Abstract
Hereditary deafness is genetically heterogeneous such that mutations of many different genes can cause hearing loss. This review focuses on the evidence and implications that several of these deafness genes encode actin-interacting proteins or actin itself. There is a growing appreciation of the contribution of the actin interactome in stereocilia development, maintenance, mechanotransduction and malfunction of the auditory system.
1. Introduction
Mutant alleles of 57 genes have been shown to cause non-syndromic deafness and 19 of them encode proteins that interact directly or indirectly with actin (Table 1). There are six different actin genes in mammalian and avian genomes. Four are muscle-specific (ACTA1, ACTC1, ACTA2, and ACTG2) and two encode β and γ cytoplasmic actins (ACTB, ACTG1). Mutations of human β-actin are associated with syndromes that include deafness as a significant feature (Nunoi et al., 1999; Procaccio et al., 2006; Riviere et al., 2011) while mutant alleles of γ-actin cause syndromic and non-syndromic progressive hearing loss (van Wijk et al., 2003; Zhu et al., 2003; Rendtorff et al., 2006; Liu et al., 2008; de Heer et al., 2009; Morin et al., 2009; Riviere et al., 2011). Despite being encoded by six different genes, mammalian actins are 90% identical to one another (reviewed in Khaitlina, 2001). β-actin and γ-actin differ from one another by only four amino acid substitutions in the first ten residues of these 375 amino acid proteins, and are each 100% conserved among mammals and birds (Sheterline et al., 1998). β-actin is the predominant actin in almost all tissues of the body except brush border cells of intestinal epithelium and hair cells of the inner ear, in which there is a 2:1 γ:β ratio in young chickens (Hofer et al., 1997) and adult guinea pig (reviewed in Khaitlina, 2001; Furness et al., 2005), although the relative amounts of these two actins may change during development and aging.
Table 1.
Mutations in actin and actin-binding proteins cause nonsyndromic hearing loss and Usher syndrome in humans.
| Gene | Hearing loss locus | MIMa | Protein | Function |
|---|---|---|---|---|
| ACTG1 | DFNA20/26 | 102560 | Cytoplasmic γ-actin | Cytoskeleton |
| TRIOBP | DFNB28 | 609823 | TRIOBP | Actin-bundler |
| ESPN | DFNB36 | 609006 | Espin | Actin-crosslinker |
| DIAPH1 | DFNA1 | 602121 | Diaphanous 1 | Actin cap/nucleator |
| DIAPH3 | AUNA1 | 609129 | Diaphanous 3 | Actin cap/nucleator |
| USH1C | DFNB18, USH1C | 605242 | Harmonin b | Scaffold |
| RDX | DFNB24 | 611022 | Radixin | Scaffold |
| MYO7A | DFNB2, DFNA11, USH1B | 600060, 601317, 276900 | Myosin 7a | Actin-activated motor |
| MYO15A | DFNB3 | 600316 | Myosin 15a | Actin-activated motor |
| MYO3A | DFNB30 | 606808 | Myosin 3a | Actin-activated motor |
| MYO6 | DFNB37, DFNA22 | 607821, 606346 | Myosin 6 | Actin-activated motor |
| MYO1A | DFNA48 | 607841 | Myosin 1a | Actin-activated motor |
| MYH14 | DFNA4 | 608568 | Myosin heavy chain 14 | Actin-activated motor |
| MYH9 | DFNA17 | 603622 | Myosin heavy chain 9 | Actin-activated motor |
| CDH23 | DFNB12, USH1D | 601386, 601067 | Cadherin 23 | Tip-link componentb |
| PDCH15 | DFNB23, USH1F | 609533, 602083 | Protocadherin 15 | Tip-link componentb |
| GRXCR1 | DFNB25 | 613283 | Glutaredoxin, cysteine-rich 1 | Predicted actin-association |
| SMPX | DFNX4 | 300066 | Small muscle protein, X-linked | Predicted actin-association |
| TPRN | DFNB79 | 613307 | Taperin | Predicted actin-association |
Mendelian Inheritance in Man (http://www.ncbi.nlm.nih.gov/omim).
Indirect interaction with actin through a complex of proteins.
Actins are 42-kDa proteins that exist in a cell as monomers (globular or G-actin) or polymers (filamentous or F-actin). Each monomer has two major domains, each with two subdomains (Schutt et al., 1993) with an ATP binding pocket near the center of the protein (Fig. 1) (Sheterline et al., 1998). Under certain conditions, ATP-G-actin polymerizes to form F-actin which is used to fabricate both stable and dynamic cell structures. Actin filaments are helical and polar, with the ends designated as barbed (plus) and pointed (minus). In dynamic cytoskeletal structures such as lamellipodia and filopodia protrusions, F-actin undergoes a polarized process known as treadmilling in which new ATP-actin monomers are added to the barbed-end of an active filament, while ADP-actin monomers are released from the pointed-end (Sheterline et al., 1998; Bugyi and Carlier, 2010). Assembly of actin filaments can produce force for directional movement or alteration of cell shape (Sheterline et al., 1998; Welch and Mullins, 2002; Pollard and Borisy, 2003; Ridley, 2011). The structural features of actin are shaped by a variety of protein partners, some of which are necessary for normal hearing. In this review we focus on actin and actin-interacting proteins in the context of hereditary hearing loss.
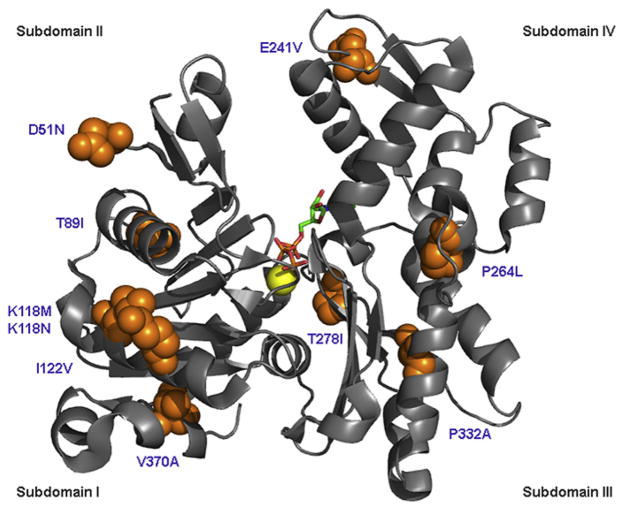
Fig. 1.
Ribbon diagram of a γ-actin monomer with the locations of 10 missense mutations associated with human DFNA20/26 deafness. These mutations are found in all subdomains of the protein and are not clustered within a single functional subdomain or protein interaction site. Calcium (yellow sphere) and ATP are bound in the center of the monomer. This structural model was generated in PyMOL using PDB 3HBT (Wang et al., 2010).
2. Cytoplasmic actins and deafness
2.1. Dominant mutations of ACTG1 are associated with non-syndromic progressive hearing loss
Progressive hearing loss segregating in a large North American family as a dominant trait was genetically mapped to chromosome 17q25 and the locus was designated DFNA20/26 (Morell et al., 2000). Genes in the 2.4-Mb DFNA20/26 interval were screened by DNA sequencing, and a missense mutation (p.T89I) that substitutes an isoleucine for the wild-type threonine at residue 89 of ACTG1 was found to co-segregate with deafness in this family (Zhu et al., 2003). Nine additional missense mutations of ACTG1 have been identified, each of which has been reported in just one family (van Wijk et al., 2003; Zhu et al., 2003; Rendtorff et al., 2006; Liu et al., 2008; de Heer et al., 2009; Morin et al., 2009).
All ten of the DFNA20/26 ACTG1 missense mutations described to date (Fig. 1) result in a similar phenotype. Hearing initially appears normal in young DFNA20/26 individuals and begins to deteriorate at high frequencies in the 1st to 3rd decade of life followed by nearly complete hearing loss at all tested frequencies by the 4th to 6th decade. Distortion product otoacoustic emissions are absent in individuals with the p.T89I mutation (Elfenbein et al., 2001), which may indicate loss of cochlear amplification due to a malfunction of outer hair cells. Given that hearing appears normal in the 1st decade in most DFNA20/26 subjects, one possibility is that ACTG1 mutations somehow affect durability of actin structures and/or interfere with a repair process. However, these observations do not rule out a developmental defect in the inner ear that may manifest in the first to second decade of life. It is tempting to speculate that the similar longitudinal audiologic phenotype observed in individuals carrying different DFNA20/26 missense mutations of ACTG1 is the result of a common molecular pathology of the mutant γ-actins.
Knowledge of the molecular defects caused by missense mutations of γ-actin is derived from a variety of biochemical analyses and studies using yeast, explant cultures of mouse inner ear sensory epithelia and Actg1 knockin and knockout mouse models. Biochemical studies investigated the folding, thermal stability, ATP-exchange rates, polymerization kinetics, and interaction with an actin-severing protein, cofilin, for the mutant actins. Each mutation affected protein function in a slightly different fashion (Bryan et al., 2006; Zhu, 2008; Bryan and Rubenstein, 2009; Morin et al., 2009). Yeast engineered to express the single yeast actin gene with DFNA20/26 mutations have diminished growth rates with the exception of yeast with the p.K118N DFNA20/26 mutation which grew as well as wild-type and had a comparable actin cytoskeleton (Bryan et al., 2006; Morin et al., 2009). These data are valuable starting points for understanding DFNA20/26 deafness, but provide no direct insight into the inner ear pathology caused by mutations of ACTG1.
In explant cultures of neonatal mouse organ of Corti, wild-type GFP-tagged γ-actin localizes to the tips of stereocilia within 4 h after biolistic transfection (our unpublished data). However, no differences in targeting were seen using some of the GFP-tagged mutant γ-actins and no obvious alterations in stereocilia morphology were observed over a period of up to 96 h (Morin et al., 2009; our unpublished data). Perhaps within the time constraints of the experiment, the high level of endogenous wild-type γ-actin masked any effect of mutant γ-actin.
2.2. To establish hearing, γ-actin is not required
A γ-actin knockout mouse initially appeared to have normal hearing. However, by 16 weeks of age a >70 dbSPL loss was observed across all frequencies tested (Belyantseva et al., 2009). At this age stereocilia in all three rows of auditory hair cell bundles begin to degenerate and there is an increased number of gaps in the actin core of stereocilia that do not stain with phalloidin. In wild-type mice phalloidin-negative gaps are found rarely and typically only in vestibular hair cell bundles (Belyantseva et al., 2009). The ultrastructure of these gaps in Actg1-null mice has not been reported. Perhaps phalloidin-negative gaps represent damage to the paracrystalline core; which might still contain F-actin but in some way the binding sites for phalloidin are obstructed.
Actg1-null mice also show reduced viability, are smaller, and develop signs of muscular myopathy. A hair cell-specific γ-actin-null mouse recapitulates only the hearing phenotype of the whole-body knockout of this gene (Perrin et al., 2010). Although the recessive null allele of Actg1 provided valuable insight into the functions of γ-actin in many tissues including the inner ear, heterozygous γ-actin knockout mice have normal hearing and do not model DFNA20/26 deafness, which is caused by dominant missense alleles of ACTG1. Wild-type hearing in mice heterozygous for the Actg1-null allele does suggest that γ-actin hap-loinsufficiency is not the mechanism of DFNA20/26 hearing loss.
To investigate the molecular pathogenesis of DFNA20/26 alleles, a knockin mouse with the orthologous human p.P264L missense mutation was engineered. Mice homozygous for the Actg1P264L knockin allele develop progressive high frequency hearing loss by 4–5 weeks of age. Hearing deteriorates rapidly to a complete loss across all frequencies by 7 weeks. Unlike γ-actin-null mice, hearing loss in Actg1P264L homozygotes is accompanied by degeneration of just the shorter rows of stereocilia within auditory hair cell bundles (Drummond et al., 2010). Furthermore, these mice have no other obvious pathological features; they are comparable in size to wild-type littermates, do not show signs of muscular myopathy, and survive as well as wild-type. These data suggest a dominant negative or gain-of-function mode of pathogenesis in which the mutant DFNA20/26 γ-actin damages hair cells but is tolerated in other cell types.
