SUMMARY
In humans, microbially-induced inflammatory periodontal diseases are the primary initiators that disrupt the functional and structural integrity of the periodontium (i.e., the alveolar bone, the periodontal ligament, and the cementum). The re-establishment of its original structure, properties and function constitutes a significant challenge in the development of new therapies to regenerate tooth-supporting defects. Preclinical models represent an important in vivo tool to critically evaluate and analyze key aspects of novel regenerative therapies including: 1) Safety, 2) Effectiveness, 3) Practicality, and 4) Functional and structural stability over time. Therefore, these models provide foundational data that supports the clinical validation and the development of novel innovative regenerative periodontal technologies. Steps are provided on the use of the root fenestration animal model for the proper evaluation of periodontal outcome measures using the following parameters: descriptive histology, histomorphometry, immunostaining techniques, three-dimensional imaging, electron microscopy, gene expression analyses and safety assessments. These methods will prepare investigators and assist them in identifying key endpoints that can then be adapted to later stage human clinical trials.
Keywords: Guided tissue regeneration, Regenerative medicine, Bone regeneration, Tissue engineering, Animal models, Periodontal diseases, Periodontal Engineering
1. INTRODUCTION
The tooth-supporting apparatus (i.e. periodontium) includes the alveolar bone, the periodontal ligament (PDL), the cementum, and the gingiva. Collectively, they represent a dynamic tissue complex with mechanical and biological functions that synergistically determine the tissue adaptive potential and its ability to sustain microbiological and mechanical challenges. Through a number of complex mechanisms involving growth factors, transcription factors, and extracellular matrix (ECM) proteins, the periodontium is able to maintain its homeostasis, structure and function, and to respond and adapt to mechanical stimuli, infectious and/or inflammatory injuries (1, 2). However, once periodontal breakdown occurs, the ideal restoration (i.e. regeneration) of its original structure and function still remains a major challenge in the clinical setting (3). In general, these efforts have focused almost exclusively on regenerating lost alveolar bone. However, by definition, regeneration of the lost periodontium involves the formation of all tooth-supporting structures including new cementum, PDL, alveolar bone and gingival tissue. Also, the appropriate PDL tissue orientation, fiber directionality and integration to both cementum and alveolar bone are required. Appropriate mechanical loading would be essential for the development of highly organized functional PDL fibers (4). Because of this critical interfacial connection of the multi-tissue complex that determines its function and stability, the use of periodontal-engineered devices have emerged as a prospective alternative to conventional treatments (5). Periodontal engineering uses life science and engineering technologies to restore the structure and function of alveolar bone, PDL, cementum and gingival tissue (4).
The regenerative potential and the plausible biological mechanism of a novel therapy are often determined in vitro. However, to test the clinical feasibility and applicability of new therapies, the value of in vitro studies is very limited and frequently inadequate for direct entry into clinical trials (6). As required by regulatory approval agencies, such as the U.S. Food and Drug Administration and European Medicines Agency (EMEA), the safety and efficacy of new materials and techniques need to be tested in preclinical studies. Moreover, biological pathways taking place in these processes can also be studied and be further validated (7). In addition, investigators can extrapolate their preclinical findings and identify important endpoints to be adapted to human clinical trial planning.
In general, the ideal preclinical model should be one that includes the following characteristics:
Standardization.
Stable and controllable genetic background.
Allow for evaluation of local and systemic safety.
Facilitate the analysis of effectiveness by multiple modalities.
Allow practical evaluation of functional and structural stability of the tissue over time.
Cost-effective.
