Abstract
Chagas disease is endemic in the state of Veracruz, Mexico, and we investigated here the dynamics of house infestation by Chagas disease vectors to understand disease transmission and design effective control interventions. Bug collections in 42 rural villages confirmed the widespread distribution of Triatoma dimidiata in central Veracruz. Unexpectedly, collection data further indicated a clear pattern of seasonal infestation by mostly adult bugs. Analysis of feeding sources with a polymerase chain reaction-heteroduplex assay indicated a frequent feeding on humans, in agreement with the high seroprevalence previously observed. Feeding sources also confirmed a significant dispersal of bugs between habitats. High dispersal capabilities and seasonal infestation may thus be a shared characteristic of several of the T. dimidiata sibling species from this complex. It would thus be critical to adapt vector control interventions to this behavior to improve their efficacy and sustainability, as the control of T. dimidiata has been notoriously challenging.
Introduction
Chagas disease is a zoonosis transmitted by triatomine insect vectors, and it is a major public health problem in most of Latin America and in particular in Mexico. The World Health Organization (WHO) estimates that about 8–11 million persons are infected worldwide,1 and in Mexico, estimates range from 2 to 6 millions, with an average national seroprevalence of 1–2%.2,3 The disease is present in most of the country, but southern states including Veracruz, seem to be particularly affected,4 although the epidemiology of the disease in Mexico is still poorly understood.
A few studies have focused on Chagas disease in the state of Veracruz and reported a seroprevalence rate of 0.9% in this state5; the highest prevalence reached 5.2% in the northern part of the state, in the jurisdiction of Tuxpan. However, we also recently identified an area of very high seroprevalence against Trypanosoma cruzi in central Veracruz, in an area that may have been overlooked in previous works, suggesting that more extensive studies are needed to fully characterize the extent of Chagas disease in the region and its transmission dynamics to humans.6
The only vector species reported so far in Veracruz is Triatoma dimidiata, which showed relatively low infestation and infection indexes (10.9% and 9.0%, respectively), but a high colonization index (50.0%).5 These data confirmed an important entomological risk of T. cruzi transmission in most parts of the state. In fact, T. dimidiata is one of the major vectors of Chagas disease, with an extensive geographic distribution ranging from the northern part of South America, through Central America, and to Southern Mexico.7,8 It is also a very diverse species in terms of its morphology, genetics, adaptation to diverse habitats, and ability to feed on a wide diversity of veterbrate hosts. For example, as mentioned previously, T. dimidiata seems well domiciliated in Veracruz and central Mexico,5 although it seasonally infests houses in the Yucatan peninsula, Mexico,9,10 and in Belize.11 In fact, recent molecular studies based on the internal transcribed spacer (ITS)-2 and cytochrome (Cyt) b markers have shown that T. dimidiata is a species complex comprising several sibling (sub)species, which may explain at least in part this extensive diversity and the different epidemiologic relevance of each taxonomic group.12,13 To date, a single taxonomic group, referred to as ITS-2 group 2, has been identified in Veracruz,12 and may correspond to a distinct morphotype compared with that of the Yucatan peninsula.14
The aim of this study was to characterize the dynamics of house infestation by T. dimidiata in central Veracruz and its feeding sources. This information is of key importance for the understanding of T. cruzi transmission in the region and for the design of effective vector control interventions.
Materials and Methods
Insect collection.
Field collections were carried out in the municipalities of Tezonapa, Amatlan de los Reyes, Zongolica, and Tierra Blanca, in the state of Veracruz, located at latitude 18°36′ north and longitude 96°41′ west. A total of 42 rural villages were included and georeferenced: Atlizacuapan, Caxapa, El Mirador, El Otate, El Suspiro, La Joya, Laguna Chica, Las Josefinas, Limonestitla, Manantiales de los Altos, Monte Alto, Palmarito, Paraíso La Reforma, Rancho Nuevo, Raya Caracol, San Agustín del Palmar, Tepecoxtla, Villa Nueva, and Almolonga from the municipality of Tezonapa; Amatepec, Aticpa, Ayojapa, Chicomapa, Cuahuixtlahuac, and Dos Caminos from the municipality of Zongolica; Ampliación Ojo de Agua, Colonia Benito Juárez, Cruz Tetela, and Rincón de Buenavista from the municipality of Omealca; El Cimarrón, El Fraile, El Tamarindo, Magueyito, General Emilio Márquez Galindo, Mata Anonilla, Mata Borrego, Matamaguey, Palmerinda, Pitalillo, San Francisco el viejo, and Zapotillo from the municipality of Tierra Blanca. Triatomines were collected by community participation following educational campaigns on Chagas disease and its vectors in the different villages.10 Briefly, households were instructed to collect bugs present inside (intradomestic) and outside (peridomestic) their houses using plastic bags to avoid direct contact with the bugs. All captured bugs were taken to local rural medical units to be labeled and stored until transported to the laboratory for further analysis.10 Insects were collected during the years 2006–2008. Entomological indexes defined by the WHO were calculated (infection, % of T. cruzi-infected triatomines; infestation, % of triatomine-positive houses, and colonization % of triatomine-positive houses with nymphs).15
Taxonomic identification by ITS-2 genotyping.
