Abstract
Group A rotaviruses (RVs) infect the young of numerous animal species and cause acute gastroenteritis. Cultivation of animal and human RVs in cells requires proteolytic activation of the viral attachment protein using trypsin. Both continuous cell lines, such as rhesus monkey kidney cells, as well as primary monkey kidney cells are routinely used for the growth and characterization of RVs. Isolation and cultivation of human RVs from clinical fecal specimens is difficult and adaptation to growth in vitro requires multiple rounds of passage in primary cells. Following growth, RV stocks can be purified by centrifugation if required and quantified using plaque assay or fluorescence focus assay. This unit describes easily applicable procedures for the culturing, storage, and quantification of RVs.
Keywords: rotavirus, RV, infection, cell culture, purification, plaque assay, fecal specimen, MA104 cells, AGMK cells, trypsin
INTRODUCTION
Group A rotaviruses (RVs) are intestinal pathogens that infect the young of virtually every avian and mammalian species (Estes, 2001). RVs replicate in intestinal tract enterocytes, leading to acute gastroenteritis and viral shedding into the stool. The successful cultivation of both animal and human RVs has been achieved through the use of primary and transformed monkey kidney cells and by proteolytic activation of the virus with trypsin prior to infection (Hasegawa et al., 1982; Sato et al., 1981; Ward et al., 1991; Ward et al., 1984; Wyatt et al., 1983; Wyatt et al., 1980). However, it is important to note that different RV strains vary in their capacity to grow in culture. In particular, animal strains that are used as laboratory models, such as RRV, SA11-4F, UK, and OSU, grow quite well in continuous cell lines, reaching titers of 107 to 108 plaque-forming units (PFU) per milliliter of medium. In contrast, culture-adapted human RVs have average titers 1- to 3-logs less than most animal strains. Without a doubt, propagation of human RVs from clinical fecal specimens is very inefficient and does not work for every sample. Growth of RVs from feces requires several blind passages in primary cells prior to growth in continuous cell lines. Once adapted to cell culture, clinical isolates are usually capable of growing as well as the prototypic culture-adapted human RV strains Wa, DS-1, ST3, and P.
This unit covers several techniques for propagating animal and human RVs in cell culture from existing stocks (see Basic Protocol 1 and Alternative Protocol 1) or from clinical fecal specimens (see Alternative Protocol 2). Following propagation, RVs can be concentrated (see Basic Protocol 2) and infectious virus particles can be purified by centrifugation in cesium chloride gradients (see Alternative Protocol 3). Methods of virus quantification are also described, including plaque assay (see Basic Protocol 3) and fluorescence focus assay (see Alternative Protocol 4). Additionally, plaque isolation techniques are outlined, allowing for the generation of clonal RV stocks or the analysis of single virus isolates (see Basic Protocol 4). RVs are very stable and are capable of being stored under a variety of conditions.
General note on tissue culture media
The propagation and quantification of RV is generally performed in rhesus monkey kidney (MA104) cells. Two types of media are commonly used for the culture of MA104 cells, Medium 199 or Eagle’s Minimal Essential Medium (EMEM). There does not appear to be any benefit or detriment to using either type of medium, and the type used is usually based on an individual laboratory’s preference. More specific information of each medium can be found in the Reagents and Solutions section.
CAUTION: The protocols presented in this unit are for use with contemporary animal and human RV strains, which must be handled under Biosafety Level 2 (BSL-2) conditions. For additional information refer to the Biosafety in Microbiological and Biomedical Laboratories manual published by the Center for Disease Control and Prevention and National Institutes of Health. All work with clinical fecal specimens should be conducted in a class II biological safety cabinet.
BASIC PROTOCOL 1-PROPAGATION OF ROTAVIRUS IN MA104 CELL CULTURE FROM VIRUS STOCKS
Rhesus monkey kidney (MA104) cells (ATTC #CRL-2378.1) are widely used for the growth and characterization of both animal and human culture-adapted RVs. Yet, depending upon the particular strain, RVs are capable of growing in other types of continuous cell lines in addition to MA104 (Table 1) (Ciarlet et al., 2002; Estes et al., 1979). If other cell types are used, infection is done in a manner identical to that described below with the exception of substituting the appropriate medium. Use the specific cell culture medium and conditions suggested by the supplier for culturing the cells prior to infection.
Table 1.
Additional Cell Types Commonly Used in Rotavirus Experiments
| Cell type | Origin | Tissue |
|---|---|---|
| BGM | Buffalo green monkey | Kidney |
| BSC-1 | African green monkey | Kidney |
| Caco-2 | Human | Colon adenocarcinoma |
| COS-7 | African green monkey | Kidney |
| CV-1 | African green monkey | Kidney |
| FRhL-2 | Rhesus monkey | Lung |
| HT-29 | Human | Colon adenocarcinoma |
| LLC-MK2 | Rhesus monkey | Kidney |
| Vero | African green monkey | Kidney |
The authors usually work with the animal RV strain SA11-4F, which grows very well and like many other RVs, causes cytopathic effect (CPE) in cultured cells. At a high multiplicity of infection (MOI ≥3), CPE is visible as cell rounding, detachment, and lysis, beginning at 8–10 h post-infection (p.i.). At very low MOI, CPE may not be visible for several days. For RV propagation, infections are commonly performed at low MOI to reduce the generation of mutations and gene rearrangements and to achieve maximal yields of infectious virus. The protocol described below is for the propagation of existing culture-adapted animal or human RV stocks. Refer to Alternative Protocols 1 and 2 for adaptation of RVs to MA104 cells or for preparation of a virus stock from a clinical fecal specimen.
Materials
RV stock vial (105 to 108 PFU/ml)
MA104 cells confluent in a 150-cm2 flask (approximately 1.5 ×107 cells)
Trypsin, porcine pancreatic type IX, Sigma Aldrich (2 mg/ml)
1 × complete Medium 199 (see recipe)
1 × serum-free Medium 199 (see recipe)
1 × serum-free Medium 199 with 0.5 μg/ml trypsin (see recipe)
Pasteur pipettes
10- and 25-ml pipettes
10-, 200- and 1000-μl pipettes and tips
15- and 50-ml conical tubes
37°C water bath
37°C incubator, 5% CO2
Inverted light microscope
Table-top centrifuge
Platform rocker (optional)
Vortex (optional)
NOTE: All equipment and solutions coming in contact with cells must be sterile and sterile technique should be used. Cell culture work should be conducted in a class II biological safety cabinet. All culture incubations should be performed in a humidified 37°C, 5% CO2 incubator.
-
Remove growth medium from a confluent monolayer of MA104 cells maintained in a 150-cm2 flask. Wash the monolayer three times using 10 ml of pre-warmed, serum-free Medium 199 each time. Take care not to add medium directly onto the monolayer as this may disrupt the cells.
Fetal bovine serum (FBS), a component in complete Medium 199, inhibits RV activation and must be removed for efficient infection of the cells. Cells can also be incubated several hours to overnight in one change of serum-free medium. -
Thaw a stock vial of RV at room temperature. Transfer the volume equivalent of an MOI ≤0.1 of RV stock into a 15-ml tube.
Calculation of the PFU needed for a MOI ≤0.1 requires knowledge of the number of cells in the culture flask. A confluent 150-cm2 flask contains approximately 1.5 × 107 MA104 cells. Use the following equation to determine the PFU:If the virus titer is 106 PFU/ml, then ≤1.5 ml would be required for an MOI ≤0.1. -
To activate the virus, add trypsin to a final concentration of 10 μg/ml. Vortex briefly or swirl to mix. Incubate the mixture for 1 h in a 37°C water bath.
Trypsin cleaves the RV attachment protein, allowing for entry of the virus into the host cell. The enzyme should be thawed on ice and not subjected to multiple rounds of freeze-thaw. Following activation, dilute the inoculum by adding serum-free Medium 199 to reach a final trypsin concentration of <2 μg/ml. For a 150-cm2 flask, the inoculum volume should be at least 3 ml.
-
Remove the serum-free Medium 199 wash from the flask. Add the inoculum and incubate the flask for 1 h at 37°C on a platform rocker to ensure equal coverage of the monolayer with the inoculum.
In the absence of a platform rocker, gently rock the flask every 10 to 15 minutes by hand.Incubation of cells >1 h or in >2 μg/ml of trypsin may cause the cell monolayer to slough off. Cells should be visualized under an inverted light microscope to ensure the monolayer is intact following virus adsorption. -
Following adsorption, remove the virus inoculum and wash the monolayer once using 10 ml of pre-warmed, serum-free Medium 199. Remove the wash and add 20 ml of pre-warmed, serum-free Medium 199 with 0.5 μg/ml trypsin.
The presence of trypsin in the culture medium allows for efficient virus propagation. However, to generate completely non-activated RV stocks, replace the inoculum with complete Medium 199 containing trypsin inhibitors. In this case, cells should be infected at an MOI ≥3. Maintain the cells at 37°C for 3 to 7 days or until CPE has progressed to the point where the cell monolayer is fully disrupted due to lysis. Transfer the flask to −80°C.
