Abstract
One approach to the study of disordered spatial attention is to carry out tests of extinction, in which stimuli are detected on the left when they are presented on the left alone, but not when both sides are stimulated simultaneously in a dual simultaneous stimulation (DSS) protocol. Extinction has been documented for multiple sensory modalities, but not for thermal pain stimuli, to our knowledge. We now test the hypothesis that subjects with visual spatial neglect (hemi-neglect) will have alterations in thermal pain sensation which are related to abnormal spatial attention. The results demonstrate that thermal pain extinction of hot and cold pain stimuli occurs in a proportion of subjects with hemi-neglect. In the subjects with visual spatial hemi-neglect but without thermal pain extinction, the sensation of the thermal pain stimulus on the affected (left) side was not extinguished but was often localized to the unaffected (right) side, and the submodality of the stimulus (cold or hot) was often misidentified. Ratios indicating the magnitude of extinction, mislocalization and misidentification were significantly larger on the left side of subjects with visual spatial neglect than in healthy controls or in controls with stroke but without hemineglect. The proportion of subjects with thermal pain extinction, mislocalization, or misidentification was significantly higher in subjects with hemi-neglect than those in either control group. These results demonstrate that disordered attention exerts a powerful effect upon the perception of both the location and the quality of thermal pain stimuli.
Keywords: Neglect, Attention, Human, Thermal pain, Mislocalization, Misidentification
1. Introduction
Attention can exert a powerful effect on the perception of thermal pain sensations, so that distraction can have an analgesic effect on acute pain equal to that of opiates [55]. The basis of analgesia by distraction may be related to attentional modulation of laser evoked potentials (LEP) which can be decreased in amplitude when the subject is distracted [4,30,36,40,42,57,60,68–71]. Subdural recordings have demonstrated that LEPs and increased activation (Event Related Desynchronization) recorded directly from primary somatic sensory cortex, parasylvian cortex, and medial frontal cortex can be powerfully modulated by attention [46,47].
Spatial attention can modulate thermal pain modalities, as demonstrated by studies in which a stimulus is presented following a cue which may indicate either the correct or the incorrect side of the stimulus. The incorrect cue leads to increased error rates and response latencies for thermal pain stimuli [7,14]. A lateralized visual discrimination task was performed more rapidly when it was preceded by a painful stimulus on the same side versus the opposite side from the visual stimulus. Similarly, eye orientation to the side of the painful stimulus leads to higher pain ratings in one study [45] and lower to pain ratings on the right among anxious subjects [28]. These results suggest that misdirected spatial attention may modulate thermal pain stimuli.
Forebrain lesions may produce abnormal spatial attention such as hemi-neglect which is failure of a subject ‘to report, respond, or orient to meaningful stimuli contralateral to the (cortical) brain lesion’ [24,61]. Visual spatial hemi-neglect is most commonly observed after lesions of the right parietal cortex, but can also occur after lesions of the prefrontal cortex, the superior temporal gyrus, the frontal operculum, or the thalamus [6,26,29,64,65].
Extinction was considered to be a subtle form of neglect [9,24,25] which was measured by a dual simultaneous stimulation (DSS) protocol [62]. Extinction is the failure to attend to stimuli on the affected (left) side of the body when an identical simultaneous stimulus occurs on the opposite (right) side, but not when the stimulus is presented on the affected side alone (DSS protocol) [5,26]. DSS protocols have been documented for multiple sensory modalities [26,27,62,63], but not for thermal pain stimuli. We now test the hypothesis that subjects with visual spatial neglect (hemi-neglect) will have attention-related alterations of sensibility for thermal pain sensation.
2. Methods
The protocol used in these studies conformed to the principles stated in the Declaration of Helsinki regarding the use of human subjects and was reviewed and approved annually by the Institutional Review Boards of the Johns Hopkins University. All subjects signed an informed consent for studies included in this protocol. All the methods used in these studies have been previously described [23,26].
2.1. Subjects
The study was carried out in nine patients (Table 1) with visual spatial hemi-neglect who were admitted to the Brain Rescue Unit at The Johns Hopkins Hospital with indisputable clinical evidence of a forebrain stroke-like event. A similar group of patients without hemi-neglect (n = 11) served as controls (Table 2). Exclusions for both stroke groups were deficits of speech, naming, cognitive, and sensory function. We also examined a population of 20 healthy subjects.
Table 1.
Characteristics of strokes in subjects with hemi-neglect. Columns as labeled. ’The right column summarizes the results by the thermal pain submodality as indicated by the words Hot or Cold, as labeled.’ The type of abnormality of thermal extinction attention is indicated as follows: Extinction ratio (ext) for thermal stimulation on the left over both sides versus the right over both sides, Mislocalization (ML) proportion of mislocalized thermal stimulation with stimulus presentation on the left versus the right side or Misidentification (MI) proporteion of mislocalized stimulation on the left versus the right side. All strokes were on the right hand side and all symptoms were on the left. See Methods: tests of extinction. Abbreviation: AF – atrial fibrillation,, DM – diabetes mellitus, FAL – face arm leg, HBP – high blood pressure, hyperchol – hypercholesterolemia, MCA – middle cerebral artery.
| Identifier | Age, sex, schooling |
Stroke | Other conditions | Stroke signs | Tests of Neglect & Extinction ratio | Extinction (EXT), Mislocalization (ML) & misidentification (Mi) by subject |
|---|---|---|---|---|---|---|
| 1:1 | 85, F, 14 (RN) | Large MCA | AF, HBP | Left FAL weak Clinical neglect |
Bisect 32% cancel visual 86%. | Hot, EXT 8/0 vs 8/8 Cold, EXT 8/8 vs 8/8 |
| 1:2 | 78, F, 12 | Extensive chronic small infarcts |
DM, hyper-chol, CRF |
Left sided weakness |
Bisect 27% cancel visual 45%. | Hot EXT 8/5 vs 8/8 Cold EXT 8/6 vs 8/8 |
| 1:3 | 56. F, 13 | New subcortical parietal & extensive small infarcts |
CAD, HBP, DM | Mild FA weakness |
Cancel tactile 17 Clock 31%. tactile extinct 2.25 |
Hot ML 8/8 vs 0/8, Hot MI 8/8 vs 0/8. |
| 1:4 | 37, F, 11 | Large Watershed | HBP, DM, migraines, depression. |
Left FAL weakness |
Clock 13% cancel visual 10. extinct tactile 2.5. |
Hot ML 6/8 vs 0/8. |
| 1:5 | 71, F, 12 | Large MCA | HBP, hyperchol | Left FAL weakness |
Bisect 19% cancel visual 20 tactile 14 clock 13. extinct tactile 8 visual 2. |
Cold ML 8/8 vs 0/8, Cold MI 7/8 vs 0/8. |
| 1:6 | 26, F, 10 | Large MCA | DM, CRF, hyperchol |
Hemiparesis, | Bisect 24% Cancel 39%. | Cold ML 4/8 vs 0/10 Cold MI 4/8 vs 0/10. |
| 1:7 | 75, M, | Large MCA | CHF | FAL weakness, neglect |
Bisect 10%, cancel 13% extinct tactile 2 | Hot ML 8/8 vs 0/8, Cold ML 7/8 vs 0/8, Cold MI 7/8 vs 0/8. |
| 1:8 | 83, F, 14 | Parietal hematoma (amyloid) |
Cholecystitis AF, polycystic kidney |
FAL weakness, neglect |
Bisect 12% Clock 63%. | Cold ML 6/8 vs 1/8, Cold MI 7/8 vs 0/8 |
| 1:9 | 71, M, 12 | Large ACA MCA | HBP, DM, Gout | FAL weakness, neglect |
Cancel 12% bisect 33. | Cold ML 8/8 vss 0/8, Cold MI 7/8 vs 0/8. |
Table 2.