Triatomine DNA was extracted from 1 of 2 legs of the bugs and 20–50 ng were used for polymerase chain reaction (PCR) genotyping of the ITS-2 sequence. We used the previously described primer sets designed to discriminate between group 3 and groups 1–2, which amplify a 250 bp partial ITS-2 sequence.16 The PCR reactions included 35 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s and were run in a Mastercycler Gradient Thermocycler Machine (Eppendorf AG, Hamburg, Germany). Some PCR products were sequenced to verify the identity for ITS-2 group 2.
Diagnosis of T. cruzi infection.
Triatomines were analyzed by PCR for the presence of T. cruzi. Samples for PCR were obtained by rinsing the abdomen in 300 mL of sterile water, and samples were boiled for 10 min, centrifuged at 13,000 × g for 10 min, and 10 μL of supernatant was used for PCR. The PCR assay used to detect T. cruzi was the amplification of a fragment of 235 pb, as described previously.9,17 The PCR products were subjected to electrophoresis on 1.8% agarose gels, stained with ethidium bromide, and visualized by using UV transillumination. All PCRs were run with a positive control of T. cruzi DNA and negative control without DNA template.
Blood meal analysis.
A 383-basepair cytochrome b gene fragment was amplified from bug abdomen samples with primers specific for vertebrate cytochrome b.18 Heteroduplex profiles were generated with PCR products from Sus scrofa domesticus (pig) cytochrome b as hybridization driver, and analyzed by electrophoresis in 10% acrylamide gels in Tris-borate-EDTA buffer following the method previously described.18 Heteroduplex profiles from samples were compared with those obtained with different DNAs from known vertebrate species and potential feeding hosts, including Homo sapiens (human), Mus musculus (mouse), Rattus rattus (rat), Felis silvestris catus (cat), Canis lupus familiaris (dog), Gallus gallus (chicken), Oryctolagus cuniculus (rabbit), Ovis canadensis (sheep), Bos taurus (cow), and Equus ferus caballus (horse). In addition, PCR products from some samples were sequenced to confirm the identification of the feeding source.
Data analysis.
All georeferenced bug collections and associated data were entered into a geographical information system database in Qgis version 1.7 to generate maps of the geographic distribution of T. dimidiata in the region. Potential differences in proportion data were analyzed by χ2 tests.
Results
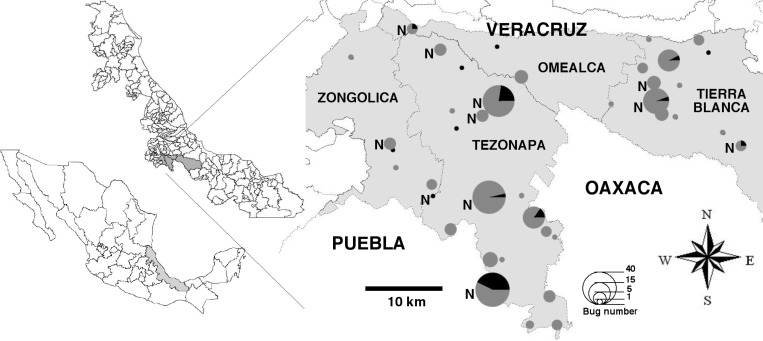
A total of 300 bugs were collected throughout the study and identified as T. dimidiata. They were further genotyped using ITS-2 group-specific primers and sequencing, which indicated that they belonged to ITS-2 group 2. Bugs were distributed among four municipalities, including Omealca, Tezonapa, Tierra Blanca, and Zongolica, and in all 42 studied villages, indicating a wide geographic distribution of the vector in the region (Figure 1). Analysis of sex ratio and developmental stage indicated that 52% were male and 48% female, and 16.7% were nymphal stages. Nymphs were collected in 12 villages (Figure 1). The abundance and proportion of nymphal stages in the population suggested some degree of colonization, and the colonization index was 18%. Infection with T. cruzi was determined by PCR and infected bugs were found in 15 of 42 villages (Figure 1), with a global T. cruzi infection rate of 13.7% (41 of 300). The infection rate did not vary significantly with sex or developmental stage (17 of 134 [12.7%] versus 15 of 111 [13.5%] and 7 of 48 [14.5%] for female, male, and nymphs, respectively, χ2 = 0.12, P = 0.94).
