Abstract
Background
Patients presenting with medically unexplained physical symptoms (MUPS) typically present with significant distress and marked impairment in functioning and pose a unique challenge to health care providers. The purpose of this study was to examine the efficacy of a psychophysiological treatment (PT) for MUPS.
Methods
Thirty-eight participants meeting criteria for subthreshold somatization disorder (abridged somatization) were randomly assigned to one of two conditions: (1) standard medical care augmented by a psychiatric consultation intervention (wait-list) or (2) a 10-session, manualized, individually-administered PT added to the psychiatric consultation intervention. Assessments were conducted at baseline, at midpoint (after four sessions), and after completing the last session. The primary outcome measure was the severity scale of the Clinical Global Impression Scale anchored for Somatic Symptoms (CGI-SD). Secondary outcome measures were responder status as determined by clinical ratings, self-report measures of mental and physical functioning.
Results
At the end of the trial, the severity (and frequency) of physical symptoms improved significantly more (p < 0.05) in the intervention group. The average improvement in the CGI-SD was 0.80 points greater in the intervention group than in the wait-list group. PT was also associated with greater improvements in self-reported functioning and depressive symptomatology. The effect sizes at the final assessment point indicate that this intervention had a robust effect on complex somatic symptom presentations.
Conclusion
For patients with high levels of MUPS (abridged somatization), PT produces significant improvements in symptoms and functional status.
Mups are comprised of physical symptoms, which are without an organic disease explanation and devoid of demonstrable structural lesion or established biochemical change.1 Such symptoms frequently include headache, fatigue, difficulty concentrating, shortness of breath, sleep problems, nausea, atypical chest pain, and musculoskeletal symptoms.2,3 Somatic symptoms appear on a continuum of severity with DSM-IV Somatization Disorder representing the most severe syndromes.4 – 6 Escobar and colleagues7 developed the construct of abridged somatization criteria (four or more symptoms for males; six or more for females) to capture for classification/research purposes individuals who did not meet full somatization criteria, yet continued to experience significant levels of distress and functional impairment. Despite having lower somatic symptom thresholds, this patient appear to have similar characteristics to patients with full somatization disorder.6,8 –10 The abridged somatization construct has been used to designate a MUPS “case” relevant to clinical and community outcomes.11
Somatic symptoms are strongly and consistently associated with psychiatric disorders. A large proportion of these patients meet criteria for major psychiatric disorders12 such as Major Depressive Disorder13–18 and Anxiety Disorders.4,7,19 –22
Treatments of MUPS Symptoms
Treatments for MUPS have largely been based on targeting the psychiatric dimensions. Effective interventions include use of antidepressant drugs and nonpharmacologic approaches such as cognitive behavior therapy (CBT)23,24 and interpersonal psychotherapy (IPT).25 A number of psychoeducational interventions leading to proper use of antidepressant medications have shown effectiveness in managing depressed primary care patients, and this benefit may also extend to the somatic symptoms.26,27
However, nonpharmacologic therapies have not been as thoroughly investigated in the primary care population, where many depressed or anxious patients present primarily with MUPS. The psychiatric consultation intervention (PCI) developed by Smith’s group28 has been shown to be an efficacious intervention for somatization disorder (higher spectrum of MUPS) in the primary care setting. The consultation intervention consists of a letter sent to primary care physicians recommending that they examine patients with somatoform disorder during regularly scheduled appointments while limiting additional diagnostic procedures and treatments. Because empirical support for any other intervention is lacking, this PCI has been considered the best evidence-based treatment for somatization spectrum disorders,1 and was employed as a comparison treatment in the present study.
Treatments Targeting Physical Dimensions of MUPS
Gevirtz and colleagues29 reported feasibility/acceptability of a biofeedback procedure incorporating a combination of psychophysiologic treatments with primary care patients complaining of somatization symptoms, and demonstrated a decrease in medical care costs compared with a no-treatment control group, who received standard medical care. In clinical experience, “medicalized” treatments, such as psychophysiologic interventions are usually better accepted than psychotherapeutic interventions in patients with MUPS, who, by definition, tend to think that the problem is in the body, not the mind.30 –32
Psychophysiologic therapy comprises a set of “self-regulation” techniques that are specifically targeted at particular symptoms or body systems.33 Biofeedback involves taking one or more physiologic measures that are thought to have a very specific relationship to the patient’s problem, and teaching the patient to control these measures voluntarily, by watching them change in real-time on a computer screen, and thereby developing a personal strategy for controlling them. Indeed, psychophysiologic therapies specifically target physiologic processes that are thought to be related to the patient’s complaint. Thus, many “psychosomatic” patients may feel that they and their physical complaints are not taken seriously when they are referred to mental health care, whereas they feel that their problems are being directly targeted by biofeedback. However, because physiologic changes often are related to emotional state, and part of biofeedback therapy involves learning to detect situations where symptoms are manifested and to control physiologic reactions in these circumstances, biofeedback can become a “Trojan horse” technique for helping such patients confront and control some of the emotional issues that may produce the physical sensations that underlie the unexplained symptoms.32
Effect of Psychophysiologic Treatments on Psychological Symptoms
Research has shown that heart rate (HR) variability biofeedback has important effects on autonomic symptoms, and the cardio-respiratory system that may be relevant for managing depression/anxiety syndromes.34 –38 Other preliminary data suggest large improvements in depression39,40 and improved quality of life in fibromyalgia on indices of pain, depression, and sleep.38 Controlled data by Humphreys and Gevirtz 41 showed that the method is helpful in managing pediatric abdominal pain and depression and anxiety among patients with post-traumatic stress disorder (PTSD).42,43
Psychophysiologic Therapies
Various psychophysiologic treatments may be applied directly to the autonomic nervous system. These include progressive muscle relaxation (PMR)44 and surface electromyographic (sEMG) biofeedback, which influence sympathetic arousal through dramatic decreases in muscle tension, and HR variability biofeedback (HRV BF), which primarily influences the parasympathetic system and produces major increases in gain in the HR baroreflex closed loop. HRV BF is designed specifically to target autonomic reactivity. It triggers the baroreflexes, and also increases respiratory gas exchange efficiency, by putting respiratory sinus arrhythmia (RSA) in phase with respiration. Previous studies from Lehrer’s laboratory36 has found that slow breathing at a specific “resonance frequency” for each individual produces increases in resting gain of the baro-reflex, which is one of the body’s important reflexes for modulating autonomic function and autonomic reactivity to stress.34,35
sEMG produces greater effects on particular muscular groups than does PMR,45,46 and sometimes produces greater clinical effects on somatic problems, depending on the types of training given.47– 49 Thermal biofeedback has greater finger temperature effects than does autogenic training.50 For the treatment of patients with somatization symptoms, Ryan and Gevirtz29 have reported positive results combining training to increase HRV amplitude, increase finger temperature and skin conductance, and decrease sEMG activity.