Characteristics of strokes in subjects with strokes but without hemi-neglect. Column 5 indicates the presence of errors > 1% which were non-significant errors (< 10%, see Section 2.5).
| Identifier | Age, sex, schooling | Stroke | Other conditions | Stroke signs | Errors on tests of neglect and extinction |
|---|---|---|---|---|---|
| 2:1 | 86, F, 12 | Large frontal opercular MCA | AVR CAD CABG hyperlip | Lt FAL weak | Bisect error2%. |
| 2:2 | 69,M, 12 | Large watershed | CAD, pancreatic CA, DM, HBP | Lt AL weak CAD sev HBP | Bisect error 2%. |
| 2:3 | 59, M, 15 | MCA infarct | Hbp glaucoma hyperchol | Lt FAL numb | Bisect error 1.5% |
| 2:4 | 25, F, 14 | Large MCA | Antiphospholip Synd,MI ITP | FAL numbness & weakness | Cancel vis 7% Bisect error 1.6% |
| 2:5 | 67, M, 10 | Extensive small infarts | Hbp ARF, obesity gout | Right arm weak | Cancel vis 7% Bisect error 6% |
| 2:6 | 45, M, | Large left MCA | HBP. DM, sinus brady | Right FAL weakness (3–4/5) | Cancel visual 6% Bisect error 3% |
| 2:7 | 62, M | Thalamic capsular | HBP, | Lt FAL weakness | |
| 2:8 | 75, m, 15 | Bilateral white matter and Lt deep frontal | AF, HBP, DM, AI | Right FAL weakness numbness | Bisect error 1.5% |
| 2:9 | 79,M. 15 | Lt fronto-insular stroke | DM, HBP, hypercholest | Right AL weakness | |
| 2:10 | 57, F | Left thalamic capsular | DM, HBP, obesity | Right AL weakness, numbness | Bisect error 6% |
| 2:11 | 74, F | Lt BG parietal | HBP, MR, AF hypercholest | FA weakness |
There is no published evidence of thermal pain inattention syndromes, to our knowledge. Therefore, our hypothesis was tested by an exploratory study of subjects with neglect syndromes, as the population most likely to demonstrate thermal pain inattention. Cognitive function was assessed by the Mini-Mental Status Examination [16]. If subjects in this study correctly identified all unilateral visual and tactile stimuli in the DSS protocol then cognitive function and sensory function in these modalities was judged satisfactory for the purposes of this study.
2.2. Tests of visual spatial neglect
Tests for visual spatial hemi-neglect included (1) a line bisection task, (2) a line cancellation task, and (3) a clock drawing task. The line bisection task required the subject to mark the center of a 20 cm line placed directly in front of the subject. The line was presented three times and the error, expressed as a percentage, was calculated from the average error to the right of the midline divided by the length of the line.
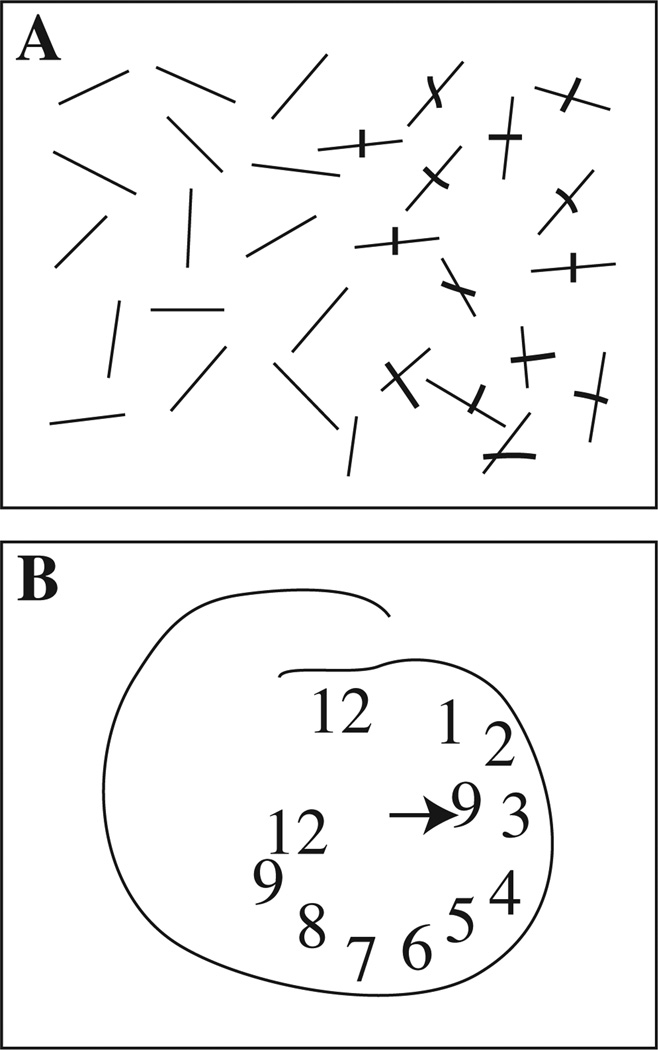
In the line cancellation task, a page was placed in front of the subject with 30 short lines at random angles; 15 lines were located to the left and 15 to the right of the midline (Fig. 1A).
Fig. 1.
Examples of the line cancellation task (Panel A), and a drawing of a clock face (Panel B) and in a subject with hemi-neglect (1.8 in Table 1). The right side of the figure corresponds to the right side of the subject’s visual field.
In a circle detection (or cancellation) task, a page containing 30 circles with or without gaps in the circle was placed directly in front of the subject [27,49]. Subjects were asked to circle all complete circles and cross out any circles with gaps.
The clock drawing task required the subject to draw a clock showing 9:00 on a blank piece of paper placed in front of the subject (Fig. 1B). This task was scored by the difference between the number of numerals on the right side minus left side of the clock face divided by the total number of numerals times 100% [11,50].
In a tactile version of the circle detection (or cancellation) task, raised circles were arranged on a board in a pattern identical to that for the visual test. With vision occluded, the subjects were asked to move their hand over the board and identify circles. The visual and tactile gap tests were administered twice for each modality, one for small (diameter of 15 mm) and the other for large stimuli (diameter of 22 mm). The results of the two tests in the same modality (large and small circles) were combined. Differences between the detection of circles and gaps were not analyzed separately because the goal of these studies was to establish the presence of hemi-neglect, not the difference between viewer versus object centered neglect.
In all of the tests, hemi-neglect was indicated by a 10% error rate on the left with a perfect score on the right [27]. An error rate of > 10% on the left with a perfect score on the right was never observed in studies of healthy controls, or of controls with stroke but without hemi-neglect. Subjects with hemi-neglect were identified by evidence for hemi-neglect on two separate tests.
2.3. Dual simultaneous stimulation (DSS)
We administered tests of visual and tactile extinction deficits in a DSS protocol, to establish the presence of visual spatial hemi-neglect. In the case of tactile extinction testing, the stimulus was a light touch on the hand with a cotton-tipped applicator, which was randomly given on the left hand (eight times), the right hand (eight times), or both hands simultaneously (eight times). The subject responded by indicating where (right or left or both) and when the stimulation occurred.