Figure 1.
Geographic distribution of Triatoma dimidiata in central Veracruz. Small maps on the left show the location of the state of Veracruz in Mexico, and of the municipalities (Zongolica, Omalca, Tierra Blanca, and Tezonapa) where bugs were collected, in light gray. The circles on the main map indicate the villages, with their size proportional to the number of bugs collected as indicated on the map, and the black areas represent the proportion of Trypanosoma cruzi-infected bugs. The N indicates the presence of nymphs.
The majority of bugs were collected inside the domiciles (225 of 300 [75%]), and the remaining in the peridomiciles. Comparison of the collections in the municipalities of Tezonapa and Tierra Blanca, indicated that a larger proportion of bugs were collected inside the domiciles in Tezonapa compared with Tierra Blanca (108 of 136 [79%] versus 25 of 55 [45%]; χ2 = 20.4, P < 0.0001). These data suggested a higher entomologic transmission risk in Tezonapa compared with Tierra Blanca.
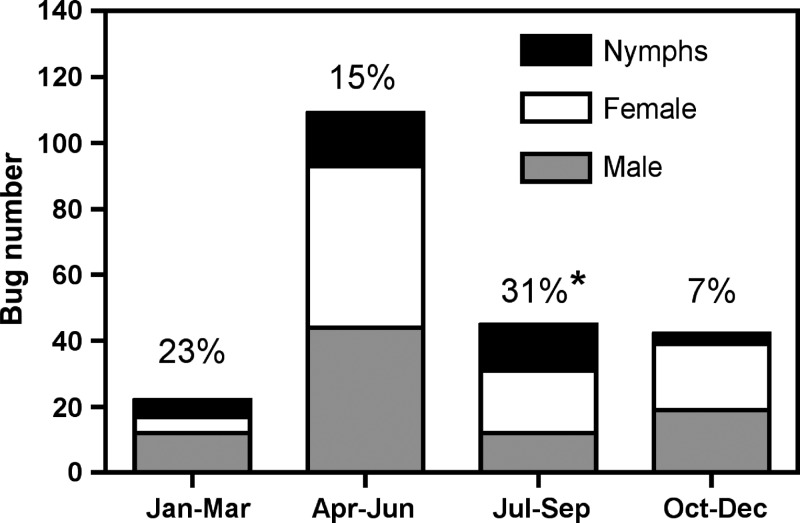
Analysis of temporal variations in bug collection revealed important seasonal variations in house infestation and in the proportion of nymphal stages. Indeed, only 10% of the yearly total of bugs was collected during the months of January–March, whereas half of the bugs were collected during the months of April–June (Figure 2). Of importance, the large increase in infesting bugs during this trimester mostly corresponded to adults (93 of 109 [85%]), suggesting a seasonal invasion by adults. On the other hand, nymphs were observed all year round, suggesting some level of domiciliation of T. dimidiata. However, there were also significant seasonal variations in the proportion of nymphs (χ2 = 9.80, P = 0.020), with the highest proportion observed during the months of July–September (14 of 45 [31%]), immediately following the maximum infestation by adults in April–June (Figure 2). Finally, there were no significant seasonal variations in the T. cruzi infection rate (χ2 = 5.17, P = 0.16), or in bug sex ratio (χ2 = 4.6, P = 0.20).
Figure 2.
Seasonal variations in Triatoma dimidiata collections. The number of bugs of the indicated sex and developmental stage collected per trimester is shown. The percentage above each bar refers to the proportion of nymphs and * indicates significant seasonal variations in this proportion (χ2 = 9.80, P = 0.020).
