Abstract
In rodents, exercise increases hippocampal neurogenesis and allows for better learning and memory performance on water maze tasks. While exercise has also been shown to be beneficial for the brain and behavior in humans, no study has examined how exercise impacts spatial learning using a directly translational water maze task, or if these relationships exist during adolescence – a developmental period which the animal literature has shown to be especially vulnerable to exercise effects. In this study, we investigated the influence of aerobic fitness on hippocampal size and subsequent learning and memory, including visuospatial memory using a human analogue of the Morris Water Task, in 34 adolescents. Results showed that higher aerobic fitness predicted better learning on the virtual Morris Water Task and larger hippocampal volumes. No relationship between virtual Morris Water Task memory recall and aerobic fitness was detected. Aerobic fitness, however, did not relate to global brain volume, or verbal learning, which might suggest some specificity of the influence of aerobic fitness on the adolescent brain. This study provides a direct translational approach to the existing animal literature on exercise, as well as adds to the sparse research that exists on how aerobic exercise impacts the developing human brain and memory.
Keywords: exercise, adolescence, neuroimaging, spatial memory, hippocampus
1. Introduction
In addition to its well-known physical benefits, aerobic exercise can positively impact the brain and improve learning and memory [For review see 1, 2]. These beneficial effects of exercise on brain and behavior have been extensively studied in the animal literature and have largely focused on the impact of exercise on hippocampal structure and learning and memory performance [3-7]. More recently, human studies have been performed to examine how physical activity and aerobic fitness relate to cognition and brain volume. However, these studies have primarily focused on exercise’s effects on executive or higher-order cognitive functions (e.g. attention, inhibition, etc.), and have largely been focused on examining these relationships at either end of the age spectrum, including the elderly and young children [8-14]. Thus, the goals of the current experiment were to 1) implement a translational approach in examining the relationships between aerobic fitness and hippocampal structure and subsequent memory behavior, and 2) to do so in a group of adolescents, as adolescence is an additional, and important developmental time period in which the brain may be especially sensitive to environmental influences [15].
Aerobic exercise has been shown to affect the hippocampus, which plays a vital role in learning and memory formation [16-19]. Wheel running results in neurogenesis in the dentate gyrus [20], as well as increases in neuron density in hippocampal regions outside the dentate gyrus, such as in areas CA1 and CA3 [6]. Furthermore, these exercise-induced changes in the hippocampus are associated with improved performance on spatial memory tasks [5, 6]. In adolescent, adult, and aged rodents, wheel running has been shown to improve performance on a variety of spatial memory tasks including the Morris Water Maze, T-maze, and Y-maze [3, 4, 6, 23]. Furthermore, inhibition of neurogenesis results in blocking exercise-induced enhancements in spatial learning and memory performance [7], suggesting that the aforementioned exercise-induced changes in hippocampal neurogenesis may directly contribute to these reported learning and memory benefits.
Although the histology underlying volumetric changes seen in the human hippocampus is less clear, human studies are in agreement with the animal literature that aerobic exercise impacts hippocampal structure and learning and memory [11, 24-28]. However, the few studies showing associations between aerobic activity, hippocampal size, and memory performance have only focused on elderly or child populations. The influence of exercise on the brain and subsequent memory has not been examined in adolescents, and previously found associations between fitness, hippocampal volumes, and behavior may not be ubiquitous across the lifespan. For example, the aerobic effects seen in previous studies on aging adults may not be applicable to younger individuals, as normal age-related processes may interact with, and contribute to, the ultimate effect of aerobic exercise on brain and behavior that have been reported. This concept that aerobic exercise may have unique actions at different parts of the lifecycle, is also likely true for adolescence. During adolescence, the brain continues to develop both structurally [29] and functionally [30, 31], with a number of changes seen in hippocampal volume between childhood and adulthood [32, 33]. Given this dynamic properties of the teenage brain, this period may be especially sensitive to the effects of exercise. In support of this idea, the effect of exercise on changes in hippocampal structural has been shown to be age-dependent, with more robust hippocampal neurogenesis seen in adolescents and young rats compared to compared to older ones [34].
Lastly, although studies have examined the relationships shown between physical activity, hippocampal volume, and memory, no study, to date, has examined these relationships using a directly translational learning and memory task. Rather, the previous human studies have examined learning and memory tasks that cannot be used in animals, as they often require additional higher-order cognitive processes such as language or other executive functions [11, 25]. Interestingly, virtual spatial navigation tasks that are analogous to the infamous Morris Water Maze rodent task have been successfully created and utilized to assess hippocampal-dependent spatial learning and memory [35, 36]. Yet, to our knowledge, no study has used such translational approaches to examine the influence of exercise on the hippocampus and subsequent memory in humans. Given that human cross-sectional studies are correlational in nature, and therefore are limited by their inability to determine causal relationships, translational designs may help to better elucidate our understanding of how exercise affects the human brain and behavior.
In the current study, we examine the relationships between aerobic fitness level, hippocampal volume, and learning and memory in adolescents, age 15 to 18. Furthermore, in an attempt to increase the translational aspect of this experiment, we utilized a validated and previously published virtual Morris Water Task (vMWT)[35] as one of our measures to assess the influence of aerobic fitness on learning and memory in these youth. Based on previous human and animal research [6, 24, 26], we hypothesized that greater aerobic fitness would be related to larger bilateral hippocampal volumes, as well as better performance on learning and memory, including visuospatial memory on the vMWT. Furthermore, in order to well as with learning and memory performance all tasks of show some regional specificity of exercise’s effect on brain and subsequent behavior, we also examined the relationships between total gray and white matter volume and aerobic fitness, as. Given that exercise induces neurogenesis, specifically in the hippocampus [4, 6], we also hypothesized that the relationship between aerobic fitness and learning and memory would be mediated by changes in hippocampal volume.
2. Materials and Methods
2.1 Participants
Participants included 34 eligible male youth, ages 15 to 18 years. All participants were recruited through fliers, advertisements, and mailings distributed throughout the community and underwent comprehensive structured interviews as part of an ongoing study focused on adolescent neurodevelopment. Briefly, following written consent and assent from all youth and one of their biological parents, both youth and parent participated in separate structured telephone interviews to determine eligibility. Inclusionary criteria for youth included being of male sex and between the ages of 15 to 18 years old. Exclusionary criteria for youth included current diagnosed DSM-IV psychiatric disorder [Diagnostic Interview Schedule for Children Predictive Scales (DISC-PS-4.32b)[37, 38]]; significant substance use (>10 lifetime alcoholic drinks or 2 drinks/occasion, > 5 uses of marijuana, any other drug use, or > 4 cigarettes per day) [Brief Lifetime version of the Customary Drinking and Drug Use Record [39]]; reported history of psychotic disorders in biological parents [Family History Assessment Module (FHAM)[40]]; no major medical condition or significant head trauma [Structured Clinical Interview (SCI)[41]]; left-handedness [Edinburgh Handedness Inventory [42]], or irremovable metal. All youth and their parent were each compensated $10 for completing the comprehensive structured interviews, and youth were further compensated $100 for completing behavioral tests and MRI scanning.