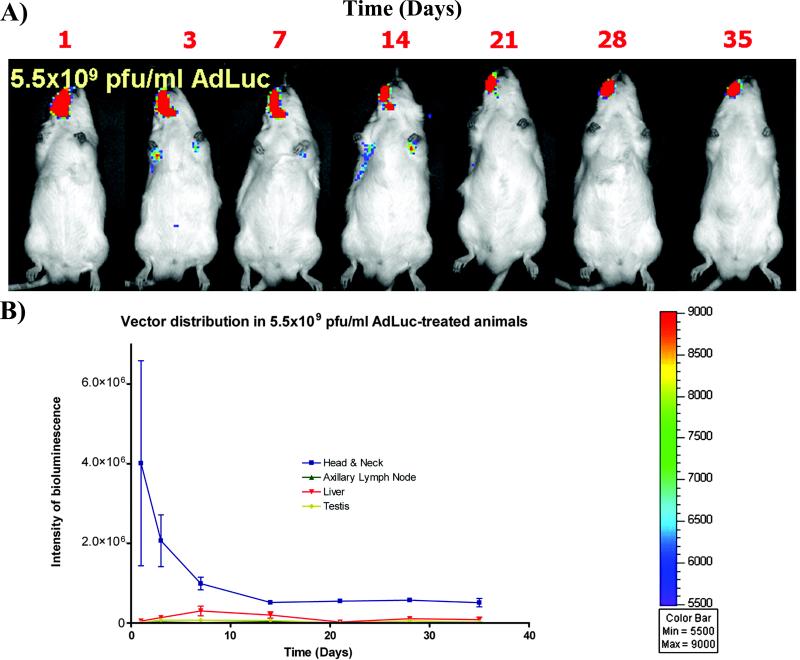
Rodents (mice and rats) are the most commonly used animal models in biomedical research. Rats are cost-effective, easy to handle, and allow for the standardization of experimental conditions in genetically similar individuals (6). They are suitable for the study of the effects of physiological alterations related to aging, systemic diseases, pharmacological therapies and immunodeficiency on tissue destruction and regeneration (8-10). Additionally, rat models allow evaluation of kinetics and biodistribution of different therapeutic agents, like adenovirus, by bioluminescence techniques. These techniques allow for safety evaluation and analysis of the short and long term biodistribution of the therapeutic agents administered both locally (11) and systemically (12) (Figure 1). In vivo bioluminescence generated by expression of the luciferase transgene allows quantification and localization of transgene expression and provides noninvasive, dynamic, accurate, and comprehensive monitoring of vector expression systemically (12-14). Furthermore, analysis of luciferase-expressing recombinant adenoviruses by bioluminescence is a highly sensitive method for evaluating the biodistribution and subsequent vector activity in the entire body (12).
Figure 1.
The safety incorporation and processing of biologics is accurately monitored in the rat fenestration animal models as shown by the vector transduction efficiency and systemic distribution by bioluminescence. After the surgery on the right side, most of the luciferin signal is restricted to the alveolar bone defect region. However, a significant vector expression can be also noticed in distant organs, with maximum expression at day 14, followed by a decrease in vector expression in the head and neck region over time as well as in the maxillary area. Reproduced with permission from (12).
To specifically study the regeneration of tooth-supporting structures, a widely used, accepted and standardized model is required. For example, an in vivo model that could assist on obtaining evidence for primary determination of the therapeutic efficacy, providing a proof of principle in a short time frame before proceeding to a larger animal model, etc. The rat fenestration model not only includes but allows for the proper standardization of a number of these important aspects (Table 1). Based on this model, cementum and bone regeneration have been evaluated following the delivery of growth factors, genes, cells and multi-phasic scaffolds (5, 15-24). The extraoral approach of this model provides isolation from the oral environment, and thus can prevent negative effects such as contamination, infection by intraoral microorganisms, or gingival tissue ingrowth.
Table 1.
| Advantages | Disadvantages |
|---|---|
| Proof-of-concept in a short time frame | Narrow healing time window |
| Well-contained defects | Small size, surgical microscopes required; technically challenging |
| No gingival tissue ingrowth | Rapid repair as kinetic healing model |
| Relatively low cost | Cannot measure healing of junctional epithelial-connective tissue interface |
| Controllable microflora | Not a “natural disease” model with microbial influence |
| Known age | Different anatomical structures compared to humans |
| Known genetic background | Different histopathologic features |
| Ease of handling and housing | Different host responses compared with humans |
For functional periodontal regeneration to occur, temporal and spatial progress in a similar sequence to that involved in the natural formation and development of the periodontium is needed (25). Although the exact cellular and molecular events are still not clear, cells must first migrate and attach to the denuded root surface. By using the rat fenestration defect model, a microenvironment that favors the proliferation, migration and maturation of mesenchymal progenitors to the defect area of the PDL or the host bone has been observed (26, 27). This process is mediated and coordinated by soluble factors, other cells, and ECM. The early healing process follows the conserved sequence of wound healing that is initiated by blood coagulation and migration of neutrophils and monocytes for wound debridement and bone resorption. Bone formation is typically initiated from the bony margins of the lesions (28). Within days after surgery, a thin cementum layer with a connective tissue attachment can be observed, particularly on the apical side of the teeth, where the cementum is thicker compared with the narrow coronal region (22). Once mineralized tissues are established, PDL fiber orientation, directionality and integration to both cementum and alveolar bone are mediated by appropriate mechanical loading (4, 29). It is therefore crucial that investigators, according to the timeline that those processes follow (Figure 2), select the appropriate time-point(s) to determine the therapeutic efficacy “window” of a candidate periodontal-engineered device or bioactive molecule. In rats, recommended study evaluation time ranges from 2 to 6 weeks to capture early healing events and wound maturation (6).
