Abstract
Most Aedes aegypti dispersal studies have focused on females because of their central role in dengue virus transmission. Only a few mark-release-recapture (MRR) studies provided insights into male Ae. aegypti dispersal. To fill this knowledge gap, we conducted five male Ae. aegypti MRR experiments in a coastal village in southern Mexico. Small and large male cohorts were marked with fluorescent dusts, released outside buildings, and recaptures were carried out by using backpack aspirators. Recapture rates ranged between 0.35% and 6.55% and median distance traveled was 12–166 meters. A statistically significant difference in median distance traveled with large males dispersing farther than small ones was detected only in one experiment (MRR5: U = 3.5, P < 0.01). Male dispersal data will be useful for constructing and estimating parameter values and validating models that will be used to plan the most effective release strategies for genetically modified male Ae. aegypti.
Introduction
Dengue virus (DENV) infection causes more human morbidity and mortality than any other vector-borne viral disease, with 2.5–3.0 billion persons at risk of infection, 50–100 million experiencing dengue fever, and 250,000–500,000 dengue hemorrhagic fever and dengue shock syndrome annually.1,2 Disease is caused by four closely related, but antigenically distinct single-strand RNA viruses belonging to the genus Flavivirus.3,4 Virus transmission to humans is mostly caused by the bite of infected Aedes aegypti, an anthropophilic mosquito that lives in close association with humans and preferentially bites people during daylight hours. Although other mosquito species have been incriminated as DENV vectors (i.e., Ae. albopictus and Ae. polynesienses), they are considered to play relatively minor roles compared with Ae. aegypti in global patterns of DENV transmission and disease burden.5
Presently, dengue control is dependent on the reduction or elimination of Ae. aegypti. Although vaccines and therapeutics are a current focus of attention, none are currently licensed for broad scale use6 and contemporary dengue control programs will continue to rely on vector control as the primary line of defense against virus transmission. When done properly, mosquito population control effectively prevents dengue.7 Unfortunately, successful dengue vector control programs are the exception, and where they are well implimented, they are difficult to sustain. The urgent need to prevent the growing dengue public health problem has motivated the exploration of novel vector control approaches, such as the use of genetically modified mosquitoes. Genetically modified mosquitoes refractory to dengue infection8 or carrying a lethal hereditable transgene9–11 have been developed and tested in the laboratory. The potential use of population replacement and/or population reduction strategies has increased awareness of how little is known about movement, spread of genes, and mating behavior in natural mosquito vector populations. Gathering above mentioned information has become critically important for testing fundamental assumptions, risk assessment, and evaluating intervention strategies of genetic-based strategies for disease prevention.
Two ecological parameters that are of crucial interest for assessing genetic control strategies and that also play a key role in dengue epidemiology are Ae. aegypti dispersal and survival in the natural environment. Most published Ae. aegypti dispersal studies have focused on females, because only females take blood meals and are involved in DENV transmission.12–16 Recent interest in the use of sterile or genetically modified male Ae. aegypti to reduce mosquito population densities and reduce dengue highlights how little is known about male biology, especially in natural settings.17
The aim of this study was to help fill this knowledge gap by studying dispersal and survival of male Ae. aegypti in a dengue-endemic area near city of Tapachula (Chiapas, Mexico) by means of mark-release-recapture (MRR) experiments. We also aimed to investigate how dispersal capabilities can be affected by different body sizes (large versus small) because recent studies18,19 have concluded that large Ae. aegypti males have a competitive mating advantage over small males. Knowledge generated from our study will be useful for the design of release strategies involving the use of transgenic Ae. aegypti males; i.e., release of insects carrying a dominant lethal (RIDL technique).20 Additionally, this information is essential for regulatory risk assessment for contained or open field testing and eventually for designing and evaluating intervention trials. Female-specific Ae. aegypti RIDL strain produce no reproductively viable female progeny unless larvae are fed with a supplement, but male progeny survive and can bear and spread into natural mosquito population the sex specific lethal dominant gene, which confers a flightless condition in female progeny.21 The RIDL strategy is intended to prevent DENV transmission by reducing or suppressing natural populations of Ae. aegypti (i.e., population reduction).
Materials and Methods
Study area.
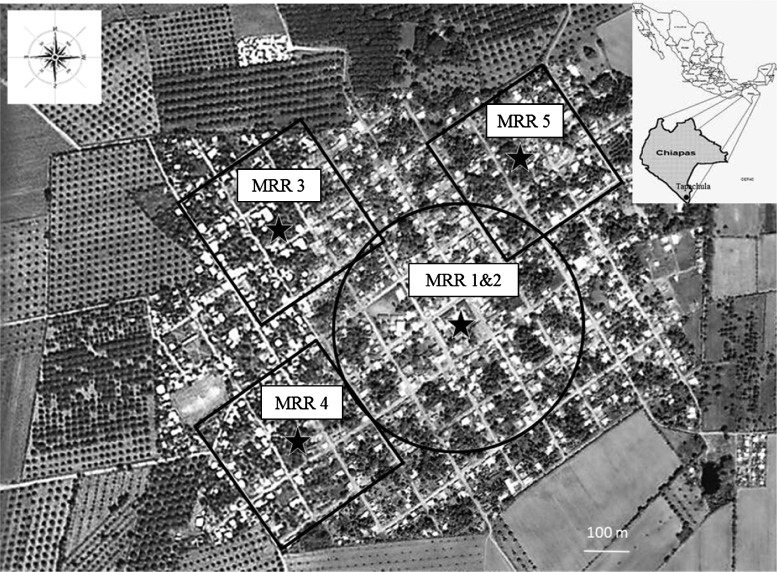
The MRR experiments were carried out in the village of Buenos Aires (Mazatan County, Chiapas, Mexico; 14°53′20.71″N, 92°28′48.96″; elevation above sea level = 13 meters) (Figure 1). The village covers approximately 1 km2 with a population of approximately 3,800 inhabitants. Soya, mango, sugar cane, and banana fields surround the village. It is located 5 km from any other village, 10 km from the Pacific Ocean, and 35 km from the city of Tapachula where our insectary facilities were located. One dengue fever case was reported in the village during the year previous to this study (2008), and one hemorrhagic dengue case was reported during the period of the study (2009).
Figure 1.
Aerial view of the village of Buenos Aires, Mexico. Study areas are indicated by black lines and release points are indicated by lack stars. Mark-release-recapture 3 (MRR3) and MRR4 were conducted simultaneously.
Houses were of medium to large size (between 80 and 160 m2 with at least /two/three bedrooms), in most cases with a garden and a courtyard where most of the human daily activities were carried out. Most houses were made of concrete and had corrugated metal roofs. A few houses were made of bamboo and had palm roofs. The municipal water supply was not constant in the village and almost all houses had water tanks for storing water for daily use in which Ae. aegypti larvae could potentially develop.
Climate at the study area is characterized by a rainy season with an average of 2.1 meters of rainfall from May through October and a dry season with an average of 0.2 meters of rainfall from November through April.
Locations in the village where MRR experiments were carried out are indicated in Figure 1. MRR1 and MRR2 were carried out in the central area of the village. MRR3, MRR4, and MRR5 were carried out in the northwest, southwest, and northeast peripheral quadrants of the village, respectively.
Approvals by institutions and community engagement
Our study design was approved by the University of California Davis Institutional Review Board (Protocol #200917036) and by the Ethics, Biosecurity and Research Commissions of the National Institute of Public Health in Mexico (INSP, Protocol #50-6344). Community engagement activities were carried out in the village to exchange knowledge regarding dengue transmission, its prevention, vector control strategies, and to seek participation and authorization for this study. Before each MRR experiment, several meetings were convened with the authorities of the village (approximately 20 persons) and with community participants in the largest government sponsored nutrition and dietary supplement program Oportunidades(http://www.sedesol.gob.mx/es/SEDESOL/Programa_de_Desarrollo_Humano_Oportunidades). Oportunidades meetings were attended primarily by women, as representatives and primary care-givers of households (100–150 women/meeting). Once collective approval was obtained, each household within each study section was visited to obtain verbal informed consent, or in the case of the last study (MRR5) written informed consent, and requested to participate in the study by allowing personnel to collect mosquitoes from outside and inside their houses before each study (by means of larval collections) and on the days after male mosquito release (by means of adult collections) until the end of each experiment. After each study, all above authorities and community members were convened again to explain study results, present the design of the next study, and to request collective participation and authorization.
Mosquitoes.
