Abstract
We investigated the use of psoralens and limes to enhance solar disinfection of water (SODIS) using an UV lamp and natural sunlight experiments. SODIS conditions were replicated using sunlight, 2 L polyethylene terephthalate (PET) bottles, and tap water with Escherichia coli, MS2 bacteriophage, and murine norovirus (MNV). Psoralens and lime acidity both interact synergistically with UV radiation to accelerate inactivation of microbes. Escherichia coli was ablated > 6.1 logs by SODIS + Lime Slurry and 5.6 logs by SODIS + Lime Juice in 30-minute solar exposures, compared with a 1.5 log reduction with SODIS alone (N = 3; P < 0.001). MS2 was inactivated > 3.9 logs by SODIS + Lime Slurry, 1.9 logs by SODIS + Lime Juice, and 1.4 logs by SODIS in 2.5-hour solar exposures (N = 3; P < 0.05). MNV was resistant to SODIS, with < 2 log reductions after 6 hours. Efficacy of SODIS against human norovirus should be investigated further.
Introduction
There are 850 million people without access to an improved drinking water source.1 Half of all hospital beds are occupied by people suffering from a water-related illness, according to the Stockholm International Water Institute.2 In low-income regions without access to adequately treated piped water, household water treatment (HWT) has been one of the most cost-effective ways to reduce the incidence of waterborne illness.3,4 Solar disinfection of water (SODIS) is one of several HWT methods that effectively reduce the incidence of diarrheal illness and is recommended by the United Nations Children's Fund (UNICEF) as a HWT method.5
The SODIS protocol, as it is most widely publicized, calls for 1–2 L polyethylene terephthalate (PET) bottles to be exposed to sunlight for 6 hours.6 In cloudy weather, 48 hours of treatment may be necessary to achieve adequate microbial inactivation.7 This long treatment time apparently has had a detrimental impact on SODIS acceptance in communities targeted by SODIS promotional campaigns and sustainable implementation has been difficult. One study in Nepal found that a mere 10% of households actively recruited for a study of SODIS efficacy routinely adopted SODIS during the study.8 In South Africa, a SODIS intervention showed significant reductions in diarrheal disease in highly motivated participants but no disease reduction in less motivated participants, indicating dependence of SODIS upon user participation.9 Although the disinfecting effects of solar radiation on drinking water were first reported 30 years ago,10 a survey of 67 low- and medium-income countries for HWT practices revealed that 0.2% of surveyed homes used SODIS, compared with 21.0% boiling, 5.7% adding bleach, and 4.2% filtering their water.3
Although shorter wavelength UV-C is capable of direct damage of nucleic acids through formation of thymine dimers, these wavelengths are almost entirely absorbed in the Earth's stratosphere, along with the majority of the sun's UV-B radiation.11 The great majority of solar ultraviolet radiation reaching Earth's surface is in the UV-A spectrum (320–400 nm), thus making UV-A irradiation the main driver of SODIS.12 The UV-A wavelengths are not absorbed by nucleic acids, and instead inactivate microorganisms by activating dissolved organic carbon (DOC) in water, which in turn leads to the formation of reactive oxygen species (ROS).13,14 This form of disinfection is more than 1,000-fold slower than direct damage of UV-C.15 Several researchers have looked for means to accelerate SODIS, using such compounds as riboflavin, TiO2, H2O2, and copper plus ascorbic acid.16–21 Many of these compounds have photoactivity in the UV-A spectrum, thereby enhancing normally inefficient insults of UV-A against microorganisms.
Psoralens (furocoumarins) are a class of photoactive cyclic biomolecules and a hitherto unexplored method to accelerate SODIS. Psoralens have been used in the sterilization of blood plasma and platelets, and are widely used to treat dermatologic conditions like psoriasis and vitiligo.22–24 These compounds intercalate into DNA double helices and in the presence of UV-A light peaking near 365 nm, form covalent cross-links between parallel DNA strands, thereby preventing replication of genetic material of the microorganism.25–27
Likely acting as antifungals or insecticides,28 psoralens are found in a variety of plants, including many citrus fruits, parsley, figs, and parsnips.29 Psoralens are found in particularly high concentrations in peel of limes, a fruit that is cultivated in many regions where SODIS may be practically implemented.30 Interestingly, lime juice has been shown to reduce levels of Vibrio cholerae in food and water, although a specific mechanism for this effect has not yet been identified.31–33 PET, the plastic that constitutes most SODIS bottles, is highly transmissive to 365 nm light, suggesting that psoralens could retain their photoreactive effects inside a PET bottle exposed to sunlight.34
We selected three microorganisms to study based on their relevance to human disease. Escherichia coli are a thermotolerant coliform widely used in laboratory studies as a representative bacterial water contaminant. The bacteriophage MS2 is a virus commonly used as a surrogate for human viruses in disinfection studies because of its physicochemical similarities to common pathogenic viruses (e.g., size and shape), the similarity of its behavior in various treatment processes to mammalian viruses, and the relative ease with which it can be studied.35 Finally, we chose to use murine norovirus (MNV) in our SODIS studies because it is the best current surrogate for human norovirus, which cannot be cultured under laboratory conditions.36 The effect of SODIS on viruses has not been adequately studied. Laboratory simulations of SODIS have evaluated various bacteriophages, adenovirus, and poliovirus,37,38 and MS2 inactivation has been studied in sunlight,39 but information on noroviruses in SODIS is very limited. Neither MNV nor any other norovirus surrogate has, as far as we know, been previously studied in SODIS. The existing SODIS literature suggests that viruses are more resistant to SODIS than bacteria such as E. coli. Because viruses are able to persist in an environment for extended periods, are often infectious to humans at very low doses, and are common contaminants of drinking water, it is vital to understand how they behave in SODIS.
To test our hypothesis that psoralens could enhance SODIS, we developed two SODIS models: the first, a laboratory simulation of sunlight using a UV-A lamp, and the second, realistic SODIS conditions using outdoor sunlight, 2 L PET bottles, and dechlorinated tap water. The bottles contained lime juice, lime slurry, or synthetic psoralen, and either E. coli, MS2, or MNV.