In previous studies from our center, CBT has been found23,24 to produce significantly greater clinical improvement than usual primary medical care and the psychiatric consultation letter. It was hypothesized that a higher rate of improvement and compliance would occur with a psychophysiologic therapy (PT) for several reasons: (1) it is more observably “medical” and “physiologic-based” treatment than CBT and, hence, should be more appealing to MUPS patients, most of whom are highly resistant to thinking of their problems as having a psychological basis and, thus, could produce a greater rate of treatment acceptance and adherence; and (2) it provides direct control of some of the symptoms of MUPS and, thus, may provide a sense of control and actual symptom relief, targeted at the underlying somatic problems that contribute to (but do not adequately explain) the existence of MUPS.
METHODS
Study Design
We conducted a randomized, controlled treatment trial in which patients diagnosed with MUPS received one of two treatments: either (1) the wait-list, which consisted of the PCI alone and (2) our 10-session PT added to the PCI (PT+PCI). In both treatment conditions, standard medical care was augmented by sending to patients’ primary care physicians the psychiatric consultation letter employed by Smith’s group28,51 with minor modifications to make it applicable to our research environment. In applying PCI to both treatment groups, the present study established a relatively conservative test of the total PT intervention package’s efficacy, in that significant findings would be obtained only if PT produced improvements over and above those resulting from PCI. Our study design was based on the Allen et al. study24 comparing a 10-week CBT intervention to PCI for somatization disorder.
Randomization and Masking
Participants were randomly assigned to PCI or PT+PCI using a computer-generated random number sequence. Neither blocking nor stratification was used. Study personnel conducting assessments were masked to participants’ treatment condition and, hereafter, are referred to as independent evaluators.
Study Population and Settings
Men and women with MUPS were recruited from primary medical clinics and through advertisements in the community. Interested potential participants gave oral informed consent to complete a telephone screening interview. Face-to-face diagnostic interviews were scheduled for patients who reported, during the telephone interview, current medical stability (no organic medical conditions reported at screening), and six or more unexplained physical symptoms during previous medical treatment for women, four or more for men.
Inclusion criteria were age 18 –70 years for individuals seeking medical care for a physical symptom (i.e., persistent fatigue, pain complaints, and gastrointestinal, cardiovascular or musculoskeletal symptoms). One would be included if no major medical illness explained symptom(s) after detailed physical and laboratory assessment. People with common disorders (such as hypertension, asthma, diabetes, low back strain) could be entered if, in the opinion of their personal treating physician, the presenting physical symptoms were not due to the underlying disorder. In order to enter the study, individuals must have met criteria for at least four medically unexplained symptoms out of the 42 somatic symptoms listed in the Composite International Diagnostic Interview (CIDI)52 rated as currently present if males and at least six medically unexplained symptoms if females (Escobar’s abridged criteria).
Exclusion criteria included individuals with a history of alcohol/drug abuse (within the last 12 months), as well as those with bipolar illness or psychotic disorders (schizophrenia). We also excluded people with any unstable major medical condition who were using medication(s) that had not been stabilized for at least 2 months prior to baseline, who were pregnant, and people with active suicidal ideation.
The study took place in the Department of Psychiatry of Robert Wood Johnson Medical School (RWJMS) between June 2006 and August 2008. The study was approved by RWJMS’s Institutional Review Board. Written informed consent was obtained from all participants.
Treatment Conditions and Therapists
The PCI was applied to all participants in each group. A standardized consultation letter was sent to the principal treating physician of every study participant (Table 1). This letter stated the patient met DSM-IV criteria for SD and made recommendations for the patient’s ongoing medical treatment. Receipt of the letter was confirmed by 54 (95.7%) of the 57 physicians to whom it was sent.
TABLE 1.
Psychiatric Consultation Letter’s Recommendations to Treating Physicians
|
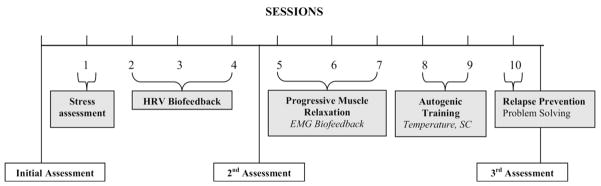
The PT is a 10-session, manualized intervention expressly designed for patients with MUPS (see Fig. 1). The treatment, developed by the authors, details guidelines for the conduct of each session (copies can be obtained by writing to the university address noted). It is described to participants as an intervention designed to assist in coping with physical discomfort and stress. While participants were introduced to the entire treatment package, specific treatment components were focused on depending on the participant’s specific symptom profile. Session 1 consisted of a psychoeducational component along with a stress assessment. Feedback was provided on how they responded to various stressors, including a mild stressor of a mental arithmetic task. A participant’s specific symptom profile was explored and they were educated on their primary mode of reactivity. For example, cardiovascular responders were more likely to experience acute and elevated reactions in their HR and peripheral skin temperature while musculoskeletal responders showed immediate changes in muscle tension. Sessions 2– 4 focused on HR variability training, which was the cornerstone component of this treatment. Participants were instructed to practice the slowed breathing at their resonance frequency throughout the protocol. Session 5–7 introduced PMR where participants were trained to progressively notice the subtlest of muscle tensing in their bodies and to “switch it off.” Feedback was provided via surface electromyography (sEMG). sEMG was used primarily to support PMR, but was used also used for focal tension problems, along with PMR in an integrated approach. Sessions 8 and 9 introduced a form of self-hypnosis called autogenic training (six standard exercises as described by Schultz and Luthe, 1969).53 When using this method, participants were provided with feedback showing distribution of blood flow to the periphery via skin temperature and skin conductance measurements. Session 10 focused on relapse prevention, where the provider and patient would collaboratively determine which treatment components the participant would continue to employ, and motivation strategies for continued practice of techniques.
FIGURE 1.
Summary of the Study Schedule.
Four therapists, either Master- or Doctoral-level psychologists, three of them being certified as biofeedback clinicians with at least 3 years of supervised training in PT, conducted the treatment. All therapists received training in PT for MUPS prior to treating a study participant. Throughout the study, therapists received weekly supervision from one of the authors during which the assessment and treatment sessions were reviewed.
Assessment
Study participants were assessed at three timepoints: on initial screening, during a mid-point session after the fourth session, as well as immediately after the treatment phase of the study. Each evaluation was conducted by a different evaluator.
All independent evaluators (licensed clinical psychologist, doctoral level psychologist, nurse, two graduate psychology students) were trained to administer the study’s structured clinical interview instruments: The Somatization Disorders (MUPS) section of the CIDI54 and the Clinical Global Impression (CGI)-SD. During the screening interview, the CIDI and the PRIME-MD55 were administered to determine diagnostic status and eligibility for inclusion in the study.
