Abstract
Objectives
To evaluate risk of all-cause mortality associated with changes in body weight, total lean mass and total fat mass in older men.
Design
Longitudinal cohort study.
Setting
Six U.S. clinical centers.
Participants
4,331 ambulatory men aged 65-93 at baseline.
Measurements
Repeated measurements of body weight, total lean and total fat mass were taken using dual energy x-ray absorptiometry 4.6±0.4 years apart. Percent changes in these measures were categorized as gain (≥ +5%), loss ≤ -5%), or stable (>-5% and <+5%). Deaths were verified centrally by death certificate reviews, and proportional hazard models estimated the risk of mortality.
Results
After accounting for baseline lifestyle factors and medical conditions, a higher risk of mortality was found for men with weight loss (HR 1.84, 95%CI 1.50, 2.26), total lean mass loss (HR 1.78, 95% CI 1.45, 2.19) and total fat mass loss (HR 1.72, 95% CI 1.34, 2.20) compared to men who were stable for each body composition measure. Men with total fat mass gain had a borderline increase in mortality risk (HR 1.29, 95% CI 0.99, 1.67) compared to those who remained stable. These associations did not differ by baseline age, obesity, or self-reported health status (p for interactions > 0.10); however, self-reported weight-loss intent did alter mortality risks with total fat mass (p for interaction=0.04) and total lean mass (p for interaction=0.09) change.
Conclusion
Older men who lost weight, total lean mass or total fat mass had a higher risk of mortality than men who remained stable.
Keywords: mortality, older men, body composition, lean mass, fat mass
INTRODUCTION
The relationship between body weight and mortality differs by age. Younger adults have a higher risk of mortality at both low and high body mass index (BMI) ranges (1-3), whereas studies consistently demonstrate that older adults have a higher risk of mortality at a low BMI, but show inconsistent results at a high BMI (4-7). Furthermore, weight loss in younger, obese adults confers health benefits, whereas, weight loss in older individuals is associated with an increased risk of mortality in epidemiologic studies (8-11).
A measure of weight change does not distinguish between alterations in lean or fat mass. While measures of weight and total body fat tend to be highest in older adults, total body weight generally remains stable or decreases because of reductions in lean mass with a concomitant increase in fat mass (12-14). Thus gross measurements of weight change in older adults do not always reflect changes in lean mass, fat mass or both (15). The individual contributions of changes in lean and fat mass to mortality, separately from weight change, require further study. Despite the wealth of knowledge about weight change and mortality (10, 11, 16), no studies have yet reported on changes in lean or fat mass in relation to mortality in older adults. Therefore, this study examined the relationship between body composition changes and mortality in a cohort of community-dwelling older men.
Declines in muscle mass are associated with higher levels of cytokines and inflammatory markers (17). Because there is an increased risk of mortality associated with greater endogenous inflammation, we hypothesize that the loss of lean mass will be a physical sign of underlying inflammation and also predict a higher risk of mortality (18, 19). Excess adiposity, especially visceral adiposity, is associated with adverse cardiometabolic health, and improvements in metabolic health are seen with fat mass loss (20, 21). Therefore a lower risk of mortality associated with fat mass loss and higher risks of mortality associated with accumulation of fat mass are expected. Intentional weight loss has been found to decrease risk of mortality, especially in younger men and overweight men, but in the setting of poor baseline health may increase the risk of mortality (22, 23). Therefore, this study also examined whether the mortality risks associated with changes in body weight, total lean mass and total fat mass would differ by age, BMI, baseline health status and weight-loss intent.
METHODS
Study Design and Participants
The Osteoporotic Fractures in Men (MrOS) study is a multicenter (6 clinical sites), longitudinal cohort of 5995 ambulatory men aged 65 and older initially enrolled between March 2000 and April 2002. Details of study design and participant recruitment have been published (24, 25). Participants without a baseline whole body dual energy x-ray absorptiometry (DXA) scan (n=39), or repeated DXA scan at the second visit between March 2005 and May 2006 due to death (n=564), failure to return to the clinic (n=884) or lack of DXA scan taken at the second visit (n=144) were excluded. Participants with deaths still pending adjudication (n=33) by July 2009 were also excluded. Thus participants included in this analysis comprised 4331 participants with complete body composition data at visits 1 and 2 and with confirmed vital status by July 2009. This study design and methods comply with the Declaration of Helsinki.
Assessment of weight, lean and fat mass
At visits 1 and 2, body weight was measured in kilograms using balance beam scales at five clinical sites and a digital scale at one site. Total lean mass, appendicular lean mass, total fat mass and truncal fat mass were measured from DXA scans using Hologic QDR 4500 scanners (Hologic, Inc., MA). Reproducibility of DXA measurements was ensured by use of a central quality control lab (San Francisco Coordinating Center), standardized scanning procedures and certification of DXA operators. In addition, a Hologic whole body phantom was scanned repeatedly at each site to monitor longitudinal changes, and correction factors applied to measurements to adjust for longitudinal drift as appropriate.
Questionnaire Data
Demographic information, lifestyle factors, and medical history collected at baseline were used for this analysis. Demographic covariates included age, race (white, African-American or other), and education (<college degree or ≥college degree). Lifestyle factors included smoking status (current use, former use, or no use), alcohol intake (none, light defined as <7 drinks/week, and moderate/high defined by ≥7 drinks/week), physical activity using the Physical Activity Scale for the Elderly score (PASE)(26), and self-reported health (excellent/good versus fair/poor/very poor). Participants were asked to self-report a number of medical conditions, including cancer, congestive heart failure, myocardial infarction, chronic obstructive pulmonary disease, diabetes and osteoporosis. Participants were asked if they were trying to lose weight during the prior 12 months on the second study visit questionnaire. Those responding in the affirmative were considered to have weight-loss intent and no weight-loss intent if the response was negative.