Aedes aegypti males used in each MRR experiment were F1 progeny from field-collected larvae and pupae. Before each MRR trial, immature Ae. aegypti collections were carried out in the village. Field-collected material was brought to the insectary and maintained at 27°C and a relative humidity of 80% until adult mosquitoes emerged. Aedes aegypti adults were then held in insectary cages and fed blood from a rabbit (University of California Davis Animal Care and Use protocol 15653, INSP Biosecurity approval CB08-205) to obtain eggs that were used to rear the Ae. aegypti males we used for our MRR experiments. This enabled us to 1) release males that were maintained in the laboratory for just one generation, 2) not introduce novel genes into the local mosquito population, and 3) provide an advantage to the community with a short-term reduction in the overall mosquito population density in the houses where we conducted our entomologic collections. During our pre-trial surveys, we eliminated potential Ae. aegypti larval development sites whenever possible and informed house owners about basic measures to avoid the presence of Ae. aegypti larvae in or around their houses.
Mosquito rearing and marking procedures.
The F1 eggs were hatched under a vacuum for synchronous hatching.22 First instar larvae were reared at a density of 0.17 larvae/mL and fed fish food (Microbites; Mascotas y Acuariofilia, Ecatepec, Mexico) by using two distinct diets that were intended to obtain adults within two non-overlapping body sizes as determined by wing length. As determined in previous trials, high and low nutrient diets consisted of 0.14 mg/larva/day and 0.04 mg/larva/day, respectively (Valerio L, unpublished data). As soon as pupae were detected, they were collected from rearing trays, sexed by size dimorphism,23 and male pupae were put in insectary cages (60 × 60 × 60 cm) and provided with cotton soaked with a 10% sucrose solution. Every 12 hours, cages were checked to eliminate any females before they could mate. This enabled us to release virgin males, which was important for two reasons: 1) release a homogenous population of males (all unmated) and 2) reproduce what would occur under conditions for a female-specific Ae. aegypti RIDL male release (i.e., all released transgenic males would be unmated).
The day before the release, 2–6-day-old males were collected from holding cages with mouth aspirators, put in 900-mL Styrofoam cups (100 males/cup), and marked with fluorescent dusts (Day-Glo Color Corp., Cleveland, OH). Dusts were applied by atomizing dust with 3-mL syringes filled to 0.3 mL for each cup. Small and large cohorts were marked with different colors. The long interval between each experiment (two months minimum; Table 1) ensured that all recaptured marked males belonged to the current release because maximum male lifespan is approximately 18 days under natural environmental conditions.24 Males released simultaneously in two different areas during MRR3 and MRR4 were marked with four different color dusts so we could distinguish between size and site of release.
Table 1.
Experimental design of each MRR experiment, Buenos Aires, Mexico*
| Characteristic | MRR1 | MRR2 | MRR3 | MRR4 | MRR5 |
|---|---|---|---|---|---|
| Date | June 2009 | September 2009 | December 2009 | December 2009 | March 2010 |
| Maximum distance (meters) between release point and most distant houses sampled | 302 | 302 | 247 | 234 | 237 |
| First collection day post-release (hours) | 4 | 2 | 2 | 7 | 7 |
| No. recapture days | 14 | 14 | 10 | 10 | 7 |
| No. houses surveyed | 480 | 496 | 183 | 172 | 182 |
| No. collected houses/ha/day | 1.27 | 1.27 | 1.47 | 1.48 | 2.24 |
| No. large males released | 1,000 | 3,000 | 1,076 | 1,068 | 3,442 |
| No. small males released | 984 | 3,000 | 1.031 | 942 | 1,689 |
| No. males released | 1,984 | 6,000 | 2,107 | 2,010 | 5,131 |
MRR = mark-release-recapture.
Effect of larval diet on survival of large and small males under insectary conditions.
To determine the effect of poor versus high larval diet on male survival, recently emerged small and large males (20 individuals of each size) obtained from larvae reared under low versus high regimen diet (as described above) were held individually in plastic cups. The internal walls of the cups were lined with filter paper that was moistened daily to maintain humidity. The top of the cup was covered with mosquito netting. Males were kept in the insectary at 27°C and a relative humidity of 80%, provided with a 10% sucrose solution, and examined daily for mortality.
Effect of fluorescent dusts on survival of males under insectary conditions.
One-day-old F1 Ae. aegypti were held in two control cages as unmarked individuals (Cage A: females = 29, males = 27; Cage B: females = 30, males = 22) and two treatment cages as dusted individuals (Cage C: females = 21, males = 27; Cage D: females = 25, males = 28). Adults were provided with a 10% sucrose solution, maintained under insectary conditions described above, and checked daily for mortality for 20 days.
Survival of large and small dusted male cohorts under semi-field conditions.
A field survival experiment was carried out during early June (rainy season) 2009. Two data-loggers (Hobo® Pro v2 temp/RH; Onset Computer Corporation, Bourne, MA) were put inside cages to collect data on temperature and relative humidity. Small and large 4–5-day-old F1 Ae. aegypti males and females (50 for each kind) were released in two large field cages (2.5 × 5 × 1.8 meters) provided with two sources of water, two balls of cotton soaked with a 10% sucrose solution, and resting sites as described by Facchinelli and others.25 Small and large cohorts were dusted with two different colors. Mosquitoes were maintained inside the cages for 14 days and then recaptured with backpack aspirators (John W. Hock Company, Gainesville, FL) to gather information on survival and to determine if markings were still detectable. Females were added to each cage to offer opportunities for mating and to better simulate open field conditions.
Climatic data.
During all MRR experiments temperature and relative humidity were recorded with one Hobo® data-logger located in the garden of a house situated in the central area of the village. On the roof of the house (4 meters from the ground) a weather station (Hobo Microstation; Onset Computer Corporation) was located to collect information on rainfall, wind speed, and wind direction. Wind data were not recorded during MRR5 because the wind sensor failed.
Mosquito collections and identification.
Before the study, we assigned a number to each block of the village. During pre-trial surveys, houses within study areas were labeled with a code indicating the number of the block and the house. Release points and each house located within the study area were georeferenced by using a global positioning system. During all trials, mosquitoes were collected indoors and outdoors by using backpack aspirators. We considered indoor locations to be those spaces surrounded by at least three walls and covered by a roof, and outdoor collections were performed within three meters from the edge of the roof. Time spent in each house was variable and ranged from 20 to 30 minutes, depending on the size and complexity of the house. Captured mosquitoes were brought to the insectary, frozen, counted, and morphologically identified to genus/species and sex by using a stereoscope. Aedes aegypti males were then examined under UV light for the presence of fluorescent dust. Marked and unmarked males were stored in different vials. The right wing of all Ae. aegypti males was removed when possible (i.e., wing was not damaged) and was dry mounted on a microscope slide. Wing length was measured to the nearest 0.01 mm as the distance from the axial incision to the R4+5 vein excluding the fringe setae26 by using a stereoscope equipped with an ocular micrometer.
Study design.
In all MRR experiments, males were released outdoors at sunrise approximately in the center of the recapture area. The three different areas of the village where MRR trials were carried out are shown in Figure 1. Details for the experimental design of each MRR trial are shown in Table 1.
MRR1 and MRR2.
Our first two experiments were carried out in June and September 2009, respectively (Table 1). The study area consisted of a total of 28.26 ha (14.97 houses/ha) with a radius of 300 meters and was divided into three concentric sectors with radii of 100, 200, and 300 meters from the release point. The number of houses sampled daily in each sector (sector 100 meters: n = 4; sector 200 meters: n = 12; sector 300 meters: n = 20) was calculated taking into account sector areas (sector 100 meters: 3.14 ha; sector 200 meters: 9.42 ha; sector 300 meters: 15.7 ha) to have an equal sampling effort in each sector (i.e., 1.3 houses/ha/sector). A total of 36 houses was randomly selected and surveyed each day across all sectors. Each subsequent day different houses were sampled until all houses inside the study area were sampled. In general, each house was sampled no more than twice during the study. During MRR1, collections started four days after the release and during MRR2, they started two days after release. In both experiments, collections were carried out for 14 days.
MRR3 and MRR4.
These two experiments were carried out simultaneously in December 2009. Each study area consisted of nine blocks at the northwest and southwest periphery of the village (Figure 1) in a total area of 12.27 ha (9.40 houses/ha) and 12.14 ha (7.66 houses/ha) for MRR3 and MRR4, respectively. Collections started two days after the release and were carried out for a total of 10 days (Table 1). Males were marked with four different dusts to distinguish between size and site of release. In these two experiments, we focused on smaller study areas because during MRR2, most marked males were collected close to the release point. A total of 18 houses was selected and surveyed each day for each study area. The criterion for collection house selection was to have an equal number of sampled houses/block/day (n = 2). Within each block, houses were randomly chosen. Each subsequent day, different houses were sampled in each block until all houses inside the study areas were sampled. In general, each house was sampled no more than twice.
