Abstract
Surveillance, research, and control of mosquito-borne diseases such as West Nile virus require efficient methods for sampling mosquitoes. We compared the efficacy of BG-Sentinel and Centers for Disease Control and Prevention (CDC)-CO2 traps in terms of the abundances of host-seeking and blood-fed female mosquitoes and the origin of mosquito bloodmeals. Our results indicate that BG-Sentinel traps that use CO2 and attractants are as effective as CDC-CO2 traps for Culex mosquito species, Ochlerotatus caspius, and they are also highly efficient at capturing Anopheles atroparvus host-seeking and blood-fed females with or without CO2. The CDC-CO2 trap is the least efficient method for capturing blood-fed females. BG-Sentinel traps with attractants and CO2 were significantly better at capturing mosquitoes that had fed on mammals than the unbaited BG-Sentinel and CDC-CO2 traps in the cases of An. atroparvus and Cx. theileri. These results may help researchers to optimize trapping methods by obtaining greater sample sizes and saving time and money.
Introduction
In recent years, the Mediterranean Basin has experienced several outbreaks of emerging mosquito-borne viruses such as Chikungunya,1 dengue,2 Usutu (USU),3 and West Nile virus (WNV).4 In particular, WNV has recently caused outbreaks in the United States and Europe, being the most relevant in Greece, Italy, France, Romania, Portugal, Spain, and Morocco.5–7 In Spain, the circulation of WNV and other closely related flaviviruses has been reported to have occurred since the 1970s,8 with high seroprevalence levels in birds9,10 and horses.11 Recently, cases of WNV-related illness have been reported in both horses and humans12 (ProMED-mail, Archive Numbers: 20101119.4203 and 20100925.3478). Additionally, WNV and USU have been detected in the mosquito species Culex perexiguus and Cx. pipiens in the Doñana Natural Area.13,14
Surveillance, research, and control of mosquito-borne diseases all require a good knowledge of mosquito populations and their interactions with the different vertebrate hosts. Sampling host-seeking females—and in particular, disease vector species—plays an important part in understanding mosquito population dynamics, spatial distribution, and arbovirus surveillance.15–17 However, the sampling of blood-fed females (i.e., engorged) is essential for assessing mosquito blood-feeding patterns, characterizing disease transmission cycles, and identifying key vectors and their hosts, and all these factors play an important role in the epidemiology of vector-borne diseases.18–20
The use of an accurate mosquito trapping method is crucial, because several studies have reported significant differences in capture efficiencies between methods.21,22 Centers for Disease Control and Prevention (CDC) light traps supplemented with CO2 (CDC-CO2) are routinely used in surveillance programs in many regions in the world and are the most common sampling method used for adult mosquito collection.22 In recent years, BG-Sentinel traps (BGS) designed by the BioGents Corporation have been used for collecting Aedes (Stegomyia) species such as Ae. aegypti, Ae. albopictus, and Ae. polinesiensis.23–25 This type of trap can be used with a variety of mosquito attractants (e.g., CO2, BG-lure, or octenol), thereby making it a versatile tool for mosquito community research and surveillance. Nevertheless, only a few studies have investigated their efficacy regarding the capture of mosquito species other than those species of the genus Aedes.26–28 However, biases related to mosquito diet in the fraction of blood-fed females captured could have major implications for the analyses and interpretation of data originating from mosquito bloodmeals. Indeed, some authors suggest that the synergistic effect of octenol (1-octen-3-ol) and CO2 significantly increases the capture of mosquitoes that feed on mammals.29–31 It has been proposed that mosquito feeding behavior (mammal or avian hosts) is the most likely explanation for the differences in catching rates generated by the different combinations of traps and/or attractants.22,29,32 However, to our knowledge, this hypothesis has never been tested by comparing the origin of vertebrate blood in mosquitoes caught with different trap configurations. Furthermore, little is known regarding the efficacy of different trapping methods for collecting fed mosquitoes19,33; likewise, the effects of mosquito feeding behavior on collection method efficiency are also poorly known.
In this paper, we evaluate the efficacy of two types of traps, BGS (with and without two specific mosquito attractants—BG-lure and octenol—and CO2) and CDC-CO2, in terms of (1) captures of host-seeking females of different mosquito species, (2) captures of blood-fed female mosquitoes, and (3) origin of mosquito bloodmeals in the Doñana Natural Area (Southwestern Spain, Europe).
Materials and Methods
Study area and experimental design.
