Abstract
The rK39 rapid immunochromatographic test (ICT) is now being widely used in the diagnosis of visceral leishmaniasis (VL) using serum. We evaluated the presence of anti-rK-39 antibody in human saliva being noninvasive to replace the invasive procedures of diagnosis. Enzyme-linked immunosorbent assay (ELISA) and ICT assays were performed in 300 subjects: 114-confirmed VL patients, 95 and 47 healthy controls from endemic and nonendemic regions, respectively, and 44 subjects with different diseases. Sensitivity in saliva was 83.3% by ELISA and 82.5% by ICT, compared with 100% for both ICT and ELISA in serum. Specificity in saliva was 100%, 90.5%, and 88.6% with ELISA, and 91.48%, 91.57%, and 84.06% using ICT, in nonendemic, endemic, and different diseases, respectively. In serum, specificity was 97%, 88.5%, and 89% by ELISA and 100%, 94.7%, and 95.5% by ICT in nonendemic, endemic, and different diseases, respectively. Saliva is not suitable for diagnosis of VL because of low sensitivity.
Introduction
Visceral leishmaniasis (VL) is one of the most important parasitic diseases in the Indian subcontinent, and India alone accounts for 40–50% of the world's burden of the disease.1 Because the disease is fatal, and most of the drugs used in its treatment carry significant adverse events, an early and accurate diagnosis is essential.2,3 Demonstration of parasites in splenic aspirates is the gold standard in the diagnosis of VL; however, these procedures are painful and associated with the risk of serious hemorrhage. Following the discovery of a 39-amino acid residue (k-39) encoded by a kinesin-related gene in the amastigotes of Leishmania chagasi, it has been used in the format of enzyme-linked immunosorbent assay (ELISA) and immunochromatographic test (ICT) for detection of antibodies with high sensitivity and specificity.1,4–6 Anti-k39 antibodies are secreted into various body fluids such as urine and sputum along with blood, and have been shown to be useful in the diagnosis of VL (Sundar and others, unpublished data).7 Saliva is absolutely non-invasive, thus we evaluated the potential of using saliva in the diagnosis of VL by antibodies against rK39, using ICT and ELISA. Here, we compared the sensitivity and specificity of saliva samples by performing rK39 ICT and rK39 ELISA both in saliva and serum of the same subject for clinical diagnosis of VL.
Material And Methods
Study site.
This prospective study was conducted at the Department of Medicine, Institute of Medical Sciences, Banaras Hindu University and at its field site at Kala-Azar Medical Research Center (KAMRC), Muzaffarpur, Bihar. The study was approved by the Ethics committee of the Banaras Hindu University, Varanasi, India and written informed consent was obtained from all subjects.
Inclusion and exclusion criteria.
All eligible patients were consecutively enrolled in the study. One hundred forty suspected VL patients with prolonged fever, splenomegaly, and weight loss were screened through a positive serological test by rK39 dipstick. Parasitological confirmation was done by presence of amastigotes (Leishman-Donovan [LD] bodies) in splenic smears, and 114 such patients were included in the study. Patients < 2 years of age, with a past history of kala-azar, positive human immunodeficiency virus serology, or a positive pregnancy test were excluded. Patients with other concurrent illnesses were also excluded. All healthy subjects with positive serology were followed for a minimum of 6 months for evolution of clinical VL.
Subjects.
In this study, 300 subjects were enrolled during August 2009 to December 2009. Parasitologically confirmed 114 VL patients, adult and pediatrics, between 5 and 65 years of age were included in the study. The median age of the subjects was 25 years. Among the recruited patients, 48% of the subjects were male and 52% were female. The group of controls (N = 186) included 47 healthy controls from an area not endemic for VL, 95 healthy controls from endemic regions for VL, and 44 subjects with other infectious diseases such as tuberculosis (N = 8), malaria (N = 10), amebic liver abscess (N = 12), typhoid (N = 8), and dengue (N = 6).
Saliva and serum sample collection.
Saliva and serum samples were collected from these 114 patients simultaneously before the start of treatment. Saliva was collected in 50 mL Falcon tubes with 2 mL of normal saline (0.9% NaCl, Merck, Mumbai, India) buffer and, stored at 4°C and used within 48 hours. Saliva was collected generally in the early morning before brushing/flossing teeth, eating, or drinking. The cap of the tube was removed and saliva was spit directly into the tube. Serum was separated from 1 mL of blood collected in parallel from different groups of controls and confirmed VL patients and stored in cryovials at −20°C.
rK-39 strip test.
