Abstract
This study investigated in utero priming as a consequence of maternal parasitic infections. Cord blood plasma samples of 63 African newborns were assessed by enzyme-linked immunosorbent assay for their content of total and schistosome-specific or filaria-specific IgE and IgG4. The frequencies of lymphocyte phenotypes in cord blood were also determined by using flow cytometry, and were compared with those of European newborns. We found significantly increased schistosome soluble egg antigen (SEA)–specific IgE in cord plasma of those born to mothers with schistosome infections and correlations between fetal and maternal SEA-specific and filaria antigen–specific IgE. These data are evidence for in utero priming of the fetal immune system to maternal helminth infections. Furthermore, we show significantly enhanced percentages of CD5– B cells in African newborns cord blood compared with Europeans, which is consistent with earlier maturation of the African fetal immune system.
Introduction
In countries where parasitic infections are endemic, pregnant women are often infected and the passage of antibodies, parasite antigens, or living parasites across the placental barrier may influence the fetal immune system.1 Human neonates are generally thought to have a reduced capacity to generate humoral immunity. In addition, it is thought that passively acquired maternal IgG mediates immunity against infectious pathogens in the first few months of life. However, there is increasing evidence of in utero sensitization as a result of maternal helminth infections.2–7 The question of how infections and/or microbial products in the mother might affect the development of the fetal immune system is of particular interest because it may explain disease patterns later in life.
Some studies have suggested that prenatal priming might be beneficial and lead to protection against infections or to reduced pathologic changes,2–6,8 and other studies have suggested that prenatal exposure might be detrimental and lead to development of allergic responses3,9 or to unresponsiveness3,8,10,11 and therefore inadequate reactivity of the immune system to infections or immunizations.8,12,13 It has also been suggested that prenatal sensitization rather than exposure to helminths during childhood is important in determining the initial immune response elicited by natural infection.4
Schistosomiasis and filariasis are chronic diseases caused by worms that can live for decades in their human host, releasing antigens continuously. In areas where these parasites are endemic, pregnant women often harbor these infections.6,10,14,15 Because IgE and IgM isotypes normally do not cross the placental barrier,3,6,16 the presence of these antibodies in umbilical cord blood is evidence of prenatal priming. It has previously been shown that in disease-endemic countries total3,7,10,12 and filarial antigen-specific3,7,10,12 fetal IgE production occurs. Only one investigation6 demonstrated a direct correlation of enhanced cord blood helminth antigen–specific IgE levels with the corresponding maternal helminth (Onchocerca volvolus) infection, and other studies found no correlation.7,10
There are few studies regarding the priming of the fetal humoral immune system as a result of maternal schistosome infection. King and others3 and Malhotra and others12 detected higher schistosome adult worm antigen (AWA)–specific IgE levels in cord blood of children in endemic countries compared with North American children. King and others3 reported that in vitro filarial and/or schistosome antigen-driven IgE production was more likely to be seen in newborns of schistosome-infected or filaria-infected mothers than in offspring of uninfected mothers. Other studies also showed enhanced levels of schistosome-specific antibodies in cord blood14,17–19 but did not discriminate between children of infected and uninfected mothers,18 did not state whether an admixture of maternal to the fetal blood was excluded,14,17–19 or did not differentiate between the distinct antibody-subtypes.14,17,19 Therefore, it is possible that the latter studies detected maternal IgG that crossed the placental barrier.20,21 At the cellular level, there are even fewer studies that directly compare cord blood from areas with high pathogen burden to countries where environmental burden of microorganisms and parasites is relatively low.22,23
To our knowledge, no study has so far identified a direct correlation between maternal schistosome infection and schistosome-specific IgE levels in cord blood. In the current study, the relationship between maternal parasitic, especially helminth infections and the fetal, especially humoral immune, response was investigated. We examined polyclonal and specific antibody levels in the umbilical cord blood of newborns in central Africa. Additionally, we performed cell surface marker analyses of circulating lymphocyte subsets in these African newborns and compared them with European newborns specifically with respect to the relative frequencies of mature and immature B cells.
Materials and Methods
Study population.
The study was approved by the ethics committee of the International Foundation of the Albert Schweitzer Hospital in Lambaréné, Gabon. The study population consisted of 63 multiparous women living in the province of Moyen-Ogooué, Gabon, in central Africa and their newborns, born at term in the Hôpital Albert Schweitzer in Lambaréné (mean age of the mothers = 27 years, range = 18–42 years; median number of previous pregnancies = 3, range = 1–12). The purpose of the study and the procedures involved were explained and only those mothers granting written informed consent were enrolled as participants. Cord and maternal peripheral blood samples were collected. Socioeconomic factors (living conditions with regard to hygiene, social status of the family) were recorded by using a standardized questionnaire.
As a control, we obtained cord blood of 15 European newborns born in a hospital (Diaconessen Ziekenhuis) in Leiden, The Netherlands, and 10 peripheral blood samples of women from the same area; all provided informed consent. The same examinations were performed in both groups.
Sample collection.
Paired umbilical cord and maternal peripheral venous blood samples were collected within minutes of delivery. To avoid contamination with maternal blood at sampling, cord blood was taken by direct needle (21 gauge) aspiration from the cord vein after cleaning the umbilical cord. All blood samples collected were treated with heparin. Plasma (2 mL) was separated by centrifugation and frozen at –20°C.