Mortality
Participants were followed for vital status from visit 1 until July 2009; assessment of vital status is >99% complete. All deaths were verified by a central physician adjudicator using death certificates and hospital discharge summaries when available. Participants who terminated the study between visit 2 and July 2009 were censored. Participant enrollment time was tracked using date of last contact or reports of death.
Statistical Analysis
To understand the relationship between all-cause mortality occurring between visit 2 and July 2009 and changes in weight, total lean mass and total fat mass, these measures were categorized as gain, defined as ≥ 5% increase; loss, defined as ≥ 5% decrease; and stable, defined as no change, or a change between < 5% increase and < 5% decrease. These categories were based on previously-reported, significant findings between mortality and 5% change categories of weight (11). Separate Cox proportional hazards regression models were used to evaluate the association between all-cause mortality after the second clinic visit and categories of weight change, total lean mass change and total fat mass change occurring between visits 1 and 2. Similar models were also used to assess mortality risks with changes in appendicular lean mass and truncal fat mass. The shapes of association between all-cause mortality and deciles of % change in weight, total lean mass and total fat mass were explored in separate Cox proportional hazard regression models using the decile with no change as the referent group.
Demographic information, lifestyle factors and medical conditions associated with weight change categories and mortality were examined in the proportional hazard models and included in the adjusted models if found to be significant in the model, as determined by backward stepwise regression with a covariate retention threshold of p<0.10. Race and clinic site were included in the models to account for known differences in measures related to these covariates. To determine whether the mortality risks associated with body composition change categories existed independent of baseline BMI, additional adjustments were made for baseline BMI in fully adjusted models. To understand whether the mortality risks associated with change in lean mass were independent of change in fat mass, and vice versa, fully-adjusted models included adjustment for continuous measures of total lean or total fat mass changes.
Effect modification by obesity status (BMI<30 kg/m2 or BMI≥30 kg/m2) was examined by testing interaction terms for obesity status and body composition change categories (weight, lean mass, fat mass) in above models with the continuous BMI covariate replaced by the categorical BMI variable representing obesity status. Similarly, interaction terms for self-reported health status, age (<75 or ≥75), and self-reported intention to lose weight were examined in fully-adjusted models. Interaction terms with p<0.10 were considered significant effect modifiers. Covariates found to be significant effect modifiers were not included in adjusted models; instead, results were stratified by the effect modifier.
RESULTS
The mean age of the study sample at baseline was 72.8 years. Most participants were Caucasian (n=3914, 90.4%), only 3.0% were current smokers, and the majority reported either excellent or good health (n=3869, 89.3%). The mean BMI was 27.3 kg/m2, mean lean body mass was 57.2 Kg and fat mass was 21.7 Kg. There was no significant difference in race, baseline lean body mass, and self-reported prevalence of cancer, myocardial infarction and osteoporosis between weight change groups (Table 1). However, several variables differed between weight change groups: baseline age, college education, smoking status, alcohol use, weight, total fat mass, truncal fat mass, physical activity, and proportion of men reporting excellent/good health status, weight-loss intent, CHF, COPD and diabetes. Specifically, compared to men who were weight stable, those with ≥5% weight loss had a significantly higher mean age, BMI, weight, total fat mass, truncal fat mass, and physical activity levels. There was a greater proportion of men with weight loss compared to men with weight stability reporting <college education, past and current smoking, no alcohol use, fair/poor/very poor baseline health status, no weight-loss intent, and diagnoses of CHF, diabetes and COPD. Men who experienced ≥5% weight gain compared to men remaining weight stable were on average younger and had greater lean body mass, and there was a greater proportion of men with weight gain reporting <college education, current smoking, no alcohol use, fair/poor/very poor health status, weight-loss intent and diagnosis of diabetes.
Table 1.
Baseline characteristics of participants by weight change categories. Mean (± SD) or proportion shown.
| Weight stable (n=2958) |
≥5% Weight loss (n=971) |
≥5% Weight gain (n=402) |
p-value | |
|---|---|---|---|---|
| Age (years) | 72.6 (5.3) | 74.2 (5.5)§ | 71.5 (5.2)¶ | <0.001 |
| Race | 0.24 | |||
| Caucasian | 90.1% | 91.7% | 89.6% | |
| African American | 3.0% | 2.9% | 4.5% | |
| Other | 6.9% | 5.5% | 6.0% | |
| ≥ College Degree | 58.4% | 51.6%§ | 49.3%¶ | <0.001 |
| Smoking | <0.001 | |||
| Never | 40.8%* | 35.0%§ | 37.6%¶ | |
| Past | 56.9% | 61.3% | 57.0% | |
| Current | 2.4% | 3.7% | 5.5% | |
| Alcohol use | 0.001 | |||
| None | 30.8%† | 37.4%†§ | 36.7%*¶ | |
| Light | 42.1% | 38.6% | 38.4% | |