We used a ready-to-use Immunochromatography strip manufactured by InBios Inc. (Seattle, WA). This strip has rK39 antigen immobilized as the lower band of the nitrocellulose pad of the strips, which contain protein A/colloidal gold as a detection reagent.8 A band 1 cm above the rK-39 band contained antibody to protein A/colloidal gold and was used as a positive control to detect normal immunoglobulin G (IgG). In this study, 0.5 mL of the saliva was taken in a test tube and rK39 strips was dipped into it. By capillary action the saliva ascended up the strip. Three drops of the chase buffer provided with the kit were added to the pad. The results were read after 10 minutes. Appearance of a red upper (control) band indicated proper functioning of the test and that of a lower red (test) band suggested the presence of anti-K39 IgG in the saliva.
rK-39 ELISA.
The rK39 antigen was received as a kind gift from S. G. Reed, Seattle, WA. The ELISA was carried out as described earlier9; briefly, flat-bottom 96-well microtiter plates were coated with 25 ng/well (100 μL) of rK39 antigen in coating buffer (0.1 M carbonate-bicarbonate buffer, pH 9.6) and incubated overnight at 4°C. The plates were then blocked with blocking buffer (1% bovine serum albumin in 0.05 M phosphate buffer) for 2 hours at room temperature. Plates were then loaded with 100 μL of biological samples (i.e., serum and saliva simultaneously) and incubated at room temperature for 1 hour. The plates were washed five times with phosphate buffered saline containing 0.1% Tween-20 (pH 7.4) and then incubated with peroxidase-conjugated goat anti-human IgG (1:16,000 dilution in serum dilution buffer) at 37°C for 1 hour. Plates were again washed five times and incubated with tetramethylbenzidine substrate (Genei, Bangalore, India) for 15 minutes at room temperature in the dark. Finally, the reaction was stopped with 0.1 N H2SO4.
The optical density was measured at 450 nm. Each sample was assayed in duplicate. Saliva and serum pools of pretreated VL patients were used as a positive control and pooled nonendemic controls were used as a negative control in each plate.
Statistical analysis.
The cutoff values for anti-rK-39 antibodies were set as the mean ± three times the standard deviation of the healthy nonendemic controls.11 The serological data thus obtained were statistically analyzed with EPi Info (version 6, CDC, Atlanta, GA) and SPSS 16 (SPSS, Inc., Chicago, IL). Sensitivity and specificity estimation was done by 95% confidence interval (CI). Agreement between two tests was done by kappa value.
Results
All the suspected VL cases that were enrolled in this study during August 2009 to December 2009 at KAMRC, Muzaffarpur, were confirmed by parasitological examination of amastigotes in splenic smears. Clinical characteristics of confirmed VL cases showed splenomegaly (mean splenic size 4.8 cm), pyrexia, and weight loss; whereas 38% of the cases suffered from thrombocytopenia and 56% cases had hepatomegaly. Other clinical and laboratory features of VL patients are mentioned in Table 1.
Table 1.
Clinical and laboratory parameters of patients with visceral leishmaniasis
| Laboratory parameters | Clinical features | No. of cases (%) N = 114 | Mean ± SD |
|---|---|---|---|
| Gender % (M/F) | – | – | 55:59 |
| Age (years) | – | – | 25.2 ± 16.4 |
| Weight (kg) | Weight loss | 114(100) | 35.9 ± 14.4 |
| Duration of illness (in days) | – | – | 51.3 ± 52.0 |
| Max. temp. (°F) | Pyrexia | 114(100) | 104.0 ± 1.1 |
| Liver size (cm) | Hepatomegaly | 64 (55.9) | 5.0 ± 1.1 |
| Spleen size (cm) | Splenomegaly | 111(97.2) | 4.8 ± 3.9 |
| RBC (×106/μL) | – | – | 3.5 ± 0.7 |
| WBC (×106/μL) | Leukopenia | 61(53.2) | 3.8 ± 1.9 |
| Platelet count (×106/μL) | Thrombocytopenia | 43(37.6) | 1.5 ± 0.9 |
| Hemoglobin (gm/dL) | Anemia | 95(83.5) | 7.91 ± 1.87 |
| Lymphocyte (×106/μL) | Lymphocytosis | 85(74.3) | 48.6 ± 14.0 |
| Infection score† | 2.7 ± 1.0 |
RBC = red blood cells; WBC = white blood cells.
Scoring of parasite load is logarithmic scale from 1 to 6, where 0 means no parasites/1,000 microscopic field (1,000×), 1 is 1–10 parasites/field, and 6 is ≥ 100 parasites/field.