Microbiologic examinations.
Thick blood smears of cord blood and maternal peripheral blood were stained with Giemsa and examined microscopically for plasmodia and filaria. Blood was squeezed out of the placenta and treated in a similar manner. From 48 mothers, a minimum of 20 mL of urine was filtered (pore size = 12 μm), and filters were examined microscopically for schistosome eggs. In addition, levels of schistosome circulating antigen (circulating anodic antigen [CAA]) were measured in cord and maternal peripheral plasma of all participants by enzyme-linked immunosorbent assay as described.24 Circulating anodic antigen is a gut-associated, species-unspecific proteoglycan, produced in relatively large quantities by the parasite that can be detected in plasma.25–27
Of the participants, 23 women provided fecal samples, which were prepared with standard methods for microscopic examination to determine the presence of intestinal helminth infections. Microbiologic examination of the mothers was only performed at the time of delivery. Therefore, maternal infections during pregnancy were not detected.
Parasite antigen preparations.
Adult Brugia malayi worms were obtained from TRS Laboratories (Athens, GA). Male and female worms were freeze-dried, ground into a powder, dissolved in phosphate-buffered saline (PBS), homogenized and slowly stirred overnight at 4°C. The protein concentration was determined by using the 2,2′-biquinoline-4,4′-dicarboxylic acid disodium salt dihydrate method before storage at –20°C. Schistosoma haematobium adult worms were collected by perfusion of golden hamsters 16 weeks after infection with 1,500 cercariae. Adult worm antigen was prepared from the supernatant fluid obtained after the worms were homogenized in 0.035 M PBS, 0.15 M NaCl, pH 7.6, and centrifuged at 25,000 × g for 1 hour at 4°C. Soluble egg antigen (SEA) was prepared from S. haematobium eggs isolated from infected hamster gut by homogenization and trypsination. Antigen preparations were dialyzed against deionized water and freeze-dried. Freeze-dried antigens were resuspended in PBS before using them to coat microtiter plates.
Enzyme-linked immunosorbent assay for detection of IgE and IgG4.
Plasma samples were transported on dry ice to Leiden where measurements were performed. For B. malayi (BmA)–specific IgE, microtiter plates (Nunc-Immuno Plate Maxisorp; Inter Med, Kamstrup, Denmark) were coated overnight at 4°C with 100 μL/well of BmA (5 μg/mL) in 0.1 M carbonate buffer, pH 9.6, and blocked for 1 hour at 37°C with 120 μL of PBS containing 2% bovine serum albumin (Organon Teknika, Boxtel, The Netherlands). Plasma samples were diluted 1:400 in 0.1 M Tris-HCl, pH 7.5, containing 0.005% Tween-20 and incubated at 100 μL/well. A positive standard plasma containing 1 × 104 arbitrary units (AU) of BmA-specific IgE from a patient was incubated on each plate at an initial dilution of 1:500 and a series of eight 3-fold dilutions. After incubation for 1 hour at 37°C, biotinylated goat anti-human IgE was diluted 1:1,000 and added (Vector Laboratories, Burlingame, CA) and incubated for 1 hour. Alkaline phosphatase–conjugated streptavidin (100 μL/well diluted 1:3,000) was added and incubated for 30 minutes.
Color was developed by addition of p-nitrophenyl phosphate (Boehringer, Mannheim, Germany) diluted in diethanolamine buffer (0.1 M diethanolamine, 0.5 mM MgCl2, pH 9.6) (Merck, Darmstadt, Germany), and optical density was measured at 405 nm after incubation for 90 minutes at room temperature on a Microplate Autoreader (Bio-Tek Instruments, Winooski, VT). The optical density value was converted into AU by extrapolation of a standard curve of the positive control plasma. Enzyme-linked immunosorbent assays for SEA-specific and AWA-specific IgE and IgG4, total IgE, and BmA-specific IgG4 were performed as described.28,29
Lymphocyte surface markers.
For lymphocyte surface marker analysis, cord blood mononuclear cells (CBMC) of 26 newborns and maternal peripheral blood mononuclear cells (PBMC) of 13 mothers were frozen in liquid nitrogen and shipped from Gabon to The Netherlands. Cells were then thawed and 1 × 105 cells were incubated with fluorescein isothiocyanate–, phycoerythrin-, or peridinin-chlorophyll-protein–labeled monoclonal antibodies specific for CD5 and CD19 according to the manufacturer's instructions (Becton Dickinson, Alphen aan den Rijn, The Netherlands). Cells were analyzed by using an FACScan flow cytometer (Becton Dickinson). The CBMC and PBMC of European donors were processed and analyzed similarly (n = 15 and n = 10, respectively).
Statistical analysis.
Statistical analysis was performed by using SPSS for Windows version 10.0 (SPSS Inc., Chicago, IL). To test the data sets for normal Gaussian distribution, the Kolmogorov-Smirnov test (Lilliefors modification) was used. Because data were not normally distributed, the Wilcoxon sgned-rank test for non-normal paired data sets and the Mann-Whitney U test for non-normal independent samples were used. In all cases, statistical significance was assumed with a P value ≤ 0.05. Multiple regression analysis was performed to detect independent associations.
Results
Parasitic infections.
