Abstract
We analyzed the development of Leishmania (Leishmania) infantum chagasi in its natural sandfly vector Lutzomyia longipalpis. In addition, we compared sandfly infections initiated with axenic amastigotes or promastigotes. Our data showed no important difference between Lu. longipalpis infection rates resulting from either type of infections. Furthermore, development of infection was equivalent in both cases. All promastigote forms were found inside the sandfly and, after blood digestion, most of the population consisted of procyclics and nectomonads. A low percentage of metacyclic forms was coincident with a high number of nectomonads during late stages of infection, but which form gives rise to metacyclic forms in L. infantum chagasi is unknown. These results also show that the promastigote infection model, at least for this situation, is suitable for obtaining of infected sandflies because it is easier and less laborious.
Introduction
Leishmaniases are neglected diseases endemic to 98 countries or territories. Leishmania infections range from mild, self-healing skin lesions to a fatal visceral form, depending on the Leishmania species involved. Approximately 500,000 cases of visceral leishmaniasis (VL) are estimated to occur per year, and leishmaniasis is the ninth most common infection disease worldwide. More than 90% of cases are concentrated in Bangladesh, Ethiopia, India, Nepal, Sudan, and Brazil.1 In developing countries of the Western Hemisphere, the urbanization of VL caused by Leishmania infantum chagasi has been increasingly reported in many cities, and its proven main vector, the sandfly Lutzomyia longipalpis,2 is becoming highly adapted to artificial environments.3–5
Development of Leishmania inside its vector is a complex process. After the sandfly has had an infected blood meal, ingested amastigotes (non-flagellate forms) differentiate into dividing promastigotes (flagellate forms) to establish the parasite life cycle. However, there are numerous adverse conditions to overcome in the midgut of the host, including digestive enzyme activities6 and the synthesis of a physical barrier (the peritrophic matrix; PM),7 and the need to bind to the midgut cells8,9 to avoid excretion. Lipophosphoglycan, the major Leishmania surface glycoconjugate, protects the parasites from the enzyme activities of its host and mediates parasite attachment to the midgut of the sandfly.8–18 After digestion, successful infection in a sandfly vector results in development of several promastigotes forms types named, according to their morphology, as procyclic, haptomonad, nectomonad, paramastigote, and metacyclic forms.19 Only metacyclic forms transmitted through sandfly bites are able to begin an infection in vertebrate hosts.20,21
Studies on Leishmania-vector interactions are needed to understand the processes involved in parasite development and transmission. Much information regarding Old World Leishmania species and their vectors is available, as reviewed recently by Sacks and others.22 In contrast, there are few published data relating to New World species; for example, there are only a few studies on L. infantum chagasi and L. mexicana in Lu. longipalpis21,23,24 and L. amazonensis and L. braziliensis in Lu. migonei and Lu. intermedia.25–27 However, there is little detailed information in the literature about the developmental biology of the L. infantum chagasi in its natural vector Lu. longipalpis. This finding is somewhat unexpected, given that L. infantum chagasi is the causative agent of the American visceral leishmaniasis, the most severe form of the disease.
Successful experimental infection of sandflies to resemble natural transmission depends on providing amastigotes (the parasitic form found in vertebrate host) during an infective blood meal. Promastigote differentiation into axenic amastigotes is achieved as a result of changes in pH, temperature, and CO2 concentration.28–31
We provide an in vivo analysis of the development L. infantum chagasi throughout its life cycle in its natural sandfly vector Lu. longipalpis from establishment of infection to the metacyclogenesis, a process that enables the parasite to be transmitted to its vertebrate host. In addition, we compared infections initiated with axenic amastigotes and promastigote forms.
Materials and Methods
Parasite cultures.
In the current study, we used L. infantum chagasi World Health Organization reference strain MHOM/BR/1970/BH46. Promastigotes was cultured in medium 199 supplemented with 10% fetal bovine serum and other components16 at 26°C. Axenic cultures of in vitro amastigotes were initiated from stationary-phase promastigotes, which were placed in a concentration of 5 × 106 cells/mL in medium 199 containing 20% fetal bovine serum and 25 μg/mL of hemin at 37°C in an atmosphere of 5% CO2 (Araújo MS and others, unpublished data). Amastigote transformation dynamics were evaluated upon observation of parasite morphology and RNA expression.
Identification of A2 amastigote-specific protein by reverse transcription–polymerase chain reaction.
Total L. infantum chagasi RNA was extracted by using Trizol® (Invitrogen, Carlsbad, CA) and treated with DNase (Invitrogen) from log-phase promastigotes at 24, 48, 72, and 96 hours and from transformed axenic amastigotes. First-strand cDNAs were generated from 2 μg of RNA by using oligo dT (15) primer (Promega, Madison, WI) and M-MLV reverse transcriptase (Promega). The cDNAs were amplified with gene-specific primers A2 (GenBank accession no. S69693), 5′-GACCGAGCACAATGAAGATC-3′ (forward), 5′-GTCACCATGCCTCATGGCAT-3′ (reverse); and α-tubulin (GenBank accession no. DQ129864.1), 5′-CGTGTGCATGATTGCCAACT-3′ (forward), 5′-GAATTGTCCGCTTCGTCTTGAT-3′ (reverse). The polymerase chain reaction mixture contained Taq Platinum DNA polymerase (1 unit) (Invitrogen), 200 mM of each dNTP (Invitrogen), 1.5 mM MgCl2, 50 mM KCl, 10 mM Tris-HCl, pH 8.5, and 10 pmol of each specific primer set in a 25-μL reaction. Thermal conditions were 94°C for 45 seconds, specific annealing temperatures (60°C for A2 and 62°C for alpha tubulin) for 45 seconds and 72°C for 45 seconds, and a final extension at 72°C for 5 minutes. Amplified products were resolved by electrophoresis on 1.5% agarose gels and stained with ethidium bromide.
Sandfly infections.
Wild-caught Lu. longipalpis sandflies were collected in the Lapinha Cave, a non-endemic leishmaniasis area located at Lagoa Santa, Brazil (43°57′W, 19°3′S) using CDC light traps. Unfed female sandflies were separated into batches of 150 insects. They were kept in an insectary of the Laboratory of Medical Entomology of the Centro de Pesquisas René Rachou for at least two days before infection experiments. The experimental infections were carried out according to the protocol of Tesh and Modi.32 Sandflies were allowed to feed through a chick skin membrane in an artificial feeding device containing heparinized mouse blood with heat-inactivated serum and seeded with 4–8×107 parasites/mL. Blood-engorged females were separated and allowed to feed ad libitum on a 50% sucrose solution at 25°C and a humidity of 95% until they were dissected for parasite development analysis.