2.3. Dominant mutations in ACTB cause syndromic hearing loss, but β-actin is not required to establish hearing
A de novo heterozygous missense mutation, p.R183W, of ACTB encoding cytoplasmic β-actin was reported in a pair of monozygotic twins that showed a broad range of abnormalities (MIM 102630) including adolescent dystonia, midline malformations, and hearing loss (Procaccio et al., 2006). When transfected into NIH3T3 cells, expression of p.R183W β-actin induced morphological changes and conferred resistance to latrunculin A-induced actin depolymerization. A second dominant mutation (p.E364K) in ACTB was reported in a child with cardiomegaly, hepatomegaly, hypothyroidism, and recurrent infection including otitis media although hearing was not tested (Nunoi et al., 1999).
Dominant missense mutations in β-actin and γ-actin are also reported to co-segregate with Baraitser-Winter syndrome (MIM 243310); a rare disorder characterized by developmental delay, facial dysmorphologies, brain malformations, colobomas and variable hearing loss (Riviere et al., 2011). It is likely that a dominant negative or gain-of-function mechanism of action is responsible for the phenotype produced by all of these ACTB mutant alleles (Nunoi et al., 1999; Procaccio et al., 2006; Riviere et al., 2011).
Homozygous Actb-null mice die as early as E7.5 (Shawlot et al., 1998; Bunnell et al., 2011), indicating that β-actin is essential for embryogenesis. A conditional Actb-flox Foxg1-cre mouse was engineered to produce hair cells devoid of β-actin and circumvent the lethality thought to be from impaired cell growth and migration (Perrin et al., 2010; Bunnell et al., 2011). Similar to Actg1-null mice, hair-cell specific Actb-null mice have high frequency hearing loss at 6 weeks of age. But unlike Actg1-null mice (Belyantseva et al., 2009) the subsequent progression of hearing loss is slower and characterized by loss of the two shorter rows of stereocilia (Perrin et al., 2010). Knockin mouse models for the human dominant ACTB missense mutations have not been generated, though they would be expected to have unique inner ear pathology.
As might be expected, stereocilia do not develop in hair cells devoid of both cytoplasmic actins (Perrin et al., 2010). Therefore, either one of the two cytoplasmic actins is sufficient for development of stereocilia and initial hearing ability, but both cytoplasmic actins are required in hair cells over the long term. Phenotypic differences between the progression of hearing loss and hair bundle pathology in hair cells of β-actin versus γ-actin knockout mouse strains also suggest non-redundant functions of these two cytoplasmic actins.
3. Differences between cytoplasmic actins
3.1. Biochemical properties
Understanding the biochemical differences between β-actin and γ-actin is an active area of research. The nucleotide exchange and ion-dependent polymerization rates of the two cytoplasmic actins are different, but cytoplasmic actins can copolymerize (Fig. 2C), and the kinetic properties reflect the β:γ ratio (Bergeron et al., 2010). In the presence of calcium, purified β-actin polymerizes in vitro nearly twice as fast as γ-actin; a difference owing to faster rates of filament nucleation and elongation. Furthermore, the rate of phosphate release and depolymerization of actin monomers from treadmilling filaments occurs rapidly for β-actin-based filaments but slowly for γ-actin-based filaments. Interestingly, these differences were less pronounced when magnesium is present in place of calcium (Bergeron et al., 2010) suggesting the possibility of tunable kinetics depending on the intracellular microenvironment.
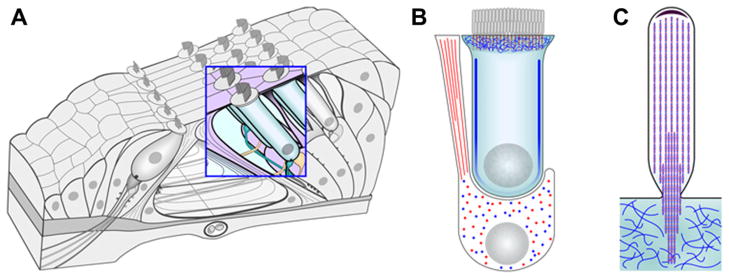
Fig. 2.
β-actin and γ-actin are differentially distributed in hair and supporting cells in the organ of Corti. The distribution of the two cytoplasmic actins in the organ of Corti (A) was determined by immuno transmission electron microscopy (TEM) (Hofer et al., 1997; Furness et al., 2005) and is summarized in this schematic. Red is used to signify β-actin and blue for γ-actin. An enlargement of an outer hair cell and Deiters’ cell (B) show that the phalanges of Deiters’ cells are primarily β-actin based, but the remainder of the cell body contains a relatively homogenous composition of the two cytoplasmic actins. In contrast, the lateral wall and cuticular plate of the hair cell is enriched for γ-actin. In adult guinea pig stereocilia (C) the total estimated ratio of γ-actin to β-actin is 2:1, though locally high concentrations of β-actin exist. The unidirectional paracrystalline array of F-actin in the stereocilium is illustrated as being composed of both γ-actin and β-actin, but the specific distribution of the two remains unclear and is represented here as a copolymer.
3.2. Interacting partners
The interactomes of β-actin and γ-actin are similar. This is not surprising since cytoplasmic actins are 99% identical at the protein level. However, there are a few exceptions that presumably directly result from or are influenced by the encoded four amino acid differences at their N-termini. In addition, after removal of the methionine at the N-terminus of β-actin, an arginine is sometimes added at the N-terminus by arginyl-tRNA-protein transferase (ATE1). For reasons not fully understood, arginylated γ-actin is not detected in cells (Karakozova et al., 2006; Zhang et al., 2010). The possible significance of this post-translational modification of β-actin in the auditory system has not been investigated or appreciated.
The only report of a γ-actin-specific interacting protein is annexin A5 (ANXA5) (Tzima et al., 2000), which is expressed abundantly in the organ of Corti (Peters et al., 2007; Shin et al., 2007). Tzima and colleagues showed that during platelet activation, the actin cytoskeleton undergoes remodeling (Tzima et al., 1999) during which γ-actin associates with ANXA5 at cell membranes (Tzima et al., 2000). ANXA5 is a member of a family containing 12 paralogous proteins, all of which bind reversibly to membranes in the presence of high Ca+2 (Moss and Morgan, 2004). Other annexins also bind F-actin, implying a calcium-sensitive role in coordinating membrane and cytoskeleton dynamics (Hayes et al., 2004). To date, only ANXA5 is thought to interact exclusively with γ-actin although a systematic search has not been reported for unique partners of each of the cytoplasmic actins.
3.3. Actin homeostasis
The regulatory mechanisms that maintain a constant total amount of actin in some cells are not understood. Up-regulation of one particular actin in response to down-regulation of another usually does not rescue the phenotype, reinforcing the conclusion that each actin has at least one exclusive function (Shawlot et al., 1998; Lloyd and Gunning, 2002; Procaccio et al., 2006; Sonnemann et al., 2006; Belyantseva et al., 2009; Bunnell and Ervasti, 2010; Perrin and Ervasti, 2010). Though expressed at relatively low levels in differentiated skeletal muscle compared to other tissues, γ-actin is required for proper muscle function, as its absence in a skeletal muscle-specific γ-actin knockout results in a progressive myopathy (Sonnemann et al., 2006). Additionally, in skeletal muscle, γ-actin was found up-regulated ten-fold in animal models of muscular dystrophy suggesting that γ-actin may be involved in a compensatory remodeling process (Hanft et al., 2006).
In the inner ear, the loss of one cytoplasmic actin and the subsequent degeneration of hair cells are not compensated for by the presence of the remaining cytoplasmic actin, or the up-regulation of total actin levels (Belyantseva et al., 2009; Perrin et al., 2010). However, some examples of compensation for the loss of one actin by a paralogous actin have been reported. Over-expression of αcardiac-actin, but not γ-actin from a transgene can rescue the lethality and muscle abnormality due to loss of αskeletal-actin (Jaeger et al., 2009; Nowak et al., 2009). Taken together, the various rescue experiments and phenotypes of actin gene mutations indicate that muscle actins have a degree of functional overlap. Although either β-actin or γ-actin can initially build a functional stereocilium, both are required for long term maintenance of hair cells (Perrin et al., 2010), which means that the two cytoplasmic actins have unique functions, at least in the inner ear.
3.4. Differential distribution of cytoplasmic actins
There are differences in subcellular localization of the cytoplasmic actins (Otey et al.,1987,1988; Hill and Gunning,1993; Hofer et al., 1997; Khaitlina, 2001; Furness et al., 2005; Dugina et al., 2009). γ-actin was reported to be preferentially located in stress fibers of myoblasts whereas β-actin was found at sites of active remodeling such as lamellipodia (Hill and Gunning, 1993). In fibroblasts and endothelial cells, β-actin is associated with the less dynamic stress fibers and γ-actin is localized to lamellipodia (Dugina et al., 2009). Differences between these studies may be due to the specificity of antibodies and methods of fixation. This topic was recently revisited. β-actin was examined during cell migration and found to be essential for membrane protrusion dynamics (Bunnell et al., 2011). From these in vivo observations comparing the two cytoplasmic actins, β-actin is the more dynamic, an observation consistent with biochemical data (Bergeron et al., 2010).
Not only are cytoplasmic actins sometimes differentially localized in the cells, but the distribution of β-actin mRNA in cells is polarized. The mechanism of this intracellular nonuniformity involves the 54 nucleotide zipcode motif in the sequence of the 3′ UTR immediately downstream of the translation stop codon (Kislauskis et al., 1993). The zipcode sequence in the β-actin mRNA interacts with ZBP1 RNA-binding protein, which then facilitates trafficking of β-actin mRNA (Ross et al., 1997; Condeelis and Singer, 2005). The 3′ UTR of γ-actin mRNA appears not to have a zipcode sequence and is presumably trafficked differently than β-actin mRNA. How regulation of mRNA distribution affects actin distribution in the inner ear is a completely unstudied phenomenon. Will an Actb bacterial artificial chromosome (BAC) transgene lacking the zipcode sequence rescue the progressive hearing loss phenotype when expressed in a conditional inner ear β-actin knockout?
3.5. Actin in hair cells
The distribution of cytoplasmic actins has been examined by transmission electron microscopy (TEM) in auditory hair cells (Hofer et al., 1997; Furness et al., 2005) and is summarized in Fig. 2. The stereocilia core is a paracrystalline array of cross-linked and bundled actin filaments with a 2:1 ratio of γ- to β-actin, perhaps as a copolymer (Tilney et al.,1980; Hofer et al.,1997; Beyer et al., 2000; Furness et al., 2005). Each stereocilium is anchored into the cuticular plate by a rootlet that is composed of more densely bundled F-actin compared to the core. Little is known about the developmental factors controlling formation of the cuticular plate (Fig. 1). Actin in the cuticular plate comprises a network of branched, primarily γ-actin-based filaments distinct from that of the F-actin paracrystalline core in the stereocilium, which is also distinct from the arrangement of F-actin in the rootlet (DeRosier and Tilney, 1989; Hofer et al., 1997; Furness et al., 2005). Formation of the rootlet begins around P0 and is largely completed by P16 (Furness et al., 2008; Kitajiri et al., 2010). A rootlet spans the entire tapered region of a stereocilium and protrudes into the cuticular plate. The overall length of a rootlet is proportional to the length of the stereocilium it anchors (Furness et al., 2008), but there is no causal connection between rootlet and stereocilia lengths (Kitajiri et al., 2010).