Materials and Methods
Definition of terms.
We have adopted a set of terms to define the contents and exposure conditions of each sample. Those samples exposed to sunlight and containing only water and test organisms are referred to as “SODIS.” To indicate the presence of an additive in a sunlight-exposed bottle, the format “SODIS + X” is used (e.g., SODIS + Lime Juice). When a lamp was the source of UV-A light, the term “SODIS” is replaced with “Modified SODIS” or “M SODIS” for brevity. The word “Dark” signifies that the sample was not exposed to light (neither sunlight nor lamp light). “Dark Control” indicates a sample with no additives and no light exposure (i.e., a dark control for SODIS).
Experiments performed.
The test conditions and parameters studied are listed for each organism in Table 1.
Table 1.
Characteristics of experiments performed
| Organism | Escherichia coli | MS2 | MNV |
|---|---|---|---|
| Exposures performed | 30 minutes solar | 2.5 hours solar | 2.5 hours solar |
| 2.5 hours solar | 6 hours solar | 6 hours solar | |
| 6 hours lamp | |||
| Additives tested | 5-MOP | 5-MOP | 5-MOP |
| Lime Slurry | Lime Slurry | Lime Slurry | |
| Lime Juice | Lime Juice | Lime Juice | |
| Lemon Slurry | Lemon Slurry | ||
| Lemon Juice | Lemon Juice | ||
| HCl | HCl |
MNV = murine norovirus; 5-MOP = 5-methoxypsoralen; HCl = hydrochloric acid.
Microbial strains, cultivation conditions, and enumeration.
Stocks of Nalidixic Acid-resistant E. coli CN-13 (ATCC 700609) were generated by inoculating tryptic soy broth (TSB) (Invitrogen, Carlsbad, CA) containing 1% Nalidixic acid solution (Sigma, St. Louis, MO) with one loop (5 μL) of frozen stock, and then incubated on a rotary shaker at 37°C with mixing at 115 rpm for 16–20 hours to produce a stationary-phase stock, which has exhibited greater resistance to SODIS than bacteria in a log-scale phase of growth.40 E. coli CN-13 stocks were enumerated by plating 100 uL or 500 uL 10-fold serial dilutions in phosphate buffered saline (PBS) on 1.5% tryptic soy agar (TSA) Petri dishes with 1% Nalidixic Acid, spreading samples evenly over the plate with glass beads, and incubating overnight at 37°C. MS2 (ATCC 16696-B1) bacteriophage were generated using the Double Agar Layer method with an E. coli Famp (ATCC 700891) bacterial host. Bacteriophage were extracted from cell lysates with an equal volume of chloroform (Sigma), centrifuged at 4,000 × g for 30 minutes at 4°C, sterile filtered through sequential 0.45, 0.2, and 0.1 μm low protein binding syringe filters (Millipore, Billerica, MA), aliquoted, and stored at –80°C. MS2 was enumerated using the bacteriophage plaque assay described previously with the exception that an F-positive log-phase Famp E. coli strain was used as host.41 We were able to spike bottles simultaneously with E. coli and MS2 because CN-13 strain of E. coli is F-negative and therefore cannot be infected by MS2. MNV stocks were generated in monolayers of RAW 264.7 cells (ATCC TIB-71), as previously described.41 Briefly, viral-infected cell lysates were extracted and purified with an equal volume of Vertrel XF (DuPont, Wilmington, DE), centrifuged at 3,000 × g for 10 minutes at 4°C, sterile filtered through sequential 0.45, 0.2, and 0.1 μm low protein binding syringe filters (Millipore), aliquoted, and stored at –80°C. MNV was enumerated by plaque assay as described previously,41 with slight modifications.42 Serial 10-fold dilutions of stock of each organism were performed and plated alongside the test sample on the same day of each experiment to determine stock titer. All plates for E. coli, MS2, and MNV were run in duplicate.
Sample preparation.
PBS was used in all UV lamp experiments (outlined below) except the lamp study of MNV, where dechlorinated tap water (see below) was used as treatment water. For 2 L PET bottle solar experiments, dechlorinated tap water was prepared by adding 1 mL of 3% Na2SO4 per liter of tap water to dechlorinate the water, which was then autoclaved to ensure sterility.43 The DOC content of this tap water is 1–2 mg C/L (Huang H, unpublished data). All sample preparation involving microbes or photoactive compounds was performed in low light to minimize incidental exposure to ultraviolet radiation. Synthetic 5-Methoxypsoralen (5-MOP) was obtained from Sigma-Aldrich (St. Louis, MO). Because 5-MOP is very weakly soluble in water, 20 mg of synthetic compound was first dissolved in 1 mL dimethyl sulfoxide (DMSO) before being added to 2 L of dechlorinated tap water to produce a concentration of 10 mg/L. These solutions were then shaken to ensure full dissolution of the compound. Persian limes (citrus latifolia) and lemons (citrus limon) were purchased from a local Baltimore, MD, supermarket either the same day or the night before each experiment and were kept refrigerated in the dark until they were used. The limes were grown in Mexico and lemons were grown in the United States. Citrus (lime or lemon) juice was prepared by hand squeezing the fruit; the juice was added to 2 L PET bottles for solar experiments and 3 mL test tubes for lamp experiments to produce a 1.5% concentration. For 2 L PET bottles, therefore, 30 mL of juice was added, roughly corresponding to juice of one-half lime or three-quarters lemon. Slurries were produced by finely chopping whole citrus fruit and adding 1.9 mL of solution (PBS for lamp experiments and dechlorinated tap water for solar experiments) to each gram of whole citrus to facilitate homogenization. This mixture was homogenized on ice in an Omni homogenizer (Kennesaw, GA) in three 1-minute intervals. The resulting slurry was centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant was added to test containers at a 3% concentration (this value includes water added before homogenization; therefore, concentration of lime was lower than 3%). Thus, 60 mL of slurry supernatant were added to each 2 L bottle in solar experiments. This procedure was followed with the intent of extracting the maximum amount of bioactive chemicals such as psoralens found predominantly in the peel of the fruit while minimizing turbidity of the water sample. For pH control in solar experiments, 5 mL of 0.6 M HCl was added to 2 L of dechlorinated tap water.