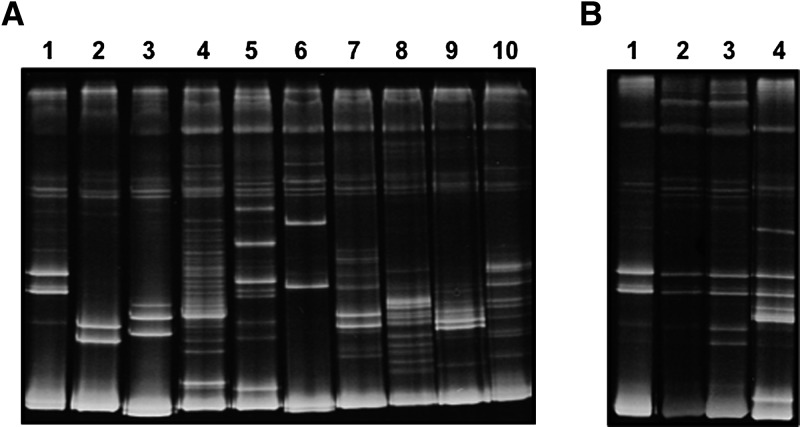
We then determined T. dimidiata feeding sources to further understand its ecology and potential T. cruzi transmission cycles. Bug samples were analyzed with a heteroduplex assay following PCR amplification of a vertebrate Cyt b sequence. Control DNA from known species gave reproducible and clearly distinct heteroduplex patterns, as in previous studies (Figure 3A). We were able to amplify a Cyt b DNA sequence from 50 of 300 (17%) of the analyzed bugs, possibly because of poor feeding, and could identify the feeding source in all of them (Figure 3B ). For confirmation, some PCR products were sequenced and sequence comparison in GeneBank validated the interpretation of the heteroduplex profiles. The majority of bugs had fed on a single blood source (33 of 50 [66%]), 14 of 50 (28%) contained blood from two species, and 3 of 50 (6%) contained blood from three species. There was a significant difference in feeding sources between bugs from the domicile and from the peridomicile (χ2 = 15.5, P = 0.016). For bugs collected inside the domiciles, human was the most frequent feeding source, representing up to 87% of blood meals (Table 1). Secondary feeding sources included rodents such as mouse and rat, accounting for 25% and 20% of blood meals, respectively. Interestingly, blood meals from domestic animals including chicken, cat, and dog were also identified in intradomiciliary bugs, suggesting that either of these animals are present inside the house, or that the bugs are able to move to the peridomiciles for feeding. Analysis of feeding sources of bugs from the peridomiciles revealed a very different feeding pattern (Table 1). Indeed, the most frequent feeding source of these bugs was the rat, accounting for 50% of the blood meals. As expected, blood from chickens, cats, dogs, and cows was also identified, but in limited proportion (12.5% each). Strikingly, 25% of the meals from peridomestic bugs contained human blood, making it the second most important feeding source in this habitat. A triple blood meal including human, chicken, and cow was even detected. These observations strongly support the dispersal of bugs from the peridomiciles to feed indoor, as it seems less likely that humans would sleep in the peridomicile.
Figure 3.
Heteroduplex patterns for Triatoma dimidiata feeding source identification. Cyt b sequences were amplified by polymerase chain reaction (PCR) and heteroduplex patterns obtained with pig PCR products as driver were resolved by electrophoresis in polyacrylamide gels. (A) Control heteroduplex patterns from known species with lanes corresponding to human (1), mouse (2), rat (3), cat (4), dog (5), chicken (6), rabbit (7), sheep (8), cow (9), and horse (10), respectively. (B) Examples of representative samples from T. dimidiata. Lanes 1 and 2 identified as human, lane 3 as dual feeding on human and mouse, and lane 4 as triple feeding on human, chicken, and cow. These samples were confirmed by sequencing of the PCR products.
Table 1.
Feeding sources of Triatoma dimidiata according to habitat
| Host | Intradomicile | Peridomicile |
|---|---|---|
| Human | 35/40 (87.5%) | 2/8 (25%) |
| Mouse | 10/40 (25%) | ND |
| Rat | 8/40 (20%) | 4/8 (50%) |
| Chicken | 2/40 (5%) | 1/8 (12.5%) |
| Cat | 2/40 (5%) | 1/8 (12.5%) |
| Dog | 1/40 (2.5%) | 1/8 (12.5%) |
| Cow | ND | 1/8 (12.5%) |
Note that for each habitat the sum of percentages is greater than 100% because of multiple blood meals in some bugs. There was a significant difference in feeding sources between the two habitats (χ2 = 15.5, P = 0.016).
ND = not detected.
Discussion
Chagas disease is endemic in the state of Veracruz, Mexico, and we previously identified a region of high seroprevalence in humans in the central region of the state.6 We thus evaluated here the dynamics of house infestation by T. dimidiata in central Veracruz, as well as its feeding sources, to provide key data on T. cruzi transmission dynamics and potential vector control interventions.
