Abstract
Objective
Chronic infections, including periodontal infections, may predispose to cardiovascular disease. We investigated the relationship between periodontal microbiota and hypertension. Methods and Results: 653 dentate men and women with no history of stroke or myocardial infarction were enrolled in INVEST. We collected 4533 subgingival plaque samples (average of 7 samples/subject). These were quantitatively assessed for 11 periodontal bacteria using DNA-DNA checkerboard hybridization. Cardiovascular risk factor measurements were obtained. Blood pressure and hypertension (systolic blood pressure≥140 mmHg, diastolic blood pressure≥90 mmHg or taking antihypertensive medication, or self-reported history) were each regressed on the level of bacteria: (1) considered causative of periodontal disease (etiologic bacterial burden); (2) associated with periodontal disease (putative bacterial burden); (3) associated with periodontal health (health associated bacterial burden). All analyses were adjusted for age, race/ethnicity, gender, education, body mass index, smoking, diabetes, LDL and HDL cholesterol. Etiologic bacterial burden was positively associated with both blood pressure and prevalent hypertension. Comparing the highest vs. lowest tertiles of etiologic bacterial burden, SBP was 9 mmHg higher, DBP was 5 mmHg higher (p for linear trend <0.001 in each case), and the odds ratio for prevalent hypertension was 3.05 (95%CI:1.60,5.82) after multivariable adjustment.
Conclusions
Our data provide evidence of a direct relationship between the levels of subgingival periodontal bacteria and both systolic and diastolic blood pressure as well as hypertension prevalence.
Keywords: infection, inflammation, hypertension, blood pressure, epidemiology, periodontitis
INTRODUCTION
Numerous studies have reported positive associations between periodontal infections and clinical cardiovascular disease (CVD). Among these studies, a pattern has emerged in which findings are markedly stronger for stroke as compared to coronary outcomes.1, 2 One possible explanation for these trends is that periodontal infections might contribute to clinical CVD through risk factors that are more strongly linked to stroke than to coronary pathophysiology. While both hypertension and abnormal cholesterol profiles are established risk factors for stroke and coronary heart disease (CHD), it is generally accepted that hypertension is a stronger risk factor for stroke3 while cholesterol profiles are more strongly linked to CHD.4 Therefore, if periodontal infections contribute to the development of hypertension but have little or no influence on lipid metabolism and cholesterol levels, one would expect periodontal infections to be more strongly associated with stroke, as compared to CHD. There is currently a need for more research on periodontal infections and hypertension to inform this hypothesis, although it is noteworthy that in regard to associations between periodontal infections and cholesterol, most studies have reported weak associations.5–7
A biological relationship between periodontal infections and hypertension is plausible in light of several findings demonstrating associations between periodontal disease and either subclinical atherosclerosis8–12 or endothelial dysfunction.13, 14 Chronically elevated levels of systemic inflammation could also mediate associations between periodontal infections and hypertension, as both conditions have been linked to inflammation.14–16 At least two studies have reported positive associations between periodontal disease and hypertension.17, 18 However, these reports have relied on surrogate markers of infectious periodontal exposure, namely tooth loss, and there are currently no studies that have directly examined bacterial species known to be strongly associated with periodontal infections in relation to hypertension.
The Oral Infections and Vascular Disease Epidemiology Study (INVEST) was specifically designed to study the hypothesis that periodontal infections predispose to accelerated progression of carotid atherosclerosis and incidence of stroke, myocardial infarction and CVD death. In this report, we investigated whether periodontal bacteria previously shown to be strongly associated with clinical periodontal disease19 were also associated with prevalent hypertension and elevated continuous blood pressure measurements. The study assessed the subgingival levels of eleven bacterial species, including, i) four species believed to be causal (or strong correlates of currently unidentified causal species) of periodontitis; ii) five species regarded as putative periodontal pathogens and known to be prevalent in states of gingivitis and periodontitis; and iii) two species that have been frequently reported to be prevalent in states of periodontal health. The latter two groups served as inherent controls, to assess the specificity of the relationship between prevalent hypertension and those bacterial species deemed etiologic of periodontal disease. Therefore, we hypothesized a priori that prevalent hypertension would be positively associated with increased etiologic bacterial burden and either unrelated, or inversely related to the putative and health associated bacterial burdens in a fashion similar to our previous findings for subclinical atherosclerosis.10 Our study also paid particular attention to the assessment of social and cardiovascular risk factors identified as potential confounders in other studies, as previously described.10
METHODS
As previously described,10 INVEST is a randomly sampled prospective population-based cohort study investigating the relationship between oral infections, carotid atherosclerosis and stroke. 1056 subjects were selected by random digit dialing from Northern Manhattan, including Hispanics, Blacks, and Whites. Participants live together in this area and have similar access to medical care. The selection process was derived from the Northern Manhattan Study (NOMAS) in which patients are also enrolled.20 Participants were ≥55 years old and had no baseline history of stroke, myocardial infarction, or chronic inflammatory conditions such as systemic lupus erythematosus, Lyme’s disease, gonococcal arthritis or bacterial endocarditis. 841 participants were dentate. Blood pressure measurements and subgingival plaque samples were available for 731 subjects. Another 78 patients were excluded from multivariate analyses because of missing body mass index (n=7), smoking information (n=15), LDL (n=60), HDL (n=57) or diabetes (n=2) data (some patients lacked several variables). Therefore 653 patients were included in the final analyses, representing 78% of the dentate patients. The Institutional Review Boards approved the study and all subjects provided informed consent.