At delivery, 21 of 63 mothers had a thick blood smear (in placental and/or peripheral blood) positive for Plasmodium falciparum parasites, 3 for filarial infections (two with Loa loa and one with Mansonella perstans). None of these parasites could be found in cord blood samples.
In 10 of 21 mothers, P. falciparum parasites were present in placental and peripheral blood. Trophozoites and/or schizonts were found in placental blood and trophozoites were found exclusively in peripheral blood (average parasitemia = 300 parasites/μL). In the other 11 mothers, P. falciparum parasites were found either exclusively in placental blood (n = 5) or exlusively in peripheral blood (n = 6) (Table 1).
Table 1.
Samples showing positive results in microbiologic examinations, Gabon*
| Sample | Maternal peripheral blood | Placental blood | Cord blood | Urine | Feces |
|---|---|---|---|---|---|
| Plasmodium falciparum | 16 | 15 | 0 | ND | ND |
| Filarial | 3 | 0 | 0 | ND | ND |
| Schistosoma | ND | 0 | ND | 4 | ND |
| CAA | 5 | ND | 1 | ND | ND |
| Nematodes | ND | ND | ND | ND | 5 |
| Total | 63 | 63 | 63 | 48 | 23 |
ND = not determined; CAA = circulating anodic antigen.
Schistosome infection was defined by the presence of eggs in urine, by detection of CAA in serum samples, or both. In 4 of 48 mothers who donated a urine sample, schistosome eggs were found (three with S. haematobium and one with mixed S. haematobium and S. intercalatum) (Table 1). In two of these mothers, CAA was detected in plasma. Three other mothers had CAA-positive plasma, one without schistosome eggs in her urine and two who refused to provide a urine sample. In addition, one newborn had detectable CAA in plasma, and the mother was negative for urinary egg counts or plasma CAA. Schistosome eggs or worms were not found in the placentas examined.
In 5 of 23 mothers who provided a stool sample, eggs of intestinal nematodes were detected (four with Ascaris lumbricoides and one with Trichuris trichiura).
Humoral responses.
To determine whether neonates were sensitized in utero, levels of parasite-specific and total (polyclonal) IgE in plasma were examined. In addition, levels of IgG4, which crosses the placenta, were measured.
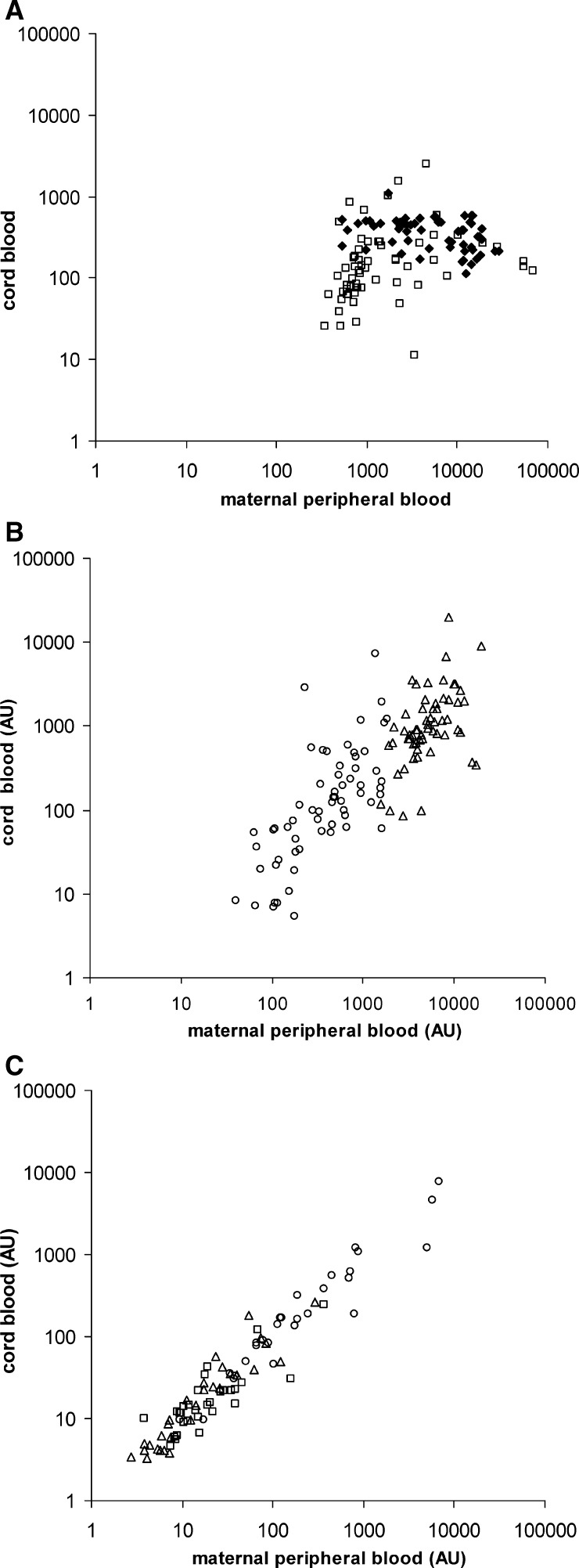
Mean levels of polyclonal IgE were increased in adult (5,003 versus 11 ng/mL) and in newborn African donors (345 versus 2 ng/mL) compared with Europeans (P < 0.001 for both groups), thus confirming the results of King and others3 and Malhotra and others.12 As expected, levels of maternal polyclonal IgE were significantly higher than those of cord blood IgE (P < 0.001). BmA-, SEA- and AWA-specific IgE was detected at higher levels in maternal blood than in cord blood (BmA-specific IgE = 378 AU versus 112 AU, i.e., more than three-fold higher; SEA-specific IgE: 6,358 AU versus 1,056 AU, i.e., more than six-fold higher; AWA-specific IgE: 1,486 AU versus 138 AU, i.e., more than 10-fold higher) (P < 0.001 for all comparisons). Differences in levels of specific IgE are relatively small when compared with the difference in total IgE (more than 12-fold higher), indicating that fetal IgE was specific for helminth antigens and not simply a reflection of polyclonal IgE production. Moreover, total and AWA-specific maternal and fetal IgE levels were not correlated (Figure 1A), whereas SEA- and BmA-specific IgE levels were correlated (P = 0.002 and P = 0.007, respectively) (Figure 1B).