In mammals, hair cells are terminally differentiated and do not normally regenerate from surrounding cells. Due to the sustained mechanical stress placed on hair cell stereocilia from nearly constant acoustic stimulation, mechanisms must have evolved to monitor and repair critical actin-based structures to maintain hearing for the lifetime of hair cells. At the cellular level, loss of stiffness and phalloidin-negative gaps within stereocilia actin cores are observed after noise damage and in aged vestibular hair cells (Tilney et al., 1982; Avinash et al., 1993; Belyantseva et al., 2009). Phalloidin-negative gaps do contain γ-actin and β-actin (Belyantseva et al., 2009) however; the organization of actin in these gaps is unclear because antibodies recognize both monomeric and filamentous actin. The absence of phalloidin labeling does not necessarily exclude the presence of F-actin in a form that does not bind this phallotoxin. Gaps are stained by DNaseI, supporting the assumption that at least some of the actin is monomeric. Other proteins found in these gaps include cofilin and espin that could be involved in remodeling of a damaged core (Belyantseva et al., 2009). Phalloidin-negative gaps observed in auditory stereocilia of Actg1-null mice may reveal a previously unknown mechanism of stress-induced dissociation and subsequent rebuilding of the F-actin core, which is regulated at the level of an individual stereocilium (Belyantseva et al., 2009). The gaps in stereocilia F-actin are strikingly similar to those that occur during disassembly of actin filaments in Drosophila bristles (Tilney et al., 1996; Guild et al., 2005). This suggests that mammals may utilize an ancient, evolutionarily conserved mechanism of actin bundle assembly and disassembly to repair and reinforce the F-actin core of stereocilia during the lifetime of hair cells.
4. Actin-binding proteins in stereocilia and associated structures
Mass spectrometry (LC-MS/MS) analyses of stereocilia bundles isolated from chicken utricles were used to identify at least 60 proteins (Shin et al., 2007). Another proteomics study of hair cell bundles identified 138 proteins (Peng, 2009). In both studies, the majority of the proteins observed are part of the actin interactome. Mutations of the genes encoding several of these proteins are associated with hereditary hearing loss and produce a variety of abnormalities of stereocilia architecture and function (Table 1).
4.1. Proteins that regulate filament elongation
De novo formation of F-actin requires a nucleation event in which three actin monomers interact to form a trimer, referred to as an actin seed, from which rapid elongation of filaments proceeds (Pollard, 1986; reviewed in Welch and Mullins, 2002). In vitro, this can be achieved by incubating actin above a critical concentration at which spontaneous filament formation occurs. Formation of an actin seed is a kinetically unfavorable event, but several actin-binding proteins facilitate this process in vivo (reviewed in Welch and Mullins, 2002; Pollard, 2007). In hair cells, actin dysregulation can cause uncontrolled filament formation producing actin-rich projections (cytocauds) that even protrude from the basal surface of cells (Probst et al., 1998; Beyer et al., 2000; Kanzaki et al., 2002).
Some proteins, such as members of the formin family of actin-cappers, serve multiple functions in regulating actin dynamics. In vitro evidence demonstrates that formins function to nucleate filaments as well as cap existing filaments (Zigmond, 2004). These caps are considered “leaky” because they also allow monomers to be added to the barbed-end of a filament (Goode and Eck, 2007). We are unaware of reports showing localization of formins in the inner ear, however a non-coding dominant point mutation in the preprocessed DIAPH1 transcript encoding diaphanous 1, a formin, co-segregated with DFNA1 hearing loss in a large Costa Rican kindred (Lynch et al., 1997). This mutation alters splicing of DIAPH1 preprocessed mRNA and is predicted to generate a protein lacking the autoregulatory domain, leading to over-activation of the mutant DIAPH1. Excessive DIAPH1 activity may be the cause of DFNA1 deafness (Lynch et al., 1997; Peng et al., 2007). A knockout Diaph1 mouse develops myeloproliferative defects, but has normal hearing (Peng et al., 2007) suggesting that the DFNA1 mutation is probably a dominant negative or gain-of-function allele. Consistent with the hypothesis that constitutive activation of a formin causes deafness, a point mutation in the 5′ UTR of DIAPH3 was associated with auditory neuropathy (AUNA1) in a North American family (Schoen et al., 2010). Functional studies in vitro and in a Drosophila model demonstrated that this non-coding point mutation induces over-expression of DIAPH3. Mouse models of DFNA1 and AUNA1 deafness in which the orthologous human mutations are knocked into mouse Diaph1 or Diaph3 might provide insight into the role of formins in the inner ear and the mechanism of pathogensis of the human mutations.
4.2. Proteins that cross-link or bundle
There are several proteins that cross-link and/or bundle F-actin in stereocilia, including espin, fimbrin, plastin-1, plastin-2, plastin-3, GRXCR1, TRIOBP, fascin-1, fascin-2, espin-like protein, and Xin-related protein-2 (Kitajiri et al., 2010; Shin et al., 2010). Mutations of human ESPN, GRXCR1, and TRIOBP genes cause autosomal recessive nonsyndromic deafness at the DFNB36, DFNB25 and DFNB28 loci, respectively (Naz et al., 2004; Riazuddin et al., 2006; Shahin et al., 2006; Odeh et al., 2010; Schraders et al., 2010). Espin is an essential actin cross-linking protein in the stereocilia core (Zheng et al., 2000), while TRIOBP is localized to rootlets and the stereocilia core proper, depending on the isoform (Kitajiri et al., 2010). As such, the phenotypes at the cellular level associated with loss of function mutations in Espn and Triobp are different. Espin cross-linking of actin filaments along the length of stereocilia cores is essential for establishing the wild-type height and thickness of a stereocilium. In the spontaneous Espn mouse mutant, jerker, a missense mutation reduces levels of espin in homozygous mice resulting in abnormally short and thin stereocilia despite the presence of espin-like protein encoded by Espnl, and several other actin cross-linkers in these structures (Sekerkova et al., 2006, 2011). The spontaneous Grxcr1 mouse mutant, pirouette, also produces abnormally thin stereocilia, though the molecular mechanisms remain to be investigated (Odeh et al., 2010).
In contrast to espin and GRXCR1, loss of TRIOBP function (iso-forms 4 and 5) in an engineered knockout mouse results in a failure to form stereocilia rootlets. Without rootlets, stereocilia lose their durability and are more flexible at the point of insertion into the cuticular plate. Triobp-deficient stereocilia are more prone to damage, fuse to one another and degenerate shortly after the onset of hearing (Kitajiri et al., 2010). Data from in vitro functional assays indicate that while espin joins actin filaments through cross-linking, TRIOBP may instead wrap around the periphery of multiple actin filaments to create a dense bundle in which each filament in the rootlet can slide past its neighbors. Rootlets also provide a majority of stiffness to the hair bundle (Kitajiri et al., 2010; reviewed in Boutet de Monvel and Petit, 2010).
4.3. Myosins and their cargo
Myosins are actin-activated ATPases that utilize ATP hydrolysis to generate force along actin filaments. Development of stereocilia requires several myosins and their associated cargo, and mutations of seven different myosins cause deafness in humans and mice (Table 1). Pathogenic alleles of MYO7A encoding myosin 7a cause recessive nonsyndromic deafness DFNB2, dominant nonsyndromic deafness DFNA11, or Type 1 Usher Syndrome (USH1B), a genetically heterogeneous deaf-blindness syndrome characterized by progressive loss of vision due to retinitis pigmentosa, vestibular dysfunction and congenital deafness (Weil et al., 1995, 1997; Liu et al., 1997; Luijendijk et al., 2004; Riazuddin et al., 2008; Ammar-Khodja et al., 2009; Ben Rebeh et al., 2010; Hildebrand et al., 2010; Friedman et al., 2011). The phenotypic outcome is hypothesized to depend on the level of residual function of the mutant allele.
Recessive mutations of MYO15A encoding myosin 15a cause DFNB3 hearing loss in individuals from many different populations world-wide. Mutant alleles of MYO15A are the third most common cause of recessive deafness after GJB2 and SLC26A4 (Wang et al., 1998; Liang et al., 1999; Liburd et al., 2001; Friedman et al., 2002; Kalay et al., 2007; Nal et al., 2007; Lezirovitz et al., 2008; Belguith et al., 2009; Hilgert et al., 2009; Shearer et al., 2009; Cengiz et al., 2010). Mutations in the unconventional myosin gene, MYO6A encoding myosin 6a, underlie DFNB37 and DFNA22 human deafness (Melchionda et al., 2001; Ahmed et al., 2003; Hilgert et al., 2008; Sanggaard et al., 2008; Topsakal et al., 2010).
Mouse models have helped to elucidate the wild-type function and mutant pathophysiology for each of these unconventional myosins. Shaker 1, shaker 2, and Snell’s waltzer mice are deficient in myosin 7a, myosin 15a, and myosin 6a, respectively, and each displays a distinctive hair cell abnormality that provides insight into the likely corresponding human pathology. Myosin 7a is found along the length of stereocilia, and missense mutations cause abnormally long and disorganized stereocilia in shaker 1 (Myo7sh1) mice (Self et al., 1998). This phenotype may be due to improper localization of twinfilin 2, an actin capping protein that co-localizes with myosin 7a in stereocilia (Rzadzinska et al., 2009) and is required for length regulation of the shorter rows of stereocilia (Peng et al., 2009). Myosin 7a may also interact with SANS and harmonin-b as a complex that participates in adaptation of mechanotransduction in hair cells (Kros et al., 2002; Grati and Kachar, 2011).
Missense and truncating mutations of myosin 15a cause abnormally short stereocilia in the shaker 2 (Myo15sh2) mouse (Probst et al., 1998; Anderson et al., 2000). Myosin 15a delivers whirlin to the tips of stereocilia where it is proposed to mediate their differential elongation (Belyantseva et al., 2003, 2005; Delprat et al., 2005; Kikkawa et al., 2005). Similar to Myo15sh2, whirler (Whrnwi) mice also have abnormally short stereocilia, and Myo15sh2;Whrnwi double homozygotes have a slightly exacerbated phenotype (Mogensen et al., 2007; Mustapha et al., 2007). Eps8 interacts with actin to regulate filament dynamics in filopodia (Roffers-Agarwal et al., 2005) and is another myosin 15 cargo. Resembling both Myo15sh2 and Whrnwi, Eps8 knockout mice are deaf and have abnormally short stereocilia (Manor et al., 2011; Zampini et al., 2011). Many of the protein partners of myosin 15a have probably not been identified and the mechanisms by which myosin 15a, whirlin and Eps8 cooperate to shape stereocilia architecture are largely unknown and remain a challenging question.
Protein tyrosine phosphatase receptor type Q (PTPRQ) is a membrane protein important for formation of the shaft connectors of stereocilia (Goodyear et al., 2003). PTPRQ is improperly localized in degenerating hair cells from Snell’s waltzer (Myo6sv) mice (Sakaguchi et al., 2008), which are deficient in myosin 6a (Avraham et al., 1995), the only reported myosin that moves toward the pointed-end of actin filaments (Wells et al., 1999; Sweeney and Houdusse, 2010). Myo6sv and PTPRQ deficient mice have fused stereocilia with occasional formation of giant stereocilia devoid of the taper at the site of insertion into the cuticular plate (Fig. 2C) (Goodyear et al., 2003). Another protein localized to the taper region in stereocilia is taperin encoded by TPRN. Mutations in TPRN were recently identified as the cause of human DFNB79 nonsyndromic deafness (Rehman et al., 2010). Of the 749 amino acids which comprise taperin, a 66 amino acid segment has 53% identity to phostensin, a pointed-end actin capping protein (Lai et al., 2009). Does taperin participate in capping the pointed-ends of peripheral F-actin of stereocilia in the taper region? Does taperin interact with the membrane and cytoskeleton, perhaps as a scaffolding protein, to regulate curvature or mechanical properties of the taper, the location where stereocilia pivot when deflected by sound (Crawford et al., 1989; Karavitaki and Corey, 2006)? Additional in vitro biochemical and biophysical studies of purified taperin in combination with mouse models deficient for taperin are essential to answer these questions.
