Abstract
During August 2009–October 2010, a multidisciplinary team investigated 14 outbreaks of animal and human anthrax in Bangladesh to identify the etiology, pathway of transmission, and social, behavioral, and cultural factors that led to these outbreaks. The team identified 140 animal cases of anthrax and 273 human cases of cutaneous anthrax. Ninety one percent of persons in whom cutaneous anthrax developed had history of butchering sick animals, handling raw meat, contact with animal skin, or were present at slaughtering sites. Each year, Bacillus anthracis of identical genotypes were isolated from animal and human cases. Inadequate livestock vaccination coverage, lack of awareness of the risk of anthrax transmission from animal to humans, social norms and poverty contributed to these outbreaks. Addressing these challenges and adopting a joint animal and human health approach could contribute to detecting and preventing such outbreaks in the future.
Introduction
Anthrax is an acute infectious zoonotic disease caused by the spore-forming, aerobic, gram-positive, non-motile bacterium Bacillus anthracis.1,2 Among the three clinical forms of anthrax in humans, more than 95% of naturally occurring infections are cutaneous anthrax.3 Gastrointestinal anthrax is usually caused by consumption of insufficiently cooked contaminated meat and it is relatively uncommon.4,5 Inhalation anthrax is rare in naturally occurring infections and it is associated with processing and handling of hides and wool in enclosed factory spaces, where aerosolized anthrax spores may be inhaled.5 Cutaneous anthrax occurs worldwide, with an estimated 20,000–100,000 human cases occurring annually,5,6 generally in low-income countries, where livestock are not routinely vaccinated. No cutaneous anthrax outbreaks have been reported from Bangladesh since 1986,7,8 but anthrax infection among livestock in Bangladesh was reported routinely,9 and cutaneous anthrax outbreaks have been reported from neighboring states in India in recent years.10,11
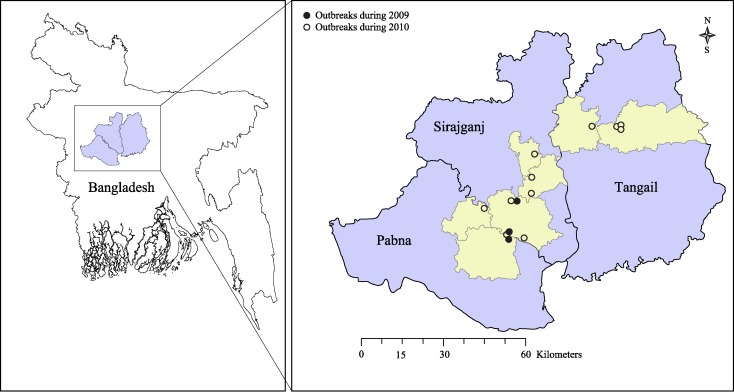
During August 2009–October 2010, we investigated 14 reported outbreaks of cutaneous anthrax in Bangladesh (Figure 1). A collaborative team of epidemiologists, physicians, veterinarians, and anthropologists from the Institute of Epidemiology, Disease Control and Research (IEDCR), the Department of Livestock Services of the Government of Bangladesh, and International Centre for Diarrheal Disease Research, Bangladesh conducted the outbreak investigations. The objectives of these outbreak investigations were to identify the etiology, modes of transmission, the social, behavioral, and cultural factors that contributed to these outbreaks, along with suggesting control and prevention measures.
Figure 1.
Bangladesh map showing 14 anthrax outbreak areas during August 2009–October 2010.
Methods
Outbreak reporting and investigations.
The first outbreak was identified in August 2009 by a researcher from the International Centre for Diarrheal Disease Research, Bangladesh, whose relatives from Pabna District in northwestern Bangladesh told him that two moribund cattle with high fever and convulsions had been slaughtered in his village and skin lesions had developed in many persons in the following days. Two more similar outbreaks were detected in the adjacent Sirajganj District during September and October 2009 (Figure 1). None of these outbreaks were reported through the formal health system in Bangladesh.
Human health authorities of Tangail District in the central part of Bangladesh reported four similar outbreaks during April–June 2010 (Figure 1). After a newspaper report of another outbreak of cutaneous anthrax in Sirajganj District in August 2010, health workers and newspapers reported a series of outbreaks and sporadic cases of cutaneous anthrax from different parts of the country.
We defined an occurrence of two or more suspected cases of cutaneous anthrax in a community that were epidemiologically linked to sick or dead livestock or slaughtering of a sick animal or handling of meat of a sick slaughtered animal as an outbreak of cutaneous anthrax. Among the 14 outbreaks we investigated during August–October 2010, 10 occurred in two adjacent districts (Pabna and Sirajganj) that had the highest cattle density in Bangladesh and were known as the main milk-producing areas.12 All outbreak sites were low-lying areas and usually flooded during and after the annual monsoon season.
For each of these outbreaks, after receiving the initial report, the investigation team members traveled to the outbreak site and collected animal, human, and anthropologic information simultaneously. Investigation teams also conducted follow-up visits in 9 of these 14 outbreak areas four weeks after initial investigations.
Animal investigation.
For each outbreak, veterinarians of the investigation team developed a list of affected cattle, goats, and sheep in the outbreak area and gathered epidemiologic and clinical information by interviewing owners of the animals by using a structured questionnaire. A suspected case of animal anthrax was defined as sudden death or convulsion of a ruminant, with or without fever, 30 days before the date of onset of first human case of cutaneous anthrax in the area until the date of investigation. We collected bone marrow smears of slaughtered sick animals from the meat stored in household refrigerators. We also collected ocular fluid from two dead goats, one from the first outbreak in August 2009 and the other from the outbreak in August 2010.