Outcome Variables
The primary outcome and one secondary outcome variable (physical functioning) were chosen in order to make this study comparable to other recent studies that tested a behavioral treatment (e.g., CBT) in this same population.23,56 Other outcomes (Hamilton Depression Scale [HAM-D]; Hamilton Anxiety Scale [HAM-A]) were chosen because the MUPS syndrome typically involves a somatic-depression-anxiety triad (Lowe et al., 2008).57 The Nijmegan Questionnaire58 was also used because it has been widely used to asses hyperventilation in a variety of clinical and research settings.58 – 60
The study’s primary outcome measure was an independent evaluator’s judgment of the severity of MUPS, measured by the CGI-SD. This is a clinician rating of overall illness severity and degree of improvement61 that was adapted to measure both the participant’s overall illness severity and degree of improvement with respect to MUPS. At the baseline session a rater (blind to condition) conducted a semistructured interview in order to rate on a 7-point scale the severity of the participant’s MUPS (‘not at all ill’ to ‘among the most extremely ill patients’). At the termination appointments, the same blinded rater re-evaluated, with the same 7-point scale, the participant’s severity of illness. The intra-class correlation for this scale is acceptable (= 0.70) and shows reasonable concurrent validity with self-assessed physical symptoms (r = 0.43, p < 0.0001) as well as with a symptom count of MUPS from the CIDI (r = 0.66, p < 0.0001). Discriminant validity is also in evidence since the partial correlations of the CGI with patient PHQ13 and the symptom count, controlling for depression (HAM-D), are 0.35 (p < 0.002) and .60 (p < 0.0001), respectively.
Secondary outcome measures included patients’ ratings of their physical functioning and severity of their somatic symptoms. Physical functioning was assessed by the physical functioning subscale of the Medical Outcomes Study Short Form General Health Survey (MOS SF-36),62 which measures impairment in physical activities, such as in climbing stairs, kneeling, and walking various distances. Depression and anxiety levels were assessed with the HAM-D59 and HAM-A. These are rating scales completed by a clinician, from a structured interview. The HAM-D and HAM-A were completed in tandem with PRIME-MD (interview modules) at baseline and at endpoint in order to assess depressive/anxiety symptoms. Kappas for HAM-D and HAM-A are 0.81 and 0.68, respectively. The Nijmegan Questionnaire58 was also used because it has been widely used to asses hyperventilation in a variety of clinical and research settings.58,63,64
Statistical Analysis
An intent-to-treat approach, based on data from all randomized participants, was used in all analyses. The PT+PCI (treatment group), PCI (wait-list group), and PCI patients receiving treatment after the wait condition (crossover group) were compared at baseline, mid-point, and final assessments on the primary and secondary outcome variables. Differences between groups on baseline characteristics and on mediator variables were tested using unpaired t-tests (for continuous variables) or χ2 tests (for categoric variables). Continuous data were analyzed using a mixed-model repeated measures analysis of variance. A fixed effects model with repeated measurement for each patient was applied to estimate the group-specific mean changes from baseline to midpoint and final assessment, and then conducted t-tests for group pair comparisons (e.g., groups 1, 2, and 3). The analyses incorporate the variable “age” as a covariate in the regression model. When the interaction was significant (p ≤ 005), planned contrasts determined whether changes in outcome measures observed in the PT+PCI group (between baseline and each of the assessments) were different from those observed in the PCI group.
All tests of statistical significance were two-tailed and interpreted with a criterion of p < 0.05. All statistical analyses were performed using SAS, ver. 8 (SAS Institute Inc, Cary, NC).
RESULTS
Participant Flow
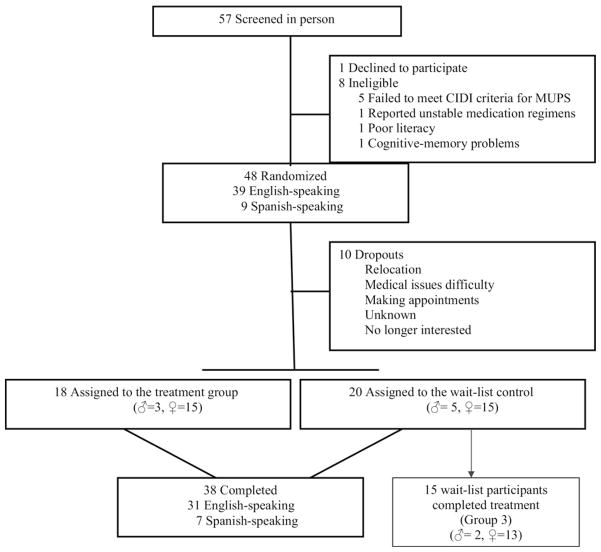
Figure 2 details study attrition. A total of 57 individuals complaining of MUPS completed a telephone screening interview. All individuals had reported at least six “possibly” unexplained physical symptoms during the phone interview and agreed to participate in a face-to-face screening interview. Forty-eight individuals were enrolled in the study. Eighteen were randomized to the PT+PCI condition; 20 were randomized to the PCI condition (wait-list). Of the 18 patients randomized to PT+PCI, the majority attended most or all of the 10 treatment sessions: 18 (75%) attended all 10 sessions, 2 (8.0%) attended 8 or 9 sessions, 2 (8.3%) attended 4 sessions, and 2 (8.3%) attended only 1 session. Fifteen of the patients in the PCI condition agreed to partake in the treatment after the wait period was completed. They received an extra two assessments while in group 3. The final assessment for their PCI condition constituted their baseline assessment for group 3 participation.
FIGURE 2.
Flow of Participants through the Study.
Treatment Groups at Baseline
The only baseline characteristic that showed between-group differences was age (p = 0.0064) (Table 2). Age was binomially categorized as those ≥40 or ≤40 years of age since 40 is the cutoff age where significant and abrupt changes occur in one’s HR variability. The majority of participants were >40 years of age (78.9%). The sample was predominately female (78.9%) and Caucasian (57.9%). Sixty percent of the control sample was >40 years of age, while 83% of the treatment group were <40 years old. Therefore, all analyses have included “age” as a covariate, thereby controlling for the effects of age in the model. Roughly 30% of the total sample consisted of Hispanic participants with an equal distribution in both study groups. Fifty-five percent of participants met HAM-D-17 criteria for severe depression, 40% for mild-moderate depression, and 85% participants met HAM-A criteria for clinically significant anxiety. Tabulation of comorbid diagnoses and psychotropic medications is presented in Table 3A, B.
TABLE 2.