4.4. Scaffolding proteins
Scaffolding proteins link the membrane to subcortical actin through the cytoplasmic domain of integral membrane proteins (Algrain et al., 1993; Bretscher et al., 2002). Radixin, which localizes to the base of stereocilia, and ezrin are two scaffolding proteins of the ezrin/radixin/moesin (ERM) family. Mutations of RDX encoding radixin are associated with human DFNB24 hearing loss (Ahmed et al., 2003; Khan et al., 2007). Rdx-null mice have hearing loss but no obvious vestibular phenotype (Kitajiri et al., 2004). Ezrin is also expressed in stereocilia, but it is unable to compensate for the loss of radixin in cochlear hair cells of Rdx-null mice. This may be due to limited expression of ezrin only at the early stages of development in auditory hair cell stereocilia (Kitajiri et al., 2004). However, ezrin expression persists through maturation in vestibular hair cells, an observation that may explain the lack of a vestibular phenotype in Rdx-null mice (Kitajiri et al., 2004).
Recessive mutations of USH1C, encoding the scaffolding protein harmonin-b, cause DFNB18 nonsyndromic deafness and Usher Syndrome type 1C in humans (Verpy et al., 2000; Ahmed et al., 2002; Ouyang et al., 2002). In mice, spontaneous deletion mutations of harmonin-b are the cause of deafness and circling behavior in deaf circler and deaf circler Jackson 2, characterized by disorganized and splayed hair cell bundles (Johnson et al., 2003). Harmonin-b interacts with F-actin and the cytoplasmic domains of protocadherin 15 and cadherin 23 (Boeda et al., 2002; Siemens et al., 2002; Adato et al., 2005; Reiners et al., 2005), thus connecting the mechanotransduction machinery of stereocilia to its F-actin core (Siemens et al., 2004; Ahmed et al., 2006; Kazmierczak et al., 2007). Together, harmonin-b, protocadherin 15 and cadherin 23 along with SANS and myosin 7a comprise a multifaceted supra-molecular complex referred to as the Usher interactome that is required not just for mechanotransduction of mature hair cells, but also transient filamentous links crucial for development of the hair bundle (reviewed in Muller, 2008; Richardson et al., 2011).
5. F-actin in stereocilia
To fully understand the mechanisms of deafness caused by mutations in actin and actin-binding proteins, it is necessary to characterize the degree of F-actin turnover in mature stereocilia. One model proposes a treadmilling mechanism featuring continuous polymerization of actin at the barbed-ends of actin filaments (stereocilia tips) with a concomitant equal rate of depolymerization from the pointed-ends (stereocilia base) so as to maintain a constant stereocilium length. This model is based upon studies using GFP-β-actin biolistically transfected into cells of cultured organ of Corti from postnatal days 1–5 (Schneider et al., 2002). These data show GFP-β-actin at the tips of stereocilia within a few hours of transfection, and subsequent labeling of the upper half of stereocilia within 24 h. The labeled actin appears distributed along the full length of stereocilia after 48 h. Subsequently, the same phenomenon was reported for GFP-espin (Rzadzinska et al., 2004). Additionally, treatment with cytochalasin D, an inhibitor of actin polymerization at the barbed-end of filaments, causes shortening of stereocilia at rates consistent with measurements of GFP-β-actin incorporation into untreated filaments (Rzadzinska et al., 2004). Based on snap-shots over time in different hair cells over-expressing GFP-β-actin, it was concluded that a stereocilium treadmills its F-actin core, renewing it every two to three days (Schneider et al., 2002; Rzadzinska et al., 2004).
Recent data of a different kind demonstrate that F-actin cores of stereocilia do not treadmill (Zhang et al., 2012). 15N-labeled leucine was fed to mice and frogs and turnover of proteins in stereocilia was then measured and found to be <0.3% per day in frogs, <2% per day in adult mice and <9% per day in neonatal mice (Zhang, et al., 2012); rates inconsistent with total renewal of the stereocilia actin paracrystal in organ of Corti hair cells after approximately two days in culture (Rzadzinska et al., 2004). Additional data reported in Zhang, et al., 2012 are also inconsistent with rapid turnover of the actin core of stereocilia. In utricle cultures from transgenic mice expressing GFP-β-actin under the control of a myosin 7a promoter, photobleached lines were introduced across hair cell bundles and monitored for possible movement. Over the course of six days the bleached line moved only 2–3% of the length of the bundle, demonstrating that a treadmill is not induced by culturing the sensory epithelium (Zhang et al., 2012). Finally, expression of β-actin was halted using an inducible Cre in combination with a floxed Actb allele in adult mice. After 34 weeks, β-actin was present along the lengths of stereocilia and absent only at the very tips (Zhang et al., 2012), thus bolstering the conclusion that the stereocilia cores are stable entities. The main conclusion from these three independent experimental methods is that stereocilia actin cores do not normally treadmill (Zhang et al., 2012).
What then are the possible explanations for the apparent movement of GFP-β-actin from the stereocilia tips to the base? If a stereocilium is a closed system as proposed (Manor and Kachar, 2008), actin monomers disassembled from the pointed-ends would be retained in this compartment and not enter into the cell body. In this case, treadmilling of a recycled and isolated pool of actin in stereocilia would not be detected by examining turnover of stereocilia proteins using 15N-labeled leucine. However, this idea is inconsistent with data from photobleaching experiments described above (Zhang et al., 2012). Perhaps over-expression of GFP-β-actin increases the intracellular concentration of cytoplasmic actin and in stereocilia induces spurious filament formation (Zhang et al., 2012). Alternatively, the reported movement of GFP-β-actin from the tips of stereocilia to the base (Schneider et al., 2002; Rzadzinska et al., 2004) may be due to diffusion of unincorporated monomers or small fragments of F-actin. Another possibility is that trauma from biolistic transfection of gold particles damage one or more stereocilia within a hair cell bundle (Di Pasquale et al., 2005; Belyantseva, 2009) and creates microbreaks in the actin core akin to phalloidin-negative gaps (Belyantseva et al., 2009). This damage may be imperceptible in the best of circumstances, but nevertheless may trigger repair of filaments by incorporation of newly synthesized actin (Zhang et al., 2012). This hypothesis could be tested by combining the gene gun method with the photobleaching technique using cultured organ of Corti to evaluate whether there is an increase in the rate of movement of the photobleached line after biolistic transfection with “empty” bullets. Nevertheless, using a variety of methodologies in hair cells of adult frogs and in cultured and freshly excised adult and neonatal mouse sensory epithelia, there appears to be no evidence of a rapid treadmill in actin cores of stereocilia (Zhang et al., 2012) as previously concluded (Schneider et al., 2002; Rzadzinska et al., 2004).
6. Conclusions
Intuitively, mutations of ubiquitously expressed genes, such as actin, would be assumed to disrupt not just the auditory system but a variety of organs and tissues causing a syndromic disorder. Yet, many mutations of γ-actin and several widely expressed actin-interacting proteins cause nonsyndromic deafness and knockout mouse models confirm that many of these proteins are only indispensible for hearing. However, knockout mouse models do not provide crucial insight into hearing loss caused by missense mutations that result in a gain-of-function, are dominant negative, or have residual function. Knockin mouse models of mutations orthologous to those causing human deafness will be instrumental in unraveling the pathophysiology of hereditary hearing loss.
Future work should also address the function and form of actin in other subcellular compartments aside from stereocilia and investigate the effect of mutations in actin and actin-binding proteins on these structures and processes. While it is appealing to focus on abnormalities of the strikingly beautiful actin-rich ster-eocilia as the cause of deafness in mouse models of actin and actin-binding proteins, the remainder of the hair cell body and other cell types of the inner ear cannot be ignored. For example, the fusion of stereocilia in the Myo6sv mouse may be a sufficient but not necessarily the sole cause of deafness (Sakaguchi et al., 2008). In Myo6sv mice there are also defects in the development and maturation of inner hair cell ribbon synapses (Roux et al., 2009). In addition, myosin VI is detected in the nucleus where it is reported to modulate transcription through an interaction with RNA polymerase II (Vreugde et al., 2006). There has been an upsurge of interest in the roles of nuclear actin and actin-binding proteins, particularly myosins, as components of the transcriptional machinery (reviewed in Louvet and Percipalle, 2009; de Lanerolle and Serebryannyy, 2011).
Mutations of actin and actin-interacting proteins may not just alter steady-state structures, but could affect rapid intermediate kinetics. This is highlighted by a recent study on the supporting cells’ cytoskeleton that revealed rapid formation of actin cables that would be difficult to detect without the use of time-lapse microscopy (Bird et al., 2010). Live cell time-lapse coupled with recent advances in super-resolution microscopy will be critical in exploring the intricacies of actin dynamics in cells of the auditory system.
Acknowledgments
We thank Jonathan Bird, Dennis Drayna, and Ben Perrin for helpful discussions, Kaillathe Padmanabhan and Pavel Belyantsev for graphics in Figs. 1 and 2, respectively. KHF was supported by NIDCD grant DC004568. MCD, IAB and TBF were supported by NIDCD intramural research funds DC000039-15.