Oral Examination
Subjects received a complete oral examination by trained, calibrated dental examiners. Assessment of periodontal status was done at six sites per tooth (mesiobuccal, midbuccal distobuccal, mesiolingual, midlingual and distolingual) for all teeth present. Probing depth (mm) and location of the gingival margin in relation to the cementoenamel junction was measured using a UNC-15 manual probe (HuFriedy, Chicago, IL).
Subgingival Plaque Collection and Bacterial Quantification
Up to eight subgingival plaque samples (mean 7; median 8) were collected from pre-determined tooth sites in each subject. 5369 bacterial plaque samples were collected independent of periodontal disease status, from the two most posterior teeth in each quadrant as available (mesiopalatal sites in the maxilla and mesiobuccal sites in the mandible). Due to the aforementioned missing covariate data, 4,533 samples are included in the present analysis. Sterile Gracey curettes were inserted into the pocket until its base was reached and subgingival plaque was collected by a single scaling stroke. The collected plaque mass from each site was transferred into an individual Eppendorf tube containing 200 μl of sterile T-E buffer (10mM Tris HCl, 1.0mm EDTA, pH 7.6). The tubes were immediately transferred into the laboratory and the plaque pellet was re-suspended, vigorously vortexed, and 200 μl of a 0.5M NaOH solution were added. The samples were kept at +4 °C until immobilization onto nylon membranes (see below), within a few days from sample collection.
Eleven bacterial species (Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola, Fusobacterium nucleatum, Prevotella intermedia, Campylobacter rectus, Parvimonas micra, Eikenella corrodens, Veillonella parvula, Actinomyces naeslundii) were assessed using checkerboard DNA-DNA hybridization as previously described.10, 21
Blood Pressure and Hypertension Assessment
Blood pressure was measured after 5 minutes quiet sitting using a calibrated standard aneroid sphygmomanometer (Omron). Two blood pressure measurementswereseparated by 15 minutes.
Hypertension was defined as a systolic blood pressure recording ≥140 mm Hg or a diastolic blood pressure recording ≥90 mm Hg (basedon the average of the two aforementioned blood pressure measurements) or thepatient’s self-report of a history of antihypertensiveuse.
C-Reactive Protein and White Blood Cell Measurements
C-reactive protein (CRP) measurements were performed at the University of Vermont.22 The assay range is 0.175–1100 mg/L. CRP was available on 538 (82%) of the analyzed patients. White blood cells (WBC) were measured with automated cell counters via standard techniques (Coulter STK-R and Coulter STK-S, Coulter Electronics, and Sysmex SE-9500, TOA Medical Electronics).23 WBC counts were available on 611 (93%) of analyzed patients.
Risk Factor Assessment
Physical and neurological examinations were conducted by study physicians. Trained physicians and research assistants administered standardized questionnaires and obtained in-person anthropomorphic measurements and fasting (overnight) blood specimens using standardized protocol. Information on sociodemographic characteristics, cardiovascular risk factors, and other medical conditions were obtained through interview using standardized questions adapted from the Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System.9 Race/ethnicity was based on self-identification.20 All assessments were conducted in English or Spanish. Height and weight were determined using calibrated scales. Blood samples were sent for complete blood count on enrollment. Fasting glucose and lipid panels were measured as described previously.24 LDL-C was computed using the Friedewald equation.24 Diabetes mellitus was defined by a history of diagnosed diabetes or the use of insulin or hypoglycemic medication, or a fasting glucose ≥126 mg/dL (7.0mmol/L). Smoking was assessed both categorically (currently smoking, former smoking or never smoking) and continuously as total pack years of cigarette smoking as previously described.9
STATISTICAL ANALYSIS
All analyses were performed using SAS for windows version 9. The laboratory reported the quantity of bacteria per subgingival plaque sample relative to known standards. For each species, bacterial values were natural log transformed, averaged within mouth, and standardized by dividing these values by the log transformed population standard deviation; we treated one standard deviation on the natural log scale as equivalent across microbes.
Standardized values for the 11 species were summed to define cumulative burden. Subsets of the cumulative burden were further defined as etiologic burden (EB), putative burden (PB), and health associated burden (HAB).10 We utilized (i) the consensus of the 1996 World Workshop in Periodontics identifying three bacterial species as causally related to periodontal disease (P. gingivalis, T. forsythia and A. actinomycetemcomitans),25 and (ii) Socransky’s Red Complex26 further identifying T. denticola as a species that closely co-varies with P. gingivalis, and T. forsythia in pathological periodontal pockets, to create an etiologic burden score, comprising the four species (A. actinomycetemcomitans, P. gingivalis, T. forsythia and T. denticola). The 5 bacterial species deemed putatively associated with periodontal disease (C. rectus, E. corrodens, F. nucleatum, M. micros and P. intermedia) were grouped as PB.25 HAB included two ‘health-associated’ bacterial species, A. naeslundii and V. parvula.19, 26
In order to compare results from the aforementioned bacterial definitions to clinical measures of periodontal disease, we also considered two additional exposure definitions based on probing depth (PD) and/or attachment loss (AL) as follows. First, using the joint CDC/American Academy of Periodontology guidelines27, periodontitis was classified as follows: 1) severe periodontitis; n=248 participants with at least two teeth having interproximal AL≥6 mm and at least one tooth having interproximal PD≥5 mm; 2) moderate periodontitis; n= 352 participants with at least two teeth having interproximal AL≥4 mm or at least two teeth having interproximal PD≥5 mm; 3) no periodontitis: n=53 participants not meeting the aforementioned criteria. In a second approach, participants were categorized into tertiles based on the percent of sites/mouth with ≥ 3 mm PD (%PD≥3). The former definition focused on accepted definitions of clinical periodontitis while the latter was intended to represent a broader spectrum of periodontal disease and was based on previous data from INVEST demonstrating that %PD≥3 better represents the underlying periodontal microbiota28.