UV-A lamp and solar exposure.
A B-100A mercury lamp (UVP, Upland, CA) with a peak emission at 365 nm (UV-A) and minimal emission in the UV-B or UV-C spectrum was used to simulate sunlight in lamp experiments. Borosilicate glass test tubes with a 3 mL capacity were placed in a rotating carousel 10 cm away from the center of the UV-A lamp during exposure and temperature was maintained near room temperature with a small fan to remove air heated by the lamp. The UV-A output of the lamp 10 cm away from the center of the bulb fluctuated around 12 mW/cm2.
Solar exposures were performed in Baltimore, MD (latitude 39.29754° N, longitude 76.59576° W) between July and early September 2010. Bottles were placed on a flat concrete surface in an open area and were rotated 180° every 15 minutes during exposures. Temperatures were measured using a thermometer probe inside a 2L PET bottle filled with tap water undergoing the same solar exposure conditions as test bottles. Dark control bottles in solar experiments were left in an empty cooler cracked open just enough to allow equilibration with outside temperature. However, because these bottles could not be exposed to infrared light from the sun, their internal temperature did not reach the temperature of bottles in sunlight. The UV data were recorded using a Solarmeter 5.0 UV-A + UV-B radiometer (Solartech, Harrison, MI), which reads a light range from 260 to 400 nm. For each time-point measured in solar experiments, a vertical and a horizontal reading were recorded, thereby estimating both direct and scattered UV incident light44; these values were summed for final UV intensity recording.
Statistical analysis.
For experiments with N > 1, differences in the level of organism inactivation between treatment bottles were examined by one-way analysis of variance (ANOVA). When differences were significant (P < 0.05), a post-hoc Tukey's test was used to determine between which treatments the difference was significant. Data were presented in table form as the mean ± SEM. Statistical calculations were performed with the software Prism 5.0 (GraphPad Software, Inc., San Diego, CA).
Results
Inactivation of E. coli.
30-minute solar exposures (N = 3) (Table 2)
Thirty-minute E. coli experiments were run on 2 days, with duplicate bottles for SODIS + Lime Slurry, SODIS + Lime Juice, and Lime Slurry Dark on the second day, for a total of three SODIS + Lime Slurry, SODIS + Lime Juice, and Lime Slurry Dark bottles. The mean UV flux was 5.7 mW/cm2 in Trial 1 and 6.2 mW/cm2 in Trial 2. The peak water temperature was 29.0°C in Trial 2 and was not recorded in Trial 1.
The results, in the form of log10 reductions, of the 30-minute and 2.5-hour solar exposures for E. coli are provided in Table 2. SODIS + Lime Slurry inactivated E. coli beyond the limit of detection in all three bottles (> 6.1 log reduction). SODIS + Lime Juice reduced E. coli by a log-average of 5.6 logs in 3 bottles over 30 minutes. Lime Slurry Dark bottles showed a log-average reduction of 5.9 logs. SODIS had a log-average reduction of 1.5 logs after the 30-minute exposures. All dark controls except for Lime Slurry Dark showed < 1 log reductions. Both SODIS + Lime Slurry and SODIS + Lime Juice showed statistically significant differences when compared with SODIS (one-way ANOVA, P < 0.0001; Tukey's test, P < 0.001 for both comparisons); the difference between SODIS and Dark Control was not significant; the difference between both SODIS + Lime Slurry and SODIS + Lime Juice and all dark bottles was significant (Tukey's test, P < 0.001 for all comparisons) except for Lime Slurry Dark, which did not differ significantly from these two treatments.
Table 2.
Average logarithmic inactivation and standard error of the mean (SEM) of Escherichia coli, MS2, and murine norovirus (MNV) in solar exposures
| -Log(N/N0) inactivation | |||||||
|---|---|---|---|---|---|---|---|
| Escherichia coli | MS2 | MNV | |||||
| Treatment | 30-minute (SEM) | 2.5-hour (SEM) | 2.5-hour (SEM) | 6-hour* | 2.5-hour (SEM) | 6-hour* | |
| Dark Control | 0.3 (0.1) | 0.1 (0.7) | 0.5 (0.3) | 1.9 | 0.1 (0.1) | 0.4 | |
| 5-MOP Dark | 0.2 (0.0) | 1.3 (0.5) | 0.1 (0.1) | ||||
| Lime Slurry Dark | 5.9 (0.1) | 5.3 (0.7) | 0.7 (0.2) | 1.3 | 0.1 (0.1) | 0.3 | |
| Lime Juice Dark | 0.5 (0.2) | 0.3 (0.1) | 0.5 (0.1) | 0.1 (0.0) | |||
| SODIS | 1.5 (0.5) | > 6.1 (–)† | 1.4 (0.7) | 5.5 | 0.2 (0.1) | 1.4 | |
| SODIS + 5-MOP | > 6.1 (–)† | 2.8 (1.4) | 0.8 (0.5) | ||||
| SODIS + Lime Slurry | > 6.1 (–)† | > 6.1 (–)† | > 3.9 (–)† | > 6.2† | 0.7 (0.4) | 1.8 | |
| SODIS + Lime Juice | 5.6 (0.3) | > 6.1 (–)† | 1.9 (0.7) | > 6.2† | 0.3 (0.2) | 1.7 | |
No replicates were performed for these exposures.
Reached limit of detection for the assay.
2.5-hour solar exposures (N = 3) (Table 2).
Three duplicate experiments of 2.5-hour exposures to sunlight in 2 L PET bottles were performed to evaluate efficacy of limes in enhancing the inactivating effects of SODIS against E. coli, MS2, and MNV. The mean UV flux was 2.9 mW/cm2 in Trial 1, 3.3 mW/cm2 in Trial 2, and 4.3 mW/cm2 in Trial 3. The peak water temperature was 36.0°C in Trial 1, 33.0°C in Trial 2, and 42.5°C in Trial 3.