The current study limited its recruitment to male adolescents for a number of reasons. Specifically, a number of intrinsic sex differences have been reported in hippocampal volumes [32, 33], aerobic capacity and physical fitness level [43, 44], and virtual maze task performance [45-47]. Furthermore, there is overwhelming evidence that aerobic exercise negatively impacts pubertal development in girls [48], which may alternatively impact brain development and behavior [49] (also see 2.4.3 Pubertal Status below). Due to these various gender-specific disparities between female and male adolescents,, we chose to begin to address the question of how exercise impacts hippocampal structure and function in one sex. By examining these relationships in males only, we aimed to reduce variability that may otherwise arise from inherent and aerobic-induced differences between the sexes.
2.1.2 Demographic Information
Information was gathered on age, ethnicity, grade point average (GPA), and socioeconomic status (SES) as part of the structured telephone interview to determine eligibility. SES was assessed by administering the Hollingshead Index of Social Position (ISP) to parents. The Hollingshead ISP determines socioeconomic status based on occupation and educational attainment of each parent [50].
2.2 Procedure
Youth completed three separate visits at Oregon Health & Science University within a 15 day time window, including 1) a learning and memory assessment session, 2) aerobic fitness and physical activity assessment, and 3) a MRI scanning session. Given that there are also acute effects of exercise that have been previously reported on learning and cognition [8, 51], aerobic fitness testing never proceeded the other two sessions on the same day.
2.2.1 Learning and Memory Assessment
2.2.1.1 Rey Auditory Verbal Learning Test (RAVLT)
The RAVLT [52, 53] is a widely accepted neuropsychological test used to evaluate verbal learning and memory. This test was chosen because recall on this task has been shown to correlate with hippocampal volume in healthy elderly individuals [54], and aerobic fitness in healthy adults [55]. Briefly, this test asks participants to learn a list (list A) of 15 unrelated words over 5 trials with an immediate recall after each trial. A second, novel 15 word list (list B) is then presented, again followed by a recall trial. Immediately after the second list, participants are asked to recall words from list A. In addition, participants are asked to recall the first word list after a 20 minute delay. The dependent measure for verbal learning was total number of words learned from list A over the 5 trials, normalized for age, whereas a retention score (computed by subtracting the immediate recall of trial 5 from the delay recall) was used as the primary dependent variable for verbal recall memory, as it takes into account the number of words initially encoded (baseline learning effects)[56].
2.2.1.2 Virtual Morris Water Task (vMWT)
Spatial learning and memory was assessed using a previously published, computerized vMWT protocol [35]. This task was designed to mirror the rodent spatial navigation test, known as the Morris Water Maze, in order to help compare assessment of spatial learning and memory between rodents and humans. The vMWT was chosen because bilateral lesions of the hippocampus impairs probe memory performance in rats and mice [57], and hippocampal size has been shown to correlate with vMWT memory performance in humans [58].
The vMWT was displayed on LCD computer monitor, and participants used a joystick (Logitech Attack 3) to move through the environment. The task consisted of 1 practice trial, 6 learning trials, a 30 minute delay probe trial, and a visual platform trial.
For the practice trial, participants were immersed into a vMWT environment that consisted of a pool of water within a larger room with ceiling, floor, walls, and four objects placed around the pool. Within the pool, there was a hidden platform that the participants were instructed to find as fast as possible. When the participant “swam” over the platform, it emerged from the water and hoisted them “out of the pool” for 10 seconds. During this 10 second period, the participant was rendered immobile except for the ability to rotate 360 degrees. This practice trial allowed participants to gain experience with the environment and sensitivity of the joystick, as well as how the vMWT platform works.
After the practice trial, the participant completed 6 learning trials. These included introducing the participant to a new vMWT environment that was similar to the practice trial, except there were six objects which were all different than the practice trial, and the hidden platform was now located in a new location. Participants were instructed to locate the hidden platform as quickly as possible during the 6 trials. They were informed that the platform would remain in the same location. Participants began each learning trial from one of six random start positions. As in the practice, they remained on the platform for 10s each time they found it and were only able to rotate 360 degrees during this time. During each trial, the computer recorded the x,y coordinate position approximately every .02 seconds and was used to calculate total distance traveled on each trial. The computer also recorded the total distance traveled in each quadrant of the pool, including the quadrant in which the platform was located, also known as the target quadrant. Percent distance traveled in the target quadrant was calculated for each subject across all 6 trials. The amount of learning that occurred across the 6 trials was determined for each participant by calculating delta (δ), or the change, between percent distance traveled in the quadrant between trial 1 and trial 6 (δ = trial 6 percent distance in target quadrant– trial 1 percent distance in target quadrant) and used as the dependent measures for spatial learning.
30 minutes following the last learning trial, a 60 second probe trial was completed. Unbeknownst to the participant, the platform was removed from the pool. All participants began the probe trial in the same start position. The percent distance traveled in the goal quadrant and the number of times the participant intercepted the platform, had it been present, were determined for each participant as the dependent variables of spatial delayed memory recall.
After the probe trial, participants were placed into the same pool used during learning, but the platform location was visible and marked by 4 red flags. Participants started from one of the six random start positions, and were instructed to navigate to the visible platform as fast as possible. Latency to travel to the visible platform was determined for each subject. This trial was used as a control trial to ensure visuomotor ability did not relate to aerobic fitness.
2.2.2 Aerobic Fitness and Physical Activity Assessment
Aerobic fitness and activity levels were examined in adolescents by collecting self-reports of aerobic exercise activity levels, as well as objectively measuring their aerobic capacity.
2.2.2.1 Youth Adolescent Activity Questionnaire
A modified version of the Youth Adolescent Activity Questionnaire (YAAQ) was administered during the structured interview with the youth to assess participation in aerobic activities over the past year. The YAAQ asks detailed questions about physical activity participation across all four seasons of the year, as well as the number of hours per week spent doing each activity [59]. This questionnaire was chosen because seasonal format questionnaires, such as the YAAQ, have been shown to increase the accuracy of self-report of physical activity in adolescents [60].
2.2.2.2 Aerobic Capacity Testing
Aerobic fitness was measured by determining the highest rate of oxygen that could be consumed during exercise (VO2 peak) for each participant using the same computerized indirect calorimetry system (VMax Series, V6200 Autobox, Sensormedics, VIASYS Healthcare) during a Bruce Protocol [61]. VO2 peak is a measure of the highest rate of an individual’s body to transport and utilize oxygen during incremental exercise, and is thought to be the most valid objective measurement of aerobic physical fitness [62]. Specifically, participants ran on a motor-driven treadmill that began at a speed of 1.7 mph and 10% grade, with increases in speed and grade every 3 minutes until volitional exhaustion. In addition, heart rate was measured throughout the fitness test, and ratings of perceived exertion were assessed every 2 minutes on a scale of 0 (very easy) to 10 (very hard). VO2 peak values were only considered valid if the participant had delivered maximal effort on the test as defined by at least one of the following the physiological criteria as outlined by Armstrong and van Mechelen [63]: 1) oxygen consumption remained at a steady state despite an increase in workload, as evidenced by a plateau in oxygen consumption, 2) heart rate reached ≥ 200 beats per minute, 3) the respiratory exchange ratio ≥ 1.0; and/or the subjective criteria of reporting a 10 on the perceived exertion scale.