-
Freeze and thaw the infected cells three times while in the flask. Transfer the cell lysate and culture supernatant into a 50-ml conical tube.
Multiple rounds of freezing and thawing releases cellular-associated RV particles into the supernatant. Loosen the cap on the culture flask to prevent it from cracking during the freeze-thaw process. Remove large cellular debris from the lysate by low-speed centrifugation (300 × g at 4°C). Transfer the clarified virus (approximately 20 ml) into a new 50-ml conical tube.
-
The clarified RV stock can now be titered, aliquoted, and stored (see Basic Protocols 3 and 4) or can be used for additional rounds of amplification.
If additional amplification is required, dilute the clarified virus stock 1:2 in serum-free Medium 199 with 0.5 μg/ml of trypsin, and then add directly onto a fresh monolayer of washed MA104 cells.
ALTERNATIVE PROTOCOL 1-PROPAGATION OF ROTAVIRUS IN PRIMARY AGMK ROLLER CELL CULTURE FROM VIRUS STOCKS
Some RV strains, in particular human isolates, are refractory to growth in MA104 cells, but grow in primary African green monkey kidney (AGMK) cells. Following several passages in AGMK cells, RVs characteristically grow more readily in culture and can then efficiently infect continuous cells (see Basic Protocol 1). The authors use roller cultures of AGMK cells in round-bottom glass tubes (Diagnostic HYBRIDS). African green monkeys are often infected with simian virus 5 (SV5) and simian virus 40 (SV40), which have the capacity to transform cultured cells. To prevent transformation, the AGMK cells are maintained in the presence of antisera against SV5 and SV40. The protocol described below is for the propagation of existing animal or human RV stocks. Refer to Alternative Protocol 2 for preparation of a RV stock from a clinical fecal specimen.
Additional Materials (also see Basic Protocol 1)
AGMK cells in a round bottom glass tube (Diagnostic HYBRIDS, catalog #46-0600A) Roller drum for 37°C incubator
NOTE: All equipment and solutions coming in contact with cells must be sterile and sterile technique should be used. Cell culture work should be conducted in a class II biological safety cabinet. All culture incubations should be performed in a humidified 37°C, 5% CO2 incubator.
-
Remove growth medium from primary AGMK cells that have been maintained for <4 days in a glass roller tube. Wash the monolayer three times using 2 ml of pre-warmed, serum-free Medium 199 each time. Take care not to add medium directly onto the monolayer on the sides of the tube as this may disrupt the cells.
FBS, a component in AGMK growth medium, inhibits RV activation and must be removed for efficient infection of the cells. Cells can also be incubated several hours to overnight in one change of serum-free medium. -
Thaw a stock vial of RV at room temperature. Transfer the volume equivalent of an MOI ≤0.1 of RV stock into a 15-ml conical tube.
Calculation of the PFU needed for a MOI ≤0.1 requires knowledge of the number of cells in the culture flask. A confluent roller tube contains approximately 1.5 × 106 AGMK cells. Use the following equation to determine the PFU:If the virus titer is 106 PFU/ml, then ≤150 μl would be required for an MOI ≤0.1. -
To activate the virus, add trypsin to a final concentration of 10 μg/ml. Vortex briefly or swirl to mix. Incubate the mixture for 1 h in a 37°C water bath.
Trypsin cleaves the RV attachment protein, allowing for entry of the virus into the host cell. The enzyme should be thawed on ice and not subjected to multiple rounds of freeze-thaw. Following activation, dilute the inoculum by adding serum-free Medium 199 to reach a final trypsin concentration of <2 μg/ml. The inoculum volume should be at least 0.5 ml.
-
Remove the serum-free Medium 199 wash from the roller tube. Add the inoculum and incubate the tube for 1 h at 37°C in a roller drum to ensure equal coverage of the monolayer with the inoculum.
Incubation of cells >1 h or in >2 μg/ml of trypsin may cause the cell monolayer to slough off. Cells should be visualized under an inverted light microscope to ensure the monolayer is intact following virus adsorption. -
Following adsorption, remove the virus inoculum and wash the monolayer once using 2 ml of pre-warmed, serum-free Medium 199. Remove the wash and add 2 ml of pre-warmed, serum-free Medium 199 with 0.5 μg/ml of trypsin.
The presence of trypsin in the culture medium allows for efficient virus propagation. However, to generate completely non-activated RV stocks, replace the inoculum with complete Medium 199 containing trypsin inhibitors. In this case, cells should be infected at an MOI ≥3. -
Maintain the cells with constant rotation in a roller drum at 37°C for 3 to 7 days or until CPE is visible, and the cell monolayer is disrupted due to lysis. Transfer the tube to −80°C.
If no CPE is detected during the first infection, the clarified supernatant should be blindly passaged every 7 days up to ten times or until CPE is visible. -
Freeze and thaw the infected cells three times while in the tube. Transfer the cell lysate and culture supernatant into a 15-ml conical tube.
Multiple rounds of freezing and thawing releases cellular-associated RV particles into the supernatant. Remove large cellular debris from the lysate by low-speed centrifugation (300 × g at 4°C). Transfer the clarified virus (approximately 2 ml) into a new 15-ml conical tube.
-
The clarified virus stock can now be titered, aliquoted, and stored (see Basic Protocols 3 and 4) or can be used for additional rounds of amplification.
If additional amplification is required, dilute the clarified virus stock 1:2 in serum-free Medium 199 with 0.5 μg/ml trypsin, and then add directly onto a fresh monolayer of washed AGMK cells. In general, 2 to 3 passages of RV in primary AGMK cells are sufficient for increasing growth efficiency in continuous MA104 cells.
ALTERNATIVE PROTOCOL 2- PROPAGATION OF ROTAVIRUS IN CELL CULTURE FROM CLINICAL FECAL SPECIMENS
Cultivation of RV from clinical fecal specimens is achieved through the use of primary AGMK cells. Bacterial contamination can be reduced by clarifying the specimen using centrifugation and by maintaining antibiotics in the culture throughout the course of infection. Following several passages of the RV clinical sample in AGMK cells, the virus is usually capable of being propagated in continuous cells. However, cultivation of RV from feces is not always possible for reasons that are not fully understood. Additionally, CPE may not be visible in the AGMK cells following infection using clinical specimens. Several blind passages, in which no CPE is detectable, may be required. Alternatively, the feces itself may be cytotoxic and dilution may be required prior to infection.
Additional Materials (also see Basic Protocol 1)
Clinical fecal sample
AGMK cells in a round bottom glass tube (Diagnostic HYBRIDS, catalog #46-0600A)
1.5-ml microcentrifuge tubes
Roller drum for 37°C incubator
NOTE: All equipment and solutions coming in contact with cells must be sterile and sterile technique should be used. Cell culture work and all handling of clinical specimens should be conducted in a class II biological safety cabinet. All culture incubations should be performed in a humidified 37°C, 5% CO2 incubator.
In a 1.5-ml microcentrifuge tube, prepare 1 ml of a 15% (w/v) homogenate of feces using serum-free Medium 199. Clarify the homogenate by centrifugation at 10,000 × g at 4°C for 15 min. Transfer the supernatant into a new 1.5-ml tube.
- To activate the virus, add trypsin to the clarified preparation to a final concentration of 10 μg/ml. Vortex briefly or swirl to mix. Incubate the mixture for 1 h in a 37°C water bath.Trypsin cleaves the RV attachment protein, allowing for entry of the virus into the host cell. The enzyme should be thawed on ice and not subjected to multiple rounds of freeze-thaw.
Following activation, dilute the inoculum by adding serum-free Medium 199 to reach a final trypsin concentration of <2 μg/ml. The inoculum volume should be at least 0.5 ml.
- Remove growth medium from primary AGMK cells that have been maintained for <4 days in a glass roller tube. Wash the monolayer three times using 2 ml of pre-warmed, serum-free Medium 199 each time. Take care not to add medium directly onto the monolayer on the sides of the tube as this may disrupt the cells.FBS, a component in AGMK growth medium, inhibits RV activation and must be removed for efficient infection of the cells. Cells can also be incubated several hours to overnight in one change of serum-free medium.
- Remove the serum-free Medium 199 wash from the roller tube. Add the inoculum and incubate the tube for 1 h at 37°C in a roller drum to ensure equal coverage of the monolayer with the inoculum.Incubation of cells >1 h or in >2 μg/ml of trypsin may cause the cell monolayer to slough off. Cells should be visualized under an inverted light microscope to ensure the monolayer is intact following virus adsorption.Additionally, the fecal sample may be cytotoxic and as a result, the cells may detach and lyse following this adsorption step. In this case, the inoculum should be diluted 1:10 to 1:100 using pre-warmed serum-free Medium 199 with 0.5 μg/ml of trypsin.
Following adsorption, remove the virus inoculum and wash the monolayer once using 2 ml of pre-warmed, serum-free Medium 199. Remove the wash and add 2 ml of pre-warmed, serum-free Medium 199 with 0.5 μg/ml of trypsin.