Parasite detection and development.
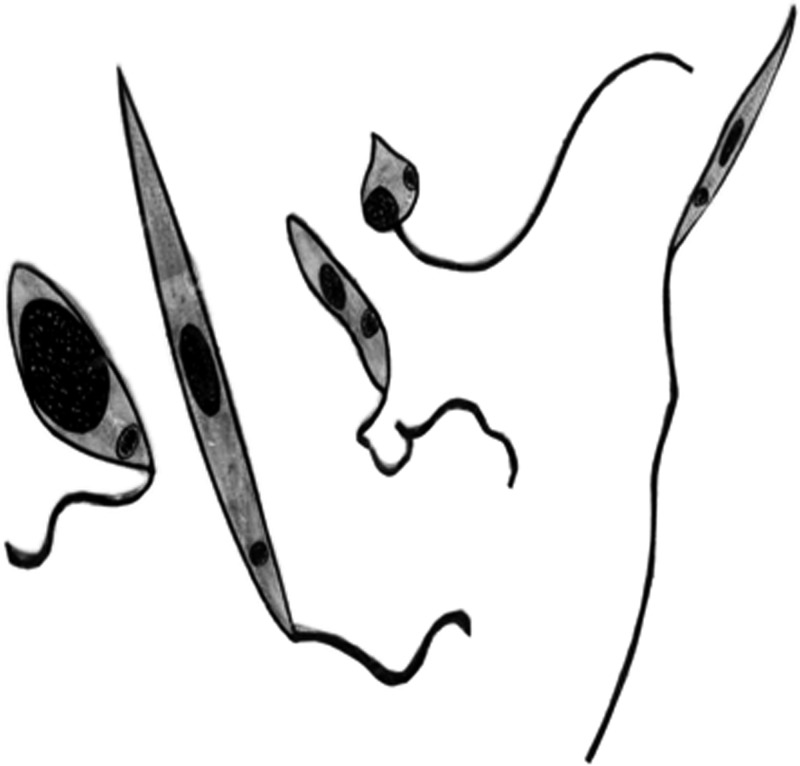
Infected flies were dissected daily from the first to the tenth day after the infective blood meal. Insects were quick immobilized in a freezer and dissected individually in drops of phosphate-buffered saline. The gut of each sandfly was also homogenized in microfuge tube containing 30 μL of phosphate-buffered saline at pH 7.2. The number of parasite was counted in a hemocytometer by using phase-contrast microscopy. The same material was used to prepare slide smears, which were stained with quick Romanovsky-type stain (Panótico Rapid; Laborclin, Pinhais, Brazil) for detection of relative proportions of developmental forms of the parasite. These forms were recognized by morphology and classified according to terminology established by Lawyer and others19 and subsequently used by several authors25–27,33 as shown in Figure 1.
Figure 1.
Developmental forms of Leishmania promastigotes showing from left to right an amastigote ingested from an infective bloodmeal; a procyclic promastigote, a short, ovoid, slightly motile, first promastigote that appears in the sandfly; a nectomonad promastigote, a long slender form; a haptomonad promastigote, a shorter and broader form; a paramastigote promastigote, a rare form with the kinetoplast adjacent to the nucleus; and a metacyclic promastigote, a short, slender, highly active, infective form for the vertebrate host.
Statistical analysis.
Results were analyzed by using the Mann Whitney test and the t-test. P values > 0.05 were considered significant.
Results
In vitro–prepared L. infantum chagasi axenic amastigotes.
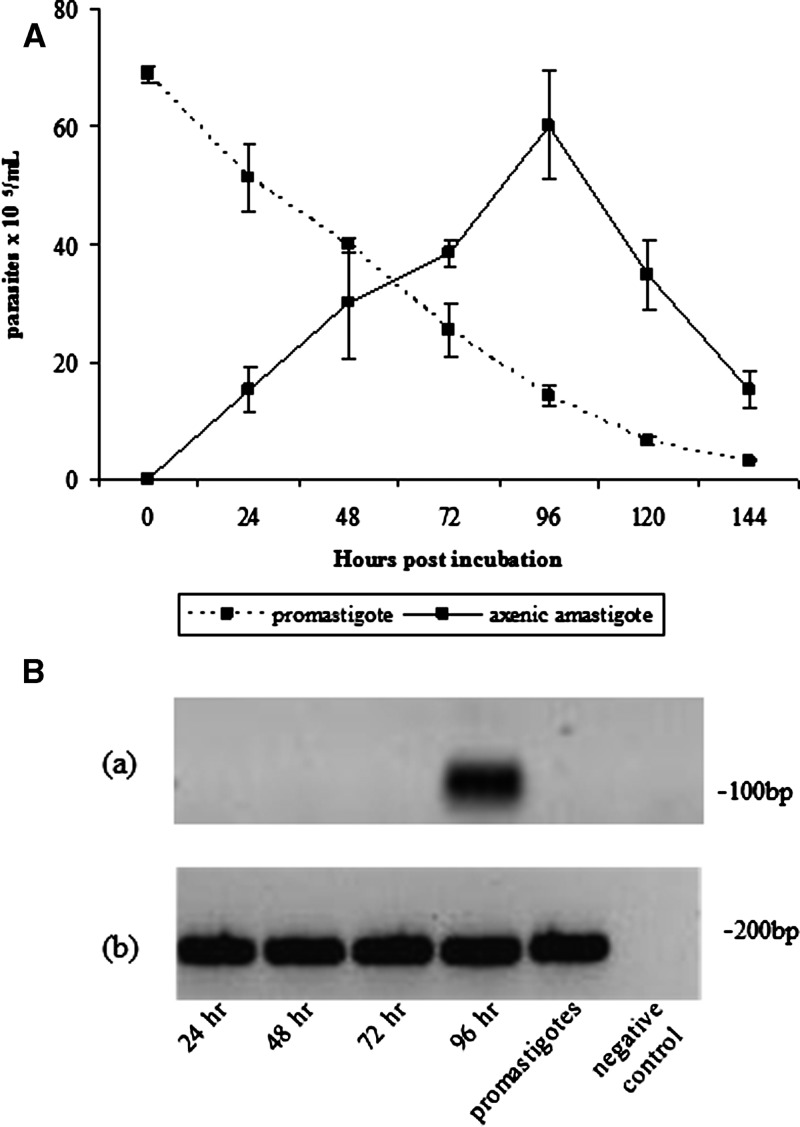
Amastigotes completed differentiation from promastigotes at 96 hours, as confirmed morphologically by light microscopy (Figure 2A). They were visualized as non-motile, round, or slightly elongated cells lacking a free flagellum. They were viably active cells, as shown by their ability to revert to promastigotes and to infect BALB/c macrophages. Moreover, A2 protein, a well-known amastigote-specific protein,34 was expressed abundantly in the axenic amastigotes, but was largely absent in promastigotes at 96 hours of cultivation, as demonstrated by reverse transcription–polymerase chain reaction (Figure 2B). These parameters ensured that axenic amastigotes would be useful for successful infection of sandflies.