Visual extinction was measured by a similar test in which the examiner’s forefingers were held in the left and right visual fields 50 cm in front of the subject’s face. The subject was asked to identify whether the finger moved on one side, or the other, or both. Subjects in our control groups rarely made errors in visual or tactile extinction tasks. The significance of extinction was evaluated by the extinction ratio which was calculated by the ratio of the number of detected stimuli on the left when the left side was stimulated separately divided by the number detected when both hands were stimulated simultaneously. Extinction was defined by a ratio of ≥ 2, or by a significant difference in the ratio on the left versus the corresponding ratio on the right hand side [27]. The rationale for these nonparametric tests is given in Section 2.5.
2.4. Thermal testing
For thermal stimuli, the pretest included determination of the pain threshold and ratings during application of heat stimuli with brass rods maintained at temperatures from 41 to 53 °C in one degree steps. The painful heat stimulus was chosen as the temperature 1° above the pain threshold. Exclusion criteria determined by this test included subjects who did not detect unilateral thermal stimuli on either side in the pretest, or in the thermal DSS protocol, or both.
In order to assess attention-related performance of the thermal pain modality, we used a thermal DSS protocol with contact heat stimuli with high specific heat (see Section 1). The contact thermal stimuli used in this study were chosen based upon our experience with stimuli of this type. Use of the same stimuli makes the present results accessible to those of our prior neuronal and psychophysical studies of the response to these stimuli [32,34,35,38,39]. The peripheral receptors involved in the response to these stimuli would be useful information for the interpretation of these results, and the activated receptors may be related to the duration of thermal, as suggested by precise studies of the response to laser stimuli [52]. However, this effect is difficult to assess in the present results because contact heat activates many receptors and is substantially different from the laser heat stimuli [3]. The tests of thermal pain attention were carried out by applying the heated brass end of a probe or the thermal neutral plastic (Delrin) end of the probe to the dorsum of the hand or forearm for three seconds, while the subject’s vision was occluded. For application of heat stimuli, a brass rod was placed in a temperature controlled water bath until immediately prior to application.
The heated end was applied to the dorsum of the left hand or forearm eight times, while the plastic end was applied to the corresponding site on the right side. Heat was applied to the right hand and forearm eight times while plastic was applied to the corresponding site on the left side eight times. Interspersed among these unilateral heat stimuli were painful heat stimuli applied to hands or forearms on both sides simultaneously. These three pairs of different stimuli were presented in pseudo-random order while the subjects’ vision was occluded. Subjects were first asked to determine whether the thermal stimulus or stimuli were applied on the right or left side, or both. They were then asked to determine whether the stimuli were hot or cold.
The same thermal DSS protocol was carried out separately for thermal stimuli in the cold submodality. For the cold stimulus, the brass rod was placed in an ice water bath at 4 °C, which all subjects judged either was painful, or would become painful if the stimulus were prolonged. The rods were taken out of the water bath, dried and applied to the skin for approximately 10 s with an interstimulus interval of approximately 20 s.
Unlike tests of visual and tactile extinction, there was a small but definite error rate on the thermal pain DSS tasks (see Section 3) among both healthy controls and controls with stroke but without hemi-neglect. Therefore, we examined differences within subjects in the proportion of detected thermal test stimuli of on the left hand when the thermal stimulus was delivered to the left side alone, versus when the stimulus was delivered to both sides simultaneously by a test of proportions (Fisher exact).
2.5. Statistical testing
2.5.1. Non-parametric within subject analyses
This testing included a non-parametric analysis of differences between the two sides within any subject. Tests of this kind are commonly done to establish whether there is significant inattention within any subject in studies of neglect and extinction [5,20,24–27,33,62]. This approach is usually adopted because extinction is conceived of as a binary variable like an upper motor neuron sign, which is either present or absent [2]. We adopted this approach because neglect and extinction are rare among controls with normal mental status, and because we wished to make the present results accessible to the literature of hemi-neglect as well as pain.
Extinction was identified by calculating the extinction ratio on the left as above and a similar ratio for the number of detected stimuli on the right when the right side was stimulated separately divided by the number detected when both hands were stimulated simultaneously. Extinction was identified within subjects when the extinction ratio for thermal pain stimuli was significantly greater for thermal stimuli on the left than on the right hand side. Non-parametric group statistics tested differences in the proportion of subjects with a sensory abnormality between different subject groups (see Section 3.4). Since we always applied eight repeats of each temperature/laterality combination of stimuli (see Section 2.3) pair non-parametric tests often included ratios with denominators of less than five. Therefore, the use of a Fisher exact test, rather than a χ2 test, is mandated to test differences in these proportions [58].
A similar approach was taken for the other sensory abnormalities. Mislocalization was measured within individual subjects by errors in the identification of the side of the thermal stimulus when it was applied on one side and the thermal neutral stimulus when it was applied on the other side (Fig. 2B). In this protocol, the mislocalization ratio was defined by the number of thermal stimuli which were localized to the side upon which the thermal neutral stimulus was applied. Mislocalization was then identified within subjects when the proportion of mislocalized thermal stimuli was significantly greater for thermal stimuli presented on the left side than when it was presented on the right side. A similar approach was adopted for misidentification.
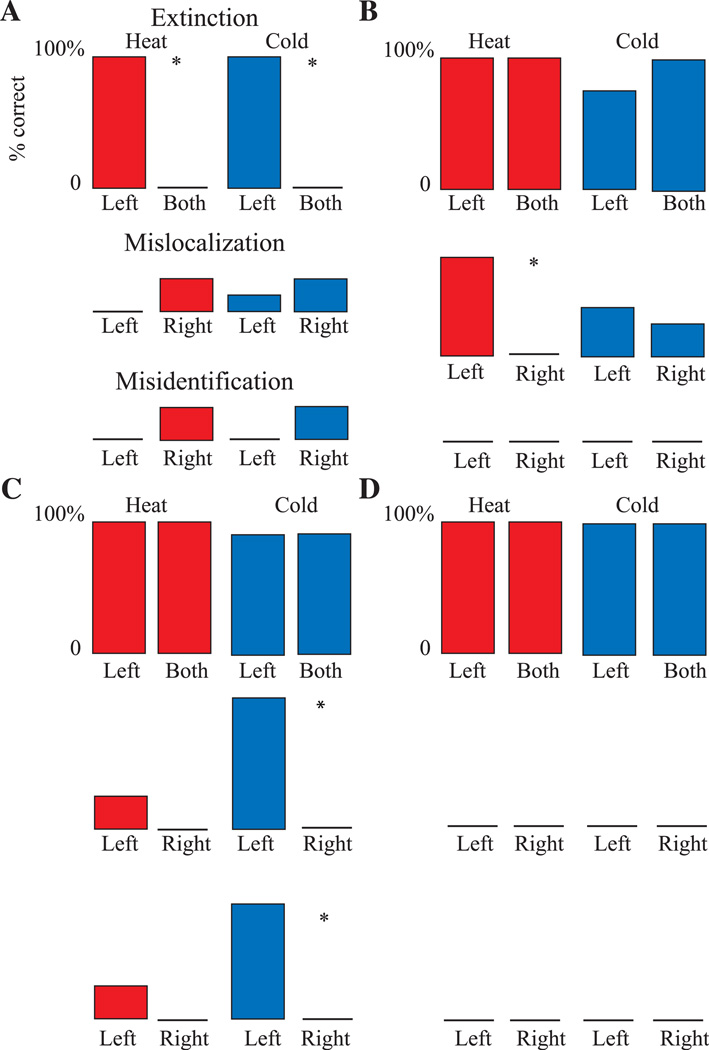
Fig. 2.