Abbreviations
- ATP
adenosine triphosphate
- ADP
adenosine diphosphate
- BAC
bacterial artificial chromosome
- DFNA
dominantly inherited nonsyndromic deafness locus
- DFNB
recessively inherited nonsyndromic deafness locus
- GFP
green fluorescent protein
- TEM
transmission electron microscopy
- USH
Usher syndrome
- UTR
untranslated region
References
- Adato A, Michel V, Kikkawa Y, Reiners J, Alagramam KN, Weil D, Yonekawa H, Wolfrum U, El-Amraoui A, Petit C. Interactions in the network of Usher syndrome type 1 proteins. Hum Mol Genet. 2005;14:347–356. doi: 10.1093/hmg/ddi031. [DOI] [PubMed] [Google Scholar]
- Ahmed ZM, Goodyear R, Riazuddin S, Lagziel A, Legan PK, Behra M, Burgess SM, Lilley KS, Wilcox ER, Riazuddin S, Griffith AJ, Frolenkov GI, Belyantseva IA, Richardson GP, Friedman TB. The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J Neurosci. 2006;26:7022–7034. doi: 10.1523/JNEUROSCI.1163-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed ZM, Morell RJ, Riazuddin S, Gropman A, Shaukat S, Ahmad MM, Mohiddin SA, Fananapazir L, Caruso RC, Husnain T, Khan SN, Griffith AJ, Friedman TB, Wilcox ER. Mutations of MYO6 are associated with recessive deafness, DFNB37. Am J Hum Genet. 2003;72:1315–1322. doi: 10.1086/375122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed ZM, Smith TN, Riazuddin S, Makishima T, Ghosh M, Bokhari S, Menon PS, Deshmukh D, Griffith AJ, Friedman TB, Wilcox ER. Nonsyndromic recessive deafness DFNB18 and Usher syndrome type IC are allelic mutations of USHIC. Hum Genet. 2002;110:527–531. doi: 10.1007/s00439-002-0732-4. [DOI] [PubMed] [Google Scholar]
- Algrain M, Turunen O, Vaheri A, Louvard D, Arpin M. Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J Cell Biol. 1993;120:129–139. doi: 10.1083/jcb.120.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammar-Khodja F, Faugere V, Baux D, Giannesini C, Leonard S, Makrelouf M, Malek R, Djennaoui D, Zenati A, Claustres M, Roux AF. Molecular screening of deafness in Algeria: high genetic heterogeneity involving DFNB1 and the Usher loci, DFNB2/USH1B, DFNB12/USH1D and DFNB23/USH1F. Eur J Med Genet. 2009;52:174–179. doi: 10.1016/j.ejmg.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Anderson DW, Probst FJ, Belyantseva IA, Fridell RA, Beyer L, Martin DM, Wu D, Kachar B, Friedman TB, Raphael Y, Camper SA. The motor and tail regions of myosin XV are critical for normal structure and function of auditory and vestibular hair cells. Hum Mol Genet. 2000;9:1729–1738. doi: 10.1093/hmg/9.12.1729. [DOI] [PubMed] [Google Scholar]
- Avinash GB, Nuttall AL, Raphael Y. 3-D analysis of F-actin in stereocilia of cochlear hair cells after loud noise exposure. Hear Res. 1993;67:139–146. doi: 10.1016/0378-5955(93)90241-r. [DOI] [PubMed] [Google Scholar]
- Avraham KB, Hasson T, Steel KP, Kingsley DM, Russell LB, Mooseker MS, Copeland NG, Jenkins NA. The mouse Snell’s waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nat Genet. 1995;11:369–375. doi: 10.1038/ng1295-369. [DOI] [PubMed] [Google Scholar]
- Belguith H, Aifa-Hmani M, Dhouib H, Said MB, Mosrati MA, Lahmar I, Moalla J, Charfeddine I, Driss N, Arab SB, Ghorbel A, Ayadi H, Masmoudi S. Screening of the DFNB3 locus: identification of three novel mutations of MYO15A associated with hearing loss and further suggestion for two distinctive genes on this locus. Genet Test Mol Biomarkers. 2009;13:147–151. doi: 10.1089/gtmb.2008.0077. [DOI] [PubMed] [Google Scholar]
- Belyantseva IA. Helios Gene Gun-mediated transfection of the inner ear sensory epithelium. Method Mol Biol (Clifton, NJ) 2009;493:103–123. doi: 10.1007/978-1-59745-523-7_7. [DOI] [PubMed] [Google Scholar]
- Belyantseva IA, Boger ET, Friedman TB. Myosin XVa localizes to the tips of inner ear sensory cell stereocilia and is essential for staircase formation of the hair bundle. Proc Natl Acad Sci USA. 2003;100:13958–13963. doi: 10.1073/pnas.2334417100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyantseva IA, Boger ET, Naz S, Frolenkov GI, Sellers JR, Ahmed ZM, Griffith AJ, Friedman TB. Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat Cell Biol. 2005;7:148–156. doi: 10.1038/ncb1219. [DOI] [PubMed] [Google Scholar]
- Belyantseva IA, Perrin BJ, Sonnemann KJ, Zhu M, Stepanyan R, McGee J, Frolenkov GI, Walsh EJ, Friderici KH, Friedman TB, Ervasti JM. Gamma-actin is required for cytoskeletal maintenance but not development. Proc Natl Acad Sci USA. 2009;106:9703–9708. doi: 10.1073/pnas.0900221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Rebeh I, Moriniere M, Ayadi L, Benzina Z, Charfedine I, Feki J, Ayadi H, Ghorbel A, Baklouti F, Masmoudi S. Reinforcement of a minor alternative splicing event in MYO7A due to a missense mutation results in a mild form of retinopathy and deafness. Mol Vis. 2010;16:1898–1906. [PMC free article] [PubMed] [Google Scholar]
- Bergeron SE, Zhu M, Thiem SM, Friderici KH, Rubenstein PA. Ion-dependent polymerization differences between mammalian beta- and gamma-nonmuscle actin isoforms. J Biol Chem. 2010;285:16087–16095. doi: 10.1074/jbc.M110.110130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer LA, Odeh H, Probst FJ, Lambert EH, Dolan DF, Camper SA, Kohrman DC, Raphael Y. Hair cells in the inner ear of the pirouette and shaker 2 mutant mice. J Neurocytol. 2000;29:227–240. doi: 10.1023/a:1026515619443. [DOI] [PubMed] [Google Scholar]
- Bird JE, Daudet N, Warchol ME, Gale JE. Supporting cells eliminate dying sensory hair cells to maintain epithelial integrity in the avian inner ear. J Neurosci. 2010;30:12545–12556. doi: 10.1523/JNEUROSCI.3042-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeda B, El-Amraoui A, Bahloul A, Goodyear R, Daviet L, Blanchard S, Perfettini I, Fath KR, Shorte S, Reiners J, Houdusse A, Legrain P, Wolfrum U, Richardson G, Petit C. Myosin VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair cell bundle. EMBO J. 2002;21:6689–6699. doi: 10.1093/emboj/cdf689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutet de Monvel J, Petit C. Wrapping up stereocilia rootlets. Cell. 2010;141:748–750. doi: 10.1016/j.cell.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- Bryan KE, Rubenstein PA. Allele-specific effects of human deafness gamma-actin mutations (DFNA20/26) on the actin/cofilin interaction. J Biol Chem. 2009;284:18260–18269. doi: 10.1074/jbc.M109.015818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan KE, Wen KK, Zhu M, Rendtorff ND, Feldkamp M, Tranebjaerg L, Friderici KH, Rubenstein PA. Effects of human deafness gamma-actin mutations (DFNA20/26) on actin function. J Biol Chem. 2006;281:20129–20139. doi: 10.1074/jbc.M601514200. [DOI] [PubMed] [Google Scholar]
- Bugyi B, Carlier MF. Control of actin filament treadmilling in cell motility. Annu Rev Biophys. 2010;39:449–470. doi: 10.1146/annurev-biophys-051309-103849. [DOI] [PubMed] [Google Scholar]
- Bunnell TM, Burbach BJ, Shimizu Y, Ervasti JM. {beta}-Actin specifically controls cell growth, migration and the G-actin pool. Mol Biol Cell. 2011 doi: 10.1091/mbc.E11-06-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnell TM, Ervasti JM. Delayed embryonic development and impaired cell growth and survival in Actg1 null mice. Cytoskeleton (Hoboken) 2010;67:564–572. doi: 10.1002/cm.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cengiz FB, Duman D, Sirmaci A, Tokgoz-Yilmaz S, Erbek S, Ozturkmen-Akay H, Incesulu A, Edwards YJ, Ozdag H, Liu XZ, Tekin M. Recurrent and private MYO15A mutations are associated with deafness in the Turkish population. Genet Test Mol Biomarkers. 2010;14:543–550. doi: 10.1089/gtmb.2010.0039. [DOI] [PubMed] [Google Scholar]
- Condeelis J, Singer RH. How and why does beta-actin mRNA target? Biol Cell. 2005;97:97–110. doi: 10.1042/BC20040063. [DOI] [PubMed] [Google Scholar]
- Crawford AC, Evans MG, Fettiplace R. Activation and adaptation of transducer currents in turtle hair cells. J Physiol. 1989;419:405–434. doi: 10.1113/jphysiol.1989.sp017878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Heer AM, Huygen PL, Collin RW, Oostrik J, Kremer H, Cremers CW. Audiometric and vestibular features in a second Dutch DFNA20/26 family with a novel mutation in ACTG1. Ann Otol Rhinol Laryngol. 2009;118:382–390. doi: 10.1177/000348940911800511. [DOI] [PubMed] [Google Scholar]
- de Lanerolle P, Serebryannyy L. Nuclear actin and myosins: life without filaments. Nat Cell Biol. 2011;13:1282–1288. doi: 10.1038/ncb2364. [DOI] [PubMed] [Google Scholar]
- Delprat B, Michel V, Goodyear R, Yamasaki Y, Michalski N, El-Amraoui A, Perfettini I, Legrain P, Richardson G, Hardelin JP, Petit C. Myosin XVa and whirlin, two deafness gene products required for hair bundle growth, are located at the stereocilia tips and interact directly. Hum Mol Genet. 2005;14:401–410. doi: 10.1093/hmg/ddi036. [DOI] [PubMed] [Google Scholar]
- DeRosier DJ, Tilney LG. The structure of the cuticular plate, an in vivo actin gel. J Cell Biol. 1989;109:2853–2867. doi: 10.1083/jcb.109.6.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale G, Rzadzinska A, Schneider ME, Bossis I, Chiorini JA, Kachar B. A novel bovine virus efficiently transduces inner ear neuroepithelial cells. Mol Ther. 2005;11:849–855. doi: 10.1016/j.ymthe.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Drummond MC, Zhu M, Belyantseva I, Halsey K, Dolan DF, Camper SA, Fri-derici KH. A knock-in mouse model for DFNA20 deafness. 33rd Midwinter Research Meeting for the Association for Research in Otolaryngology.2010. [Google Scholar]
- Dugina V, Zwaenepoel I, Gabbiani G, Clement S, Chaponnier C. Beta and gamma-cytoplasmic actins display distinct distribution and functional diversity. J Cell Sci. 2009;122:2980–2988. doi: 10.1242/jcs.041970. [DOI] [PubMed] [Google Scholar]
- Elfenbein JL, Fisher RA, Wei S, Morell RJ, Stewart C, Friedman TB, Friderici K. Audiologic aspects of the search for DFNA20: a gene causing late-onset, progressive, sensorineural hearing loss. Ear Hear. 2001;22:279–288. doi: 10.1097/00003446-200108000-00003. [DOI] [PubMed] [Google Scholar]
- Friedman TB, Hinnant JT, Ghosh M, Boger ET, Riazuddin S, Lupski JR, Potocki L, Wilcox ER. DFNB3, spectrum of MYO15A recessive mutant alleles and an emerging genotype-phenotype correlation. Adv Otorhinolaryngol. 2002;61:124–130. doi: 10.1159/000066824. [DOI] [PubMed] [Google Scholar]
- Friedman TB, Schultz JM, Ahmed ZM, Tsilou ET, Brewer CC. Usher syndrome: hearing loss with vision loss. Adv Otorhinolaryngol. 2011;70:56–65. doi: 10.1159/000322473. [DOI] [PubMed] [Google Scholar]