Figure 1.
Absolute values of IgE in 48 persons in Gabon. A, Absolute values of total (♦) and adult worm antigen (AWA)–specific (□) IgE (ng/mL and arbitrary units [AU], respectively) are significantly higher in maternal peripheral blood than in cord blood without a correlation between the two groups. B, Absolute values of Brugia malayi antigen (BmA)– (ο) and soluble egg antigen (SEA)–specific (Δ) IgE are significantly higher in maternal peripheral blood than in cord blood, but are positively correlated between the two groups. C, Absolute values of BmA– (ο), SEA– (Δ), and AWA-specific (□) IgG4 are nearly identical in cord and the respective maternal peripheral blood.
In contrast to IgE and in accordance with free placental passage, all maternal and fetal IgG4 levels were highly correlated (P < 0.001) (Figure 1C) and levels were nearly identical (P = 0.92 for BmA-specific IgG4, P = 0.73 for SEA-specific IgG4, and P = 0.079 for AWA-specific IgG4).
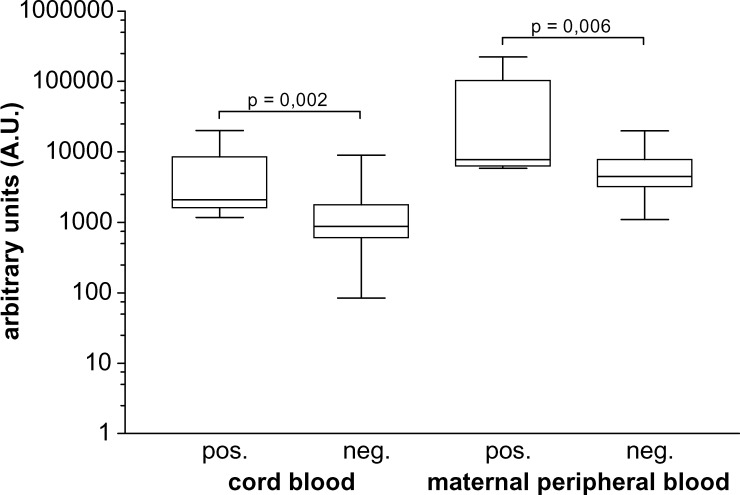
SEA-specific IgE was significantly higher in S. haematobium-infected mothers and their newborns (n = 8) than in non-infected persons (P = 0.006 for mothers and P = 0.002 for newborns) (Figure 2). The level of SEA-specific IgE in infected mothers (geometric mean = 16,511 AU) was on average three times higher than that of non-infected mothers (geometric mean = 4,857 AU), but was on average more than five times higher in children of infected mothers than in their counterparts born to non-infected mothers (geometric means = 3,419 AU and 804 AU, respectively).
Figure 2.
Levels of Soluble egg antigen (SEA)–specific IgE are significantly higher in cord and maternal peripheral blood of Gabonese women depending on the presence of maternal schistosome infections (indicated as positive [pos.], n = 8, or negative (neg.), n = 40). Box-whisker plots illustrate medians with 25th and 75th percentiles and whiskers for 10th and 90th percentiles; P values are for Mann-Whitney U tests.
AWA-specific IgE was not increased in cord samples of children born to mothers infected with schistosomes. Moreover, no correlation was found between cord IgE levels specific for SEA, AWA, or BmA. None of the other parasitic infections we detected, such as P. falciparum, had an influence on the antibody responses measured, and none of the socioeconomic factors recorded affected the results obtained.
Lymphocyte surface markers.
B cells carrying the CD5 surface marker (B-1 cells) are considered immature, functionally naive B cells. CD5+ B cells are the predominant cell type in the European newborn B cell repertoires. To determine the relative state of maturity of B cells of newborns delivered in an environment with a high burden of parasitic infections, we measured the percentages of CD19 and CD5 markers on the surfaces of CBMC and PBMC in Gabonese and European donors.
The percentages of CD19+ mononuclear cells were equivalent in cord blood of Gabonese and European newborns (8% versus 7% of all mononuclear cells; P = 0.38), in Gabonese and European adults (6% versus 9%; P = 0.65), in newborns and adults of Gabon (P = 0.57), and in their European counterparts (P = 0.28).
