Abstract
The non-heme iron enzyme cysteine dioxygenase (CDO) catalyzes the S-oxygenation of cysteine by O2 to cysteine sulfinic acid. The synthesis of a new structural and functional model of the cysteine-bound CDO active site, [FeII(N3PyS)(CH3CN)]BF4 (1) is reported. This complex is prepared with a new facially chelating 4N/1S(thiolate) pentadentate ligand. Reaction of 1 with O2 results in oxygenation of the thiolate donor to afford the doubly-oxygenated sulfinate product [FeII(N3PySO2)(NCS)] (2), which was crystallographically characterized. The thiolate donor provided by the new N3PyS ligand has a dramatic influence on the redox potential and O2 reactivity of this iron(II) model complex.
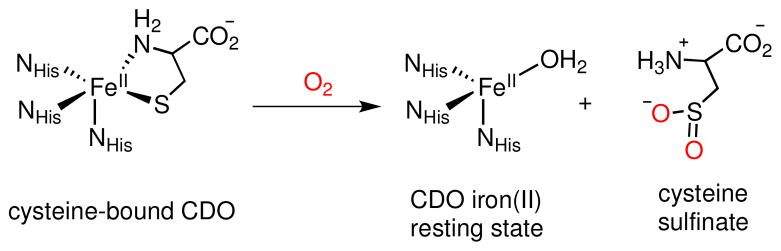
Metalloenzymes containing mononuclear non-heme iron centers are implicated in a variety of biological roles, including the activation of O2 for substrate oxidation. These non-heme iron oxygenases form a class of enzymes that utilize dioxygen for catalytic oxidations and are typically comprised of a 2-His-1-carboxylate donor set bound to the iron center. Cysteine dioxygenase (CDO), on the other hand, differs from the norm in that its active site consists of a mononuclear high-spin FeII center with three His residues bound in a “facial triad” (Scheme 1).1 Upon substrate binding,2 CDO utilizes O2 to catalyze the S-oxygenation of cysteine to cysteine sulfinic acid (Cys-SO2H), which is essential for the biosynthesis of pyruvate and taurine. This enzyme is also vital for the maintenance of healthy levels of cysteine in the body. While information on the mechanism of O2 activation in CDO is just emerging,3a,b several key X-ray crystal structures of CDO have been determined, including a substrate-bound form where the Cys is coordinated bidentate through the S and NH2 donors.1e,3c
Scheme 1.
Sulfur Oxygenation of Cysteine by CDO with O2
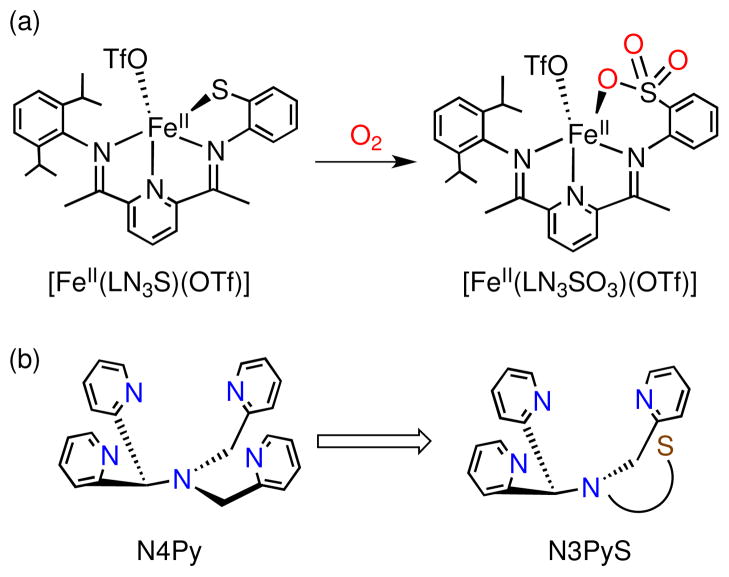
Mononuclear non-heme iron model complexes have proven beneficial for addressing questions regarding enzyme mechanism, but the use of biologically relevant O2 as oxidant with these models remains quite rare.4 Previously, we described the syntheses of two N3S(thiolate)FeII model complexes of CDO, which react with O2 to yield sulfonato products.5 In one case, the thiolate donor was covalently tethered to the N3 platform (Figure 1a), whereas in the other case an exogenous arylthiolate ligand was employed. In each of these models, a high-spin FeII center was ligated by three planar N donors from a bis(imino)pyridine (BIP) framework in a distorted square pyramidal geometry with a triflate anion occupying the axial position. One finding to result from these studies was that there appears to be a requirement for the S donor to be positioned cis to the potential binding site for O2 to obtain S-oxygenation. These analogs contain only three N donors bound to the iron(II) center, unlike the four N donors ligated in the Cys-bound form of the enzyme. 1e The three nitrogen ligands are also constricted to one plane by the BIP framework, leading to a meridional configuration, unlike the facial arrangement of the 3 His donors found in CDO. Furthermore, while there was some indirect evidence for S-oxygenation giving sulfinato intermediates in these model systems, the final, isolated S-oxygenated iron complexes were found to be triply-oxygenated sulfonato products, reaching a level of oxygenation beyond that observed in the biochemical reaction.