Our data first confirm that T. dimidiata is widely distributed in the region, because it was detected in all the villages studied. The T. cruzi infection rate we observed is slightly higher but similar to that previously reported for the entire state of Veracruz (13.7% versus 9.0%5), whereas the colonization index is lower (18% versus 50%5). Interestingly, these entomologic indexes are rather similar to those reported for T. dimidiata in the Yucatan peninsula, Mexico,9 and Belize.11 Taken together, these data confirm that there is a widespread risk for Chagas disease in the region, and are in good agreement with our previous observation of a high seroprevalence in humans (16.8%) in central Veracruz.6 Indeed, our data indicate a higher abundance of bugs inside the domiciles in the municipality of Tezonapa compared with Tierra Blanca, suggesting a higher risk in the former, which coincides very well with the higher seroprevalence observed in Tezonapa and the lower seroprevalence in Tierra Blanca.6 These discrepancies between the two municipalities may be related to differences in ecological conditions as suggested before,6 because villages in Tezonapa valley are surrounded by dry soil of open fields for agriculture, with large and numerous patches of evergreen tropical forest, whereas in the coastal plain of Tierra Blanca the soil is humid and almost exclusively covered by fields and pasture. Alternatively, there might be differences in housing structure and conditions between the municipalities that could also contribute to differences in triatomine infestation.
Analysis of the seasonal variations in house infestation further allowed assessing infestation dynamics. The observation of nymphal stages all year long clearly indicated that T. dimidiata is able to colonize some houses, as determined before in Veracruz.5 On the other hand, we also observed important seasonal variations in the abundance of bugs, that are very similar to previous observations in the Yucatan peninsula, Mexico,9,10,19 Belize,11 and Guatemala (Monroy C, personal communication). These data strongly indicate a seasonal infestation by mostly adult bugs attempting to colonize the houses. Indeed, as reported in the Yucatan peninsula,9 there is first a dramatic increase in adult bugs during the months of April–June, followed by an increase in nymphal stages during the following months of July–September, as the adults reproduce and try to colonize houses. Thus, it appears that T. dimidiata in central Veracruz largely behaves as an invasive bug causing seasonal infestation of houses.
Our data on feeding sources further support this scenario, as these can be used to assess bug dispersal.20–22 We observed several cases of bugs collected inside the houses that fed on domestic animals such as chicken, dog, or cat, which are most frequently located in the peridomiciles, and peridomestic bugs feeding on humans and mixed feedings. These data suggest that T. dimidiata is able to disperse in both directions between the peridomicile and the domicile, similarly to previous observations in Colombia and Costa Rica.21,22 Further population genetics studies should help assess the extent of dispersal between these habitats.23 The abundant feeding on humans inside the domiciles also confirms the high risk of T. cruzi transmission, in agreement with the elevated seroprevalence observed in the area.6 On the other hand, the feeding on several domestic animals suggests that these may play a role as risk factors for house infestation and T. cruzi transmission. Further studies on the determinants for house infestation should help clarify this point.24 Finally, the observation of different feeding profiles between bugs from the domiciles and the peridomiciles suggest that T. dimidiata is highly adaptable to different food sources. Previous studies have similarly shown that T. dimidiata is very eclectic and may feed on a large variety of hosts.21,22,25–27 Feeding on multiple sources was also relatively frequent, accounting for 34% of the blood meals in our study, compared with 20% in T. dimidiata from Guatemala.25 Taken together, these data suggest that T. dimidiata may not have marked preferences for specific hosts, but simply takes advantage of whatever food sources are readily available in its habitat.
Taken together, our data strongly suggest that high dispersal capabilities and seasonal infestation may be a shared characteristics of several of the T. dimidiata taxonomic groups identified in this species complex.12 Indeed, we clearly confirmed that ITS-2 group 2 is present in central Veracruz, and that it behaves similarly as groups 2 and 3 in the Yucatan peninsula, Mexico, causing seasonal infestation of houses. Thus, T. dimidiata ITS-2 genotypes may not be relevant or associated with different domiciliation capabilities of T. dimidiata subspecies. Rather, as suggested before,16 local ecologic factors may determine the extent of domiciliation of T. dimidiata. Additional studies on the determinants of infestation and domiciliation may help clarify these aspects.
Finally, our observations have important implications for T. dimidiata vector control in the region. Indeed, it is now well established that the efficacy of conventional insecticide spraying is short-lived in the case of non-domiciliated triatomines seasonally infesting houses.28–31 Previous control efforts in Veracruz indicated that three insecticide applications 8 months apart were required to reduce T. dimidiata infestation,32 but there was no follow-up to assess potential re-infestation after interrupting control interventions. Re-infestation by T. dimidiata following insecticide spraying has been frequently observed in the Yucatan peninsula, Mexico,28,31 as well as in Guatemala.33 Thus, alternative control interventions focusing on non-domiciliated triatomines30,31 may be more appropriate for the region, and more generally, for all or most of the taxonomic groups of the T. dimidiata complex.