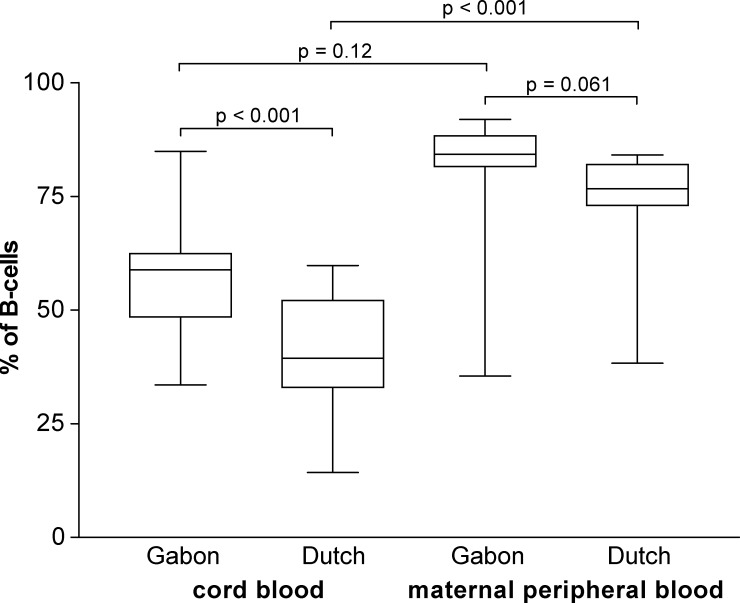
As previously shown,22 we found a significantly increased mean level of CD5– B cells in the cord blood of Gabonese newborns compared with European newborns (55% and 38%, respectively, of all CD19+ mononuclear cells; P < 0.001) (Figure 3), whereas no difference in the percentages of CD5– B cells was seen in peripheral blood of African and European adults (75% versus 78%; P = 0.06). Multiple regression analysis did not show correlations between antibody levels or maternal parasitic infections for any of the cell subsets.
Figure 3.
Percentages of CD5– B-cells (of all B cells) are significantly higher in Gabonese newborns than in Dutch newborns (n = 15), but not in adults (n = 13). Box-whisker plots illustrate medians with 25th and 75th percentiles and whiskers for 10th and 90th percentiles. P values are for Mann-Whitney U tests.
Discussion
This study investigated in utero priming of the fetal immune system caused by maternal parasitic infections in an area where helminth infections are endemic. We found significantly enhanced levels of schistosome egg (SEA)-specific IgE in cord blood of persons born to schistosome-infected mothers than in the control uninfected group.
Other studies that have shown enhanced levels of schistosome-specific antibodies in cord blood either did not show a correlation with a maternal infection3,12,18 or did not provide information about how the cord blood was collected and whether an admixture of maternal antibodies was excluded.14,17–19 As expected, we found a high correlation between specific IgG4 from mothers and their newborns, and a similarity between absolute levels of IgG4; this finding can be explained by the free placental passage of maternal IgG. In contrast, we found no correlation between mothers and newborns regarding AWA-specific and total IgE. For SEA- and BmA-specific IgE, which were correlated in cord and maternal peripheral blood, the absolute antibody levels were significantly distinct and always lower in cord compared with maternal samples. In addition, for all different IgE types, ratios of mother:newborn levels were distinct. This finding indicates that the IgE detected in fetal and maternal blood originated from different blood pools (to avoid admixture of maternal and cord blood, cord blood was obtained not by squeezing the umbilical cord but instead by direct needle aspiration, taking care to clean the cord of maternal blood beforehand). Therefore, we conclude that the significantly enhanced SEA-specific IgE levels in cord blood of children born to mothers with schistosome infections are not a result of an admixture of maternal and cord blood but instead of a prenatal exposure to egg antigens and intrauterine priming of fetal B cells, followed by production of specific antibody.
The correlation between fetal and maternal BmA-specific IgE levels also suggests that in utero priming may occur in this population. However, the number of persons in whom we could detect a filarial infection at the time point of delivery was too small (n = 3) to make a firm conclusion. Further studies should be conducted to clarify this issue.
Although maternal schistosome infection was associated with enhanced levels of fetal cord blood SEA-specific IgE, we found no influence on cord blood AWA-specific IgE. This difference may result from the higher immunogenicity of SEA. Many studies show that antibody responses to SEA are higher than those to AWA in infected persons.30–33 Thus, an inherent inferior immunogenicity of AWA may explain these results. Alternatively, the levels of egg antigens may be much higher than those of adult worm antigens in infected persons. Adult worms do not excrete large amounts of antigenic molecules and mask their surface tegument with host antigens.34–36 They can live in the blood stream for many years, whereas eggs die in the host continuously and release their antigens. Additionally, adult worm antigens may not cross the placenta as well as or in the same quantity as egg-related antigens.
The transplacental passage of helminth antigens resulting in in utero sensitization has been reported in several studies.3,4,6,7,19 The intravascular location of schistosomes ensures that antigens excreted or secreted by worms and eggs directly enter the maternal vasculature.14,26,37 These antigens may thus reach the placenta and the fetal vessels.14,15,19,25,38 For the schistosome antigen CAA, it has already been shown in an animal model that it can be transferred to the offspring via the placenta.25 In the present study, we detected CAA in the cord blood of one newborn. Thus, antigen transfer via the placenta is a likely trigger for priming of fetal immune cells.