Baseline Characteristics of the Participants*
| Treatment Group n = 18 |
Control n = 20 |
p | |
|---|---|---|---|
| Age, no. (%) | |||
| <40 years old | 15 (83.3) | 8 (40.0) | 0.0064* |
| ≥40 years old | 3 (16.7) | 12 (60.0) | |
| Female, no. (%) | 15 (83.3) | 15 (75.0) | 0.5292 |
| Male, no. (%) | 3 (16.7) | 515 (25.0) | |
| Race/ethnicity, no. (%) | |||
| White | 11 (61.1) | 11 (55.0) | 0.7032 |
| African American | 0 | 0 | – |
| Asian | 2 (11.1) | 3 (15.0) | 0.7233 |
| Hispanic | 5 (27.8) | 6 (30.0) | 0.8801 |
| Other | 0 | 0 | – |
| Married, no. (%) | 8 (44.4) | 9 (45) | 0.9726 |
| Employment status no. (%) | |||
| Full-time employment | 6 (40.0) | 6 (37.5) | 0.8864 |
| Part-time employment | 1 (6.7) | 2 (12.5) | 0.5830 |
| Unemployed | 8 (53.3) | 8 (50.0) | 0.8528 |
| Receiving disability, no. (%) | 1 (5.6) | 2 (10.0) | 0.6119 |
HAM-D.
Data are presented as no. (%) unless otherwise indicated.
TABLE 3.
| (A) Tabulation for Co-Morbid Disorders
| |
|---|---|
| Type of Co-Morbid Disorders | Percentage |
| Multisomatoform | 50.00% |
| Anxiety NOS | 36.84% |
| Somatoform | 34.21% |
| Generalized anxiety | 31.58% |
| Major depression | 28.95% |
| Dysthymia | 15.79% |
| Hypochondriasis | 10.53% |
| Panic disorder | 10.53% |
| Partial depression | 10.53% |
| Major anxiety | 10.53% |
| Minor depression | 2.63% |
| Rule-out anxiety | 2.63% |
| Binge eating | 2.63% |
| (B) Tabulation for Psychotropic Medications
| |
|---|---|
| Psychotropic medications name | Percentage |
| Paroxetine | 7.89% |
| Alprazolam | 7.89% |
| Duloxetine | 5.26% |
| Escitalopram | 2.63% |
| Trazodone | 2.63% |
| Lorazepam | 2.63% |
| Fluvoxamine | 2.63% |
| Diazepam | 2.63% |
Treatment Outcome
After approximately 10 weeks of psychophysiologic treatment, significantly greater improvement was found in the treatment than control group on the primary outcome measure of general somatic symptomatology the CGI, a clinician rating of overall illness severity and degree of improvement61 that we have adapted to measuring both the patient’s overall illness severity and degree of improvement with respect to MUPS. At baseline, participants in both groups met a CGI rating of 4 = moderate somatization: exceeds abridged criteria, frequent complaints or some functional impairment but, in the treatment group, that decreased to 2 = borderline signs of somatization: few somatic complaints, below threshold for the abridged criteria (Table 4A, B, C for means and standard errors).
TABLE 4.
| (A) Summary Statistics of Baseline Scores by Group
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment Group (Group 1)
|
Control Group (Group 2)
|
Crossover Group (Group 3)
|
|||||||
| n | Mean | SE | n | Mean | SE | n | Mean | SE | |
| CGI-SD | 18 | 4.00 | 0.84 | 20 | 4.00 | 0.79 | 14 | 3.71 | 0.83 |
| HAM-D | 18 | 17.28 | 4.90 | 20 | 17.70 | 5.00 | 11 | 14.18 | 4.53 |
| BDI-II Total | 18 | 20.67 | 11.33 | 20 | 17.10 | 8.75 | 13 | 15.31 | 10.96 |
| BDI-II Cognitive | 18 | 5.17 | 4.27 | 20 | 4.45 | 3.69 | 14 | 3.79 | 3.09 |
| BDI-II Neurovegetative | 18 | 8.22 | 4.08 | 20 | 6.90 | 3.68 | 13 | 6.08 | 4.79 |
| Hamilton Anxiety | 18 | 22.72 | 7.23 | 20 | 23.30 | 9.19 | 14 | 15.86 | 7.04 |
| MOS SF-36 | 18 | 40.19 | 17.36 | 16 | 47.43 | 15.90 | 11 | 50.34 | 18.21 |
| MOS SF-36 (Transformed) | 18 | 35.36 | 6.95 | 16 | 38.04 | 5.94 | 11 | 39.00 | 6.89 |
| MOS SF-36-Physical | 18 | 34.43 | 7.65 | 16 | 36.51 | 7.18 | 12 | 37.86 | 8.58 |
| MOS SF-36-Mental | 18 | 36.29 | 8.27 | 18 | 39.01 | 6.46 | 12 | 40.82 | 7.11 |
| (B) Summary Statistics of Midpoint Assessment by Group
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment Group (Group 1)
|
Control Group (Group 2)
|
Crossover Group (Group 3)
|
|||||||
| n | Mean | SE | n | Mean | SE | n | Mean | SE | |
| CGI-SD | 18 | 3.56 | 0.86 | 18 | 3.83 | 1.04 | 15 | 3.40 | 0.83 |
| HAM-D | 17 | 12.41 | 7.21 | 16 | 13.50 | 6.21 | 15 | 11.40 | 6.90 |
| BDI-II Total | 16 | 15.13 | 12.08 | 17 | 16.88 | 10.17 | 14 | 12.50 | 12.02 |
| BDI-II Cognitive | 18 | 3.83 | 4.08 | 18 | 4.56 | 3.17 | 15 | 3.07 | 3.49 |
| BDI-II Neurovegetative | 16 | 5.94 | 3.36 | 17 | 5.24 | 3.87 | 14 | 5.00 | 3.98 |
| Hamilton anxiety | 17 | 15.24 | 9.81 | 18 | 16.78 | 8.28 | 15 | 14.87 | 7.64 |
| MOS SF-36 | 17 | 54.84 | 21.55 | 17 | 43.00 | 20.85 | 12 | 59.80 | 19.88 |
| MOS SF-36 (Transformed) | 17 | 41.32 | 8.48 | 17 | 36.47 | 8.26 | 12 | 42.97 | 7.93 |
| MOS SF-36-Physical | 18 | 41.19 | 7.32 | 17 | 35.59 | 8.15 | 13 | 40.01 | 9.71 |
| MOS SF-36-Mental | 18 | 40.66 | 10.07 | 18 | 37.65 | 9.35 | 13 | 43.25 | 9.45 |
| (C) Summary Statistics of Last Assessment by Group
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment Group (Group 1)
|
Control Group (Group 2)
|
Crossover Group (Group 3)
|
|||||||
| n | Mean | SE | n | Mean | SE | n | Mean | SE | |
| CGI-SD | 18 | 2.78 | 0.94 | 19 | 3.63 | 0.83 | 14 | 3.21 | 1.25 |
| HAM-D | 17 | 8.82 | 6.01 | 15 | 14.53 | 4.27 | 13 | 8.69 | 6.33 |
| BDI-II Total | 15 | 11.13 | 12.69 | 18 | 14.89 | 9.29 | 13 | 8.69 | 9.44 |
| BDI-II Cognitive | 18 | 2.83 | 3.50 | 19 | 4.05 | 2.91 | 14 | 1.36 | 2.02 |
| BDI-II Neurovegetative | 15 | 3.87 | 3.76 | 18 | 5.61 | 4.10 | 13 | 4.00 | 3.96 |
| Hamilton Anxiety | 17 | 12.06 | 7.30 | 19 | 16.00 | 6.29 | 13 | 12.00 | 7.35 |
| MOS SF-36 | 16 | 63.46 | 19.14 | 15 | 48.39 | 16.71 | 12 | 59.48 | 25.55 |
| MOS SF-36 (Transformed) | 16 | 44.47 | 7.92 | 15 | 38.37 | 6.28 | 12 | 42.84 | 10.43 |
| MOS SF-36-Physical | 17 | 43.78 | 7.39 | 17 | 38.37 | 7.56 | 13 | 40.41 | 7.11 |
| MOS SF-36-Mental | 17 | 44.64 | 9.54 | 17 | 39.61 | 6.82 | 13 | 44.41 | 9.74 |
CGI-SD = Clinical Global Impression Scale anchored for Somatic Symptoms; HAM-D = Hamilton Depression Scale; BDI-II = Beck Depression Inventory-II; MOS SF-36 = Medical Outcomes Study Short Form General Health Survey.