| Moderate/High | 27.2% | 24.1% | 24.9% | |
| BMI (Kg/m2) | 27.2 (3.5)† | 27.9 (4.0)§ | 27.3 (4.1) | <0.001 |
| Weight (Kg) | 82.9 (12.4) | 84.5 (13.3)§ | 83.8 (14.4) | 0.003 |
| Total lean mass (Kg) | 57.1 (6.9) | 57.3 (7.2) | 57.9 (7.9)¶ | 0.12 |
| Appendicular lean mass (Kg) | 24.4 (3.3) | 24.4 (3.5) | 24.7 (3.7) | 0.26 |
| Total fat mass (Kg) | 21.3 (6.7) | 22.7 (7.3)§ | 21.6 (7.7) | <0.001 |
| Truncal fat mass (Kg) | 12.0 (4.0) | 12.9 (4.4)§ | 12.1 (4.6) | <0.001 |
| Physical Activity Scale in the Elderly score | 154.6 (67.0)† | 144.9 (66.1)*§ | 154.0 (68.0) | <0.001 |
| Excellent/Good Health | 90.9% | 85.5%§ | 87.1%¶ | <0.001 |
| Weight-loss Intent‡ | 35.7%* | 29.9%§ | 48.5%¶ | <0.001 |
| Cancer | 27.5% | 28.1% | 27.4% | 0.93 |
| Congestive heart failure | 3.4% | 6.3%§ | 3.2% | <0.001 |
| Myocardial infarction | 11.7% | 12.5% | 10.2% | 0.49 |
| Diabetes mellitus | 8.2% | 10.3%§ | 15.7%¶ | <0.001 |
| Osteoporosis | 2.8% | 3.5% | 3.7% | 0.39 |
| Chronic obstructive pulmonary disease | 8.7% | 12.8%§ | 9.7% | 0.001 |
Data missing for 1 participant
Data missing for 2 participants
Reported at Visit 2
p-value < 0.05 for unpaired t-test comparing weight loss and weight stable groups
p-value < 0.05 for unpaired t-test comparing weight gain and weight stable groups
The average time between the baseline visit and visit 2 was 4.6 years (range, 3.5 to 5.9 years). The participants studied at both visits had an average -1.3 Kg loss (-1.6%) in weight, +0.14 Kg gain (1.5%) in total fat mass and -1.2 Kg loss (-2.0%) in total lean mass (results not shown). While mean changes in lean and fat mass add up to a given weight change for an individual, the average changes in lean and fat mass across a cohort are influenced by the distribution of individual measures and do not sum up to an average weight change for the cohort. While a high proportion of men remained stable in weight or total lean mass, fewer men remained stable in total fat mass (Table 2). Loss of ≥5% weight, total fat or total lean mass occurred in 20% or more men. In contrast, few men gained ≥ 5% body weight or total lean mass, whereas 37% of men had ≥5% total fat mass gain. Of the participants who were in the weight stable category, 9.9% (n=292) lost lean mass and 40.2% (n=1190) gained fat mass (results not shown). For those with ≥5% weight loss, the median weight loss was -6.5 kg (IQR: -8.9, -5.0). The median weight gain for men who gained ≥5% body weight was 6.0 kg (IQR: 4.8, 7.8).
Table 2.
Number and proportion of men and absolute change of the measure within categories of body composition change (Stable defined by <+5 gain and <-5% loss; Loss defined by ≥-5% loss; Gain defined by ≥+5% gain)
| n | % | Median change in kg (Interquartile range) | |
|---|---|---|---|
| Δ Weight | |||
| Stable | 2958 | 68.3 | -0.50 (-2.1, 1.2) |
| Loss | 971 | 22.4 | -6.5 (-8.9, -5.0) |
| Gain | 402 | 9.3 | 6.0 (4.8, 7.8) |
| Δ Lean mass | |||
| Stable | 3331 | 76.9 | -0.7 (-1.6, 0.4) |
| Loss | 868 | 20.0 | -4.0 (-5.1, -3.3) |
| Gain | 132 | 3.0 | 3.6 (3.1, 4.5) |
| Δ Fat mass | |||
| Stable | 1379 | 31.8 | -0.004 (-0.5, 0.5) |
| Loss | 1349 | 31.1 | -2.5 (-4.1, -1.7) |
| Gain | 1603 | 37.0 | 2.6 (1.7, 3.9) |
There were 433 deaths after visit 2 during a mean follow-up of 3.2 years (12 days to 4.2 years). Death rates were highest for men with ≥ 5% loss in weight, total body lean mass, appendicular lean mass, total body fat mass and truncal fat mass (Table 3). There was a non-linear association between the risk of all-cause mortality and changes in weight, total lean mass and total fat mass (Figure 1a-c). A higher risk of mortality exists for 3 out of the 4 lowest deciles for weight change compared to the referent decile encompassing no change in weight. Similarly, mortality risk increases with a greater loss in lean mass starting with decile 3 (average % lean mass loss of -4.4%). Both greater fat mass loss (below decile 4) and fat mass gain (above decile 8) were associated with a higher risk of mortality.
Table 3.
Risk of all-cause mortality by categories of ≥ 5% body composition loss or gain
| Deaths/1000 person-yr (95% CI) |
Unadjusted HR (95% CI) |
Adjusted* HR (95% CI) |
|
|---|---|---|---|
| Δ Weight | |||
| Stable | 23.8 (20.9, 27.0) | 1.0 ref | 1.0 ref |
| Loss | 56.0 (48.2, 65.1) | 2.39 (1.96, 2.91) | 1.84 (1.50, 2.26) |
| Gain | 24.6 (17.4, 34.9) | 1.00 (0.69, 1.45) | 1.04 (0.71, 1.51) |
| Δ Lean mass | |||
| Stable | 24.2 (21.5, 27.3) | 1.0 ref | 1.0 ref |
| Loss | 57.5 (49.2, 67.3) | 2.43 (2.00, 2.97) | 1.78 (1.45, 2.19) |
| Gain | 28.9 (16.4, 51.0) | 1.25 (0.70, 2.22) | 1.18 (0.66, 2.11) |
| Δ Appendicular lean | |||
| Stable | 21.1 (18.3, 24.3) | 1.0 ref | 1.0 ref |
| Loss | 50.2 (44.1, 57.1) | 2.43 (2.00, 2.95) | 1.84 (1.51, 2.24) |
| Gain | 28.5 (17.4, 46.5) | 1.39 (0.84, 2.32) | 1.31 (0.79, 2.20) |
| Δ Fat mass | |||
| Stable | 21.6 (17.7, 26.3) | 1.0 ref | 1.0 ref |
| Loss | 42.8 (37.0, 49.4) | 2.01 (1.58, 2.57) | 1.72 (1.34, 2.20) |
| Gain | 29.0 (24.7, 34.0) | 1.30 (1.00, 1.67) | 1.29 (0.99, 1.67) |
| Δ Truncal fat | |||
| Stable | 18.7 (14.9, 23.5) | 1.0 ref | 1.0 ref |
| Loss | 43.3 (37.8, 49.7) | 2.37 (1.82, 3.08) | 2.03 (1.55, 2.65) |
| Gain | 28.8 (24.6, 33.8) | 1.50 (1.14, 1.98) | 1.44 (1.09, 1.91) |
Model was adjusted for age, race, site, smoking status, alcohol use, education, physical activity, baseline health, baseline BMI, congestive heart failure, chronic obstructive pulmonary disease, diabetes
Figure 1.