Using linear regression models, systolic blood pressure, diastolic blood pressure and hypertension prevalence were separately regressed as dependent variables across tertiles of the aforementioned bacterial burden scores (EB, PB and HAB). All adjusted models included the following covariates: age, body-mass index, sex, race/ethnicity (Hispanic, Black, White), education (defined dichotomously as completed high school yes or no), smoking (defined as never, former or current), physical activity level (low, light, moderate or heavy) diabetes, LDL-cholesterol, HDL-cholesterol. We also considered models adjusting for WBC and CRP in addition to the aforementioned CVD risk factors to assess the evidence that inflammation might mediate the association between bacterial burden and either hypertension or blood pressure. Similarly, logistic regression models were utilized to obtain odds ratios for prevalent hypertension across tertiles of bacterial burden scores. A set of identical analyses were conducted modeling either periodontitis (healthy, moderate, severe) or %PD≥3 (tertiles) as the periodontal exposure.
All analyses were conducted among gender subgroups to examine whether the associations differed by gender. We also performed a subgroup analysis among 405 participants without a self-report history of hypertension and who were therefore not taking hypertension medications.
RESULTS
General Characteristics
Sixty percent of the 653 participants were females, and males were younger (67±8 vs. 70±9 years) (p<0.001). The study population was predominantly tri-ethnic with 56% Hispanics, 23% Black non-Hispanic and 18% White non-Hispanic (the remaining 3% reported ‘other’). Ninety-five percent of Hispanics were foreign born with most from the Dominican Republic.9 Table 1 presents additional characteristics of the study participants across tertiles of etiologic bacterial burden.
Table 1.
Characteristics across Etiologic Bacterial Burden Tertiles, Adjusted for Age and Gender (% or mean±SE)
| Variable | Tertile I N=217 |
Tertile II N=218 |
Tertile III N=218 |
|---|---|---|---|
| Socio-Demographic Variables | |||
| Age* | 70±0.6 | 70±0.6 | 67±0.6 |
| Female | 63% | 56% | 62% |
| Completed high school | 52% | 57% | 51% |
| Hispanic | 54% | 54% | 52% |
| Black | 22% | 21% | 26% |
| White | 21% | 23% | 18% |
| Other | 2% | 3% | 2% |
| Life-style and Behavioral Variables | |||
| Never smokers | 52% | 51% | 51% |
| Former smokers | 32% | 40% | 35% |
| Current smokers | 15% | 11% | 13% |
| Pack years | 111.6 | 12±1.6 | 13±1.6 |
| No physical activity* | 35% | 40% | 50% |
| Light physical activity* | 53% | 45% | 41% |
| Moderate/Heavy physical activity | 13% | 12% | 12% |
| Brushing at least 1/day | 97% | 97% | 99% |
| Flossing at least 1/day* | 52% | 44% | 39% |
| Medical Variables | |||
| Diabetes† | 19% | 15% | 21% |
| Serum total cholesterol (mmol/l) | 5.15±0.06 | 5.19±70.06 | 5.18±0.06 |
| HDL-cholesterol (mmol/l) | 1.35±0.03 | 1.33±0.03 | 1.28±0.03 |
| LDL-cholesterol (mmol/l) | 3.15±0.06 | 3.20±0.06 | 3.27±0.06 |
| Body Mass Index (Kg/M2) | 28.3±0.4 | 29.0±0.4 | 28.0±0.4 |
| WBC* | 5.58±0.13 | 6.08±0.13 | 6.01±0.13 |
| hs-CRP | 4.07±0.59 | 4.31±0.58 | 4.15±0.59 |
p < 0.05 for any difference in tertiles
p < 0.10 for any difference in tertiles
Mean(±SD) systolic and diastolic blood pressures were 139±19 and 79±12 mmHg respectively and 62% (n=406) of participants were hypertensive, 39% of whom were undiagnosed. Among participants who reported blood pressure medication the prevalence of drug class was as follows: 39% ACE inhibitors, 37% calcium-channel blockers, 32% diuretics, 27% used beta-blockers and 20% other. After age and body mass index adjustment, systolic blood pressure was equivalent in males and females, while males had slightly elevated diastolic blood pressure (80 vs. 78 mmHg p=0.01). There were no gender differences in the prevalence of hypertension.
After adjusting for age, gender and BMI, average systolic blood pressure was highest among Blacks (144 mmHg), followed by Hispanics (139 mmHg) and Whites (135 mmHg) (p for any difference=0.001). Diastolic blood pressure was equal among Blacks and Hispanics (80 mmHg) but lower among Whites (77 mmHg) (p for any difference=0.05). 72% of Blacks were hypertensive, as compared to 63% and 51% for Hispanics and Whites respectively (p=0.0004).
The mean±SD of %PD≥3 was 43%±27%. Mean±SD values of etiologic, putative and health associated burden were 31±4, 57±3 and 26±2 (units are sum of standard deviations of ln(bacterial counts over species groupings), respectively and there were no gender differences in these distributions. An analysis of the periodontal microbial profiles showed an unequal distribution with a predominance of A. naeslundii (34%) followed by P. intermedia (20%), those two bacteria accounting for 54% of the subgingival microbiota assessed. The 4 etiologic bacteria accounted for 23% of the microbiota assessed in absolute numbers. In the standardized values presented above, each contributing species represented ~9% of the cumulative burden and the four species comprising the etiologic burden accounted for 35% of the cumulative burden.10
Cumulative Periodontal Bacterial Burden
After adjustment for conventional risk factors, mean systolic blood pressure increased across tertiles of cumulative bacterial burden from 136 mmHg to 138 mmHg to 143 mmHg (p for trend =0.0004). Diastolic blood pressure also increased as follows: 77 mmHg, 79 mmHg and 81 mmHg (p for trend <0.0001). The prevalence of hypertension increased from 57% to 62% to 68% across tertiles (p for trend =0.02). These trends remained essentially unchanged in analyses of the subsample (n=453) with additional adjustments for both white blood cell count and CRP.