- Furness DN, Katori Y, Mahendrasingam S, Hackney CM. Differential distribution of beta- and gamma-actin in guinea-pig cochlear sensory and supporting cells. Hear Res. 2005;207:22–34. doi: 10.1016/j.heares.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Furness DN, Mahendrasingam S, Ohashi M, Fettiplace R, Hackney CM. The dimensions and composition of stereociliary rootlets in mammalian cochlear hair cells: comparison between high- and low-frequency cells and evidence for a connection to the lateral membrane. J Neurosci. 2008;28:6342–6353. doi: 10.1523/JNEUROSCI.1154-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- Goodyear RJ, Legan PK, Wright MB, Marcotti W, Oganesian A, Coats SA, Booth CJ, Kros CJ, Seifert RA, Bowen-Pope DF, Richardson GP. A receptor-like inositol lipid phosphatase is required for the maturation of developing cochlear hair bundles. J Neurosci. 2003;23:9208–9219. doi: 10.1523/JNEUROSCI.23-27-09208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grati M, Kachar B. Myosin VIIa and sans localization at stereocilia upper tip-link density implicates these Usher syndrome proteins in mechano-transduction. Proc Natl Acad Sci USA. 2011;108:11476–11481. doi: 10.1073/pnas.1104161108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guild GM, Connelly PS, Ruggiero L, Vranich KA, Tilney LG. Actin filament bundles in Drosophila wing hairs: hairs and bristles use different strategies for assembly. Mol Biol Cell. 2005;16:3620–3631. doi: 10.1091/mbc.E05-03-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanft LM, Rybakova IN, Patel JR, Rafael-Fortney JA, Ervasti JM. Cytoplasmic gamma-actin contributes to a compensatory remodeling response in dystrophin-deficient muscle. Proc Natl Acad Sci USA. 2006;103:5385–5390. doi: 10.1073/pnas.0600980103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MJ, Rescher U, Gerke V, Moss SE. Annexin-actin interactions. Traffic. 2004;5:571–576. doi: 10.1111/j.1600-0854.2004.00210.x. [DOI] [PubMed] [Google Scholar]
- Hildebrand MS, Thorne NP, Bromhead CJ, Kahrizi K, Webster JA, Fattahi Z, Bataejad M, Kimberling WJ, Stephan D, Najmabadi H, Bahlo M, Smith RJ. Variable hearing impairment in a DFNB2 family with a novel MYO7A missense mutation. Clin Genet. 2010;77:563–571. doi: 10.1111/j.1399-0004.2009.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgert N, Smith RJ, Van Camp G. Forty-six genes causing nonsyndromic hearing impairment: which ones should be analyzed in DNA diagnostics? Mutat Res. 2009;681:189–196. doi: 10.1016/j.mrrev.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgert N, Topsakal V, van Dinther J, Offeciers E, Van de Heyning P, Van Camp G. A splice-site mutation and overexpression of MYO6 cause a similar phenotype in two families with autosomal dominant hearing loss. Eur J Hum Genet. 2008;16:593–602. doi: 10.1038/sj.ejhg.5202000. [DOI] [PubMed] [Google Scholar]
- Hill MA, Gunning P. Beta and gamma actin mRNAs are differentially located within myoblasts. J Cell Biol. 1993;122:825–832. doi: 10.1083/jcb.122.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer D, Ness W, Drenckhahn D. Sorting of actin isoforms in chicken auditory hair cells. J Cell Sci. 1997;110 (Pt 6):765–770. doi: 10.1242/jcs.110.6.765. [DOI] [PubMed] [Google Scholar]
- Jaeger MA, Sonnemann KJ, Fitzsimons DP, Prins KW, Ervasti JM. Context-dependent functional substitution of alpha-skeletal actin by gamma-cytoplasmic actin. Faseb J. 2009;23:2205–2214. doi: 10.1096/fj.09-129783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Gagnon LH, Webb LS, Peters LL, Hawes NL, Chang B, Zheng QY. Mouse models of USH1C and DFNB18: phenotypic and molecular analyses of two new spontaneous mutations of the Ush1c gene. Hum Mol Genet. 2003;12:3075–3086. doi: 10.1093/hmg/ddg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalay E, Uzumcu A, Krieger E, Caylan R, Uyguner O, Ulubil-Emiroglu M, Erdol H, Kayserili H, Hafiz G, Baserer N, Heister AJ, Hennies HC, Nurnberg P, Basaran S, Brunner HG, Cremers CW, Karaguzel A, Wollnik B, Kremer H. MYO15A (DFNB3) mutations in Turkish hearing loss families and functional modeling of a novel motor domain mutation. Am J Med Genet Part A. 2007;143A:2382–2389. doi: 10.1002/ajmg.a.31937. [DOI] [PubMed] [Google Scholar]
- Kanzaki S, Beyer LA, Canlon B, Meixner WM, Raphael Y. The cytocaud: a hair cell pathology in the waltzing Guinea pig. Audiol Neurootol. 2002;7:289–297. doi: 10.1159/000064447. [DOI] [PubMed] [Google Scholar]
- Karakozova M, Kozak M, Wong CC, Bailey AO, Yates JR, 3rd, Mogilner A, Zebroski H, Kashina A. Arginylation of beta-actin regulates actin cytoskeleton and cell motility. Science. 2006;313:192–196. doi: 10.1126/science.1129344. [DOI] [PubMed] [Google Scholar]
- Karavitaki KD, Corey DP. Hair bundle mechanics at high frequencies: a test of series or parallel transduction. In: Nutall AL, editor. Auditory Mechanisms: Processes and Models. World Scientific; Singapore: 2006. pp. 286–292. [Google Scholar]
- Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Muller U, Kachar B. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 2007;449:87–U59. doi: 10.1038/nature06091. [DOI] [PubMed] [Google Scholar]
- Khaitlina SY. Functional specificity of actin isoforms. Int Rev Cytol. 2001;202:35–98. doi: 10.1016/s0074-7696(01)02003-4. [DOI] [PubMed] [Google Scholar]
- Khan SY, Ahmed ZM, Shabbir MI, Kitajiri S, Kalsoom S, Tasneem S, Shayiq S, Ramesh A, Srisailpathy S, Khan SN, Smith RJ, Riazuddin S, Friedman TB, Riazuddin S. Mutations of the RDX gene cause nonsyndromic hearing loss at the DFNB24 locus. Hum Mutat. 2007;28:417–423. doi: 10.1002/humu.20469. [DOI] [PubMed] [Google Scholar]
- Kikkawa Y, Mburu P, Morse S, Kominami R, Townsend S, Brown SD. Mutant analysis reveals whirlin as a dynamic organizer in the growing hair cell stereocilium. Hum Mol Genet. 2005;14:391–400. doi: 10.1093/hmg/ddi035. [DOI] [PubMed] [Google Scholar]
- Kislauskis EH, Li Z, Singer RH, Taneja KL. Isoform-specific 3′-untranslated sequences sort alpha-cardiac and beta-cytoplasmic actin messenger RNAs to different cytoplasmic compartments. J Cell Biol. 1993;123:165–172. doi: 10.1083/jcb.123.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajiri S, Fukumoto K, Hata M, Sasaki H, Katsuno T, Nakagawa T, Ito J, Tsukita S. Radixin deficiency causes deafness associated with progressive degeneration of cochlear stereocilia. J Cell Biol. 2004;166:559–570. doi: 10.1083/jcb.200402007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajiri S, Sakamoto T, Belyantseva IA, Goodyear RJ, Stepanyan R, Fujiwara I, Bird JE, Riazuddin S, Riazuddin S, Ahmed ZM, Hinshaw JE, Sellers J, Bartles JR, Hammer JA, 3rd, Richardson GP, Griffith AJ, Frolenkov GI, Friedman TB. Actin-bundling protein TRIOBP forms resilient rootlets of hair cell stereocilia essential for hearing. Cell. 2010;141:786–798. doi: 10.1016/j.cell.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kros CJ, Marcotti W, van Netten SM, Self TJ, Libby RT, Brown SD, Richardson GP, Steel KP. Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nat Neurosci. 2002;5:41–47. doi: 10.1038/nn784. [DOI] [PubMed] [Google Scholar]
- Lai NS, Wang TF, Wang SL, Chen CY, Yen JY, Huang HL, Li C, Huang KY, Liu SQ, Lin TH, Huang HB. Phostensin caps to the pointed end of actin filaments and modulates actin dynamics. Biochem Biophys Res Commun. 2009;387:676–681. doi: 10.1016/j.bbrc.2009.07.086. [DOI] [PubMed] [Google Scholar]
- Lezirovitz K, Pardono E, de Mello Auricchio MT, de Carvalho ESFL, Lopes JJ, Abreu-Silva RS, Romanos J, Batissoco AC, Mingroni-Netto RC. Unexpected genetic heterogeneity in a large consanguineous Brazilian pedigree presenting deafness. Eur J Hum Genet. 2008;16:89–96. doi: 10.1038/sj.ejhg.5201917. [DOI] [PubMed] [Google Scholar]
- Liang Y, Wang A, Belyantseva IA, Anderson DW, Probst FJ, Barber TD, Miller W, Touchman JW, Jin L, Sullivan SL, Sellers JR, Camper SA, Lloyd RV, Kachar B, Friedman TB, Fridell RA. Characterization of the human and mouse unconventional myosin XV genes responsible for hereditary deafness DFNB3 and shaker 2. Genomics. 1999;61:243–258. doi: 10.1006/geno.1999.5976. [DOI] [PubMed] [Google Scholar]
- Liburd N, Ghosh M, Riazuddin S, Naz S, Khan S, Ahmed Z, Liang Y, Menon PS, Smith T, Smith AC, Chen KS, Lupski JR, Wilcox ER, Potocki L, Friedman TB. Novel mutations of MYO15A associated with profound deafness in consanguineous families and moderately severe hearing loss in a patient with Smith-Magenis syndrome. Hum Genet. 2001;109:535–541. doi: 10.1007/s004390100604. [DOI] [PubMed] [Google Scholar]
- Liu P, Li H, Ren X, Mao H, Zhu Q, Zhu Z, Yang R, Yuan W, Liu J, Wang Q, Liu M. Novel ACTG1 mutation causing autosomal dominant non-syndromic hearing impairment in a Chinese family. J Genet Genomics. 2008;35:553–558. doi: 10.1016/S1673-8527(08)60075-2. [DOI] [PubMed] [Google Scholar]
- Liu XZ, Walsh J, Mburu P, Kendrick-Jones J, Cope MJ, Steel KP, Brown SD. Mutations in the myosin VIIA gene cause non-syndromic recessive deafness. Nat Genet. 1997;16:188–190. doi: 10.1038/ng0697-188. [DOI] [PubMed] [Google Scholar]
- Lloyd C, Gunning P. Beta- and gamma-actin genes differ in their mechanisms of down-regulation during myogenesis. J Cell Biochem. 2002;84:335–342. doi: 10.1002/jcb.10014. [DOI] [PubMed] [Google Scholar]
- Louvet E, Percipalle P. Transcriptional control of gene expression by actin and myosin. In: Jeon KW, editor. International Review of Cell and Molecular Biology. Vol. 272. 2009. p. 107. [DOI] [PubMed] [Google Scholar]
- Luijendijk MW, Van Wijk E, Bischoff AM, Krieger E, Huygen PL, Pennings RJ, Brunner HG, Cremers CW, Cremers FP, Kremer H. Identification and molecular modelling of a mutation in the motor head domain of myosin VIIA in a family with autosomal dominant hearing impairment (DFNA11) Hum Genet. 2004;115:149–156. doi: 10.1007/s00439-004-1137-3. [DOI] [PubMed] [Google Scholar]
- Lynch ED, Lee MK, Morrow JE, Welcsh PL, Leon PE, King MC. Non-syndromic deafness DFNA1 associated with mutation of a human homolog of the Drosophila gene diaphanous. Science. 1997;278:1315–1318. [PubMed] [Google Scholar]
- Manor U, Disanza A, Grati M, Andrade L, Lin H, Di Fiore PP, Scita G, Kachar B. Regulation of stereocilia length by myosin XVa and whirlin depends on the actin-regulatory protein Eps8. Curr Biol. 2011;21:167–172. doi: 10.1016/j.cub.2010.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor U, Kachar B. Dynamic length regulation of sensory stereocilia. Semin Cell Dev Biol. 2008;19:502–510. doi: 10.1016/j.semcdb.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchionda S, Ahituv N, Bisceglia L, Sobe T, Glaser F, Rabionet R, Arbones ML, Notarangelo A, Di Iorio E, Carella M, Zelante L, Estivill X, Avraham KB, Gasparini P. MYO6, the human homologue of the gene responsible for deafness in Snell’s waltzer mice, is mutated in autosomal dominant nonsyndromic hearing loss. Am J Hum Genet. 2001;69:635–640. doi: 10.1086/323156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen MM, Rzadzinska A, Steel KP. The deaf mouse mutant whirler suggests a role for whirlin in actin filament dynamics and stereocilia development. Cell Motil Cytoskeleton. 2007;64:496–508. doi: 10.1002/cm.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell RJ, Friderici KH, Wei S, Elfenbein JL, Friedman TB, Fisher RA. A new locus for late-onset, progressive, hereditary hearing loss DFNA20 maps to 17q25. Genomics. 2000;63:1–6. doi: 10.1006/geno.1999.6058. [DOI] [PubMed] [Google Scholar]