Microbiologists of the Central Disease Investigation Laboratory (CDIL), Bangladesh prepared bone marrow smears stained with polychrome methylene blue and performed microscopic examination from animal specimens collected from four outbreak areas. We sent animal specimens to the Centers for Disease Control and Prevention (CDC) for laboratory confirmation and genotyping of isolates. We defined those suspected animal anthrax cases as confirmed cases that had laboratory evidence of acute B. anthracis infection shown by microscopy of bone marrow smears stained with Loeffler's polychrome methylene blue and/or isolation of the bacilli.
Human investigation.
We visited slaughtering sites and spoke to owners of the slaughtered animals, local health workers, and neighbors to obtain identifying, demographic, and clinical information of suspected cutaneous anthrax cases. In August–September 2010, we also visited door to door in the affected communities in three outbreak areas to identify cases and record exposure status of members of the households who received meat of a sick slaughtered animal. We defined a suspected human case of cutaneous anthrax as any person who had acute onset of a skin lesion with papule or vesicle or skin ulceration with a raised margin and central black eschar from three weeks before the date of slaughtering the first sick animal to three weeks after the slaughtering of last sick animal in the area. The field team investigated the epidemiologic, clinical, and exposure history of the identified cases by using a structured questionnaire.
We collected vesicular fluid for slide smears and vesicular swabs in nutrient broth for culture. We obtained skin biopsy specimens from three patients from the first outbreak. In eight of the 14 outbreaks, we collected paired serum samples from human cases for antibody testing. Microbiologists from IEDCR conducted microscopy of field-prepared vesicular smears by using staining with Loeffler's polychrome methylene blue. They also cultured material from vesicular swabs on nutrient agar and blood agar media, followed by colony morphology study, staining, and microscopy. We sent human specimens from eight outbreaks to CDC for laboratory confirmation and genotyping of the isolates. We categorized those suspected cutaneous anthrax cases as confirmed cases that had laboratory evidence of acute B. anthracis infection shown by either microscopy of vesicular smears stained with Loeffler's polychrome methylene blue, and/or isolation of bacilli, and/or a four-fold increase in anti-protective antigen (PA) antibody titer.
In the first three of the outbreaks investigated in August–September 2010, four weeks after the initial investigations, we searched for suspected cases of gastrointestinal anthrax by using the list of household members developed in preliminary investigations. We defined a suspected case of gastrointestinal anthrax as a person in the affected communities who ate meat or handled the raw meat of cattle, goats, or sheep and showed development of a febrile illness associated with either oral ulcer, sore throat, vomiting, or diarrhea from the date of slaughtering the first sick animal to three weeks after the slaughtering of last suspected animal anthrax case in the area. To interview suspected cases of gastrointestinal anthrax, we modified the questionnaire used in the preliminary investigation to include exposure and clinical information related to gastrointestinal anthrax. From the suspected cases of gastrointestinal anthrax without cutaneous lesions, we collected single serum samples for antibody testing.
Through unstructured discussion with local health officials and community people we explored death of any suspected cutaneous or suspected gastrointestinal anthrax case in the outbreak community.
Anthropologic investigation.
In four outbreak areas, two outbreaks in 2009 and two outbreaks in 2010, a team of anthropologists conducted 13 in-depth interviews and 30 short interviews with suspected case-patients in the community to understand their knowledge and perception regarding contagiousness, illness, and treatment history. They also conducted 20 informal and formal group discussions with affected persons in the community to explore beliefs, rituals, and usual practices of sick animal slaughtering, disposal of dead animals, and their knowledge regarding disease transmission (animal to animal, animal to human, and human to human). Building on this in-depth exploration by the anthropologic team, the epidemiologists continued to explore social, behavioral, and cultural factors in all other outbreaks.
Data analysis.
We analyzed quantitative data by using SPSS version 12.0 for Windows (SPSS, Chicago, IL) and descriptive statistics to calculate frequencies of variables. We calculated attack rates and risk ratios for different exposures among the exposed and unexposed groups in three outbreak areas. We reviewed anthropologic data to identify themes and their context. Using the identified patterns, we developed and finalized a code list. We later summarized coded data according to the study objectives and themes.
Ethical issues.
For all outbreaks, we obtained informed consent from adult respondents and guardians of children and assent for children 11–17 years of age. Because these investigations were outbreak investigations, a study protocol was not reviewed by a human subjects committee, but the investigation plan was approved by the Government of Bangladesh.
Results
Animal investigation.
We identified 140 sick animals in the 14 outbreaks. Among these animals, 96 (69%) were cattle, 41 (29%) were goats, and 3 (2%) were sheep. Ninety-six (69%) animals were female. The median age was 36 months for cattle, 24 months for goats, and 9 months for sheep. Ninety eight (70%) of 140 animals died of their illness and 35 (25%) were slaughtered (Table 1). Twenty four (25%) of the 96 cattle had a history of anthrax vaccination in the previous year. The median period from onset of illness to death of an animal anthrax case was 5 hours (range = 5 minutes–8.5 days). The median period from onset of illness to slaughtering of an animal anthrax case was 7 hours (range = 15 minutes–6.2 days). We collected disposal history for the 59 death cases in the 11 outbreaks in 2010. Of these cases, 29 (49%) were thrown into flood or river waters, 10 (17%) were abandoned in open fields, and 20 (34%) were buried. The median duration from the date of slaughtering of the first sick animal in an outbreak area to the date of investigation of the outbreak was 17 days (range = 6–34 days). All 35 slaughtered sick animals were associated with human cases.
Table 1.