Lean body mass (LBM) was determined just prior to aerobic testing by conducting a bioelectrical impedance test on each subject using the Body Composition Analyzer, Model 310e (Biodynamics Corp, Seattle, WA). Bioelectrical impedance analysis examines the body’s impedance or resistance to a generated low-level electrical current (<1 milliamp), accurately and reliably allowing for an estimate of lean and fat tissue content to determine percentage of body fat [For review see 64]. Peak oxygen consumption was then expressed in mL/kg LBM/min used as the independent measure of aerobic fitness. Scaling by lean body mass was used as it has been found to be a more accurate way of expressing fitness in relation to metabolism and reduces the possibility of body fat as a confounding variable in youth and aerobic [65].
2.2.3 MRI Scanning Session
Images were acquired on a 3.0 Tesla Siemens Magnetom Tim Trio system (Siemens Medical Solutions, Erlangen, Germany) with a twelve channel head coil at OHSU’s Advanced Imaging Research Center. Whole-brain, high-resolution structural anatomical images were acquired in the sagittal plane using a T1 weighted MPRAGE scanning sequence (TI = 900ms, Flip Angle = 10 degrees, TE = 3.58 ms, TR = 2300 ms, acquisition matrix = 256×240, resolution = 1mm × 1mm × 1.1mm).
2.3 Brain Volumes
2.3.1 Hippocampal Volumes
Bilateral hippocampi were manually defined (traced) on each individual’s high resolution anatomical image based on modification of a previously established method [66-69]. The tracer (MH) was blind to participant characteristics, and had established high intra and inter-rater reliability (all intraclass correlations ≥.95) on an independent sample using this previously published protocol prior to tracing. Specifically, bilateral hippocampi were traced on contiguous coronal slices, perpendicular to the anterior-posterior commissure plane using Analysis of Functional NeuroImages (AFNI) software, and were confirmed in axial and sagittal view. The anatomical boundaries used included: the anterior boundary: demarcated by the white matter of the alveus and the anterior recess of the temporal horn; the posterior boundary: demarcated by the columns of the fornix and the pulvinar of the thalamus, followed until the tail is no longer visible; the inferior boundary: white matter of the parahippocampal gyrus; the superior and lateral boundary: determined by temporal horn and alveus/fimbria; and the medial boundary: ambient cistern. Right and left hippocampal volumes were then calculated by multiplying the number of voxels by the associated voxel dimensions (1.1 mm3) and were expressed as a ratio to overall intracranial volume (ICV) to account for individual differences in brain size.
2.3.2 Gray, White, and Total Intracranial Volume
Gray matter and white matter volume, as well as ICV (the sum of gray, white, and cerebrospinal fluid) were calculated from each subject’s high-resolution T1 weighted anatomical using the Functional Magnetic Resonance Imaging of the Brain (FMRIB)’s automated segmentation tool (FAST) from the FMRIB’s Software Library, version 4.1 [70, 71]. Specifically, anatomical images were first skull-stripped using a combination of a hybrid watershed and deformable surface skull-stripping semi-automated program [72] and manual editing. Next, skull-stripped anatomical images were used as input to FAST, which uses a Markov random field model and an associated expectation-maximization algorithm to segment 3D brain images into gray matter, white matter, and cerebrospinal fluid, while also correcting for spatial intensity variations. This algorithm has been shown to be superior to other automated and semi-automated segmentation programs for segmenting tissue types [73]. Gray matter, white matter, and total ICV were then calculated by multiplying the number of voxels for that tissue type by the associated voxel dimensions (1.1 mm3). Finally, similar to the hippocampus, gray and white matter volumes were analyzed as a ratio to overall ICV to control for individual variability in brain size.
2.4 Addressing Potential Theoretical Confounds
A number of potential theoretical confounds were assessed and used as covariates during analyses if they met both theoretical and statistical criteria for being considered a potential confound. Theoretical explanations for these confounds can be found below. These potential confounds were then included in statistical testing if they showed a significant (p < .05) or trend level correlation (p <.09) with both the independent (aerobic fitness) and dependent variables of interest.
2.4.1 Age
Developmental changes are well documented in brain and behavior across adolescence
[For review see 74], including changes in hippocampal volume [33]. Thus, age was examined as a potential covariate for analyses for examining relationships between variables that were not already normalized for age.
2.4.2 General Intelligence and Grade Point Average
Positive relationships have been reported between aerobic fitness levels and general intelligence (IQ), as well as academic achievement, in children and adults [For meta-analysis review see 28, 75]. In addition, relationships have been reported between memory performance and IQ in teenagers and adults [76] Participants were administered the 2-subtest version of the Wechsler Abbreviated Scale of Intelligence [77] to provide an estimate of overall intellectual functioning, and grade point average (GPA) was collected from youth during the initial phone interview.
2.4.3 Pubertal Status
Although more commonly reported in girls, intensive physical exercise has been reported to delay pubertal maturation in some athletes [78]. Puberty and its related sex hormones have also been shown to relate to brain structure in adolescents [79, 80], including hippocampal size, in a sex dependent manner [81]. For these reasons, pubertal development was measured in male subjects and assessed as a potential confound. Specifically, pubertal maturation was assessed for each subject using the self-rating Pubertal Development Scale (PDS) [82]. The PDS consists of a total of 5 distinct questions for boys in regards to height growth, body hair, skin changes, vocal changes, and facial hair. For each of the 5 questions, participants were asked to rate their development on a 4 point scale (1 = has not begun yet, 2 = barely begun, 3 = definitely begun, 4 = seems complete). An average was then calculated from these responses for a pubertal development score ranging from 1 (prepubertal) to 4 (postpubertal). This questionnaire was chosen as a relatively noninvasive assessment of pubertal development, and because self-reports on this scale have been shown to correlate significantly with other measures of pubertal status, including physician ratings [82].
2.4.4 Video Games Questionnaire
Physical activity has been shown to be negatively associated with time spent playing video games in adolescents [83], and relationships have been seen between brain volumes and video game abilities [84]. Furthermore, the nature of the spatial navigation task is similar to modern video games played by youth, as it requires manual navigation around a 3-dimensional virtual world. Thus, video game experience was also assessed as a potential confound. A brief video game questionnaire was utilized to determine average amount of time spent playing video games per week for each subject. Subjects reported the video game console type used (e.g. Wii, PlayStation, PC computer), the average number of hours playing video games on this console type per day (i.e. sessions), and the number of video game sessions per console type per week. Number of hours and number of sessions were summed across video game console types and multiplied together to assess the average number of hours of video games played per week.
2.5 Analyses
Exploratory data analyses were performed to determine normality, and when necessary, appropriate transformations were used to correct for violations of this parametric assumption. When transformations were not successful in obtaining normality, non-parametric statistics were employed. Outliers were determined as values exceeding 3 standard deviations (SD) from the mean. Spearman’s correlation was used to examine the relationship between activity self-reports and fitness (VO2 peak) test results. Pearson’s correlations were used to assess the relationships between the 3 theoretical confounds and the independent and dependent variables of interest. To ensure learning occurred across the 6 vMWT learning trials in this sample, a repeated-measures ANOVA was used to examine changes in percent distance in the target quadrant for all subjects. Simple regression analyses were performed to examine if aerobic fitness (as measured by VO2 peak) predicted verbal and spatial learning and memory. Simple regression analyses were also performed to examine if aerobic fitness predicted left and right hippocampal volumes. When one of the above variables was determined to be a potential confound variable of interest based on the criteria outlined, follow-up multiple regression analyses were performed for identified significant relationships to determine if the results held after controlling for the potentially confounding variable. Given the number of regression analyses performed, Bonferroni corrections were applied to reduce reporting Type I errors and these results are mentioned when applicable.