- Maintain the cells with constant rotation in a roller drum at 37°C for 3 to 7 days or until CPE is visible, and the cell monolayer is disrupted due to lysis. Transfer the tube to −80°C.If no CPE is detected during the first infection, the virus should be blindly passaged every 7 days up to ten times or until CPE is visible.
- Freeze and thaw the infected cells three times while in the tube. Transfer the cell lysate and culture supernatant into a 15-ml conical tube.Multiple rounds of freezing and thawing releases cellular-associated RV particles into the supernatant.
Remove large cellular debris from the lysate by low-speed centrifugation (300 × g at 4°C). Transfer the clarified virus (approximately 2 ml) into a new 15-ml conical tube.
- The clarified virus stock can now be titered, aliquoted, and stored (see Basic Protocols 3 and 4) or can be used for additional rounds of amplification.If additional amplification is required, dilute the clarified virus stock 1:2 in serum-free Medium 199 containing 0.5 μg/ml trypsin, and then add directly onto a fresh monolayer of washed AGMK cells. In general, 2 to 3 passages of RV in primary AGMK cells are sufficient for increasing growth efficiency in continuous MA104 cells.
BASIC PROTOCOL 2-SEMI-PURIFICATION OF ROTAVIRUSES USING ULTRACENTRIFUGATION
The quantity of RV recovered from the clarified cell supernatant is sufficient for most experimental procedures. However, some experiments may require the concentration or semi-purification of RV stocks, in particular for very low titer human RV stocks. Such concentration is achieved by pelleting the virus particles through a 35% sucrose cushion using ultracentrifugation. For RV stocks amenable to biochemical and structural assays, the virus can be further purified using cesium chloride gradients (see Alternative Protocol 3). RV particles should be maintained in buffer containing calcium (such as TNC buffer), which is essential for the stability of outer capsid. Likewise, buffers containing calcium-chelation agents (such as EDTA) should be avoided.
Materials
ml of clarified RV stock (from twelve 150-cm2 flasks of infected MA104 cells)
TNC buffer (see recipe)
35% sucrose (w/v) in TNC buffer
2-ml and 50-ml pipettes
Beckman Ultra-Clear 25×89 mm ultracentrifuge tubes
15-ml conical tube
Beckman ultracentrifuge with an SW32Ti rotor (or equivalent)
Sonicator (optional)
NOTE: If preparing a sterile RV stock, the purification protocol should be performed in a class II biological safety cabinet and all equipment and solutions should be sterile. If preparing a non-sterile RV stock, the purification protocol can be performed on the laboratory bench.
- Aliquot 35 ml of clarified RV stock into each of six Beckman Ultra-Clear 25×89 mm ultracentrifuge tubes.Leave approximately 1 inch of space from the top of the tube to provide room for the volume expansion that occurs when underlying the sucrose solution.
- Using a 2-ml pipette, carefully underlay 2 ml of 35% sucrose below the 35 ml of RV stock. Gently fill the remainder of the tube with virus supernatant. Balance the tubes by weight.Because sucrose is denser than the virus, an interface should be visible near the bottom of the tube. Care should be taken to avoid disrupting this interface prior to centrifugation.
Centrifuge at 100,000 × g for 1.5 h at 4°C using an SW32Ti rotor to pellet virus particles.
Pour the supernatant into an appropriate waste container and resuspend each pellet in 1 ml of TNC buffer, making 6 ml total volume.
- Transfer the 6 ml of resuspended pellets into a clean 15-ml conical tube. Sonicate briefly using a microtip probe to create a homogenous suspension of particles.The virus should be kept on ice during sonication to avoid protein denaturation. In the absence of a sonicator, a homogenous suspension can be created by passing the material through a 25-gauge needle several times.
This concentrated, semi-purified RV stock can be titered or used directly for biological assays. Semi-purified virus can be stored at 4°C in TNC buffer for up to a year with minimal loss in titer.
ALTERNATIVE PROTOCOL 3-PURIFICATION OF ROTAVIRUS USING CESIUM CHLORIDE GRADIENTS
Further purification of RV particles using cesium chloride (CsCl) gradients is required for some biochemical or structural assays. The starting material for this purification protocol is non-clarified, freeze-thawed RV-infected cell lysate (see Basic Protocol 1). Cellular lipids are removed from the preparation using trichlorotrifluoroethane (Freon), prior to banding virion particles in CsCl. As noted in Basic Protocol 2, RV particles should be maintained in buffer containing calcium (such as TNC buffer), and calcium-chelation agents should be avoided.
Materials
240 ml of RV-infected cell lysate (from twelve 150-cm2 flasks of infected MA104 cells)
Trichlorotrifluoroethane (Freon)
TNC buffer (see recipe)
Cesium chloride, Ultra-Pure, Optical Grade
50-ml conical tubes
Beckman Ultra-Clear 25×89 mm ultracentrifuge tubes
Beckman Ultra-Clear 13×51 mm ultracentrifuge tubes
Beckman ultracentrifuge with SW32Ti, and SW55Ti rotors (or equivalent)
Table-top centrifuge
Refractometer
Sorvall Omni-mixer or vortex
NOTE: If preparing a sterile RV stock, the purification protocol should be performed in a class II biological safety cabinet and all equipment and solutions should be sterile. If preparing a non-sterile RV stock, the purification protocol can be performed on the laboratory bench.
CAUTION: This protocol requires the use of Freon, which is a colorless liquid that has known toxic properties and is an ozone-depleting substance. Freon should be handled in a chemical fume hood and according to the Materials Safety and Data Sheet (MSDS) provided by the manufacturer. In addition, Freon should be disposed of appropriately.
Aliquot 40 ml of RV cell lysate into each of six Beckman Ultra-Clear 25×89 mm ultracentrifuge tubes. Balance the tubes by weight.
Centrifuge at 100,000 × g for 1.5 h at 4°C using an SW32Ti rotor to pellet virus particles and cellular debris.
- Pour the supernatant into an appropriate waste container and resuspend each pellet in 10 ml of TNC buffer, making 60 ml total volume.The pellet may be difficult to resuspend. The authors use a 25-ml pipette for several rounds, followed by a 10-ml pipette for several rounds. Brief sonication can be used to achieve a more homogenous mixture. The virus should be kept on ice during sonication to avoid protein denaturation.
- Combine the 60 ml pellet suspension with an equal volume of Freon in the Sorvall mixing vessel. Emulsify the mixture while on ice using a Sorvall Omni-mixer 3 min at speed 7.In the absence of a Sorvall Omni-mixer, the Freon/virus mixture can be vortexed in a 50-ml conical tube three times for 3 min each until emulsified, with 1 min incubations on ice between each interval.
Transfer the emulsion into two 50-ml conical tubes. Balance the tubes by weight. Separate the organic and aqueous phases by centrifuging the emulsion at 4,100 × g for 10 min at 4°C.
Transfer 30 ml of the upper, aqueous phase into each of two Beckman Ultra-Clear 25×89 mm centrifuge tubes. Fill the remainder of the tubes with TNC buffer. Balance the tubes by weight.
Centrifuge at 100,000 × g for 1.5 h at 4°C using an SW32Ti rotor to pellet virus particles.
Pour the supernatant into an appropriate waste container and resuspend each pellet in 4 ml of TNC buffer, making 8 ml total volume.
- Transfer the 8 ml pellet suspension into a clean 50-ml conical tube. Sonicate briefly using a microtip probe to create a homogenous suspension of particles.The virus should be kept on ice during sonication to avoid protein denaturation.
- Dissolve 4 g of CsCl in the virus suspension to achieve a density of 1.37g/cm3 as determined by refractometry.Precise determination of the density by refractometry is critical for proper separation and purification of virus particles using CsCl.
Transfer 5 ml each of CsCl/virus solution into two Beckman Ultra-Clear 13×51 mm ultracentrifuge tubes. Balance the tubes by weight.
- Centrifuge at 110,000 × g for 18 h at 12°C using an SW55Ti rotor to create gradients and band virus particles.Use the slowest deceleration profile (i.e., no brake) to prevent mixing of the gradients.
- Inspect the gradients in a darkened room with an inverted light source. Two cloudy, whitish-colored bands should be visible (see Figure 1).The upper band consists of infectious, triple-layered RV virions, while the lower band consists of non-infectious, double-layered particles.
- Using a pipette, carefully remove 100 to 500 μl fractions from the gradient. Save all fractions in separate 1.5 ml microcentrifuge tubes at 4°C. Remove the upper, virus band in the smallest possible volume to achieve a maximum concentration.If necessary, the upper band containing infectious RV particles can be re-purified on another CsCl gradient to remove traces of double-layered particles. In that case, dilute the material from the upper band in a CsCl/TNC solution that has a density of 1.37g/cm3 and centrifuge at 110,000 × g for 18 h at 12°C using an SW55Ti rotor.
- Dialyze the virus sample exhaustively against several changes of TNC buffer. Purified virus can be titered and stored at 4°C for up to a year with minimal loss in titer.Following dialysis the sample should also be analyzed by SDS-PAGE to verify recovery, purity, and relative concentration of RV particles. Collected fractions can be discarded once virus sample is obtained.