Statistical analyses were based on the changes from the initial assessment to the mid-point and third assessment with the variable “age” as a covariate in the model. The mixed-model repeated measures analysis of variance (ANOVA) on the primary outcome measure, CGI-SD severity, resulted in statistically significant effects for the treatment-by-time interaction (p = 0.04, Table 5). Planned contrasts revealed significantly greater reductions in CGI-SD severity scores for PT+PCI participants than for PCI participants between baseline and each of the other assessments. All parametric secondary endpoints were analyzed with same mixed-model repeated measures ANOVA described above, all of which resulted in significant treatment-by-time interactions. Significant improvements were noted for the BDI-II, SF-36, and the HAM-A (p ≤ 0.02, Table 5 and Table 4A, B, C for mean and standard error of the mean).
TABLE 5.
Changes From Baseline to Mid-Point and Final Assessment Points on Outcome Measures
| Assessment Point | Estimated Difference | Stardard Error of the Mean | Degrees of Freedom | t Value | Prob t | |
|---|---|---|---|---|---|---|
| CGI-SD Severity (score range, 1–7)† | ||||||
| Group 1 vs. group 2 | Final | −0.77 | 0.36 | 35 | −2.14 | 0.04 |
| BDI-II Total score(score range, 1–25) | ||||||
| Group 1 vs. group 2 | Final | −5.70 | 2.56 | 34 | −2.23 | 0.03 |
| BDI-II Neurovegetative subscale | ||||||
| Group 1 vs. group 2 | Final | −2.21 | 1.06 | 34 | −2.08 | 0.045 |
| HAM-A | ||||||
| Group 3 vs. group 2 | Midpoint | 6.10 | 2.33 | 10 | 2.62 | 0.03 |
| MOS SF-36 Total (score range, 0 –100)‡ | ||||||
| Group 1 vs. group 2 | Midpoint | 17.07 | 4.57 | 31 | 3.74 | 0.0008 |
| Group 1 vs. group 2 | Final | 21.57 | 6.02 | 31 | 3.58 | 0.0011 |
| MOS SF-36 Physical Functioning | ||||||
| Group 1 vs. group 2 | Midpoint | 5.94 | 1.82 | 33 | 3.26 | 0.0026 |
| Group 1 vs. group 2 | Final | 6.83 | 2.19 | 33 | 3.12 | 0.0037 |
| MOS SF-36 Mental Functioning | ||||||
| Group 1 vs. group 2 | Midpoint | 6.52 | 2.21 | 33 | 2.95 | 0.01 |
| Group 1 vs. group 2 | Final | 8.27 | 2.55 | 33 | 3.25 | 0.0027 |
| MOS SF-36 Normal Group 1 vs. group 2 | Midpoint | 6.53 | 1.74 | 31 | 3.75 | 0.0007 |
| MOS SF-36 Normal Group 1 vs. group 2 | Final | 8.18 | 2.23 | 31 | 3.67 | 0.0009 |
CGI-SD = Clinical Global Impression Scale anchored for Somatic Symptoms; BDI-II = Beck Depression Inventory-II; HAM-A = Hamilton Anxiety Scale; MOS SF-36 = Medical Outcomes Study Short Form General Health Survey.
The odds ratio of patients who are labeled “improved” in terms of HAM-D in the treatment group is about 8.3 times of that of the control group after the last visit. The proportion of the “improved” patients in the treatment group is 59%, yielding the odds (i.e., the ratio of the probability of “improved” over the probability of “not-improved”) 1.44 [= 59%/(1%–59%)]; while the proportion of “improved” in the control group is 13% with the odds equal to 0.15 (= 13%/(1%–13%). The improvement in the treatment group was clinically significant, 59%, χ2 (2, n = 38) = 5.19, p = 0.023. The cross-over group (group 3) also evidenced clinically significant improvements compared with the wait-list condition by both the mid-point, 80%, χ2 (2, n = 31) = 4.99, p = 0.025; and final assessment, 80%, χ2 (2, n = 28) = 10.52, p = 0.0012.
Effect Sizes
Further analysis of effects sizes, generated from the between-group differences at the final assessment point65 indicated medium to large effect sizes for the primary outcome measure. Significant effect sizes were noted in the primary outcome measure, CGI-SD (d = 0.8), the BDI-II total score (d = 0.81), and in the BDI-II neurovegetative subscale (d = 0.70). While there was no significant difference in the average reduction of the Hamilton Anxiety Rating Scale in the treatment group and the control group, there was a significant difference between the crossover group and the control group, with a reduction of 6.1 after mid-visit, yielding an effect size of d = 0.84.
DISCUSSION
Our data suggest that a 10-session psychophysiologic intervention produces clinically significant improvements in somatization symptoms and functioning over and above those obtained from PCI alone. Amelioration of symptoms was observed as early as 5 weeks after the intervention and continued after the intervention phase was completed. To our knowledge, our PT treatment package is the only somatic-based therapy to show efficacy with patients, presenting with complex somatic complaints, in a methodologically adequate randomized controlled trial.
The effect of PT+PCI on MUPS was clinically significant and durable within the time frame studied. In this non-pharmacologic study where, at baseline, the average CGI-SD levels met were of moderate somatization (exceeding abridged criteria, frequent complaints, or some functional impairment), the effect size for the primary outcome measure was impressive. The results provide preliminary evidence that this PT treatment package provides clinically important benefits to individuals suffering from abridged somatization.