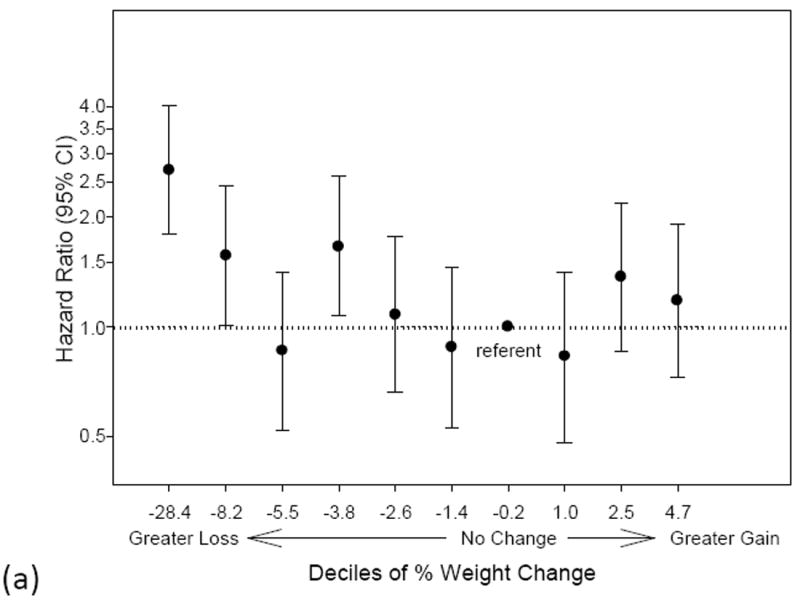
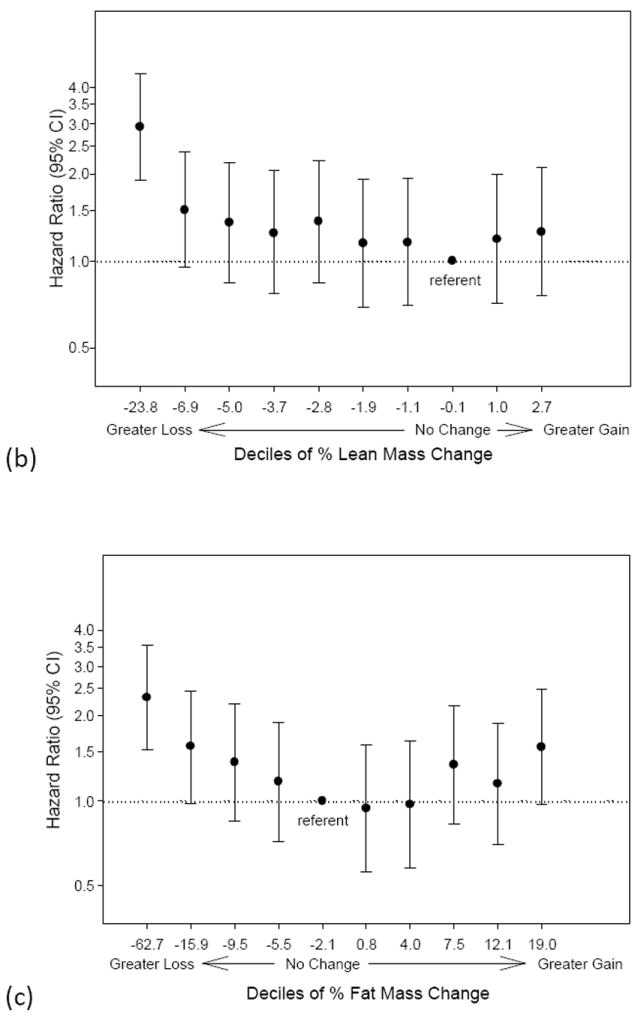
a-c. Risk of mortality by deciles of (a) % weight change (b) % lean mass change or (c) % fat mass change. The x-axis labels specify the lower limit of each decile group. The decile encompassing no change is designated as the referent group. Hazard ratios are adjusted for age, race, clinic site, smoking status, alcohol use, education, physical activity, baseline health status, body mass index, congestive heart failure, chronic obstructive pulmonary disease and diabetes.
Compared to men who remained stable, men with ≥ 5% loss in weight, total lean mass or total fat mass had higher risks of all-cause mortality (HR for weight loss 2.39, 95% CI 1.96, 2.91; lean loss 2.43, 95% CI 2.00, 2.97; and fat loss 2.01 95% CI 1.58, 2.57;). These risks were attenuated by adjustment for lifestyle factors and medical conditions, but remained significant (Table 3). Similarly, there was a higher risk of mortality for men with ≥ 5% loss in appendicular lean mass and truncal fat mass (Table 3). However, in multivariate-adjusted models, there was also higher risk of mortality for men with total fat mass gain that was borderline significant (HR 1.29, p=0.06) and statisticially significant for truncal fat mass gain (HR 1.44, 95% CI 1.09, 1.91) compared to men who were stable in these measures. There remained an increased risk of mortality associated with fat mass loss (HR 1.54, 95% CI 1.20, 1.98) and gain (HR 1.33, 95% CI 1.03, 1.72) even with further adjustment for change in lean mass. Lean mass loss remained associated with a higher risk of mortality after additional adjustment for change in fat mass (HR 1.69, 95% CI 1.37, 2.09).