Demographic and clinical characteristic of 140 anthrax-infected animals in outbreaks investigated during 2009–2010 in Bangladesh
| Characteristic | Value |
|---|---|
| Age (months) | |
| Cattle: median (range) | 36 (1–120) |
| Goats: median (range) | 24 (3–96) |
| Sheep: median (range) | 9 (9–48) |
| Females, no. (%) | |
| Cattle | 71 (74) |
| Goats | 24 (59) |
| Sheep | 1 (33) |
| Clinical presentation, no. (%) | |
| Fever | 103 (74) |
| Fallen down | 91 (65) |
| Sudden death | 71 (51) |
| Anorexia | 70 (50) |
| Convulsion | 67 (48) |
| Depression | 49 (35) |
| Respiratory distress | 24 (17) |
| Listlessness | 14 (10) |
| Diarrhea | 13 (9) |
| Muscle tremor | 6 (4) |
| Decease in milk production | 6 (4) |
| Edema | 3 (2) |
| Abortion | 3 (2) |
| Outcome, no. (%) | |
| Death from illness | 98 (70) |
| Slaughtered | 35 (25) |
| Sick at the time of interview | 5 (4) |
| Sold during illness | 1 (1) |
| Recovered | 1 (1) |
| Median (range) onset of illness to death | 5 hours (5 minutes–8.5 days) |
| Median (range) onset of illness to slaughter | 7 hours (15 minutes–6.2 days) |
Microbiologists of CDIL identified gram-positive, chain-forming, spore-containing, capsulated bacilli from smears of bone marrow of five slaughtered cattle. The CDC isolated B. anthracis from the ocular fluid of two dead goats, and also detected B. anthracis from bone marrow smears collected from a slaughtered cow and a disposed dead goat by M'Fadyean staining. Based on the laboratory evidence from CDIL and CDC, we identified seven confirmed animal anthrax cases, five in cattle and two in goats.
Human investigation.
Combining all 14 outbreak investigations, we interviewed 273 cases of cutaneous anthrax, 39 confirmed cases and 234 suspected cases. In the three outbreak investigations in 2010 in which we inquired about gastrointestinal symptoms, we identified 25 cases of suspected gastrointestinal anthrax, 10 of which also had cutaneous anthrax. In all 14 outbreaks, human cases occurred after slaughtering of anthrax-infected animals (Figure 2 ). Of the 273 cutaneous anthrax cases, 165 (60%) were in male patients. The median age of cutaneous anthrax case-patients was 26 years (range = 1–90 years) (Table 2). The median duration from exposure to onset of illness was 2 days (range = 0–22 days). Skin lesions were characterized by ulcers (86%), papules (85%), vesicles (84%), central black eschars (75%), erythema (65%), and surrounding edema (60%) (Table 2 and Figure 3). Lesions were mostly distributed on the upper limbs (81%), but were also present on lower limbs (17%), face (5%), chest (4%), back (3%), neck (2%), abdomen (2%) and scalp (2%). A significant difference was observed between confirmed and suspected cutaneous anthrax cases with regard to surrounding erythema (82% versus 62%; P = 0.02), surrounding edema (84% versus 56%; P = 0.01), nausea (30% versus 14%; P = 0.01), and oropharyngeal ulceration (5% versus 1%; P = 0.04) (Table 2).
Figure 2.
A, Distribution of cutaneous anthrax outbreaks in relation to animal slaughtering over time in Bangladesh during August 2009–June 2010. B, Distribution of seven overlapping cutaneous anthrax outbreaks in relation to animal slaughtering over time in Bangladesh during July–September 2010.
Table 2.
Demographic and clinical presentation of 273 suspected and confirmed human cutaneous anthrax cases in outbreaks investigated during 2009–2010 in Bangladesh
| Characteristic | Suspected cutaneous anthrax cases (n = 234)* | Confirmed cutaneous anthrax cases (n = 39)† | P | Total (n = 273)‡ |
|---|---|---|---|---|
| Demographic | ||||
| Age (years) | ||||
| Median (range) | 28 (1–90) | 25 (4–65) | 26 (1–90) | |
| Age group, no. (%) | ||||
| 1–10 | 34 (15) | 6 (15) | 0.89 | 40 (15) |
| 11–20 | 48 (21) | 11 (28) | 0.28 | 59 (22) |
| 21–30 | 59 (25) | 8 (20) | 0.53 | 67 (25) |
| 31–40 | 45 (19) | 5 (13) | 0.34 | 50 (18) |
| > 40 | 48 (21) | 9 (23) | 0.72 | 57 (21) |
| Sex, no. (%) | ||||
| Male | 140 (60) | 25 (64) | 0.61 | 165 (60) |
| Clinical presentation, no. (%) | ||||
| Ulcer | 201 (86) | 33 (85) | 0.83 | 234 (86) |
| Papule | 201 (86) | 30 (77) | 0.18 | 231 (85) |
| Vesicle | 199 (85) | 31 (79) | 0.42 | 230 (84) |
| Central black eschar | 180 (77) | 25 (64) | 0.08 | 205 (75) |
| Itching around skin lesion | 154 (66) | 30 (77) | 0.17 | 184 (67) |
| Surrounding erythema | 146 (62) | 32 (82) | 0.02 | 178 (65) |
| Surrounding edema | 130 (56) | 33 (85) | 0.01 | 163 (60) |
| Fever | 149 (64) | 27 (69) | 0.50 | 176 (65) |
| Nausea | 32 (14) | 12 (30) | 0.01 | 44 (16) |
| Abdominal pain | 20 (9) | 5 (13) | 0.39 | 25 (9) |
| Vomiting | 13 (6) | 4 (10) | 0.26 | 17 (6) |
| Diarrhea | 5 (2) | 1 (3) | 0.87 | 6 (2) |
| Oropharyngeal ulceration | 2 (1) | 2 (5) | 0.04 | 4 (2) |
Includes eight cases of suspected cutaneous and suspected gastrointestinal anthrax.