Examples of the characteristic responses of different subjects to thermal pain stimuli presented in a DSS protocol. (A) A subject 1:2 with a significant extinction ratio but without mislocalization or misidentification. In this subject the number of detected stimuli, heat or cold, was greater when the stimulus was presented on the left side versus both sides, as labeled. In all panels the color indicates the submodality of the stimulus (heat, red; cold, blue). (B) A subject 1:4 with mislocalization of heat stimuli so that heat applied to the left was mislocalized to the right where the thermal neutral stimulus was applied (middle line). (C) A subject (1:5) who misidentified as heat (lower line) the cold stimulus which was applied on the left but mislocalized to the right (middle line). (D) DSS protocol results in control subjects without hemi-neglect.
Misidentification within individual subjects was based upon errors in identification of the submodality of the thermal stimulus (hot versus cold) when it was applied on one side and the thermal neutral stimulus was applied on the other side (Fig. 2C). Misidentification was then identified within subjects when the proportion of misidentified thermal stimuli was significantly greater when the thermal stimulus was presented on the left side than when it was presented on the right side.
2.5.2. Parametric group analyses
Parametric tests (ANOVA) were carried out because the ratios used to assess the sensory abnormalities in this study may reflect the severity of neglect or extinction above the threshold for making the diagnosis. Extinction ratios were tested in an ANOVA as a function of the subject group (hemi-neglect, stroke without hemi-neglect, healthy controls), laterality of the ratio (left versus right), and submodality (hot and cold) (see Section 2.3). Similarly misidentification ratios (see above) and mislocalization ratios were tested in separate ANOVAs as a function of the subject group, laterality, and submodality (see Section 2.3).
3. Results
3.1. Subjects
These studies were carried out in a target group of subjects (n = 9) with hemi-neglect, and without exclusionary criteria (see Table 1); all were right handed. Strokes were ischemic or hemorrhagic, and were all located on the right side of the brain. These strokes included five subjects with large strokes of the middle cerebral artery (MCA) territory. One subject (1:4) had a stroke of the right posterior and anterior cerebral artery watershed territory, which involved the parasagittal aspect of the frontal and parietal lobes. One subject had a parietal lobar hemorrhage, two had extensive small vessel infarcts, and one had a new subcortical stroke. All nine subjects had left sided weakness.
Eleven subjects in the Brain Rescue Unit with a diagnosis of a stroke-like event but without hemi-neglect served as controls (Table 2). The sample included subjects with acute hemispheric strokes, one hemorrhagic stroke, and extensive chronic small vessel infarcts with neurologic decompensation. We also examined twenty healthy controls, (n = 20, 10 men aged 25–58 years old; 10 women aged 27–59 years old) without any history of neurologic disorders or of chronic medical disease. The proportion of subjects with diabetes mellitus was not significantly different between subjects with strokes with (5/9) or without hemi-neglect (5/12, p = 0.67).
In controls with strokes, there were occasional errors in the description of stimulus in the heat or cold submodality of the DSS protocol divided by the total number of trials (n = 48 trials/subject, error rate = 3.9 ± 8.2%, mean ± SD) (cf. [41]). The percentage of errors was not significantly different from that among healthy controls (see above, 3.3 ± 3.2%, t = 0.27, p = 0.79). The number of errors were not significantly different between women (3.3 ± 3.6) and men in the healthy control population (3.4 ± 5.7, t = 0.02, p = 1).
3.2. Hemi-neglect
Fig. 1 shows a subject (Table 1, 1:8) who had dramatic neglect of her left side, which is on the reader’s left. This subject cancelled only lines on the right, and drew the clock almost entirely on the right, including the hand indicating nine o’clock. All nine subjects with hemi-neglect had significant abnormalities of clock drawing, line bisection, or line cancellation, or some combination of these (see Table 1). Four subjects had visual or tactile extinction, or both; among these four, three had mislocalization or misidentification of thermal pain stimuli (Table 1: subjects 1:3, 1:5, 1:7), one had only mislocalization (1:4), and none had thermal pain extinction. Therefore, these results do not point to a clear relationship between visual spatial extinction and thermal pain extinction.
3.3. Thermal pain extinction
Fig. 2 shows examples of subjects with the three types of attention-related thermal pain sensory abnormalities as identified by the thermal pain DSS protocol (see Section 2): A extinction, B mislocalization, and C mislocalization. The significance of attention-related sensory abnormalities was tested within all subjects as outlined in Section 2.5. The upper row of all panels in Fig. 2 (labeled Extinction) shows the percentage identification of the thermal/pain stimulus on the left hand when presented on the left (labeled left), and on the left when presented on both hands simultaneously (labeled both), for heat (red) and cold (blue). Fig. 2A shows results in a subject (Table 1, subject 1:1) with thermal pain extinction for heat by testing within individuals.
3.3.1. Parametric group analysis
Extinction ratios were significantly higher in the hemi-neglect group of subjects than in the control groups (F = 6.9, p = 0.0036, ANOVA). There was a strong trend toward higher extinction ratios on the left than on the right (F = 3.5, p = 0.063). Extinction ratios were larger for the left side in subjects with hemi-neglect (side:group interaction term, F = 6.0, p = 0.003). The difference in thermal pain extinction between the hot and cold submodality were not significant (F = 0.08, p = 0.78).
3.3.2. Non-parametric within subject analysis
A non-parametric analysis was carried out after the ANOVA described above. Fig. 2A shows a within subject analysis which demonstrates a significant extinction ratio for heat because the proportion of stimuli identified on the left with left sided heat stimulation (8/8) was significantly greater than the proportion on the left with bilateral heat stimulation (0/8, p = 0.0001, Fisher). Significance is indicated by an asterisk in the upper row of Fig. 2A. In this subject, the analysis was identical for the cold sub-modality. Extinction ratios for both hot and cold stimuli were found in another subject (Table 1, subject 1:2), but in none of the other subjects with hemi-neglect (Table 1, column 6).
3.3.3. Non-parametric group analysis
The responses evoked by thermal/pain stimuli in the subjects with thermal pain extinction had neither mislocalization nor misidentification (0/2, see Table 1) while subjects without such extinction all had either mislocalization or misidentification or both (7/7, p = 0.0278, Fisher).
3.4. Mislocalization
The middle row of all panels in Fig. 2 (mislocalization) shows the localization of the thermal stimulus on the side opposite to that upon which it was presented. Localization to the left when the stimulus is presented on the right is labeled left; localization of the thermal stimulus on the right when presented on the left is labeled right. In each of these trials analyzed for mislocalization, the thermal-neutral control stimulus was applied opposite to the thermal stimulus. These conventions also apply to misidentification, which is shown in the lower row of each panel of Fig. 2 (see Section 3.3).
3.4.1. Parametric group analysis
Mislocalization ratios were significantly higher in the hemineglect group of subjects than in the two control groups (F = 60, p = 2.2 × 10−16, ANOVA). Across all subjects, mislocalization ratios were significantly higher among stimuli presented on the left (affected, 5.78 + 0.76, average + variance) versus the right side of the body while the thermal neutral stimulus was presented on the opposite side (0.78 + 0.33) (F = 37.9, p = 0.000003, ANOVA). The difference in mislocalization between the hot and cold modality was not significant (F = 0.001, p = 0.38) as tested by comparing the number of mislocalized stimuli among hot versus cold submodalities. In the post hoc analysis, the side:group interaction term showed significance due to higher mislocalization ratios for the left side in subjects with hemi-neglect (side:group interaction F = 65, p = 2.0 × 10−15).