The schistosome egg is a major stimulus39 for the production of IgE that cross-reacts39,40 with surface antigens of the larval schistosomula, itself a major target of protective immune responses.39,41 In several animal models, littermates born to mothers with schistosomiasis show limited infection and reduced pathologic changes when exposed for the first time.2,5,11,17,42 Furthermore, the effector phase components43,44 required for IgE-mediated immunity are already functional.42 Therefore, SEA-specific IgE may also play an important role in the immune system of human newborns.
Further evidence of in utero priming of the fetal immune system is in the higher relative frequencies of CD5– B cells in the Gabonese newborns than in the European newborns. It has been hypothesized that the functionally naive low-affinity, polyreactive antibodies produced spontaneously by CD5+ B cells45–47 could provide a relatively primitive antimicrobial first-line defense in the fetus and newborn.46,48–51 Their gradual decrease in frequency with age, accompanied by an increase in the frequency of CD5– B cells, may represent an ontologic shift towards use of cells capable of mounting a high-affinity, fine-specificity, memory antibody response.46 All published data for Europeans showed a significantly higher relative frequency of CD5– B cells in adults than in newborns, and CD5– B cells were the major B cell type in adults (median range = 60–75% of all B cells) but a minor B cell type in cord blood (range = 10–25%).45,46,52–55 Our results with European newborns and adults confirm these data.
Strikingly, however, Gabonese newborns had significantly higher frequencies of CD5– B cells than their European counterparts, and most Gabonese newborn B cell populations lacked surface CD5. This finding supports the notion that CD5 is a marker for immaturity of B cells, and furthermore indicates enhanced maturation of the humoral immune system of Gabonese newborns, likely a result of a regular contact of the fetus with antigens in utero throughout pregnancy. Unfortunately, in this study, it was not possible to perform a broad spectrum of microbiologic examinations (including examinations for bacterial infections) throughout the whole pregnancy to diagnose the potentially encountered infections.
The few studies that have examined differences in cellular compartment of the immune system between high-income countries and areas of the world where infections are rampant have also concluded that there is considerable differences seen in the maturity of the immune system.22,23 The studies in Gabon have shown that T cells have an activated phenotype that is typical of antigen-experienced T cells. Interestingly, in a geographically distinct area in Papua New Guinea, studies of cord blood when compared with studies of cord blood in Australia, showed higher antigen-specific interferon-γ and interleukin-10 responses in newborns in Papua New Guinea, Moreover, recent studies of differences in response to vaccination of newborns in Malawi and the United Kingdom have indicated that there might be a large functional differences in how in utero period impacts development of the immune system of the unborn child.56,57
In conclusion, we have shown increased relative frequencies of mature CD5– B cells in newborns in an area where parasitic diseases are endemic, suggesting that in utero exposure in environments with high microbial loads can lead to a more rapid maturation of the immune system in general. We have shown specifically that priming of the fetal immune system to schistosome antigens occurs in utero, and speculate that this priming could shape the development of a schistosome-specific immune response, potentially affecting immunity and/or susceptibility to infection. The latter hypothesis requires investigation in further follow-up studies.
ACKNOWLEDGMENTS
We thank all women who consented to participate in the study, the midwifes of the Hôpital Albert Schweitzer for logistical support, Ronald Binder and Mike Mueller for their co-operation in the field, Cynthia Naus for performing the CAA assays, and Caroline Michielse for critically reading the manuscript.
Footnotes
Authors' addresses: Larsen S. Seydel, St.-Johannes-Hospital, Johannesstraße 9-17, 44135 Dortmund, Germany, E-mail: larsen.seydel@gmx.de. Annika Petelski and Peter G. Kremsner, Unite de Recherches Medicales, Hôpital du Dr. Albert Schweitzer, Lambaréné, Gabon and Institut für Tropenmedizin, Universität Tübingen, Wilhelmstrasse 27, 72074 Tübingen, Germany, E-mails: Annipetelski@yahoo.de and peter.kremsner@uni-tuebingen.de. Govert J. van Dam and Yvonne C. M. Kruize-Hoeksma, Department of Parasitology, Leiden University Medical Center, Albinusdreef 2, 2333 ZA Leiden, The Netherlands, E-mails: G.J.van_Dam@lumc.nl and Y.C.M.Hoeksma-Kruize@lumc.nl. Desiree van der Kleij, Sanquin Diagnostic Services, Laboratory for Monoclonal Therapeutics, Allergy and Immunochemistry, Plesmanlaan 125, 1066CX Amsterdam, The Netherlands, E-mail: d.vanderkleij@sanquin.nl. Adrian J. F. Luty, Unités Mixtes de Recherche, Institut de Recherche pour le Développement, Mère et Enfant Face aux Infections Tropicales, Faculté de Pharmacie, 4 Avenue de l'Observatoire, 75270 Paris Cedex 06, France, E-mail: adrian.luty@ird.fr. Maria Yazdanbakhsh, Department of Parasitology, Leiden University Medical Center, Albinusdreef 2, 2333 ZA Leiden, The Netherlands and Unite de Recherches Medicales, Hôpital du Dr. Albert Schweitzer, Lambaréné, Gabon, E-mail: M.Yazdanbakhsh@lumc.nl.