An E. coli assay was not performed in Trial 1; however, in Trials 2 and 3, E. coli was ablated to the limit of detection (> 6.1 log reduction in Trial 2, > 6.0 log reduction in Trial 3) in all bottles exposed to sunlight. Dark controls showed little or no reduction in counts except in the case of Lime Slurry Dark, which exhibited a 5.3 log inactivation. In contrast to E. coli tests, Lime Slurry Dark did not show any substantial reduction in viral titers, either for MS2 or MNV. There were no statistically significant differences between bottles exposed to sunlight, nor between any of the sun-exposed bottles and Lime Slurry Dark. The differences between all these bottles and all the dark control bottles besides Lime Slurry Dark were significant (one-way ANOVA, P < 0.0001; Tukey's test, P < 0.001 for all comparisons).
Lemons, limes, and pH controls (N = 1).
To determine whether inactivating effects of SODIS + Lime were due primarily to presence of psoralen in lime or the reduced pH of water with lime, experiments with lemons and HCl for pH control were conducted. Because lemons contain much less psoralen than limes, any observed effect of lemons on this system is likely not caused by psoralen.45,46 The pH of the bottle containing lime slurry was 3.6 and lemon slurry was 4.4; for lime juice it was 3.4 and for lemon juice it was 3.3; and a 2 L solution of dechlorinated tap water containing 5 mL of 0.6 M HCl had a pH of 3.4.
Lime slurry was not tested because it had been demonstrated to consistently inactivate E. coli beyond the limit of detection after 30 minutes. SODIS exhibited a 0.6 log reduction. SODIS + Lime Juice exhibited a 5.0 log reduction. SODIS + Lemon Juice was ablated beyond the limit of detection after 30 minutes (> 6.0 logs). SODIS + Lemon Slurry reduced E. coli counts by 4.5 logs. The SODIS + HCl control inactivated E. coli beyond the limit of detection in 30 minutes (> 6.0 logs). Lemon Slurry Dark inactivated E. coli by 2.6 logs. All other dark controls exhibited little reduction (0.3 logs for Dark Control, 0.3 logs for Lime Juice Dark, 0.3 logs for Lemon Juice Dark, and 0.2 logs for HCl Dark).
Inactivation of MS2.
2.5-hour solar exposure (N = 3) (Table 2).
In the case of MS2 bacteriophage, the logarithmic mean reduction over three 2.5-hour trials was 1.4 logs for SODIS, 2.8 logs for SODIS + 5-MOP, > 3.9 logs for SODIS + Lime Slurry (reached limit of detection in Trial 3), and 1.9 logs for SODIS + Lime Juice. The inactivation varied substantially in relation to different intensities of sunlight over the three trials. In Trial 1, only the SODIS + Lime Slurry bottle exhibited a reduction in viral plaque count of > 1 log. In Trial 2, SODIS + Lime Slurry inactivated MS2 beyond the limit of detection (> 4.8 logs), and SODIS + 5-MOP and SODIS + Lime Juice also exhibited strong inactivation. In Trial 3, SODIS + Lime Slurry, SODIS + Lime Juice, and SODIS + 5-MOP showed strongly inactivating effects. SODIS also showed an improved inactivation on this day of higher solar intensity, with a 2.7 log reduction. The only treatment comparisons that were statistically significant were between SODIS + Lime Slurry and Dark Control, Lime Slurry Dark, and Lime Juice Dark (one-way ANOVA P = 0.019; Tukey's test, P < 0.05 for all three comparisons).
6-hour solar exposure (N = 1) (Table 2).
The widely promoted SODIS protocol involves a 6-hour solar exposure on a sunny day. To evaluate effects of SODIS and SODIS + Lime on MS2 and MNV under the conditions of the recommended SODIS protocol, we performed a 6-hour solar exposure in 2 L PET bottles. We did not evaluate E. coli in this experiment because we had previously found that SODIS completely ablated E. coli in 2.5-hour solar exposures. The mean UV flux during the exposure was 5.0 mW/cm2 and water temperature increased during the exposure from 25.0 to 42.5°C.
In this experiment, the Dark Control for SODIS exhibited a 1.9 log reduction from the expected titer. The Lime Slurry Dark bottle had a 1.3 log reduction in MS2. The SODIS also was strongly inactivating, showing a 5.5 log inactivation of MS2 from original titer. The MS2 was completely ablated in both SODIS + Lime Slurry and SODIS + Lime Juice after the 6-hour exposure (> 6.2 log reduction).
Lemons, limes, and pH controls (N = 1).
The effects of other citrus preparations and of pH on MNV were studied in a 2.5-hour exposure in strong sunny conditions. SODIS + Lime Slurry reduced MS2 beyond the limit of detection (> 5.3 logs) in sunlight. The SODIS + Lemon Slurry and SODIS + HCl showed no effect on MS2 compared with SODIS; all three had a < 1 log reduction compared with the Dark Control.
Inactivation of MNV.
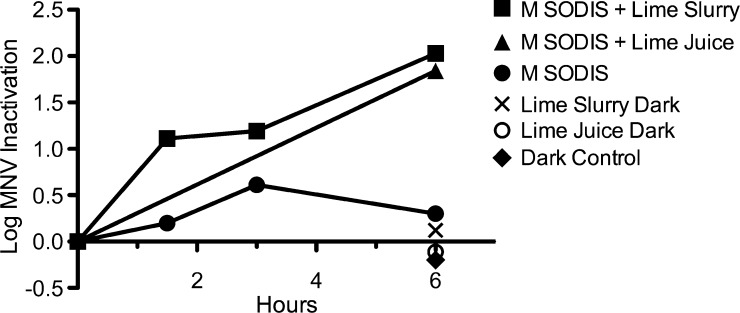
Lamp time-series (N = 1) (Figure 1).
Figure 1.
Logarithmic inactivation of murine norovirus (MNV) in a UV-A lamp exposure.