Etiologic, Putative and Health Associated Bacterial Burden
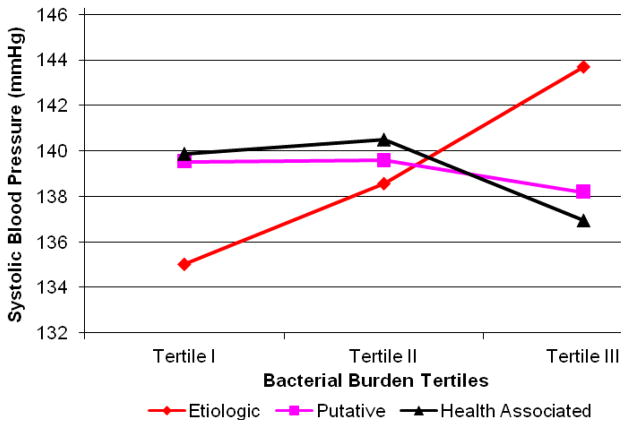
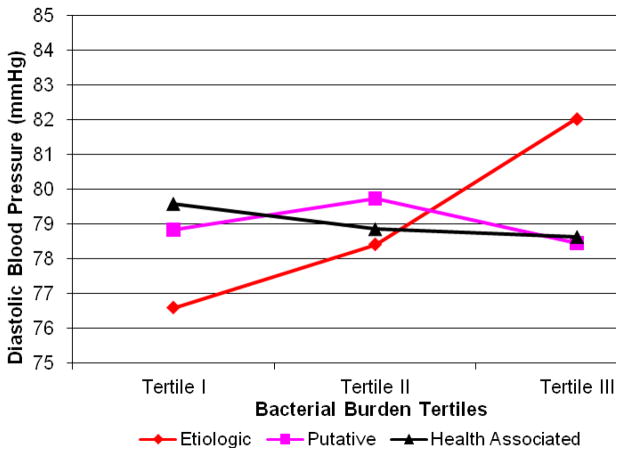
After adjustment for health-associated and putative bacterial burden in addition to conventional risk factors, both mean systolic and diastolic blood pressure increased across tertiles of etiologic bacterial burden (Figures 1 & 2, Table 2). These trends strengthened after further adjustment for white blood cell count and C-reactive protein. There was no association between blood pressure and either health-associated or putative bacterial burden (Figures 1 & 2). There was evidence for an inverse association between putative burden and hypertension among the reduced sample (model 4, Table 2).
Figure 1.
Mean Systolic Blood Pressure across Tertiles of Bacterial Burden: adjusted for etiologic, putative and health-associated bacterial burdens, age, body mass index, smoking, race/ethnicity, gender, diabetes, education, LDL-C, HDL-C (n=653). P for linear trend: causal<0.001, putative=0.54 and protective=0.10
Figure 2.
Mean Diastolic Blood Pressure across Tertiles of Bacterial Burden: adjusted for etiologic, putative and health-associated bacterial burdens, age, body mass index, smoking, race/ethnicity, gender, diabetes, education, LDL-C, HDL-C (n=653). P for linear trend: causal<0.001, putative=0.84 and protective=0.37
Table 2.
Prevalence and Odds Ratios of Hypertension across Increasing Tertiles of Bacterial Burden
| Model | Tertile I N=217 |
Tertile II N=218 |
Tertile III N=218 |
P for linear trend | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Prevalence | OR (95%CI) | Prevalence | OR (95%CI) | Prevalence | OR (95%CI) | ||
| Etiologic Burden | |||||||
|
| |||||||
| 1. | 56% | 1.0 | 64% | 1.40(0.95,2.05) | 67% | 1.61(1.09,2.37) | 0.02 |
| 2. | 51% | 1.0 | 63% | 1.66(1.05,2.60) | 72% | 2.51(1.39,4.53) | 0.002 |
| 3. | 50% | 1.0 | 63% | 1.83(1.11,3.02) | 74% | 3.13(1.62,6.03) | <0.001 |
| 4. | 46% | 1.0 | 64% | 2.48(1.33,4.62) | 74% | 3.93(1.76,8.76) | <0.001 |
| Putative Burden | |||||||
|
| |||||||
| 1. | 60% | 1.0 | 64% | 1.18(0.80,1.74) | 62% | 1.07(0.73,1.57) | 0.74 |
| 2. | 69% | 1.0 | 64% | 0.82(0.52,1.29) | 54% | 0.52(0.29,0.94) | 0.03 |
| 3. | 67% | 1.0 | 64% | 0.87(0.53,1.43) | 55% | 0.54(0.28,1.03) | 0.07 |
| 4. | 72% | 1.0 | 61% | 0.58(0.32,1.07) | 53% | 0.38(0.17,0.85) | 0.02 |
| Health Associated Burden | |||||||
|
| |||||||
| 1. | 59% | 1.0 | 63% | 1.15(0.78,1.70) | 64% | 1.22(0.83,1.80) | 0.31 |
| 2. | 64% | 1.0 | 61% | 1.01(0.67,1.52) | 61% | 1.15(0.75,1.75) | 0.50 |
| 3. | 65% | 1.0 | 60% | 0.83(0.53,1.29) | 61% | 0.86(0.54,1.36) | 0.48 |
| 4. | 61% | 1.0 | 60% | 0.98(0.56,1.69) | 63% | 1.12(0.63,1.99) | 0.65 |
Unadjusted
Adjusted for etiologic, health associated and putative bacterial burden as appropriate
Model 2 plus age, body-mass index, sex, race/ethnicity, education, smoking, physical activity, diabetes, LDL-cholesterol, HDL-cholesterol
Model 3 plus C-reactive protein and white blood cell count (n=200 participants missing CRP or WBC data or CRP >10 were excluded;)
The odds of hypertension were 3.05 (95%CI: 1.60,5.82) times greater among participants in the third vs. first tertile of etiologic burden; after further adjustment for WBC and CRP, the odds ratio increased to 3.93 (95%CI: 1.76, 8.76) (Table 2).