Figure 1.
(a) S-oxygenation reaction of a previous CDO model with a covalently tethered phenylthiolate donor and (b) N3PyS ligand design.
To improve our model design, we set out to prepare a pentadentate N4S ligand that would be constrained to provide three neutral N donors in a facial arrangement, as well as a fourth N donor to mimic the amino coordination from Cys, and a single S donor held cis to the putative O2 binding site at the metal center. The N4Py ligand established previously for non-heme iron models appeared to be an ideal scaffold for building the desired N4S system (Figure 1b).6 The pentadentate N4Py ligand provides a rigid structure that binds iron(II) in a square-pyramidal fashion, and has allowed for the characterization and isolation of several key iron-oxygen (e.g. Fe=O, Fe-OO(H)) intermediates. These beneficial properties have provided motivation for the synthesis of derivatives of the N4Py ligand by several groups, but thus far none of these efforts have led to the incorporation of a thiolato donor.7 Herein we report the synthesis of a new pentadentate ligand, N3PySH, and its corresponding iron(II) complex [FeII(N3PyS)(CH3CN)]BF4 (1) (Scheme 2). It is shown that 1 reacts with O2 via sulfur oxygenation to generate the doubly-oxygenated sulfinato complex, [FeII(N3PySO2)]+ (Scheme 3). Thus 1 is a close structural and functional model of CDO, and provides a rare example of an iron(II)-thiolate complex reacting with O2 to give a sulfinato complex. This product was characterized by X-ray crystallography, and is, to our knowledge, the first example of a structurally characterized mononuclear FeII-sulfinate complex.
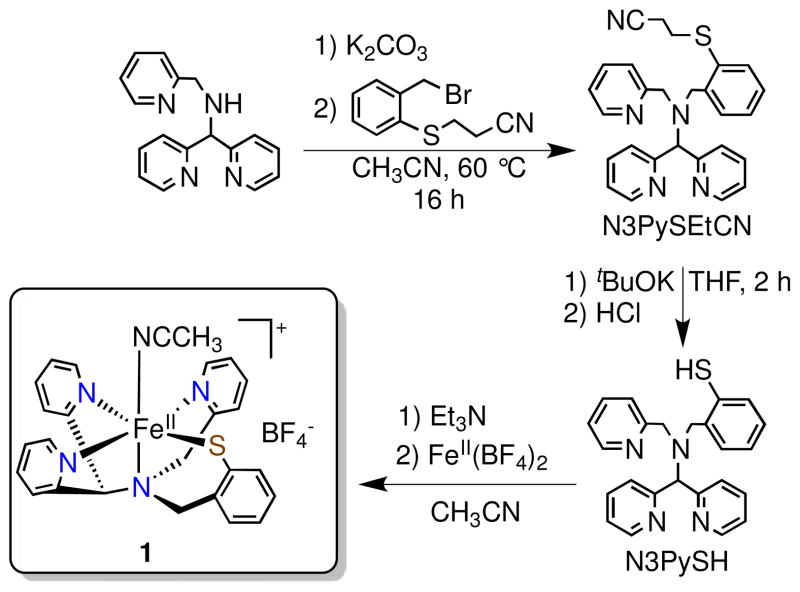
Scheme 2.