Figure 2.
A, Evaluation of amastigote transformation dynamics of parasite morphology and cell viability in samples stained with trypan blue. B, reverse transcription polymerase chain reaction analysis of expression of A2 amastigote-specific protein in axenic Leishmania infantum chagasi amastigotes. a, A2 primer; b, α-tubulin primer. Lanes 1–4 = axenic amastigotes 24, 48, 72, and 96 hours post in vitro incubation, respectively; lane 5 = cultivated promastigotes; lane 6 = negative control. The dataset represent the analysis of two independent experiments. bp = basepairs. Error bars indicate mean ± SD.
Lutzomyia longipalpis infection with L. infantum chagasi.
Lutzomyia longipalpis was able to sustain L. infantum chagasi infection for the experimental period (10 days post-feeding) when the metacyclogenesis is completed and the parasites can be transmitted to vertebrate hosts. Infection rates beginning with either promastigotes or axenic amastigotes ranged from 79% to 94% and from 83% to 100%, respectively. The decreased infection rates was observed at days 3 and 4 (79% and 83%), respectively for promastigotes and amastigotes. After digestion, the parasite number increased again with migration to midgut regions, as observed until the end of the analysis (Figure 3). No significant difference was observed in the infection rates of Lu. longipalpis initiated with either promastigote or axenic amastigote (P > 0.05), except on day 6. The parasite number at 48 hours, before excretion of the blood meal, was 2.4×104/sandfly. This number decreased by day 3 because of digestion of the blood meal, increased again to approximately 2.4×104/sandfly until days 6 and 7, respectively, to promastigotes and amastigotes, and was finally maintained at approximately 1×104/sandfly by day 10 (Figure 3). The significant difference observed in the infection rates on day 6 can be explained by the transformation time of axenic amastigotes (slower) and promastigotes (faster) needed to achieve the maximum density of parasites.
Figure 3.
Lutzomyia longipalpis infected midgut with A, promastigote and B, axenic amastigote forms of Leishmania infantum chagasi. Numbers above the points indicate the number of dissected females. The dataset represent the analysis of four independent experiments. Horizontal lines indicate means.
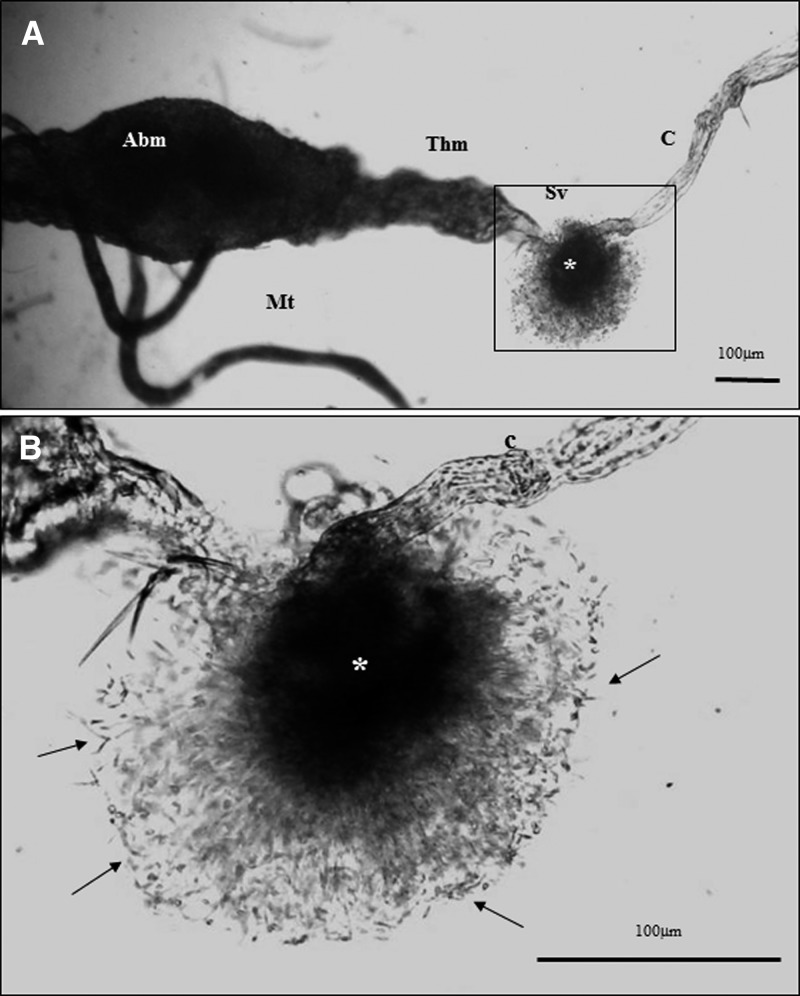
Colonization of the stomodeal valve and a gelatinous substance similar to the promastigote secretory gel described by Rogers and others21 in Lu. longipalpis infected with L. amazonensis, a non-natural vector-parasite pair, were observed at day 5 in most of the L. infantum chagasi females infected with either promastigotes or amastigotes (Figure 4).
Figure 4.
A, Dissected midgut of Lutzomyia longipalpis showing the promastigote secretory gel plug (*) in a sectioned stomodeal valve (Sv). Abm = abdominal midgut; Thm = thoracic midgut; C = crop, Mt = midgut. B, Enlargement showing details of the promastigote secretory gel plug (*) with a massive concentration of promastigotes (arrows). C = crop.