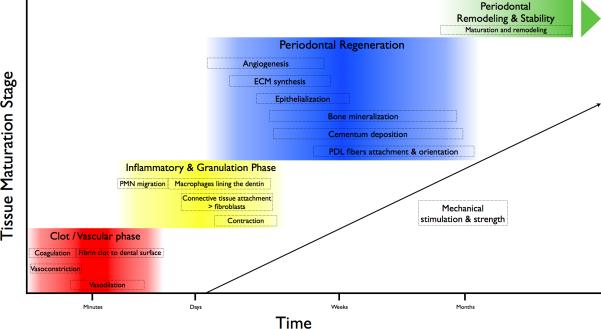
Figure 2.
Phases during periodontal healing and regeneration. Periodontal regeneration requires different processes in a sequential manner. After the initial coagulation phase, inflammatory reaction and granulation tissue formation events, progenitor cells involved in multi-tissue regeneration are locally recruited and mediate the bioavailability of important growth factors. As the healing progresses, mechanical stimuli increase and promote an organized ECM synthesis as well as cementum and bone formation and maturation. Once those structures are established, PDL fibers are organized and oriented. Progressively, the tissues mature and ultimately increase its mechanical strength. Remodeling processes continue in the regenerated periodontium as an essential mechanism that monitors the adaptation potential to the challenging local and systemic environment.
Briefly, based on previously published procedures reviewed by Pellegrini et al. (6), Rios & Giannobile (30) and Seol et al. (31), we provide an overview of the rat fenestration model for the evaluation of periodontal outcome measures using descriptive histology, histomorphometry, immunostaining techniques, three-dimensional imaging, electron microscopy, gene expression analyses and safety assessments.
The procedure starts with an extraoral incision to reach the buccal aspect of the inferior molars. Buccal bone is removed up to the roots of the teeth to create a defect with standardized dimensions (e.g. 3 × 2 × 1 mm). Roots are denuded, including the superficial dentin. Testing-material can subsequently be applied to the defect and the flap be repositioned and secured back in place.
2. MATERIALS
- Surroundings:
- ○ Sterile and sanitized surgical area.
- ■ Disinfectants such as sodium hypochlorite, chlorine dioxide, dimethyl ammonium chloride, chlorine dioxide, or glutaraldehyde-based solutions.
- ■ Handwashing, sterile gloves, gowns, and masks.
- ■ Hot-bead instrument sterilizer, autoclave.
- ■ Hood and adequate ventilation system to assure aseptic conditions during the surgery.
- ○ Magnification/amplification system such as a magnifying stereoscope (×2 to 10).
- Anesthesia, analgesics and antibiotics will vary with animal weight and must be according to veterinary instructions. In general:
- ○ Ketamine (IP, 40–90 mg/kg) and xylazine (IP 5–10 mg/kg), as anesthetics.
- ○ Buprenorphine (0.01–0.05 mg/ kg) and Ketoprofen (5 mg/kg), as analgesics.
- ○ Ampicillin 268 mg/L added to a 5% to 10% dextrose solution, as antibiotic.
- ○ 20–27-gauge needle.
Animal restraint and tissue retraction systems adaptable to animal size.
External heat source(s) (e.g., recirculating water blanket, microwaveable heating packs, or self-regulating heating pad).
Ophthalmic ointment (lubricant).
Povidoneiodine topical antiseptic, sterile saline, water, and/or 70% ethanol.
Hair removal blade, shaver or cream.