In conclusion, we confirmed here the widespread distribution of T. dimidiata ITS-2 group 2 in central Veracruz, Mexico and the presence of an important entomologic risk for T. cruzi transmission, as indicated by frequent feeding on humans. In addition, we show for the first time that T. dimidiata sibling species in Veracruz behave very similar to those present in the Yucatan peninsula, Mexico, and in Belize with a strong seasonal infestation of houses by mostly adult bugs. The analysis of feeding sources suggested a lack of feeding preferences, and confirmed a significant dispersal of bugs between the peridomiciles and the domiciles. It would thus be critical to adapt vector control interventions to this behavior to improve their efficacy and sustainability, as control of T. dimidiata has been notoriously challenging in many regions.
ACKNOWLEDGMENTS
We thank QFB Daniel Guzmán-Gómez for technical assistance.
Disclaimer: The authors have declared that no conflicts of interest exist.
Footnotes
Financial support: JTM was a recipient of a PhD fellowship from CONACyT, México (No. 215333). This work was supported by grant FOMIX CONACyT-Gobierno del Estado de Veracruz (VER-2008-C02-108783).
Authors' addresses: Jesús Torres-Montero, Aracely López-Monteon, and Angel Ramos-Ligonio, LADISER Inmunología y Biología Molecular, Facultad de Ciencias Químicas, Universidad Veracruzana, Orizaba, Veracruz, Mexico, E-mails: jetorres@uv.mx, aralopez@uv.mx, and angramos@uv.mx. Eric Dumonteil, Laboratorio de Parasitología, Centro de Investigaciones Regionales Dr. Hideyo Noguchi, Universidad Autónoma de Yucatán, Merida, Yucatan, Mexico and Department of Tropical Medicine, School of Public Health and Tropical Medicine, Tulane University, New Orleans, LA, E-mail: edumonte@tulane.edu.
References
- 1.Schofield CJ, Jannin J, Salvatella R. The future of Chagas disease control. Trends Parasitol. 2006;22:583–588. doi: 10.1016/j.pt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 2. Ramsey J, Ordonez R, Tello-Lopez A, Phols JL, Sanchez V, Peterson AT. 2003. Actualization on the epidemiology of Chagas disease in MexicoIn: Initiative for the Surveillance and Control of Chagas Disease in the Mexican Republic Cuernavaca, Morelos: Instituto Nacional de Salud Pública; 85 103 [Google Scholar]
- 3.Bottazzi ME, Dumonteil E, Valenzuela JG, Betancourt-Cravioto M, Tapia-Conyer R, Hotez PJ. Bridging the innovation gap for neglected tropical diseases in Mexico: capacity building for the development of a new generation of antipoverty vaccines. Bol Med Hosp Infant Mex. 2011;68:130–138. [Google Scholar]
- 4.Cruz-Reyes A, Pickering-Lopez JM. Chagas disease in Mexico: an analysis of geographical distribution during the past 76 years–a review. Mem Inst Oswaldo Cruz. 2006;101:345–354. doi: 10.1590/s0074-02762006000400001. [DOI] [PubMed] [Google Scholar]
- 5.Salazar PM, Rojas G, Bucio M, Cabrera M, Garcia G, Ruiz A, Guevara Y, Tapia R. Seroprevalence of Trypanosoma cruzi antibodies and associated risk factors among the population under 18 years of age in Veracruz, Mexico. Rev Panam Salud Publica. 2007;22:75–82. doi: 10.1590/s1020-49892007000700001. [DOI] [PubMed] [Google Scholar]
- 6.Ramos-Ligonio A, López-Monteon A, Guzmán-Gómez D, Rosales-Encina JL, Limón-Flores Y, Dumonteil E. Identification of a hyper-endemic area for Trypanosoma cruzi infection in central Veracruz, Mexico. Am J Trop Med Hyg. 2010;83:164–170. doi: 10.4269/ajtmh.2010.09-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorn PL, Monroy C, Curtis A. Triatoma dimidiata (Latreille, 1811): a review of its diversity across its geographic range and the relationship among populations. Infect Genet Evol. 2007;7:343–352. doi: 10.1016/j.meegid.