Synthesis of N3PySH and [FeII(N3PyS)(CH3CN)]BF4 (1)
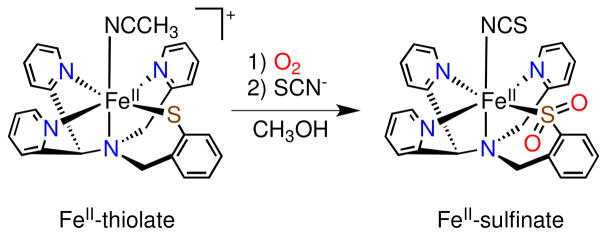
Scheme 3.
O2 Reactivity of [FeII(N3PyS)(CH3CN)]+ in CH3OH
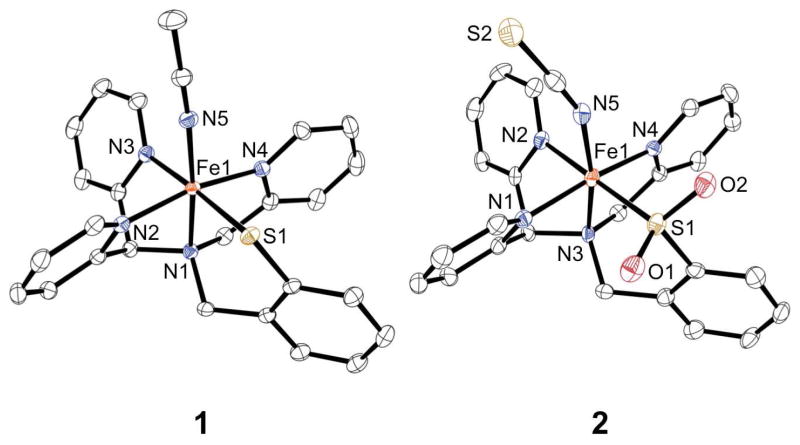
Reaction of the protected phenylthiolate 3-(2-bromomethylphenylsulfanyl)propionitrile (BrCH2PhSEtCN)8 with N-[di(2-pyridinyl)methyl]-N-(2-pyridinylmethyl)amine7c in the presence of K2CO3 at 60 °C in acetonitrile affords the protected pentadentate ligand N3PySEtCN (Scheme 2). Addition of tBuOK in THF yields after workup the deprotected ligand, N3PySH, in moderate yield (43%). Addition of FeII(BF4)2 to N3PySH in CH3CN in the presence of 1 equiv Et3N affords the dark red FeII complex [FeII(N3PyS)(CH3CN)]BF4 (1). X-ray quality crystals were grown from vapor diffusion of diethyl ether into an acetonitrile solution of 1. The X-ray structure of 1 reveals the pentadentate ligand bound in a pseudo-square pyramidal geometry about the FeII center as designed, with the phenylthiolate donor held cis to the sixth site occupied by a labile acetonitrile ligand (Figure 2). The four neutral nitrogen and one thiolate donor motif corresponds closely to the cysteine-bound active site of CDO.
Figure 2.
Displacement ellipsoid plots (50% probability level) of the cation of 1 and complex 2. The H atoms are omitted for clarity.
The Fe-N bond distances (average Fe-Npy = 1.9594(13) Å) in 1 are consistent with low-spin iron(II) (ls-FeII) complexes utilizing pyridyl ligands.6a,9 Likewise, the Fe-S distance is consistent with other FeII-thiolate complexes (Fe-S = 2.3018(4) Å).5a,b,10 The diamagnetic 1H NMR spectrum in CD3CN is characteristic of a low-spin iron(II) ion. When dissolved in acetonitrile, intense UV-vis features at 325, 418, and 493 nm (ε = 4475 M−1 cm−1, 4550 M−1 cm−1, and 4820 M−1 cm−1, respectively) are observed (Figure S1a). However, upon dissolution in methanol, 1 converts to a high-spin FeII complex, as indicated by a solution state magnetic moment (μeff = 4.5 μB, Evan’s method) and less intense UV-vis bands (Figure S1b). It is concluded that CH3OH, a relatively weak-field ligand, likely displaces CH3CN in the labile site of 1, providing a convenient switch to access the biologically relevant hs-iron(II) state that mimics resting CDO.