Initial incision: Surgical blade (#11, 15), periosteal elevator (Pritchard).
- Defect creation:
- Surgical retractors, periodontal probe, 17/23 dental explorer.
- Small, sharp, hand instruments such as Gracey curettes, hoes, or chisels.
- Number ¼, and 4 round burs, low-speed and high-speed handpiece with engine and chisel.
- Wound closure:
- Needle holder (Crile-Wood).
- Suture material (resorbable).
- Scissors (LaGrange double curved).
- Surgical clips (e.g. metal staples).
Sterile, clean cages for post-surgery animal recovery.
Staple remover.
Tissue harvesting: Scissors, round disc and low speed engine.
- Tissue analyses:
- Micro-computed tomography (CT) system for three-dimensional analysis of the mineralized tissues.
- Histological, immunohistochemical and immunofluorescence staining methods for specific molecules.
- Optical microscope with imaging analysis apparatus. Ideally, a high-definition charge-coupled device (CCD) color camera capable of taking microscopic images is recommended.
- Optical immunofluorescence microscope with imaging analysis apparatus or confocal microscope for capturing immunofluorescence images.
- Histomorphometric and image analysis software.
3. METHODS
3.1 Preparation for the surgery
Acclimatisation period of the animal of approximately 2 days to 1 week after arrival in a new housing facility.
Aseptic surgical area to perform an aseptic surgery (must not be used for any other purpose during the time of the surgery) (see Note 1).
Anesthesia with a combination of ketamine and xylazine via intraperitoneal (IP) injection, lasting for 45–90 minutes (see Note 2).
Apply ophthalmic ointment (lubricant) to the eyes of the animal to prevent drying.
Hair removal around the surgical area.
Skin disinfection with three alternating scrubs of povidoneiodine topical antiseptic, and warm, sterile saline, water, or 70% ethanol (ethanol is less desirable) scrubbing in an outward and spiral direction.
Surgeons’ preparation including, but not limited to, handwashing, sterile gloves, gowns, and masks for each animal's surgical procedures.
3.2. Surgical procedures
Animal restraint and retraction of soft tissues.
Surgery should be performed under a magnifying stereoscope (×2 to 10) to allow proper identification of anatomic landmarks and site preparation.
Identification of epithelial and hard tissue landmarks for the initial incision: parotid gland, masseter muscle, labial angle, inferior border of the mandible, molar teeth, etc.
First incision should be very superficial (only dermal layers) to expose the masseter muscle and gain access to a ligamentous landmark that extends in a postero-anterior direction approximating the lower border of the mandible (see Note 3).
Second incision is meant to dissect the area of interest through the masseter muscle slightly under the lower ligamentous line that could be visualized in an anterior-posterior direction until the body of the mandible is reached (buccal plate) (see Note 4).
Dissection of a distinct ligament that covers the area lateral to the first molar in order to ensure proper flap refection and adequate surgical access is required.
Once the bone is exposed and access to the first molar region is gained, the operator will be able to distinguish a more opaque and bulbous bone region with a tear-like shape, which is characteristic of the buccal plate.
The target defect creation area is the distal root of the mandibular first molar (buccal roots of the first and second molars can be included in the surgical defect). Initiate access with a no. 4 round bur and continue with a ¼ bur to complete the osteotomy and remove the cementum once roots become visible. Standard bony defects should have 3 × 2 × 1 mm (see Note 5) (Figure 3).
Apply the test agent(s) or regenerative device(s) into the created defect area according to the specific instruction that each material could require.
Reposition the muscle by using resorbable sutures.
Finally, reposition the skin by surgical clips.
Figure 3.
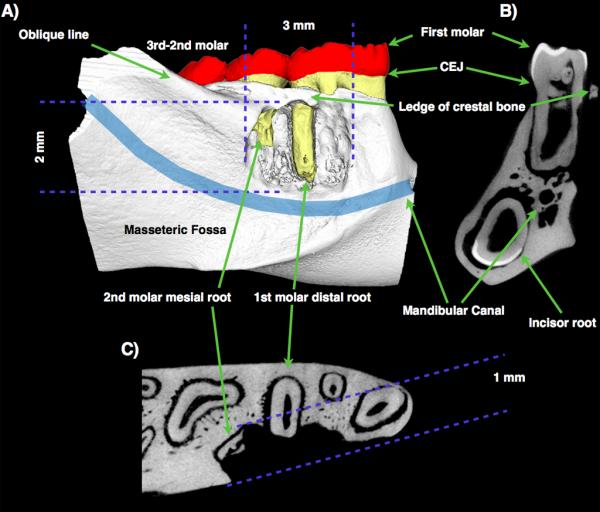
μ-CT 3D reconstruction (A) and 2D sections (B: coronal; C: transversal) of a rat fenestration defect. Location, characteristics, and anatomical landmarks from different views are shown (18 × 18 × 18 μm3 voxel size).