Figure 1. Example of RV plaque assay.
These images depict the results of a plaque assay using the animal RV strain SA11-4F, which grows well in cell culture. At 48 h post-infection, the cells were stained with neutral red. The image on the top was taken 4 h after staining. The image on the bottom is a plate that was incubated an additional 16 h after staining. The sizes of the plaques for this virus grew substantially during the overnight incubation, and are considered large at 3 days post-infection. Many strains of rotavirus will make plaques that take additional days to form and are smaller in size. To calculate the titer for this plaque assay, the plate on the right was used, and the titer was calculated as: 15 plaques × 1/(10−7) × 1/(1 ml) = 1.5 × 108 PFU/ml.
BASIC PROTOCOL 3-QUANTIFICATION OF ROTAVIRUS BY PLAQUE ASSAY
The plaque assay method is one of two common methods for quantifying RV. This assay is used to determine the viral titer, in plaque-forming units per milliliter of virus (PFU/ml) and is based on the CPE caused by RV in cultured cells. A single cell initially infected by RV spreads only to adjacent cells due to an overlay of 0.6% agarose in 1× EMEM. As the infection progresses, cell detachment and lysis causes the formation of localized clearings, or plaques. RV strains that grow well in cell culture will form visible plaques within a few days, while those strains that do not grow as well can take up to a week for plaques to become visible. Neutral red is used to visualize the plaques in this assay. Living cells take up the neutral red stain, whereas the lysed cells of the plaques do not, leaving the plaques visible as a clear area surrounded by the remaining cell monolayer. In some cases, plaques can be so small as to be difficult to see with neutral red staining. If this occurs, cells can be fixed with formaldehyde and stained with crystal violet to enhance the contrast between plaques and uninfected cells.
Plaque assays for RV are performed in MA104 cells in six-well tissue culture plates. The cells must form tightly packed monolayers in order to see well-defined plaques. The cells are sensitive to drying, so when removing medium from wells at any step, work quickly to prevent cells from drying out. This protocol uses an inoculating volume of 1 ml of virus per well. Generally, if experimental infections require a larger inoculating volume, then this is appropriate to use. However, if experimental infections use a small inoculating volume, then plaque assays should be performed similarly. Inoculating volumes as low as 100 μl per well of a six-well plate can be used, as long as care is taken to prevent the monolayer from drying out. Though the methods as written here use Medium 199 for growth of the cells and dilution of the virus, EMEM can be used as an equivalent substitute.
The authors generally use one six-well plate per dilution series. A dilution series ranging from 10−3 to 10−8 is usually sufficient to see individual plaques, though a lower or higher range may be required. Plaque assays are commonly performed in duplicate, along with an uninfected control in duplicate. This would require four six-well plates of MA104 cells to titer a single RV stock.
Materials
RV stock (unknown titer)
MA104 cells confluent in a 150-cm2 flask (approximately 1.5 ×107 cells)
Trypsin, porcine pancreatic type IX, Sigma Aldrich (2 mg/ml)
1 × complete Medium 199 (see recipe)
1 × serum-free Medium 199 (see recipe)
2 × serum-free EMEM (see recipe)
1.2% agarose (see recipe)
Neutral red (0.33% in PBS)
Six-well tissue culture plates
5-, 10-, and 25-ml pipettes
Pasteur pipettes
10-, 200- and 1000-μl pipettes and tips
15- and 50-ml conical tubes
37°C water bath
37°C incubator, 5% CO2
Vortex
Rocking platform (optional)
Light box
NOTE: All equipment and solutions coming in contact with cells must be sterile and sterile technique should be used. Cell culture work should be conducted in a class II biological safety cabinet. All culture incubations should be performed in a humidified 37°C, 5% CO2 incubator unless otherwise specified.
Prepare MA104 cells
-
1
Determine the number of plaque assays to be performed. Seed MA104 cells into six-well plates at a density of 3.0 × 105 cells per well, and incubate for 3 to 5 days.
One confluent 150-cm2 flask of MA104 cells contains approximately 1.5 × 107 cells. For simplified plating, one flask will be sufficient to seed eight six-well plates. For example, the trypsinized cells from one flask can be resuspended in 150 ml of complete Medium 199, and 3 ml of that cell suspension is plated per well.If cells are confluent before you are ready to use them, they may be incubated overnight at 30°C.
Activate virus and prepare dilutions
-
2
Thaw the vial of RV to be assayed at room temperature.
-
3
Activate the virus by mixing 400 μl of RV with 2 μl of trypsin (2 mg/ml stock) for a final trypsin concentration of 10 μg/ml.
-
4
Incubate the virus for 1 h in a 37°C water bath.
-
5
Prepare dilution tubes by adding 2.7 ml of serum-free Medium 199 into each 15-ml conical tube. One tube is needed for each dilution, usually requiring eight tubes/virus for an endpoint of 10−8.
-
6
Prepare serial ten-fold dilutions of the virus by adding 300 μl of the trypsin-activated RV into the first tube (10−1), bringing the final volume to 3 ml. Vortex to mix and then transfer 300 μl of the 10−1 dilution into the 10−2 tube. Continue to prepare serial ten-fold dilutions by adding 300 μl of each dilution into the next tube until the desired endpoint is reached (usually to 10−8). Change pipette tips between each dilution to ensure virus is not carried over.
Only 300 μl of the activated RV is used in dilutions, though 400 μl was activated to account for error in pipetting.
Infect cells
-
7
Wash MA104 cells in six-well plates three times using 2 ml/well of pre-warmed, serum-free Medium 199 each time.
FBS, a component in MA104 growth medium, inhibits RV activation and must be removed for efficient infection of the cells. Cells can also be incubated several hours to overnight in one change of serum-free medium. -
8
Remove the wash and add 1 ml of virus dilution (usually 10−3, 10−4, 10−5, 10−6, 10−7, and 10−8) to each of two wells. Add virus from most to least dilute, which allows the same pipette tip to be used.
-
9
Incubate the plates for 1 h at 37°C on a rocking platform to allow the virus to adsorb.
Alternatively, if a rocking platform is not available, plates may be rocked by hand every 10 to 15 minutes of the incubation period. -
10
Remove the inoculum and wash the cells once using 2 ml pre-warmed, serum-free Medium 199.
Assay for plaques
-
11
Prepare a 1:1 mixture of 1.2% agarose and complete 2 × EMEM containing 0.5 μg/ml trypsin as follows:
Warm 2× EMEM to 37°C. Melt pre-sterilized 1.2% agarose by microwaving, and allow agarose to cool in a 55°C water bath. Combine equal volumes of the pre-warmed 2 × EMEM and 1.2% agarose, followed by addition of trypsin to a final concentration of 0.5 μg/ml. Be sure the agarose/medium mixture is less than 42°C to avoid heat inactivation of the trypsin. Swirl the agarose/medium overlay several times to ensure equal mixing. -
12
Remove the wash medium and gently overlay the cells in each well with 3 ml of the agarose/medium mixture. Immediately swirl the plate one time to incorporate any residual wash medium into the overlay.
Do not swirl the plate once the agarose begins to solidify as this may damage the cell monolayer. -
13
Once the agarose has solidified, transfer the plates to a 37°C incubator for 3 to 4 days or until plaques are visible.
The authors have found that inverting the plates helps prevent the agarose from drying out during the incubation period.At this stage, prior to staining, the plaques will appear whitish or opaque against the rest of the monolayer when held up to light. -
14
For the neutral red overlay, prepare a 1:1 mixture of 1.2% agarose with an equal volume of serum-free 2× EMEM containing 50 μg/ml neutral red. Add 2 ml per well of stain overlay on top of the first agarose/medium overlay. Cover the six-well plates to protect from light and allow agarose to harden.
-
15
Once the neutral red overlay has solidified, cover the plates to protect them from light and incubate at 37°C until plaques can be seen (approximately 4 to 24 h) (see Figure 2).
-
16Count individual plaques using a light box. Calculate the titer of the virus as follows:For instance, if the 10−7 dilution yielded 30 plaques, then the virus titer would be: 30 × 1/(10−7) × 1/(1 ml) = 3.0 × 108 PFU/ml
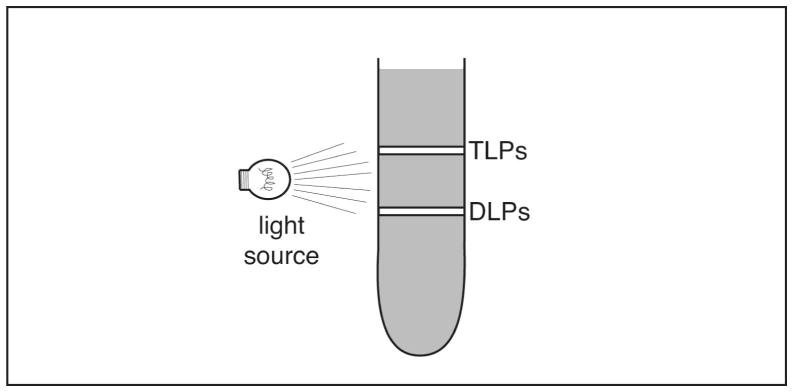
Figure 2. Cartoon of two types of RV particles in a CsCl gradient.