The study was conducted from July 12 to July 16 in the Doñana Natural Area (Southwestern Spain) (Figure 1), one of the most important wetlands in Europe for migratory birds. Three localities were chosen as replicates for our experimental design: the Doñana Palace (Palacio de Doñana), surrounded by freshwater marshes and heathlands; the Jose Antonio Valverde Visitor Center (FAO), consisting of wetlands with important breeding colonies of herons and ibis and fields with horses; and the Wildlife Breeding Center of Cañada de los Pajaros, a former gravel pit surrounded by rice fields with a great biodiversity of aquatic bird species (exotic and native) and few mammals. In parallel studies34 (Roiz D and others, unpublished results), we detected 11 mosquito species, with Cx. theileri being the most common followed by Cx. perexiguus, Cx. modestus, Cx. pipiens, and Anopheles atroparvus. Ochlerotatus caspius is detected flying from the tidal marshes in the coast (mouth of river Gualdalquivir) at 20–30 km apart of the studied localities. Occasionally, we have detected some specimens of Culiseta longiareolata, An. algeriensis, Cs. annulata, Oc. detritus, and Uranotaenia unguiculata. The experiment was developed in an optimal climate for mosquitoes in the peak period for Culex species abundance in the area in crescent moon with an average temperature of 23.8°C (mean minimum of 17.0°C and mean maximum of 31.3°C) and without any rainfall event.
Figure 1.
Map of the study area and photographs of the different types of traps used: (A) CDC with dry ice; (B) BGS with dry ice; and (C) unbaited BGS without dry ice.
Four trap/attractant configurations were tested: (1) unbaited BGS traps (BioGents, Regensbourg, Germany), (2) BGS traps with CO2 (generated using dry ice) and BG-Lureâ sachets (BioGents, GmbH, Regensbourg, Germany; supplied by AgriSense, Pontypridd, SouthWales, UK), (3) BGS with CO2 and 1-octen-3-ol (Octenol-Bioquip, Bioquip Products, Rancho Dominguez, CA) sachets, and (4) CDC mosquito traps with CO2. The CO2 used in CDC traps attracts mosquitoes, which are then sucked up with a fan. Octenol is a chemical contained in human breath and sweat, whereas BG-Lure contains a combination of substances found on human skin (lactic acid, ammonia, and fatty acids). BGS traps consist of a white cylindrical container covered with gauze in which ascending currents of the attractant are generated in the center of the trap, where there is a catch bag and a fan that sucks up the mosquitoes. A container with a capacity for around 3 kg dry ice was used with the BGS traps (Figure 1). The CDC-CO2 traps were hung on low trees, whereas the various different configurations of BGS traps were placed on the ground.
Each of the four different trap configurations were placed at least 200 m from each other in four different sampling points in each of the four localities and thus, generated three sets of a 4×4 Latin square experimental design. To eliminate any position-specific effect, all traps were rotated to the next position every 24 hours four times during the trapping cycle such that each trap at each locality occupied all four positions during the capture period, making a total of 48 traps per nights. Every 24 hours, mosquitoes were collected, transported in dry ice, and stored at −80°C until processed in the laboratory.
Mosquito and bloodmeal identification.
Frozen mosquitoes were placed on a piece of white filter paper in a Petri plate on a chill table and identified to species level using appropriate taxonomic keys and a stereo microscope.35 Specimens belonging to the Univittatus complex were identified as Cx. perexiguus on the basis of male genitalia.35 Blood-fed females were identified visually by their dilated red abdomens and stored individually at −80°C until molecular bloodmeal identification could be performed. DNA was isolated from abdominal contents using the HotSHOT protocol as described by Alcaide and others.34 DNA extracts from the bloodmeals were used as the DNA template in a standard polymerase chain reaction (PCR) assay. PCR products were subsequently used for a nested PCR to amplify a fragment of the vertebrate cytochrome c oxidase subunit I (COI) mitochondrial gene using previously described primers (M13BC-FW/BCV-RV1 and M13-FW/BCV-RV2) and thermal cycling conditions.34 PCR reactions were carried out using a PTC-100 (Programmable Thermal Controller, MJ Research). PCR-amplified products were cleaned up using ExoSAP-IT (GE Healthcare Life Sciences). Sequencing reactions were performed using BigDye 1.1 technology (Applied Biosystems) with BCV-RV2 primer. Labeled DNA fragments were analyzed using an ABI 3130xl automated sequencer (Applied Biosystems). Sequences were checked using Sequencher v.4.5 (Gene Codes Corp.), and COI sequences were assigned to particular vertebrate species using the Barcode of Life Data (BOLD) Systems platform (http://www.boldsystems.org/views/login.php). Positive identifications of host species were based on exact or nearly exact matches (> 98%).
Statistical analysis.