3. Results
3.1 Participant Characteristics
Participant characteristics and aerobic fitness results can be found in Table 1. All subjects had IQ values well-above the normal range and came from upper middle class families. Physical activity self-reports and aerobic fitness (VO2 peak) test results were significantly related [Spearman’s rho(34) = .57, p =.001]. Given this high colinearity between self-report and aerobic fitness, and that VO2 peak results is the current gold standard in assessing aerobic fitness [62], VO2 peak was selected as the independent variable used to examine the relationships between aerobic exercise and brain structure and behavior.
Table 1.
| Participants | |
| Age | 16.4 (.82) |
| % Caucasian | 82.4 |
| GPA | 3.4 (.4) |
| SESa | 22.4 (10.8) |
| IQb | 117.6 (10.75) |
| Aerobic Exercise (hrs/week)c | 6.2 (5.8); range: 0-18 |
| VO2 peak (mL/kg LBM/min) | 72 (10.5) |
| RAVLT d | |
| Total words learned (z-score) | 1.7 (.8) |
| Memory Retention Score | −.4 (1.5) |
| vMWT e | |
| Learning (δ) | .35 (.2) |
| Delay Memory (Probe) | |
| % Distance in target quadrant | 67 (21) |
| Probe Crossings | 3.7 (2.3) |
| Swim Time (seconds) | 4.8 (1.8) |
| Brain Volumes(mm3) | |
| Left Hippocampus | 3942.5 (445.9) |
| Right Hippocampus | 4126.5 (485.9) |
| Gray Matter | 707579.9 (43137.3) |
| White Matter | 550300.4 (39717.9) |
Hollingshead Index of Social Position
Wechsler Abbreviated Scale of Intelligence
Youth Adolescent Activity Questionnaire
Rey Auditory Verbal Learning Test
virtual Morris Water Task
3.2 Assessment of Potential Confounds
Statistical results for the potential theoretical confounds can be found in supplemental material (Supplemental Table 1). Only one of the proposed theoretical confounds met the aforementioned criterion to be included as a statistical covariate during hypothesis testing. That is, a significant negative relationship was seen between pubertal status and VO2 peak fitness results [r(34) = −.37, p = .03], with higher aerobic fitness values related to lower self-ratings of pubertal development. In addition, negative relationships were seen between pubertal status and hippocampal volumes [left: r(34) = −.44, p = .01; right: r(34) = −.34, p = .05]. No relationship was seen between puberty and ICV alone [r(34) = .22, p = .21]. Thus, pubertal status was used as a covariate in follow-up analyses examining aerobic fitness and hippocampal and gray matter brain volumes.
3.3 Aerobic Fitness and Learning and Memory
3.3.1 RAVLT
Data for 1 subject were outlying on both learning and memory trials, and excluded from subsequent RAVLT analyses. Behavioral results for the group can be found in Table 1. Aerobic fitness did not significantly relate to either learning [total words learned: R2 = .01, F(1,31) = .17, p =.68] or recall memory [retention memory score: R2 = .03, F(1,31) = 1.09, p =.31] on the RAVLT.
3.3.2 vMWT
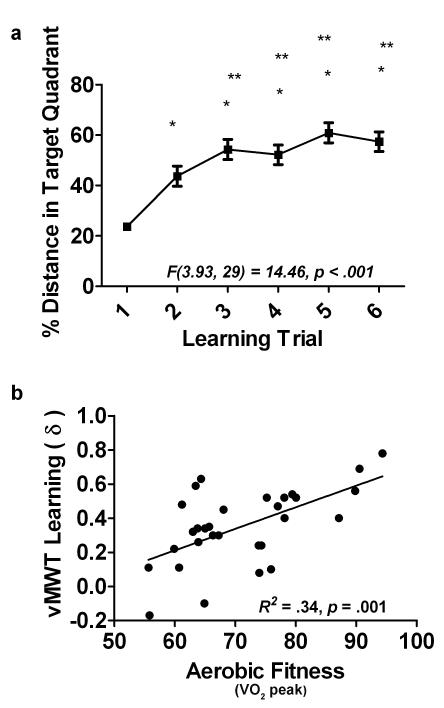
Data were not collected on all 6 learning trials for 3 participants due to technical errors, and 1 subject was an outlier on all trials, leaving N = 30 for all subsequent analyses for the vMWT. Behavioral results for the group can be found in Table 1 and Figure 1a. Results showed that percent distance traveled in the target quadrant was significantly different between the 6 vMWT trials [F(3.93, 29) = 14.46, p < .001](Figure 1a). Post-hoc tests revealed a significantly larger percent of distance traveled in the target quadrant in trials 2 thru 6 when compared to trial 1 [p’s < .001], as well as between trial 2 and trials 3, 4, 5, and 6 [p’s < .05]. A trend was also seen between trial 3 and trial 5 (p =.08). Simple regression analyses showed aerobic fitness to be a significant predictor of the amount of learning that occurred (δ) during the vMWT task [R2 = .34, F(1,29) = 14.64, p =.001](Figure 1b). However, aerobic fitness did not predict delayed memory recall performance on this task, as aerobic fitness did not relate to either number of platform crossings [R2 = .05, F(1,29) = 1.36, p =.25] or percent distance traveled in the target quadrant during the probe trial [R2 = .03, F(1,29) = .91, p =.35]. Importantly, no relationship was seen between aerobic fitness and latency to travel to the platform in the visible platform control trial [R2 = .001, F(1,29) = .04, p =.84, suggesting no relationship between aerobic fitness and visual or motor processes, as assessed by this task.
Figure 1.
Virtual Morris Water Task Learning a) Percent distance traveled in the target quadrant across the 6 trials. * denotes significantly different from trial 1 (p’s < .05); ** denotes significantly different from trial 2 (p’s < .05). F-statistic of the repeated measures ANOVA and its associated p-value. b) Aerobic fitness (as measured by VO2 peak) predicts the amount of learning that occurred (δ =percent distance in target quadrant in trial 6 –percent distance in target quadrant in trial 1) during the vMWT task. R2 and associated p-value is also reported for this relationship. Higher δ reflects better learning.