3.4.2. Non-parametric within subject analysis
Mislocalization was assessed within individual subjects by the mislocalization ratio (Section 2.5). In subject 1:4 (Table 1), the mislocalization ratio for heat stimuli was significantly more common with presentation of the stimulus on the left (6/8) than on the right (0/8, p = 0.007, Fisher, Fig. 2B, Table 1). Significant mislocalization was never observed in any subject in either control group.
3.4.3. Non-parametric group analysis
In the group non-parametric analysis, the proportion of subjects with mislocalization among those with hemi-neglect (7/9, Table 1 column 6) was significantly greater than in the controls with stroke (0/11, p = 0.0003, Table 2, column 5), or without stroke (0/20, p < 0.00001). These results provide strong evidence for mislocalization of thermal pain stimuli in subjects with hemi-neglect.
3.5. Misidentification
The lower row in each panel in Fig. 2 shows the misidentification of the submodality of the stimulus (i.e. reporting heat stimuli as cold, or cold as heat) which occurred in situations where the stimulus was mislocalized to the side opposite the side of the thermal pain stimulus. The proportion of misidentification of the thermal stimulus on the left was compared with that on the right.
3.5.1. Parametric group analysis
Misidentification ratios were significantly between subject groups due to higher ratios in the hemi-neglect group of subjects than in the two control groups (F = 7.0, p = 0.0013, ANOVA). Misidentification ratios for the modality of the thermal stimulus were significantly higher (F = 7.0, p = 0.04, ANOVA) among stimuli presented on the left (4.32 + 1.25, average + variance) versus the right side (0.57 + 0.21). The misidentification ratios were not significantly different between the hot and cold modalities (F = 1.1, p = 0.31). On post hoc analysis, the side:group interaction term showed significance (F = 6.8, p = 0.0015) due to higher misidentification ratios for the left side in subjects with hemi-neglect.
3.5.2. Non-parametric within subject analysis
An example of within subject analysis is shown in Fig. 2C (Table 1, subject 1:5), in which misidentification of cold stimuli as heat stimuli was more common with presentation of the stimulus on the left (8/8) than on the right (0/8, p = 0.0002). In each trial, the thermal-neutral control stimulus was applied opposite to the thermal stimulus.
3.5.3. Non-parametric group analysis
Significant misidentification was not identified on analysis within subjects in any of the healthy controls or in controls without hemi-neglect (Table 2 column 5). Therefore, the proportion of misidentification among subjects with hemi-neglect (Table 1 column 6) was greater than among controls with stroke without hemi-neglect (6/9 versus 0/11, p = 0.0015). These results provide strong evidence for misidentification for the heat and cold submodalities in subjects with hemi-neglect.
3.6. Analysis of large hemispheric strokes
Large hemispheric strokes were more common among subjects with hemi-neglect than controls with stroke-like events, so that thermal pain inattention in these strokes may have influenced the results of this study. We tested this possibility by comparing attention- related sensory abnormalities among subjects in both groups. Among subjects with large hemispheric strokes, the proportion of subjects with mislocalization for hot or cold or both was greater in those with hemi-neglect (6/7) versus those without hemi-neglect (0/7, p = 0.0023, Fisher). The same was true for misidentification (6/7 versus 0/7); differences in the incidence of thermal pain extinction were not significant (1/7 versus 0/7, p = 1). Therefore, among subjects with large hemispheric strokes, those with hemi-neglect had higher attention-related abnormalities of thermal/pain sensations than subjects with strokes without hemi-neglect.
4. Discussion
These results demonstrate that extinction for thermal pain stimuli can occur in subjects with hemi-neglect. More commonly, these subjects have mislocalization which occurs when the sensation on the affected (left) side is detected, but is referred to the unaffected (right) side. Among stimuli which are referred to the affected side, the submodality (hot or cold) of the stimulus is often misidentified. Mislocalization and misidentification are significantly more common with application of thermal stimuli to the affected side than the unaffected side, among subjects with hemi-neglect than in either control group. These results demonstrate that disordered spatial attention exerts a powerful effect upon the perception of location as well as the identity of thermal pain stimuli. This is consistent with evidence that processing of experimental painful stimuli depends, in part, upon the location of the focus of spatial attention on the body [37], and may be dependent on the orientation of the limbs in peripersonal space [43].
4.1. Methodological considerations
It could be argued that the mechanical control stimulus used in thermal pain extinction testing might confound the results. However, the same kind of contralateral control stimulus is used in many tests of extinction, such as the vibratory modality. In this modality, the control stimulus is a non-vibrating tuning fork. Stimuli of this kind allow for reliable detection of extinction in multiple modalities including vibration, proprioception, graphesthesia, movement, and electrocutaneous stimuli through electrodes applied to the skin [24–27,33]. Therefore, the presence of a control stimulus is not standard but has been found to be a useful part of many tests for extinction.
In the present study, we applied the thermal neutral control stimuli in all populations and the differences between groups were highly significant. In order to understand the effect of the control modality (mechanical) we considered studies of cross-modal spatial attention. In this study an extinction protocol for visual stimuli was perturbed by auditory stimuli which were directed toward one side or the other [18,51]. If the auditory signal was on the affected (neglected) side then it led to increased detection of the visual stimulus on that side; if the auditory stimulus was located on the unaffected side it did not bias the results.
This result suggests that the mechanical stimulus on the affected side, in our study, would increase the identification of thermal and mechanical stimuli on that side but not when same stimuli are applied on the unaffected side. Therefore, mechanical stimuli might lead to a decreased thermal pain extinction ratio, although the effect of bilateral thermal and mechanical stimuli is unknown. Therefore, this effect might lead to decreased identification of thermal pain extinction, although it cannot explain mislocalization or misidentification of the thermal stimulus (Fig. 2B). Pain DSS protocols could best be carried out by use of bilateral radiant heat stimuli which could identify extinction of a pure pain stimulus.
4.2. Mislocalization
The present results showed evidence of mislocalization of thermal pain stimulation to the attended (right) side when the thermal stimulus was applied to the unattended side as shown in the middle rows of Fig. 2B and C. Mislocalization, also termed alloesthesia, has been observed in studies of cordotomy of the spinothalamic tract in which painful stimuli at the spinal level and side of the lesion may be felt on the unaffected side or below that lesion of stimulation [15]. Thermal pain mislocalization may involve the thermal signaling component of the STT to the ipsilateral hemisphere [67].
The presence of an ipsilateral pain system is supported by physiological evidence of monkey thalamic [8,66], and parietal cortical neurons with bilateral receptive fields to painful stimuli [13,31]. Some of these neurons are multimodal and respond to the appearance of a painful stimulus in a particular region of the space around the subject. This effect of location may or may not apply to present study of cutaneous space, although neuronal responses to ipsilateral painful stimuli provide a substrate which might be modulated by location of the painful stimulus in the space around the subject [12].
Evidence of ipsilateral brain activation is also found in PET studies which demonstrate that intensity-dependent activation can occur ipsilateral to an acute painful stimulus [10,53]. This stimulation protocol evokes bilateral activation of secondary somatic sensory cortex, insula and anterior cingulate cortex, although these activations might not be related to pain sensations [44]. Ipsilateral activation is also observed in the cerebellum, putamen, thalamus, anterior cingulate cortex, and frontal operculum.