Addition of excess O2 to a methanolic solution of high-spin 1 results in an immediate color change from the dark brown of 1 (470 nm, 1000 M−1 cm−1) to a new deep green (674 nm, 1300 M−1 cm−1, Figure S3). Efforts to obtain X-ray quality crystals of this green species for characterization have thus far been unsuccessful. However, preliminary experiments suggest this species may be an S-oxygenated intermediate (vide infra). To isolate a crystalline product, SCN− was added as a potentially good ligand for the labile site on the iron product. Immediately upon addition of one equivalent of KSCN, the green solution turns brown. Vapor diffusion of diisopropyl ether results in the isolation of orange-red lath-like crystals of 2 in 6 days. Structural determination of 2 by X-ray crystallography (Figure 2) revealed a low-spin iron(II) complex wherein the chelated S atom has been doubly oxygenated to give the sulfinate product, as well as a thiocyanate anion bound in place of the acetonitrile ligand of 1.
The average Fe-N distance is consistent with other ls-FeII complexes and is only slightly larger than that of complex 1 (average Fe-Npy for 2 = 1.971(3) Å). The Fe-S bond distance decreases upon S-oxygenation (Fe-S for 2 = 2.1812(9) Å), which is consistent with the decrease in other metal-sulfur (M-S) bond lengths observed upon S-oxygenation. 11a–e The reduction in M-S bond length going from the thiolate to the sulfinate donor corresponds to both a contraction in the size of the sulfur atom in the sulfinate due to the increase in formal oxidation state, and an elimination of the repulsive interaction between the metal’s filled d-orbitals and the sulfur lone pairs on the thiolate donor. The S-O distances (S1-O1 = 1.488(2) Å, S1-O2 = 1.476(2) Å) are consistent with other sulfinate bond lengths.11 Spectroscopic characterization of crystalline 2 shows two intense UV-vis bands at 380 and 451 nm (ε = 4600 M −1 cm−1 and 4200 M −1 cm−1, respectively, Figure S2) in CH3CN, similar to the spectrum for the ls-FeII starting material 1 in CH3CN. The attenuated total reflectance infrared (ATR-IR) spectrum of neat 2 reveals two prominent peaks at 1129 and 1012 cm−1. These peaks fall in the observed range for metal-sulfinate complexes,11 and therefore we assign these bands to the asymmetric and symmetric S-O stretching modes. Thus addition of O2 to the CDO model 1 leads to the isolation and characterization of an FeII-sulfinate complex, wherein the thiolate donor has been doubly oxygenated in a manner parallel with the enzymatic chemistry. In addition, although there are a few examples of structurally characterized FeIII-sulfinates,11f,12 to our knowledge 2 is the first example of a crystallographically characterized mononuclear FeII-sulfinate complex.
The influence of the thiolate donor on non-heme iron active sites is of fundamental interest, and the N3PySH ligand provides us with an ideal system to address this issue by comparison with the all-nitrogen N4Py analog. Complex 1 and [FeII(N4Py)]2+ show a dramatic difference in O2 reactivity. Complex 1 reacts with O2 without the use of added reductants, whereas [FeII(N4Py)]2+ reacts with O2 only in the presence of BNAH (BNAH = 1-benzyl-1,4-dihydronicotinamide) to generate [(N4Py)FeIII(OOH)]2+.13 As we have reported, a prerequisite for O2 reactivity is E1/2(FeIII/II) < −0.1 V vs Fc+/Fc.5b Cyclic voltammetry of 1 in CH3CN showed a single quasi-reversible wave at −0.226 V vs Fc+/Fc (Figure S4a), which we assign to the FeIII/II couple. This potential is shifted significantly more negative compared to the same couple for the non-thiolate analog [FeII(N4Py)]2+ (E1/2 = +0.61 V vs Fc+/Fc in CH3CN).14 These results show that replacement of a pyridyl donor with a single thiolate ligand causes a dramatic cathodic shift of over 800 mV compared to N4Py. The redox potential of 1 was also measured in CH3OH, revealing two quasireversible waves at E1/2 = −233 mV and −583 mV vs Fc+/Fc. The wave at E1/2 = −233 mV is assigned to some CH3CNbound 1 that remains in equilibrium with the methanol complex. The FeIII/II couple of the high-spin CH3OHbound 1 is assigned to the more negative potential, which clearly falls well within the previously noted range for O2 reactivity. As expected, the redox potential for 2 (−0.001 V, Figure S4b) is shifted more positive than 1 by 225 mV, consistent with less electron donation from the S-bound sulfinate group. However, the E1/2 for S-bound 2 remains significantly more negative than that of [FeII(N4Py)]2+.