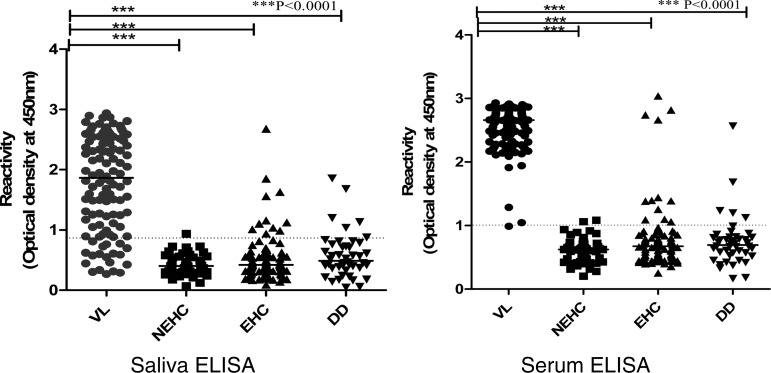
The level of anti-rK-39 antibodies in saliva and serum of 300 subjects with confirmed VL cases and different control groups by ELISA, is demonstrated in Figure 1. All VL cases were positive by sera samples with ELISA and ICT assay. There was excellent agreement between results with ELISA and ICT (Kappa 1, 95% CI = 0.97–1.0) for VL patients. There was absolute specificity in nonendemic healthy controls by ICT assay, however, only one subject showed positivity with ELISA (specificity 97.9%) in sera samples. In 44 subjects with other infectious diseases, 42 were negative with ICT and 39 were negative with ELISA. In the case of healthy subjects of the endemic region, more were positive with ELISA (84 of 95) than ICT assay (90 of 95, Table 2).
Figure 1.
Anti-rK-39 antibody levels in saliva and serum determined by enzyme-linked immunosorbent assay (ELISA) in parasitologically confirmed cases of visceral leishmaniasis (VL) and three groups of controls; namely, nonendemic healthy (NEHC), endemic healthy (EHC), and different diseases (DD). Results are expressed as optical density at 450 nm. Dotted line represents cutoff values between cases and control groups. Each dot represents an individual subject; bars display median value.
Table 2.
Comparative sensitivity and specificity by ICT and ELISA using rK39 antigen in saliva and serum samples in VL cases and different controls groups*
| Subjects | ICT | ELISA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Saliva | Serum | Saliva | Serum | Kappa | |||||
| Sensitivity % (95% CI) | Specificity % (95%CI) | Sensitivity % (95% CI) | Specificity % (95%CI) | Sensitivity % (95% CI) | Specificity % (95%CI) | Sensitivity % (95% CI) | Specificity % (95%CI) | ||
| VL cases | 82.5% | 100% | 83.3% | 100% | |||||
| (N = 114) | (74.5–88.3) | – | (96.7–100) | – | (75.4–89.1) | – | (96.7–100) | – | 0.9–1 |
| Non-endemic healthy | 91.5% | 100% | 100% | 97.9% | |||||
| (N = 47) | – | (80.1–96.6) | – | (92.4–100) | – | (92.4–100) | – | (88.9–99.6) | 0.8 |
| Endemic healthy | 91.6% | 94.7% | 90.5%) | 88.4% | |||||
| (N = 95) | – | (84.3–9.7) | – | (88.3–97.7) | – | (82.9–94.9 | – | (80.5–93.4) | 0.7 |
| Different diseases | 84.1% | 95.5% | 88.6% | 88.6% | |||||
| (N = 44) | – | (70.6–92.1) | – | (84.9–98.7) | – | (76.0–95.0) | – | (76.0–95.0) | 0.6 |
ICT = immunochromatographic test; ELISA = enzyme-linked immunosorbent assay; VL = visceral leishmaniasis; CI = confidence interval.
In saliva samples, almost equal numbers of subjects were positive with ELISA (94 of 114) and ICT assay (95 of 114). Agreement between ICT and ELISA results for VL patients was also good (kappa 0.88, 95% CI = 0.74–0.88). In healthy subjects of nonendemic regions there was absolute specificity by ELISA in saliva samples, whereas by ICT assay four subjects showed false positivity. In endemic controls specificity was almost equal by both the assays in saliva (> 90%). Although in the panel of 44 different diseases, 37 were negative with ICT and 39 were negative with ELISA in saliva (Table 2). None of the healthy subjects with positive rK39 serology developed clinical VL up to 6 months of the follow-up period.