These results were unchanged in analyses adjusting for brushing, flossing, time of last dental visit and family history of stroke (data not shown). In addition, the findings were consistent when restricting the analysis to never smokers (n=335). Specifically, among the never smokers, when comparing first and third tertiles of etiologic burden the prevalence of hypertension increased from 49% to 72% (p<0.01), while systolic and diastolic blood pressure increased by 6 mmHg (p=0.03) and 4 mmHg (p=0.08) respectively.
Analyses focused on systolic and diastolic blood pressure among participants without a self-report history of blood pressure medication use (n=405) were similar: systolic and diastolic blood pressure increased by 7 mmHg (p=0.02) and 4 mmHg (p=0.05), respectively. Similarly, results were unchanged among participants without a history of beta-blocker use (as the indication for beta-blocker use is not always hypertension).
Clinical Periodontal Status
After multivariable adjustment, hypertension prevalence, mean systolic blood pressure or mean diastolic blood pressures across tertiles of %PD≥3 were 57%, 59% and 70% (p for trend=0.004), 136, 138, 143 mmHg (p for trend<0.0001) and 77, 77, 82 mmHg (p for trend<0.0001), respectively. The prevalence of hypertension among participants defined as “healthy” or having either moderate or severe periodontitis was 72%, 58% and 66% (p for linear trend=0.64), respectively. Mean systolic and diastolic blood pressures across these same periodontitis categories were 141, 138, 141 mmHg (p=0.26) and 76, 78, 81 mmHg (p=0.001), respectively.
Sex Specific Findings
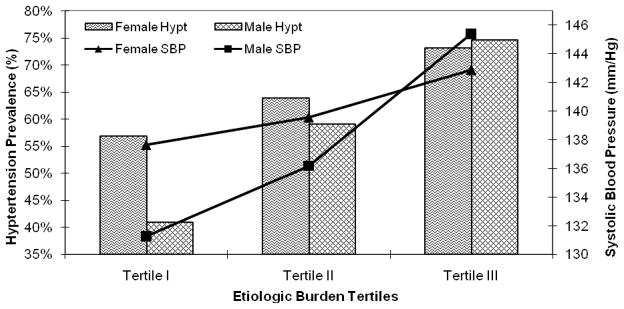
Positive associations between etiologic burden and either hypertension or blood pressure were observed in both men and women after multiple risk factor adjustment. However, the increase in both hypertension prevalence and mean diastolic blood pressure between the first and third tertiles of etiologic burden appeared to be over twice as large for males when compared to females. Similarly, the increase in systolic blood pressure among men was nearly three times larger when compared to women (Figure 3), although the statistical interaction of blood pressure predicted from etiologic burden with gender did not achieve statistical significance. The sex-specific results were nearly identical when defining periodontal status clinically (%PD≥3; data not shown).
Figure 3.
Sex Specific Systolic Blood Pressure and Hypertension Prevalence across Tertiles of Etiologic Bacterial Burden: Adjusted for health associated and protective bacterial burdens, age, body-mass index, race/ethnicity, smoking, education, diabetes, HDL-C and LDL-C; P for linear hypertension trend among females=0.09 & males=0.01; p for gender interaction = 0.44 P for linear systolic blood pressure trend among females=0.05 & males<0.001; p for gender interaction = 0.15
DISCUSSION
Our current findings from INVEST add important information to the nascent literature concerning periodontal infections and hypertension. These data provide the first direct evidence of a relationship between periodontal infections and hypertension, by using direct assessments of periodontal bacterial burden as our exposure as opposed to using clinical surrogates of past infection such as tooth loss, attachment loss and pocket depth. We report a strong positive association between increased subgingival colonization by A. actinomycetemcomitans, P. gingivalis, T. forsythia and T. denticola (etiologic bacterial burden) and prevalent hypertension. After multivariable adjustment, participants in the highest vs. lowest tertile of etiologic bacterial burden experienced an more than a 3-fold increase in the odds of prevalent hypertension. Accordingly, systolic blood pressure increased by 9 mm/Hg and diastolic blood pressure increased by 5 mm/Hg when comparing the highest and lowest tertiles of etiologic burden. These associations remained positive in gender subgroups, although the findings were stronger among men than women. Results were also consistent when using a low threshold clinical periodontal definition reflecting the percent of sites/mouth with ≥ 3 mm pocket depth. However, consistent with previous reports,29 definitions using a higher threshold clinical periodontal definition yielded null or weak results. Previous reports have suggested an association between tooth loss and hypertension. Taguchi and colleagues found tooth loss to be associated with hypertension among n=98 postmenopausal women.18 Data from the large, population-based Study of Health in Pomerania (SHIP) also demonstrated an association between tooth loss and both systolic blood pressure and the prevalence of hypertension.17 However, in contrast to the findings by Taguchi et al., the associations in SHIP were confined to men, while no association was observed among women.