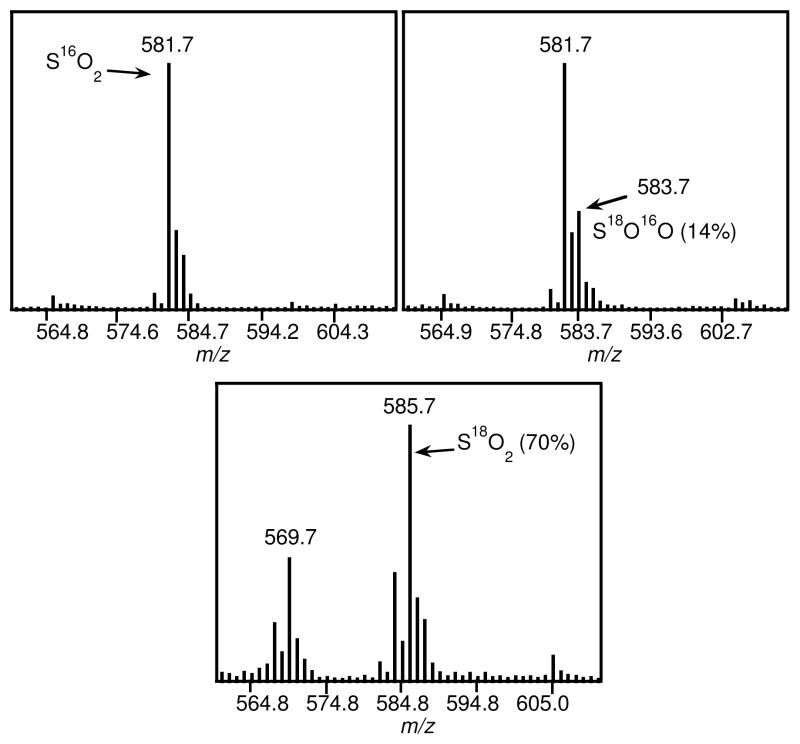
Isotope labeling studies with 18O2 were conducted to gain insight into the mechanism of the reaction of 1 with O2. Analysis of reaction mixtures with natural abundance O2 by electrospray ionization mass spectrometry (ESI-MS) revealed a prominent isotopic cluster centered at m/z 581.7 which can be assigned to [(K)(FeII(N3PyS16O2)(NCS))]+ ([M+K]+, Figure 3). Upon running the reaction with 18O2 (98% isotope-enriched), a shift in the isotopic pattern is observed. Simulations of this pattern indicate that 14% of the sulfinate-iron(II) complex contains one labeled oxygen atom (S16O18O), while approximately 2% of the product is labeled in both oxygen positions (S18O2). These results suggest that O2 is a source of O atoms for the S-oxygenation, but there is likely significant exchange with exogenous H2O during the course of the reaction. To test this idea, the reaction was carried out with the addition of H2 18O (50 μL) followed by ESI-MS analysis, which revealed 70% S18O2 and 28% S16O18O. This experiment suggests that the O atoms in the sulfinate complex are susceptible to exchange during S-oxygenation. However, independent experiments with pure 2 and H2 18O revealed that the O atoms of the lowspin FeII-sulfinate were not exchangeable with H2 18O under the same conditions (data not shown). Thus the isotope-labeling results implicate one or more intermediates in the S-oxygenation pathway that undergo facile O exchange with H2O. One such intermediate could include a mono-oxygenated sulfenato-iron species. Sulfenato-metal (RS(O)-M) complexes are rare, but have been shown to undergo facile O-atom exchange with H2O.15
Figure 3.
ESI-MS spectra after the reaction of 1 with O2 (top left), 18O2 (top right), and O2/H2 18O (bottom).