There was no evidence of effect modification by categories of age, baseline health status, and obesity for the relationships between mortality risk and body composition change categories (p-value for interaction terms > 0.10). However, there was significant effect modification on the relationship between mortality risks with fat and lean change categories by weight-loss intent (p=0.04 and p=0.09 for respective interaction terms). Among men reporting weight-loss intent, loss of fat mass (compared to stability in fat mass) was associated with a very high risk of mortality (Figure 2a, HR 3.09, 95% CI 1.80, 5.31), while among men without weight-loss intent, the relation between fat mass loss (compared to fat mass stability) and mortality was only modestly elevated (Figure 2b, HR 1.43, 95% CI 1.08, 1.90). Fat mass gain vs. fat mass stability for men with weight-loss intent was also associated with a higher risk of mortality (HR 2.36, 95% CI 1.39, 4.03), whereas men without weight-loss intent did not have a higher risk of mortality associated with fat mass gain (HR 1.10, 95% CI 0.81, 1.48). In contrast, men without weight-loss intent had a higher risk of mortality with lean mass loss compared to those with lean mass stability (HR 2.09, 95%CI 1.65, 2.65), while men with weight-loss intent did not have an increased risk of mortality with lean mass loss compared to those who remained stable (HR 1.06, 95%CI 0.69, 1.62).
Figure 2.
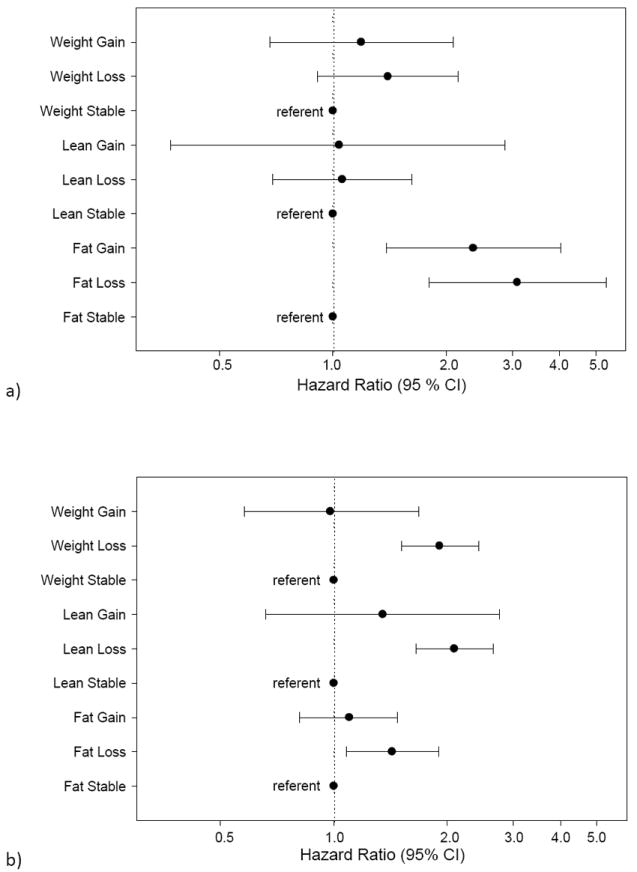
a-b. Risk of mortality with categories of body composition change for (a) men with weight-loss intent and for (b) men without weight-loss intent. Stable is defined by <+5% gain and >-5% loss. Loss is defined by ≥-5% loss. Gain is defined by ≥+5% gain. Hazard ratios are adjusted for age, race, clinic site, smoking status, alcohol use, education, physical activity, baseline health, body mass index, congestive heart failure, chronic obstructive pulmonary disease and diabetes.
Men who reported intent to lose weight compared to those reporting no weight-loss intent were more likely to report former smoking history, fair/poor self-rated health and diabetes. They were on average younger and had higher mean BMI, total body fat and total body lean mass compared to men with no weight-loss intent. Between visits 1 and 2, men with weight-loss intent lost -0.9 % body weight and -1.8% total body lean mass, but actually gained +3.1% total body fat, while men without weight-loss intent lost -1.9% body weight and -2.2% total body lean mass, but had no significant change in total body fat.
DISCUSSION
In older men, loss of weight, total lean mass, appendicular lean mass, total fat mass or truncal fat mass was associated with an increased risk of all-cause mortality compared to those who remained stable in these measures. Older men who gained fat mass, particularly truncal fat mass, had a modest elevation in their risk of mortality, whereas a gain in total weight was not associated with increased mortality risk. The associations between changes in weight, lean mass and fat mass with all-cause mortality did not differ by categories of baseline BMI, health status or age.
Prior studies using a single measurement of weight or BMI in older adults demonstrated that those with low BMI have an increased risk of mortality (5, 27-29). The use of longitudinal measures to identify changes in body composition in this study provided further, novel information. While the findings of increased mortality risk with weight loss in older men are consistent with previous reports (10, 11, 30), this study contributes additional results on the increased mortality risk associated with changes in body composition, specifically, total lean and fat mass, separately. The increased risk of mortality with total body fat gain and especially truncal fat gain builds on data from prior cross-sectional studies showing an increased risk of mortality with high levels of adiposity, particularly central adiposity (27, 31-33). However, the finding of increased mortality risk in older men with fat mass loss was somewhat surprising given health benefits associated with losing fat mass from exercise and diet (20, 34). However, these are intervention studies with obese participants who are on average 10-20 years younger than the mean age of this study’s population. Similarly, an epidemiologic study of younger cohorts shows that a loss of fat mass is associated with decreased mortality risk (35). Because cross-sectional studies of older adults demonstrate an increased risk of all-cause mortality with low BMI (5, 8), a progression towards lower fat mass may have adverse health consequences or be indicative of poor underlying health status in older men.