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Gourbière S, Dorn P, Tripet F, Dumonteil E. Genetics and evolution of triatomines: from phylogeny to vector control. Heredity. 2011;108:190–202. doi: 10.1038/hdy.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumonteil E, Gourbière S, Barrera-Perez M, Rodriguez-Felix E, Ruiz-Piña H, Baños-Lopez O, Ramirez-Sierra MJ, Menu F, Rabinovich JE. Geographic distribution of Triatoma dimidiata and transmission dynamics of Trypanosoma cruzi in the Yucatan peninsula of Mexico. Am J Trop Med Hyg. 2002;67:176–183. doi: 10.4269/ajtmh.2002.67.176. [DOI] [PubMed] [Google Scholar]
- 10.Dumonteil E, Ferral J, Euan-García M, Chavez-Nuñez L, Ramirez-Sierra MJ. Usefulness of community participation for the fine-scale monitoring of non-domiciliated triatomines. J Parasitol. 2009;95:469–471. doi: 10.1645/GE-1712.1. [DOI] [PubMed] [Google Scholar]
- 11.Polonio R, Ramirez-Sierra MJ, Dumonteil E. Dynamics and distribution of house infestation by Triatoma dimidiata in central and southern Belize. Vector Borne Zoonotic Dis. 2009;9:19–24. doi: 10.1089/vbz.2008.0002. [DOI] [PubMed] [Google Scholar]
- 12.Bargues MD, Klisiowicz DR, Gonzalez-Candelas F, Ramsey J, Monroy C, Ponce C, Salazar Schettino PM, Panzera F, Abad F, Sousa OE, Schofield C, Dujardin JP, Guhl F, Mas-Coma S. Phylogeography and genetic variations of Triatoma dimidiata, the main Chagas disease vector in Central America, and its position within the genus Triatoma. PLoS Negl Trop Dis. 2008;2:e233. doi: 10.1371/journal.pntd.0000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorn PL, Calderon C, Melgar S, Moguel B, Solorzano E, Dumonteil E, Rodas A, de la Rua N, Garnica R, Monroy C. Two distinct Triatoma dimidiata (Latreille, 1811) taxa are found in sympatry in Guatemala and Mexico. PLoS Negl Trop Dis. 2009;3:e393. doi: 10.1371/journal.pntd.0000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehmann P, Ordonez R, Ojeda-Baranda R, de Lira JM, Hidalgo-Sosa L, Monroy C, Ramsey JM. Morphometric analysis of Triatoma dimidiata populations (Reduviidae:Triatominae) from Mexico and northern Guatemala. Mem Inst Oswaldo Cruz. 2005;100:477–482. doi: 10.1590/s0074-02762005000500006. [DOI] [PubMed] [Google Scholar]
- 15.WHO Control of Chagas disease. World Health Organ Tech Rep Ser. 2002;905:1–109. [PubMed] [Google Scholar]
- 16.Herrera-Aguilar M, Be-Barragán LA, Ramirez-Sierra MJ, Tripet F, Dorn P, Dumonteil E. Identification of a large hybrid zone between sympatric sibling species of Triatoma dimidiata in the Yucatan peninsula, Mexico, and its epidemiological importance. Infect Genet Evol. 2009;9:1345–1351. doi: 10.1016/j.meegid.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Dorn PL, Engelke D, Rodas A, Rosales R, Melgar S, Brahney B, Flores J, Monroy C. Utility of the polymerase chain reaction in detection of Trypanosoma cruzi in Guatemalan Chagas' disease vectors. Am J Trop Med Hyg. 1999;60:740–745. doi: 10.4269/ajtmh.1999.60.740. [DOI] [PubMed] [Google Scholar]
- 18.Bosseno MF, Garcia LS, Baunaure F, Gastelum EM, Gutierrez MS, Kasten FL, Dumonteil E, Breniere SF. Identification in triatomine vectors of feeding sources and Trypanosoma cruzi variants by heteroduplex assay and a multiplex miniexon polymerase chain reaction. Am J Trop Med Hyg. 2006;74:303–305. [PubMed] [Google Scholar]
- 19.Payet V, Ramirez-Sierra MJ, Rabinovich J, Menu F, Dumonteil E. Variations in sex-ratio, feeding and fecundity of Triatoma dimidiata between habitats in the Yucatan Peninsula, Mexico. Vector Borne Zoonotic Dis. 2009;9:243–251. doi: 10.1089/vbz.2008.0078. [DOI] [PubMed] [Google Scholar]