MRR5.
This experiment was carried out in March 2010. The study area consisted of nine blocks at the northeast section of the village (Figure 1), with a total area of 11.62 ha (8.91 houses/ha). During this experiment, the number of collection houses/ha/day was almost double compared with previous experiments (Table 1) to gather finer-scale information on marked mosquito distribution. Collections started seven hours after the release and were carried out for seven days (Table 1). During the first two days of collections, we collected only from houses located at the edge of the study area from 12 houses/day to gather information on rapid, early male dispersal. From day 3 until the end of the experiment, 36 houses were surveyed daily. When possible, we tried to maintain the same number of houses surveyed per block (n = 4), but this was not always feasible because house owners were not always available. In general, each house was sampled no more than twice.
Statistical analysis.
Data sets were checked for normality by using the Kolmogorov-Smirnov test, and when data did not follow a normal distribution, non-parametric statistics were applied. Survival under insectary conditions of dusted and not dusted large versus small males was compared by using Kaplan-Meier survival curves that were then compared by log-rank test. The Fisher exact probability test was used to analyze differences in the number of small versus large males that survived under semi-field conditions after a two-week period. Recapture rates of small and large males were calculated for each MRR as the proportion of the number of recaptured marked mosquitoes over the total number of released for each size cohort.
Wing length data were normally distributed (P > 0.05) and differences in wing length between small, large, and wild collected males in each MRR experiment were analyzed with analysis of variance, followed by the Tukey post-hoc test.
Dispersal distances were calculated for each recaptured male as the linear distance from the release point to the house where it was recaptured. Thus, the dispersal estimates do not account for indirect flight patterns, but represent net movement from the release point until recapture. Dispersal distances of the two different size cohorts were calculated as the median distance traveled (MedianDT); i.e., the median linear distance from the release point. Maximum distance traveled (MaxDT) was established as the linear distance from the release site to the most distant point where marked males were recaptured. Dispersal distances did not follow a normal distribution (P < 0.001), and differences in MedianDT and in MaxDT between small and large cohorts within each MRR were evaluated with the Mann Whitney U test. To investigate movements of marked males through time, linear regression analysis was applied to the MedianDT of marked and recaptured males against days of collections.
For analysis of survival of small and large males recaptured during each MRR study, regression lines were computed by fitting the log-transformed number of recaptured males against the day of collection. To analyze differences in survival between small and large males during each MRR study, the slopes of regression lines were compared.27 The probability of daily survival (PDS) for marked male cohorts in each MRR experiment was estimated by fitting the exponential model28 to log-transformed data for recaptured males against the day of collection. The antilogarithm of the slope of the regression line gives an estimate of PDS.29 The average life expectancy was calculated from the PDS as 1/–logePDS.29 Despite the fact that the exponential model has two fundamental drawbacks (it assumes a priori that mosquito mortality is age independent and it does not consider removal of individuals by the capturing method), it has been traditionally used to describe mortality patterns in mark release recapture experiments with Ae. aegypti.30–32
Results
Effect of larval diet on survival of large and small males under insectary conditions.
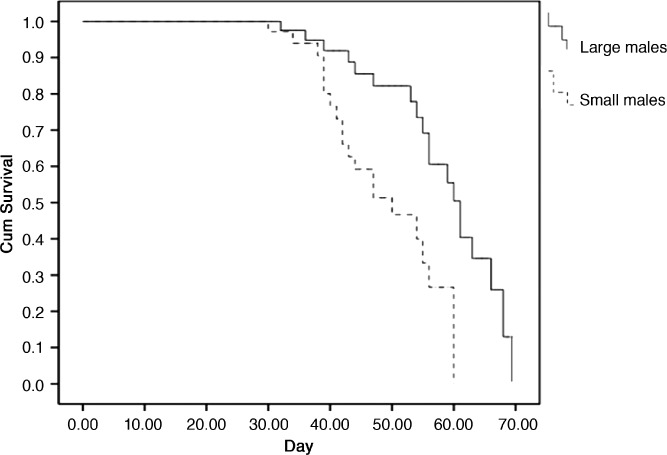
Survival of large and small males was 100% for 30 days. Survival curves for the two size cohorts, however, were significantly different (Mantel-Cox χ2 = 9.36 degrees of freedom [df] = 1, P = 0.002) with large males having a higher median survival time (61 days) than small males (50 days) (Figure 2). We do not expect shorter median survival time of small male cohorts to have affected results of MRR experiments because the longest trials (MRR1 and MRR2) lasted only 18 and 16 days, respectively, which correspond to a maximum age of 24 and 22 days.
Figure 2.
Survival curves of large and small males under insectary conditions, Buenos Aires, Mexico.
Effect of fluorescent dusts on survival of males under insectary conditions.
No difference in survival between marked and unmarked males was detected (Mantel-Cox χ2 = 3.66 df = 3 P = 0.301); i.e., survival in the insectary was not affected by the marking method evaluated over a 20-day period.
Survival of large and small dusted male cohorts under semi-field conditions.
The mean ± SD temperature and mean ± SD relative humidity registered during the two weeks of the experiment were 26.35 ± 2.50°C and 83.73 ± 7.45%.
No differences in the survival of either small or large males were detected between replicates (small: Fisher exact P = 0.44; large: Fisher exact P = 0.74). Overall, no difference in survival between small versus large cohorts was detected (Fisher exact P = 0.72). After the two-week period, a mean number of three small and five large males were found surviving inside the cages, which correspond to a daily survival of 0.82 and 0.85, respectively, and markings on all survived mosquitoes were still clearly detectable.
Climatic data.
Climatic data of MRR1 are not shown because only seven marked males were recaptured. Mean ± SD temperature was 27.56 ± 4.45°C, 26.89 ± 4.05°C, and 26.70 ± 0.29°C during MRR2, MRR3 + 4, and MRR5, respectively. Mean ± SD relative humidity was 82.43 ± 10.37%, 76.87 ± 15.07%, and 77.67 ± 2.38% during MRR2, MRR3 + 4, and MRR5, respectively. During MRR2, 149.80 mm of rainfall fell over nine days; most fell on day 9 and day 13 of collections (84.80 mm and 51.00 mm each day, respectively). No rainfall occurred during MRR3, 4, and 5.
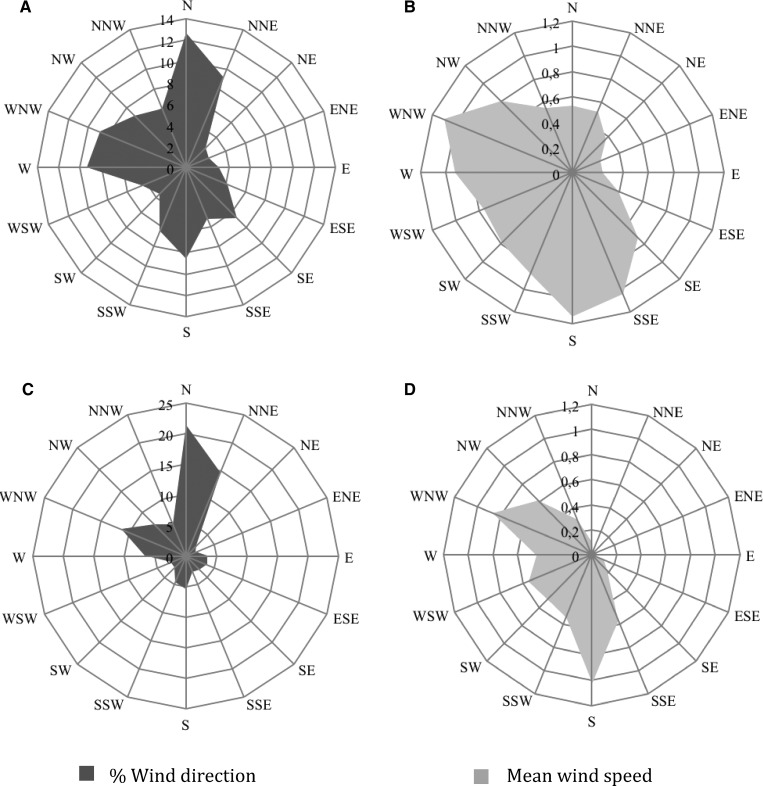
Wind direction and mean wind speed recorded during MRR2 and MRR3 + 4 are shown in Figure 3, respectively. Mean wind speed was in the range of 0.24–1.14 meters/second (Figure 3B) and 0.02–1.02 meters/second (Figure 3D) during MRR2 and MRR3 + 4, respectively. The maximum wind gusts registered were 9.09 meters/second and 6.31 meters/second during MRR2 and MRR3 + 4, respectively. In general, the predominant wind blew from the south to the north during MRR2 and MRR3 + 4 (MRR2: mean wind speed = 0.53 meters/second and MRR3 + 4: mean wind speed = 0.10 meters/second). Aedes aegypti has been reported to fly upwind at air speed up to 1.5 meters/second.33 Mean wind speed recorded during MRR experiments was < 1.14 meters/second, which indicated that the wind had in general no effect on Ae. aegypti male dispersal patterns observed during the experiments. Moreover, gust directions did not overlap with the direction recorded for high dispersal males, which excludes their effects on released male dispersal.