The effects of the trapping method on estimates of the relative abundance of host-seeking females, blood-fed females, and origin of bloodmeals (mammal or avian) were analyzed in the five most commonly captured species using generalized linear mixed models (GLMM). GLMM allow dependent variables with error structures that differ from normal distributions (as expected for binary and count data) to be modeled while controlling for independent random variables (in this case, sampling site was nested within locality) to test the statistical significance of a fixed independent variable (trapping method). Negative binomial error distribution and logarithmic link were used for the models; the number of host-seeking or blood-fed females was included as a dependent variable, a procedure that is appropriate for count data. We used a negative binomial rather than Poisson-distributed error to reduce model overdispersion caused by the aggregation of captures.36 Because we did not capture any Oc. caspius with unbaited BGS, we sum one individual to one randomly chosen observation from this category to facilitate model convergence. The presence of mammal blood in bloodmeals was modeled with a binomial distributed error and a logit link in a single model for all mosquito species, with species identity included as a fixed factor. In addition, separate analyses were conducted for the two species that had fed mainly on mammals (An. atroparvus and Cx. theileri) and those species that had fed mainly on birds (Cx. modestus, Cx. perexiguus, and Cx. pipiens). Least square mean estimates and standard errors of the model were back-transformed before plotting. Statistical analyses were performed in SAS 9.2 with PROC GLIMMIX (SAS-Institute, Cary, NC) fitted by pseudolikelihood.37
Results
We collected and identified to species level a total of 33,033 female mosquitoes belonging to 10 species: 15 An. algeriensis Theobald, 1903; 4,301 An. atroparvus Van Thiel, 1927; 2,454 Cx. modestus Ficalbi, 1890; 5,035 Cx. perexiguus Theobald, 1903; 219 Cx. pipiens Linnaeus, 1758; 20,563 Cx. theileri Theobald, 1903; 1 Cs. longiareolata Macquart, 1838; 426 Oc. caspius Pallas, 1771; 18 Oc. detritus Haliday, 1833; and 1 Ur. unguiculata Edwards, 1913. In all, 781 of these females were visually identified as blood-fed.
Comparison of traps for capturing host-seeking females.
Six mosquito species were captured in sufficient quantity in a representative number of traps to allow for statistical analysis (An. atroparvus, Cx. pipiens, Cx. theileri, Cx. perexiguus, Cx. modestus, and Oc. caspius). In general, the unbaited BGS captured the fewest females (mean ± standard deviation [SD]: 81.5 ± 96) followed by the CDC-CO2 trap (737.23 ± 669.8), the BG-octenol-CO2 trap (808.8 ± 642.2), and the BG-lure-CO2 trap (1,273.7 ± 1,797). Culex species and Oc. caspius did not show any significant difference between the traps supplied with CO2 (i.e., CDC and BGS with both attractants) (Figure 2). By contrast, unbaited BGS traps were significantly less efficient than the other three trap configurations. However, for An. atroparvus, there were no significant differences between the unbaited BG and the BGS traps with attractants and CO2; for this Anopheles species, the CDC-CO2 traps caught significantly fewer host-seeking females (Figure 2). The CDC-CO2 and the BG-lure-CO2 traps collected the most species (N = 11) followed by the BG-octenol-CO2 trap (N = 10). The unbaited BGS traps captured the fewest species (N = 6).
Figure 2.
Least square means and standard errors of the number of host-seeking females per trap/night. Columns with the same letter are not significantly different (P < 0.05).
Comparison of traps for collecting blood-fed females.
The CDC-CO2 traps captured the fewest blood-fed females of all species (8.7 ± 12.6) followed by the unbaited BGS (15 ± 29.9), the BG-lure-CO2 (21.3 ± 26.1), and the BG-octenol-CO2 traps, of which the latter captured almost three times as many species (23.3 ± 36.1). For the Culex species, the unbaited BGS traps captured fewer blood-fed mosquitoes than BGS traps with attractants and CO2, whereas for An. atroparvus, unbaited BGS traps performed as well as BGS with attractants and CO2 (Figure 3). Oc. caspius was not analyzed, because we captured very few blood-fed females.
Figure 3.
Least square means and standard errors of the number of blood-fed females per trap/night. Columns with the same letter are not significantly different (< 0.05).
Host identification and bloodmeal origin.