To obtain preliminary information regarding the inactivation of MNV by solar disinfection processes, we performed a time-series experiment using the UV-A lamp. The results of the study are given in Figure 1. Modified SODIS (M SODIS) + Lime Slurry reduced MNV counts by 1.1 logs after 1.5 hours, 1.2 logs after 3 hours, and 2.0 logs after a 6-hour exposure to the UV-A lamp at an average intensity of 11.7 mW/cm2. The M SODIS + Lime Juice produced a reduction of 1.8 logs after 6 hours, whereas M SODIS test tubes showed only a 0.3 log reduction after 6 hours. There were minimal reductions in MNV in the dark controls, with log reductions of –0.2 for Dark Control, 0.1 for Lime Slurry Dark, and –0.1 for Lime Juice Dark after 6 hours.
2.5-hour solar exposure (N = 3) (Table 2).
In the 2.5-hour solar exposures, MNV was more resistant to SODIS than MS2. The log-average reductions were 0.2 logs for SODIS, 0.9 logs for SODIS + 5-MOP, 0.7 logs for SODIS + Lime Slurry, and 0.3 logs for SODIS + Lime Juice. No bottles in Trials 1 or 2 had a reduction of > 1 log in MNV. In Trial 3, SODIS + Lime Slurry and SODIS + 5-MOP were inactivated by 1.4 logs and 1.7 logs, respectively. Comparison of all treatments yielded no statistically significant differences.
6-hour solar exposure (N = 1) (Table 2).
In a replication of standard 6-hour SODIS protocol, MNV counts were reduced by 1.8 and 1.7 logs for SODIS + Lime Slurry and SODIS + Lime Juice, respectively, whereas the SODIS bottle showed a 1.3 log reduction in MNV counts. Dark Control and Lime Slurry Dark were inactivated by 0.4 logs and 0.3 logs, respectively.
Discussion
In this study, we sought to evaluate the efficacy of SODIS in inactivating surrogates for human viral pathogens and the role psoralens in limes play in enhancing effects of SODIS. We used a synthetic form of 5-MOP, the predominant psoralen in limes, to isolate the effects of this chemical. Initial UV lamp studies indicated that 5-MOP inactivated E. coli at concentrations as low as 5 mg/L. We moved to outdoor solar experiments using 2 L soda bottles and dechlorinated tap water because conditions such as temperature, UV intensity, water turbidity, water volume, and material of the container can significantly alter SODIS outcomes.
Our field findings, consistent with those observed in the laboratory, indicate that SODIS + Lime Slurry and SODIS + Lime Juice have strong inactivating effects against E. coli, intermediate inactivation of MS2, and modest effects against MNV (Table 2). The SODIS + Lime Juice is slightly less effective than SODIS + Lime Slurry. However, because of the ease with which lime juice can be prepared, it represents a more realistic addition to SODIS than lime slurry. None of the SODIS treatments used in this study was able to reduce MNV titers by 2 logs after a 6-hour exposure on a sunny day.
Finally, we compared the effects of limes, which have high psoralen content, to lemons, with lower psoralen content, and HCl. Our results indicate that acidification (pH < 4) is sufficient to observe substantial reductions in E. coli levels in just 30 minutes in the sun, but these effects are not observed with viruses such as MS2, suggesting that inactivation of MS2 observed with limes is likely due primarily to effects of psoralens.
MNV is highly resistant to SODIS.
Other studies have reported that viruses are more resistant to SODIS than bacteria.37,38 However, we are not aware of any previous studies investigating effects of SODIS on norovirus, a leading cause of acute gastroenteritis worldwide, or any of its surrogates. We used MNV, which has been shown to be an applicable surrogate for human norovirus in other applications.47–49 We found that after a 2.5-hour exposure in bright sun (Trial 3), SODIS only registered a 0.4 log reduction in infectious MNV by plaque assay. After 6 hours, SODIS achieved a 1.4 log reduction in viable MNV particles. The SODIS + Lime Slurry and SODIS + Lime Juice fared only slightly better, with 1.8 log and 1.7 log reductions after 6-hour exposure, respectively. There were no significant differences between any samples of MNV in the 2.5-hour exposures, including the dark control bottles.
These results contrast with those for MS2, which, although still more resistant to SODIS + Lime than E. coli, was reduced below the limit of detection (> 6.2 log) by SODIS + Lime Slurry and SODIS + Lime Juice in the same 6-hour exposure (Table 2). SODIS also achieved better reductions in MS2 than MNV. The differences between SODIS + Lime Slurry and the dark controls in the 2.5 hours of exposure were statistically significant, although no other treatments exhibited statistically significant differences.
Previous studies have shown MS2 to be more resistant to simulated solar irradiation than human poliovirus or other bacteriophages and roughly equal to human adenovirus in resistance to simulated solar irradiation.37 Our study, therefore, suggests that MNV is exceptionally resistant to damage by SODIS. Because human norovirus is a prominent cause of waterborne gastrointestinal illness, our findings raise questions about the efficacy of SODIS for preventing viral gastroenteritis and warrant further investigation.
Because DOCs represent the principal sensitizer for formation of ROSs in SODIS, it is possible that water with higher levels of DOCs than the tap water used in these experiments might display higher rates of inactivation of MNV and other organisms.14 Therefore, it will be important to study MNV inactivation in other water sources to determine whether inactivation remains low.
Psoralens and limes accelerate SODIS.
In a series of 30-minute solar exposures, we showed that SODIS + 5-MOP, SODIS + Lime Slurry, and SODIS + Lime Juice consistently achieved near 6 log reductions of E. coli, compared with 1.5 log inactivation by SODIS. These differences were statistically significant.
Killing effects of SODIS + Lime appear to have multiple mechanisms.