Figure 3.
Wind direction (left) showing the percentage of time that the wind comes from a given direction and the mean wind speed (right) during mark-release-recapture 2 (MRR2) (A and B) and MRR3 and 4 (C and D), Buenos Aires, Mexico.
Mosquito collection and identification.
Mosquitoes collected in each MRR experiment are shown in Table 2. A total of 35,696 Culicidae were collected during all experiments, of which 18.88% were Ae. aegypti (n = 6,741), 64.37% were Culex spp. (n = 22,978), 16.15% were Ochlerotatus taeniorhyncus (n = 5,764) and 0.60% were Ae. albopictus (n = 213). High numbers of Culex spp. were captured indoors and outdoors while resting in dark and humid places in all MRR experiments, which indicated that these mosquitoes emerged from larval development sites in and around the community. High numbers of Oc. taeniorhyncus were captured only during MRR1 while resting indoors and outdoors. MRR1 was performed at the beginning of the rainy season when the first heavy rains and southern tropical storms promoted egg hatching of this species in the salt marshes of the costal area located close to the village of Buenos Aires. Females of this species are strong fliers and are able to migrate to communities located miles away from the salt water marshes where they develop seeking for hosts. This finding might explain the higher number of females of this species with respect to the number of male Oc. taeniorhyncus collected in MRR1. Only 213 Ae. albopictus were recaptured in all experiments, although this species is widely spread in the area (Bond G and others, unpublished data).
Table 2.
Numbers of mosquitoes by species according to location collected during each MRR experiment, Buenos Aires, Mexico*
| Species | Location | Sex | MRR1 | MRR2 | MRR3 | MRR4 | MRR5 | Total |
|---|---|---|---|---|---|---|---|---|
| Aedes aegypti | In | M | 895 | 858 | 409 | 166 | 393 | 2,721 |
| F | 990 | 720 | 387 | 129 | 313 | 2,539 | ||
| Out | M | 382 | 246 | 92 | 22 | 65 | 807 | |
| F | 415 | 174 | 39 | 18 | 28 | 674 | ||
| Total | 2,682 | 1,998 | 927 | 335 | 799 | 6,741 | ||
| Ae. albopictus | In | M | 2 | 14 | 6 | 15 | 3 | 40 |
| F | 15 | 14 | 4 | 5 | 1 | 39 | ||
| Out | M | 17 | 29 | 5 | 8 | 6 | 65 | |
| F | 44 | 15 | 1 | 5 | 4 | 69 | ||
| Total | 78 | 72 | 16 | 33 | 14 | 213 | ||
| Ochlerotatus taeniorhyncus | In | M | 317 | 11 | 29 | 72 | 7 | 436 |
| F | 1,046 | 66 | 105 | 156 | 24 | 1,397 | ||
| Out | M | 1,214 | 24 | 43 | 40 | 3 | 1,324 | |
| F | 2,427 | 48 | 58 | 68 | 8 | 2,607 | ||
| Total | 5,004 | 147 | 235 | 336 | 42 | 5,764 | ||
| Culex spp. | In | M | 2,556 | 371 | 189 | 525 | 286 | 3,927 |
| F | 2,827 | 532 | 352 | 597 | 194 | 4,502 | ||
| Out | M | 5,783 | 455 | 144 | 190 | 102 | 6,674 | |
| F | 6,574 | 694 | 256 | 280 | 71 | 7,875 | ||
| Total | 17,740 | 2,052 | 941 | 1,592 | 653 | 22,978 | ||
| Total no. of mosquitoes | 25,504 | 4,269 | 2,119 | 2,296 | 1,508 | 35,696 |
MRR = mark-release-recapture; In = indoors; Out = outdoors.
Although the number of houses sampled per ha/day was the same during MRR1 and MRR2 (Table 1), more mosquitoes were collected during MRR1 than MRR2 (25,504 versus 4,269) (Table 2) (Yates χ2 = 8,674 df = 1, P < 0.0001). In MRR3 and MRR4, the number of houses sampled per ha/day was similar (Table 1), and no differences were detected in the total number of mosquitoes collected (2,119 versus 2,296) (Table 2) (Yates χ2 = 3.47, df = 1, P = 0.06). During MRR5, 1,508 mosquitoes were collected, of which 53% were Ae. aegypti (Table 2).
More Ae. aegypti were always collected indoors than outdoors. This finding was observed for females (79.02%) and males (77.13%). The number of Ae. aegypti females/house fluctuated between 0.85 in MRR4 and 2.93 in MRR1, and the number of males/house ranged between 1.09 in MRR4 and 2.72 in MRR3.
Release and recapture data.
A total of 17,232 Ae. aegypti marked males were released during all five MRR experiments (1,984 in MRR1, 6,000 in MRR2, 2,107 in MRR3, 2,010 in MRR4, and 5,131 in MRR5), of which 55.63% were large and 44.37% were small (Table 1).
Highly significant differences were observed in the mean wing length of large, small, and wild Ae. aegypti males in each MRR experiment (Table 3). Mean wing length ranged between 2.2 and 2.3 mm, 1.9 and 2.1 mm, and 2.0 and 2.1 mm for large, small, and wild males, respectively. During MRR2 and MRR3 + 4, small males were similar in size to wild-collected males (Table 3) (P = 0.11 for MRR2 and P = 0.92 for MRR3 + 4, by post hoc Tukey test).
Table 3.
Mean wing length (mm) ± 95% confidential interval of large, small, and wild-collected males during each MRR experiment, Buenos Aires, Mexico*
| Trial | Large males | Small males | Wild males | ANOVA | ||||
|---|---|---|---|---|---|---|---|---|
| No. | Mean wing length (mm) ± 95% CI | No. | Mean wing length (mm) ± 95% CI | No. | Mean wing length (mm) ± 95% CI | F | P | |
| MRR1 | 100† | 2.26 ± 0.01a | 100 | 2.09 ± 0.02b | 42 | 2.04 ± 0.04c | 113.01 | < 0.001 |
| MRR2 | 130 | 2.30 ± 0.01a | 167 | 2.05 ± 0.02b | 1,005 | 2.08 ± 0.01b | 129.35 | < 0.001 |
| MRR3 + 4 | 27 | 2.31 ± 0.01a | 25 | 1.99 ± 0.02b | 382 | 2.09 ± 0.01b | 28.45 | < 0.001 |
| MRR5 | 129 | 2.21 ± 0.01a | 72 | 1.95 ± 0.09b | 383 | 2.08 ± 0.01c | 122.76 | < 0.001 |
MRR = mark-release-recapture; CI = confidence interval; ANOVA = analysis of variance. ANOVA followed by the Tukey post-hoc test was performed for each MRR experiment. Different letters indicate significant difference between variables within each MRR experiment at P = 0.05.
In MRR1 we did not recollect enough males to gather information on wing length. Thus, wing length data for MRR1 were obtained from a sample of males that emerged from the same rearing trays as the released males. Wing length data of MRR2, MRR3, MRR4 and MRR5 pertain to large and small males that were recaptured.
A total of 390 (4.07%) and 332 (4.34%) large and small males were recaptured, respectively, and in both most (> 84%) males were recaptured indoors. Numbers of recaptured males and observed recapture rates for each MRR experiment are summarized in Table 4. Recapture rates during MRR1 were low (a cumulative recapture of 0.35%) because we waited relatively long (4 days) after the release to initiate collections. During our other MRR experiments, recapture rates ranged between 3.09% (95% confidence interval [CI] = 2.21–4.31% in MRR4) and 7.81% (95% CI = 6.35–9.57% in MRR3) for large males and between 2.12% (95% CI = 1.38–3.25% in MRR4) and 5.93% (95% CI = 5.14–6.83% in MRR2) for small males.