Developmental forms of L. infantum chagasi in Lu. longipalpis.
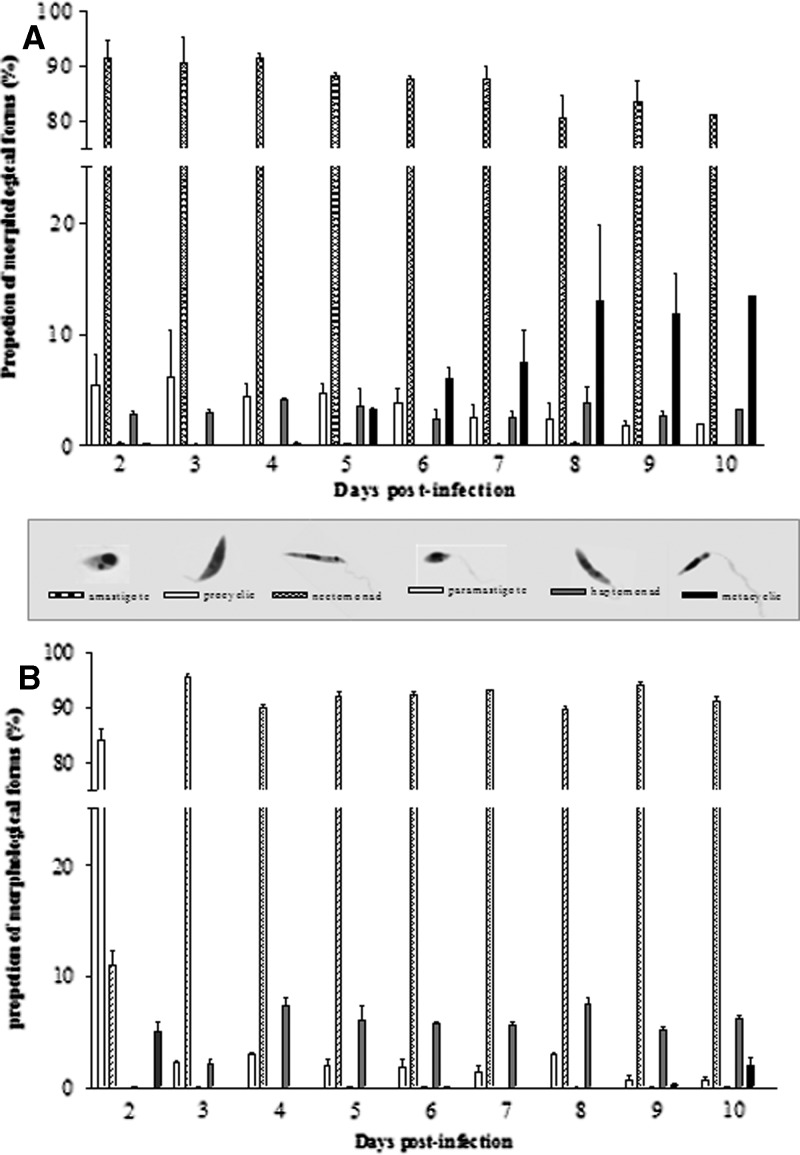
The proportion of L. infantum chagasi morphotypes also changed during development of infection within Lu. longipalpis (Figure 5 and Table 1). In infections initiated with promastigotes, procyclic forms were observed on day 2, but at low proportions (4.7%). At day 4, the number of procyclic forms was approximately the same (5%) but decreased after day 6. Nectomonads were seen on day 2 and were the predominant form (> 80%) until day 10, which was the last day of the experiments. In contrast, paramastigotes were rarely seen (0.1%). Haptomonads were observed on day 2 (2.8%) and never exceeded 4%. Only a few metacyclic forms were detected until day 4. However, they accounted for 13% of the entire population by day 10.
Figure 5.
Lutzomyia longipalpis infected with either A, promastigotes or B, axenic amastigotes of Leishmania infantum chagasi. Samples of 12 sandflies were prepared for morphologic analyses and at least 300 parasites were analyzed in each dissected midgut. Data represent the geometric mean of flies analyzed from two independent experiments. The data set represents the analysis of approximately 30,000 individual parasites. Different forms are all displayed at the same magnification. Error bars indicate mean ± SD.
Table 1.
Lutzomyia longipalpis infected with promastigotes (Pro) or axenic amastigotes (Ama) of Leishmania infantum chagasi*
| Days post-infection | Morphologic forms (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amastigote | Procyclic | Nectomonad | Paramastigote | Haptomonad | Metacyclic | |||||||
| Pro | Ama | Pro | Ama | Pro | Ama | Pro | Ama | Pro | Ama | Pro | Ama | |
| 2 | – | 5.9 | 4.7 | 81.7 | 92.2 | 12.3 | 0.1 | 0.0 | 2.8 | 0.0 | 0.2 | 0.0 |
| 3 | – | 0.0 | 5.3 | 2.1 | 91.5 | 96.2 | 0.1 | 0.1 | 3.0 | 1.7 | 0.1 | 0.0 |
| 4 | – | 0.0 | 4.6 | 3.1 | 91.2 | 90.4 | 0.0 | 0.0 | 4.0 | 6.4 | 0.2 | 0.0 |
| 5 | – | 0.0 | 4.7 | 2.5 | 88.3 | 92.7 | 0.1 | 0.1 | 3.6 | 4.7 | 3.3 | 0.0 |
| 6 | – | 0.0 | 3.7 | 2.6 | 87.4 | 91.8 | 0.1 | 0.1 | 2.6 | 5.5 | 6.2 | 0.0 |
| 7 | – | 0.0 | 2.5 | 1.9 | 87.5 | 92.8 | 0.0 | 0.0 | 2.5 | 5.3 | 7.4 | 0.0 |
| 8 | – | 0.0 | 2.5 | 3.0 | 80.8 | 90.1 | 0.1 | 0.0 | 3.9 | 6.9 | 12.7 | 0.0 |
| 9 | – | 0.0 | 1.9 | 1.2 | 83.5 | 93.2 | 0.0 | 0.0 | 2.7 | 5.4 | 11.9 | 0.2 |
| 10 | – | 0.0 | 2.0 | 0.9 | 81.3 | 90.4 | 0.0 | 0.2 | 3.3 | 5.9 | 13.5 | 2.7 |
Samples were prepared for morphologic analyses and at least 300 parasites were analyzed. Data represent the geometric mean of flies analyzed from two independent experiments. Data set represents the analysis of approximately 30,000 individual parasites.