Men with lean mass loss also had an increased risk of mortality even after adjustments for baseline lifestyle factors and medical conditions. Given that older men who lose weight disproportionately lose lean mass (36), this finding was expected to correspond with the mortality risks associated with weight loss. Low thigh muscle area and loss of extremity fat-free mass are already related to a higher risk of declining physical function and disability (14, 37). These data now also show that loss of lean mass in older men confers a higher risk of all-cause mortality.
The association of lean mass loss with mortality risk existed independent of fat mass change; associations of fat change categories with mortality risk were also independent of lean mass change. Because the changes in fat and lean are independently associated with mortality risk, there may be different mechanisms behind these changes that contribute to a decline in health and require further investigation. While concurrent lean and fat mass loss can occur with anorexic and cachectic processes, a greater loss of fat mass than lean mass may be due to insufficient dietary intake (38, 39), whereas a greater loss of lean mass than fat mass may occur with a cachectic process related to cytokines (40, 41). Age-related decreases in physical activity or androgens in elderly men may contribute to both lean mass loss and fat mass gain (42, 43). Further studies are needed to understand the mechanisms underlying separate and joint changes in fat and lean mass in the elderly and their contributions to mortality.
Secondary analyses showed that the risks of mortality associated with fat gain or loss in weight, fat or lean mass did not differ by baseline BMI, health status and age. The higher mortality risk associated with lean mass loss in the absence of weight-loss intention is consistent with the concept that involuntary lean mass loss may occur due to cachexia from disease occurrence or progression. On the other hand, results showing increased risk of mortality with fat mass loss in men who expressed weight-loss intent were surprising based on prior studies which reported that intentional weight loss is associated with a decreased risk of mortality in younger adults (22, 23). A higher proportion of men with weight-loss intent also reported poor or fair self-rated health, as compared to men without intent to lose weight. Although a number of health conditions associated with mortality was accounted for in adjusted analyses, the men who intended to lose weight may have had undiagnosed medical problems that resulted in fat mass loss and increased their risk of death. Fat mass gain for men reporting weight-loss intent was also associated with a higher risk of mortality compared to men with no weight-loss intent. Because the men with weight-loss intent had higher average BMI, fat mass, lean mass, fat gain and greater proportion of self-reported diabetes, metabolic abnormalities may play a role in the increased risk of mortality with further fat mass gain. Overall, mechanisms for fat loss or gain for men in this study who reported weight-loss intent are unknown; therefore intentional weight loss is best examined with controlled, intervention studies which have shown intentional weight and fat mass loss in older adults to be associated with a decreased risk of mortality (44, 45).
This study is unique because of its size, its use of longitudinal measures of body composition and the close follow-up of participants. The advantages of using DXA to measure body composition include its ability to estimate total and subcompartments of lean and fat mass, lower cost, and lower radiation exposure compared to techniques such as deuterium dilution or CT for visceral adiposity, respectively. While the measurement of truncal fat by DXA correlates highly with total abdominal fat by CT, it has a weaker correlation with CT measurements of visceral fat (46). In contrast, there is a high agreement between DXA measurements of total lean and fat mass and measurements using deuterium dilution in older men (47, 48). Because MrOS is a cohort of ambulatory, older males and approximately 28% of participants who did not follow-up at visit 2 for repeat measures of body composition or had missing data were excluded, this analytic cohort is likely healthier than the general population of older men. Therefore, the findings cannot be generalized to ailing, dependent older men. Furthermore, these results cannot be generalized to older women. This study was limited in power to assess cause-specific mortality associated with categories of body composition change, and further studies of cause-specific mortality risk are needed. There was also insufficient power to analyze mortality risk associated with joint changes in fat and lean mass. Weight-loss intent was obtained from self-report at visit 2 and may be subject to reporting bias. It is possible that men reported weight-loss intent at visit 2 as a consequence of underlying poor health or as a result of gaining weight. This self-report of weight-loss intent may be just a marker for underlying illness or progressing metabolic disease. Given the observational nature of the study, mechanisms behind changes in body composition are unknown; therefore, recommendations for interventions to modify body composition in preventing mortality cannot be made.
In conclusion, the findings demonstrate higher mortality associated with loss of weight, fat mass and lean mass, and a slight increase in risk of death with fat mass gain in older men. These risks do not vary based on obesity status, age or baseline health status. Because these associations were independent of baseline BMI, attention should be given to the trajectory of weight change and not to just a one-time measurement of weight. Therefore, further studies are needed to evaluate the health effects and potential mechanisms for changes in fat and lean mass in older men.
Acknowledgments
Dr. Cawthon has served as a consultant to Merck & Co. and Amgen. Dr. Ensrud has received funding from the NIH (AG05394) as listed under Funding Sources on title page.
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH roadmap for Medical Research under the following grant numbers: U01AR45580, U01AR45614, U01AR45632, U01AR45647, U01AR45654, U01AR45583, U01AG18197, U01AG027810, and UL1RR024140. Dr. Ensrud has received funding from the NIH (AG05394). Dr. Boyko is supported by the Department of Veterans Affairs.
Sponsor’s Role: The funding agencies had no role in the concept, design, analysis or preparation of this manuscript.
Footnotes
The data was presented at the American Geriatrics Society Annual Scientific Meeting. Orlando, FL. May 2010.