Studies in subjects with hemispherectomy or callosotomy demonstrate that ipsilateral inputs can subserve the sensory aspect of pain [48,59]. Subjects in both these groups could detect the presence of a painful and a non-painful thermal stimulus but made errors in localization of the stimulus. In subjects with hemispherectomy, localization of tactile stimuli to the medial or the lateral side of the foot was impaired on the affected but not the unaffected side [48]. Furthermore, in the subjects with hemispherectomy, blood flow activation by ipsilateral stimulation was weaker but involved the same structures as activated by contralateral stimulation, i.e. anterior cingulate, primary and secondary somatic sensory cortex.
In subjects with hemi-neglect, the mislocalized stimuli may be the result of callosal transmission of sensory inputs from the affected (right) to the unaffected hemisphere. Transmission to the unaffected (left) hemisphere of sensory input from the contralateral thermal neutral stimulus may be essential for mislocalization. The importance of the stimulus on the unaffected side has been demonstrated in a DSS protocol with two tactile stimuli in which mislocalization occurred in healthy subjects [41].
In a subject with a total callosotomy, a thermal pain stimulus was applied to produce input to the contralateral hemisphere. The subject rated sensations using the hand controlled by that hemisphere on a visual analog scale which was presented in the visual field of that hemisphere (responding hemisphere) [59]. In this subject, the somatotopic localization of a painful stimulus was unreliable when the stimulus was ipsilateral to the responding hemisphere. The localization was significantly more reliable when the stimulus was contralateral to the responding hemisphere. Therefore, the localization in the present results might reflect inputs to the unaffected hemisphere through either ipsilateral connections from the spinal cord, or callosal connections to the unaffected hemisphere.
4.3. Misidentification of thermal stimuli
The present results were consistent with thalamic and (parietal) cortical neuronal responses to a range of thermal and mechanical stimuli applied across large bilateral receptive fields [8,12,13, 31,59]. These neurons could be the substrate for misidentification of the stimulus applied to the affected hand (misidentification). This suggestion is consistent with results in subjects with hemispherectomy who could identify the presence of a thermal stimulus on the affected side of the body but could not correctly identify the modality of the stimulus as hot or cold [59]. Unlike subjects with hemispherectomy the misidentification of thermal pain stimuli in subjects with hemi-neglect could be due, in part, to input to the contralateral hemisphere from the ipsilateral hemisphere through the corpus callosum.
Studies in a subject with a total callosotomy demonstrated that mildly painful or non-painful thermal sensations on the side ipsilateral to the responding hemisphere were rated as less intense than that on the other side or the normal population [1,17,19]. The present results indicate that input to the unaffected (right) hemisphere from the neglected (left) hand may mediate the sensory component of thermal pain sensations evoked by a stimulus applied to the left hand. The transcallosal mechanism of misidentification assumes that the unaffected hemisphere can still support transmission of thermal pain signals to the affected hemisphere.
It has been proposed that inattention to the limb affected with chronic regional pain syndrome may be an underestimated component of chronic pain syndromes. These subjects show multiple abnormalities including spatial shifts in tactile perception [21,22], pain on watching the mirror image of the unaffected hand [56], and information processing difficulties [54]. Inattention might also explain phenomenon which are currently ascribed to reflex or involuntary immobility of the affected limb, or to a movement disorder associated with chronic pain, such as dystonia in reflex sympathetic dystrophy. Therefore, important features of chronic pain can be mediated through the ipsilateral processes which support mislocalization and misidentification. This mechanism may also support sensory or psychological interventions in the case of pain with associated inattention.
Acknowledgement
This work is supported by the National Institutes of Health –National Institute of Neurological Disorders and Stroke (NS38493 and NS40059 to FAL, NS-39337 to JDG). The authors would like to thank LH Rowland for excellent technical assistance.
Footnotes
Conflict of interest statement
None of the authors has any conflict of interest with respect to this manuscript.
References
- 1.Acerra NE, Moseley GL. Dysynchiria: watching the mirror image of the unaffected limb elicits pain on the affected side. Neurology. 2005;65:751–753. doi: 10.1212/01.wnl.0000178745.11996.8c. [DOI] [PubMed] [Google Scholar]
- 2.Adams RD, Victor M, Ropper AH. Principles of neurology. New York: McGraw-Hill; 1996. [Google Scholar]
- 3.Arendt-Nielsen L, Chen AC. Lasers and other thermal stimulators for activation of skin nociceptors in humans. Neurophysiol Clin. 2003;33:259–268. doi: 10.1016/j.neucli.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Becker DE, Yingling CD, Fein G. Identification of pain, intensity, and P300 components in the pain evoked potential. EEG Clin Neurophysiol. 1993;88:290–301. doi: 10.1016/0168-5597(93)90053-r. [DOI] [PubMed] [Google Scholar]
- 5.Bender MB. Extinction and other patterns of sensory interaction. In: Weinstein EA, Friedland RP, editors. Hemi-inattention and hemispheric specialization. New York: Raven Press; 1977. pp. 106–110. [Google Scholar]
- 6.Berthier M, Starkstein S, Leiguarda R. Asymbolia for pain: a sensory-limbic disconnection syndrome. Ann Neurol. 1988;24:41–49. doi: 10.1002/ana.410240109. [DOI] [PubMed] [Google Scholar]
- 7.Bushnell MC, Duncan GH, Dubner R, Jones RL, Maixner W. Attentional influences on noxious and innocuous cutaneous heat detection in humans and monkeys. J Neurosci. 1985;5:1103–1110. doi: 10.1523/JNEUROSCI.05-05-01103.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casey KL. Unit analysis of nociceptive mechanisms in the thalamus of the awake squirrel monkey. J Neurophysiol. 1966;29:727–750. doi: 10.1152/jn.1966.29.4.727. [DOI] [PubMed] [Google Scholar]
- 9.Cicek M, Gitelman D, Hurley RS, Nobre A, Mesulam M. Anatomical physiology of spatial extinction. Cereb Cortex. 2007;17:2892–2898. doi: 10.1093/cercor/bhm014. [DOI] [PubMed] [Google Scholar]
- 10.Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82:1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- 11.di-Pellegrino G. Clock-drawing in a case of left visuo-spatial neglect: a deficit of disengagement? Neuropsychologia. 1995;33:353–358. doi: 10.1016/0028-3932(94)00106-y. [DOI] [PubMed] [Google Scholar]