- Morin M, Bryan KE, Mayo-Merino F, Goodyear R, Mencia A, Modamio-Hoybjor S, del Castillo I, Cabalka JM, Richardson G, Moreno F, Rubenstein PA, Moreno-Pelayo MA. In vivo and in vitro effects of two novel gamma-actin (ACTG1) mutations that cause DFNA20/26 hearing impairment. Hum Mol Genet. 2009;18:3075–3089. doi: 10.1093/hmg/ddp249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss SE, Morgan RO. The annexins. Genome Biol. 2004;5:219. doi: 10.1186/gb-2004-5-4-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U. Cadherins and mechanotransduction by hair cells. Curr Opin Cell Biol. 2008;20:557–566. doi: 10.1016/j.ceb.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustapha M, Beyer LA, Izumikawa M, Swiderski DL, Dolan DF, Raphael Y, Camper SA. Whirler mutant hair cells have less severe pathology than shaker 2 or double mutants. J Assoc Res Otolaryngol. 2007;8:329–337. doi: 10.1007/s10162-007-0083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nal N, Ahmed ZM, Erkal E, Alper OM, Luleci G, Dinc O, Waryah AM, Ain Q, Tasneem S, Husnain T, Chattaraj P, Riazuddin S, Boger E, Ghosh M, Kabra M, Morell RJ, Friedman TB. Mutational spectrum of MYO15A: the large N-terminal extension of myosin XVA is required for hearing. Hum Mutat. 2007;28:1014–1019. doi: 10.1002/humu.20556. [DOI] [PubMed] [Google Scholar]
- Naz S, Griffith AJ, Riazuddin S, Hampton LL, Battey JF, Jr, Khan SN, Riazuddin S, Wilcox ER, Friedman TB. Mutations of ESPN cause autosomal recessive deafness and vestibular dysfunction. J Med Genet. 2004;41:591–595. doi: 10.1136/jmg.2004.018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak KJ, Ravenscroft G, Jackaman C, Filipovska A, Davies SM, Lim EM, Squire SE, Potter AC, Baker E, Clement S, Sewry CA, Fabian V, Crawford K, Lessard JL, Griffiths LM, Papadimitriou JM, Shen Y, Morahan G, Bakker AJ, Davies KE, Laing NG. Rescue of skeletal muscle alpha-actin-null mice by cardiac (fetal) alpha-actin. J Cell Biol. 2009;185:903–915. doi: 10.1083/jcb.200812132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunoi H, Yamazaki T, Tsuchiya H, Kato S, Malech HL, Matsuda I, Kanegasaki S. A heterozygous mutation of beta-actin associated with neutrophil dysfunction and recurrent infection. Proc Natl Acad Sci USA. 1999;96:8693–8698. doi: 10.1073/pnas.96.15.8693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeh H, Hunker KL, Belyantseva IA, Azaiez H, Avenarius MR, Zheng L, Peters LM, Gagnon LH, Hagiwara N, Skynner MJ, Brilliant MH, Allen ND, Riazuddin S, Johnson KR, Raphael Y, Najmabadi H, Friedman TB, Bartles JR, Smith RJ, Kohrman DC. Mutations in Grxcr1 are the basis for inner ear dysfunction in the pirouette mouse. Am J Hum Genet. 2010;86:148–160. doi: 10.1016/j.ajhg.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otey CA, Kalnoski MH, Bulinski JC. Identification and quantification of actin isoforms in vertebrate cells and tissues. J Cell Biochem. 1987;34:113–124. doi: 10.1002/jcb.240340205. [DOI] [PubMed] [Google Scholar]
- Otey CA, Kalnoski MH, Bulinski JC. Immunolocalization of muscle and nonmuscle isoforms of actin in myogenic cells and adult skeletal muscle. Cell Motil Cytoskeleton. 1988;9:337–348. doi: 10.1002/cm.970090406. [DOI] [PubMed] [Google Scholar]
- Ouyang XM, Xia XJ, Verpy E, Du LL, Pandya A, Petit C, Balkany T, Nance WE, Liu XZ. Mutations in the alternatively spliced exons of USH1C cause non-syndromic recessive deafness. Hum Genet. 2002;111:26–30. doi: 10.1007/s00439-002-0736-0. [DOI] [PubMed] [Google Scholar]
- Peng AW. PhD. Massachusettes Institute of Technology; 2009. A Hair Bundle Proteomics Approach to Discovering Actin Regulatory Proteins in Inner Ear Stereocilia. Speech Hearing Biosciences Technol. [Google Scholar]
- Peng AW, Belyantseva IA, Hsu PD, Friedman TB, Heller S. Twinfilin 2 regulates actin filament lengths in cochlear stereocilia. J Neurosci. 2009;29:15083–15088. doi: 10.1523/JNEUROSCI.2782-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Kitchen SM, West RA, Sigler R, Eisenmann KM, Alberts AS. Myeloproliferative defects following targeting of the Drf1 gene encoding the mammalian diaphanous related formin mDia1. Cancer Res. 2007;67:7565–7571. doi: 10.1158/0008-5472.CAN-07-1467. [DOI] [PubMed] [Google Scholar]
- Perrin BJ, Ervasti JM. The actin gene family: function follows isoform. Cytoskeleton (Hoboken) 2010;67:630–634. doi: 10.1002/cm.20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin BJ, Sonnemann KJ, Ervasti JM. beta-actin and gamma-actin are each dispensable for auditory hair cell development but required for Stereocilia maintenance. PLoS Genet. 2010;6:e1001158. doi: 10.1371/journal.pgen.1001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters LM, Belyantseva IA, Lagziel A, Battey JF, Friedman TB, Morell RJ. Signatures from tissue-specific MPSS libraries identify transcripts preferentially expressed in the mouse inner ear. Genomics. 2007;89:197–206. doi: 10.1016/j.ygeno.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD. Rate constants for the reactions of ATP- and ADP-actin with the ends of actin filaments. J Cell Biol. 1986;103:2747–2754. doi: 10.1083/jcb.103.6.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–477. doi: 10.1146/annurev.biophys.35.040405.101936. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Probst FJ, Fridell RA, Raphael Y, Saunders TL, Wang A, Liang Y, Morell RJ, Touchman JW, Lyons RH, Noben-Trauth K, Friedman TB, Camper SA. Correction of deafness in shaker-2 mice by an unconventional myosin in a BAC transgene. Science. 1998;280:1444–1447. doi: 10.1126/science.280.5368.1444. [DOI] [PubMed] [Google Scholar]
- Procaccio V, Salazar G, Ono S, Styers ML, Gearing M, Davila A, Jimenez R, Juncos J, Gutekunst CA, Meroni G, Fontanella B, Sontag E, Sontag JM, Faundez V, Wainer BH. A mutation of beta -actin that alters depolymerization dynamics is associated with autosomal dominant developmental malformations, deafness, and dystonia. Am J Hum Genet. 2006;78:947–960. doi: 10.1086/504271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman AU, Morell RJ, Belyantseva IA, Khan SY, Boger ET, Shahzad M, Ahmed ZM, Riazuddin S, Khan SN, Friedman TB. Targeted capture and next-generation sequencing identifies C9orf75, encoding taperin, as the mutated gene in nonsyndromic deafness DFNB79. Am J Hum Genet. 2010;86:378–388. doi: 10.1016/j.ajhg.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiners J, Marker T, Jurgens K, Reidel B, Wolfrum U. Photoreceptor expression of the Usher syndrome type 1 protein protocadherin 15 (USH1F) and its interaction with the scaffold protein harmonin (USH1C) Mol Vis. 2005;11:347–355. [PubMed] [Google Scholar]
- Rendtorff ND, Zhu M, Fagerheim T, Antal TL, Jones M, Teslovich TM, Gillanders EM, Barmada M, Teig E, Trent JM, Friderici KH, Stephan DA, Tranebjaerg L. A novel missense mutation in ACTG1 causes dominant deafness in a Norwegian DFNA20/26 family, but ACTG1 mutations are not frequent among families with hereditary hearing impairment. Eur J Hum Genet. 2006;14:1097–1105. doi: 10.1038/sj.ejhg.5201670. [DOI] [PubMed] [Google Scholar]
- Riazuddin S, Khan SN, Ahmed ZM, Ghosh M, Caution K, Nazli S, Kabra M, Zafar AU, Chen K, Naz S, Antonellis A, Pavan WJ, Green ED, Wilcox ER, Friedman PL, Morell RJ, Riazuddin S, Friedman TB. Mutations in TRIOBP, which encodes a putative cytoskeletal-organizing protein, are associated with nonsyndromic recessive deafness. Am J Hum Genet. 2006;78:137–143. doi: 10.1086/499164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazuddin S, Nazli S, Ahmed ZM, Yang Y, Zulfiqar F, Shaikh RS, Zafar AU, Khan SN, Sabar F, Javid FT, Wilcox ER, Tsilou E, Boger ET, Sellers JR, Belyantseva IA, Friedman TB. Mutation spectrum of MYO7A and evaluation of a novel nonsyndromic deafness DFNB2 allele with residual function. Hum Mutat. 2008;29:502–511. doi: 10.1002/humu.20677. [DOI] [PubMed] [Google Scholar]
- Richardson GP, de Monvel JB, Petit C. How the genetics of deafness illuminates auditory physiology. Annu Rev Physiol. 2011;73:311–334. doi: 10.1146/annurev-physiol-012110-142228. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Riviere JB, van Bon BWM, Hoischen A, O’Roak BJ, Kholmanskikh SS, Verloes A, Pilz D, Siu VM, Rossi M, Abdul-Rahman OA, Atkin JF, Nowaczyk MJM, Mancini GMS, Ross ME, Shendure J, Veltman JA, Brunner HG, Dobyns WB. Exome sequencing implicates de novo mutations in the actin genes ACTB and ACTG1 in Baraitser-Winter syndrome. The 12th International Congress of Human Genetics and the American Society of Human Genetics 61st Annual Meeting; Montreal, Canada. 2011. [Google Scholar]
- Roffers-Agarwal J, Xanthos JB, Miller JR. Regulation of actin cytoskeleton architecture by Eps8 and Abi1. BMC Cell Biol. 2005;6:36. doi: 10.1186/1471-2121-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AF, Oleynikov Y, Kislauskis EH, Taneja KL, Singer RH. Characterization of a beta-actin mRNA zipcode-binding protein. Mol Cell Biol. 1997;17:2158–2165. doi: 10.1128/mcb.17.4.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux I, Hosie S, Johnson SL, Bahloul A, Cayet N, Nouaille S, Kros CJ, Petit C, Safieddine S. Myosin VI is required for the proper maturation and function of inner hair cell ribbon synapses. Hum Mol Genet. 2009;18:4615–4628. doi: 10.1093/hmg/ddp429. [DOI] [PubMed] [Google Scholar]
- Rzadzinska AK, Nevalainen EM, Prosser HM, Lappalainen P, Steel KP. MyosinVIIa interacts with Twinfilin-2 at the tips of mechanosensory stereocilia in the inner ear. PLoS One. 2009;4:e7097. doi: 10.1371/journal.pone.0007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzadzinska AK, Schneider ME, Davies C, Riordan GP, Kachar B. An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal. J Cell Biol. 2004;164:887–897. doi: 10.1083/jcb.200310055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi H, Tokita J, Naoz M, Bowen-Pope D, Gov NS, Kachar B. Dynamic compartmentalization of protein tyrosine phosphatase receptor Q at the proximal end of stereocilia: implication of myosin VI-based transport. Cell Motil Cytoskeleton. 2008;65:528–538. doi: 10.1002/cm.20275. [DOI] [PubMed] [Google Scholar]