A similar trend was seen in infection initiated with amastigotes but with some delay in their differentiation to the promastigote forms. On day 2, undifferentiated amastigotes accounted for 5.9% of the parasite population, but they had disappeared completely by day 3, when procyclic forms became predominant (81.7%). In contrast, nectomonads accounted for 12.3% of forms by day 2 and became the predominant form by day 3 (96.2%). Procyclic promastigotes decreased to 2.1% of the total forms by day 3 and persisted at low levels throughout the course of infection. Metacyclic promastigotes were only found at day 9 (0.2%), but represented 2.7% of the population by day 10 (Figure 5 and Table 1).
Discussion
Promastigotes are the easiest parasite form to obtain from Leishmania parasites in culture to use in laboratory experiments. Amastigotes of some species were originally cultivated axenically in cell-free media.30,31,35–37 Acidic pH and a higher temperature induce developmentally regulated changes in shape and gene expression of promastigotes, which generate amastigotes that resemble the animal tissue-derived amastigotes.28,30,31,38 In vitro cultivation of amastigotes provides an excellent source of parasites that are free from host-derived components. They have been used in drug evaluation, molecular cloning, identification of developmentally regulated genes, and vaccine production.39 In this study, we have used axenically cultivated Leishmania amastigotes to infect sandfly vectors. To verify L. infantum chagasi amastigote viability, we used criteria such as morphology, ability to revert into promastigotes in culture-dependent temperature, ability to infect macrophages, and expression of A2 protein, a well-known amastigote-specific protein.34 Our data show that axenic L. infantum chagasi amastigotes resemble morphologically and physiologically animal tissue-derived amastigotes, including their expressing of the A2 protein and their ability to infect Lu. longipalpis, the natural vector of the parasite. In addition, infection with amastigote, the natural mode of infection, was used to compare their development with infection initiated with cultured promastigotes. Interestingly, no significant difference was observed between Lu. longipalpis infection rates initiated with either promastigote or amastigote forms. This result shows that the infection model using promastigotes, at least for the L. infantum chagasi–Lu. longipalpis model system, is suitable for obtaining infected sandflies. It is also easier and less laborious than other options.
Experimental infections in sandflies exhibit different developmental patterns of Leishmania species inside the host insect gut. These patterns are determined by colonization of different anatomic regions of the gut and the appearance of distinct promastigote forms. Differentiation of infective metacyclic forms is crucial to determine the vectorial capacity of a sandfly.25 To evaluate the impact of the type of infection (amastigotes versus promastigotes) on L. infantum chagasi development, sandfly midguts were examined daily. Parasite multiplication was higher on the second day of infection. However, in all sandflies, there was a significant decrease in parasite number during the early events of blood meal digestion. Transformed promastigotes inside the sandfly gut have to overcome potentially lethal conditions; for example, approximately 50% of L. major ingested by Phlebotomus papatasi during initial infection died during this early stage.7 Borovsky and Schlein6 suggested that trypsin-like activity in the midgut of the P. papatasi prevented survival of L. donovani. Pimenta and others7 observed that the midgut environment, in the first few hours after blood feeding, is harmful even for a strain of L. major that is capable of complete development in the sandfly. These studies also showed that the addition of soybean trypsin inhibitor to the blood meal prevent much of the early parasite deaths. In Lu. longipalpis, trypsin-like proteins were identified that displaying high sequence similarities to those from P. papatasi.40–42 Also, mutants lacking lipophosphoglycan were more susceptible to digestion by enzymes.10 Our data consistently showed high parasite mortality at the early stages of L. infantum chagasi infection, which is probably caused by enzymatic activity.
The PM is a semi-permeable barrier that enables gradually diffusion of sandfly hydrolytic enzymes43 and, for this reason, protects the parasites from them.7 Although the PM eventually disintegrates, this process was described to occur more quickly in infected sandflies,24,44 which suggested a contribution of insect and Leishmania chitinases.45 Parasite escape from PM is an essential step to avoid their expulsion with the non-digested blood meal. At this point, appearance and variation of distinct promastigote forms are considered sequential steps necessary for metacyclogenesis.25 This process culminates with development of thin, high-motile and infective metacyclic forms46 that are able to infect the vertebrate host. Morphology of L. infantum chagasi parasites within the Lu. longipalpis midgut changed during infection process. After blood digestion (day 2), the parasite population consisted almost entirely of procyclic forms and nectomonads in infections started either with amastigotes or promastigotes. Procyclic forms have the capacity to multiply, initiate, and sustain infection during the digestion process.
In the sandflies fed with amastigotes, the new transformed procyclic population decreased on day 3. According to Pimenta and others,7 the susceptibility to gut proteases is extremely high in transitional-stage parasites during transformation of amastigotes to promastigotes. After expulsion of the blood meal, nectomonads became the main promastigote form present in the gut until the end of experiment. In contrast, in the sandflies infected with promastigotes, the procyclic population decreased early, before expulsion of the blood meal and, on the second day, the predominant forms were nectomonads.
In the current study, all promastigote forms described and classified by Lawyer and others19 were found. However, we could not find a so-called haptomonad (a morphotype with disc-like expansion of flagellar tip), proposed as a new morphologic category by Rogers and others.21 This finding could be caused by differences in the Leishmania strain and sandfly vector pair used because these Rogers and others used a non-natural model: Lu. longipalpis infected with L. mexicana. Nevertheless, similarly to the results reported by Rogers and others, we also observed formation of a promastigote secretory gel–like substance located in the stomedeal valve, but we were not able to determine the morphotype involved in its formation.
The appearance of L. infantum chagasi metacyclic forms in Lu. longipalpis was coincident with the presence of a high number of nectomonads during late stages of infection. In other studies using different vector-parasite pairs, appearance of metacyclic forms overlapped with paramastigotes in the foregut.25,47 However, we did not observe this phenomenon. Despite careful sequential observation on the appearance of morphologic promastigotes types, the question of which form gives rise to L. infantum chagasi metacyclic forms remains unanswered. However, L. infantum chagasi and other New World Leishmania species25,27,48 develop low percentages of metacyclic forms, a feature that differs from the Old World species,33 which appear to be better adapted to generate higher numbers of infective parasite forms.