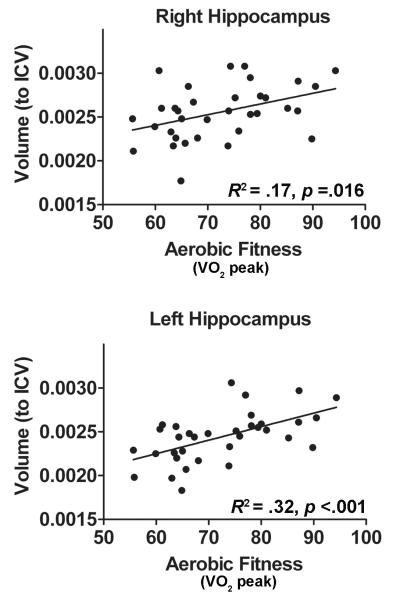
3.4 Aerobic Fitness and Hippocampal Volumes
Bilateral hippocampal, gray, and white matter volumes are reported in Table 1. Aerobic fitness showed a significant positive relationship with both the left [R2 = .32, F(1,32) = 15.3, p <.001] and the right hippocampus [R2 = .17, F(1,32) = 6.5, p =.016] (Figure 2). No significant relationships were seen between aerobic fitness and total gray [R2 = .03, F(1,32) = 1.0, p =.32], white matter volume [R2 = .01, F(1,32) = .19, p =.66], or ICV [R2 = .05, F(1,32) = .09, p =.76] . Follow-up analyses were run to determine if aerobic fitness remained a significant predictor of hippocampal volume after covarying for pubertal status (Supplemental Table 2). While aerobic fitness showed positive relationships with the volume of the right and left hippocampus, negative relationships were seen between pubertal status and hippocampal volume. With both in the model, aerobic fitness remained a significant predictor of hippocampal volume for only the left hippocampus (Supplemental Table 2).
Figure 2.
Aerobic fitness (as measured by VO2 peak) predicts right and left hippocampal volumes. Volumes (in mm3) reported controlling for Intracranial Volume (ICV). R2 and associated p-values are also reported for each relationship.
3.5 Brain Volumes and Learning and Memory
3.5.1 RAVLT
Significant positive relationships were seen between learning on the RAVLT and bilateral hippocampal volumes [left: R2 = .12, F(1,31) = 4.14, p =.05; right: R2 = .15, F(1,31) = 5.26, p =.029]. While no relationship was seen with total gray matter volume [R2 = .08, F(1,31) = 2.78, p =.11], larger total white matter volume was related to greater learning performance on the RAVLT [R2 = .25, F(1,31) = 10.2, p =.003]. However, only the relationship between white matter volume and learning passed Bonferroni correction (p < .013). No significant relationships were seen between verbal recall and brain volumes [left hippocampus: R2 = .00, F(1,31) = .002, p =.96; right hippocampus: R2 = .006, F(1,31) = .18, p =.67; gray matter: R2 = .02, F(1,31) = .50, p =.49; white matter: R2 = .02, F(1,31) = .63, p =.44].
3.5.2 vMWT
Significant positive relationships were also seen between the amount of learning (δ) on the vMWT and bilateral hippocampal volumes [left: R2 = .26, F(1,29) = 10.06, β = .514, p =.004; right: R2 = .18, F(1,29) = 6.07, β = .422, p =.02]. On the contrary, total gray matter [R2 = .08, F(1,29) = 2.49, p =.13] or white matter [R2 = .004, F(1,29) = .10, p =.75] volume did not significantly predict spatial learning on the vMWT. After employing Bonferroni correction (p < .013), only the relationship between left hippocampal volume and spatial learning (δ) remained significant. Brain volumes did not, however, predict delayed memory performance on the vMWT probe trial [platform crossings: left hippocampus: R2 = .00, F(1,29) = .006, p =.96; right hippocampus: R2 = .00, F(1,29) = .01, p =.92; gray matter: R2 = .01, F(1,29) = .27, p =.61; white matter: R2 = .001, F(1,29) = .04, p =.85; percent distance in target quadrant: left hippocampus: R2 = .016, F(1,29) = .45, p =.51; right hippocampus: R2 = .007, F(1,29) = .20, p =.66; gray matter: R2 = .00, F(1,29) = .001, p =.97; white matter: R2 = .05, F(1,29) = 1.49, p =.23].
Given that the above results showed that aerobic exercise predicts learning on the vMWT, as well as hippocampal volume, and hippocampal volume predicts vMWT learning (δ), follow-up multiple regression analyses were performed to determine if hippocampal volume predicts vMWT learning (δ), while controlling for aerobic fitness. Both relationships between the bilateral hippocampus and vMWT learning (δ) were no longer significant [left: β = .277, p =.13; right: β = .237, p =.16], suggesting that the relationship seen between the hippocampus and spatial learning was driven by their individual associations with aerobic fitness. Although it was hypothesized that hippocampal volume would mediate the relationship between aerobic fitness and spatial learning, the absence of a relationship between the hippocampus and learning when controlling for aerobic fitness violates the necessary criteria for a potential mediating variable using the classic causal steps approach, as outlined by Baron and Kenny [85]. Thus, hippocampal volume was not a mediator of the relationship between aerobic fitness and vMWT learning (δ) in the current sample.
4 Discussion
This is the first study of its kind to show that associations between exercise and learning on the Morris Water Maze translate to humans on a virtual Morris Water Task. In addition, this is the first study to examine the influence of aerobic exercise on brain structure and function in an adolescent sample, a population which has been previously overlooked in the exercise literature. The results show that aerobic fitness relates to hippocampal volume and spatial learning on the vMWT. Similar results were not found for verbal memory, or for total gray or white matter volume, perhaps suggesting some specificity in the influence of aerobic exercise on the adolescent brain and behavior.
As hypothesized, aerobic fitness predicted larger hippocampal volumes in adolescents. This finding is in agreement with the animal literature showing exercise-induced neurogenesis in the hippocampus. Specifically, aerobic exercise has been shown to promote cell proliferation and survival in the dentate gyrus of the hippocampus [3]. Although MRI technology does not have the ability to detect such changes at the cellular level, increases in neurogensis may contribute to larger hippocampal volumes, which can subsequently be detected by structural MRI. Beyond neurogenesis, exercise may also lead to larger hippocampal volumes by increasing dendritic spines and arborization of pyramidal cells outside the dentate gyrus, such as neurons in CA1 [86]. Regardless of the underlying neural mechanism, these findings extend previous work showing that physical activity levels and aerobic capacity relate to larger hippocampal volumes in elderly adults [26, 27] and young children [24]. Taken together with the current study, these findings strongly suggest that aerobic exercise influences hippocampal size at all stages of life.
In addition to hippocampal volume, aerobic exercise was associated with better visuospatial learning in adolescents. The creation of virtual spatial navigation tasks that mirror commonly utilized tests to assess learning and memory in animals [87, 88], such as the vMWT [35, 36], are an invaluable tool in allowing for truly translational research to be performed. By examining how aerobic exercise relates to performance on a vMWT, we were able to replicate previous work, showing that exercise training enhances learning behavior in young rodents [6, 23], in human adolescents. Together, these findings suggest that exercise impacts spatial learning across both human and rodent species. However, in terms of memory performance, aerobic exercise did not relate to either spatial delayed recall memory on the vMWT, or verbal learning or delayed memory recall on the RAVLT in the current study. These findings are contrary to previous research. For example, one animal study found exercise to increase spatial memory retention using the Morris Water Maze; although this relationship was shown in aging rodents [3]. In addition, a number of associations have been reported between physical activity levels and improved performance on a wide variety of other learning and memory tasks in children [11, 24] and adults [25-27], including learning novel vocabulary words [25], item and relational memory for visual stimuli [11, 24], and a visual short-term delayed-match to sample task [26, 27].