Includes two cases of suspected cutaneous and suspected gastrointestinal anthrax.
Includes 10 cases of suspected cutaneous and suspected gastrointestinal anthrax.
Figure 3.
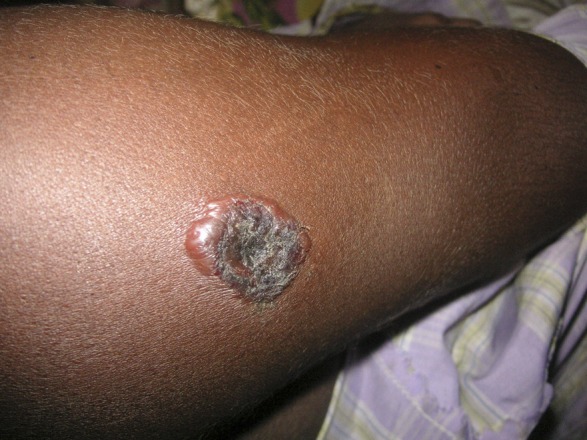
Laboratory-confirmed cutaneous anthrax case identified during 2009 in Bangladesh.
Of 273 cutaneous anthrax case-patients, 225 (82%) handled raw meat from sick animals, 172 (63%) were present at a slaughtering site, 89 (33%) handled skin of a sick slaughtered animal, 86 (32%) were involved in butchering sick animals, and 71 (26%) had contact with a sick animal. Ninety-one percent of 273 cutaneous anthrax cases reported a history of at least one of these exposures. Two hundred thirty-five (86%) cutaneous anthrax case-patients had a history of consuming meat of a sick slaughtered animal. Local health authorities treated all cutaneous anthrax cases with ciprofloxacin for at least 10 days. Of 169 cutaneous anthrax case-patients who received ciprofloxacin before the date of investigation, 10 (6%) reported vomiting, compared with 7 (7%; P = 0.79) of 104 case-patients who received it on or after the date of investigation. Two (1%) of 169 cutaneous anthrax case-patients who received ciprofloxacin before the date of investigation reported diarrhea, compared with 4 (4%; P = 0.15) of 104 case-patients who received it on or after the date of investigation. Local health workers followed-up case-patients and ensured that they had medication. No case-patient required hospitalization, and all case-patients recovered uneventfully. We did not identify any reported deaths of suspected cutaneous anthrax or suspected gastrointestinal anthrax cases in the outbreak communities.
Through door-to-door visits of 437 households in three outbreak areas in 2010, we collected information about case status and exposure history of 1,665 persons. Among them, 67 (4%) were identified as case-patients. Persons involved in butchering a sick animal at the slaughtering site were at a significantly higher risk of contracting cutaneous anthrax (relative risk = 21.9, 95% confidence interval = 14.5–32.9) than those who did not participate in butchering a sick animal at the slaughtering site. Involvement in handling raw meat of a sick animal at home or being present at the slaughtering site without participating in butchering was not significantly associated with development of cutaneous lesions (Table 3).
Table 3.
Relationship between exposure variables and cutaneous anthrax case status (n = 1,665), Bangladesh
| Exposure | Exposed | Not exposed | Risk ratio | No. missing | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Cases | Attack rate, % | Total | Cases | Attack rate, % | ||||
| Butchered sick animal at slaughtering site but did not handle raw meat of sick animal at home | 63 | 31 | 49.21 | 1,600 | 36 | 2.25 | 21.9 | 2 | 14.5–32.9 |
| Handled raw meat of sick animal at home but did not participate in butchering at slaughtering site and was not present at slaughtering site | 495 | 27 | 5.45 | 1,165 | 41 | 3.51 | 1.6 | 5 | 1–2.5 |
| Present at slaughtering site but did not participate in butchering or in handling raw meat of sick animal at home | 45 | 2 | 4.44 | 1,618 | 66 | 4.08 | 1.1 | 2 | 0.3–4.3 |
CI = confidence interval.
Microbiologists of IEDCR identified blue-stained bacilli with squared ends by microscopy of vesicular smears by using Loeffler's polychrome methylene blue stain for 23 of 28 suspected cutaneous anthrax case-patients whose specimen they tested. At CDC, B. anthracis was detected in three skin biopsy specimens by immunohistochemical analysis and from 10 vesicular swab smears by M'Fadyean staining. Bacillus anthracis was isolated at CDC from vesicular swabs of three cases, two from the first outbreak in 2009 and one from the outbreaks in August 2010.
Nineteen of 45 persons in whom a case of suspected cutaneous anthrax developed and whose paired serum samples were tested at CDC showed a four-fold increase in anti-PA titer. The first serum sample from 31 (69%) suspected cutaneous anthrax case-patients was collected within eight days of their onset of illness. Those persons who had their first serum samples collected within eight days of their onset of illness were more likely to have a four-fold increase in anti-PA titer than those whose first serum sample was collected after eight days of their onset of illness (58% versus 7%; odds ratio = 18, 95% confidence interval = 2–806, P < 0.001). Fifteen of 26 case-patients who did not have a four-fold increase had a detectable anti-PA titer.
Multiple-locus variable-number tandem repeat analysis (MLVA) was performed for B. anthracis isolated from human and animal specimens at CDC. The MLVA-8 genotype of 2009 isolates confirmed that animals and humans were infected by isolates of the same genotype. The MLVA-8 genotype of 2010 isolates that caused infection in animals and humans in 2010 differed from the 2009 isolate at one locus (pXO1 locus) (Table 4).