Lutzomyia longipalpis has been the focus of studies because of its importance as a vector of VL in Latin America. Therefore, interaction studies with this sandfly are needed to understand its competence as a vector.
A study comparing sandfly infection with amastigotes from lesion and promastigotes of L. infantum did not show any difference in parasite development in Lu. longipalpis (Jacobina).49 Similarly, using amastigotes and promastigotes and another Lu. longipalpis population (Lapinha) and L. infantum chagasi strain (BH46), we also observed this trend. Comparative studies are of interest because differences in development of parasites might exist if Lu. longipalpis is considered a species complex,50 with currently unknown underlying differences between geographically distinct populations with regard to Leishmania interaction and, more importantly, vectorial competence.
Despite minor differences in morphotypes, it is clear that infection of Lu. longipalpis with L. infantum chagasi promastigotes is an easier way to obtain infected vectors. Importantly, the proportion of metacyclic forms inside the sandfly midgut was higher in promastigote-initiated infection than in amastigote-initiated infection. This finding is probably caused by a two-day delay needed for transformation of amastigotes into promastigotes inside the vector. In conclusion, promastigote infection can be a reliable tool for obtaining infected sandflies and for interaction studies. It is less laborious, quicker, and enables metacyclic differentiation before considerable vector mortality occurs.
ACKNOWLEDGMENTS
We thank Márcio Sobreira for helping with the establishment of the axenic culture of L. infantum chagasi amastigotes, Rafael Gonçalves da Silva and Carolina Cunha Monteiro for helping with the initial experiments with sandfly infections, and Dr. Ana Paula Madureira for helping with the statistical analysis.
Footnotes
Financial support: This study was supported by grants from Fundação Oswaldo Cruz (FIOCRUZ), Fundação de Amparo à Pesquisa do Estado de Minas Gerais, Conselho Nacional de Desenvolvimento Científico e Tecnológico), Programa Estratégico de Apoio à Pesquisa em Saúde of FIOCRUZ, and AMSURD (Pôle Amériques). Vanessa C. Freitas is supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais FAPEMIG (process 00260/09).
Authors' addresses: Vanessa C. Freitas, Klívia P. Parreiras, Ana Paula M. Duarte, Nágila F. C. Secundino, and Paulo F. P. Pimenta, Laboratory of Medical Entomology, Centro de Pesquisas René Rachou, Fundação, Oswaldo Cruz, Belo Horizonte, Minas Gerais, Brazil, E-mails: vanesfreitas@cpqrr.fiocruz.br, klivia_paty@hotmail.com, apduarte@cpqrr.fiocruz.br, nagila@cpqrr.fiocruz.br, and pimenta@cpqrr.fiocruz.br.
References
- 1.World Health Organization Control of the Leishmaniases. Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, March 22–26, 2010. 2010. http://whqlibdoc.who.int/trs/WHO_TRS_949_eng.pdf Available at. Accessed March 16, 2011.
- 2.Lutz A, Neiva A. Contribuição para o conhecimento das espécies do gênero Phlebotomus no Brasil. Mem Inst Oswaldo Cruz. 1912;4:84–95. [Google Scholar]
- 3.Michalsky EM, Rocha MF, Lima AC, França-Silva JC, Pires MQ, Oliveira FS, Pacheco RS, dos Santos SL, Barata RA, Romanha AJ, Fortes-Dias CL, Dias ES. Infectivity of seropositive dogs, showing different clinical forms of leishmaniasis, to Lutzomyia longipalpis phlebotominae sandflies. Vet Parasitol. 2007;147:67–76. doi: 10.1016/j.vetpar.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Michalsky M, França-Silva JC, Barata RA, Lara-Silva FO, Loureiro AM, Fortes-Dias CL, Dias ES. Phlebotominae distribution in Janaúba, an area transmission for visceral leishmaniasis in Brazil. Mem Inst Oswaldo Cruz. 2009;104:56–61. doi: 10.1590/s0074-02762009000100009. [DOI] [PubMed] [Google Scholar]
- 5.Cerbino Neto J, Werneck GL, Costa CH. Factors associated to the incidence of urban visceral leishmaniasis: an ecologic study in Teresina, Brazil. Cad Saude Publica. 2009;25:1543–1551. doi: 10.1590/s0102-311x2009000700012. [DOI] [PubMed] [Google Scholar]
- 6.Borovsky D, Schlein Y. Trypsin and chymotrypsin-like enzymes of the sandfly Phlebotomus papatasi infected with Leishmania and their possible role in vector competence. Med Vet Entomol. 1987;1:235–242. doi: 10.1111/j.1365-2915.1987.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 7.Pimenta PF, Modi GB, Pereira ST, Shahabuddin M, Sacks DL. A novel role for the peritrophic matrix in protecting Leishmania from the hydrolytic activities of the sandfly midgut. Parasitol. 1997;115:359–369. doi: 10.1017/s0031182097001510. [DOI] [PubMed] [Google Scholar]
- 8.Pimenta PF, Turco SJ, McConville M, Lawyer PG, Perkins PV, Sacks DL. Stage-specific adhesion of Leishmania promastigotes to sandfly midgut. Science. 1992;256:1812–1815. doi: 10.1126/science.1615326. [DOI] [PubMed] [Google Scholar]
- 9.Kamhawi S, Ramalho-Ortigão M, Pham VM, Kumar S, Lawyer PG, Turco SJ, Barrillas-Mury C, Sacks DL, Valenzuela JG. A role for insect galectins in parasite. Cell. 2004;119:329–341. doi: 10.1016/j.cell.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Pimenta PF, Saraiva EM, Rowton E, Modi GG, Garraway LA, Beverley SM, Turco S, Sacks DL. Evidence that the vectorial competence of phlebotomine sandflies for different species of Leishmania is controlled by structural polymorphisms in the surface lipophosphoglycan. Proc Natl Acad Sci USA. 1994;91:9155–9159. doi: 10.1073/pnas.91.19.9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sacks DL, Saraiva EM, Rowton E, Turco SJ, Pimenta PF. The role of lipophosphoglycan of Leishmania in vector competence. Parasitol. 1994;108:55–62. doi: 10.1017/s0031182000075727. [DOI] [PubMed] [Google Scholar]