Following centrifugation of RV in a CsCl gradient, two distinct, cloudy, whitish-colored bands will be visible. Shining a light on the gradient will facilitate visualization of the bands. The upper band consists of infectious, triple-layered particles (TLPs; density ~1.36 g/cm3) and the lower band consists of non-infectious, double-layered particles (DLPs; density ~1.38 g/cm3). Harvest the upper band as a source of purified RV.
ALTERNATIVE PROTOCOL 4-QUANTIFICATION OF ROTAVIRUS BY FLUORESCENCE FOCUS ASSAY
Quantifying RVs by fluorescence focus assay is a more rapid approach than the plaque assay. This assay detects fluorescence foci through the use of an antibody that reacts with RV-infected cells. Most investigators use antibodies that were made in or have been well characterized in their laboratories. However, there are also commercially available RV antibodies. Polyclonal antibodies generated against intact virions will usually cross-react with several RV strains and provide a strong fluorescence signal. Monoclonal antibodies offer antigenic specificity, but may only recognize some strains of RV. Thus, if using a monoclonal antibody, its antigenicity must cross-react with the strain of RV being tested. A common antibody to use would be directed against the viral capsid protein VP6 of group A RVs (see Commentary). As with all antibodies, the primary and secondary antibodies must be titrated prior to use to determine the optimal working dilution.
NOTE: All equipment and solutions coming in contact with cells must be sterile and sterile technique should be used. Cell culture work should be conducted in a class II biological safety cabinet. All culture incubations should be performed in a humidified 37°C, 5% CO2 incubator.
Additional Materials (also see Basic Protocol 3)
Anti-RV antibody
FITC-conjugated secondary antibody
1 × Phosphate-buffered saline (PBS), pH 7.4
3% bovine serum albumin (BSA) in PBS
100% methanol
96-well tissue culture plates
1.5 ml microcentrifuge tubes
Multi-channel pipette
Inverted fluorescence microscope with fluorescein isothiocyanate (FITC) filter
-
Seed MA104 cells into 96-well plates at a density of 6.4 × 103 cells per well, and incubate for 3 to 5 days.
One confluent 150-cm2 flask of MA104 cells contains approximately 1.5 × 107 cells. For simplified plating, the trypsinized cells from one flask can be resuspended in 10 ml of complete medium, and 500 μl of the cell suspension is diluted in 20 ml of complete medium. Then, 200 μl of the diluted suspension is plated per well. -
Using a multi-channel pipette, wash the MA104 cells in 96-well plates two times using 200 μl/well of pre-warmed, serum-free Medium 199 each time.
FBS, a component in MA104 growth medium, inhibits RV activation and must be removed for efficient infection of the cells. Cells can also be incubated several hours to overnight in one change of serum-free medium. -
Remove the second wash and add 100 μl/well of pre-warmed, serum-free Medium 199 containing 1 μg/ml trypsin.
Medium containing trypsin is added to the cells to prevent them from drying out. The activated virus inoculum will be added directly to this medium. Thaw the vial of RV to be assayed at room temperature.
Activate the virus by mixing 200 μl of RV with 1 μl of trypsin (2 mg/ml stock) for a final trypsin concentration of 10 μg/ml.
Incubate the virus for 1 h in a 37°C water bath.
Prepare dilution tubes by adding 900 μl of serum-free Medium 199 into each 1.5-ml microcentrifuge tube. One tube is needed for each dilution, usually requiring eight tubes/virus for an endpoint of 10−8.
Prepare serial ten-fold dilutions of the virus by adding 100 μl of the trypsin-activated RV into the first tube (10−1), bringing the final volume to 1 ml. Vortex to mix and then transfer 100 μl of the 10−1 dilution into the 10−2 tube. Continue to prepare serial ten-fold dilutions by adding 100 μl of each dilution into the next tube until the desired endpoint is reached (usually to 10−8). Change pipette tips between each dilution to ensure virus is not carried over.) to
Add 100 μl of each dilution of virus (usually 10−3, 10−4, 10−5, 10−6, 10−7, and 10−8 each of two wells that already contain 100 μl of serum-free Medium 199 with 1 μg/ml trypsin. Add virus from most to least dilute, which allows the same pipette tip to be used.
Incubate the plates for 1 h at 37°C.
-
Remove the inoculum and add 200 μl/well of pre-warmed, complete Medium 199. Incubate the infected cells at 37°C for 12 to 18 h.
Complete medium containing FBS is added to prevent the virus from spreading in the culture. A neutralizing antibody can also be added into the medium. Wash the infected MA104 cells once using 200 μl of PBS. Fix the cells by adding 200 μl of 100% methanol and incubating for 2 min at room temperature. Wash the fixed cells again using 200 μl of PBS.
-
Remove the PBS and add the anti-RV primary antibody diluted in PBS containing 3% BSA (final volume of 200 μl). Incubate for 30 min at room temperature with gentle rotation.
The anti-RV primary antibody must be titrated prior to use to determine the optimal working dilution. Remove the antibody solution and wash the cells two times using 200 μl of PBS each time.
-
Remove the PBS and add the secondary antibody conjugate to fluorescein isothiocyanate (FITC) diluted in PBS containing 3% BSA (final volume of 200 μl). Incubate for 30 min at room temperature with gentle rotation.
The secondary antibody must be directed against the species the primary antibody was raised in, and must be titrated prior to use to determine the optimal working dilution, which usually ranges from 1:500 to 1:5000. Remove the secondary antibody solution and wash the cells four times using 200 μl of PBS each time.
- Count individual fluorescence foci on an inverted fluorescence microscope with a FITC-compatible filter. Calculate the titer of the virus as follows:For instance, if the 10−6 dilution yielded 30 foci, then the virus titer would be: 30 × 1/(10−6) × 1/(0.1 ml) = 3 × 108 FFU/ml
BASIC PROTOCOL 4-PURIFICATION OF ROTAVIRUS FROM PLAQUES
Plaque purification is a technique used to prepare a clonal RV stock. The protocol below describes how to isolate a single plaque, which can then be amplified in a 25-cm2 tissue culture flask. To ensure purity, three sequential rounds of plaque purification are generally performed prior to large-scale propagation (see Basic Protocol 1).
Materials (also see Basic Protocol 3)
RV stock to be plaque purified
1 × serum-free Medium 199 (see recipe)
Pasteur pipettes with rubber bulbs
1.5 ml microcentrifuge tubes
NOTE: All equipment and solutions coming in contact with cells must be sterile and sterile technique should be used. Cell culture work should be conducted in a class II biological safety cabinet. All culture incubations should be performed in a humidified 37°C, 5% CO2 incubator.
Prepare a plaque assay as in Basic Protocol 3 such that several individual wells of each six-well plate contain 10 to 30 plaques.
Identify plaques that are completely separated from one another to ensure that only a single plaque is isolated. On the bottom of the plate, use a marker to draw a circle around the plaques to be picked.
Add 500 μl of serum-free Medium 199 to 1.5 ml microcentrifuge tubes so that each plaque to be picked has its own tube.
For each plaque, insert a Pasteur pipette into the agarose above the plaque, staying perpendicular to the agarose surface. Push the pipette tip through the agarose until it contacts the cell monolayer. Using the rubber bulb, gently expel a little air to disrupt the monolayer around the plaque. Slowly suck the infected cell material and the agarose plug up into the pipette. Try to avoid the agarose plug from moving too far up into the pipette.
Move the agarose plug to an individual 1.5 ml microcentrifuge tube containing 500 μl of serum-free Medium 199. Dislodge the agarose into the media by pipetting up and down.
Incubate the tubes containing the picked plaque/medium overnight at 4°C to allow the virus to elute from the agarose. The tubes may be rocked overnight at 4°C to facilitate virus elution. Transfer the tubes to −80°C.
- Freeze and thaw the tubes two times at −80°C. The supernatant can now be plaque purified again or used to infect MA104 cells in a 25-cm2 flask (see Basic Protocol 1).For sequential rounds of plaque purification, a plaque assay can be performed with medium from which the agarose plug was soaked. A dilution series of 10−1, 10−2, and 10−3 will allow for single plaque isolation again. This procedure is usually repeated for the third and final time.
STORAGE OF ROTAVIRUSES
RV stocks should be stored long-term (>1 year) at −70 to −80°C. RV does not require the presence of a cryoprotectant. Avoid repeated freezing and thawing to prevent loss of infectivity by storing the virus in small aliquots of 1 to 5 ml. Semi-purified or purified RV can also be stored in TNC buffer at 4°C for up to 1 year with minimal loss of titer.
SUPPORT PROTOCOL 1-GROWTH AND MAINTENANCE OF MA104 CELLS
MA104 cells are African green monkey kidney cells and are available from ATCC as MA104 clone 1. Early isolates of MA104 cells were found to be a mixture of African green monkey and Rhesus monkey cells. ATCC isolated a pure MA104 cell population in 2001. If obtaining cells from sources other than ATCC, the history of the cells should be taken into account. Though MA104s can be cultured beyond 80 passages, the authors do not recommend using them for RV growth and quantification beyond 50 passages.