These divergent results in the human literature may stem from the fundamental differences between learning versus memory processes, as well as the specific tasks used in each study. Memory can be defined as a behavioral change following an experience, whereas learning is the process that allows for acquiring new information. Learning and memory can also be broken down further into a number of interdependent, but unique, processes including encoding, retention (consolidation and storage), and retrieval; all of which have distinct neural and molecular mechanisms [89]. For example, hippocampal NMDA receptors are important for encoding of associative place memory, whereas delayed memory retrieval depends on AMPA receptors [90]. At a systems level, memory encoding requires the hippocampus, whereas as retrieval is subserved by a number of brain regions, including the hippocampus, but also the parietal and prefrontal cortices [91]. Therefore, the above discrepancies in the literature, and the current findings that aerobic exercise relates to spatial learning but not delayed memory recall on the vWMT, may result from aerobic exercise having different effects on the neural and/or molecular pathways underlying each component of the learning and memory process. Beyond the differences in learning versus memory mechanisms, the differences seen between our study and previous research may be due to the tasks used to assess learning and/or memory. For example, unlike in the current study, previously published findings employed memory tasks that do not specifically assess episodic memory, but rather short-term memory [26, 27] or in some cases involved memory tasks that also tap executive functions [11]. Moreover, it is also possible that aerobic fitness was not found to relate to memory performance in the current study, as both tasks utilized had a relatively short retrieval delay (≤ 30 minutes), and longer retrieval delays may be more sensitive to exercise effects. Lastly, there are differences between the current study and other work in study design, particularly regarding the length of time between physical exercise testing or intervention and cognitive assessment. In the current study, we ensured that memory performance was not directly influenced by any carryover effects of acute exercise from aerobic fitness testing by not allowing aerobic exercise testing to proceed the MRI scan or the memory assessment sessions, whereas in the previous study showing the beneficial effect of exercise on verbal learning and memory, novel words were learned within 15 minutes of the exercise intervention [25]. Other studies did not report the time between aerobic activity and cognitive assessment [11, 24, 26, 27]. However, the idea of “time-dependent benefits” of exercise is likely an important factor, as aerobic-induced benefits in learning for animals tend to peak following a delay, whereas the benefits of physical activity on memory performance are best seen immediately following exercise [92]. Thus, more research manipulating time between physical activity and cognitive assessment is warranted. Future research should also focus on which types of memory, as well as what subcomponents (encoding, retention, retrieval), are specifically influenced by exercise, and if these change across development.
Contrary to our hypothesis, hippocampal volume did not mediate the relationship between aerobic fitness and learning on the vMWT. This was surprising, as the hippocampus has been found to mediate relationships seen between cardiovascular aerobic fitness and learning and memory in studies of children (ages 9-10, N = 41) [24] and elderly participants (N = 109) [27]. Notably, the effect sizes of the hippocampus as a mediation variable were small to medium (Cohen’s f2 = .24 – .34). For the sample size used for mediation analyses in the current study (n = 30), an effect size of at least .40 would have been necessary to detect a significant hippocampal mediation effect, suggesting that the current study may have been somewhat underpowered for detection of mediation. There are also a number of additional mechanism(s) that occur outside the hippocampus that may help to explain the beneficial impact of aerobic exercise on spatial learning. For example, changes in neurotransmitter levels, including those important for learning and memory such as dopamine and acetylcholine, are up-regulated following exercise and may mediate exercise’s induced actions on learning [For review see 93]. Furthermore, neurotrophic factors, such as brain derived neurotrophic factor (BDNF), are also thought to be important for the beneficial cognitive effects of exercise, and increases in BDNF mRNA are not limited to the hippocampus, but have been shown in the cortex following physical activity in rodents [94]. Therefore, exercise induced chances in neurotransmitter or neurotrophic factors may be important mechanisms for the relationships seen between aerobic fitness and vMWT learning in adolescents.
Beyond aerobic fitness, we found that verbal learning in adolescents was positively associated with total white matter volume. These findings are similar to previous work showing white matter volume to relate to other cognitive functions in youth [95]. Using structural MRI, increases in white matter volume have been repeatedly shown across adolescence [29, 96]. These increases in white matter volume are thought to reflect increases in myelination, as postmortem-studies have found that neurons in the frontal cortices [97] and parahippocampus [98] continue to myelinate during adolescence and into adulthood. Given that myelination increases the rate of neuronal signaling conduction, these findings might suggest that improved verbal learning is achieved through more efficient and faster neural processing. Further research is needed to determine if more specific relationships may exist between the white matter volume relationship with verbal learning detected here, including if it is driven by specific increases in myelination in brain regions that subserve memory processes such as the hippocampus and frontal lobes [19].
5 Conclusion
This study employed a translational approach to examine how exercise relates to hippocampal structure and learning and memory in adolescents. These results show that aerobic fitness relates to larger hippocampal volumes and better learning on a virtual Morris Water Task in adolescence. Taken together with previous studies on young children and aged individuals, these findings suggest that aerobic exercise may benefit the brain and learning at all stages across the lifespan. Future studies should address what additional types of memory may benefit from aerobic fitness during adolescence, and if similar relationships are seen in adolescent girls. Lastly, this study was correlational in nature, thus longitudinal exercise intervention studies are essential to confirm that aerobic exercise results in hippocampal structural changes and enhanced spatial learning in adolescents.
Supplementary Material
Highlights.