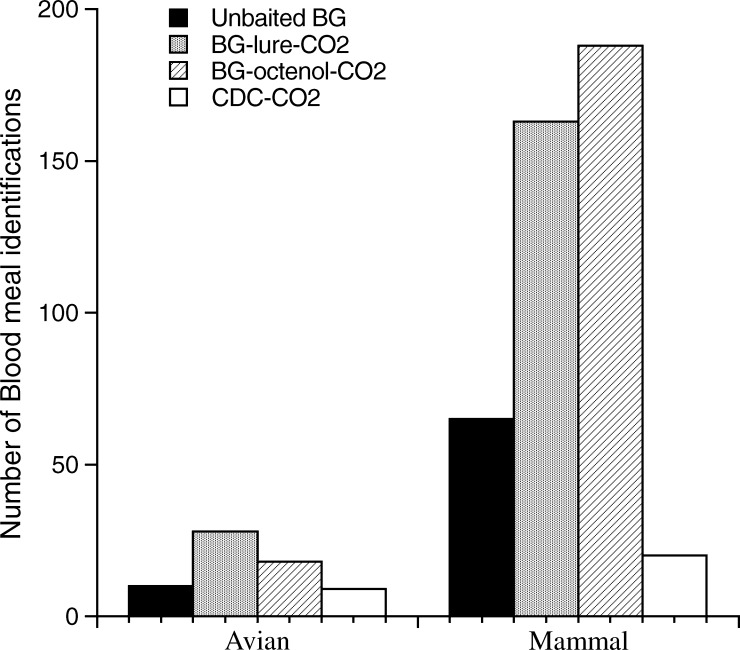
Of the 781 captured blood-fed females, 651 were processed for DNA blood identification. Nevertheless, bloodmeals can be partially digested, and a deterioration of vertebrate DNA occurs (depending on the time elapsed since the mosquito fed20). Consequently, only 507 mosquito bloodmeals (77.8%) could be identified to host species level (440 mammals, including 1 human, 65 birds, and 1 reptile). A total of 35 different host species were identified (23 birds, 11 mammals, and 1 reptile). The BG-lure-CO2 trap was the best for bird bloodmeal trapping (43% host identifications), whereas the BG-octenol-CO2 trap was the best for mammal bloodmeals (43% host identifications); nevertheless, these results were not statistically significant (data not shown). The GLMM analyses of the origin of the bloodmeals (avian or mammal) in relation to the type of trap used indicate a significant effect for the mosquito species (F4,24 = 7.07, P < 0.001), which means that some species feed more on mammals than others, but no significant effect for the trap used (F3,19 = 2.02, P = 0.14). An analysis of the two mosquito species that fed mainly on mammals, Cx. theileri and An. atroparvus, reveals a significant effect of the species (F1,11 = 10.93, P = 0.007) and the trap (F3,19 = 4.88, P = 0.01). BGS traps with CO2 and attractants were significantly better at capturing mammal bloodmeals than the unbaited BGS and CDC-CO2 traps for both these species (Figure 4). However, we detected no significant differences between traps for captures of ornitophilic species (Cx. perexiguus, Cx. modestus, and Cx. pipiens). Despite some differences (Figure 4), BGS traps with octenol and CO2 did not attract significantly more mammal-fed mosquitoes than BGS traps with BG-lure and CO2.
Figure 4.
Number of mammal or bird meal identifications in the five studied mosquito species.
Discussion
Our results show that, for Culex species, there were no significant differences in the relative abundance of host-seeking female mosquitoes trapped in CDC-CO2 and BGS traps with CO2 and attractants. This finding confirms that the different WNV vector species (Cx. pipiens, Cx. perexiguus, Cx. modestus, and Cx. theileri) can be sampled with a similar efficacy using either BGS or CDC-CO2 traps and that BGS traps are as useful for capturing Culex species (WNV vectors) as Aedes (Stegomyia) species.23–25 The same conclusion is valid for Oc. caspius. In addition, the effect of the carbon dioxide in both types of CO2-baited traps was more important for capturing host-seeking mosquitoes than the type of trap itself or the attractants.21 However, there were no significant differences between the unbaited BG and the BGS traps with attractants and CO2 for An. atroparvus host-seeking females; the CDC-CO2 traps caught significantly fewer specimens of this mosquito species. These findings, together with data on An. gambiae38 reported by other researchers, could have important implications for the capture of malaria mosquito vectors,39 especially in areas where access to CO2 is difficult.
Interestingly, the CDC-CO2 traps are, in general, the least efficient way of capturing fed females. In fed Culex females, BGS traps with attractants and CO2 are the best trapping method, although for An. atroparvus, unbaited BGS traps perform as well as BGS traps with CO2. Our findings highlight the importance of choosing the type of traps to be used when designing a field study. Given the abundance of mosquitoes and the relative abundance of fed females during our study, the collection of 1,000 blood-fed females using BG traps with CO2 and an attractant should be completed in 43–47 trap-nights. However, more than the double (115 trap-nights) the number would have been necessary if trapping with CDC-CO2 traps. In the same way, 10,000 unfed females could be easily trapped using 8 trap-nights with BG-Lure CO2 traps, but 122 trap-nights would be necessary if trapping with unbaited BG traps.