References
- 1.Loke YW. Transmission of parasites across the placenta. Adv Parasitol. 1982;21:155–228. doi: 10.1016/s0065-308x(08)60276-6. [DOI] [PubMed] [Google Scholar]
- 2.Attallah AM, Abbas AT, Dessouky MI, El-emshaty HM, Elsheikha HM. Susceptibility of neonate mice born to Schistosoma mansoni-infected and noninfected mothers to subsequent S. mansoni infection. Parasitol Res. 2006;99:137–145. doi: 10.1007/s00436-006-0127-x. [DOI] [PubMed] [Google Scholar]
- 3.King CL, Malhotra I, Mungai P, Wamachi A, Kioko J, Ouma JH, Kazura JW. B cell sensitization to helminthic infection develops in utero in humans. J Immunol. 1998;160:3578–3584. [PubMed] [Google Scholar]
- 4.Malhotra I, Mungai P, Wamachi A, Kioko J, Ouma JH, Kazura JW, King CL. Helminth- and Bacillus Calmette-Guerin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J Immunol. 1999;162:6843–6848. [PubMed] [Google Scholar]
- 5.Othman AA, Shoheib ZS, Saied EM, Soliman RH. Congenital exposure to Schistosoma mansoni infection: impact on the future immune response and the disease outcome. Immunobiology. 2010;215:101–112. doi: 10.1016/j.imbio.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Soboslay PT, Geiger SM, Drabner B, Banla M, Batchassi E, Kowu LA, Stadler A, Schulz-Key H. Prenatal immune priming in onchocerciasis: Onchocerca volvulus-specific cellular responsiveness and cytokine production in newborns from infected mothers. Clin Exp Immunol. 1999;117:130–137. doi: 10.1046/j.1365-2249.1999.00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weil GJ, Hussain R, Kumaraswami V, Tripathy SP, Phillips KS, Ottesen EA. Prenatal allergic sensitization to helminth antigens in offspring of parasite-infected mothers. J Clin Invest. 1983;71:1124–1129. doi: 10.1172/JCI110862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malhotra I, Mungai PL, Wamachi AN, Tisch D, Kioko JM, Ouma JH, Muchiri E, Kazura JW, King CL. Prenatal T cell immunity to Wuchereria bancrofti and its effect on filarial immunity and infection susceptibility during childhood. J Infect Dis. 2006;193:1005–1013. doi: 10.1086/500472. [DOI] [PubMed] [Google Scholar]
- 9.Noureldin MS, Shaltout AA. Anti-schistosomal IgE and its relation to gastrointestinal allergy in breast-fed infants of Schistosoma mansoni infected mothers. J Egypt Soc Parasitol. 1998;28:539–550. [PubMed] [Google Scholar]
- 10.Hitch WL, Eberhard ML, Lammie PJ. Investigation of the influence of maternal infection with Wuchereria bancrofti on the humoral and cellular responses of neonates to filarial antigens. Ann Trop Med Parasitol. 1997;91:461–469. doi: 10.1080/00034989760824. [DOI] [PubMed] [Google Scholar]
- 11.Lewert RM, Mandlowitz S. Schistosomiasis: prenatal induction of tolerance to antigens. Nature. 1969;224:1029–1030. doi: 10.1038/2241029a0. [DOI] [PubMed] [Google Scholar]
- 12.Malhotra I, Ouma J, Wamachi A, Kioko J, Mungai P, Omollo A, Elson L, Koech D, Kazura JW, King CL. In utero . 1997;99:1759–1766. doi: 10.1172/JCI119340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarz NG, Adegnika AA, Breitling LP, Gabor J, Agnandji ST, Newman RD, Lell B, Issifou S, Yazdanbakhsh M, Luty AJ, Kremsner PG, Grobusch MP. Placental malaria increases malaria risk in the first 30 months of life. Clin Infect Dis. 2008;47:1017–1025. doi: 10.1086/591968. [DOI] [PubMed] [Google Scholar]
- 14.Carlier Y, Nzeyimana H, Bout D, Capron A. Evaluation of circulating antigens by a sandwich radioimmunoassay, and of antibodies and immune complexes, in Schistosoma mansoni-infected African parturients and their newborn children. Am J Trop Med Hyg. 1980;29:74–81. doi: 10.4269/ajtmh.1980.29.74. [DOI] [PubMed] [Google Scholar]
- 15.Hassan MM, Hassounah OA, Hegab M, Salah K, el-Mahrouky L, Galal N. Transmission of circulating schistosomal antigens from infected mothers to their newborns. J Egypt Soc Parasitol. 1997;27:773–780. [PubMed] [Google Scholar]
- 16.Avrech OM, Samra Z, Lazarovich Z, Caspi E, Jacobovich A, Sompolinsky D. Efficacy of the placental barrier for immunoglobulins: correlations between maternal, paternal and fetal immunoglobulin levels. Int Arch Allergy Immunol. 1994;103:160–165. doi: 10.1159/000236622. [DOI] [PubMed] [Google Scholar]