- 20.Pizarro JC, Stevens L. A new method for forensic DNA analysis of the blood meal in chagas disease vectors demonstrated using Triatoma infestans from Chuquisaca, Bolivia. PLoS ONE. 2008;3:e3585. doi: 10.1371/journal.pone.0003585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farfan-Garcia AE, Angulo-Silva VM. Triatoma dimidiata populations' (Hemiptera: Reduviidae: Triatominae) feeding behaviour in an endemic zone and related epidemiological implications. Rev Salud Publica (Bogota) 2011;13:163–172. doi: 10.1590/s0124-00642011000100014. [DOI] [PubMed] [Google Scholar]
- 22.Zeledon R, Calvo N, Montenegro VM, Lorosa ES, Arevalo C. A survey on Triatoma dimidiata in an urban area of the province of Heredia, Costa Rica. Mem Inst Oswaldo Cruz. 2005;100:607–612. doi: 10.1590/s0074-02762005000600002. [DOI] [PubMed] [Google Scholar]
- 23.Dumonteil E, Tripet F, Ramirez-Sierra MJ, Payet V, Lanzaro G, Menu F. Assessment of Triatoma dimidiata dispersal in the Yucatan peninsula of Mexico using morphometry and microsatellite markers. Am J Trop Med Hyg. 2007;76:930–937. [PubMed] [Google Scholar]
- 24.Gurevitz JM, Ceballos LA, Gaspe MS, Alvarado-Otegui JA, Enriquez GF, Kitron U, Gurtler RE. Factors affecting infestation by Triatoma infestans in a rural area of the humid Chaco in Argentina: a multi-model inference approach. PLoS Negl Trop Dis. 2011;5:e1349. doi: 10.1371/journal.pntd.0001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasaki H, Rosales R, Tabaru Y. Host feeding profiles of Rhodnius prolixus and Triatoma dimidiata in Guatemala (Hemiptera: Reduviidae: Triatominae) Med Entomol Zool. 2003;54:283–289. [Google Scholar]
- 26.Quintal RE, Polanco G. Feeding preferences of Triatoma dimidiata maculipennis in Yucatan, Mexico. Am J Trop Med Hyg. 1977;26:176–178. doi: 10.4269/ajtmh.1977.26.176. [DOI] [PubMed] [Google Scholar]
- 27.Arzube Rodriguez M. Investigation of the feeding source of T. dimidiata, Lart. 1811 (Hemiptera: Reduvidae), using the precipitin reaction. Rev Ecuat Hig Med Trop. 1966;23:137–152. [PubMed] [Google Scholar]
- 28.Dumonteil E, Ruiz-Pina H, Rodriguez-Felix E, Barrera-Perez M, Ramirez-Sierra MJ, Rabinovich JE, Menu F. Re-infestation of houses by Triatoma dimidiata after intra-domicile insecticide application in the Yucatan peninsula, Mexico. Mem Inst Oswaldo Cruz. 2004;99:253–256. doi: 10.1590/s0074-02762004000300002. [DOI] [PubMed] [Google Scholar]
- 29.Barbu C, Dumonteil E, Gourbiere S. Optimization of control strategies for non-domiciliated Triatoma dimidiata, Chagas disease vector in the Yucatán peninsula, Mexico. PLoS Negl Trop Dis. 2009;3:e416. doi: 10.1371/journal.pntd.0000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbu C, Dumonteil E, Gourbière S. Optimization of spatially targeted vector control interventions for non-domiciliated triatomines. PLoS Negl Trop Dis. 2011;5:e1045. doi: 10.1371/journal.pntd.0001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferral J, Chavez-Nuñez L, Euan-Garcia M, Ramirez-Sierra MJ, Najera-Vasquez MR, Dumonteil E. Comparative field trial of alternative vector control strategies for non-domiciliated Triatoma dimidiata in the Yucatan peninsula, Mexico. Am J Trop Med Hyg. 2010;82:60–66. doi: 10.4269/ajtmh.2010.09-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wastavino GR, Cabrera-Bravo M, Garcia De La Torre G, Vences-Blanco M, Hernandez AR, Torres MB, Gomez YG, Mesa A, Schettino PM. Insecticide and community interventions to control Triatoma dimidiata in localities of the State of Veracruz, Mexico. Mem Inst Oswaldo Cruz. 2004;99:433–437. doi: 10.1590/s0074-02762004000400015. [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa J, Hashimoto K, Cordon-Rosales C, Abraham Juarez J, Trampe R, Marroquin Marroquin L. The impact of vector control on Triatoma dimidiata in the Guatemalan department of Jutiapa. Ann Trop Med Parasitol. 2003;97:288–297. doi: 10.1179/000349803235001895. [DOI] [PubMed] [Google Scholar]