- 12.Sacks DL, Pimenta PF, McConville MJ, Schneider P, Turco SJ. Stage-specific binding of Leishmania donovani to the sandfly vector midgut is regulated by conformational changes in the abundant surface lipophosphoglycan. J Exp Med. 1995;181:685–697. doi: 10.1084/jem.181.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sacks DL, Modi G, Rowton E, Spath G, Epstein L, Turco SJ, Berveley SM. The role of phosphoglycans in Leishmania sandfly interations. Proc Natl Acad Sci USA. 2000;97:406–411. doi: 10.1073/pnas.97.1.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butcher BA, Turco SJ, Hilty BA, Pimenta PF, Panunzio M, Sacks DL. Deficiency in β1,3-galactosyltranferase of a Leishmania major lipophosphoglycan mutant adversely influences the Leishmania-sandfly interaction. J Biol Chem. 1996;271:20573–20579. doi: 10.1074/jbc.271.34.20573. [DOI] [PubMed] [Google Scholar]
- 15.Kamhawi S. The biological and immunomodulatory properties of sandfly saliva and its role in the establishment of Leishmania infections. Microbes Infect. 2000;2:1765–1773. doi: 10.1016/s1286-4579(00)01331-9. [DOI] [PubMed] [Google Scholar]
- 16.Soares RP, Macedo ME, Ropert C, Gontijo NF, Almeida IC, Gazzinelli RT, Pimenta PF, Turco SJ. Leishmania chagasi: lipophosphoglycan characterization and binding to the midgut of the sandfly Lutzomyia longipalpis. Mol Biochem Parasitol. 2002;121:213–224. doi: 10.1016/s0166-6851(02)00033-6. [DOI] [PubMed] [Google Scholar]
- 17.Soares RP, Margonari C, Secundino NF, Macedo ME, Costa SM, Rangel EF, Pimenta PF, Turco SJ. Differential midgut attachment of Leishmania (Viannia) braziliensis in the sandflies Lutzomyia (Nyssomyia) whitmani and Lutzomyia (Nyssomyia) intermedia. J Biomed Biotechnol. 2010;2010(439174) doi: 10.1155/2010/439174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coelho-Finamore JM, Freitas VC, Assis RR, Melo MN, Novozhilova N, Secundino NF, Pimenta PF, Turco SJ, Soares RP. Leishmania infantum: lipophosphoglycan intraspecific variation and interaction with vertebrate and invertebrate hosts. Int J Parasitol. 2011;41:333–342. doi: 10.1016/j.ijpara.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Lawyer PG, Ngumbi PM, Anjili CO, Odongo SO, Mebrahtu YB, Githure JI, Koech DK, Roberts CR. Development of Leishmania major in Phlebotomus duboscqi and Sergentomyia schwetzi (Diptera: Psychodidae) Am J Trop Med Hyg. 1990;43:31–43. doi: 10.4269/ajtmh.1990.43.31. [DOI] [PubMed] [Google Scholar]
- 20.Sacks DL. Metacyclogenesis in Leishmania promastigotes. Exp Parasitol. 1989;69:100–103. doi: 10.1016/0014-4894(89)90176-8. [DOI] [PubMed] [Google Scholar]
- 21.Rogers ME, Chance ML, Bates PA. The role of promastigote secretory gel in the origin and transmission of the infective stage of Leishmania mexicana by the sandfly Lutzomyia longipalpis. Parasitol. 2002;124:498–507. doi: 10.1017/s0031182002001439. [DOI] [PubMed] [Google Scholar]
- 22.Sacks DL, Lawyer P, Kamhawi S. In: Leishmania: After the Genome. Myler P, Fasel N, editors. Norfolk, United Kingdom: Caister Academic Press; 2008. pp. 205–238. (The biology of Leishmania-sandfly interactions). [Google Scholar]
- 23.Lainson R, Shaw JJ. Observations on the development of Leishmania (L.) chagasi Cunha and Chagas in the midgut of the sandfly vector Lutzomyia longipalpis (Lutz and Neiva) Ann Parasitol Hum Comp. 1988;63:134–145. doi: 10.1051/parasite/1988632134. [DOI] [PubMed] [Google Scholar]
- 24.Walters LL, Modi GB, Chaplin GL, Tesh RB. Ultrastructural development of Leishmania chagasi in its vector Lutzomyia longipalpis (Diptera: Psychodidae) Am J Trop Med Hyg. 1989;41:259–317. [PubMed] [Google Scholar]
- 25.Nieves E, Pimenta PF. Development of Leishmania (Viannia) braziliensis and Leishmania (Leishmania) amazonensis in the sandfly Lutzomyia migonei (Diptera: Psycodidae) J Med Entomol. 2000;37:134–140. doi: 10.1603/0022-2585-37.1.134. [DOI] [PubMed] [Google Scholar]
- 26.Nieves E, Pimenta PF. Influence of vertebrate blood meals on the development of Leishmania (Viannia) braziliensis and Leishmania (Leishmania) amazonensis in the sandfly Lutzomyia migonei (Diptera: Psychodidae) Am J Trop Med Hyg. 2002;67:640–647. doi: 10.4269/ajtmh.2002.67.640. [DOI] [PubMed] [Google Scholar]
- 27.Miranda JC, Secundino NF, Nieves E, Souza AP, Bahia-Nascimento AC, Prates DB, Pimenta RN, Pinto LC, Barral A, Pimenta PF. Studies of the influence of the presence of domestic animals on increasing the transmission probabilities of leishmaniasis. Ann Med Entomol. 2008;17:9–15. [Google Scholar]
- 28.Zilberstein D, Shapira M. The role of pH and temperature in the development of Leishmania parasites. Annu Rev Microbiol. 1994;48:449–470. doi: 10.1146/annurev.mi.48.100194.002313. [DOI] [PubMed] [Google Scholar]
- 29.Saar Y, Ransford A, Waldman E, Mazareb S, Amim-Spector S, Plumblee J, Turco S, Ziberstein D. Characterization of developmentally-regulated activities in amastigote of Leishmania donovani. Mol Biochem Parasitol. 1998;95:9–20. doi: 10.1016/s0166-6851(98)00062-0. [DOI] [PubMed] [Google Scholar]