One of the suppositions of this study was that the therapeutic effect would be greater and the treatment would be more accepted than the most recent empirically-validated treatment for somatic symptoms. The best effect sizes achieved, prior to this study, have been from a cognitive-behavioral intervention where relaxation techniques were diffusely included and where modest effect sizes were achieved. This 10-week cognitive-behavioral trial for somatization disorder66 had an identical primary outcome measure and study design yielding between-group differences on the CGI-SD of d = 0.51 at 3 months after baseline and d = 0.66 at the 12-month follow-up assessment (i.e., 15 months after baseline). In the current study, the effect size of d = 0.80 on the CGI-SD at 3 months after baseline suggests that the PT treatment package is effective in the treatment of somatic symptoms. Given the structure of the experimental design, however, we cannot conclude that PT is a superior treatment than CBT for his population, nor can we determine its effectiveness if administered without PCI.
This is the first study reporting on a psychophysiologic treatment that was acceptable to patients with abridged somatization, showed low attrition, and was associated with amelioration of pathology. The degree to which our treatment was effective due to factors specific to PT, and theorized to underlie its efficacy, also cannot be answered here, given the limitations of the present experimental design. This is a difficult population to engage and retain in treatment. However, we were able to recruit and retain (80% retention rate). We attributed such success to the close collaboration with the medical clinic’s ancillary staff, acceptance by the clinic’s professionals, and facilitation of services insofar as patients with severe psychopathologies received proper evaluation and referral. Our experience with patients also indicated good acceptance, adherence, and even preference for a nonpharmacologic approach.
In general, the “practical” advantages of this intervention were that it served as a medicalized, user-friendly method that was liked by patients. One of the key features of the treatment was the individual flexibility it allowed in each session. While there was a structured timeline for using each of the four treatment components (HRV BF), PMR (using sEMG BF), autogenic training (peripheral skin temperature, skin conductance), and education on hyperventilation (ETCO2 BF), patients presenting with a predominant somatic complaint would receive more focused training using a treatment module specifically targeting that symptom. Additionally, treatment sessions and assessments were conducted in both a department of psychiatry clinic setting and at a primary care setting. Disadvantaged participants were seen in locations that were easily accessible to them with limited transportation. We believe that this individualized, somatic-based tactic contributed to adequate retention, user “likeability,” and statistical improvements noted. A treatment focusing on the physiologic process potentially removes some of the stigma associated with depression and psychosomatic symptoms and enables patients to become active participants, thereby returning some level of control to patients.
This efficacy trial suggests that PT contributes to producing clinically meaningful benefits in patients with MUPS. PT+PCI recipients manifested a reduction in somatization symptoms and improved physical and psychological functioning. Additional research is needed both to replicate our findings and to examine underlying cause and effect relationships.
There are some limitations to our findings. We did not treat a demographic cross-section of the U.S. population, nor a randomly selected sample of MUPS patients, but rather a group of MUPS patients willing to accept referral into this treatment study. The study had a relatively small sample size and a heterogeneous population with regard to symptoms. Additionally, there was an inability to match effectiveness to symptoms, or individual treatment components to symptoms. Lastly, the use of a control group that consisted of a wait period is yet another limitation. Future research should attempt to utilize more complex designs to systematically assess components of the PT treatment package, compare the efficacy of PT with that of competing treatments, such as CBT, and determine whether therapeutic benefits endure.
Acknowledgments
The authors acknowledge the contributions made by Alejandro Gonzalez-Restrepo, M.D., Esperanza Diaz, M.D., Jane Mayers, R.N., and Nasya Breach.
Footnotes
Disclosure: The study was supported by research grants P20 MH074634 support Multiple Unexplained Physical Symptoms in Primary Care Research Center. The sponsor played no role in the design or conduct of the study; in the collection, management, analysis, or interpretation of data; or in the preparation, review or approval of the manuscript.
References
- 1.Mayou R, Kirmayer LJ, Simon G, et al. Somatoform disorders: time for a new approach in DSM-V. Am J Psychiatry. 2005;162:847– 855. doi: 10.1176/appi.ajp.162.5.847. [DOI] [PubMed] [Google Scholar]
- 2.Kroenke K, Mangelsdorff D. Common symptoms in ambulatory care: incidence, evaluation, therapy, and outcome. Arch Int Med. 1989;86:262–266. doi: 10.1016/0002-9343(89)90293-3. [DOI] [PubMed] [Google Scholar]
- 3.Kroenke K, Spitzer RL, Williams JB. Physical symptoms in primary care. Predictors of psychiatric disorders and functional impairment. Arch Family Med. 1994;3:774–779. doi: 10.1001/archfami.3.9.774. [DOI] [PubMed] [Google Scholar]
- 4.Fiedler N, Kipen H, DeLuca J, et al. A controlled comparison of multiple chemical sensitivities and chronic fatigue syndrome. Psychosom Med. 1996;58(1):38– 49. doi: 10.1097/00006842-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Fink P, Toft T, Hansen M, et al. Symptoms and syndromes of bodily distress: an exploratory study of 978 internal medical, neurological, and primary care patients. Psychosom med. 2007;69:30–39. doi: 10.1097/PSY.0b013e31802e46eb. [DOI] [PubMed] [Google Scholar]
- 6.Katon W, Lin E, Von Korff M, et al. Somatization: a spectrum of severity. Am J Psychiatry. 1991;48:34– 40. doi: 10.1176/ajp.148.7.A34. [DOI] [PubMed] [Google Scholar]