Table 4.
MLVA-8 genotyping of Bacillus anthracis isolated during 2009 and 2010 in Bangladesh
| B. anthracis strain | Outbreak year | pXO1 | pXO2 | CG3 | vrrB2 | vrrB1 | vrrA | vrrC2 | vrrC1 |
|---|---|---|---|---|---|---|---|---|---|
| GT152 | 2009 | 132 | 141 | 158 | 162 | 229 | 313 | 532 | 554 |
| GT155 | 2010 | 129 | 141 | 158 | 162 | 229 | 313 | 532 | 554 |
MLVA = multiple-locus variable-number tandem repeat analysis.
Fifteen (60%) of 25 suspected gastrointestinal anthrax cases were in female patients. The median age of these case-patients was 20 years (range = 2–50 years). Suspected gastrointestinal anthrax case-patients reported fever (100%), abdominal pain (76%), nausea (56%), vomiting (56%), diarrhea (36%), and ulceration in oral mucosa (19%). All suspected gastrointestinal anthrax case-patients had a history of consuming meat of slaughtered sick animals. All case-patients recovered and did not require hospitalization. Among 15 case-patients with suspected gastrointestinal anthrax who did not have cutaneous anthrax lesions, two had a detectable anti-PA titer.
Anthropologic investigation.
Persons in the affected community were aware of anthrax in livestock and identified it by using the local term torka. Persons were not aware that they could become infected with anthrax because of butchering, handling or eating the meat of sick animals.
After observing the deteriorating condition of the sick cattle and realizing that livestock would not survive, neighbors and relatives of the cattle owners recommended slaughtering them. In some cases, neighbors and relatives slaughtered the cattle without even seeking consent from the owner. Slaughtering sick livestock and selling the meat at a price below its normal value was a common practice in these outbreak areas because owners were attempting to recover some of their financial investment. Some sick cattle and goats were not slaughtered because the owners believed that slaughtering a pregnant or an animal less than one year of age is against Islamic customs. Because of the sudden onset and unpredictable course of illness, some cattle and goats died before their owners were able to decide whether to slaughter the animal. Because residents of affected communities perceived that river water washes everything away with its current, they usually discarded dead cattle in the river or in flood waters.
According to the community members, eating meat of slaughtered sick cattle and goats is a common practice. The price of this meat is low, and because most persons cannot afford to buy meat regularly, they were eager to purchase it. Despite selling meat of a slaughtered sick animal, owners of these animals had substantial economic losses. In the first outbreak in 2009, the market price of a healthy cow was reported as 50,000 Taka (US$ 714), but the owner received only 20,000 Taka (US$ 285) by selling the meat. In each of these outbreaks, cattle owners could only recover 10–30% of the market price of a healthy animal of the same size by selling its meat.
Discussion
Epidemiologic, clinical, and laboratory findings suggest that these outbreaks in animals and humans were caused by B. anthracis. Participation in butchering anthrax-infected animals and exposure to infected meat and animal by-products were responsible for the human outbreaks. There are several environmental factors in Bangladesh that favor the presence of anthrax, including high ambient temperature and relative humidity.13 Because persons are not aware of proper disposal practices, anthrax-infected dead animals were frequently disposed in open fields and rivers, which can contaminate grazing land with anthrax bacilli that can sporulate rapidly on exposure to air and high temperatures.14 Therefore, unvaccinated animals might have acquired anthrax by ingestion of spores while grazing.
The three districts where these 14 outbreaks occurred during August 2009–September 2010 shared similar environmental characteristics. With animal anthrax cases reported in Bangladesh in previous years9 and a low coverage of livestock anthrax vaccination and disposal of 66% of dead animals in flood waters or surrounding open fields, these outbreaks were likely separate outbreaks in which animals were infected from spores spread in the grazing lands around those outbreak areas. Difference in genotype of B. anthracis isolates during 2009 and 2010 outbreaks also suggest these were separate outbreaks, which were unlikely to be the result of a spread of the organism from one outbreak area to the other.
Up to 2009, no human anthrax cases had been reported in Bangladesh for more than 25 years, and most of these recent outbreaks were not detected through the routine reporting system of the health or livestock departments. This finding likely indicates substantial under-diagnosis and under-reporting of human anthrax in Bangladesh, which might be influenced by the medical education system in the country, where cutaneous anthrax is not considered a common disease and so is not emphasized within the medical curriculum.
In the wave of outbreaks during August–October 2010, 607 suspected cases of cutaneous anthrax were reported by the health authorities from 12 of the 64 districts.15 These outbreaks were much more widespread compared with previous reported anthrax outbreaks in Bangladesh. This increase in the number of outbreaks and suspected cases might have been caused by increased awareness among health workers and the general population because of the unprecedented media attention to the outbreaks, but we cannot rule out an underlying increase in cases.
Although there were no deaths of human case-patients, reports of anthrax outbreaks in different parts of the country during August–September 2010 created fear among the general population, which reduced meat consumption. Newspapers reported a 92% decrease in number of cattle slaughtered in a day in the capital city of Dhaka. Reduction in sales of animals ahead of Eid-ul-Fitr, a major Muslim festival, had a major negative impact on the livestock industry of the country.16
A number of social, cultural, and economic factors contributed to these human outbreaks. In all of these outbreaks, neighbors and relatives of the owners of the sick animals actively participated in butchering sick animals and processing raw meat. Community participation in such activities is common in rural Bangladesh where collective, not individual, decision making is the norm. Voluntary participation in butchering resulted in exposure of more persons to infected meat and other by-products of the sick slaughtered animal.