The use of direct bacterial assessments in INVEST minimizes potential biases related to the imprecision of traditional clinical assessments, as there are several noninfectious causes of tooth loss. Moreover, while AL and PD clearly have infectious etiologies26, 30, other noninfectious risk factors, such as smoking,31 exist.
Our findings were specific to the etiologic bacterial cluster previously reported to demonstrate strong positive associations with clinical periodontal disease in our population19 and others.26, 32 Accordingly, both blood pressure level and hypertension prevalence were unrelated to putative or health associated bacterial burden levels. This specificity reduces the likelihood of confounding by healthy lifestyle as previously discussed.10 It is also noteworthy that the clinical definition of periodontal disease based on the percent of sites per mouth with 3 mm pocket depth yielded results nearly identical to our etiologic bacterial exposure definition. These findings are consistent with prior methodological studies regarding appropriate periodontal definitions in studies of infection-induced systemic risk28, 29. As previously discussed29, the concept of subclinical periodontal disease/infection might be important because in population-based settings such as INVEST, substantial exposure to pathological periodontal microbiology likely occurs in shallow periodontal pockets that do not yet exhibit commonly accepted clinical signs of frank periodontal disease28.
The observation that the association between etiologic burden and hypertension was stronger among men than women, despite not achieving statistical significance, is consistent with previous reports regarding periodontal infections and cardiovascular disease.2, 8, 17 While this finding could be due to chance, gender differences in the association between clinical periodontal disease, tooth loss and systemic disease have several potential explanations8, 29 such as: i) differential biological susceptibility to infection induced systemic disease; ii) gender variation in historical infectious exposure; iii) more aggressive periodontal treatment practices in women than in men preventing women from realizing infectious thresholds necessary for systemic effects; iv) gender differences in causes of periodontal disease and tooth loss. Notably, the gender difference reported presently was observed despite comparable levels of prevalent hypertension, blood pressure and bacterial colonization between genders. This may suggest the possibility of a gender differential in biological susceptibility to infectious etiologies of hypertension.
The potential for smoking behaviors to confound periodontal/systemic disease associations is a prominent concern. However, the current and former smoking experience of INVEST participants did not vary across levels of etiologic burden. Moreover, results from subgroup analyses among never smokers were consistent with findings from the full sample. Therefore the potential for spurious findings related to smoking behaviors are substantially minimized in these data.
Systemic inflammation as assessed via WBC and CRP did not explain the current findings between etiologic bacterial burden and hypertension. WBC and CRP are nonspecific markers of systemic inflammation and while several previous studies have reported positive associations between periodontal disease and both WBC and CRP, the associations are generally weak.5, 10, 16, 33 Therefore, elevations in WBC and CRP in the INVEST cohort are likely to be largely attributable to factors other than oral bacterial colonization. Moreover, the relatively high mean CRP levels observed in INVEST (as in the ARIC cohort34) might limit the predictive ability of CRP, as previously described.10
Direct assessments of bacterial levels readily reflect the infectious nature of periodontal disease and are easier to interpret than serological assessments of antibody titers to infective agents that are known to be influenced by multiple factors.35 Assessments of periodontal bacterial profiles by means of DNA-DNA hybridization are well suited for epidemiological studies with large samples sizes and have been shown to correlate well with culture-based data.36 In addition, they have the advantage of not requiring microbial viability, which might be important in the study of chronic bacterial exposures.
We assessed only eleven species out of the over 700 species that have been identified in subgingival biofilms to date.37, 38 However, we assessed the bacteria thought to be causally linked to periodontal disease. While a more comprehensive microbiological assessment might be more informative, the large sample sizes common to epidemiologic investigations precludes this approach for practical and financial reasons. Indeed, even a moderate expansion (by current population-based research standards39) of bacterial assessment to 30 or 40 species would still leave us far short of a truly comprehensive representation of the periodontal flora while at the same time leaving limited analytical options based on a priori hypotheses. We therefore believe to have reached a reasonable compromise with these 11 species. Whether the proposed species are causally related to periodontal disease or are simply strong markers for unmeasured causal species is a separate issue to be resolved by detailed microbiological studies. Nevertheless, additional pathogens might have been informative and could conceivably modify the overall relationship.
Our decision to standardize microbiological collection for all subjects (8 most posterior sites/mouth), irrespective of clinical disease, might have attenuated our results by diluting the degree of exposure to pathogenic bacteria. Although more conservative, this standardized approach yields results more reflective of the “average” pathogen exposure in our study population and therefore reduces the potential for overestimation of the association between infection and hypertension.
This study shares with others the limitations of cross-sectional data. Because both hypertension and periodontal microbiology were measured concurrently, the time sequence cannot be established and causal inferences cannot be made. We must await the prospective results of INVEST and other studies to make firmer conclusions. It is also possible that the variation in bacterial colonization levels observed in INVEST might reflect other risk factors not properly measured or identified, such as salt intake or dietary pattern in general. However, we extensively adjusted for confounders and the relationship strengthened after statistical adjustment, which minimizes this possibility.
These data provide the first direct microbiological evidence of a possible contributory role for periodontal infections in hypertension etiology. Participants with a relative excess of oral pathogens strongly related to clinical periodontal disease in INVEST19 had both elevated blood pressure (systolic and diastolic) levels and increased hypertension odds after adjustment for conventional risk factors. These findings strengthen the hypothesis that periodontal infections may contribute to clinical CVD, and provide insights regarding a mediating mechanism that might explain why periodontal infections have been reported to be a stronger risk factor for stroke than for CHD. While these results require confirmation in prospective settings, they could be of public health importance as both periodontal infections and hypertension are common and hypertension etiology is not completely understood.