Cell cultures should be maintained in an actively growing state (log phase or exponential growth) to ensure optimum health. Using an inverted phase contrast microscope, check the general appearance of your culture. It is also important to examine the culture with the unaided eye to look for signs of microbial contamination. Your standard protocol routinely used for subculturing cell cultures can generally be used to maintain MA104 cells. The amounts of reagents recommended in this procedure are for a 150-cm2 flask; volumes should be adjusted accordingly for other culture vessel sizes.
Materials
MA104 cells (ATTC #CRL-2378.1)
1 × complete Medium 199 (see recipe)
1 × Phosphate-buffered saline (PBS), pH 7.4
0.05% Trypsin-0.1% EDTA solution (or alternative disassociating reagent)
5-, 10-, and 25-ml pipettes
Tissue culture flasks
Inverted light microscope
Trypan blue solution (optional)
NOTE: All equipment and solutions coming in contact with cells must be sterile and sterile technique should be used. Cell culture work should be conducted in a class II biological safety cabinet. All culture incubations should be performed in a humidified 37°C, 5% CO2 incubator unless otherwise specified.
Using a sterile pipette, remove and discard the culture medium.
Wash the cell monolayer once using 10 ml of pre-warmed PBS to remove all traces of FBS.
Remove the PBS and wash the cell monolayer once using 5 ml of trypsin-EDTA.
- Remove the trypsin-EDTA wash. Add 1 ml of trypsin-EDTA and rock the flask by hand to ensure coverage of the cell monolayer. Incubate the flask at 37°C to detach the cells from the culture dish.Pre-warming the trypsin-EDTA solution will decrease the time needed to detach the cells.
Look at the cells every 2 to 3 minutes using an inverted light microscope. Once the cells have rounded up, gentle tapping of the flask should detach them from the plastic surface.
- Add 9 ml of pre-warmed, complete Medium 199 to the flask to inactivate the trypsin-EDTA solution. The detached cells are now resuspended in 10 ml total volume.Vigorous pipetting may be required to detach any remaining cells from the bottom of the culture vessel or to create a single cell suspension.
If desired, trypan blue staining can be performed to determine the viability and number of MA104 cells.
For a 1:5 dilution, transfer 2 ml of the cell suspension into a new 150-cm2 tissue culture flask. Add 28 ml of pre-warmed, complete Medium 199 to bring the volume up to 30 ml. Place the flask in the incubator with cap loosened. Cells will produce a confluent monolayer in 4 to 7 days.
REAGENTS AND SOLUTIONS
Agarose, 1.2%
Prepare 1.2% w/v cell culture grade agarose (SeaPlaque or SeaKem) with cell culture grade water. Autoclave to sterilize. Store at room temperature for up to 1 year.
Before use, melt pre-sterilized 1.2% agarose by microwaving, and allow agarose to cool in a 55°C water bath.
Cesium Chloride, Ultra-pure Optical Grade
Available commercially
Eagle’s Minimal Essential Medium (EMEM), 1×, incomplete
Contains Earle’s balanced salts, without L-glutamine.
EMEM, 1 ×, serum-free
Supplement 500 ml incomplete 1 × EMEM with:
5 ml 200 mM L-glutamine stock (2 mM L-glutamine)
5 ml penicillin/streptomycin stock (100 Units/ml penicillin, 100 ug/ml streptomycin)
0.5 ml 250 μg/ml Amphotericin B stock (0.25 μg/ml Amphotericin B)
Store at 4°C for up to 3 months.
EMEM, 1×, complete
Supplement 500 ml incomplete 1 × EMEM with:
5 ml 200 mM L-glutamine stock (2 mM L-glutamine)
5 ml penicillin/streptomycin stock (100 Units/ml penicillin, 100 ug/ml streptomycin)
0.5 ml 250 μg/ml Amphotericin B stock (0.25 μg/ml Amphotericin B)
57 ml fetal bovine serum (10% FBS)
Store at 4°C for up to 3 months.
EMEM, 2×, incomplete
Contains Earle’s balanced salts, without L-glutamine, without Phenol Red.
EMEM, 2×, serum-free
Supplement 500 ml incomplete 2 × EMEM with:
10 ml 200 mM L-glutamine stock (4 mM L-glutamine)
10 ml penicillin/streptomycin stock (200 Units/ml penicillin, 200 ug/ml streptomycin)
1 ml 250 μg/ml Amphotericin B stock (0.5 μg/ml Amphotericin B)
Store at 4°C for up to 3 months.
Medium 199, 1×, incomplete
Contains Earle’s salts, 2,200 mg/L sodium bicarbonate, L-glutamine, and 25 mM HEPES buffer.
Medium 199, 1×, serum-free
Supplement 500 ml incomplete Medium 199 with:
5 ml penicillin/streptomycin stock (100 Units/ml penicillin, 100 ug/ml streptomycin)
0.5 ml 250 μg/ml Amphotericin B stock (0.25 μg/ml Amphotericin B)
Store at 4°C for up to 3 months.
Medium 199, 1×, complete
Supplement 500 ml incomplete Medium 199 with:
5 ml penicillin/streptomycin stock (100 Units/ml penicillin, 100 ug/ml streptomycin)
0.5 ml 250 μg/ml Amphotericin B stock (0.25 μg/ml Amphotericin B)
27 ml fetal bovine serum (5% FBS)
Store at 4°C for up to 3 months.
Neutral Red Solution (0.33%)
Available commercially
Contains 3.3 g neutral red/L PBS, sterile filtered, cell culture grade
Store at 4°C for up to 1 year.
Penicillin/Streptomycin stock solution
Available commercially
10,000 Units/ml penicillin
10,000 ug/ml streptomycin
Store at −20°C for up to 1 year.
Sucrose 35% (w/v) in TNC buffer
Dissolve 35 g sucrose in 50 ml TNC buffer
Bring volume up to 100 ml using TNC buffer
Store at 4°C for up to 1 year.
TNC buffer (pH 8.0)
To make a 500 ml solution, add:
10 ml of 1M Tris-HCl, pH 8.0 (20 mM Tris-HCl)
10 ml of 5M NaCl (100 mM NaCl)
0.5 ml of 1M CaCl2 (1 mM CaCl2)
479.5 ml distilled, deionized water
Store at 4°C for up to 1 year.
Trypsin, porcine pancreatic type IX, Sigma Aldrich
Dilute 2 g trypsin in 100 ml PBS to make a stock solution of 2 mg/ml. Filter sterilize. Aliquot into small volumes (≤200 μl). Store at −20°C for up to 1 year. Avoid thawing and re-freezing aliquots more than twice.
COMMENTARY
Background Information
RVs are members of the Reoviridae family and major pathogens associated with acute gastroenteritis in humans and animals. The RV genome consists of eleven segments of double-stranded RNA (dsRNA) contained within a non-enveloped, triple-layered capsid (Pesavento et al., 2006). In general, each gene segment encodes a single polypeptide, allowing RVs to express five or six nonstructural proteins (NSP1-5 and sometimes NSP6) and six capsid proteins (VP1-4, VP6, and VP7). The inner layer of the virion is composed of the core lattice protein (VP2) and encases the viral RNA-dependent RNA polymerase (VP1), the RNA capping enzyme (VP3), and genomic RNA. The intermediate layer is made entirely of VP6, and the outermost layer of the RV particle is comprised of capsid proteins VP7 and VP4. The structural proteins within each shell can vary among strains, leading to antigenic differences that can be detected using immunological assays. As such, the reactivity pattern of antibodies against certain rotavirus capsid proteins is the primary method by which viruses in this family are classified (Desselberger et al., 2001; Estes, 2001; Kapikian, 2001). Specifically, the serogroups described for RVs (groups A to G) are based on the binding of non-neutralizing monoclonal antibodies to VP6 (Estes, 2001; Kapikian, 2001). Though group A, B, and C have been isolated from humans, group A RVs represent by far the most common cause of infantile gastroenteritis (Parashar et al., 2003).