3.3. Postoperative care
Analgesics should be administered for at least 24 hours after the periodontal defect surgery (see Note 6).
Antibiotics dispensed via the water supply (see Note 7).
Animals should be mobile and fed freely following surgical recovery, and housed individually (see Note 8).
Re-evaluation of the sutured wound at least three times per week is recommended until removal of clips at 2 weeks after surgery.
Treatments and monitoring must fit with the animal surgery guidelines given by the researcher's institution in accordance with regulations and in compliance with animal housing authorities (see Note 9).
3.4. Timing
At the designated endpoint, rats will be sacrificed using carbon dioxide overdose or according to institutional guidelines. A secondary method should be employed in order to confirm animal death prior to resuming tissue collection. This can be removal of vital organs.
Harvest tissues depending on the process desired to analyze and accordingly to the healing timeline (Figure 2) (see Note 10).
Harvested samples should be fixed immediately to prevent degradation without damaging the tissues according to the procedure to be performed.
3.5. Endpoint Measurements
- Structural analysis:
- ○ μ-CT evaluation or radiographic methods could be used to establish mineralized tissue lineal measurement parameters.
- ○ In addition, volumetric parameters could also be determined (see Note 11): bone volume (BV), bone volume fraction (BVF), tissue mineral content (TMC), tissue mineral density (TMD), bone mineral density (BMD).
- Biochemical analysis:
- ○ Whole tissue dissection or Laser Capture Microdissection (LCM) could be used to obtain tissue/cell samples from specific areas, such as PDL, bone or cementum, for RNA or protein analysis (mRNA analysis, Western-Blot, or ELISA techniques can be done to detect specific molecules relevant in periodontal regeneration).
- Cellular characterization:
- ○ Histology and histomorphometry: The distal root of first molar is the main target for histologic and histomorphometric evaluation. PDL fibroblasts, osteoblast, cementoblasts, and fiber orientation from cementum to bone can also be studied (32).
- ○ Immunohistochemical and immunofluorescence techniques allow the detection and immunolocalization of specific markers among the regenerated tissues.
ACKNOWLEDGEMENTS
The authors appreciate Sarah L. Volk for assisting with the preparation of the manuscript. We also acknowledge the technical contributions of Chan Ho Park, Scott Lim and Jim Sugai. This work was supported by the NIH Grants K23DE019872 (HFR) and DE13397 (WVG). MPM was also supported by the Talentia Scholarship Program from the Regional Ministry for Innovation, Science and Enterprise, Junta de Andalusia (Spain). The authors report no conflicts of interest.
Footnotes
Ideally, the surgical area can be located within the housing facilities, therefore limiting stress and potential health hazards to the animals. Disinfectants such as sodium hypochlorite, chlorine dioxide, dimethyl ammonium chloride, or glutaraldehyde-based solutions can be used to clean and disinfect the surgery area, although some may not be as effective at eliminating all contaminants. Animals and instruments must also be prepared in a way to prevent contamination and ensure success of the survival surgery.
All instruments should be cleaned and sterilized (e.g., autoclaved) prior to surgery. Disinfection/sterilization of multiple sets of instruments should be carried out for successive surgeries. Following use, instruments should be thoroughly cleaned before sterilization. Hot bead sterilization is a fast, dry method to prevent cross contamination between animals during surgery. Alternative sterilization methods may incorporate the use of glutaraldehyde or chlorine dioxide immersion followed by a sterile water or saline rinse. Aseptic techniques and sterile environments are critical to animal survival and positive experimental results.
Working in a laminar airflow hood can ensure the environment aseptic requirements.