Acknowledgments
ACKNOWLEDGMENTS AND FUNDING SOURCES
This research is supported by NIH grants R01 DE-13094 (Dr. Desvarieux), NS-29993 (Dr. Sacco). Dr. Desvarieux is also supported by a Chair of Excellence award from the French Agency for Research and the Institut National de la Santé et de la Recherche Médicale (R05115DD). Dr. Demmer is supported by K99 DE-018739. Dr. Rundek is also supported by NINDS R01 NS-047655. Dr. Jacobs receives support from the Mayo Chair Endowment, School of Public Health, University of Minnesota. We thank the INVEST staff Mr. George Loo, Drs. Mariana Cukier, Yira Flores, Shantanu Lal and Ms. Janet DeRosa for their devoted patient care; Ms. Miriam Herrera-Abreu, Ms. Romi Celenti and Mr. Jun Yang for the laboratory analysis of the dental plaque samples; and most importantly the patients. Patients were seen at the Columbia University General Clinical Research Center, NIH grant 1UL1RR024156.
References
- 1.Janket SJ, Baird AE, Chuang SK, Jones JA. Meta-analysis of periodontal disease and risk of coronary heart disease and stroke. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(5):559–569. doi: 10.1067/moe.2003.107. [DOI] [PubMed] [Google Scholar]
- 2.Demmer RT, Desvarieux M. Periodontal infections and cardiovascular disease: The heart of the matter. J Am Dent Assoc. 2006;137 (Suppl):14S–20S. doi: 10.14219/jada.archive.2006.0402. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, Culebras A, DeGraba TJ, Gorelick PB, Guyton JR, Hart RG, Howard G, Kelly-Hayes M, Nixon JV, Sacco RL. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113(24):e873–923. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 4.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 5.Wu T, Trevisan M, Genco RJ, Falkner KL, Dorn JP, Sempos CT. Examination of the relation between periodontal health status and cardiovascular risk factors: serum total and high density lipoprotein cholesterol, C-reactive protein, and plasma fibrinogen. American Journal of Epidemiology. 2000;151(3):273–282. doi: 10.1093/oxfordjournals.aje.a010203. [DOI] [PubMed] [Google Scholar]
- 6.D’Aiuto F, Nibali L, Parkar M, Suvan J, Tonetti MS. Short-term effects of intensive periodontal therapy on serum inflammatory markers and cholesterol. J Dent Res. 2005;84(3):269–273. doi: 10.1177/154405910508400312. [DOI] [PubMed] [Google Scholar]
- 7.Katz J, Flugelman MY, Goldberg A, Heft M. Association between periodontal pockets and elevated cholesterol and low density lipoprotein cholesterol levels. J Periodontol. 2002;73(5):494–500. doi: 10.1902/jop.2002.73.5.494. [DOI] [PubMed] [Google Scholar]
- 8.Desvarieux M, Schwahn C, Volzke H, Demmer RT, Ludemann J, Kessler C, Jacobs DRJ, John U, Kocher T. Gender differences in the relationship between periodontal disease, tooth loss, and atherosclerosis. Stroke. 2004;35(9):2029–2035. doi: 10.1161/01.STR.0000136767.71518.36. [DOI] [PubMed] [Google Scholar]
- 9.Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DRJ, Papapanou PN, Sacco RL. Relationship between periodontal disease, tooth loss, and carotid artery plaque: the Oral Infections and Vascular Disease Epidemiology Study (INVEST) Stroke. 2003;34(9):2120–2125. doi: 10.1161/01.STR.0000085086.50957.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DRJ, Sacco RL, Papapanou PN. Periodontal microbiota and carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST) Circulation. 2005;111(5):576–582. doi: 10.1161/01.CIR.0000154582.37101.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck JD, Elter JR, Heiss G, Couper D, Mauriello SM, Offenbacher S. Relationship of periodontal disease to carotid artery intima-media wall thickness: the atherosclerosis risk in communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2001;21(11):1816–1822. doi: 10.1161/hq1101.097803. [DOI] [PubMed] [Google Scholar]
- 12.Beck JD, Eke P, Lin D, Madianos P, Couper D, Moss K, Elter J, Heiss G, Offenbacher S. Associations between IgG antibody to oral organisms and carotid intima-medial thickness in community-dwelling adults. Atherosclerosis. 2005 doi: 10.1016/j.atherosclerosis.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Amar S, Gokce N, Morgan S, Loukideli M, Van Dyke TE, Vita JA. Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation. Arterioscler Thromb Vasc Biol. 2003;23(7):1245–1249. doi: 10.1161/01.ATV.0000078603.90302.4A. [DOI] [PubMed] [Google Scholar]
- 14.Tonetti MS, D’Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356(9):911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 15.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290(22):2945–2951. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 16.Slade GD, Ghezzi EM, Heiss G, Beck JD, Riche E, Offenbacher S. Relationship between periodontal disease and C-reactive protein among adults in the Atherosclerosis Risk in Communities study. Arch Intern Med. 2003;163(10):1172–1179. doi: 10.1001/archinte.163.10.1172. [DOI] [PubMed] [Google Scholar]
- 17.Volzke H, Schwahn C, Dorr M, Schwarz S, Robinson D, Doren M, Rettig R, Felix SB, John U, Kocher T. Gender differences in the relation between number of teeth and systolic blood pressure. J Hypertens. 2006;24(7):1257–1263. doi: 10.1097/01.hjh.0000234104.15992.df. [DOI] [PubMed] [Google Scholar]