Limes contain a variety of compounds that may have microbicidal effects. In addition to psoralens, discussed previously, they also contain ascorbic and citric acids and limonene, a terpene found in many citrus fruits, including lemons.50–53 The effective inactivation of microbes by SODIS + 5-MOP likely accounts for part of the inactivating effects observed with limes. However, our research and that of previous studies using artificial UV sources suggest the possibility of other mechanisms.21
We performed two experiments comparing effects of adding lime, lemon, and HCl to the SODIS protocol. In a 30-minute exposure, all these preparations strongly inactivated E. coli. These results suggest that a pH below 4 alone may be sufficient to dramatically reduce E. coli viability in the presence of sunlight. These results are consistent with findings of some other investigators,21 but are in contrast to investigators who did not observe any changes in killing of E. coli with a solar lamp between pH 4–9.54 Interestingly, none of the bottles with low pH showed substantial inhibition of E. coli in the dark except for Lime Slurry Dark and Lemon Slurry Dark. This finding indicates that a synergistic antimicrobial relationship is present between a low pH solution and solar radiation.
The lemon and lime slurries both showed a substantially reduced E. coli count in the dark. However, this finding can be attributed to the presence of limonene in the peel of both fruits, which can have a bactericidal effect regardless of UV exposure.53 Because dark controls of slurries ablated E. coli, none of the ablation of E. coli in the slurry samples can be attributed to an effect of sunlight. However, the dark control slurries did not reduce either MS2 or MNV counts, indicating that sunlight does in fact play an important synergistic role with slurry in inactivating viruses. This synergy may be caused by the presence of psoralen in lime bottles.
Weather alters efficacy of SODIS.
In our comparison of 2.5-hour solar exposures on 3 days with different levels of cloud cover, we found that rates of viral inactivation depended heavily upon solar flux during the experiment. Although there were some brief periods of strong solar intensity during Trial 1, the sun was obstructed by a thick cloud cover for most of the experiment. In contrast, the sky was almost entirely clear during Trial 3, with only brief periods of light cloud cover. There was considerable variability in results with MNV and MS2 that appears to correlate with intensity of UV radiation during each trial. These results indicate that current conservative recommendations for solar exposures in cloudy weather are warranted.
Conclusions
SODIS can be practiced in regions receiving 3–5 hours of > 500 W/m2 solar radiation daily.7 There is a large overlap between these high solar intensity regions of the world and areas where limes and lemons are cultivated. The preliminary results of this study show SODIS + Citrus to be effective at greatly reducing E. coli levels in just 30 minutes, a treatment time on a par with boiling and other HWT methods. The 30 mL juice per 2 L water amounts to about one-half Persian lime per bottle, a quantity that will likely not be prohibitively expensive or create an unpleasant flavor. Furthermore, many cultures already practice treatment with citrus juice (e.g., preparation of ceviche in Latin America), perhaps indicating that this treatment method will be more appealing to potential SODIS users than other additives such as TiO2 or H2O2.
However, a number of issues should be investigated before field implementation of SODIS + Lime can begin. The large contribution to morbidity of diarrheal disease attributed to human norovirus indicates that the absence of a statistically significant reduction of MNV by SODIS and SODIS + Citrus should be further investigated, as this may represent a major obstacle to SODIS programs. The effects of SODIS + Lime on other viruses and spore-forming organisms should be studied. Additional research should be done to evaluate the use of lemon or other acidic fruit instead of Persian limes, as these other fruits may be more easily obtained in certain regions. An analysis of psoralen content of bottles containing lime juice should be performed to assess potential toxicity risks, although these are not anticipated to be substantial.30
ACKNOWLEDGMENTS
We thank James Schissler and Cecilia Larocca for their assistance, Jon Lorsch and Timothy Julian for their invaluable insights and constructive review, and Paul Miller for generously allowing us to use his ultraviolet lamp and laboratory space.
Footnotes
Financial support: This research was supported in part by the Osprey Foundation of Maryland, the JHU Global Water Program, the JHU SOM Dean's Funding for Summer Research, and the JHU SOM Scholarly Concentrations.
Authors' addresses: Alexander S. Harding, The Johns Hopkins University School of Medicine, Baltimore, MD, E-mail: ahardin4@jhmi.edu. Kellogg J. Schwab, Johns Hopkins University Bloomberg School of Public Health, Department of Environmental Health Sciences, Division of Environmental Health Engineering and the Johns Hopkins Global Water Program, Baltimore, MD, E-mail: kschwab@jhsph.edu.
References
- 1.WHO/UNICEF . World Health Organization/UNICEF; France: 2009. p. 28. (Water for Life: Making It Happen). [Google Scholar]
- 2.Stockholm International Water Institute Stockholm Statement and Reflections on the MDG Summit. 2010. http://www.siwi.org/sa/node.asp?node=52&sa_content_url=%2Fplugins%2FResources%2Fresource.asp&id=232 Available at. Accessed May 14, 2011.
- 3.Rosa G, Clasen T. Estimating the scope of household water treatment in low- and medium-income countries. Am J Trop Med Hyg. 2010;82:289–300. doi: 10.4269/ajtmh.2010.09-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clasen T, Haller L, Walker D, Bartram J, Cairncross S. Cost-effectiveness of water quality interventions for preventing diarrhoeal disease in developing countries. J Water Health. 2007;5:599–608. doi: 10.2166/wh.2007.010. [DOI] [PubMed] [Google Scholar]
- 5.UNICEF Promotion of Household Water Treatment and Safe Storage in UNICEF WASH Programmes. 2008. http://www.unicef.org/was Available at. Accessed May 14, 2011.
- 6.SODIS an initiative of The Swiss Federal Institute of Aquatic Science and Technology. 2011. www.SODIS.ch Available at. Accessed May 14, 2011.