Author Contributions:
Dr. Lee: study concept, data analysis, interpretation of data, and preparation of manuscript
Dr. Boyko: interpretation of data and critical review of the manuscript
Dr. Nielson: interpretation of data and critical review of the manuscript
Dr. Stefanick: interpretation of data and critical review of the manuscript
Dr. Bauer: interpretation of data and critical review of the manuscript
Dr. Hoffman: interpretation of data and critical review of the manuscript
Dr. Dam: interpretation of data and critical review of the manuscript
Dr. Lapidus: interpretation of data
Dr. Cawthon: data acquisition, interpretation of data and critical review of the manuscript
Dr. Ensrud: acquisition of data and subjects, interpretation of data and critical review of the manuscript
Dr. Orwoll: acquisition of data and subjects, interpretation of data and critical review of the manuscript
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
References
- 1.Rissanen A, Heliovaara M, Knekt P, et al. Weight and mortality in Finnish men. J Clin Epidemiol. 1989;42:781–789. doi: 10.1016/0895-4356(89)90076-0. [DOI] [PubMed] [Google Scholar]
- 2.Lee IM, Manson JE, Hennekens CH, et al. Body weight and mortality. A 27-year follow-up of middle-aged men. JAMA. 1993;270:2823–2828. doi: 10.1001/jama.270.23.2823. [DOI] [PubMed] [Google Scholar]
- 3.Manson JE, Willett WC, Stampfer MJ, et al. Body weight and mortality among women. N Engl J Med. 1995;333:677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 4.Janssen I, Mark AE. Elevated body mass index and mortality risk in the elderly. Obes Rev. 2007;8:41–59. doi: 10.1111/j.1467-789X.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- 5.Kuk JL, Ardern CI. Influence of age on the association between various measures of obesity and all-cause mortality. J Am Geriatr Soc. 2009;57:2077–2084. doi: 10.1111/j.1532-5415.2009.02486.x. [DOI] [PubMed] [Google Scholar]
- 6.Grabowski DC, Ellis JE. High body mass index does not predict mortality in older people: analysis of the Longitudinal Study of Aging. J Am Geriatr Soc. 2001;49:968–979. doi: 10.1046/j.1532-5415.2001.49189.x. [DOI] [PubMed] [Google Scholar]
- 7.Heiat A, Vaccarino V, Krumholz HM. An evidence-based assessment of federal guidelines for overweight and obesity as they apply to elderly persons. Arch Intern Med. 2001;161:1194–1203. doi: 10.1001/archinte.161.9.1194. [DOI] [PubMed] [Google Scholar]
- 8.Woo J, Ho SC, Sham A. Longitudinal changes in body mass index and body composition over 3 years and relationship to health outcomes in Hong Kong Chinese age 70 and older. J Am Geriatr Soc. 2001;49:737–746. doi: 10.1046/j.1532-5415.2001.49150.x. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415. [PubMed] [Google Scholar]
- 10.Wallace JI, Schwartz RS, LaCroix AZ, et al. Involuntary weight loss in older outpatients: incidence and clinical significance. J Am Geriatr Soc. 1995;43:329–337. doi: 10.1111/j.1532-5415.1995.tb05803.x. [DOI] [PubMed] [Google Scholar]
- 11.Newman AB, Yanez D, Harris T, et al. Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49:1309–1318. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
- 12.Howard BV, Manson JE, Stefanick ML, et al. Low-fat dietary pattern and weight change over 7 years: The Women’s Health Initiative Dietary Modification Trial. JAMA. 2006;295:39–49. doi: 10.1001/jama.295.1.39. [DOI] [PubMed] [Google Scholar]
- 13.Droyvold WB, Nilsen TI, Kruger O, et al. Change in height, weight and body mass index: Longitudinal data from the HUNT Study in Norway. Int J Obes (Lond) 2006;30:935–939. doi: 10.1038/sj.ijo.0803178. [DOI] [PubMed] [Google Scholar]
- 14.Fantin F, Di Francesco V, Fontana G, et al. Longitudinal body composition changes in old men and women: Interrelationships with worsening disability. J Gerontol A Biol Sci Med Sci. 2007;62:1375–1381. doi: 10.1093/gerona/62.12.1375. [DOI] [PubMed] [Google Scholar]
- 15.Newman AB, Lee JS, Visser M, et al. Weight change and the conservation of lean mass in old age: The Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82:872–878. doi: 10.1093/ajcn/82.4.872. quiz 915-876. [DOI] [PubMed] [Google Scholar]
- 16.Arnold AM, Newman AB, Cushman M, et al. Body weight dynamics and their association with physical function and mortality in older adults: The Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 65:63–70. doi: 10.1093/gerona/glp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 18.Alley DE, Crimmins E, Bandeen-Roche K, et al. Three-year change in inflammatory markers in elderly people and mortality: The Invecchiare in Chianti study. J Am Geriatr Soc. 2007;55:1801–1807. doi: 10.1111/j.1532-5415.2007.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaptoge S, Di Angelantonio E, Lowe G, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janssen I, Fortier A, Hudson R, et al. Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care. 2002;25:431–438. doi: 10.2337/diacare.25.3.431. [DOI] [PubMed] [Google Scholar]
- 21.Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: The Framingham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 22.Wannamethee SG, Shaper AG, Lennon L. Reasons for intentional weight loss, unintentional weight loss, and mortality in older men. Arch Intern Med. 2005;165:1035–1040. doi: 10.1001/archinte.165.9.1035. [DOI] [PubMed] [Google Scholar]
- 23.Gregg EW, Gerzoff RB, Thompson TJ, et al. Intentional weight loss and death in overweight and obese U.S. adults 35 years of age and older. Ann Intern Med. 2003;138:383–389. doi: 10.7326/0003-4819-138-5-200303040-00007. [DOI] [PubMed] [Google Scholar]