To our knowledge, this study is the first to combine an analysis of mosquito trapping efficacy with an analysis of the origin of bloodmeals for two commonly used types of traps. All sampling devices used to survey mosquito populations possess different levels of efficacy and potentially target different mosquito species; they are more selective for a specific fraction of the mosquito community.22 In fact, it has been proposed that the addition of octenol and/or lactic acid (one of the components of the BG-lure attractant) increases the efficiency of traps with dry ice.40 Octenol is a common volatile in the emanations of herbivorous mammals41 and therefore, has been proposed as an attractant for mosquitoes that feed predominately on those vertebrates.42 Additionally, several researchers state that the combination of octenol and CO2 increases the collection rates for certain species31,40,43 but not for others,40,42,44,45 and therefore, in general, results are not uniform.22 However, we did not detect any differences in the responses of these mosquito species to BGS traps baited with the combination CO2-octenol or CO2–BG-lure. In the Doñana area, our data indicate that Cx. perexiguus, Cx. pipiens, and Cx. modestus are generalist species with a preference for birds (70–80% of bloodmeals); Cx. theileri is also generalist but has a preference for mammals (87% of the bloodmeals), whereas An. atroparvus is a specialist in mammals (Muñoz J, and others, unpublished results). In fact, BGS traps with CO2 and attractants performed significantly better in capturing mammal bloodmeals than the unbaited BGS and CDC-CO2 traps for both mammophilic species (An. atroparvus and Cx. theileri). These differences could be because of the ability of octenol and lactic acid to simulate mammal hosts and may bias studies of diet comparison.18,40,46–48 However, we detected no differences among trap configurations in relation to the captures of bird bloodmeals from ornitophilic species, and consequently, no important bias for analyzing bloodmeal diets for those species was detected. Interestingly, we also detected several bird bloodmeals in An. atroparvus (12 of 185) from La Cañada; this finding is unusual, because this species is always described as mammophilic,35 although it is worth remarking that the bloodmeal origin in mosquitoes depends on not only the mosquito species but also, the composition of the vertebrate community. The opportunistic feeding behavior of Culex species together with the heterogeneity of host communities have important consequences for the epidemiology of WNV and other arboviruses.18 Our study contributes new insights that could improve knowledge of zoonotic vector-borne disease patterns through an optimization of trapping tools.
In conclusion, BGS traps with CO2 are highly suitable for monitoring the Culex mosquito species that are vectors of WNV in Mediterranean wetlands and similar habitats, which are priority areas for monitoring virus introduction and amplification.49 BGS traps are suitable for monitoring Oc. caspius and are also highly efficient for capturing An. atroparvus with or without CO2. Comparison with other traps, such as Mosquito Magnet or Zumba traps50,51 and attractants, with and without CO2 and development of ornitophilic lures are important keystones to the ultimate objective of improving trapping efficiency for several mosquito-borne diseases such as WNV and USU. Such an evaluation of the efficacy of the different trapping methods and their biases is essential if we are to provide researchers and fieldworkers with accurate tools for targeted trapping (that is, to point to the fraction of the mosquito community that is of greatest ecoepidemiological importance).
ACKNOWLEDGMENTS
We would like to thank Esmeralda Perez Morueta from the Doñana Biological Station, Seville, Spain, and Juani Moreno from the Mosquito Control Service, Huelva, Spain, for their help and technical support.
Footnotes
Financial support: This work was partially supported by Projects P07-RNM-02511, RNM118, and RNM157 of the Junta de Andalucia and European Commission EuroWestNile FP7 Project 261391.
Authors' addresses: David Roiz, Marion Roussel, Joaquin Muñoz, Ramón Soriguer, and Jordi Figuerola, Department of Wetland Ecology, Estación Biologica de Doñana (EBD-CSIC), Seville, Spain, E-mails: davidroiz@gmail.com, marion.roussel1@gmail.com, quini@ebd.csic.es, soriguer@ebd.csic.es, and jordi@ebd.csic.es. Santiago Ruiz, Mosquito Control Service, Huelva, Spain, E-mail: sruiz@diphuelva.org.