Of 60 countries reporting anthrax in 2004, nearly 60% were low-income countries.17 Poor persons are more at risk of contracting anthrax because of traditional raising practices involving close contact with animals, even when they are sick.17 In Bangladesh, the average per capita annual income is US $599 (42,000 Taka),18 and 43% of rural persons live below the poverty line.19 With more than one-third of rural women identified as undernourished,18 buying inexpensive meat is viewed as a favorable opportunity when animals become sick and must be slaughtered. Moreover, livestock play a vital component in the economy of these outbreak areas. Therefore, the fear of impending financial loss can influence the cattle owners to slaughter the sick animal and sell the meat.
Although we did not systematically search for gastrointestinal anthrax cases during initial investigations, we identified 25 suspected gastrointestinal anthrax cases during a follow-up visit in three outbreak areas in 2010. Anti-PA titer of collected single serum samples failed to confirm any of those cases as anthrax. The absence of reports of hospitalization or deaths in these outbreak areas suggests that these cases were unlikely to be cases of gastrointestinal anthrax, especially if one considers the usually reported high case-fatality rate of this illness.3,13,20 The local practice of overcooking meat also significantly reduces the risk of gastrointestinal anthrax, which usually results from consuming undercooked meat.1,5
Suspected cutaneous anthrax cases in these outbreaks had characteristic skin lesions of cutaneous anthrax, starting with a papule, which gradually progressed to formation of a vesicle, ulcer, and eschar. Although the absence of surrounding edema in 44% of suspected cases might be related to the time gap between the onset of illness of cases and date of investigation, there is also evidence of similar characteristic in other settings.21 Enteric symptoms such as nausea, abdominal pain, vomiting, and diarrhea are unlikely with cutaneous anthrax, and cutaneous anthrax cases who also met the case definition of suspected gastrointestinal anthrax in these outbreaks could not be confirmed as gastrointestinal anthrax. Because 86% of the cutaneous anthrax case-patients had a history of consuming meat of sick slaughtered animals, reported symptoms such as nausea and abdominal pain might have been influenced by subjective feelings of the respondents after consuming meat from a sick animal.
Delayed collection of the first serum sample might explain the absence of a four-fold increase in anti-PA titer in 26 of the 45 suspected cutaneous anthrax case-patients whose paired serum samples were tested. Cutaneous anthrax patients have a lower anti-PA response than inhalation anthrax patients,22 which might be the reason for a detectable anti-PA titer in 15 cases without a four-fold increase. In another study, anti-PA IgG was detected only in a subset of cutaneous anthrax patients.23 Therefore, absence of a four-fold increase in anti-PA titer does not exclude the possibility that a suspected cutaneous anthrax case was infected by anthrax bacilli.
Poor coverage of livestock anthrax vaccination and ineffective vaccination in some animals contributed to the animal outbreaks. The Livestock Research Institute of the Government of Bangladesh is the only supplier of livestock anthrax vaccine in Bangladesh. There are approximately 23 million cattle, 1 million buffalo, 21 million goats, and 3 million sheep in Bangladesh.19 However, the total annual vaccine production is only 3.8 million doses,24 which the Department of Livestock Services distributes at a subsidized rate of US $ 0.74 (50 Taka) per 100 doses. In comparison, a vial of 50 doses of livestock anthrax vaccine costs approximately $49 in the United States.25 If one considers the economic impact of animal and human anthrax, if this vaccine was available in the local market, even at a higher price, it might be feasible for individual cattle raisers to purchase it. Increasing the availability of anthrax vaccine in the market, either through increasing domestic production or through importation, and increasing the demand through raising awareness among the cattle owners, would be one strategy to increase coverage.
A history of anthrax vaccination within the previous year among 25% of the suspected cattle cases of anthrax suggests either over-reporting of vaccination or vaccination failure. Successful development of protective immunity against anthrax in animals requires effective vaccine and proper vaccination technique. Also, a single dose of Sterne vaccine may not be sufficient to ensure protective immunity in the animal to last for a year, and more than one initial dose of the Sterne vaccine may be necessary.13,26,27 The Department of Livestock Services, should assess vaccine efficacy and develop a strategy to improve coverage. If one considers the scarcity of resources, the vaccination strategy should identify target areas for vaccination based on the understanding of the spatial ecology and the geographic distribution of B. anthracis in Bangladesh, as has been done in other settings.28,29
Because some human cases were reported more than one month after the onset of illness, imperfect recall by the case-patients might be a reason that 24 (9%) of the 273 cutaneous anthrax case-patients did not report a history of any of the exposures related to contact with a sick animal, meat of a sick slaughtered animal, or animal by-products. There might also have been some unknown exposure because the surrounding environment was contaminated by disposed animal carcasses, and stable flies and mosquitoes may play a role in transmission of animal and human anthrax.5,30–32
Lack of awareness of the cattle owners and the community regarding transmission of this disease from animals to humans, scarcity of the livestock vaccine, social norms, and poverty all contributed to these cutaneous anthrax outbreaks. The anthrax outbreaks affected households through livestock deaths and morbidity of humans. Improved treatment of human cases of anthrax can be achieved through short-term training for practicing physicians and by modifying the undergraduate medical curriculum to include information on detecting and managing cutaneous anthrax cases. Although anthrax affects human and animal health, there is no legal, policy or institutional framework in Bangladesh to ensure joint action of the Directorate General of Health Services and Department of Livestock Services to control anthrax. A joint human and animal health approach, or the one health concept, can be beneficial in controlling zoonotic diseases such as anthrax because they can be better surveyed, diagnosed, and controlled.17 This one health approach could improve coordination between human and animal health departments and thereby facilitate early detection, control, and prevention of anthrax outbreaks.