- 7.Oates P, Shanahan P, Polz M. Solar disinfection (SODIS): simulation of solar radiation for global assessment and application for point-of-use water treatment in Haiti. Water Res. 2003;37:47–54. doi: 10.1016/s0043-1354(02)00241-5. [DOI] [PubMed] [Google Scholar]
- 8.Rainey R, Harding A. Drinking water quality and solar disinfection: effectiveness in peri-urban households in Nepal. J Water Health. 2005;3:239–248. doi: 10.2166/wh.2005.036. [DOI] [PubMed] [Google Scholar]
- 9.Du Preez M, Mcguigan KG, Conroy RM. Solar disinfection of drinking water in the prevention of dysentery in South African children aged under 5 years: the role of participant motivation. Environ Sci Technol. 2010;44:8744–8749. doi: 10.1021/es103328j. [DOI] [PubMed] [Google Scholar]
- 10.Acra A, Karahagopian Y, Raffoul Z, Dajani R. Disinfection of oral rehydration solutions by sunlight. Lancet. 1980;2:1257–1258. doi: 10.1016/s0140-6736(80)92530-1. [DOI] [PubMed] [Google Scholar]
- 11.Madronich S, Flocke S, editors. Solar Ultraviolet Radiation: Modelling, Measurement, and Effects. Berlin: Springer; 1997. p. 23. [Google Scholar]
- 12.Wegelin M, Canonica S, Fleischmann T, Pesaro F, Metzler A. Solar water disinfection: scope of the process and analysis of radiation experiments. J Water SRT-Aqua. 1994;43:154–169. [Google Scholar]
- 13.Reed R. Solar inactivation of fecal bacteria in water: the critical role of oxygen. Lett Appl Microbiol. 1997;24:276–280. doi: 10.1046/j.1472-765x.1997.00130.x. [DOI] [PubMed] [Google Scholar]
- 14.Abele Oeschger D, Tug H, Rottgers R. Dynamics of UV-driven hydrogen peroxide formation on an intertidal sand flat. Limnol Oceanogr. 1997;42:1406–1415. [Google Scholar]
- 15.Jagger J. New Jersey: Prentice-Hall; 1967. p. 114. (Introduction to Research in Ultraviolet Photobiology). [Google Scholar]
- 16.Heaselgrave W, Kilvington S. Antimicrobial activity of simulated solar disinfection against bacterial, fungal, and protozoan pathogens and its enhancement by riboflavin. Appl Environ Microbiol. 2010;76:6010–6012. doi: 10.1128/AEM.00445-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunlop P, Byrne J, Manga N, Eggins B. The photocatalytic removal of bacterial pollutants from drinking water. J Photochem Photobiol B. 2002;148:355–363. [Google Scholar]
- 18.Gelover S, Gomez L, Reyes K, Leal M. A practical demonstration of water disinfection using TiO2 films and sunlight. Water Res. 2006;40:3274–3280. doi: 10.1016/j.watres.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Sciacca F, Rengifo-Herrera J, Wethe J, Pulgarin C. Dramatic enhancement of solar disinfection (SODIS) of wild Salmonella spp. in PET bottles by H2O2 addition on natural water in Burkina Faso containing dissolved iron. Chemosphere. 2010;78:1186–1191. doi: 10.1016/j.chemosphere.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Spuhler D, Rengifo-Herrera J, Pulgarin C. The effect of Fe2+, Fe3+, H2O2 and the photo-Fenton reagent at near neutral pH on the solar disinfection (SODIS) at low temperatures of water containing Escherichia coli K12. Appl Catal B. 2010;96:126–141. [Google Scholar]
- 21.Fisher M, Keenan C, Nelson K, Voelker B. Speeding up solar disinfection (SODIS): effects of hydrogen peroxide, temperature, pH, and copper plus ascorbate on the photoinactivation of E. coli. J Water Health. 2008;6:35–51. doi: 10.2166/wh.2007.005. [DOI] [PubMed] [Google Scholar]
- 22.Wollowitz S. Fundamentals of the psoralen-based Helinx technology for inactivation of infectious pathogens and leukocytes in platelets and plasma. Semin Hematol. 2001;38:4–11. doi: 10.1016/s0037-1963(01)90118-0. [DOI] [PubMed] [Google Scholar]
- 23.Lin L, Wiesehahn G, Morel P, Corash L. Use of 8-Methoxypsoralen and long-wavelength ultraviolet radiation for platelet decontamination. Blood. 1989;74:517–525. [PubMed] [Google Scholar]
- 24.Parrish J. Photochemotherapy of psoriasis. Arch Dermatol. 1976;112:35–36. [PubMed] [Google Scholar]
- 25.Dall'Acqua F, Marciani S, Vedaldi D, Rodighiero G. Studies on the photoreactions (365 nm) between DNA and some methylpsoralens. Biochim Biophys Acta. 1974;353:267–273. doi: 10.1016/0005-2787(74)90019-7. [DOI] [PubMed] [Google Scholar]
- 26.Scott B, Pathak M, Mohn G. Molecular and genetic basis of furocoumarin reactions. Mutat Res. 1976;39:29–74. doi: 10.1016/0165-1110(76)90012-9. [DOI] [PubMed] [Google Scholar]
- 27.Cole R. Light-induced cross-linking of DNA in the presence of furocoumarin (psoralen) Biochim Biophys Acta. 1970;217:30–39. doi: 10.1016/0005-2787(70)90119-x. [DOI] [PubMed] [Google Scholar]
- 28.Schuler M. The role of Cytochrome P450 monooxygenases in plant-insect interactions. Plant Physiol. 1996;112:1411–1419. doi: 10.1104/pp.112.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pathak M, Daniels F, Fitzpatrick T. The presently known distribution of furocoumarins (psoralens) in plants. J Invest Dermatol. 1962;1:225–239. doi: 10.1038/jid.1962.106. [DOI] [PubMed] [Google Scholar]
- 30.Gorgus E, Lohr C, Raquet N, Guth S, Schrenk D. Limettin and furocoumarins in beverages containing citrus juices or extracts. Food Chem Toxicol. 2010;48:93–98. doi: 10.1016/j.fct.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 31.Rowe AK, Angulo FJ, Tauxe RV. A lime in a litre rapidly kills toxogenic Vibrio cholerae O1. Trop Doct. 1998;28:247–248. doi: 10.1177/004947559802800427. [DOI] [PubMed] [Google Scholar]
- 32.Rodrigues A, Sandstrom A, Ca T, Steinsland H, Jensen H, Aaby P. Protection from cholera by adding lime juice to food-results from community and laboratory studies in Guinea-Bissau, West Africa. Trop Med Int Health. 2000;5:418–422. doi: 10.1046/j.1365-3156.2000.00575.x. [DOI] [PubMed] [Google Scholar]