References
- 1.Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, Cordioli P, Fortuna C, Boros S, Magurano F, Silvi G, Angelini P, Dottori M, Ciufolini MG, Majori GC, Cassone A. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- 2.La Ruche G, Souares Y, Armengaud A, Peloux-Petiot F, Delaunay P, Despres P, Lenglet A, Jourdain F, Leparc-Goffart I, Charlet F, Ollier L, Mantey K, Mollet T, Fournier JP, Torrents R, Leitmeyer K, Hilairet P, Zeller H, Van Bortel W, Dejour-Salamanca D, Grandadam M, Gastellu-Etchegorry M. First two autochthonous dengue virus infections in metropolitan France, September 2010. Euro Surveill. 2010;15:19676. [PubMed] [Google Scholar]
- 3.Weissenbock H, Kolodziejek J, Url A, Lussy H, Rebel-Bauder B, Nowotny N. Emergence of Usutu virus, an African mosquito-borne Flavivirus of the Japanese Encephalitis virus group, Central Europe. Emerg Infect Dis. 2002;8:652–656. doi: 10.3201/eid0807.020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Annu Rev Entomol. 2008;53:61–81. doi: 10.1146/annurev.ento.53.103106.093258. [DOI] [PubMed] [Google Scholar]
- 5.Papa A, Danis K, Baka A, Bakas A, Dougas G, Lytras T, Theocharopoulos G, Chrysagis D, Vassiliadou E, Kamaria F, Liona A, Mellou K, Saroglou G, Panagiotopoulos T. Ongoing outbreak of West Nile virus infections in humans in Greece, July–August 2010. Euro Surveill. 2010;15:19644. doi: 10.2807/ese.15.34.19644-en. [DOI] [PubMed] [Google Scholar]
- 6.Angelini P, Tamba M, Finarelli AC, Bellini R, Albieri A, Bonilauri P, Cavrini F, Dottori M, Gaibani P, Martini E, Mattivi A, Pierro AM, Rugna G, Sambri V, Squintani G, Macini P. West Nile virus circulation in Emilia-Romagna, Italy: the integrated surveillance system 2009. Euro Surveill. 2010;15:19547. [PubMed] [Google Scholar]
- 7.Sirbu A, Ceianu CS, Panculescu-Gatej RI, Vazquez A, Tenorio A, Rebreanu R, Niedrig M, Nicolescu G, Pistol A. Outbreak of West Nile virus infection in humans, Romania, July to October 2010. Euro Surveill. 2011;16:19762. [PubMed] [Google Scholar]
- 8.Gonzalez MT, Filipe AR. Antibodies to arboviruses in northwestern Spain. Am J Trop Med Hyg. 1977;26:792–797. doi: 10.4269/ajtmh.1977.26.792. [DOI] [PubMed] [Google Scholar]
- 9.Figuerola J, Jimenez-Clavero MA, Rojo G, Gomez-Tejedor C, Soriguer R. Prevalence of West Nile virus neutralizing antibodies in colonial aquatic birds in southern Spain. Avian Pathol. 2007;36:209–212. doi: 10.1080/03079450701332329. [DOI] [PubMed] [Google Scholar]
- 10.Figuerola J, Soriguer R, Rojo G, Gomez Tejedor C, Jimenez-Clavero MA. Seroconversion in wild birds and local circulation of West Nile virus, Spain. Emerg Infect Dis. 2007;13:1915–1917. doi: 10.3201/eid1312.070343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jimenez-Clavero MA, Llorente F, Sotelo E, Soriguer R, Gomez-Tejedor C, Figuerola J. West Nile virus serosurveillance in horses in Donana, Spain, 2005 to 2008. Vet Rec. 2010;167:379–380. doi: 10.1136/vr.c3155. [DOI] [PubMed] [Google Scholar]
- 12.Kaptoul D, Viladrich PF, Domingo C, Niubo J, Martinez-Yelamos S, De Ory F, Tenorio A. West Nile virus in Spain: report of the first diagnosed case (in Spain) in a human with aseptic meningitis. Scand J Infect Dis. 2007;39:70–71. doi: 10.1080/00365540600740553. [DOI] [PubMed] [Google Scholar]
- 13.Vazquez A, Sanchez-Seco MP, Ruiz S, Molero F, Hernandez L, Moreno J, Magallanes A, Tejedor CG, Tenorio A. Putative new lineage of west nile virus, Spain. Emerg Infect Dis. 2010;16:549–552. doi: 10.3201/eid1603.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vazquez A, Ruiz S, Herrero L, Moreno J, Molero F, Magallanes A, Sanchez-Seco MP, Figuerola J, Tenorio A. West Nile and Usutu viruses in mosquitoes in Spain, 2008–2009. Am J Trop Med Hyg. 2011;85:178–181. doi: 10.4269/ajtmh.2011.11-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roiz D, Rosa R, Arnoldi D, Rizzoli A. Effects of temperature and rainfall on the activity and dynamics of host-seeking Aedes albopictus females in northern Italy. Vector Borne Zoonotic Dis. 2010;10:811–816. doi: 10.1089/vbz.2009.0098. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez-Seco MP, Vazquez A, Collao X, Hernandez L, Aranda C, Ruiz S, Escosa R, Marques E, Bustillo MA, Molero F, Tenorio A. Surveillance of arboviruses in Spanish wetlands: detection of new flavi- and phleboviruses. Vector Borne Zoonotic Dis. 2010;10:203–206. doi: 10.1089/vbz.2008.0188. [DOI] [PubMed] [Google Scholar]