Exercise was related to translational virtual Morris Water Task (vWMT) behavior
Aerobic fitness relates to learning on the vWMT in adolescents
Fitness also relates to hippocampal, but not total gray matter, volume in youth
These findings suggest some specificity of exercise’s effect on the adolescent brain
Acknowledgments
This research was supported by the National Institute of Alcohol Abuse and Addiction Grant (F31AA019866 – Herting; R01 AA017664 – Nagel), the Dana Foundation (Nagel), the National Institute of Neurological Disorders and Stroke (K08 NS052147 – Nagel), the Oregon Clinical and Translational Research Institute, the OHSU Tartar Trust Research Fellowship (Herting), American Psychological Association Science Directorate’s Dissertation Research Award (Herting), and ARCS Foundation, Inc. Portland Chapter (Herting). A special thanks to Madison Stroup, Khadiya Chinnarath, Jill Waldman, Stephanie Sasse, Jenny Peraza, and Kristen Seghete for their assistance in data collection and data entry. Thank you to Dr. Jacob Raber, Dr. Joel Nigg, and Dr. Diane Elliot for their time and guidance with data analyses, and Dr. Elliot for her help with aerobic fitness testing. Lastly, we would also like to thank Dr. Scott Moffat for sharing his virtual spatial navigation task with us.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nature reviews. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- [2].van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009;32:283–90. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- [5].Van der Borght K, Havekes R, Bos T, Eggen BJ, Van der Zee EA. Exercise improves memory acquisition and retrieval in the Y-maze task: relationship with hippocampal neurogenesis. Behav Neurosci. 2007;121:324–34. doi: 10.1037/0735-7044.121.2.324. [DOI] [PubMed] [Google Scholar]
- [6].Uysal N, Tugyan K, Kayatekin BM, Acikgoz O, Bagriyanik HA, Gonenc S, et al. The effects of regular aerobic exercise in adolescent period on hippocampal neuron density, apoptosis and spatial memory. Neurosci Lett. 2005;383:241–5. doi: 10.1016/j.neulet.2005.04.054. [DOI] [PubMed] [Google Scholar]
- [7].Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–58. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- [8].Hillman CH, Pontifex MB, Raine LB, Castelli DM, Hall EE, Kramer AF. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience. 2009;159:1044–54. doi: 10.1016/j.neuroscience.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hillman CH, Castelli DM, Buck SM. Aerobic fitness and neurocognitive function in healthy preadolescent children. Medicine and science in sports and exercise. 2005;37:1967–74. doi: 10.1249/01.mss.0000176680.79702.ce. [DOI] [PubMed] [Google Scholar]
- [10].Hillman CH, Buck SM, Themanson JR, Pontifex MB, Castelli DM. Aerobic fitness and cognitive development: Event-related brain potential and task performance indices of executive control in preadolescent children. Dev Psychol. 2009;45:114–29. doi: 10.1037/a0014437. [DOI] [PubMed] [Google Scholar]
- [11].Chaddock L, Hillman CH, Buck SM, Cohen NJ. Aerobic fitness and executive control of relational memory in preadolescent children. Medicine and science in sports and exercise. 2011;43:344–9. doi: 10.1249/MSS.0b013e3181e9af48. [DOI] [PubMed] [Google Scholar]
- [12].Buck SM, Hillman CH, Castelli DM. The relation of aerobic fitness to stroop task performance in preadolescent children. Medicine and science in sports and exercise. 2008;40:166–72. doi: 10.1249/mss.0b013e318159b035. [DOI] [PubMed] [Google Scholar]
- [13].Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–9. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- [14].Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–30. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- [15].Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neuroscience and Biobehavioral Reviews. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- [16].Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clinical neurosurgery. 1972;19:421–46. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- [17].Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- [18].Squire LR. The neuropsychology of human memory. Annual review of neuroscience. 1982;5:241–73. doi: 10.1146/annurev.ne.05.030182.001325. [DOI] [PubMed] [Google Scholar]
- [19].Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiology of learning and memory. 2004;82:171–7. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- [20].van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular medicine. 2008;10:128–40. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- [21].Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–9. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- [22].O’Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behavioural brain research. 2007;176:362–6. doi: 10.1016/j.bbr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- [23].Lou SJ, Liu JY, Yang RY, Chen PJ. Treadmill running enhances the ability of learning in young rats. Sheng Li Xue Bao. 2006;58:365–9. [PubMed] [Google Scholar]
- [24].Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, Vanpatter M, et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010;1358:172–83. doi: 10.1016/j.brainres.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, et al. High impact running improves learning. Neurobiology of learning and memory. 2007;87:597–609. doi: 10.1016/j.nlm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- [26].Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–22. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–9. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Etnier JL, Nowell PM, Landers DM, Sibley BA. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev. 2006;52:119–30. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- [29].Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, et al. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cerebral Cortex. 1996;6:551–60. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- [30].Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, et al. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- [31].Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, et al. Functional brain networks develop from a “local to distributed” organization. PLoS computational biology. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gogtay N, Nugent TF, 3rd, Herman DH, Ordonez A, Greenstein D, Hayashi KM, et al. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–72. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- [33].Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. Journal of Comparitive Neurology. 1996;366:223–30. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- [34].Kim YP, Kim H, Shin MS, Chang HK, Jang MH, Shin MC, et al. Age-dependence of the effect of treadmill exercise on cell proliferation in the dentate gyrus of rats. Neurosci Lett. 2004;355:152–4. doi: 10.1016/j.neulet.2003.11.005. [DOI] [PubMed] [Google Scholar]
- [35].Nowak NT, Moffat SD. The relationship between second to fourth digit ratio, spatial cognition, and virtual navigation. Archives of sexual behavior. 2010;40:575–85. doi: 10.1007/s10508-010-9668-2. [DOI] [PubMed] [Google Scholar]
- [36].Burkitt J, Widman D, Saucier DM. Evidence for the influence of testosterone in the performance of spatial navigation in a virtual water maze in women but not in men. Horm Behav. 2007;51:649–54. doi: 10.1016/j.yhbeh.2007.03.007. [DOI] [PubMed] [Google Scholar]
- [37].Hoven CW, Duarte CS, Lucas CP, Wu P, Mandell DJ, Goodwin RD, et al. Psychopathology among New York city public school children 6 months after September 11. Arch Gen Psychiatry. 2005;62:545–52. doi: 10.1001/archpsyc.62.5.545. [DOI] [PubMed] [Google Scholar]
- [38].Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, et al. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry. 2001;40:443–9. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- [39].Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59:427–38. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- [40].Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism Clin Exp Res. 1995;19:1018–23. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- [41].Brown SA, Myers MG, Mott MA, Vik PW. Correlates of success following treatment for adolescent substance abuse. Applied & Preventive Psychology. 1994;3:61–73. [Google Scholar]
- [42].Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- [43].Riddoch CJ, Bo Andersen L, Wedderkopp N, Harro M, Klasson-Heggebo L, Sardinha LB, et al. Physical activity levels and patterns of 9- and 15-yr-old European children. Medicine and science in sports and exercise. 2004;36:86–92. doi: 10.1249/01.MSS.0000106174.43932.92. [DOI] [PubMed] [Google Scholar]
- [44].Krahenbuhl GS, James SS, Kohrt WM. Developmental Aspects of Maximal Aerobic Power in Children. Exercise and Sport Sciences Reviews. 1985;13:503–38. [PubMed] [Google Scholar]
- [45].Newhouse P, Newhouse C, Astur RS. Sex differences in visual-spatial learning using a virtual water maze in pre-pubertal children. Behavioural brain research. 2007;183:1–7. doi: 10.1016/j.bbr.2007.05.011. [DOI] [PubMed] [Google Scholar]