ACKNOWLEDGMENTS
We thank Dr. Labib Imran Faruque, Dr. Abu Mohammed. Naser Titu, Dr. Hossain Mohammed, Shahed Sazzad, Dr. Kazi Mohammed, Hassan Ameen, and Dr. Selina Khatun for contributions during field investigations; Robyn A. Stoddard, Chung K. Marston, Wun-Ju Shieh, and Cari A. Beesley for contributions during laboratory investigations at CDC; all study participants for their contributions; civil surgeons and district livestock officers of Pabna, Sirajganj, and Tangail Districts and sub-district health and family planning officers and livestock officers of Santhia, Shahjadpur, Kamarkhand, Faridpur, Belkuchi, Ghatail, and Bhuapur sub-districts for assistance; and Dorothy Southern for reviewing the manuscript.
Footnotes
Financial support: This study was supported by CDC and the Government of Bangladesh. The International Center for Diarrheal Disease Research, Bangladesh acknowledges with gratitude the commitment of the Government of Bangladesh and CDC to research efforts of the Centre.
Authors' addresses: Apurba Chakraborty, Salah Uddin Khan, Mohammed Abul Hasnat, Shahana Parveen, M. Saiful Islam, Andrea Mikolon, Najmul Haider, Stephen P. Luby, and M. Jahangir Hossain, Centre for Communicable Diseases, International Centre for Diarrheal Disease Research, Bangladesh, 68, Shaheed Tajuddin Ahmed Sarani, Mohakhali, Dhaka 1212, Bangladesh, E-mails: achakraborty@icddrb.org, khansu@icddrb.org, hasnatvet1@gmail.com, shahana@icddrb.org, saiful@icddrb.org, amikolon@icddrb.org, nazmulh@icddrb.org, sluby@icddrb.org, and jhossain@icddrb.org. Ranjit Kumar Chakraborty, Department of Livestock Services, Ministry of Fisheries and Livestock, Dhaka, Bangladesh, E-mail: chakraborty.ranjit@yahoo.com. Be-Nazir Ahmed, Khorsed Ara, and Mahmudur Rahman, Institute of Epidemiology Disease Control and Research, Mohakhali, Dhaka 1212, Bangladesh, E-mails: benazir1959@gmail.com, khorsedara1957@yahoo.com, and mrahman@citechco.net. Sherif R. Zaki and Alex R. Hoffmaster, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: sxz1@cdc.gov and amh9@cdc.gov.
References
- 1.Woods CW, Ospanov K, Myrzabekov A, Favorov M, Plikaytis B, Ashford DA. Risk factors for human anthrax among contacts of anthrax-infected livestock in Kazakhstan. Am J Trop Med Hyg. 2004;71:48–52. [PubMed] [Google Scholar]
- 2.Demirdag K, Ozden M, Saral Y, Kalkan A, Kilic SS, Ozdarendeli A. Cutaneous anthrax in adults: a review of 25 cases in the eastern Anatolian region of Turkey. Infection. 2003;31:327–330. doi: 10.1007/s15010-003-3169-3. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention . In: Epidemiology and Prevention of Vaccine-Preventable Diseases. Atkinson W, Wolfe S, Hamborsky J, McIntyre L, editors. Washington, DC: Public Health Foundation; 2009. p. 308. (Anthrax). [Google Scholar]
- 4.Sirisanthana T, Brown AE. Anthrax of the gastrointestinal tract. Emerg Infect Dis. 2002;8:649–651. doi: 10.3201/eid0807.020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swartz MN. Recognition and management of anthrax: an update. N Engl J Med. 2001;345:1621–1626. doi: 10.1056/NEJMra012892. [DOI] [PubMed] [Google Scholar]
- 6.Freedberg IM, Eisen AZ, Wolff K, Austen K, Goldsmith LA, Katz SI. Fitzpatrick's Dermatology in General Medicine. 6th edition. New York: McGraw-Hill; 2003. pp. 1918–1921. [Google Scholar]
- 7.International Centre for Diarrhoeal Diseases Research, Bangladesh Cutaneous anthrax outbeak in two districts of North-Western Bangladesh. Health and Science Bulletin. 2009;7:1–8. [Google Scholar]
- 8.Samad MA, Hoque ME. Anthrax in man and cattle in Bangladesh. J Trop Med Hyg. 1986;89:43–45. [PubMed] [Google Scholar]
- 9.World Organization for Animal Health World Animal Health Information Database. 2010. http://www.oie.int/wahis/public.php?WAHIDPHPSESSID=adce2e623aeedddaa4b858ff84f59f Available at. Accessed August 12, 2010.
- 10.Ray TK, Hutin YJ, Murhekar MV. Cutaneous anthrax, West Bengal, India, 2007. Emerg Infect Dis. 2009;15:497–499. doi: 10.3201/eid1503.080972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datta KK, Singh J. Anthrax. Indian J Pediatr. 2002;69:49–56. doi: 10.1007/BF02723777. [DOI] [PubMed] [Google Scholar]
- 12.Jabbar MA, Islam SMF, Delgado C, Ehui S, Akanda MAI, Khan MI, Kamruzzaman M. International Livestock Research Institute, Nairobi, Kenya, Systemwide Livestock Programme, Addis Ababa, Ethiopia, and Bangabandhu Sheikh Mujibur Rahman Agricultural University, Salana, Gazipur, Bangladesh: 2005. p. 76. (Policy and Scale Factors Influencing Efficiency in Dairy and Poultry Production in Bangladesh). [Google Scholar]
- 13.World Health Organization Anthrax in Animals and Humans. 2008. http://www.who.int/csr/resources/publications/anthrax_webs.pdf Available at. Accessed November 25, 2011.