Group A RVs are further classified into subgroups and serotypes. Subgroups are based on the immunoreactivity pattern of non-neutralizing monoclonal antibodies against VP6 of the same serogroup, while serotypes are based on the reactivity of outer capsid proteins (VP7; G-types and VP4; P-types) to neutralizing antibodies (Estes, 2001; Kalica et al., 1981; Kapikian et al., 1981). Along with the more recently described genotypes, serotypes remain the most common method of classifying group A RVs in epidemiological studies (Desselberger et al., 2001). To date, a total of 14 G-types (19 G-genotypes) and 14 P-types (27 P-genotypes) have been identified in nature (Matthijnssens et al., 2008). In humans, G1P1A[8], G2P1B[4], G3P1A[8], and G4P1A[8] strains are commonly associated with disease (Desselberger et al., 2001). Recently, a classification system has been developed to assign genotypes for each of the eleven RV genes based on nucleotide sequence (Matthijnssens et al., 2008). Group A RV infections cause severe diarrhea in infants and young children and are responsible for up to 600,000 deaths each year worldwide (Parashar et al., 2003). Although morbidity and mortality are usually associated with the very young, asymptomatic and symptomatic infections of adults can occur (Ramig, 2007). RVs are transmitted via the fecal-oral route and replicate in the mature enterocytes covering the tips of intestinal tract villi (Estes et al., 2001). The incubation period of the virus is 24 to 48 hours, and the onset of symptoms is abrupt and characterized by watery diarrhea, vomiting, and fever (Kapikian, 2001). While the mechanism of RV-induced gastroenteritis is not fully understood, CPE caused by viral replication is thought to play a role. Infected enterocytes are visibly damaged, and defective in digestive and absorptive functions, resulting in an osmotic imbalance and development of diarrhea (Estes et al., 2001). Additionally, several lines of evidence suggest that a RV-encoded nonstructural protein (NSP4) has endotoxin-like properties and may contribute to the development of disease (Ball et al., 2005). In developed countries, the acute gastroenteritis is often self-limiting and with proper rehydration therapy, infected individuals generally make a full recovery (Parashar et al., 2003). However, children in developing countries, who lack access to clean water and hospital care, often die from dehydration (Parashar et al., 2003). Emerging data suggests that RV infection is systemic, where both viral antigens and infectious virus enter into the bloodstream; yet, the contribution of such antigenemia and viremia to RV pathogenesis and spread is unknown (Ramig, 2007). Two live-attenuated vaccines, RotaTeq and Rotarix, are available for use in the United States as well as other countries. Both vaccines have been proven safe and effective in preventing RV disease. RotaTeq is a bovine-derived multivalent vaccine that contains the G1–G4 and P1A[8] components of human RVs. Rotarix is monovalent, attenuated human vaccine with G1P1A[8] specificity (Ward, 2008). Vaccination is considered the best strategy to decrease the disease burden associated with RV infections.
The discovery that RV infectivity is dependent upon proteolytic activation was a major breakthrough in the routine cultivation of these viruses (Estes, Graham, and Mason, 1981; Graham and Estes, 1980; Sato et al., 1981). Prior to this discovery, few animal strains had been propagated, and infection of cultured cells using a human RV (strain Wa) first required several passages in gnotobiotic piglets (Wyatt et al., 1980). Early studies utilized a mix of gastrointestinal enzymes to increase RV infectivity. Further studies demonstrated that the enzyme trypsin is solely responsible for cleaving the attachment protein VP4 into products VP8* and VP5*, promoting the entry of the virus into a host cell (Arias et al., 1996; Dormitzer et al., 2004; Estes, Graham, and Mason, 1981). The mechanism of RV entry is not fully understood and the search for cellular receptors is continuing. Because non-activated RV is capable of binding to cells, trypsin activation is probably required following the initial viral attachment step (Clark et al., 1981). Studies have shown that RV is capable of entering cells either by directly penetrating the plasma membrane, or by penetrating an endosome following receptor-mediated endocytosis (Jayaram et al., 2004). While the usage of these distinct entry pathways may be cell-type or strain dependent, both require the virus to be trypsin-activated. In addition to VP4 cleavage, efficient RV entry is also contingent upon the integrity of the outer capsid protein VP7, which is stabilized by calcium (Shirley et al., 1981). The concentration of calcium is low (10 to 20 nM) in the intracellular environment, leading to RV uncoating and initiation of the viral replication cycle (Jayaram et al., 2004). Treatment of RV with calcium-chelating agents, such as EDTA, will result in disruption of the VP7 layer and loss of infectivity. It is particularly important when cultivating RV using medium other than those described in this chapter that the calcium concentration is taken into account.
Critical Parameters and Troubleshooting
Most difficulties encountered when cultivating RVs result from problems with cell culture. To maintain the health and quality of MA104 cells, optimal growth conditions should be used. Cells should be subcultured when the monolayer is just confluent and diluted 1:5 each passage to ensure log-phase or exponential growth. The optimal monolayer is one in which the cells are just touching, but with no visible spaces in between. Over a number of passages, MA104 cells can lose their susceptibility to RV infection; the authors recommend starting a fresh culture around passage 50. Also, if a monolayer is subconfluent or overgrown, it is less supportive of infection. For primary AGMK cells, infections should be performed on a healthy cell monolayer that is not more than 4 days old.
Proper trypsin activation is of paramount importance when amplifying or quantifying RV. For best results, the authors recommend using porcine pancreatic trypsin type IX (Sigma Aldrich), which can be prepared as a 2 mg/ml stock solution in PBS and frozen in small aliquots. Trypsin is a proteolytically-active enzyme, and is sensitive to changes in temperature. To prevent inactivation, the enzyme should be thawed on ice and not subjected to multiple rounds of freeze-thaw. Because FBS will inhibit trypsin and may contain RV-neutralizing antibodies, problems with RV infection can arise when cells are not washed sufficiently to remove serum-containing growth medium. To facilitate the complete removal of FBS, MA104 cells can be incubated for several hours to overnight in one change of serum-free medium. Once activated by trypsin, RV infection of cells may proceed. Presence of trypsin in the viral inoculum at a concentration exceeding 2 μg/ml may cause the cell monolayer to slough off. The authors advise diluting virus activated with 10 μg/ml of trypsin to <2 μg/ml to prevent loss of the cell monolayer during infection.
The presence of trypsin in the culture medium at a concentration of 0.5 μg/ml allows for efficient virus propagation. However, to generate a completely non-activated RV stock, replace the inoculum with complete Medium 199 containing trypsin inhibitors (such as aprotinin). In this case, cells should be infected at an MOI ≥3. Due to the presence of trypsin inhibitors in the resulting clarified lysate, virus cannot be reactivated by incubation with trypsin. The non-activated RV stock must be semi-purified by pelleting (see Basic Protocol 2) or purified in a cesium chloride gradient (see Alternative Protocol 3) to remove trypsin inhibitors if reactivation is necessary.
When growing RV from clinical specimens, bacteria present in the feces are a significant source of contamination. Such bacterial contamination can be reduced by clarifying the specimen using centrifugation and by maintaining antibiotics in the culture throughout the course of infection. Additionally, the clarified fecal material can be passed through a 0.45-micron filter to remove most bacteria.
The plaque assay can be a difficult technique to master, with many critical steps that could potentially cause the assay to fail. Please refer to Table 2 for solutions to frequently encountered problems with the RV plaque assay. Some of the same problems may be encountered in the fluorescence focus assay, but most difficulties are due to the primary antibody used to detect RV antigen. The optimal antibody concentration must be determined in advance, and it must immuno-react with the strain of RV being tested.
Table 2.
Troubleshooting Guide for the RV Plaque Assay
| Problem | Possible Cause | Solution |
|---|---|---|
| No plaques | Cells dried out | Work quickly at each step when removing medium from wells |
| Unhealthy cells | Monolayers should be confluent but do not allow cells to overgrow | |
| Agarose overlay too hot, inactivating trypsin or killing cells | Equilibrate molten agarose to 55°C before mixing with 2 × EMEM | |
| Entire monolayer of cells lysed | Make more serial dilutions so less virus is present in the inoculum | |
| Virus too dilute | Make fewer serial dilutions so more virus is present in the inoculum | |
| Infection failed | Repeat assay in parallel with a positive control | |
| Plaques too small to be visible with neutral red staining | Stain plaque assays with crystal violet to enhance contrast between plaques and uninfected cells | |
| Clumpy agarose | 2 × EMEM too cold | Warm 2 × EMEM to 37°C before adding molten agarose |
| Agarose too cool | Equilibrate molten agarose to 55°C before mixing with 2× EMEM | |
| Contamination | Improperly sterilized reagents | Prepare all reagents fresh, ensuring solutions are autoclaved or filter sterilized; use sterile technique |
| Fungal growth | Increase Amphotericin B concentration to 2.5 μg/ml |
Anticipated Results
Animal RVs that are cell culture-adapted should be expected to grow to titers of approximately 107 to 108 PFU/ml. Cell culture-adapted human RVs grow to titers of approximately 104 to 106 PFU/ml. Human RVs from clinical fecal specimens do not grow efficiently in cell culture, and must be passaged in primary cells prior to growth in continuous cell lines. Once adapted to cell culture, clinical isolates usually grow as well as the prototypic cell culture-adapted human RV strains. It is of note that the RV titer obtained by plaque assay will likely differ from that obtained by the fluorescence focus assay. Usually, one method of quantification is chosen and used for all RVs of interest to maintain consistent results.
Time Considerations
For growth and maintenance of MA104 cells, solutions coming into contact with cells should be warmed to 37°C. This time will vary from 30 min to 2 h, depending on the size of the solution vessel. The time it takes to physically manipulate the cells from splitting is less than 30 min. MA104 cells must be plated 3 to 5 days in advance of the infection for RV propagation.
The time necessary for thawing the RV sample will vary, depending on the size of the frozen aliquot. The RV activation step requires 1 hour, followed by an adsorption time of 1 hour. For propagation at a low MOI, infected MA104 cultures can take 3 to 7 days before CPE is visible. Clinical specimens may need to be passaged in primary cells every 7 days up to 10 times (i.e., 70 days) before CPE is seen.