Rat anesthetics and analgesics: A combination of ketamine (IP, 40–90 mg/kg) and xylazine (IP 5– 10 mg/kg) can be used as a general, injectable anesthesia for oral procedures. For prolonged anesthesia, supplement with one-third dose of ketamine only. IP injections should be performed using a 20–27-gauge needle that is inserted into the lower left abdominal quadrant with the animal in a head-down position. Anesthesia depth is typically monitored by the loss of response to external stimuli, such as a limb pinch.
All animals should be provided an external heat source (e.g., recirculating water blanket, microwaveable heating packs, or self-regulating heating pad) in indirect contact with the animal to prevent hypothermia during the entire anesthesia and recovery period.
Effective drug dosage may vary from animal to animal according to body weight, metabolism, and age. There are no exact calculations to relate the effective dose between animal and humans. Dosage can be determined by previous study results, published literature, and veterinary guidelines.
At this time it is also important to separate the skin from the muscle around the incision line to allow proper closure and space for the use of surgical staples at the end of the surgery.
In some cases, the parotid gland duct (Stenson's) can be involved, causing postsurgical buccal swelling (mucoceles). This swelling can affect tissue regeneration as it produces mechanical pressure to the surgical area. Drainage of salivary secretions with 10 mL syringe and 25-gauge needle may provide temporary relief from mechanical pressure. Parotid gland needs to be completely removed to eliminate swelling. Antibiotic water should be administered after removal of parotid gland.
During bone and cementum removal, it is difficult to irrigate with saline due to the small defect size, thin bone, and cementum. Special care should be taken to not generate heat damage at the surgical site, as it prevents a normal healing process. The PDL, cementum, and superficial dentin can be removed by a combination of hand instrumentation and careful use of rotatory instruments. A very small ledge of crestal bone must remain coronally to maintain the integrity of the ridge and prevent communication with the oral cavity.
Buprenorphine (subcutaneous or intraperitoneal, 0.01–0.05 mg/ kg) can be used for 8–12 hours for post-operative pain relief, or for 24-hour pain management, 5 mg/kg ketoprofen (subcutaneous) may be selected. Buprenorphine has negative interaction (which can lead to death of animal) with ketamine/xylazine cocktail if administered before animal recovers from anesthesia. So, if buprenorphine is used, must be administered after rat awakens from anesthesia.
Ampicillin 268 mg/L added to a 5% to 10% dextrose solution can be used. Colored water bottles should be used with light sensitive antibiotics.
Animal recovery time will vary with the type and dose of anesthesia, and may also vary between animals of similar sex, size, body mass, and genetic background.
For example, if biohazardous materials, such as viral vectors, are applied the animal must be kept in biohazard facility until viral shedding has completed. Each virus will have a specific shedding period for which it can be considered contagious, and contact should be limited during this period. For example, adenovirus should be considered biohazardous for at least 72 hours following application/inoculation.
By using this model, 3, 10, 21, and 35 days post surgery time-points are usually selected to harvest the tissue samples for analyzing bone and cementum regeneration.
Digital subtraction of teeth in the region of interest has to be done in order to exclude these structures from the analysis. Complete guidelines can be found in Park et al. (33).