Table 4.
Indoor and outdoor recapture rates for large and small male mosquitoes collected in each MRR experiment, Buenos Aires, Mexico*
| Size cohort | Location | MRR1 | MRR2 | MRR3 | MRR4 | MRR5 |
|---|---|---|---|---|---|---|
| Large | In | 4 (0.40) | 128 (4.27) | 52 (4.83) | 28 (2.62) | 119 (3.46) |
| Out | 0 (0.00) | 3 (0.10) | 32 (2.97) | 5 (0.37) | 19 (0.55) | |
| Total | 4 (0.40) | 131 (4.37) | 84 (7.81) | 33 (3.09) | 138 (4.01) | |
| Small | In | 1 (0.10) | 171 (5.70) | 34 (3.30) | 16 (1.70) | 60 (3.55) |
| Out | 2 (0.20) | 7 (0.23) | 20 (1.84) | 4 (0.42) | 17 (1.01) | |
| Total | 3 (0.30) | 178 (5.93) | 54 (5.24) | 20 (2.12) | 77 (4.56) | |
| Large plus small | In plus out | 7 (0.35) | 309 (5.15) | 138 (6.55) | 53 (2.64) | 215 (4.19) |
Values are no. (%). MRR = mark-release-recapture.
Overall, 99%, 92%, and 79% of marked males were collected during the first 4 days of collection in MRR2, MRR3, and MRR4, respectively. In MRR5, collections were initiated seven hours after the release and during the first two days (day 0 and day 1), and sampling was performed only on the periphery of the study area and yielded seven marked males. Most (94.88%) recaptured males were collected between day 2 and day 5 of collection, which corresponded to the first 4 days of MRR2, MRR3, and MRR4 collections.
Dispersal and survival.
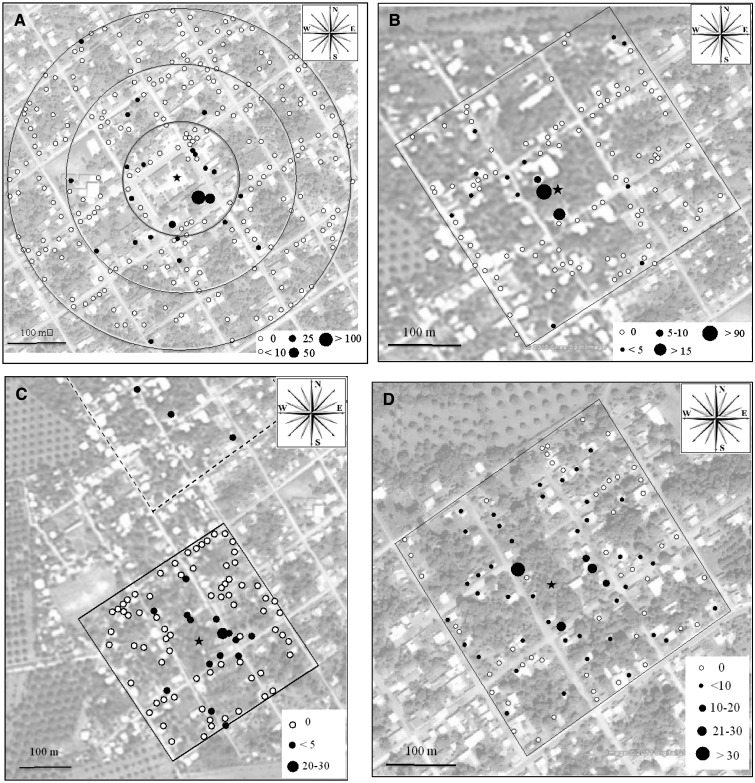
The distribution of marked males that were collected during MRR2, MRR3, MRR4, and MRR5 is shown in Figure 4. Dispersal data from MRR1 are not shown because only seven marked males were collected during that experiment. Of the total recaptured marked males, 94.5% (n = 309), 93.48% (n = 138), 71.70% (n = 53), and 79.5% (n = 215) were collected < 100 meters from the release point during MRR2, MRR3, MRR4, and MRR5, respectively. As shown in Figure 4, most recaptured males were collected in the same block where they were released (MRR2 = 88.67%; MRR3 = 88.41%; MRR4 = 54.71%; MRR5 = 67.91%). Spatial distribution of recaptured marked males was highly aggregated in all experiments; more than 50% of the recaptured marked males were collected in just three houses that were close to the release point. In MRR2 and MRR3, this percentage was > 86%.
Figure 4.
Study areas and marked recaptured males for mark-release-recapture 2 (MRR2) (A), MRR3 (B), MRR4 (C), and MRR5 (D), Buenos Aires, Mexico. The black star indicates the release point. Black dots indicate houses where marked males were collected. White dots indicate houses where collections were carried out but no marked males were encountered. The size of black circle is representative of number of mosquitoes collected (see individual figure legends).
Daily and cumulative MedianDT and MaxDT of large and small male cohorts for each MRR experiment is shown in Table 5. The cumulative MedianDT was < 100 meters for large and small males in all experiments, except in MRR1. No differences were observed in the cumulative MedianDT between large and small males during MRR2, MRR3, and MRR4, (see P values in Table 5). During MRR5, large males dispersed farther than small males (U = 4075.00, P < 0.01). The same situation was observed for the difference between the MaxDT of large and small male cohorts (see P values in Table 5); a significant difference was detected only during MRR5 (U = 3.5, P < 0.01) when large males had a higher MaxDT than small males.
Table 5.
Median and maximum distance traveled (MedianDT and MaxDT) for large and small cohorts, Buenos Aires, Mexico*
| MRR1 | MRR2 | MRR3 | MRR4 | MRR5 | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MedianDT (meters) | MaxDT (meters) | MedianDT (m) | MaxDT (meters) | MedianDT (meters) | MaxDT (meters) | MedianDT (meters) | MaxDT (meters) | MedianDT (meters) | MaxDT (meters) | |||||||||||||||||||||
| Large | Small | Large | Small | Large | Small | Large | Small | Large | Small | Large | Small | Large | Small | Large | Small | Large | Small | Large | Small | |||||||||||
| Days after release | No. | No. | No. | No. | No. | No. | No. | No. | No. | No. | ||||||||||||||||||||
| 0 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | 168 | 1 | 173 | 173 | 173 |
| 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 3 | 134 | 1 | 153 | 181 | 153 |
| 2 | – | – | – | – | – | – | 86 | 53 | 117 | 53 | 302 | 138 | 71 | 12 | 41 | 12 | 36 | 201 | 1* | 48 | 0 | ND | 48 | ND | 63 | 53 | 45 | 48 | 237 | 112 |
| 3 | – | – | – | – | – | – | 34 | 52 | 44 | 54 | 110 | 130 | 4 | 31 | 5 | 22 | 138 | 134 | 17 | 50 | 12 | 50 | 156 | 57 | 18 | 81 | 12 | 67 | 190 | 116 |
| 4 | 1 | 265 | 0 | ND | 265 | ND | 4 | 70 | 7 | 70 | 178 | 285 | 0 | ND | 2 | 64 | ND | 75 | 2 | 52 | 1 | 123 | 60 | 123 | 24 | 76 | 8 | 48 | 160 | 136 |
| 5 | 2 | 131 | 1 | 70 | 192 | 70 | 5 | 130 | 8 | 75 | 164 | 130 | 2 | 80 | 2 | 176 | 103 | 247 | 0 | ND | 1 | 90 | ND | 90 | 24 | 68 | 10 | 83 | 154 | 138 |
| 6 | 0 | ND | 1 | 131 | ND | 131 | 0 | ND | 0 | ND | ND | ND | 0 | ND | 0 | ND | ND | ND | 4 | 89 | 2 | 67 | 110 | 73 | 4 | 138 | 0 | ND | 156 | ND |
| 7 | 0 | ND | 1 | 143 | ND | 143 | 1 | 61 | 0 | ND | 61 | ND | 4 | 107 | 0 | ND | 246 | ND | 2 | 55 | 3 | 56 | 61 | 61 | – | – | – | – | – | – |
| 8 | 0 | ND | 0 | ND | ND | ND | 1 | 61 | 0 | ND | 61 | ND | 1 | 12 | 3 | 12 | 12 | 12 | 0 | ND | 1 | 123 | ND | 123 | – | – | – | – | – | – |
| 9 | 0 | ND | 0 | ND | ND | ND | 0 | ND | 0 | ND | ND | ND | 2 | 85 | 0 | ND | 117 | ND | 0 | ND | 0 | ND | ND | ND | – | – | – | – | – | – |
| 10 | 0 | ND | 0 | ND | ND | ND | 0 | ND | 1 | 53 | ND | 53 | 0 | ND | 1 | 22 | ND | 22 | 0 | ND | 0 | ND | ND | ND | – | – | – | – | – | – |
| 11 | 0 | ND | 0 | ND | ND | ND | 0 | ND | 1 | 190 | ND | 190 | 0 | ND | 0 | ND | ND | ND | 0 | ND | 0 | ND | ND | ND | – | – | – | – | – | – |
| 12–14 | 1 | 200 | 0 | ND | 200 | ND | 0 | ND | 0 | ND | ND | ND | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Cum. | 4 | 166 | 3 | 131 | 265 | 131 | 131 | 53 | 178 | 53 | 302 | 285 | 84 | 12 | 54 | 12 | 246 | 247 | 26 | 57 | 20 | 52 | 533 | 123 | 138 | 68 | 77 | 53 | 237 | 173 |
| U test, large vs. small | Not performed | U = 11016.50, P = 0.33 | U = 17.00, P = 0.94 | U = 2105.50, P = 0.38 | U = 17.50, P = 0.94 | U = 303.00, P = 0.61 | U = 11.50, P = 0.54 | U = 4075.00, P = 0.004 | U = 3.50, P = 0.008 | |||||||||||||||||||||
The first day of collection 7 large males were recaptured in the study area of MRR3 at an MDT of 533.48 meters. MRR = mark-release-recapture; ND = not determined because no males were recaptured; Cum. = cumulative. P values refer to Mann-Whitney U test results when comparing differences between large and small males in MedianDT and MaxDT.