- 12.Dong WK, Chudler EH, Sugiyama K, Roberts VJ, Hayashi T. Somatosensory, multisensory, and task-related neurons in cortical area 7b (PF) of unanesthetized monkeys. J Neurophysiol. 1994;72:542–564. doi: 10.1152/jn.1994.72.2.542. [DOI] [PubMed] [Google Scholar]
- 13.Dong WK, Salonen LD, Kawakami Y, Shiwaku T, Kaukoranta EM, Martin RF. Nociceptive responses of trigeminal neurons in SII-7b cortex of awake monkeys. Brain Res. 1989;484:314–324. doi: 10.1016/0006-8993(89)90375-2. [DOI] [PubMed] [Google Scholar]
- 14.Dowman R. Electrophysiological indices of orienting attention toward pain. Psychophysiology. 2004;41:749–761. doi: 10.1111/j.1469-8986.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 15.Eidelberg E, Schwartz AS. Experimental analysis of the extinction phenomenon in monkeys. Brain. 1971;94:91–108. doi: 10.1093/brain/94.1.91. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh P. ‘Mini-mental state’. a practical method for grading the cognitive state of patients for the clinician. J Psychiat Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Forderreuther S, Sailer U, Straube A. Impaired self-perception of the hand in complex regional pain syndrome (CRPS) Pain. 2004;110:756–761. doi: 10.1016/j.pain.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Frassinetti F, Pavani F, Ladavas E. Acoustical vision of neglected stimuli: interaction among spatially converging audiovisual inputs in neglect patients. J Cogn Neurosci. 2002;14:62–69. doi: 10.1162/089892902317205320. [DOI] [PubMed] [Google Scholar]
- 19.Frettloh J, Huppe M, Maier C. Severity and specificity of neglect-like symptoms in patients with complex regional pain syndrome (CRPS) compared to chronic limb pain of other origins. Pain. 2006;124:184–189. doi: 10.1016/j.pain.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Gainotti G, De BC, Daniele A, Caltagirone C. Contralateral and ipsilateral tactile extinction in patients with right and left focal brain damage. Int J Neurosci. 1989;45:81–89. doi: 10.3109/00207458908986219. [DOI] [PubMed] [Google Scholar]
- 21.Galer BS, Butler S, Jensen MP. Case reports and hypothesis: a neglect-like syndrome may be responsible for the motor disturbance in reflex sympathetic dystrophy (complex regional pain syndrome-1) J Pain Symptom Manage. 1995;10:385–391. doi: 10.1016/0885-3924(95)00061-3. [DOI] [PubMed] [Google Scholar]
- 22.Galer BS, Jensen M. Neglect-like symptoms in complex regional pain syndrome: results of a self-administered survey. J Pain Symptom Manage. 1999;18:213–217. doi: 10.1016/s0885-3924(99)00076-7. [DOI] [PubMed] [Google Scholar]
- 23.Greenspan JD, Ohara S, Sarlani E, Lenz FA. Allodynia in patients with post-stroke central pain (CPSP) studied by statistical quantitative sensory testing within individuals. Pain. 2004;109:357–366. doi: 10.1016/j.pain.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Heilman KM, Watson RT, Valenstein E. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinical neuropsychology. NewYork: Oxford; 1993. pp. 279–336. [Google Scholar]
- 25.Hillis AE, Chang S, Heidler-Gary J, Newhart M, Kleinman JT, Davis C, Barker PB, Aldrich E, Ken L. Neural correlates of modality-specific spatial extinction. J Cogn Neurosci. 2006;18:1889–1898. doi: 10.1162/jocn.2006.18.11.1889. [DOI] [PubMed] [Google Scholar]
- 26.Hillis AE, Lenz FA, Zirh TA, Dougherty PM, Eckel TS, Jackson K. Hemispatial somatosensory and motor extinction after stereotactic thalamic lesions. Neurocase. 1998;4:21–34. [Google Scholar]
- 27.Hillis AE, Newhart M, Heidler J, Barker PB, Herskovits EH, Degaonkar M. Anatomy of spatial attention: insights from perfusion imaging and hemispatial neglect in acute stroke. J Neurosci. 2005;25:3161–3167. doi: 10.1523/JNEUROSCI.4468-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honore J, Henon H, Naveteur J. Influence of eye orientation on pain as a function of anxiety. Pain. 1995;63:213–218. doi: 10.1016/0304-3959(95)00050-3. [DOI] [PubMed] [Google Scholar]
- 29.Husain M, Rorden C. Non-spatially lateralized mechanisms in hemispatial neglect. Nat Rev Neurosci. 2003;4:26–36. doi: 10.1038/nrn1005. [DOI] [PubMed] [Google Scholar]
- 30.Kanda M, Fujiwara N, Xu X, Shindo K, Nagamine T, Ikeda A, Shibisaki H. Pain-related and cognitive components of somatosensory evoked potentials following CO2 laser stimulation. EEG Clin Neurophysiol. 1996;100:105–114. doi: 10.1016/0013-4694(95)00190-5. [DOI] [PubMed] [Google Scholar]
- 31.Kenshalo DR, Iwata K, Sholas M, Thomas DA. Response properties and organization of nociceptive neurons in area 1 of monkey primary somatosensory cortex. J Neurophysiol. 2000;84:719–729. doi: 10.1152/jn.2000.84.2.719. [DOI] [PubMed] [Google Scholar]
- 32.Kim JH, Greenspan JD, Coghill RC, Ohara S, Lenz FA. Lesions limited to the human thalamic principal somatosensory nucleus (ventral caudal) are associated with loss of cold sensations and central pain. J Neurosci. 2007;27:4995–5004. doi: 10.1523/JNEUROSCI.0716-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kluger BM, Meador KJ, Garvan CW, Loring DW, Townsend DT, Heilman KM. A test of the mechanisms of sensory extinction to simultaneous stimulation. Neurology. 2008;70:1644–1645. doi: 10.1212/01.wnl.0000310988.11575.fa. [DOI] [PubMed] [Google Scholar]
- 34.Lee J, Dougherty PM, Antezana D, Lenz FA. Responses of neurons in the region of human thalamic principal somatic sensory nucleus to mechanical and thermal stimuli graded into the painful range. J Comp Neurol. 1999;410:541–555. doi: 10.1002/(sici)1096-9861(19990809)410:4<541::aid-cne3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Lee JI, Ohara S, Dougherty PM, Lenz FA. Pain and temperature encoding in the human thalamic somatic sensory nucleus (ventral caudal): inhibition-related bursting evoked by somatic stimuli. J Neurophysiol. 2005;94:1676–1687. doi: 10.1152/jn.00343.2005. [DOI] [PubMed] [Google Scholar]
- 36.Legrain V, Guerit JM, Bruyer R, Plaghki L. Attentional modulation of the nociceptive processing into the human brain: selective spatial attention, probability of stimulus occurrence, and target detection effects on laser evoked potentials. Pain. 2002;99:21–39. doi: 10.1016/s0304-3959(02)00051-9. [DOI] [PubMed] [Google Scholar]
- 37.Legrain V, Plaghki L, Garcia-Larrea L. Cognitive modulation of pain-related brain responses. Pain. 2005;114:524–526. doi: 10.1016/j.pain.2005.01.025. Comments on Seminowicz et al (Pain 2004; 112: 48–58). [DOI] [PubMed] [Google Scholar]
- 38.Lenz FA, Dougherty PM. Neurons in the human thalamic somatosensory nucleus (ventralis caudalis) respond to innocuous cool and mechanical stimuli. J Neurophysiol. 1998;79:2227–2230. doi: 10.1152/jn.1998.79.4.2227. [DOI] [PubMed] [Google Scholar]
- 39.Lenz FA, Seike M, Lin YC, Baker FH, Rowland LH, Gracely RH, Richardson RT. Neurons in the area of human thalamic nucleus ventralis caudalis respond to painful heat stimuli. Brain Res. 1993;623:235–240. doi: 10.1016/0006-8993(93)91433-s. [DOI] [PubMed] [Google Scholar]