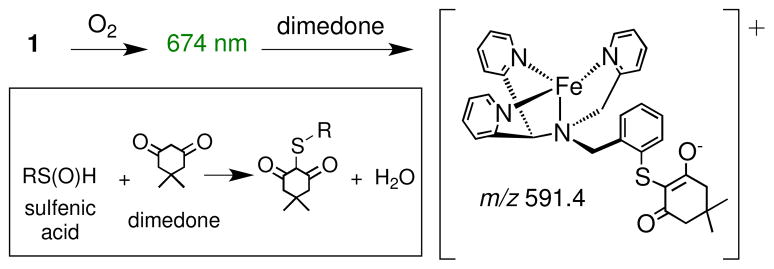
Given these results, we speculated that the green intermediate (674 nm) may contain a singly-oxygenated sulfenate donor. In previous studies, it has been shown that dimedone reacts selectively with sulfenic acids, but not thiols or sulfinic acids.16 Addition of dimedone (50 equiv) caused the complete decay of the band at 674 nm over 2 h. Mass spectral analysis revealed a major peak at m/z 591.4, corresponding to [Fe-N3PyS-dimedone – H]+, the expected product from reaction of dimedone with a sulfenic acid group (Scheme 4). Metal-sulfenates are also known to react with PR3.17 Anaerobic addition of PPh3 (2 equiv) to the green intermediate resulted in a color change to brown and the production of OPPh3 (50%, 31P NMR, based on total Fe). Addition of H2 18O to the green intermediate followed by reaction with PPh3 led to 49% 18O-labeled OPPh3. In contrast, the sulfinato product 2 does not react with either dimedone or PPh3 under the same conditions. These data are consistent with the presence of a sulfenato-iron intermediate that contains an exchangeable O atom. However, these experiments do not rule out other intermediates being present at this stage, including doubly-oxygenated sulfinato species.
Scheme 4.
Reaction of Dimedone with the Green Species
In summary, we report the synthesis of a novel N4S(thiolate) ligand, which functions as designed to give [FeII(N3PyS)]+, a structural analog of substrate-bound CDO. A single thiolate donor is incorporated into the coordination sphere to mimic the Cys sulfur ligation in the enzyme. This substitution is critical for the reactivity of the iron(II) complex with dioxygen, in contrast to the all-nitrogen N4Py analog, which is completely inert to O2 in the absence of co-reductants. The findings presented here, in comparison with our previous CDO model complexes, suggest that a facial arrangement of the N donors about the iron(II) center biases the O2 reactivity toward production of the biologically relevant sulfinato product. Although more work needs to be done to understand the mechanism of S-oxygenation for 1, the preliminary data suggest that the O2 addition proceeds through monooxygenase-like steps, as indicated by calculations for the CDO mechanism.3b
Supplementary Material
Acknowledgments
The NIH (GM62309) is gratefully acknowledged for financial support and Y.J. is grateful for the Ernest M. Marks Fellowship.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information. Experimental details, spectra, electrochemical data, and X-ray crystallographic files (CIF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Joseph CA, Maroney MJ. Chem Commun. 2007:3338–3349. doi: 10.1039/b702158e. [DOI] [PubMed] [Google Scholar]; (b) McCoy JG, Bailey LJ, Bitto E, Bingman CA, Aceti DJ, Fox BG, Phillips GN., Jr Proc Natl Acad Sci USA. 2006;103:3084–3089. doi: 10.1073/pnas.0509262103. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Simmons CR, Liu Q, Huang QQ, Hao Q, Begley TP, Karplus PA, Stipanuk MH. J Biol Chem. 2006;281:18723–18733. doi: 10.1074/jbc.M601555200. [DOI] [PubMed] [Google Scholar]; (d) Stipanuk MH, Ueki I, Dominy JE, Jr, Simmons CR, Hirschberger LL. Amino Acids. 2009;37:55–63. doi: 10.1007/s00726-008-0202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Ye S, Wu X, Wei L, Tang DM, Sun P, Bartlam M, Rao ZH. J Biol Chem. 2007;282:3391–3402. doi: 10.1074/jbc.M609337200. [DOI] [PubMed] [Google Scholar]