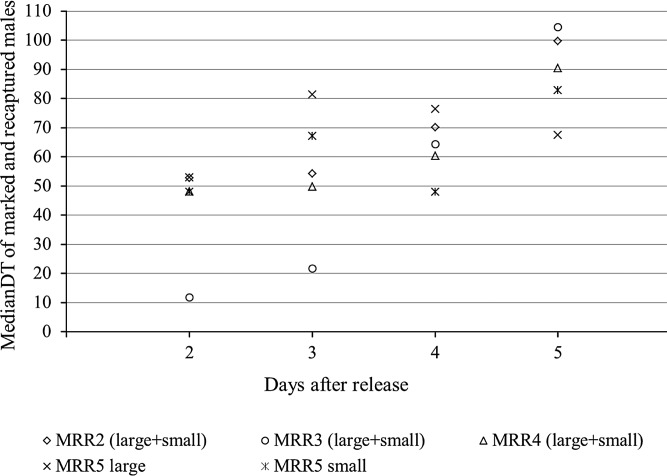
Daily MedianDT for each MRR experiment, except MRR1, calculated from two to five days after release when most males were recaptured is shown in Figure 5. Data from large and small males were merged in MRR2, MRR3, and MRR4. For MRR5, data were maintained separately because the two sets of data were statistically different. The seven marked males released in the MRR4 area and recaptured during the first day of collection outside that area, at a MedianDT of 533 meters, were excluded from dispersal analysis because although these data are interesting, they cannot be combined with data belonging to the rest of the MRR4 area. Overall, the daily MedianDT slowly increases through time from two to five days after the release. In all experiments, values of slopes of equations obtained from linear regression analysis applied to the MedianDT by marked and recaptured through time indicate that males move only short distances each day (MRR2: y = 15.71x + 29.95, R2 = 0.86, P = 0.06; MRR3: y = 32.08x – 29.64, R2 = 0.95, P = 0.03; MRR4: y = 13.74x + 27.84, R2 = 0.82, P = 0.09; MRR5 large males: y = 3.87x + 56.02, R2 = 0.16, P = 0.60; and small males: y = 8.54x + 31.58, R2 = 0.43, P = 0.35).
Figure 5.
Movement of marked males through time computed by daily median distance traveled of marked and recaptured males during mark-release-recapture 2 (MRR2), MRR3, MRR4 and MRR5, Buenos Aires, Mexico. For MRR2, MRR3, and MRR4 dispersal, data are merged for large and small males because no difference was observed in the median distance traveled.
Although most recaptured males did not disperse far from the release point, our results indicate that some have striking dispersal tendencies; within 48 hours after release, 2 males were collected 301 meters from the release point in MRR2, 1 male was collected 200 meters from the release point in MRR3, and 7 males were collected at 533 meters from the release point in MRR4. Results from early collections carried out during MRR5 show that 7 males were collected between 134 and 181 meters from the release point during the first 48 hours after release. These data indicate that some individuals exhibit relatively strong flight abilities for this species shortly after release.
No differences were detected in the survival of small and large male cohorts for any MRR; slopes obtained from the regression lines of the log-transformed number of small and large recaptured males against days of collection were not significantly different (Table 6). Therefore, we concluded that assuming a common slope for the two size cohorts was reasonable. The PDS ranged between 0.48 (during MRR5) and 0.82 (during MRR4) and provided an estimate of average life expectancy in the range of 1.36 and 5.10 days (Table 7).
Table 6.
Comparison between two regression lines obtained by fitting the log-transformed number of large and small marked Aedes aegypti males recaptured against days of collection in MRR2, MRR3, MRR4, and MRR5, Buenos Aires, Mexico*
| Characteristic | MRR2 | MRR3 | MRR4 | MRR5 |
|---|---|---|---|---|
| Comparison | Large vs. small | Large vs. small | Large vs. small | Large vs. small |
| Blarge – bsmall | –0.005 | –0.001 | 0.075 | –0.021 |
| SEdiff | 0.040 | 0.068 | 0.049 | 0.101 |
| T | –0.125 | 0 | 1.525 | –0.212 |
| Df | 24 | 16 | 16 | 8 |
| P | 0.902 | 1 | 0.147 | 0.837 |
MRR = mark-release-recapture; b = slope of regression; SEdiff = standard error of difference between the two slopes, t = tests the null hypothesis that the slopes of the two regression lines are the same; df = degrees of freedom.
Table 7.
Components of regression analyses of log-transformed marked Aedes aegypti males recaptured against days of collection in MRR2, MRR3, MRR4, and MRR5, and the derived probability of daily survival and average life expectancy, Buenos Aires, Mexico*
| Characteristic | MRR2 | MRR3 | MRR4 | MRR5 |
|---|---|---|---|---|
| R2 | 0.610 | 0.417 | 0.363 | 0.781 |
| R | 0.781 | 0.645 | 0.603 | 0.884 |
| A | 1.346 | 1.066 | 0.726 | 2.062 |
| B | –0.124 | –0.116 | –0.085 | –0.319 |
| T | –6.382 | –3.585 | –3.203 | –5.970 |
| P | 9.26 × 10–7† | 0.002‡ | 0.005‡ | 1.38 × 10–4† |
| PDS | 0.752 | 0.766 | 0.822 | 0.480 |
| ALE (days) | 3.511 | 3.744 | 5.102 | 1.361 |
MRR = mark-release-recapture; R2 = coefficient of determination; R = correlation coefficient; a = y – intercept (constant); b = slope of regression; t = tests the null hypothesis that the coefficient of the independent variable (day of recapture) is zero; PDS = probability of daily survival; ALE = average life expectancy.
P < 0.001.
P < 0.01.
Discussion
Previous Ae. aegypti dispersal studies focused on adult females and indicated that the species has limited dispersal tendencies.24,29,32,34,35 In our study, males showed a similar propensity to disperse short distances, with a minimum of 83.02% of recaptures occurring within 100 meters of the release point and > 50% of marked males being collected in the same block where they were released.
The age interval of released males (a maximum of four days between young and old males) was narrow enough that we expect it did not affect dispersal distances. This finding is consistent with results of the study of Harrington and others,35 which did not detect an age-dependent effect on dispersal distances of males Ae. aegypti during MRR experiments in Thailand and Puerto Rico.
Low mean wind speed registered during MRR2 and MRR3 + 4 indicate that males flew actively, and wind direction and speed did not substantially affect their dispersal. However, it is worth noting that 7 males that were collected 533 meters from the release point during MRR4 flew north in the direction of prevailing winds. In this experiment, these individuals may have exploited southern winds for rapid dispersal. The MaxDT recorded during MRR4 represents the highest value reported for MaxDT that we could find for Ae. aegypti males. Harrington and others35 reported a MaxDT of 453 meters, and other studies detected a MaxDT in the range of 100–160 meters.24,29,32
Male size did not affect dispersal, except in MRR5, when large males dispersed farther than small males. The number of houses sampled/ha/day was almost double in MRR5 than in other MRR experiments. This finding enables higher resolution and statistical power to detect dispersal differences between large and small males. Results indicate that larger males could have relatively higher dispersal abilities than smaller males. Similarly, Maciel-de-Freitas and others32 assessed the effect of body size on displacement of male Ae. aegypti and found that although it was not statistically significantly different, larger males tended to disperse farther than smaller males (42 meters versus 32 meters).