- Sanggaard KM, Kjaer KW, Eiberg H, Nurnberg G, Nurnberg P, Hoffman K, Jensen H, Sorum C, Rendtorff ND, Tranebjaerg L. A novel nonsense mutation in MYO6 is associated with progressive nonsyndromic hearing loss in a Danish DFNA22 family. Am J Med Genet Part A. 2008;146A:1017–1025. doi: 10.1002/ajmg.a.32174. [DOI] [PubMed] [Google Scholar]
- Schneider ME, Belyantseva IA, Azevedo RB, Kachar B. Rapid renewal of auditory hair bundles. Nature. 2002;418:837–838. doi: 10.1038/418837a. [DOI] [PubMed] [Google Scholar]
- Schoen CJ, Emery SB, Thorne MC, Ammana HR, Sliwerska E, Arnett J, Hortsch M, Hannan F, Burmeister M, Lesperance MM. Increased activity of Diaphanous homolog 3 (DIAPH3)/diaphanous causes hearing defects in humans with auditory neuropathy and in Drosophila. Proc Natl Acad Sci USA. 2010;107:13396–13401. doi: 10.1073/pnas.1003027107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraders M, Lee K, Oostrik J, Huygen PL, Ali G, Hoefsloot LH, Veltman JA, Cremers FP, Basit S, Ansar M, Cremers CW, Kunst HP, Ahmad W, Admiraal RJ, Leal SM, Kremer H. Homozygosity mapping reveals mutations of GRXCR1 as a cause of autosomal-recessive nonsyndromic hearing impairment. Am J Hum Genet. 2010;86:138–147. doi: 10.1016/j.ajhg.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutt CE, Myslik JC, Rozycki MD, Goonesekere NC, Lindberg U. The structure of crystalline profilin-beta-actin. Nature. 1993;365:810–816. doi: 10.1038/365810a0. [DOI] [PubMed] [Google Scholar]
- Sekerkova G, Richter CP, Bartles JR. Roles of the espin actin-bundling proteins in the morphogenesis and stabilization of hair cell stereocilia revealed in CBA/CaJ congenic jerker mice. PLoS Genet. 2011;7:e1002032. doi: 10.1371/journal.pgen.1002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekerkova G, Zheng L, Loomis PA, Mugnaini E, Bartles JR. Espins and the actin cytoskeleton of hair cell stereocilia and sensory cell microvilli. Cell Mol Life Sci. 2006;63:2329–2341. doi: 10.1007/s00018-006-6148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self T, Mahony M, Fleming J, Walsh J, Brown SD, Steel KP. Shaker-1 mutations reveal roles for myosin VIIA in both development and function of cochlear hair cells. Development. 1998;125:557–566. doi: 10.1242/dev.125.4.557. [DOI] [PubMed] [Google Scholar]
- Shahin H, Walsh T, Sobe T, Abu Sa’ J, Abu Rayan A, Lynch ED, Lee MK, Avraham KB, King MC, Kanaan M. Mutations in a novel isoform of TRIOBP that encodes a filamentous-actin binding protein are responsible for DFNB28 recessive nonsyndromic hearing loss. Am J Hum Genet. 2006;78:144–152. doi: 10.1086/499495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawlot W, Deng JM, Fohn LE, Behringer RR. Restricted beta-galactosidase expression of a hygromycin-lacZ gene targeted to the beta-actin locus and embryonic lethality of beta-actin mutant mice. Transgenic Res. 1998;7:95–103. doi: 10.1023/a:1008816308171. [DOI] [PubMed] [Google Scholar]
- Shearer AE, Hildebrand MS, Webster JA, Kahrizi K, Meyer NC, Jalalvand K, Arzhanginy S, Kimberling WJ, Stephan D, Bahlo M, Smith RJ, Najmabadi H. Mutations in the first MyTH4 domain of MYO15A are a common cause of DFNB3 hearing loss. Laryngoscope. 2009;119:727–733. doi: 10.1002/lary.20116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheterline P, Clayton J, Sparrow JC. Actin. Oxford University Press; New York: 1998. [Google Scholar]
- Shin JB, Longo-Guess CM, Gagnon LH, Saylor KW, Dumont RA, Spinelli KJ, Pagana JM, Wilmarth PA, David LL, Gillespie PG, Johnson KR. The R109H variant of fascin-2, a developmentally regulated actin crosslinker in hair-cell stereocilia, underlies early-onset hearing loss of DBA/2J mice. J Neurosci. 2010;30:9683–9694. doi: 10.1523/JNEUROSCI.1541-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JB, Streijger F, Beynon A, Peters T, Gadzala L, McMillen D, Bystrom C, Van der Zee CE, Wallimann T, Gillespie PG. Hair bundles are specialized for ATP delivery via creatine kinase. Neuron. 2007;53:371–386. doi: 10.1016/j.neuron.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemens J, Kazmierczak P, Reynolds A, Sticker M, Littlewood-Evans A, Muller U. The Usher syndrome proteins cadherin 23 and harmonin form a complex by means of PDZ-domain interactions. Proc Natl Acad Sci USA. 2002;99:14946–14951. doi: 10.1073/pnas.232579599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemens J, Lillo C, Dumont RA, Reynolds A, Williams DS, Gillespie PG, Muller U. Cadherin 23 is a component of the tip link in hair-cell ster-eocilia. Nature. 2004;428:950–955. doi: 10.1038/nature02483. [DOI] [PubMed] [Google Scholar]
- Sonnemann KJ, Fitzsimons DP, Patel JR, Liu Y, Schneider MF, Moss RL, Ervasti JM. Cytoplasmic gamma-actin is not required for skeletal muscle development but its absence leads to a progressive myopathy. Dev Cell. 2006;11:387–397. doi: 10.1016/j.devcel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Sweeney HL, Houdusse A. Myosin VI rewrites the rules for myosin motors. Cell. 2010;141:573–582. doi: 10.1016/j.cell.2010.04.028. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Connelly P, Smith S, Guild GM. F-actin bundles in Drosophila bristles are assembled from modules composed of short filaments. J Cell Biol. 1996;135:1291–1308. doi: 10.1083/jcb.135.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Derosier DJ, Mulroy MJ. The organization of actin filaments in the stereocilia of cochlear hair cells. J Cell Biol. 1980;86:244–259. doi: 10.1083/jcb.86.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Saunders JC, Egelman E, DeRosier DJ. Changes in the organization of actin filaments in the stereocilia of noise-damaged lizard cochleae. Hear Res. 1982;7:181–197. doi: 10.1016/0378-5955(82)90013-2. [DOI] [PubMed] [Google Scholar]
- Topsakal V, Hilgert N, van Dinther J, Tranebjaerg L, Rendtorff ND, Zarowski A, Offeciers E, Van Camp G, van de Heyning P. Genotype-phenotype correlation for DFNA22: characterization of non-syndromic, autosomal dominant, progressive sensorineural hearing loss due to MYO6 mutations. Audiol Neurootol. 2010;15:211–220. doi: 10.1159/000255339. [DOI] [PubMed] [Google Scholar]
- Tzima E, Trotter PJ, Orchard MA, Walker JH. Annexin V binds to the actin-based cytoskeleton at the plasma membrane of activated platelets. Exp Cell Res. 1999;251:185–193. doi: 10.1006/excr.1999.4553. [DOI] [PubMed] [Google Scholar]
- Tzima E, Trotter PJ, Orchard MA, Walker JH. Annexin V relocates to the platelet cytoskeleton upon activation and binds to a specific isoform of actin. Eur J Biochem. 2000;267:4720–4730. doi: 10.1046/j.1432-1327.2000.01525.x. [DOI] [PubMed] [Google Scholar]
- van Wijk E, Krieger E, Kemperman MH, De Leenheer EM, Huygen PL, Cremers CW, Cremers FP, Kremer H. A mutation in the gamma actin 1 (ACTG1) gene causes autosomal dominant hearing loss (DFNA20/26) J Med Genet. 2003;40:879–884. doi: 10.1136/jmg.40.12.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verpy E, Leibovici M, Zwaenepoel I, Liu XZ, Gal A, Salem N, Mansour A, Blanchard S, Kobayashi I, Keats BJ, Slim R, Petit C. A defect in harmonin, a PDZ domain-containing protein expressed in the inner ear sensory hair cells, underlies Usher syndrome type 1C. Nat Genet. 2000;26:51–55. doi: 10.1038/79171. [DOI] [PubMed] [Google Scholar]
- Vreugde S, Ferrai C, Miluzio A, Hauben E, Marchisio PC, Crippa MP, Bussi M, Biffo S. Nuclear myosin VI enhances RNA polymerase II-dependent transcription. Mol Cell. 2006;23:749–755. doi: 10.1016/j.molcel.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Wang A, Liang Y, Fridell RA, Probst FJ, Wilcox ER, Touchman JW, Morton CC, Morell RJ, Noben-Trauth K, Camper SA, Friedman TB. Association of unconventional myosin MYO15 mutations with human non-syndromic deafness DFNB3. Science. 1998;280:1447–1451. doi: 10.1126/science.280.5368.1447. [DOI] [PubMed] [Google Scholar]
- Wang H, Robinson RC, Burtnick LD. The structure of native G-actin. Cytoskeleton (Hoboken) 2010;67:456–465. doi: 10.1002/cm.20458. [DOI] [PubMed] [Google Scholar]
- Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, Walsh J, Mburu P, Varela A, Levilliers J, Weston MD, et al. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature. 1995;374:60–61. doi: 10.1038/374060a0. [DOI] [PubMed] [Google Scholar]
- Weil D, Kussel P, Blanchard S, Levy G, Levi-Acobas F, Drira M, Ayadi H, Petit C. The autosomal recessive isolated deafness, DFNB2, and the Usher 1B syndrome are allelic defects of the myosin-VIIA gene. Nat Genet. 1997;16:191–193. doi: 10.1038/ng0697-191. [DOI] [PubMed] [Google Scholar]
- Welch MD, Mullins RD. Cellular control of actin nucleation. Annu Rev Cell Dev Biol. 2002;18:247–288. doi: 10.1146/annurev.cellbio.18.040202.112133. [DOI] [PubMed] [Google Scholar]
- Wells AL, Lin AW, Chen LQ, Safer D, Cain SM, Hasson T, Carragher BO, Milligan RA, Sweeney HL. Myosin VI is an actin-based motor that moves backwards. Nature. 1999;401:505–508. doi: 10.1038/46835. [DOI] [PubMed] [Google Scholar]
- Zampini V, Ruttiger L, Johnson SL, Franz C, Furness DN, Waldhaus J, Xiong H, Hackney CM, Holley MC, Offenhauser N, Di Fiore PP, Knipper M, Masetto S, Marcotti W. Eps8 regulates hair bundle length and functional maturation of mammalian auditory hair cells. PLoS Biol. 2011;9:e1001048. doi: 10.1371/journal.pbio.1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DS, Piazza V, Perrin BJ, Rzadzinska AK, Poczatek JC, Wang M, Prosser HM, Ervasti JM, Corey DP, Lechene CP. Multi-isotope imagining mass spectrometry reveals slow protein turnover in hair-cell stereocilia. Nature. 2012;418:521–524. doi: 10.1038/nature10745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Saha S, Shabalina SA, Kashina A. Differential arginylation of actin isoforms is regulated by coding sequence-dependent degradation. Science. 2010;329:1534–1537. doi: 10.1126/science.1191701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Sekerkova G, Vranich K, Tilney LG, Mugnaini E, Bartles JR. The deaf jerker mouse has a mutation in the gene encoding the espin actin-bundling proteins of hair cell stereocilia and lacks espins. Cell. 2000;102:377–385. doi: 10.1016/s0092-8674(00)00042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M. Cell and Molecular Biology. Michigan, East Lansing: 2008. Examining the folding and stability of in vitro synthesized gamma-actin mutations. Vol. Ph.D. [Google Scholar]
- Zhu M, Yang T, Wei S, DeWan AT, Morell RJ, Elfenbein JL, Fisher RA, Leal SM, Smith RJ, Friderici KH. Mutations in the gamma-actin gene (ACTG1) are associated with dominant progressive deafness (DFNA20/26) Am J Hum Genet. 2003;73:1082–1091. doi: 10.1086/379286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond SH. Formin-induced nucleation of actin filaments. Curr Opin Cell Biol. 2004;16:99–105. doi: 10.1016/j.ceb.2003.10.019. [DOI] [PubMed] [Google Scholar]