- 14.Christie AB. Infectious Diseases: Epidemiology and Clinical Practice. 4th edition. Edinburgh: Churchill Livingstone; 1987. pp. 100–164. [Google Scholar]
- 15.Institute of Epidemiology, Disease Control and Research Anthrax Update. 2010. http://www.iedcr.org Available at. Accessed January 11, 2010.
- 16.Akter S. The Daily Star. Dhaka: 2010. Anthrax scare hurts leather industry.http://www.thedailystar.net/newDesign/news-details.php?nid=155128 Available at. Accessed July 21, 2011. [Google Scholar]
- 17.World Health Organization . Geneva: World Health Organization; 2005. (The Control of Neglected Zoonotic Diseases—A Route to Poverty Alleviation). [Google Scholar]
- 18.National Institute of Population Research and Training . Dhaka, Bangladesh and Calverton, MD: Mitra and Associates and Macro International; 2009. (Bangladesh Demographic and Health Survey 2007). [Google Scholar]
- 19.Bangladesh Bureau of Statistics Bangladesh Data Sheet. 2008. http://www.bbs.gov.bd/ Available at. Accessed May 20, 2010.
- 20.Beatty ME, Ashford DA, Griffin PM, Tauxe RV, Sobel J. Gastrointestinal anthrax: review of the literature. Arch Intern Med. 2003;163:2527–2531. doi: 10.1001/archinte.163.20.2527. [DOI] [PubMed] [Google Scholar]
- 21.Maguina C, Flores Del Pozo J, Terashima A, Gotuzzo E, Guerra H, Vidal JE, Legua P, Solari L. Cutaneous anthrax in Lima, Peru: retrospective analysis of 71 cases, including four with a meningoencephalic complication. Rev Inst Med Trop Sao Paulo. 2005;47:25–30. doi: 10.1590/s0036-46652005000100005. [DOI] [PubMed] [Google Scholar]
- 22.Quinn CP, Dull PM, Semenova V, Li H, Crotty S, Taylor TH, Steward-Clark E, Stamey KL, Schmidt DS, Stinson KW, Freeman AE, Elie CM, Martin SK, Greene C, Aubert RD, Glidewell J, Perkins BA, Ahmed R, Stephens DS. Immune responses to Bacillus anthracis protective antigen in patients with bioterrorism-related cutaneous or inhalation anthrax. J Infect Dis. 2004;190:1228–1236. doi: 10.1086/423937. [DOI] [PubMed] [Google Scholar]
- 23.Brenneman KE, Doganay M, Akmal A, Goldman S, Galloway DR, Mateczun AJ, Cross AS, Baillie LW. The early humoral immune response to Bacillus anthracis toxins in patients infected with cutaneous anthrax. FEMS Immunol Med Microbiol. 2011;62:164–172. doi: 10.1111/j.1574-695X.2011.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livestock Research Institute . Dhaka: Livestock Research Institute; 2010. (Annual Report on Production, Distribution and Balance of Vaccines in the Financial Year 2009–2010, Bangladesh). [Google Scholar]
- 25.Colorado Serum Company Anthrax Spore Vaccine. 2008. https://ssl1001.qwestoffice.com/colorado-serum.com/merchantmanager/advanced_search_result.php?keywords=Anthrax&x=0&y=0 Available at. Accessed January 8, 2010.
- 26.Ndiva Mongoh M, Dyer NW, Stoltenow CL, Hearne R, Khaitsa ML. A review of management practices for the control of anthrax in animals: the 2005 anthrax epizootic in North Dakota–case study. Zoonoses Public Health. 2008;55:279–290. doi: 10.1111/j.1863-2378.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- 27.Turnbull PC, Tindall BW, Coetzee JD, Conradie CM, Bull RL, Lindeque PM, Huebschle OJ. Vaccine-induced protection against anthrax in cheetah (Acinonyx jubatus) and black rhinoceros (Diceros bicornis) Vaccine. 2004;22:3340–3347. doi: 10.1016/j.vaccine.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 28.Joyner TA, Lukhnova L, Pazilov Y, Temiralyeva G, Hugh-Jones ME, Aikimbayev A, Blackburn JK. Modeling the potential distribution of Bacillus anthracis under multiple climate change scenarios for Kazakhstan. PLoS ONE. 2010;5:e9596. doi: 10.1371/journal.pone.0009596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blackburn JK, McNyset KM, Curtis A, Hugh-Jones ME. Modeling the geographic distribution of Bacillus anthracis, the causative agent of anthrax disease, for the contiguous United States using predictive ecological [corrected] niche modeling. Am J Trop Med Hyg. 2007;77:1103–1110. [PubMed] [Google Scholar]
- 30.Turell MJ, Knudson GB. Mechanical transmission of Bacillus anthracis by stable flies (Stomoxys calcitrans) and mosquitoes (Aedes aegypti and Aedes taeniorhynchus) Infect Immun. 1987;55:1859–1861. doi: 10.1128/iai.55.8.1859-1861.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fasanella A, Scasciamacchia S, Garofolo G, Giangaspero A, Tarsitano E, Adone R. Evaluation of the house fly Musca domestica as a mechanical vector for an anthrax. PLoS ONE. 2010;5:e12219. doi: 10.1371/journal.pone.0012219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hugh-Jones M, Blackburn J. The ecology of Bacillus anthracis. Mol Aspects Med. 2009;30:356–367. doi: 10.1016/j.mam.2009.08.003. [DOI] [PubMed] [Google Scholar]