- 33.Ulate-Rodriguez J, Schafer H, Zottola E, Davidson P. Inhibition of Listeria monocytogenes, Escherichia coli O157:H7, and Micrococcus luteus by linear furanocoumarins in a model food system. J Food Prot. 1997;60:1050–1054. doi: 10.4315/0362-028X-60.9.1050. [DOI] [PubMed] [Google Scholar]
- 34.Wegelin M, Canonica S, Alder A, Marazuela D, Suter M, Bucheli T, Haefliger O, Zenobi R, McGuigan K, Kelly M, Ibrahim P, Larroque M. Does sunlight change the material and content of polyethylene terephthalate (PET) bottles? J Water SRT-Aqua. 2001;5:125–133. [Google Scholar]
- 35.Dawson DJ, Paish A, Staffell LM, Seymour IJ, Appleton H. Survival of viruses on fresh produce, using MS2 as a surrogate for norovirus. J Appl Microbiol. 2005;98:203–209. doi: 10.1111/j.1365-2672.2004.02439.x. [DOI] [PubMed] [Google Scholar]
- 36.Wobus CE, Thackray LB, Virgin HW IV. Murine norovirus: a model system to study norovirus biology and pathogenesis. J Virol. 2006;80:5104–5112. doi: 10.1128/JVI.02346-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Love D, Silverman A, Nelson K. Human virus and bacteriophage inactivation in clear water by simulated sunlight compared to bacteriophage inactivation at a southern California beach. Environ Sci Technol. 2010;44:6965–6970. doi: 10.1021/es1001924. [DOI] [PubMed] [Google Scholar]
- 38.Heaselgrave W, Patel N, Kilvington S, Kehoe SC, McGuigan KG. Solar disinfection of poliovirus and Acanthamoeba polyphaga cysts in water–a laboratory study using simulated sunlight. Adv Appl Microbiol. 2006;43:125–130. doi: 10.1111/j.1472-765X.2006.01940.x. [DOI] [PubMed] [Google Scholar]
- 39.Carey-Walker D, Len SV, Sheehan B. Development and evaluation of a reflective solar disinfection pouch for treatment of drinking water. Appl Environ Microbiol. 2004;70:2545–2550. doi: 10.1128/AEM.70.4.2545-2550.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berney M, Weilenmann HU, Ihssen J, Bassin C, Egli T. Specific growth rate determines the sensitivity of Escherichia coli to thermal, UVA and solar disinfection. Appl Environ Microbiol. 2006;72:2586–2593. doi: 10.1128/AEM.72.4.2586-2593.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bae J, Schwab K. Evaluation of murine norovirus, feline calicivirus, poliovirus, and MS2 as surrogates for human norovirus in a model for viral persistence in surface water and groundwater. Appl Environ Microbiol. 2008;74:477–484. doi: 10.1128/AEM.02095-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibson KE, Schwab KJ. Tangential-flow ultrafiltration with integrated inhibition detection for recovery of surrogates and human pathogens from large-volume source water and finished drinking water. Appl Environ Microbiol. 2011;77:385–391. doi: 10.1128/AEM.01164-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eaton A. Washington, DC: American Public Health Association Washington; 2005. (Standard Methods for the Examination of Water and Waste Water). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sichel C, Tello J, de Cara M, Fernández-Ibáñez P. Effect of UV solar intensity and dose on the photocatalytic disinfection of bacteria and fungi. Catal Today. 2007;129:152–216. [Google Scholar]
- 45.Wagstaff D. Dietary exposure to furocoumarins. Regul Toxicol Pharmacol. 1991;14:261–272. doi: 10.1016/0273-2300(91)90029-u. [DOI] [PubMed] [Google Scholar]
- 46.Stanley W, Vannier S. Psoralens and substituted coumarins from the expressed oil of lime. Phytochemistry. 1967;6:585–596. [Google Scholar]
- 47.Cannon J, Papafragkou E, Park G, Osborne J, Jaykus L, Vinje J. Surrogates for the study of norovirus stability and inactivation in the environment: a comparison on murine norovirus and feline calicivirus. J Food Prot. 2006;69:2761–2765. doi: 10.4315/0362-028x-69.11.2761. [DOI] [PubMed] [Google Scholar]
- 48.Hewitt J, Rivera-Aban M, Greening G. Evaluation of murine norovirus as a surrogate for human norovirus and hepatitis A virus in heat inactivation studies. J Appl Microbiol. 2009;107:65–71. doi: 10.1111/j.1365-2672.2009.04179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nappier S, Graczyk T, Schwab K. Bioaccumulation, retention, and depuration of enteric viruses by Crassotrea virginica and Crassotrea ariakensis oysters. Appl Environ Microbiol. 2008;74:6825–6831. doi: 10.1128/AEM.01000-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minh Tu N, Thanh L, Une A, Ukeda H, Sawamura M. Volatile components of Vietnamese pummelo, orange, tangerine, and lime peel oils. Flavour Fragrance J. 2002;17:169–174. [Google Scholar]
- 51.Wattenberg L, Coccia J. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone carcinogenesis in mice by D-limonene and citrus fruit oils. Carcinogenesis. 1991;12:115–117. doi: 10.1093/carcin/12.1.115. [DOI] [PubMed] [Google Scholar]
- 52.Inouye S, Takizawa T, Yamaguchi H. Antibacterial activity of the essential oils and their major constituents against upper respiratory tract infections by gaseous contact. J Antimicrob Chemother. 2001;47:565–573. doi: 10.1093/jac/47.5.565. [DOI] [PubMed] [Google Scholar]
- 53.Duke J. Dr. Duke's Phytochemical and Ethnobotanical Database. 2011. http://www.ars-grin.gov/duke Available at. Accessed February 17, 2011.
- 54.Rincon AG, Pulgarin C. Effect of pH, inorganic ions, organic matter and H2O2 on E. coli K12 photocatalytic inactivation by TiO2: implications in solar water disinfection. Appl Catal B. 2004;51:283–300. [Google Scholar]