- 18.Taguchi A, Sanada M, Suei Y, Ohtsuka M, Lee K, Tanimoto K, Tsuda M, Ohama K, Yoshizumi M, Higashi Y. Tooth loss is associated with an increased risk of hypertension in postmenopausal women. Hypertension. 2004;43(6):1297–1300. doi: 10.1161/01.HYP.0000128335.45571.ce. [DOI] [PubMed] [Google Scholar]
- 19.Demmer RT, Papapanou PN, Jacobs DR, Jr, Desvarieux M. Bleeding on probing differentially relates to bacterial profiles: the Oral Infections and Vascular Disease Epidemiology Study. J Clin Periodontol. 2008;35(6):479–486. doi: 10.1111/j.1600-051X.2008.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, Paik MC, Hauser WA. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol. 1998;147(3):259–268. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 21.Socransky SS, Smith C, Martin L, Paster BJ, Dewhirst FE, Levin AE. “Checkerboard” DNA-DNA hybridization. Biotechniques. 1994;17(4):788–792. [PubMed] [Google Scholar]
- 22.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clinical Chemistry. 1997;43(1):52–58. [PubMed] [Google Scholar]
- 23.Elkind MS, Cheng J, Boden-Albala B, Paik MC, Sacco RL The Northern Manhattan Stroke S. Elevated white blood cell count and carotid plaque thickness: the northern manhattan stroke study. Stroke (Online) 2001;32(4):842–849. doi: 10.1161/01.str.32.4.842. [DOI] [PubMed] [Google Scholar]
- 24.Sacco RL, Elkind M, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, Shea S, Paik MC. The protective effect of moderate alcohol consumption on ischemic stroke. JAMA. 1999;281(1):53–60. doi: 10.1001/jama.281.1.53. [DOI] [PubMed] [Google Scholar]
- 25.Consensus report. Periodontal diseases: pathogenesis and microbial factors. Ann Periodontol. 1996;1(1):926–932. doi: 10.1902/annals.1996.1.1.926. [DOI] [PubMed] [Google Scholar]
- 26.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RLJ. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25(2):134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 27.Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(7 Suppl):1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 28.Demmer RT. ProQuest Digital Dissertations Publication Number: AAT 3198088. University of Minnesota; Infections and Systemic Disease: Methodological Issues for Periodontal Infection Models and an Application to Type 2 Diabetes Mellitus Risk Factor Epidemiology. [Google Scholar]
- 29.Demmer RT, Kocher T, Schwahn C, Volzke H, Jacobs DR, Jr, Desvarieux M. Refining exposure definitions for studies of periodontal disease and systemic disease associations. Community Dent Oral Epidemiol. 2008;36(6):493–502. doi: 10.1111/j.1600-0528.2008.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Socransky SS, Haffajee AD. Evidence of bacterial etiology: a historical perspective. Periodontol 2000. 1994;5:7–25. doi: 10.1111/j.1600-0757.1994.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 31.Grossi SG, Zambon JJ, Ho AW, Koch G, Dunford RG, Machtei EE, Norderyd OM, Genco RJ. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. Journal of Periodontology. 1994;65(3):260–267. doi: 10.1902/jop.1994.65.3.260. [DOI] [PubMed] [Google Scholar]
- 32.Papapanou PN, Teanpaisan R, Obiechina NS, Pithpornchaiyakul W, Pongpaisal S, Pisuithanakan S, Baelum V, Fejerskov O, Dahlen G. Periodontal microbiota and clinical periodontal status in a rural sample in southern Thailand. Eur J Oral Sci. 2002;110(5):345–352. doi: 10.1034/j.1600-0722.2002.21361.x. [DOI] [PubMed] [Google Scholar]
- 33.Slade GD, Offenbacher S, Beck JD, Heiss G, Pankow JS. Acute–phase inflammatory response to periodontal disease in the US population. J Dent Res. 2000;79(1):49–57. doi: 10.1177/00220345000790010701. [DOI] [PubMed] [Google Scholar]
- 34.Folsom AR, Aleksic N, Catellier D, Juneja HS, Wu KK. C-reactive protein and incident coronary heart disease in the Atherosclerosis Risk In Communities (ARIC) study. Am Heart J. 2002;144(2):233–238. doi: 10.1067/mhj.2002.124054. [DOI] [PubMed] [Google Scholar]
- 35.Dye BA, Herrera-Abreu M, Lerche-Sehm J, Vlachojannis C, Pikdoken L, Pretzl B, Schwartz A, Papapanou PN. Serum antibodies to periodontal bacteria as diagnostic markers of periodontitis. J Periodontol. 2009;80(4):634–647. doi: 10.1902/jop.2009.080474. [DOI] [PubMed] [Google Scholar]
- 36.Papapanou PN, Madianos PN, Dahlen G, Sandros J. “Checkerboard” versus culture: a comparison between two methods for identification of subgingival microbiota. Eur J Oral Sci. 1997;105(5 Pt 1):389–396. doi: 10.1111/j.1600-0722.1997.tb02135.x. [DOI] [PubMed] [Google Scholar]
- 37.Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, Sahasrabudhe A, Dewhirst FE. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183(12):3770–3783. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000. 2006;42:80–87. doi: 10.1111/j.1600-0757.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 39.Feng Z, Weinberg A. Role of bacteria in health and disease of periodontal tissues. Periodontol 2000. 2006;40:50–76. doi: 10.1111/j.1600-0757.2005.00148.x. [DOI] [PubMed] [Google Scholar]