- 24.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 27.Kalmijn S, Curb JD, Rodriguez BL, et al. The association of body weight and anthropometry with mortality in elderly men: The Honolulu Heart Program. Int J Obes Relat Metab Disord. 1999;23:395–402. doi: 10.1038/sj.ijo.0800832. [DOI] [PubMed] [Google Scholar]
- 28.Janssen I, Katzmarzyk PT, Ross R. Body mass index is inversely related to mortality in older people after adjustment for waist circumference. J Am Geriatr Soc. 2005;53:2112–2118. doi: 10.1111/j.1532-5415.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 29.Price GM, Uauy R, Breeze E, et al. Weight, shape, and mortality risk in older persons: elevated waist-hip ratio, not high body mass index, is associated with a greater risk of death. Am J Clin Nutr. 2006;84:449–460. doi: 10.1093/ajcn/84.1.449. [DOI] [PubMed] [Google Scholar]
- 30.Wedick NM, Barrett-Connor E, Knoke JD, et al. The relationship between weight loss and all-cause mortality in older men and women with and without diabetes mellitus: The Rancho Bernardo study. J Am Geriatr Soc. 2002;50:1810–1815. doi: 10.1046/j.1532-5415.2002.50509.x. [DOI] [PubMed] [Google Scholar]
- 31.Oppert JM, Charles MA, Thibult N, et al. Anthropometric estimates of muscle and fat mass in relation to cardiac and cancer mortality in men: The Paris Prospective Study. Am J Clin Nutr. 2002;75:1107–1113. doi: 10.1093/ajcn/75.6.1107. [DOI] [PubMed] [Google Scholar]
- 32.Heitmann BL, Erikson H, Ellsinger BM, et al. Mortality associated with body fat, fat-free mass and body mass index among 60-year-old swedish men-a 22-year follow-up. The study of men born in 1913. Int J Obes Relat Metab Disord. 2000;24:33–37. doi: 10.1038/sj.ijo.0801082. [DOI] [PubMed] [Google Scholar]
- 33.Reis JP, Araneta MR, Wingard DL, et al. Overall obesity and abdominal adiposity as predictors of mortality in U.S. White and black adults. Ann Epidemiol. 2009;19:134–142. doi: 10.1016/j.annepidem.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Solomon TP, Haus JM, Kelly KR, et al. Randomized trial on the effects of a 7-d low-glycemic diet and exercise intervention on insulin resistance in older obese humans. Am J Clin Nutr. 2009;90:1222–1229. doi: 10.3945/ajcn.2009.28293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allison DB, Zannolli R, Faith MS, et al. Weight loss increases and fat loss decreases all-cause mortality rate: Results from two independent cohort studies. Int J Obes Relat Metab Disord. 1999;23:603–611. doi: 10.1038/sj.ijo.0800875. [DOI] [PubMed] [Google Scholar]
- 36.Hughes VA, Frontera WR, Roubenoff R, et al. Longitudinal changes in body composition in older men and women: Role of body weight change and physical activity. Am J Clin Nutr. 2002;76:473–481. doi: 10.1093/ajcn/76.2.473. [DOI] [PubMed] [Google Scholar]
- 37.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 38.Katzel LI, Bleecker ER, Colman EG, et al. Effects of weight loss vs aerobic exercise training on risk factors for coronary disease in healthy, obese, middle-aged and older men. A randomized controlled trial. JAMA. 1995;274:1915–1921. doi: 10.1001/jama.1995.03530240025035. [DOI] [PubMed] [Google Scholar]
- 39.Meckling KA, Gauthier M, Grubb R, et al. Effects of a hypocaloric, low-carbohydrate diet on weight loss, blood lipids, blood pressure, glucose tolerance, and body composition in free-living overweight women. Can J Physiol Pharmacol. 2002;80:1095–1105. doi: 10.1139/y02-140. [DOI] [PubMed] [Google Scholar]
- 40.Schaap LA, Pluijm SM, Deeg DJ, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64:1183–1189. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morley JE, Thomas DR, Wilson MM. Cachexia: Pathophysiology and clinical relevance. Am J Clin Nutr. 2006;83:735–743. doi: 10.1093/ajcn/83.4.735. [DOI] [PubMed] [Google Scholar]
- 42.Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 43.Luhrmann PM, Bender R, Edelmann-Schafer B, et al. Longitudinal changes in energy expenditure in an elderly German population: A 12-year follow-up. Eur J Clin Nutr. 2009;63:986–992. doi: 10.1038/ejcn.2009.1. [DOI] [PubMed] [Google Scholar]
- 44.Berentzen T, Sorensen TI. Effects of intended weight loss on morbidity and mortality: possible explanations of controversial results. Nutr Rev. 2006;64:502–507. doi: 10.1111/j.1753-4887.2006.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 45.Shea MK, Houston DK, Nicklas BJ, et al. The Effect of Randomization to Weight Loss on Total Mortality in Older Overweight and Obese Adults: The ADAPT Study. J Gerontol A Biol Sci Med Sci. 2010;65:519–25. doi: 10.1093/gerona/glp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snijder MB, Visser M, Dekker JM, et al. The prediction of visceral fat by dual-energy X-ray absorptiometry in the elderly: A comparison with computed tomography and anthropometry. Int J Obes Relat Metab Disord. 2002;26:984–993. doi: 10.1038/sj.ijo.0801968. [DOI] [PubMed] [Google Scholar]
- 47.Visser M, Fuerst T, Lang T, et al. Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. Health, Aging, and Body Composition Study--Dual-Energy X-ray Absorptiometry and Body Composition Working Group. J Appl Physiol. 1999;87:1513–1520. doi: 10.1152/jappl.1999.87.4.1513. [DOI] [PubMed] [Google Scholar]
- 48.Salamone LM, Fuerst T, Visser M, et al. Measurement of fat mass using DEXA: A validation study in elderly adults. J Appl Physiol. 2000;89:345–352. doi: 10.1152/jappl.2000.89.1.345. [DOI] [PubMed] [Google Scholar]


