111/j.1475-097X.1989.tb00958.x. [DOI] [PubMed] [Google Scholar]
- Fagius J, Berne C. Increase in muscle nerve sympathetic activity in humans after food intake. Clinical Science (London, England) 1994;86(2):159–167. doi: 10.1042/cs0860159. [DOI] [PubMed] [Google Scholar]
- Faulk KE, Bartholomew JB. The moderating effect of physical activity on cardiovascular reactivity following single fat feedings. Psychophysiology. 2011 doi: 10.1111/j.1469-8986.2011.01283.x. [DOI] [PubMed] [Google Scholar]
- Giannattasio C, Zoppo A, Gentile G, Failla M, Capra A, Maggi FM, Mancia G. Acute effect of high-fat meal on endothelial function in moderately dyslipidemic subjects. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(2):406–410. doi: 10.1161/01.ATV.0000152231.93590.17. [DOI] [PubMed] [Google Scholar]
- Ginsberg HN, Karmally W, Siddiqui M, Holleran S, Tall AR, Rumsey SC, Ramakrishnan R. A dose-response study of the effects of dietary cholesterol on fasting and postprandial lipid and lipoprotein metabolism in healthy young men. Arteriosclerosis, Thrombosis, and Vascular Biology. 1994;14(4):576–586. doi: 10.1161/01.atv.14.4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GISSI-Prevenzione. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354(9177):447–455. doi: 10.1016/S0140-6736(99)07072-5. [DOI] [PubMed] [Google Scholar]
- Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology. 2007;74(2):263–285. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Heseltine D, Potter JF, Hartley G, Macdonald IA, James OF. Blood pressure, heart rate and neuroendocrine responses to a high carbohydrate and a high fat meal in healthy young subjects. Clinical Science (London, England) 1990;79(5):517–522. doi: 10.1042/cs0790517. [DOI] [PubMed] [Google Scholar]
- Host U, Kelbaek H, Rasmusen H, CourtPayen M, Christensen NJ, PedersenBjergaard U, Lorenzen T. Haemodynamic effects of eating: The role of meal composition. Clinical Science (London, England) 1996;90(4):269–276. doi: 10.1042/cs0900269. [DOI] [PubMed] [Google Scholar]
- Jakulj F, Zernicke K, Bacon SL, van Wielingen LE, Key BL, West SG, Campbell TS. A high-fat meal increases cardiovascular reactivity to psychological stress in healthy young adults. Journal of Nutrition. 2007;137(4):935–939. doi: 10.1093/jn/137.4.935. [DOI] [PubMed] [Google Scholar]
- Kaufman LN, Peterson MM, Smith SM. Hypertension and sympathetic hyperactivity induced in rats by high-fat or glucose diets. The American Journal of Physiology. 1991;260(1 Pt 1):E95–100. doi: 10.1152/ajpendo.1991.260.1.E95. [DOI] [PubMed] [Google Scholar]
- Kearney MT, Cowley AJ, Stubbs TA, Macdonald IA. Effect of a physiological insulin infusion on the cardiovascular responses to a high fat meal: Evidence supporting a role for insulin in modulating postprandial cardiovascular homoeostasis in man. Clinical Science (London, England) 1996;91(4):415–423. doi: 10.1042/cs0910415. [DOI] [PubMed] [Google Scholar]
- Liao D, Cai J, Rosamond WD, Barnes RW, Hutchinson RG, Whitsel EA, Heiss G. Cardiac autonomic function and incident coronary heart disease: a population-based case-cohort study. The ARIC Study. Atherosclerosis Risk in Communities Study. American Journal of Epidemiology. 1997;145(8):696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- Luciano GL, Brennan MJ, Rothberg MB. Postprandial hypotension. American Journal of Medicine. 2010;123(3):281 e281–286. doi: 10.1016/j.amjmed.2009.06.026. [DOI] [PubMed] [Google Scholar]
- MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Stamler J. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335(8692):765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Salomon K, Brady SS, Allen MT. Cardiovascular reactivity to stress predicts future blood pressure in adolescence. Psychosomatic Medicine. 2003;65(3):410–415. doi: 10.1097/01.PSY.0000057612.94797.5F. [DOI] [PubMed] [Google Scholar]
- Morris MC, Sacks FM. Dietary fats and blood pressure. In: Swales JD, editor. Textbook of Hypertension. Oxford, UK: Blackwell; 1994. pp. 605–618. [Google Scholar]
- Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: A meta-analysis of randomized controlled trials. Circulation. 2005;112(13):19451952. doi: 10.1161/CIRCULATIONAHA.105.556886. [DOI] [PubMed] [Google Scholar]
- Potter JF, Heseltine D, Hartley G, Matthews J, MacDonald IA, James OF. Effects of meal composition on the postprandial blood pressure, catecholamine and insulin changes in elderly subjects. Clinical Science (London, England) 1989;77(3):265–272. doi: 10.1042/cs0770265. [DOI] [PubMed] [Google Scholar]
- Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, Head GA. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2010;55(4):862–868. doi: 10.1161/HYPERTENSIONAHA.109.141119. [DOI] [PubMed] [Google Scholar]