- 2.(a) Gardner JD, Pierce BS, Fox BG, Brunold TC. Biochemistry. 2010;49:6033–6041. doi: 10.1021/bi100189h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tchesnokov EP, Wilbanks SM, Jameson GNL. Biochemistry. 2012;51:257–264. doi: 10.1021/bi201597w. [DOI] [PubMed] [Google Scholar]
- 3.(a) Crawford JA, Li W, Pierce BS. Biochemistry. 2011;50:10241–10253. doi: 10.1021/bi2011724. [DOI] [PubMed] [Google Scholar]; (b) Kumar D, Thiel W, de Visser SP. J Am Chem Soc. 2011;133:3869–3882. doi: 10.1021/ja107514f. [DOI] [PubMed] [Google Scholar]; (c) Simmons CR, Krishnamoorthy K, Granett SL, Schuller DJ, Dominy JE, Jr, Begley TP, Stipanuk MH, Karplus PA. Biochemistry. 2008;47:11390–11392. doi: 10.1021/bi801546n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SO, Sastri CV, Seo MS, Kim J, Nam W. J Am Chem Soc. 2005;127:4178–4179. doi: 10.1021/ja043083i.Lee YM, Hong S, Morimoto Y, Shin W, Fukuzumi S, Nam W. J Am Chem Soc. 2010;132:10668–10670. doi: 10.1021/ja103903c.MacBeth CE, Golombek AP, Young VG, Jr, Yang C, Kuczera K, Hendrich MP, Borovik AS. Science. 2000;289:938–941. doi: 10.1126/science.289.5481.938.Thibon A, England J, Martinho M, Young VG, Jr, Frisch JR, Guillot R, Girerd JJ, Münck E, Que L, Jr, Banse F. Angew Chem, Int Ed. 2008;47:7064–7067. doi: 10.1002/anie.200801832.A CDO model study in which O2 was utilized was recently described. See Sallmann M, Siewert I, Fohlmeister L, Limberg C, Knispel C. Angew Chem, Int Ed. 2012;51:2234–2237. doi: 10.1002/anie.201107345.
- 5.(a) Jiang YB, Widger LR, Kasper GD, Siegler MA, Goldberg DP. J Am Chem Soc. 2010;132:12214–12215. doi: 10.1021/ja105591q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Badiei YM, Siegler MA, Goldberg DP. J Am Chem Soc. 2011;133:1274–1277. doi: 10.1021/ja109923a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kumar D, Sastry GN, Goldberg DP, de Visser SP. J Phys Chem A. 2012;116:582–591. doi: 10.1021/jp208230g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Lubben M, Meetsma A, Wilkinson EC, Feringa B, Que L., Jr Angew Chem, Int Ed. 1995;34:1512–1514. [Google Scholar]; (b) Ho RYN, Roelfes G, Hermant R, Hage R, Feringa BL, Que L., Jr Chem Commun. 1999:2161–2162. [Google Scholar]; (c) Klinker EJ, Kaizer J, Brennessel WW, Woodrum NL, Cramer CJ, Que L., Jr Angew Chem, Int Ed. 2005;44:3690–3694. doi: 10.1002/anie.200500485. [DOI] [PubMed] [Google Scholar]
- 7.(a) Roelfes G, Vrajmasu V, Chen K, Ho RYN, Rohde JU, Zondervan C, la Crois RM, Schudde EP, Lutz M, Spek AL, Hage R, Feringa BL, Münck E, Que L., Jr Inorg Chem. 2003;42:2639–2653. doi: 10.1021/ic034065p. [DOI] [PubMed] [Google Scholar]; (b) Ligtenbarg AGJ, Oosting P, Roelfes G, La Crois RM, Lutz M, Spek AL, Hage R, Feringa BL. Chem Commun. 2001:385–386. [Google Scholar]; (c) Roelfes G, Branum ME, Wang L, Que L, Jr, Feringa BL. J Am Chem Soc. 2000;122:11517–11518. [Google Scholar]; (d) Sen Soo H, Komor AC, Iavarone AT, Chang CJ. Inorg Chem. 2009;48:10024–10035. doi: 10.1021/ic9006668. [DOI] [PubMed] [Google Scholar]; (e) van den Heuvel M, van den Berg TA, Kellogg RM, Choma CT, Feringa BL. J Org Chem. 2004;69:250–262. doi: 10.1021/jo035157z. [DOI] [PubMed] [Google Scholar]