- 7.Escobar JI, Waitzkin H, Cohen Silver R, et al. Abridged somatization: a study in primary care. Psychosom Med. 1998;60:466–472. doi: 10.1097/00006842-199807000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Deary IJ. A taxonomy of medically unexplained symptoms. J Psychosom Res. 2000;47:51–59. doi: 10.1016/s0022-3999(98)00129-9. [DOI] [PubMed] [Google Scholar]
- 9.Escobar JI, Burnam MA, Karno M, et al. Somatization in the community. Arch Gen Psychiatry. 1987;44:713–718. doi: 10.1001/archpsyc.1987.01800200039006. [DOI] [PubMed] [Google Scholar]
- 10.Kroenke K, Spitzer RL, deGruy FV, et al. Multisomatoform disorder: an alternative to undifferentiated somatoform disorder for the somatizing patient in primary care. Arch Gen Psychiatry. 1997;54:352–358. doi: 10.1001/archpsyc.1997.01830160080011. [DOI] [PubMed] [Google Scholar]
- 11.Escobar JI, Gara M. DSM-IV Somatoform disorders: Do we need a new classification? Gen Hospital Psychiatry. 1999;21:154–156. doi: 10.1016/s0163-8343(99)00008-0. [DOI] [PubMed] [Google Scholar]
- 12.Fleischhacker W, Cetkovich-Bajmas M, De Hert M, et al. Co-morbid somatic illnesses in patients with severe mental disorders: clinical, policy, and research challenges. J Clin Psychiatry. 2008;69:514–519. doi: 10.4088/jcp.v69n0401. [DOI] [PubMed] [Google Scholar]
- 13.Karasz A. The development of valid subtypes for depression in primary care settings: a preliminary study using an exploratory model approach. J Nerv Ment Dis. 2008;196:289–296. doi: 10.1097/NMD.0b013e31816a496e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katon WJ, Buchwald DS, Simon GE, et al. Psychiatric illness in patients with chronic fatigue and those with rheumatoid arthritis. J Gen Internal Med. 1991;6:277–285. doi: 10.1007/BF02597420. [DOI] [PubMed] [Google Scholar]
- 15.Simon GE, VonKorff M. Somatization and psychiatric disorder in the NIMH epidemiologic catchment area study. Am J Psychiatry. 1991;148:1494–1500. doi: 10.1176/ajp.148.11.1494. [DOI] [PubMed] [Google Scholar]
- 16.Walker E, Katon W, Harrop-Griffiths J, et al. Relationship of chronic pelvic pain to psychiatric diagnoses and childhood sexual abuse. Am J Psychiatry. 1988;145:75– 80. doi: 10.1176/ajp.145.1.75. [DOI] [PubMed] [Google Scholar]
- 17.Von Korff M, Le Resche L, Dworkin SF. First onset of common pain symptoms: a prospective study of depression as a risk factor. Pain. 1993;55:251–258. doi: 10.1016/0304-3959(93)90154-H. [DOI] [PubMed] [Google Scholar]
- 18.Walker EA, Roy-Byrne PP, Katon WJ, et al. Psychiatric illness and irritable bowel syndrome: a comparison with inflammatory bowel disease. Am J Psychiatry. 1990;147:1656–1661. doi: 10.1176/ajp.147.12.1656. [DOI] [PubMed] [Google Scholar]
- 19.Andreski P, Chilcoat H, Breslau N. Post-traumatic stress disorder and somatization symptoms: a prospective study. Psychiatry Res. 1998;79:131–138. doi: 10.1016/s0165-1781(98)00026-2. [DOI] [PubMed] [Google Scholar]
- 20.Engel C, Liu X, McCarthy B, Miller R, et al. Relationship of physical symptoms to posttraumatic stress disorder among veterans seeking care for Gulf War-related health concerns. Psychosom Med. 2000;62:739–745. doi: 10.1097/00006842-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Fiedler N, Kipen H. Chemical sensitivity: the scientific literature environmental health perspective. 1997;105:409– 415. doi: 10.1289/ehp.97105s2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Interian A, Gara MA, Diaz-Martinez A, et al. The value of pseudoneurologic symptoms for assessing psychopathology in primary care. Psychosom Med. 2004;66:141–146. doi: 10.1097/01.psy.0000107883.14385.ec. [DOI] [PubMed] [Google Scholar]
- 23.Allen LA, Woolfolk RL, Lehrer PM, et al. Cognitive behavior therapy for somatization disorder: a preliminary investigation. J Behav Ther Exp Psychiatry. 2001;32:53– 62. doi: 10.1016/s0005-7916(01)00020-9. [DOI] [PubMed] [Google Scholar]
- 24.Allen LA, Escobar JI, Lehrer PM, et al. Psychosocial treatments for multiple unexplained physical symptoms: a review of the literature. Psychosom Med. 2002 Nov-Dec;64(6):939–950. doi: 10.1097/01.psy.0000024231.11538.8f. [DOI] [PubMed] [Google Scholar]
- 25.Weissman M, Markowitz J, Klerman G. Comprehensive Guide to Interpersonal Psychotherapy. New York: Basic Books; 2000. [Google Scholar]
- 26.Wells K, Sherbourne C, Schoenbaum M, et al. Impact of disseminating quality improvement programs for depression in managed primary care: A randomized controlled trial. J Am Med Assoc. 2000;262:914–919. doi: 10.1001/jama.283.2.212. [DOI] [PubMed] [Google Scholar]
- 27.Simon G, Manning W, Katzlenick D, et al. Cost-effectiveness of systematic depression treatment for high utilizers of general medical care. Arch Gen Psychiatry. 2001;58:181–187. doi: 10.1001/archpsyc.58.2.181. [DOI] [PubMed] [Google Scholar]
- 28.Smith GR, Monson RA, Ray DC. Patients with multiple unexplained symptoms: their characteristics, functional health, and health care utilization. Arch Int Med. 1986;146:69–72. [PubMed] [Google Scholar]
- 29.Ryan M, Gevirtz R. Biofeedback-based psychophysiological treatment in a primary care setting: an initial feasibility study. Appl Psychophysiol Biofeedback. 2004;29:79–93. doi: 10.1023/b:apbi.0000026635.03016.ef. [DOI] [PubMed] [Google Scholar]
- 30.Bridges K, Goldberg D, Evans B, et al. Determinants of somatization in primary care. Psychol Med. 1991;21:473– 483. doi: 10.1017/s0033291700020584. [DOI] [PubMed] [Google Scholar]
- 31.Kirmayer LJ, Robbins JM. Patients who somatize in primary care: a longitudinal study of cognitive and social characteristics. Psychol Med. 1996;26:937–951. doi: 10.1017/s0033291700035273. [DOI] [PubMed] [Google Scholar]
- 32.Wickramasekera I. Secrets kept from the mind but not the body or behavior: the unsolved problems of identifying and treating somatization and psychophysiological disease. Adv Mind-Body Med. 1998;14:81–98. [Google Scholar]
- 33.Schwartz MD, Andrasik F, editors. Biofeedback. A Practitioner’s Guide. 3. New York: Guilford Publications; 2003. [Google Scholar]
- 34.Hamer M, Boutcher YN, Boutcher SH. The role of cardiopulmonary baroreceptors during the forearm vasodilatation response to mental stress. Psychophysiology. 2003;40:249–253. doi: 10.1111/1469-8986.00026. [DOI] [PubMed] [Google Scholar]
- 35.Lipman RD, Grossman P, Bridges SE, et al. Mental stress response, arterial stiffness, and baroreflex sensitivity in healthy aging. J Gerontol Series A–Biol Sci Med Sci. 2002;57A:B279–B284. doi: 10.1093/gerona/57.7.b279. [DOI] [PubMed] [Google Scholar]
- 36.Lehrer, Vaschillo E, Vaschillo B, et al. Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosom Med. 2003;65:796– 805. doi: 10.1097/01.psy.0000089200.81962.19. [DOI] [PubMed] [Google Scholar]