REFERENCES
- 1.Bartold PM, Narayanan AS. Molecular and cell biology of healthy and diseased periodontal tissues. Periodontol 2000. 2006;40:29–49. doi: 10.1111/j.1600-0757.2005.00140.x. [DOI] [PubMed] [Google Scholar]
- 2.Rios HF, Ma D, Xie Y, Giannobile WV, Bonewald LF, Conway SJ, Feng JQ. Periostin is essential for the integrity and function of the periodontal ligament during occlusal loading in mice. J Periodontol. 2008;79:1480–1490. doi: 10.1902/jop.2008.070624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartold PM, Shi S, Gronthos S. Stem cells and periodontal regeneration. Periodontol 2000. 2006;40:164–172. doi: 10.1111/j.1600-0757.2005.00139.x. [DOI] [PubMed] [Google Scholar]
- 4.Rios HF, Lin Z, Oh B, Park CH, Giannobile WV. Cell- and gene-based therapeutic strategies for periodontal regenerative medicine. J Periodontol. 2011;82:1223–1237. doi: 10.1902/jop.2011.100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park CH, Rios HF, Jin Q, Bland ME, Flanagan CL, Hollister SJ, Giannobile WV. Biomimetic hybrid scaffolds for engineering human tooth-ligament interfaces. Biomaterials. 2010;31:5945–5952. doi: 10.1016/j.biomaterials.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pellegrini G, Seol YJ, Gruber R, Giannobile WV. Pre-clinical models for oral and periodontal reconstructive therapies. J Dent Res. 2009;88:1065–1076. doi: 10.1177/0022034509349748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaigler D, Fuller K, Giannobile WV. Regulatory process for the evaluation of dental drugs, devices and biologics (Chapter 4). In: Giannobile WV, Burt BA, Genco RJ, editors. Clinical Research in Oral Health. 1 ed. Wiley-Blackwell; Ames: 2010. pp. 55–78. [Google Scholar]
- 8.Benatti BB, Neto JBC, Casati MZ, Sallum EA, Sallum AW, Nociti FH., Jr Periodontal healing may be affected by aging: A histologic study in rats. J Periodontal Res. 2006;41:329–333. doi: 10.1111/j.1600-0765.2006.00872.x. [DOI] [PubMed] [Google Scholar]
- 9.Graves DT, Fine D, Teng YTA, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klausen B. Microbiological and immunological aspects of experimental periodontal disease in rats: a review article. J Periodontol. 1991;62:59–73. doi: 10.1902/jop.1991.62.1.59. [DOI] [PubMed] [Google Scholar]
- 11.Jin Q, Anusaksathien O, Webb SA, Printz MA, Giannobile WV. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol Ther. 2004;9:519–526. doi: 10.1016/j.ymthe.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang PC, Cirelli JA, Jin Q, Seol YJ, Sugai JV, D'Silva NJ, Danciu TE, Chandler LA, Sosnowski BA, Giannobile WV. Adenovirus encoding human platelet-derived growth factor-B delivered to alveolar bone defects exhibits safety and biodistribution profiles favorable for clinical use. Hum Gene Ther. 2009;20:486–496. doi: 10.1089/hum.2008.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson M, Huyn S, Burton J, Sato M, Wu L. Differential biodistribution of adenoviral vector in vivo as monitored by bioluminescence imaging and quantitative polymerase chain reaction. Hum Gene Ther. 2006;17:1262–1269. doi: 10.1089/hum.2006.17.1262. [DOI] [PubMed] [Google Scholar]
- 14.Wood M, Perrotte P, Onishi E, Harper ME, Dinney C, Pagliaro L, Wilson DR. Biodistribution of an adenoviral vector carrying the luciferase reporter gene following intravesical or intravenous administration to a mouse. Cancer Gene Ther. 1999;6:367–372. doi: 10.1038/sj.cgt.7700090. [DOI] [PubMed] [Google Scholar]
- 15.Giannobile WV, Lee CS, Tomala MP, Tejeda KM, Zhu Z. Platelet-derived growth factor (PDGF) gene delivery for application in periodontal tissue engineering. J Periodontol. 2001;72:815–823. doi: 10.1902/jop.2001.72.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howell TH, Fiorellini JP, Paquette DW, Offenbacher S, Giannobile WV, Lynch SE. A phase I/II clinical trial to evaluate a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J Periodontol. 1997;68:1186–1193. doi: 10.1902/jop.1997.68.12.1186. [DOI] [PubMed] [Google Scholar]
- 17.Huang KK, Shen C, Chiang CY, Hsieh YD, Fu E. Effects of bone morphogenetic protein-6 on periodontal wound healing in a fenestration defect of rats. J Periodontal Res. 2005;40:1–10. doi: 10.1111/j.1600-0765.2004.00752.x. [DOI] [PubMed] [Google Scholar]