- [46].Lovden M, Herlitz A, Schellenbach M, Grossman-Hutter B, Kruger A, Lindenberger U. Quantitative and qualitative sex differences in spatial navigation. Scandinavian journal of psychology. 2007;48:353–8. doi: 10.1111/j.1467-9450.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- [47].Astur RS, Tropp J, Sava S, Constable RT, Markus EJ. Sex differences and correlations in a virtual Morris water task, a virtual radial arm maze, and mental rotation. Behavioural brain research. 2004;151:103–15. doi: 10.1016/j.bbr.2003.08.024. [DOI] [PubMed] [Google Scholar]
- [48].Sherman RT, Thompson RA. The female athlete triad. J Sch Nurs. 2004;20:197–202. doi: 10.1177/10598405040200040301. [DOI] [PubMed] [Google Scholar]
- [49].Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–74. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- [50].Hollingshead AB. Four factor index of social status. Yale University; New Haven, CT: 1975. [Google Scholar]
- [51].Coles K, Tomporowski PD. Effects of acute exercise on executive processing, short-term and long-term memory. Journal of sports sciences. 2008;26:333–44. doi: 10.1080/02640410701591417. [DOI] [PubMed] [Google Scholar]
- [52].Rey A. L’examen clinique en psychologie. Presses Universitaires de France; Paris: 1964. [Google Scholar]
- [53].Rey A. L’examen psychologique dans les cas d’encephalopathie traumatique.(Les problems.) Archives de Psychologie. 1941;18:215–85. [Google Scholar]
- [54].Reiman EM, Uecker A, Caselli RJ, Lewis S, Bandy D, de Leon MJ, et al. Hippocampal volumes in cognitively normal persons at genetic risk for Alzheimer’s disease. Ann Neurol. 1998;44:288–91. doi: 10.1002/ana.410440226. [DOI] [PubMed] [Google Scholar]
- [55].Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–43. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. Journal of the International Neuropsychological Society. 2001;7:312–22. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- [57].Eichenbaum H, Stewart C, Morris RG. Hippocampal representation in place learning. J Neurosci. 1990;10:3531–42. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, et al. The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex. 2003;13:1344–51. doi: 10.1093/cercor/bhg081. [DOI] [PubMed] [Google Scholar]
- [59].Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. International journal of epidemiology. 1994;23:991–9. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- [60].Rifas-Shiman SL, Gillman MW, Field AE, Frazier AL, Berkey CS, Tomeo CA, et al. Comparing physical activity questionnaires for youth: seasonal vs annual format. American journal of preventive medicine. 2001;20:282–5. doi: 10.1016/s0749-3797(01)00296-3. [DOI] [PubMed] [Google Scholar]
- [61].Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. American heart journal. 1973;85:546–62. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- [62].Armstrong N, Welsman JR. Aerobic fitness: what are we measuring? Medicine and sport science. 2007;50:5–25. doi: 10.1159/000101073. [DOI] [PubMed] [Google Scholar]
- [63].Armstrong N, van Mechelen W. Paediatric Exercise Science and Medicine. Second Edition ed Oxford University Press Inc.; New York: 2008. [Google Scholar]
- [64].Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gomez JM, et al. Bioelectrical impedance analysis--part I: review of principles and methods. Clinical nutrition (Edinburgh, Scotland) 2004;23:1226–43. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- [65].Dencker M, Bugge A, Hermansen B, Froberg K, Andersen LB. Aerobic fitness in prepubertal children according to level of body fat. Acta Paediatr. 2010;99:1854–60. doi: 10.1111/j.1651-2227.2010.01952.x. [DOI] [PubMed] [Google Scholar]
- [66].Nagel BJ, Palmer SL, Reddick WE, Glass JO, Helton KJ, Wu S, et al. Abnormal Hippocampal Development in Children with Medulloblastoma Treated with Risk-Adapted Irradiation. American Journal of Neuroradiology. 2004;25:1575–82. [PMC free article] [PubMed] [Google Scholar]
- [67].Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicology and teratology. 2007;29:141–52. doi: 10.1016/j.ntt.2006.10.010. Epub 2006 Dec 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Research: Neuroimaging. 2005;139:181–90. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hanson KL, Medina KL, Nagel BJ, Spadoni AD, Gorlick A, Tapert SF. Hippocampal volumes in adolescents with and without a family history of alcoholism. The American journal of drug and alcohol abuse. 2010;36:161–7. doi: 10.3109/00952991003736397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- [71].Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- [72].Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–75. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- [73].Clark KA, Woods RP, Rottenberg DA, Toga AW, Mazziotta JC. Impact of acquisition protocols and processing streams on tissue segmentation of T1 weighted MR images. Neuroimage. 2006;29:185–202. doi: 10.1016/j.neuroimage.2005.07.035. [DOI] [PubMed] [Google Scholar]
- [74].Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–57. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- [75].Sibley BA, Etnier JL. The relationship between physical activity and cognition in children: a meta-analysis. Pediatric Exercise Science. 2003;15:243–56. [Google Scholar]
- [76].Wechsler D. Wechsler Memory Scale. Fourth Edition Psychological Corp.; San Antonio, TX: 2009. [Google Scholar]
- [77].Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corp.; San Antonio, TX: 1999. [Google Scholar]
- [78].Georgopoulos NA, Roupas ND, Theodoropoulou A, Tsekouras A, Vagenakis AG, Markou KB. The influence of intensive physical training on growth and pubertal development in athletes. Annals of the New York Academy of Sciences. 2010;1205:39–44. doi: 10.1111/j.1749-6632.2010.05677.x. [DOI] [PubMed] [Google Scholar]
- [79].Peper JS, Hulshoff Pol HE, Crone EA, van Honk J. Sex steroids and brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience. 2011;191:28–37. doi: 10.1016/j.neuroscience.2011.02.014. [DOI] [PubMed] [Google Scholar]
- [80].Herting MM, Maxwell EC, Irvine C, Nagel BJ. The Impact of Sex, Puberty, and Hormones on White Matter Microstructure in Adolescents. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, et al. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex. 2011;21:636–46. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–33. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- [83].Janz KF, Mahoney LT. Maturation, Gender, and Video Game Playing Are Related to Physical Activity Intensity in Adolescents. The Muscatine Study Pediatric Exercise Science. 1997;9:353–63. [Google Scholar]
- [84].Erickson KI, Boot WR, Basak C, Neider MB, Prakash RS, Voss MW, et al. Striatal volume predicts level of video game skill acquisition. Cereb Cortex. 2010;20:2522–30. doi: 10.1093/cercor/bhp293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of personality and social psychology. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- [86].Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–22. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Levy LJ, Astur RS, Frick KM. Men and women differ in object memory but not performance of a virtual radial maze. Behav Neurosci. 2005;119:853–62. doi: 10.1037/0735-7044.119.4.853. [DOI] [PubMed] [Google Scholar]
- [88].Baker TE, Holroyd CB. Which way do I go? Neural activation in response to feedback and spatial processing in a virtual T-maze. Cereb Cortex. 2009;19:1708–22. doi: 10.1093/cercor/bhn223. [DOI] [PubMed] [Google Scholar]
- [89].Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11:180–7. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- [90].Bast T, da Silva BM, Morris RG. Distinct contributions of hippocampal NMDA and AMPA receptors to encoding and retrieval of one-trial place memory. J Neurosci. 2005;25:5845–56. doi: 10.1523/JNEUROSCI.0698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Rugg MD, Otten LJ, Henson RN. The neural basis of episodic memory: evidence from functional neuroimaging. Philosophical transactions of the Royal Society of London. 2002;357:1097–110. doi: 10.1098/rstb.2002.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Berchtold NC, Castello N, Cotman CW. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167:588–97. doi: 10.1016/j.neuroscience.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Meeusen R, Piacentini MF, De Meirleir K. Brain microdialysis in exercise research. Sports medicine (Auckland, NZ. 2001;31:965–83. doi: 10.2165/00007256-200131140-00002. [DOI] [PubMed] [Google Scholar]
- [94].Llorens-Martin M, Torres-Aleman I, Trejo JL. Growth factors as mediators of exercise actions on the brain. Neuromolecular medicine. 2008;10:99–107. doi: 10.1007/s12017-008-8026-1. [DOI] [PubMed] [Google Scholar]
- [95].Silveri MM, Tzilos GK, Yurgelun-Todd DA. Relationship between white matter volume and cognitive performance during adolescence: effects of age, sex and risk for drug use. Addiction. 2008;103:1509–20. doi: 10.1111/j.1360-0443.2008.02272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, et al. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Progress in Neuropsychopharmacology and Biological Psychiatry. 1999;23:571–88. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- [97].Yakovlev PI, Lecours A. The myelogenetic cycles of regional maturation in the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. Blackwell Scientific Publications; Paris: 1967. pp. 3–70. [Google Scholar]
- [98].Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Archives of General Psychiatry. 1994;51:477–84. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.