- 37.Lehrer P, Vaschillo E. Heart rate variability biofeedback: A new tool for improving autonomic homeostasis and treating emotional and psychosomatic diseases. Jpn J Biofeedback. 2004;30:7–16. [Google Scholar]
- 38.Hassett AL, Radvanski DC, Vaschillo EG, et al. A pilot study of the efficacy of heart rate variability (HRV) biofeedback in patients with fibromyalgia. Appl Psychophysiol Biofeedback. 2007;32:1–10. doi: 10.1007/s10484-006-9028-0. [DOI] [PubMed] [Google Scholar]
- 39.Karavidas MK, Lehrer PM, Vaschillo E, et al. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Appl Psychophysiol Biofeedback. 2007;32:19–30. doi: 10.1007/s10484-006-9029-z. [DOI] [PubMed] [Google Scholar]
- 40.Siepmann M, Aykac V, Unterdorfer JP, et al. A pilot study on the effects of heart rate variability biofeedback in patients with depression and in healthy subjects. Appl Psychophysiol Biofeedback. 2008;33:195–201. doi: 10.1007/s10484-008-9064-z. [DOI] [PubMed] [Google Scholar]
- 41.Humphreys P, Gevirtz RN. Treatment of recurrent abdominal pain: components analysis of four treatment protocols. J Pediatr Gastroenterol Nutr. 2000;31:47–51. doi: 10.1097/00005176-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Zucker T, Gevirtz R, Samuelson K, et al. The effects of respiratory sinus arrhythmia biofeedback on posttraumatic stress disorder symptoms. Proceedings of the 39th annual meeting of Applied Psychophysiology and Biofeedback; Daytona Beach, FL. 2008. [DOI] [PubMed] [Google Scholar]
- 43.Zucker T. Dissertation Abstracts International: Section B: The Sciences and Engineering. 2008. The effects of respiratory sinus arrhythmia biofeedback on posttraumatic stress disorder symptoms. [Google Scholar]
- 44.Jacobson E. The course of relaxation in muscles of athletes. Am J Psychol. 1936;48:98–108. [Google Scholar]
- 45.Appelbaum K, Blanchard E, Andrasik F. Muscle discrimination ability at three muscle sites in three headache groups. Biofeedback Self Regul. 1984;9:421– 430. doi: 10.1007/BF01000559. [DOI] [PubMed] [Google Scholar]
- 46.Neff D, Blanchard E, Andrasik F. The relationship between capacity for absorption and chronic headache patients response to relaxation and biofeedback treatment. Biofeedback Self Regul. 1983;8:177–183. doi: 10.1007/BF01000547. [DOI] [PubMed] [Google Scholar]
- 47.Arena JG, Bruno GM, Hannah SL, et al. A comparison of frontal electromyographic biofeedback training, trapezius electromyographic biofeedback training, and progressive muscle relaxation therapy in the treatment of tension headache. Headache. 1995;35:411–419. doi: 10.1111/j.1526-4610.1995.hed3507411.x. [DOI] [PubMed] [Google Scholar]
- 48.Hahn YB, Ro YJ, Song HH, et al. The effect of thermal biofeedback and progressive muscle relaxation training in reducing blood pressure of patients with essential hypertension. Image–J Nursing Scholarship. 1993;25:204–207. doi: 10.1111/j.1547-5069.1993.tb00782.x. [DOI] [PubMed] [Google Scholar]
- 49.Lehrer PM, Carr R, Sargunaraj D, et al. Stress management techniques: are they all equivalent, or do they have specific effects? Biofeedback Self Regul. 1994;19:353– 401. doi: 10.1007/BF01776735. [DOI] [PubMed] [Google Scholar]
- 50.Freedman RR. Quantitative measurements of finger blood flow during behavioral treatments for Raynaud’s disease. Psychophysiology. 1989;26:437– 441. doi: 10.1111/j.1469-8986.1989.tb01948.x. [DOI] [PubMed] [Google Scholar]
- 51.Rost K, Kashner TM, Smith GR. Effectiveness of psychiatric intervention with somatization disorder patients: improved outcomes at reduced costs. Gen Hosp Psychiatry. 1994;16:381–387. doi: 10.1016/0163-8343(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 52.Escobar JI, Waitzkin H, Cohen Silver R, et al. Abridged somatization: a study in primary care. Psychosoma Med. 1998;60:466–472. doi: 10.1097/00006842-199807000-00012. [DOI] [PubMed] [Google Scholar]
- 53.Luthe W, Schultz JH. Autogenic Therapy. Grune and Stratton, Inc; New York: 1969. Republished in (2001) by The British Autogenic Society. [Google Scholar]
- 54.Robins E, editor. Completed Suicide. Baltimore, MD: Williams and Williams; 1986. Suicide. [Google Scholar]
- 55.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 56.Escobar JI, Gara MA, Diaz-Martinez AM, et al. Effectiveness of a time-limited cognitive behavior therapy-type intervention among primary care patients with medically unexplained symptoms. Ann Family Med. 2007;5:328–335. doi: 10.1370/afm.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lowe B, Spitzer RL, Williams JB, et al. Depression, anxiety, and somatization in primary care: syndrome overlap and functional impairment. Gen Hosp Psychiatry. 2008;30:191–199. doi: 10.1016/j.genhosppsych.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 58.van Dixhoorn J, Duivenvoorden HJ. Efficacy of Nijmegen Questionnaire in recognition of the hyperventilation syndrome. J Psychosom Res. 1985;29:199–206. doi: 10.1016/0022-3999(85)90042-x. [DOI] [PubMed] [Google Scholar]
- 59.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56– 62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Doorn P, Folgering H, Colla P. Control of the endtidal PCO2 in the hyperventilation syndrome: effects of biofeedback and breathing instructions compared. Bull Eur Physiopathol Respir. 1982;18:829– 836. [PubMed] [Google Scholar]
- 61.Guy W. Clinical Global Impressions. In: Guy W, editor. ECDEU Assessment Manual of Psychopharmacology. Rockville, MD: NIMH; 1976. pp. 202–212. [Google Scholar]
- 62.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36) Med Care. 1992;30:473– 483. [PubMed] [Google Scholar]
- 63.Vansteenkiste J, Rochette F, Demedts M. Diagnostic tests of hyperventilation syndrome. Eur Respir J. 1991;4:393–399. [PubMed] [Google Scholar]
- 64.Humphriss R, Baguley D, Andersson G, et al. Hyperventilation in the vestibular clinic: use of the Nijmegen Questionnaire. Clin Otoilaryngol. 2004;29:232–237. doi: 10.1111/j.1365-2273.2004.00798.x. [DOI] [PubMed] [Google Scholar]
- 65.Cohen . Statistical Power Analysis for the Behavioral Sciences. 2. Hillside, New Jersey: Lawrence Erlbaum Associates Publishers; 1988. [Google Scholar]
- 66.Allen LA, Woolfolk RL, Escobar JI, et al. Cognitive-behavioral therapy for somatization disorder: a randomized controlled trial. Arch Int Med. 2006;166:1512–1518. doi: 10.1001/archinte.166.14.1512. [DOI] [PubMed] [Google Scholar]