- 18.Jin QM, Anusaksathien O, Webb SA, Rutherford RB, Giannobile WV. Gene therapy of bone morphogenetic protein for periodontal tissue engineering. J Periodontol. 2003;74:202–213. doi: 10.1902/jop.2003.74.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin QM, Zhao M, Webb SA, Berry JE, Somerman MJ, Giannobile WV. Cementum engineering with three-dimensional polymer scaffolds. J Biomed Mater Res A. 2003;67:54–60. doi: 10.1002/jbm.a.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King GN, Hughes FJ. Effects of occlusal loading on ankylosis, bone, and cementum formation during bone morphogenetic protein-2-stimulated periodontal regeneration in vivo. J Periodontol. 1999;70:1125–1135. doi: 10.1902/jop.1999.70.10.1125. [DOI] [PubMed] [Google Scholar]
- 21.King GN, Hughes FJ. Bone morphogenetic protein-2 stimulates cell recruitment and cementogenesis during early wound healing. J Clin Periodontol. 2001;28:465–475. doi: 10.1034/j.1600-051x.2001.028005465.x. [DOI] [PubMed] [Google Scholar]
- 22.King GN, King N, Cruchley AT, Wozney JM, Hughes FJ. Recombinant human bone morphogenetic protein-2 promotes wound healing in rat periodontal fenestration defects. J Dent Res. 1997;76:1460–1470. doi: 10.1177/00220345970760080801. [DOI] [PubMed] [Google Scholar]
- 23.Talwar R, Di Silvio L, Hughes FJ, King GN. Effects of carrier release kinetics on bone morphogenetic protein-2-induced periodontal regeneration in vivo. J Clin Periodontol. 2001;28:340–347. doi: 10.1034/j.1600-051x.2001.028004340.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhao M, Jin Q, Berry JE, Nociti FH, Jr, Giannobile WV, Somerman MJ. Cementoblast delivery for periodontal tissue engineering. J Periodontol. 2004;75:154–161. doi: 10.1902/jop.2004.75.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen FM, An Y, Zhang R, Zhang M. New insights into and novel applications of release technology for periodontal reconstructive therapies. J Control Release. 2011;149:92–110. doi: 10.1016/j.jconrel.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Lekic P, Sodek J, McCulloch CAG. Osteopontin and bone sialoprotein expression in regenerating rat periodontal ligament and alveolar bone. Anat Rec. 1996;244:50–58. doi: 10.1002/(SICI)1097-0185(199601)244:1<50::AID-AR5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 27.Lekic P, Sodek J, McCulloch CAG. Relationship of cellular proliferation to expression of osteopontin and bone sialoprotein in regenerating rat periodontium. Cell Tissue Res. 1996;285:491–500. doi: 10.1007/s004410050665. [DOI] [PubMed] [Google Scholar]
- 28.Rajshankar D, McCulloch CAG, Tenenbaum HC, Lekic PC. Osteogenic inhibition by rat periodontal ligament cells: Modulation of bone morphogenic protein-7 activity in vivo. Cell Tissue Res. 1998;294:475–483. doi: 10.1007/s004410051199. [DOI] [PubMed] [Google Scholar]
- 29.Mine K, Kanno Z, Muramoto T, Soma K. Occlusal forces promote periodontal healing of transplanted teeth and prevent dentoalveolar ankylosis: an experimental study in rats. Angle Orthod. 2005;75:637–644. doi: 10.1043/0003-3219(2005)75[637:OFPPHO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Rios HF, Giannobile WV. Preclinical protocols for periodontal regeneration (Chapter 7). In: Giannobile WV, Nevins M, editors. Osteology Guidelines for oral and maxillofacial regeneration: Preclinical models for translational research. Quintessence Publishing Co, Inc; Berlin: 2011. pp. 77–104. [Google Scholar]
- 31.Seol YJ, Pellegrini G, Franco LM, Chang PC, Park CH, Giannobile WV. Preclinical methods for the evaluation of periodontal regeneration in vivo. Methods Mol Biol. 2010;666:285–307. doi: 10.1007/978-1-60761-820-1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park CH, Rios HF, Jin Q, Sugai JV, Padial-Molina M, Taut AD, Flanagan CL, Hollister SJ, Giannobile WV. Tissue engineering bone-ligament complexes using fiber-guiding scaffolds. Biomaterials. 2012;33:137–145. doi: 10.1016/j.biomaterials.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park CH, Abramson ZR, Taba M, Jr, Jin Q, Chang J, Kreider JM, Goldstein SA, Giannobile WV. Three-dimensional micro-computed tomographic imaging of alveolar bone in experimental bone loss or repair. J Periodontol. 2007;78:273–281. doi: 10.1902/jop.2007.060252. [DOI] [PMC free article] [PubMed] [Google Scholar]