RV purification requires several days, mostly as a result of the centrifugation and dialysis steps. If using the sucrose protocol to semi-purify RV, the spin time is only 1.5 hours; yet, if banding virus in a CsCl gradient, >18 hours is required. The authors recommend dialyzing the purified RV against TNC buffer for 2 to 3 days, changing the buffer at least once a day. Thus, it may take up to a week to go from RV-infected cell lysate to purified RV particles amenable to biochemical or structural studies.
For quantifying RV by plaque assay, MA104 cells must be plated 3 to 5 days in advance of the infection. The RV activation step requires 1 hour, followed by preparing serial dilutions of the virus, which requires approximately 30 min. After the 1-hour adsorption time, the agarose overlay requires 30 min to solidify. Cell-culture adapted animal RVs form plaques 3 to 4 days post-infection, while human RVs may take as long as 7 days for plaques to form. The fluorescence focus assay takes less time than the plaque assay. The infection proceeds for only 12 to 18 hours and the antibody-binding steps can be completed in a single day. Thus, while a plaque assay takes approximately 4 days (from infection to titer determination), the fluorescence focus assay only takes 2 days.
Acknowledgments
The authors would like to thank Taka Hoshino, LaShanda Long-Croal, and Shane Trask of the Laboratory of Infectious Diseases, NIAID/NIH, for their helpful suggestions and critical reading of the chapter. The authors are supported by the Intramural Research Program of the NIAID/NIH.
Contributor Information
Michelle Arnold, Email: arnoldm@niaid.nih.gov.
John T. Patton, Email: jpatton@niaid.nih.gov.
Sarah M. McDonald, Email: mcdonaldsa@niaid.nih.gov.
LITERATURE CITED
- Arias CF, Romero P, Alvarez V, Lopez S. Trypsin activation pathway of rotavirus infectivity. Journal of virology. 1996;70(9):5832–9. doi: 10.1128/jvi.70.9.5832-5839.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball JM, Mitchell DM, Gibbons TF, Parr RD. Rotavirus NSP4: a multifunctional viral enterotoxin. Viral immunology. 2005;18(1):27–40. doi: 10.1089/vim.2005.18.27. [DOI] [PubMed] [Google Scholar]
- Ciarlet M, Crawford SE, Cheng E, Blutt SE, Rice DA, Bergelson JM, Estes MK. VLA-2 (alpha2beta1) integrin promotes rotavirus entry into cells but is not necessary for rotavirus attachment. Journal of virology. 2002;76(3):1109–23. doi: 10.1128/JVI.76.3.1109-1123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SM, Roth JR, Clark ML, Barnett BB, Spendlove RS. Trypsin enhancement of rotavirus infectivity: mechanism of enhancement. Journal of virology. 1981;39(3):816–22. doi: 10.1128/jvi.39.3.816-822.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desselberger U, Iturriza-Gomara M, Gray JJ. Rotavirus epidemiology and surveillance. Novartis Foundation symposium. 2001;238:125–152. doi: 10.1002/0470846534.ch9. [DOI] [PubMed] [Google Scholar]
- Dormitzer PR, Nason EB, Prasad BV, Harrison SC. Structural rearrangements in the membrane penetration protein of a non-enveloped virus. Nature. 2004;430(7003):1053–8. doi: 10.1038/nature02836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes MK. Rotaviruses and their replication. In: Knipe DM, Howley PM, editors. Fields virology. 4. Lippincott/The Williams & Wilkins Co; Philadelphia, PA: 2001. pp. 1747–1785. [Google Scholar]
- Estes MK, Graham DY, Gerba CP, Smith EM. Simian rotavirus SA11 replication in cell cultures. Journal of virology. 1979;31(3):810–5. doi: 10.1128/jvi.31.3.810-815.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes MK, Graham DY, Mason BB. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. Journal of virology. 1981;39(3):879–88. doi: 10.1128/jvi.39.3.879-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes MK, Kang G, Zeng CQ, Crawford SE, Ciarlet M. Pathogenesis of rotavirus gastroenteritis. Novartis Foundation symposium. 2001;238:82–96. doi: 10.1002/0470846534.ch6. discussion 96–100. [DOI] [PubMed] [Google Scholar]
- Graham DY, Estes MK. Proteolytic enhancement of rotavirus infectivity: biology mechanism. Virology. 1980;101(2):432–9. doi: 10.1016/0042-6822(80)90456-0. [DOI] [PubMed] [Google Scholar]
- Hasegawa A, Matsuno S, Inouye S, Kono R, Tsurukubo Y, Mukoyama A, Saito Y. Isolation of human rotaviruses in primary cultures of monkey kidney cells. Journal of clinical microbiology. 1982;16(2):387–90. doi: 10.1128/jcm.16.2.387-390.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram H, Estes MK, Prasad BV. Emerging themes in rotavirus cell entry, genome organization, transcription and replication. Virus research. 2004;101(1):67–81. doi: 10.1016/j.virusres.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Kalica AR, Greenberg HB, Wyatt RG, Flores J, Sereno MM, Kapikian AZ, Chanock RM. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology. 1981;112(2):385–90. doi: 10.1016/0042-6822(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Kapikian AZ, Cline WL, Greenberg HB, Wyatt RG, Kalica AR, Banks CE, James HD, Jr, Flores J, Chanock RM. Antigenic characterization of human and animal rotaviruses by immune adherence hemagglutination assay (IAHA): evidence for distinctness of IAHA and neutralization antigens. Infection and immunity. 1981;33(2):415–25. doi: 10.1128/iai.33.2.415-425.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian AZ, Hoshino Y, Chanock RM. Rotaviruses. In: Knipe DM, Howley PM, editors. Fields virology. 4. Lippincott/The Williams & Wilkins Co; Philadelphia, PA: 2001. pp. 1787–1833. [Google Scholar]
- Matthijnssens J, Ciarlet M, Heiman E, Arijs I, Delbeke T, McDonald SM, Palombo EA, Iturriza-Gomara M, Maes P, Patton JT, Rahman M, Van Ranst M. Full genome-based classification of rotaviruses reveals a common origin between human Wa-Like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. Journal of virology. 2008;82(7):3204–19. doi: 10.1128/JVI.02257-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerging infectious diseases. 2003;9(5):565–72. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesavento JB, Crawford SE, Estes MK, Prasad BV. Rotavirus proteins: structure and assembly. Current topics in microbiology and immunology. 2006;309:189–219. doi: 10.1007/3-540-30773-7_7. [DOI] [PubMed] [Google Scholar]
- Ramig RF. Systemic rotavirus infection. Expert review of anti-infective therapy. 2007;5(4):591–612. doi: 10.1586/14787210.5.4.591. [DOI] [PubMed] [Google Scholar]
- Sato K, Inaba Y, Shinozaki T, Fujii R, Matumoto M. Isolation of human rotavirus in cell cultures: brief report. Archives of virology. 1981;69(2):155–60. doi: 10.1007/BF01315159. [DOI] [PubMed] [Google Scholar]
- Shirley JA, Beards GM, Thouless ME, Flewett TH. The influence of divalent cations on the stability of human rotavirus. Archives of virology. 1981;67(1):1–9. doi: 10.1007/BF01314596. [DOI] [PubMed] [Google Scholar]
- Ward RL. Rotavirus vaccines: how they work or don’t work. Expert reviews in molecular medicine. 2008;10:e5. doi: 10.1017/S1462399408000574. [DOI] [PubMed] [Google Scholar]
- Ward RL, Clemens JD, Sack DA, Knowlton DR, McNeal MM, Huda N, Ahmed F, Rao M, Schiff GM. Culture adaptation and characterization of group A rotaviruses causing diarrheal illnesses in Bangladesh from 1985 to 1986. Journal of clinical microbiology. 1991;29(9):1915–23. doi: 10.1128/jcm.29.9.1915-1923.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RL, Knowlton DR, Pierce MJ. Efficiency of human rotavirus propagation in cell culture. Journal of clinical microbiology. 1984;19(6):748–53. doi: 10.1128/jcm.19.6.748-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt RG, James HD, Jr, Pittman AL, Hoshino Y, Greenberg HB, Kalica AR, Flores J, Kapikian AZ. Direct isolation in cell culture of human rotaviruses and their characterization into four serotypes. Journal of clinical microbiology. 1983;18(2):310–7. doi: 10.1128/jcm.18.2.310-317.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt RG, James WD, Bohl EH, Theil KW, Saif LJ, Kalica AR, Greenberg HB, Kapikian AZ, Chanock RM. Human rotavirus type 2: cultivation in vitro. Science (New York, NY) 1980;207(4427):189–91. doi: 10.1126/science.6243190. [DOI] [PubMed] [Google Scholar]
KEY REFERENCES
- Gray J, Desselberger U. Rotaviruses: Methods and Protocols. Humana Press; Totowa, New Jersey: 2000. This book describes several useful methods and protocols for studying rotavirus biology. [Google Scholar]