Excluding results from MRR1, the cumulative MedianDT was low (≤ 68 meters) and in the range of other studies. Muir and Kay29 in northern Australia found that males moved an average of 35 meters. Trpis and Hauserman24 in Kenya calculated a mean distance traveled of 44 meters. Tsuda and others34 in China found that the greatest mean distance traveled was 60 meters. Harrington and others35 reported that 72% of marked males in Thailand were found in the house adjacent to where they were released.
In our MRR experiments, the MedianDT increased slowly over time, which indicated that male daily dispersal is low (average = 15 meters/day), which is identical to the approximately 15 meters/day reported by Harrington and others12 for female Ae. aegypti. Interestingly, a small number of males in all of our experiments moved considerably longer distances. This finding was most evident in MRR5, when 3 marked males moved approximately 170 meters during the first 7 hours after release. The fact that a few males rapidly moved relatively long distances in a short period could have been caused by a crowding effect from cages where males had been maintained just before release.36 To test if crowding can affect dispersal capacity, additional experiments using different holding densities are required. Results will be useful in planning optimal release strategies for RIDL males. However, current data indicate that most males do not engage in rapid long-range dispersal.
The spatial distribution of recaptured marked males in our experiments was highly aggregated. More than 50% of recaptured males were always collected in only three houses that were near the release point, and most large and small marked males were collected together in the same houses. These findings are consistent with results from spatial analyses by Getis and others.37 After two months of intensive entomologic surveys in Iquitos, Peru, they found that adult Ae. aegypti tended to cluster within single households, implying that most adult Ae. aegypti do not fly far from the container where they developed as larvae. Therefore, we speculate that without physical/ecological barriers and low wind speed, males released outdoors in groups, as was performed in our experiments, will tend to disperse short distances in any direction, and then select some preferred sites where they tend to persist and spend the great part of their adult life. This scenario is supported by the fact that in those buildings where high numbers of marked males were collected during the first visit, a few marked individuals were collected during the second visit, indicating that they possibly escaped the first sampling and remained in the same building. Most of these buildings were dark and humid, with dark objects (such as buckets, chairs, boxes with clothes over them, or shoes) where males were frequently collected.
Our overall recapture rate of 4.19% is in the range of that found in other studies. Muir and Kay29 used sticky traps and had a recapture rate of 14.8% and 4.6% for two cohorts of Ae. aegypti dusted males in northern Australia. Using backpack aspirators and BG traps in Brazil, Maciel-de-Freitas and others32 reported a recapture rate of 12.27% and 7.35% for small and large Ae. aegypti males, respectively. Tsuda and others34 in Hainan Island, China, had a recapture rate ranging between 2.8% and 1.3%. Harrington and others35 reported a higher recapture rate (17%), and most (72%) males were recaptured in the house adjacent to the outdoor release location. It is worth noting that in all of the above cited studies, recaptures started the same day or the day after the release, and most males were recaptured during the first day of collection. In our study, we waited a minimum of two days in most experiments before starting collections near the release point. This procedure gave males time to disperse or die and may help to explain why our recapture rates were somewhat reduced compared with those reported by our predecessors. Four days after release, the number of marked males collected per day decreased dramatically. In MRR4 we had a relatively low recapture rate of 2.64%. An interview with the owner of the property where males were released led us to suspect that he had used insecticide just after the release. Insecticide application could explain our low recapture rate in that experiment and why a few males were collected at relatively longer distances from the release point. Longer-range dispersal in this case could be the consequence of the repellent effect of synthetic pyrethroids38 that are commonly available in markets near our study area.
High mortality in natural environmental conditions is probably an important issue affecting our ability to detect Ae. aegypti marked males. We infer dispersal behavior on the basis of less than 5% of released Ae. aegypti males; no information is available for the remaining 95%. Results from our pre-trial test that was carried out to investigate survival of marked mosquitoes in field cage conditions, indicated that after 2 weeks, only 8% of males survived with a mean daily survival rate of 0.84. Results from our MRR experiments indicate that survival is much lower under natural conditions, ranges between 0.48 and 0.82, and is similar for the two size cohorts evaluated. The PDS values were similar to those calculated by Muir and Kay29 by using the same method (0.57); by Trpis and Hauserman24 by using the Jolly-Seber stochastic method (0.53); and by McDonald,39 who compared observed to estimated survivorship curves (0.77); and are in the range of those obtained by Sheppard and others,40 who used a modification of the Fisher and Ford deterministic model (0.55–0.87). High mortality in open field conditions is a possible explanation for the low number of recaptured males six days after release and suggests that most Ae. aegypti males were not recaptured because they did not survive, not because they flew out of the recapture area.
Results from our Ae. aegypti male dispersal study provide valuable information relevant to development of male release strategies for genetic approaches to prevent dengue. Our dispersal and survival data indicate that males will need to be released frequently at a relatively fine geographic scale because they tend not to live long or move far. This kind of information is important for predictive modeling, such as the one developed by Magori and others41 to estimate patterns of transgene spread from genetically modified mosquitoes into wild mosquito populations and the impact of released males on population suppression or population replacement. This information will help predict and evaluate, in different ecologic settings, which genetically modified mosquito delivery/release strategy is most effective.
ACKNOWLEDGMENTS
We thank Fred Gould, Laura C. Harrington, and Guillermo J. Bond for valuable suggestions during the protocol approval process; Ana Laura Pacheco and Abraham Marcoschamer for precious help during community engagement activities; Carlos N. Ibarra-Cerdeña for advice about statistical analysis; Luís Alberto García Rodas, Juan Carlos Joo Chang, Hugo Cigarroa de Los Reyes, and Crystian Citalán Uriel Hidalgo for technical assistance; and Leslie Sandberg, Susana Lemus Camarena, and Yadira Garay for administrative support.
Footnotes
Financial support: This study was supported by the Pasteur Institute–Cenci Bolognetti Foundation and the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative (GC7 #316).
Authors' addresses: Laura Valerio, Pasteur Institute–Cenci Bolognetti Foundation, University of Rome La Sapienza, Rome, Italy; and Department of Entomology, University of California, Davis, CA, E-mail: lvalerio@ucdavis.edu. Luca Facchinelli, Department of Entomology, University of California, Davis CA 95616, E-mail: lfacchinelli@ucdavis.edu. Janine M. Ramsey, Centro Regional de Investigacion en Salud Pública/INSP 30700 Tapachula, Chiapas Mexico, E-mail: jramsey@insp.mx. Thomas W. Scott, Department of Entomology, University of California, Davis, CA; and Fogarty International Center, National Institutes of Health, Bethesda, MD, E-mail: twscott@ucdavis.edu.