- 8.Thapper A, Behrens A, Fryxelius J, Johansson MH, Prestopino F, Czaun M, Rehder D, Nordlander E. Dalton Trans. 2005:3566–3571. doi: 10.1039/b505180k. [DOI] [PubMed] [Google Scholar]
- 9.Zang Y, Kim J, Dong YH, Wilkinson EC, Appelman EH, Que L., Jr J Am Chem Soc. 1997;119:4197–4205. [Google Scholar]
- 10.(a) Namuswe F, Kasper GD, Sarjeant AAN, Hayashi T, Krest CM, Green MT, Moënne-Loccoz P, Goldberg DP. J Am Chem Soc. 2008;130:14189–14200. doi: 10.1021/ja8031828. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kitagawa T, Dey A, Lugo-Mas P, Benedict JB, Kaminsky W, Solomon E, Kovacs JA. J Am Chem Soc. 2006;128:14448–14449. doi: 10.1021/ja064870d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Buonomo RM, Font I, Maguire MJ, Reibenspies JH, Tuntulani T, Darensbourg MY. J Am Chem Soc. 1995;117:963–973. [Google Scholar]; (b) Mullins CS, Grapperhaus CA, Frye BC, Wood LH, Hay AJ, Buchanan RM, Mashuta MS. Inorg Chem. 2009;48:9974–9976. doi: 10.1021/ic901246w. [DOI] [PubMed] [Google Scholar]; (c) Grapperhaus CA, Darensbourg MY. Acc Chem Res. 1998;31:451–459. [Google Scholar]; (d) Masitas CA, Kumar M, Mashuta MS, Kozlowski PM, Grapperhaus CA. Inorg Chem. 2010;49:10875–10881. doi: 10.1021/ic101221z. [DOI] [PubMed] [Google Scholar]; (e) Masitas CA, Mashuta MS, Grapperhaus CA. Inorg Chem. 2010;49:5344–5346. doi: 10.1021/ic100414c. [DOI] [PubMed] [Google Scholar]; (f) Galardon E, Giorgi M, Artaud I. Chem Commun. 2004:286–287. doi: 10.1039/b312318a. [DOI] [PubMed] [Google Scholar]; (g) Lee CM, Hsieh CH, Dutta A, Lee GH, Liaw WF. J Am Chem Soc. 2003;125:11492–11493. doi: 10.1021/ja035292t. [DOI] [PubMed] [Google Scholar]
- 12.(a) Heinrich L, Li Y, Vaissermann J, Chottard G, Chottard JC. Angew Chem, Int Ed. 1999;38:3526–3528. doi: 10.1002/(sici)1521-3773(19991203)38:23<3526::aid-anie3526>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]; (b) Lugo-Mas P, Taylor W, Schweitzer D, Theisen RM, Xu L, Shearer J, Swartz RD, Gleaves MC, DiPasquale A, Kaminsky W, Kovacs JA. Inorg Chem. 2008;47:11228–11236. doi: 10.1021/ic801704n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Noveron JC, Olmstead MM, Mascharak PK. J Am Chem Soc. 2001;123:3247–3259. doi: 10.1021/ja001253v. [DOI] [PubMed] [Google Scholar]; (d) Tyler LA, Noveron JC, Olmstead MM, Mascharak PK. Inorg Chem. 1999;38:616–617. doi: 10.1021/ic990794m. [DOI] [PubMed] [Google Scholar]
- 13.Hong S, Lee YM, Shin W, Fukuzumi S, Nam W. J Am Chem Soc. 2009;131:13910–13911. doi: 10.1021/ja905691f. [DOI] [PubMed] [Google Scholar]
- 14.Collins MJ, Ray K, Que L., Jr Inorg Chem. 2006;45:8009–8011. doi: 10.1021/ic061263i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinrich L, Mary-Verla A, Li Y, Vaissermann J, Chottard JC. Eur J Inorg Chem. 2001:2203–2206. [Google Scholar]
- 16.(a) Heinecke J, Ford PC. J Am Chem Soc. 2010;132:9240–9243. doi: 10.1021/ja102221e. [DOI] [PubMed] [Google Scholar]; (b) Seo YH, Carroll KS. Angew Chem, Int Ed. 2011;50:1342–1345. doi: 10.1002/anie.201007175. [DOI] [PubMed] [Google Scholar]
- 17.(a) Farmer PJ, Verpeaux JN, Amatore C, Darensbourg MY, Musie G. J Am Chem Soc. 1994;116:9355–9356. [Google Scholar]; (b) Lugo-Mas P, Dey A, Xu L, Davin SD, Benedict J, Kaminsky W, Hodgson KO, Hedman B, Solomon EI, Kovacs JA. J Am Chem Soc. 2006;128:11211–11221. doi: 10.1021/ja062706k. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.