- Puska P, Iacono JM, Nissinen A, Korhonen HJ, Vartianinen E, Pietinen P, Huttunen J. Controlled, randomised trial of the effect of dietary fat on blood pressure. Lancet. 1983;1(8314–5):1–5. doi: 10.1016/s0140-6736(83)91556-8. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Wylie-Rosett J. Heart disease and stroke statistics-2011 update: A report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/ CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros E, Nunez I, Perez-Heras A, Serra M, Gilabert R, Casals E, Deulofeu R. A walnut diet improves endothelial function in hypercholesterolemic subjects: A randomized crossover trial. Circulation. 2004;109(13):1609–1614. doi: 10.1161/01.CIR.0000124477.91474.FF. 01.CIR.0000124477.91474.FF. [DOI] [PubMed] [Google Scholar]
- Schwartz JH, Young JB, Landsberg L. Effect of dietary fat on sympathetic nervous system activity in the rat. The Journal of Clinical Investigation. 1983;72(1):361–370. doi: 10.1172/JCI110976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, Vandoornen LJP. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27(1):1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Sidery MB, Cowley AJ, Macdonald IA. Cardiovascular responses to a high-fat and a high-carbohydrate meal in healthy elderly subjects. Clinical Science (London, England) 1993;84(3):263–270. doi: 10.1042/cs0840263. [DOI] [PubMed] [Google Scholar]
- Sidery MB, Macdonald IA, Cowley AJ, Fullwood LJ. Cardiovascular responses to high-fat and high-carbohydrate meals in young subjects. American Journal of Physiology. 1991;261(5):H1430–1436. doi: 10.1152/ajpheart.1991.261.5.H1430. [DOI] [PubMed] [Google Scholar]
- Singh JP, Larson MG, Tsuji H, Evans JC, O’Donnell CJ, Levy D. Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: The Framingham Heart Study. Hypertension. 1998;32(2):293–297. doi: 10.1161/01.hyp.32.2.293. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Marmot M. Impaired cardiovascular recovery following stress predicts 3-year increases in blood pressure. Journal of Hypertension. 2005;23(3):529–536. doi: 10.1097/01.hjh.0000160208.66405.a8. [DOI] [PubMed] [Google Scholar]
- Straznicky NE, Louis WJ, McGrade P, Howes LG. The effects of dietary lipid modification on blood pressure, cardiovascular reactivity and sympathetic activity in man. Journal of Hypertension. 1993;11(4):427437. doi: 10.1097/00004872-199304000-00014. [DOI] [PubMed] [Google Scholar]
- Task Force. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065. doi: 10.1161/01.CIR.93.5.1043. [DOI] [PubMed] [Google Scholar]
- Tentolouris N, Tsigos C, Perea D, Koukou E, Kyriaki D, Kitsou E, Katsilambros N. Differential effects of high-fat and high-carbohydrate isoenergetic meals on cardiac autonomic nervous system activity in lean and obese women. Metabolism. 2003;52(11):1426–1432. doi: 10.1016/S0026-0495(03)00322-6. [DOI] [PubMed] [Google Scholar]
- Tortora GJ, Grabowski SR. Principles of anatomy and physiology. New York: John Wiley and Sons; 2000. [Google Scholar]
- Tsuji H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94(11):2850–2855. doi: 10.1161/01.CIR.94.11.2850. [DOI] [PubMed] [Google Scholar]
- Turner J, Sherwood A, Light KC, editors. Individual differences in cardiovascular response to stress. New York: Plenum Press; 1992. [Google Scholar]
- Uijtdehaage SHJ, Shapiro D, Jaquet F. Effects of carbohydrate and protein meals on cardiovascular levels and reactivity. Biological Psychology. 1994;38(1):53–72. doi: 10.1016/0301-0511(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. American Journal of Cardiology. 1997;79(3):350–354. doi: 10.1016/S0002-9149(96)00760-6. [DOI] [PubMed] [Google Scholar]
- West SG, Hecker KD, Mustad VA, Nicholson S, Schoemer SL, Wagner P, Kris-Etherton P. Acute effects of monounsaturated fatty acids with and without omega-3 fatty acids on vascular reactivity in individuals with type 2 diabetes. Diabetologia. 2005;48(1):113–122. doi: 10.1007/s00125-004-1600-7. [DOI] [PubMed] [Google Scholar]
- West SG, Krick AL, Klein LC, Zhao G, Wojtowicz TF, McGuiness M, Kris-Etherton PM. Effects of diets high in walnuts and flax oil on hemodynamic responses to stress and vascular endothelial function. Journal of the American College of Nutrition. 2010;29(6):595–603. doi: 10.1080/07315724.2010.10719898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West SG, Likos-Krick A, Brown P, Mariotti F. Oral L-arginine improves hemodynamic responses to stress and reduces plasma homocysteine in hypercholesterolemic men. Journal of Nutrition. 2005;135(2):212–217. doi: 10.1093/jn/135.2.212. [DOI] [PubMed] [Google Scholar]
- Williams MJ, Sutherland WH, McCormick MP, de Jong SA, Walker RJ, Wilkins GT. Impaired endothelial function following a meal rich in used cooking fat. Journal of the American College of Cardiology. 1999;33(4):1050–1055. doi: 10.1016/S0735-(98)00681-0. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Global health risks: Mortality and burden of disease attributable to selected major risks. Geneva: World Health Organization; 2009. [Google Scholar]