References
- 1.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10:98–109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 3.Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10:100–103. doi: 10.1016/s0966-842x(01)02288-0. [DOI] [PubMed] [Google Scholar]
- 4.Halstead SB. Dengue virus-mosquito interactions. Annu Rev Entomol. 2008;53:273–291. doi: 10.1146/annurev.ento.53.103106.093326. [DOI] [PubMed] [Google Scholar]
- 5.Lambrechts L, Scott TW, Gubler DJ. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis. 2010;25:e646. doi: 10.1371/journal.pntd.0000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee BY, Connor DL, Kitchen SB, Bacon KM, Shah M, Brown ST, Bailey RR, Laosiritaworn Y, Burke DS, Cummings DA. Economic value of dengue vaccine in Thailand. Am J Trop Med Hyg. 2011;84:764–772. doi: 10.4269/ajtmh.2011.10-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison AC, Scott TW, Rosenberg R. Defining challenges and proposing solutions for control of the virus vector Aedes albopictus. PLoS Med. 2008;18:e68. doi: 10.1371/journal.pmed.0050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franz AWE, Sanchez-Vargas I, Adelman ZN, Blair CD, Barry J, Beaty BJ, James AA, Olson KE. Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc Natl Acad Sci USA. 2006;103:4198–4203. doi: 10.1073/pnas.0600479103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alphey L, Nimmo D, O'Connell S, Alphey N. Insect population suppression using engineered insects. Adv Exp Med Biol. 2008;627:93–103. doi: 10.1007/978-0-387-78225-6_8. [DOI] [PubMed] [Google Scholar]
- 10.Alphey L, Benedict M, Bellini R, Clark GG, Dame DA, Service MW, Dobson SL. Sterile-insect methods for control of mosquito-borne diseases: an analysis. Vector Borne Zoonotic Dis. 2010;10:295–311. doi: 10.1089/vbz.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wise de Valdez MR, Nimmo D, Betz J, Gong HF, James AA, Alphey L, Black WC IV. Genetic elimination of dengue vector mosquitoes. Proc Natl Acad Sci USA. 2011;108:4772–4775. doi: 10.1073/pnas.1019295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrington LC, Buonaccorsi JP, Edman JD, Costero A, Kittayapong P, Clark GG, Scott TW. Analysis of survival of young and old Aedes aegypti from Puerto Rico and Thailand. J Med Entomol. 2001;38:537–547. doi: 10.1603/0022-2585-38.4.537. [DOI] [PubMed] [Google Scholar]
- 13.Harrington LC, Evermeylen F, Jones JJ, Kitthawee S, Sithiprasasna R, Edman JD, Scott TW. Age-dependent survival of the dengue vector Aedes aegypti demonstrated by simultaneous release–recapture of different age cohorts. J Med Entomol. 2008;45:307–313. doi: 10.1603/0022-2585(2008)45[307:asotdv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Rocha David M, Lourenço-de-Oliveira R, Maciel de Freitas R. Container productivity, daily survival rates and dispersal of Aedes aegypti mosquitoes in a high income dengue epidemic neighborhood of Rio de Janeiro: presumed influence of differential urban structure on mosquito biology. Mem Inst Oswaldo Cruz. 2009;104:927–932. doi: 10.1590/s0074-02762009000600019. [DOI] [PubMed] [Google Scholar]
- 15.Liew C, Curtis CF. Horizontal and vertical dispersal of dengue vector mosquitoes, Aedes aegypti and Aedes albopictus, in Singapore. Med Vet Entomol. 2004;18:351–360. doi: 10.1111/j.0269-283X.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 16.Maciel-de-Freitas R, Lourenço-de-Oliveira R. Presumed unconstrained dispersal of Aedes aegypti in the city of Rio de Janeiro, Brazil. Rev Saude Publica. 2009;43:8–12. doi: 10.1590/s0034-89102009000100002. [DOI] [PubMed] [Google Scholar]
- 17.Scott TW, Takken W, Knols BG, Boete C. The ecology of genetically modified mosquitoes. Science. 2002;298:117–119. doi: 10.1126/science.298.5591.117. [DOI] [PubMed] [Google Scholar]
- 18.Ponlawat A, Harrington LC. Age and body size influence male sperm capacity of the dengue vector Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2007;44:422–426. doi: 10.1603/0022-2585(2007)44[422:aabsim]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Ponlawat A, Harrington LC. Factors associated with male mating success of the dengue vector mosquito, Aedes aegypti. Am J Trop Med Hyg. 2009;80:395–400. [PubMed] [Google Scholar]
- 20.Thomas DD, Donnelly CA, Wood RJ, Alphey LS. Insect population control using a dominant, repressible, lethal genetic system. Science. 2000;287:2474–2476. doi: 10.1126/science.287.5462.2474. [DOI] [PubMed] [Google Scholar]
- 21.Fu G, Lees RS, Nimmo D, Aw D, Jin L, Gray P, Berendonk TU, White-Cooper H, Scaife S, Phuc HK, Marinotti O, Jasinskiene N, James AA, Alphey L. Female-specific flightless phenotype for mosquito control. Proc Natl Acad Sci USA. 2010;107:4550–4554. doi: 10.1073/pnas.1000251107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbosa P, Peters TM. A comparative study of egg hatching techniques for Aedes aegypti (L.) Mosq News. 1969;29:548–551. [Google Scholar]
- 23.Cantrell W. Relation of size to sex in pupae of Aedes aegypti, Ae. triseriatus and Ae. vexans. J Parasitol. 1939;25:448–449. [Google Scholar]
- 24.Trpis M, Hauserman W. Dispersal and other population parameters of Aedes aegypti in an African village and their possible significance in epidemiology of vector-borne diseases. Am J Trop Med Hyg. 1986;35:1263–1279. doi: 10.4269/ajtmh.1986.35.1263. [DOI] [PubMed] [Google Scholar]
- 25.Facchinelli L, Valerio L, Bond JG, Wise de Valdez MR, Harrington LC, Ramsey JM, Casas-Martinez M, Scott TW. Development of a semi-field system for contained field trials with Aedes aegypti in southern Mexico. Am J Trop Med Hyg. 2011;85:248–256. doi: 10.4269/ajtmh.2011.10-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landry SV, DeFoliart GR, Hogg DB. Adult body size and survivorship in a field population of Aedes triseriatus. J Am Mosq Control Assoc. 1988;4:121–128. [PubMed] [Google Scholar]
- 27.Armitage P. Oxford, United Kingdom: Blackwell Scientific Publications; 1980. pp. 147–166. (Statistical Methods in Medical Research). [Google Scholar]
- 28.Gillies MT. Studies on the dispersion and survival of Anopheles gambiae in east Africa, by means of marking and release experiments. Bull Entomol Res. 1961;52:99–127. [Google Scholar]
- 29.Muir LE, Kay BH. Aedes aegypti survival ad dispersal estimated by mark-release-recapture in northern Australia. Am J Trop Med Hyg. 1998;58:277–282. doi: 10.4269/ajtmh.1998.58.277. [DOI] [PubMed] [Google Scholar]
- 30.Niebylski ML, Craig GB., Jr Dispersal and survival of Aedes albopictus at a scrap tire yard in Missouri. J Am Mosq Control Assoc. 1994;10:339–343. [PubMed] [Google Scholar]
- 31.Clements AN, Paterson GD. The analysis of mortality and survival rates in wild populations of mosquitoes. J Appl Ecol. 1981;18:373–399. [Google Scholar]
- 32.Maciel-de-Freitas M, Codeco CT, Lourenco De Oliveira R. Body size-associated survival and dispersal rates of Aedes aegypti in Rio de Janeiro. Med Vet Entomol. 2007;21:284–292. doi: 10.1111/j.1365-2915.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- 33.Mullen GR, Mullen G, Durden LA. San Diego, CA: Academic Press; 2009. (Medical and Veterinary Entomology). [Google Scholar]
- 34.Tsuda Y, Takagi M, Wang S, Wang Z, Tang L. Movement of Aedes aegypti (Diptera: Culicidae) released in a small isolated village on Hainan Island, China. J Med Entomol. 2001;38:93–98. doi: 10.1603/0022-2585-38.1.93. [DOI] [PubMed] [Google Scholar]
- 35.Harrington LC, Scott TW, Lerdthusnee K. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am J Trop Med Hyg. 2005;72:209–220. [PubMed] [Google Scholar]
- 36.Silver JB. Third ed. New York: Springer; 2008. (Mosquito Ecology: Field Sampling Method). [Google Scholar]
- 37.Getis A, Morrison AC, Gray K, Scott TW. Characteristics of the spatial patterns of the dengue vector, Aedes aegypti, in Iquitos, Peru. Am J Trop Med Hyg. 2003;69:494–505. [PubMed] [Google Scholar]
- 38.Mongkalangoon P, Grieco JP, Achee NL, Suwonkerd W, Chareonviriyaphap T. Irritability and repellency of synthetic pyrethroids on an Aedes aegypti population from Thailand. J Vector Ecol. 2009;34:217–224. doi: 10.1111/j.1948-7134.2009.00029.x. [DOI] [PubMed] [Google Scholar]
- 39.McDonald PT. Population characteristics of domestic Aedes aegypti (Diptera: Culicidae) in villages on the Kenya coast. II. Dispersal within and between villages. J Med Entomol. 1977;14:49–53. doi: 10.1093/jmedent/14.1.49. [DOI] [PubMed] [Google Scholar]
- 40.Sheppard PM, MacDonald WW, Tonn RJ, Grabs B. The dynamics of an adult population of Aedes aegypti in relation to dengue haemorrhagic fever in Bangkok. J Anim Ecol. 1969;38:661–702. [Google Scholar]
- 41.Magori K, Legros M, Puente ME, Focks DA, Scott TW, Lloyd AL, Gould F. Skeeter buster: a stochastic, spatially explicit modeling tool for studying Aedes aegypti population replacement and population suppression strategies. PLoS Negl Trop Dis. 2009;3:e508. doi: 10.1371/journal.pntd.0000508. [DOI] [PMC free article] [PubMed] [Google Scholar]