- 17.Almeida AP, Galao RP, Sousa CA, Novo MT, Parreira R, Pinto J, Piedade J, Esteves A. Potential mosquito vectors of arboviruses in Portugal: species, distribution, abundance and West Nile infection. Trans R Soc Trop Med Hyg. 2008;102:823–832. doi: 10.1016/j.trstmh.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006;4:606–610. doi: 10.1371/journal.pbio.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kent RJ, Reiche AS, Morales-Betoulle ME, Komar N. Comparison of engorged Culex quinquefasciatus collection and blood-feeding pattern among four mosquito collection methods in Puerto Barrios, Guatemala, 2007. J Am Mosq Control Assoc. 2010;26:332–336. doi: 10.2987/09-5953.1. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Diaz E, Figuerola J. New perspectives in tracing vector-borne interaction networks. Trends Parasitol. 2010;26:470–476. doi: 10.1016/j.pt.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Kline DL. Traps and trapping techniques for adult mosquito control. J Am Mosq Control Assoc. 2006;22:490–496. doi: 10.2987/8756-971X(2006)22[490:TATTFA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Silver JB, Service MW. London, United Kingdom: Springer; 2008. (Mosquito Ecology: Field Sampling Methods). [Google Scholar]
- 23.Krockel U, Rose A, Eiras AE, Geier M. New tools for surveillance of adult yellow fever mosquitoes: comparison of trap catches with human landing rates in an urban environment. J Am Mosq Control Assoc. 2006;22:229–238. doi: 10.2987/8756-971X(2006)22[229:NTFSOA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Williams CR, Long SA, Russell RC, Ritchie SA. Field efficacy of the BG-Sentinel compared with CDC Backpack Aspirators and CO2-baited EVS traps for collection of adult Aedes aegypti in Cairns, Queensland, Australia. J Am Mosq Control Assoc. 2006;22:296–300. doi: 10.2987/8756-971X(2006)22[296:FEOTBC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Schmaedick MA, Ball TS, Burkot TR, Gurr NE. Evaluation of three traps for sampling Aedes polynesiensis and other mosquito species in American Samoa. J Am Mosq Control Assoc. 2008;24:319–322. doi: 10.2987/5652.1. [DOI] [PubMed] [Google Scholar]
- 26.Molnar T. Regensburg, Germany: University of Regensburg; 2006. (Comparative Studies of Two Trapping Systems for Mosquito Surveillance in Baviera, Germany). [Google Scholar]
- 27.Meeraus WH, Armistead JS, Arias JR. Field comparison of novel and gold standard traps for collecting Aedes albopictus in northern Virginia. J Am Mosq Control Assoc. 2008;24:244–248. doi: 10.2987/5676.1. [DOI] [PubMed] [Google Scholar]
- 28.Obenauer PJ, Kaufman PE, Allan SA, Kline DL. Host-seeking height preferences of Aedes albopictus (Diptera: Culicidae) in north central Florida suburban and sylvatic locales. J Med Entomol. 2009;46:900–908. doi: 10.1603/033.046.0424. [DOI] [PubMed] [Google Scholar]
- 29.Kline DL. Semiochemicals, traps/targets and mass trapping technology for mosquito management. J Am Mosq Control Assoc. 2007;23:241–251. doi: 10.2987/8756-971X(2007)23[241:STAMTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Silver JB. SpringerLink (Online Service) Dordrecht, The Netherlands: Springer Science+Business Media B.V.; 2008. (Mosquito Ecology Field Sampling Methods). [Google Scholar]
- 31.Irish SR, Chandre F, N'Guessan R. Comparison of octenol- and BG Lure-baited biogents sentinel traps and an encephalitis virus surveillance trap in Portland, OR. J Am Mosq Control Assoc. 2008;24:393–397. doi: 10.2987/5682.1. [DOI] [PubMed] [Google Scholar]
- 32.Kline DL. Semiochemicals, traps/targets and mass trapping technology for mosquito management. J Am Mosq Control Assoc. 2007;23:241–251. doi: 10.2987/8756-971X(2007)23[241:STAMTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Odiere M, Bayoh MN, Gimnig J, Vulule J, Irungu L, Walker E. Sampling outdoor, resting Anopheles gambiae and other mosquitoes (Diptera: Culicidae) in western Kenya with clay pots. J Med Entomol. 2007;44:14–22. doi: 10.1603/0022-2585(2007)44[14:soraga]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alcaide M, Rico C, Ruiz S, Soriguer R, Munoz J, Figuerola J. Disentangling vector-borne transmission networks: a universal DNA barcoding method to identify vertebrate hosts from arthropod bloodmeals. PLoS One. 2009;4:e7092. doi: 10.1371/journal.pone.0007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becker N, Petric D, Zgomba M, Boase C, Madon M, Dahl C, Kaiser A, SpringerLink (Online Service) Berlin, Germany: Springer-Verlag; 2010. (Mosquitoes and Their Control). [Google Scholar]