Discussion
In this study, anti-rK39 antibodies by rapid ICT and ELISA assays performed well using sera, consistent with previous studies,5 although results with ELISA were slightly better than ICT. However, with saliva, the sensitivity, though greater than 80% by both techniques, were much below the acceptable limit of > 95%.11 Because we are measuring IgG, these findings are not surprising because the reported level of anti-rK-39 IgG antibodies is 600 times lower in saliva than in serum. Human serum contains ∼1,200 mg of IgG/100 mL, whereas saliva contains about 2 mg of IgG/100 mL.12 The low sensitivity 83.3% makes saliva an unlikely candidate to be used for the diagnosis of VL. The specificity of using serum was along the expected lines, being perfect among the nonendemic controls and patients with other diseases, and slightly lower with endemic controls. The presence of antibody in nonendemic controls might be because of subclinical infection or an impending clinical illness.4 With saliva, about 10% subjects tested positive across the categories of controls despite being negative in serology, and this suggests non-specific binding of antibodies to rK39 antigen. Contrary to these results, not any of the serological studies using rK39, a non-specific positive reaction has been reported in controls from nonendemic regions.4
Although this study was conducted in an endemic area of Bihar, but because of the poor sensitivity and specificity of saliva, it cannot be used in normal clinical practice, notwithstanding an isolated report of extremely high sensitivity (> 99%) and specificity (100%) reported from Patna,7 which could not be reproduced in this study.
Footnotes
Financial support: This study was partially funded by the Tropical Medicine Research Center grant number: P50AI074321 from National Institute of Allergy and Infectious disease (NIAID), DMID funding mechanism.
Authors' addresses: Manisha Vaish, Om Prakash Singh, Jaya Chakravarty, and Shyam Sundar, Department of Medicine, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India, E-mails: vaish.manisha@gmail.com, opsingh@imsbhu.com, tapadar@gmail.com, and drshyamsundar@hotmail.com.
References
- 1.Sundar S, Pai K, Sahu M, Kumar V, Murray HW. Immunochromatographic strip-test detection of anti-K39 antibody in Indian visceral leishmaniasis. Ann Trop Med Parasitol. 2002;96:19–23. doi: 10.1179/000349802125000466. [DOI] [PubMed] [Google Scholar]
- 2.Sundar S, Rai M. Advances in the treatment of leishmaniasis. Curr Opin Infect Dis. 2002;15:593–598. doi: 10.1097/00001432-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Davies CR, Kaye P, Croft SL, Sundar S. Leishmaniasis: new approaches to disease control. BMJ. 2003;326:377–382. doi: 10.1136/bmj.326.7385.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sundar S, Maurya R, Singh RK, Bharti K, Chakravarty J, Parekh A, Rai M, Kumar K, Murray HW. Rapid, noninvasive diagnosis of visceral leishmaniasis in India: comparison of two immunochromatographic strip tests for detection of anti-K39 antibody. J Clin Microbiol. 2006;44:251–253. doi: 10.1128/JCM.44.1.251-253.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sundar S, Reed SG, Singh VP, Kumar PC, Murray HW. Rapid accurate field diagnosis of Indian visceral leishmaniasis. Lancet. 1998;351:563–565. doi: 10.1016/S0140-6736(97)04350-X. [DOI] [PubMed] [Google Scholar]
- 6.Sundar S, Singh RK, Maurya R, Kumar B, Chhabra A, Singh V, Rai M. Serological diagnosis of Indian visceral leishmaniasis: direct agglutination test versus rK39 strip test. Trans R Soc Trop Med Hyg. 2006;100:533–537. doi: 10.1016/j.trstmh.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Singh D, Pandey K, Das VN, Das S, Kumar S, Topno RK, Das P. Novel noninvasive method for diagnosis of visceral leishmaniasis by rK39 testing of sputum samples. J Clin Microbiol. 2009;47:2684–2685. doi: 10.1128/JCM.00988-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welch RJ, Anderson BL, Litwin CM. Rapid immunochromatographic strip test for detection of anti-K39 immunoglobulin G antibodies for diagnosis of visceral leishmaniasis. Clin Vaccine Immunol. 2008;15:1483–1484. doi: 10.1128/CVI.00174-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badaro R, Benson D, Eulalio MC, Freire M, Cunha S, Netto EM, Pedral-Sampaio D, Madureira C, Burns JM, Houghton RL, David JR. Reed SG, 1996. rK39: a cloned antigen of Leishmania chagasi that predicts active visceral leishmaniasis. J Infect Dis. 173:758–761. doi: 10.1093/infdis/173.3.758. [DOI] [PubMed] [Google Scholar]
- 10.Romero HD, Silva Lde A, Silva-Vergara ML, Rodrigues V, Costa RT, Guimaraes SF, Alecrim W, Moraes-Souza H, Prata A. Comparative study of serologic tests for the diagnosis of asymptomatic visceral leishmaniasis in an endemic area. Am J Trop Med Hyg. 2009;81:27–33. [PubMed] [Google Scholar]
- 11.Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, Alvar J, Boelaert M. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5:873–882. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- 12.Masum MA, Evans DA. Agglutinating anti-leishmanial antibodies in the saliva of kala-azar patients. Trans R Soc Trop Med Hyg. 1994;88:660. doi: 10.1016/0035-9203(94)90216-x. [DOI] [PubMed] [Google Scholar]