- 30.Debrabante A, Joshi MB, Pimenta PF, Dwyer DM. Generation of Leishmania donovani amastigotes: their growth and biological characteristics. Int J Parasitol. 2004;34:205–217. doi: 10.1016/j.ijpara.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Dias Costa J, Soares R, Finkelstein LC, Corte-Real S, Meirelles MN, Porozzi R. Fast high yield of pure Leishmania (Leishmania) infantum axenic amastigotes and their infectivity to mouse macrophages. Parasitol Res. 2009;105:227–236. doi: 10.1007/s00436-009-1390-4. [DOI] [PubMed] [Google Scholar]
- 32.Tesh RB, Modi GB. A simple method for experimental infection of phlebotomine sandflies with Leishmania. Am J Trop Med Hyg. 1984;33:41–46. doi: 10.4269/ajtmh.1984.33.41. [DOI] [PubMed] [Google Scholar]
- 33.Saraiva EM, Pimenta PF, Brodin TN, Rowton E, Modi GB, Sacks DL. Changes in lipophosphoglycan and gene expression associated with the development of Leishmania major on Phelebotomus papatasi. Parasitol. 1995;111:275–287. doi: 10.1017/s003118200008183x. [DOI] [PubMed] [Google Scholar]
- 34.Charest H, Matlashewski G. Developmental gene expression in Leishmania donovani: differential cloning and analysis of an amastigote-stage-specific gene. Mol Cell Biol. 1994;14:2975–2984. doi: 10.1128/mcb.14.5.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan AA. Leishmania mexicana: serial cultivation of intracellular stages in a cell-free medium. Exp Parasitol. 1984;58:72–80. doi: 10.1016/0014-4894(84)90022-5. [DOI] [PubMed] [Google Scholar]
- 36.Bates PA, Robertson CD, Tetley L, Coombs GH. Axenic cultivation and characterization of Leishmania mexicana amastigote-like forms. Parasitol. 1992;105:193–202. doi: 10.1017/s0031182000074102. [DOI] [PubMed] [Google Scholar]
- 37.Bates PA. The development biology of Leishmania promatigotes. Exp Parasitol. 1994;79:215–218. doi: 10.1006/expr.1994.1084. [DOI] [PubMed] [Google Scholar]
- 38.Doyle PS, Engel JC, Pimenta PFP, Da Silva PP, Dweyer DM. Leishmania donovani: long term culture of axenic amastigotes at 37°C. Exp Parasitol. 1991;73:326–334. doi: 10.1016/0014-4894(91)90104-5. [DOI] [PubMed] [Google Scholar]
- 39.Gupta N, Goyal N, Rastogi AK. In vitro cultivation and characterization of axenic amastigote of Leishmania. Trends Parasitol. 2001;17:150–153. doi: 10.1016/s1471-4922(00)01811-0. [DOI] [PubMed] [Google Scholar]
- 40.Dillon RJ, Ivens AC, Churcher C, Holroyd N, Quail MA, Rogers ME, Soares MB, Bonaldo MF, Casavant TL, Lehane MJ, Bates PA. Analysis of ESTs from Lutzomyia longipalpis sandflies and their contribution toward understanding the insect-parasite relationship. Genomics. 2006;88:831–840. doi: 10.1016/j.ygeno.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Telleria EZ, Pitaluga AN, Ortigão-Farias JR, Araújo APO, Ramalho Ortigão JM, Traub-Cseko YM. Constitutive and blood meal-induced trypsin genes in Lutzomyia longipalpis. Arch Insect Biochem Physiol. 2007;66:53–63. doi: 10.1002/arch.20198. [DOI] [PubMed] [Google Scholar]
- 42.Pitaluga AN, Beteille V, Lobo AR, Ortigão-Farias JR, Davila AMR, Souza AA, Ramalho-Ortigão JM, Traub-Cseko YN. EST sequencing of blood-fed and Leishmania-infected midgut of Lutzomyia longipalpis, the principal visceral leishmaniasis vector in the Americas. Mol Genet Genomics. 2009;282:307–317. doi: 10.1007/s00438-009-0466-2. [DOI] [PubMed] [Google Scholar]
- 43.Lehane MJ. Peritrophic matrix structure and function. Annu Rev Entomol. 1997;42:525–550. doi: 10.1146/annurev.ento.42.1.525. [DOI] [PubMed] [Google Scholar]
- 44.Schlein Y, Jacobson RL, Schlomai J. Chitinase secreted by Leishmania functions in the sandfly vector and implement parasite transmission by bite. Proc R Soc Lond. 1991;245:121–126. doi: 10.1098/rspb.1991.0097. [DOI] [PubMed] [Google Scholar]
- 45.Ramalho-Ortigão JM, Kamhawi S, Joshi MB, Reynoso D, Lawyer PG, Dwyer DM, Sacks DL, Valenzuela JG. Characterization of an activated chitinolytic system in the midgut of the sandfly vectors Lutzomyia longipalpis and Phlebotomus papatasi. Insect Mol Biol. 2005;14:703–712. doi: 10.1111/j.1365-2583.2005.00601.x. [DOI] [PubMed] [Google Scholar]
- 46.Sacks DL, Perkins PV. Development of infective stage Leishmania promastigotes within phebotomine sandflies. Am J Trop Med Hyg. 1985;34:456–459. doi: 10.4269/ajtmh.1985.34.456. [DOI] [PubMed] [Google Scholar]
- 47.Warburg A, Schlein Y. The effect of post-bloodmeal nutrition of Phlebotomus papatasi on the transmission of Leishmania major. Am J Trop Med Hyg. 1986;35:926–930. doi: 10.4269/ajtmh.1986.35.926. [DOI] [PubMed] [Google Scholar]
- 48.Elnaeim DA, Ward RD, Young PE. Development of Leishmania chagasi (Kinetoplastida: Trypanosomatidae) in the second blood-meal of its vector Lutzomyia longipalpis (Diptera: Psychodidae) Parasitol Res. 1994;80:414–419. doi: 10.1007/BF00932379. [DOI] [PubMed] [Google Scholar]
- 49.Gossage SM, Rogers ME, Bates PA. Two separate growth phases during the development of Leishmania in sandflies: implications for understanding the life cycle. Int J Parasitol. 2003;33:1027–1034. doi: 10.1016/s0020-7519(03)00142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bauzer LG, Souza NA, Maingon RD, Peixoto AA. Lutzomyia longipalpis in Brazil: a complex or a single species? A mini-review. Mem Inst Oswaldo Cruz. 2007;102:1–12. doi: 10.1590/s0074-02762007000100001. [DOI] [PubMed] [Google Scholar]