- 36.Ver Hoef JM, Boveng PL. Quasi-Poisson vs. negative binomial regression: how should we model overdispersed count data? Ecology. 2007;88:2766–2772. doi: 10.1890/07-0043.1. [DOI] [PubMed] [Google Scholar]
- 37.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol. 2009;24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Schmied WH, Takken W, Killeen GF, Knols BG, Smallegange RC. Evaluation of two counterflow traps for testing behaviour-mediating compounds for the malaria vector Anopheles gambiae s.s. under semi-field conditions in Tanzania. Malar J. 2008;7:230. doi: 10.1186/1475-2875-7-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okumu FO, Madumla EP, John AN, Lwetoijera DW, Sumaye RD. Attracting, trapping and killing disease-transmitting mosquitoes using odor-baited stations—The Ifakara Odor-Baited Stations. Parasit Vectors. 2010;3:12. doi: 10.1186/1756-3305-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kline DL, Dame DA, Meisch MV. Evaluation of 1-octen-3-ol and carbon dioxide as attractants for mosquitoes associated with irrigated rice fields in Arkansas. J Am Mosq Control Assoc. 1991;7:165–169. [PubMed] [Google Scholar]
- 41.Kline DL, Takken W, Wood JR, Carlson DA. Field studies on the potential of butanone, carbon dioxide, honey extract, 1-octen-3-ol, L-lactic acid and phenols as attractants for mosquitoes. Med Vet Entomol. 1990;4:383–391. doi: 10.1111/j.1365-2915.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- 42.Mboera LE, Takken W, Sambu EZ. The response of Culex quinquefasciatus (Diptera: culicidae) to traps baited with carbon dioxide, 1-octen-3-ol, acetone, butyric acid and human foot odour in Tanzania. Bull Entomol Res. 2000;90:155–159. doi: 10.1017/s0007485300000262. [DOI] [PubMed] [Google Scholar]
- 43.Shone SM, Ferrao PN, Lesser CR, Glass GE, Norris DE. Evaluation of carbon dioxide- and 1-octen-3-ol-baited Centers for Disease Control Fay-Prince traps to collect Aedes albopictus. J Am Mosq Control Assoc. 2003;19:445–447. [PMC free article] [PubMed] [Google Scholar]
- 44.Shone SM, Ferrao PN, Lesser CR, Norris DE, Glass GE. Analysis of mosquito vector species abundances in Maryland using geographic information systems. Ann N Y Acad Sci. 2006;951:364–368. doi: 10.1111/j.1749-6632.2001.tb02720.x. [DOI] [PubMed] [Google Scholar]
- 45.Rueda LM, Harrison BA, Brown JS, Whitt PB, Harrison RL, Gardner RC. Evaluation of 1-octen-3-ol, carbon dioxide, and light as attractants for mosquitoes associated with two distinct habitats in North Carolina. J Am Mosq Control Assoc. 2001;17:61–66. [PubMed] [Google Scholar]
- 46.Van Essen PH, Kemme JA, Ritchie SA, Kay BH. Differential responses of Aedes and Culex mosquitoes to octenol or light in combination with carbon dioxide in Queensland, Australia. Med Vet Entomol. 1994;8:63–67. doi: 10.1111/j.1365-2915.1994.tb00387.x. [DOI] [PubMed] [Google Scholar]
- 47.Cohen SB, Lewoczko K, Huddleston DB, Moody E, Mukherjee S, Dunn JR, Jones TF, Wilson R, Moncayo AC. Host feeding patterns of potential vectors of eastern equine encephalitis virus at an epizootic focus in Tennessee. Am J Trop Med Hyg. 2009;81:452–456. [PubMed] [Google Scholar]
- 48.Mackay AJ, Kramer WL, Meece JK, Brumfield RT, Foil LD. Host feeding patterns of Culex mosquitoes (Diptera: Culicidae) in East Baton Rouge Parish, Louisiana. J Med Entomol. 2010;47:238–248. doi: 10.1603/me09168. [DOI] [PubMed] [Google Scholar]
- 49.Hubalek Z, Halouzka J. West Nile fever—a reemerging mosquito-borne viral disease in Europe. Emerg Infect Dis. 1999;5:643–650. doi: 10.3201/eid0505.990505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xue RD, Doyle MA, Kline DL. Field evaluation of CDC and Mosquito Magnet X traps baited with dry ice, CO2 sachet, and octenol against mosquitoes. J Am Mosq Control Assoc. 2008;24:249–252. doi: 10.2987/5701.1. [DOI] [PubMed] [Google Scholar]
- 51.Bhalala H, Arias JR. The Zumba mosquito trap and BG-Sentinel trap: novel surveillance tools for host-seeking mosquitoes. J Am Mosq Control Assoc. 2009;25:134–139. doi: 10.2987/08-5821.1. [DOI] [PubMed] [Google Scholar]