- 40.Lorenz J, Garcia-Larrea L. Contribution of attentional and cognitive factors to laser evoked brain potentials. Neurophysiol Clin. 2003;33:293–301. doi: 10.1016/j.neucli.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Marcel A, Postma P, Gillmeister H, Cox S, Rorden C, Nimmo-Smith I, Mackintosh B. Migration and fusion of tactile sensation – premorbid susceptibility to allochiria, neglect and extinction? Neuropsychologia. 2004;42:1749–1767. doi: 10.1016/j.neuropsychologia.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 42.Miltner W, Johnson R, Braun C, Larbig W. Somatosensory event related potentials to painful and non-painful stimuli: effects of attention. Pain. 1989;38:303–312. doi: 10.1016/0304-3959(89)90217-0. [DOI] [PubMed] [Google Scholar]
- 43.Moseley GL, Gallace A, Spence C. Space-based, but not arm-based, shift in tactile processing in complex regional pain syndrome and its relationship to cooling of the affected limb. Brain. 2009;132:3142–3151. doi: 10.1093/brain/awp224. [DOI] [PubMed] [Google Scholar]
- 44.Mouraux A, Iannetti GD. Nociceptive laser-evoked brain potentials do not reflect nociceptive-specific neural activity. J Neurophysiol. 2009;101:3258–3269. doi: 10.1152/jn.91181.2008. [DOI] [PubMed] [Google Scholar]
- 45.Naveteur J, Mars F, Crombez G. The effect of eye orientation on slowly increasing pain. Eur J Pain. 2005;9:79–85. doi: 10.1016/j.ejpain.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Ohara S, Crone NE, Weiss N, Lenz FA. Attention to a painful cutaneous laser stimulus modulates electrocorticographic event-related desynchronization in humans. Clin Neurophysiol. 2004;115:1641–1652. doi: 10.1016/j.clinph.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 47.Ohara S, Crone NE, Weiss N, Vogel H, Treede RD, Lenz FA. Attention to pain is processed at multiple cortical sites in man. Exp Brain Res. 2004;156:513–517. doi: 10.1007/s00221-004-1885-2. [DOI] [PubMed] [Google Scholar]
- 48.Olausson H, Ha B, Duncan GH, Morin C, Ptito A, Ptito M, Marchand S, Bushnell MC. Cortical activation by tactile and painful stimuli in hemispherectomized patients. Brain. 2001;124:916–927. doi: 10.1093/brain/124.5.916. [DOI] [PubMed] [Google Scholar]
- 49.Ota H, Fujii T, Suzuki K, Fukatsu R, Yamadori A. Dissociation of body-centered and stimulus-centered representations in unilateral neglect. Neurology. 2001;57:2064–2069. doi: 10.1212/wnl.57.11.2064. [DOI] [PubMed] [Google Scholar]
- 50.Oxbury JM, Campbell DC, Oxbury SM. Unilateral spatial neglect and impairments of spatial analysis and visual perception. Brain. 1974;97:551–564. doi: 10.1093/brain/97.1.551. [DOI] [PubMed] [Google Scholar]
- 51.Pavani F, Ladavas E, Driver J. Auditory and multisensory aspects of visuospatial neglect. Trends Cogn Sci. 2003;7:407–414. doi: 10.1016/s1364-6613(03)00189-x. [DOI] [PubMed] [Google Scholar]
- 52.Plaghki L, Mouraux A. How do we selectively activate skin nociceptors with a high power infrared laser? Physiology and biophysics of laser stimulation. Neurophysiol Clin. 2003;33:269–277. doi: 10.1016/j.neucli.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Rainville P, Bushnell MC, Duncan GH. PET studies of the subjective experience of pain. In: Casey KL, Bushnell MC, editors. Pain imaging. vol. 18. Seattle: IASP Press; 2000. pp. 123–156. [Google Scholar]
- 54.Ramachandran VS, Altschuler EL, Stone L, Al Aboudi M, Schwartz E, Siva N. Can mirrors alleviate visual hemineglect? Med Hypotheses. 1999;52:303–305. doi: 10.1054/mehy.1997.0651. [DOI] [PubMed] [Google Scholar]
- 55.Scharein E, Bromm B. The intracutaneous pain model in the assessment of analgesic efficacy. Pain Rev. 1998;5:216–246. [Google Scholar]
- 56.Schwartzman RJ, Kerrigan J. The movement disorder of reflex sympathetic dystrophy. Neurology. 1990;40:57–61. doi: 10.1212/wnl.40.1.57. [DOI] [PubMed] [Google Scholar]
- 57.Siedenberg R, Treede RD. Laser-evoked potentials: exogenous and endogenous components. Electroencephalogr Clin Neurophysiol. 1996;100:240–249. doi: 10.1016/0168-5597(95)00255-3. [DOI] [PubMed] [Google Scholar]
- 58.Snedecor GW, Cochran WG. Statistical methods. Ames: Iowa State University Press; 1967. [Google Scholar]
- 59.Stein B, Price D, Gazzaniga M. Pain perception in a man with total corpus callosum transection. Pain. 1989;38:51–56. doi: 10.1016/0304-3959(89)90072-9. [DOI] [PubMed] [Google Scholar]
- 60.Towell AD, Boyd SG. Sensory and cognitive components of the CO2 laser evoked cerebral potential. EEG Clin Neurophysiol. 1993;88:237–239. doi: 10.1016/0168-5597(93)90009-e. [DOI] [PubMed] [Google Scholar]
- 61.Vallar G, Perani D. The anatomy of unilateral neglect after right-hemisphere stroke lesions. A clinical/CT-scan correlation study in man. Neuropsychologia. 1986;24:609–622. doi: 10.1016/0028-3932(86)90001-1. [DOI] [PubMed] [Google Scholar]
- 62.Vallar G, Rusconi ML, Bignamini L, Geminiani G, Perani D. Anatomical correlates of visual and tactile extinction in humans: a clinical CT scan study. J Neurol Neurosurg Psychiat. 1994;57:464–470. doi: 10.1136/jnnp.57.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Villardita C. Tactile exploration of space and visual neglect in brain-damaged patients. J Neurol. 1987;234:292–297. doi: 10.1007/BF00314283. [DOI] [PubMed] [Google Scholar]
- 64.Watson RT, Heilman KM. Thalamic neglect. Neurology. 1979;29:690–694. doi: 10.1212/wnl.29.5.690. [DOI] [PubMed] [Google Scholar]
- 65.Watson RT, Valenstein E, Day A, Heilman KM. Posterior neocortical systems subserving awareness and neglect. Neglect associated with superior temporal sulcus but not area 7 lesions. Arch Neurol. 1994;51:1014–1021. doi: 10.1001/archneur.1994.00540220060015. [DOI] [PubMed] [Google Scholar]
- 66.Whitlock DG, Perl ER. Thalamic projections of spinothalamic pathways in monkey. Exp Neurol. 1961;3:240–255. doi: 10.1016/0014-4886(61)90015-2. [DOI] [PubMed] [Google Scholar]
- 67.Willis WD. The pain system. Basel: Karger; 1985. [Google Scholar]
- 68.Yamasaki H, Kakigi R, Watanabe S, Hoshiyama M. Effects of distraction on pain-related somatosensory evoked magnetic fields and potentials following painful electrical stimulation. Brain Res Cogn Brain Res. 2000;9:165–175. doi: 10.1016/s0926-6410(99)00056-7. [DOI] [PubMed] [Google Scholar]
- 69.Yamasaki H, Kakigi R, Watanabe S, Naka D. Effects of distraction on pain perception: magneto- and electro-encephalographic studies. Brain Res Cogn Brain Res. 1999;8:73–76. doi: 10.1016/s0926-6410(99)00003-8. [DOI] [PubMed] [Google Scholar]
- 70.Zaslansky R, Sprecher E, Katz Y, Rozenberg B, Hemli JA, Yarnitsky D. Pain-evoked potentials: what do they reallymeasure? EEG Clin Neurophysiol. 1996;100:384–392. [PubMed] [Google Scholar]
- 71.Zaslansky R, Sprecher E, Tenke CE, Hemli JA, Yarnitsky D. The P300 in pain evoked potentials. Pain. 1995;66:39–49. doi: 10.1016/0304-3959(96)03020-5. [DOI] [PubMed] [Google Scholar]