- 17.el-Nahal HM, Kaddah MA, Hassan SI, Abdel Ghany A, Ibrahim AM, Ramzy RM, Mostafa EA. Effect of Schistosoma mansoni infection on offsprings born from infected mothers. J Egypt Soc Parasitol. 1998;28:523–538. [PubMed] [Google Scholar]
- 18.Novato-Silva E, Gazzinelli G, Colley DG. Immune responses during human schistosomiasis mansoni. XVIII. Immunologic status of pregnant women and their neonates. Scand J Immunol. 1992;35:429–437. doi: 10.1111/j.1365-3083.1992.tb02878.x. [DOI] [PubMed] [Google Scholar]
- 19.Romia SA, Handoussa AE, Youseff SA, el Zayat MM. Transplacental transfer of schistosomal antigens and antibodies. J Egypt Soc Parasitol. 1992;22:575–582. [PubMed] [Google Scholar]
- 20.Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36:248–255. doi: 10.1111/j.1600-0897.1996.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 21.Pitcher-Wilmott RW, Hindocha P, Wood CB. The placental transfer of IgG subclasses in human pregnancy. Clin Exp Immunol. 1980;41:303–308. [PMC free article] [PubMed] [Google Scholar]
- 22.Kohler C, Adegnika AA, Van der Linden R, Agnandji ST, Chai SK, Luty AJ, Szepfalusi Z, Kremsner PG, Yazdanbakhsh M. Comparison of immunological status of African and European cord blood mononuclear cells. Pediatr Res. 2008;64:631–636. doi: 10.1203/PDR.0b013e31818718ba. [DOI] [PubMed] [Google Scholar]
- 23.van den Biggelaar AH, Prescott SL, Roponen M, Nadal-Sims MA, Devitt CJ, Phuanukoonnon S, Pomat W, Tulic MK, Lehmann D, Siba PM, Richmond PC, Holt PG. Neonatal innate cytokine responses to BCG controlling T-cell development vary between populations. J Allergy Clin Immunol. 2009;124:544–550. doi: 10.1016/j.jaci.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 24.Kremsner PG, Enyong P, Krijger FW, De Jonge N, Zotter GM, Thalhammer F, Muhlschlegel F, Bienzle U, Feldmeier H, Deelder AM. Circulating anodic and cathodic antigen in serum and urine from Schistosoma haematobium-infected Cameroonian children receiving praziquantel: a longitudinal study. Clin Infect Dis. 1994;18:408–413. doi: 10.1093/clinids/18.3.408. [DOI] [PubMed] [Google Scholar]
- 25.Gabriel S, De Bont J, Phiri IK, Masuku M, Riveau G, Schacht AM, Deelder AM, Van Dam GJ, Vercruysse J. Transplacental transfer of schistosomal circulating anodic antigens in cows. Parasite Immunol. 2002;24:521–525. doi: 10.1046/j.1365-3024.2002.00494.x. [DOI] [PubMed] [Google Scholar]
- 26.Naus CW, van Dam GJ, Kremsner PG, Krijger FW, Deelder AM. Human IgE, IgG subclass, and IgM responses to worm and egg antigens in schistosomiasis haematobium: a 12-month study of reinfection in Cameroonian children. Clin Infect Dis. 1998;26:1142–1147. doi: 10.1086/520310. [DOI] [PubMed] [Google Scholar]
- 27.Qian ZL, Deelder AM. Circulating antigens in Schistosoma infections. Acta Leiden. 1982;49:71–80. [PubMed] [Google Scholar]
- 28.Grogan JL, Kremsner PG, van Dam GJ, Metzger W, Mordmuller B, Deelder AM, Yazdanbakhsh M. Antischistosome IgG4 and IgE responses are affected differentially by chemotherapy in children versus adults. J Infect Dis. 1996;173:1242–1247. doi: 10.1093/infdis/173.5.1242. [DOI] [PubMed] [Google Scholar]
- 29.Haarbrink M, Terhell AJ, Abadi K, Asri M, de Medeiros F, Yazdanbakhsh M. Anti-filarial IgG4 in men and women living in Brugia malayi-endemic areas. Trop Med Int Health. 1999;4:93–97. doi: 10.1046/j.1365-3156.1999.00367.x. [DOI] [PubMed] [Google Scholar]
- 30.Butterworth AE, Bensted-Smith R, Capron A, Capron M, Dalton PR, Dunne DW, Grzych JM, Kariuki HC, Khalife J, Koech D. Immunity in human schistosomiasis mansoni: prevention by blocking antibodies of the expression of immunity in young children. Parasitology. 1987;94:281–300. doi: 10.1017/s0031182000053956. [DOI] [PubMed] [Google Scholar]
- 31.El Ridi R, Ismail S, Gaafar T, El Demellawy M. Differential responsiveness of humans with early-stage schistosomiasis haematobium to Schistosoma haematobium soluble adult-worm and egg antigens. Parasitol Res. 1997;83:471–477. doi: 10.1007/s004360050282. [DOI] [PubMed] [Google Scholar]
- 32.Hillyer GV, Pacheco E. Isolation and characterization of Schistosoma haematobium egg antigens. Am J Trop Med Hyg. 1986;35:777–785. doi: 10.4269/ajtmh.1986.35.777. [DOI] [PubMed] [Google Scholar]
- 33.Iskander R, Das PK, Aalberse RC. IgG4 antibodies in Egyptian patients with schistosomiasis. Int Arch Allergy Appl Immunol. 1981;66:200–207. doi: 10.1159/000232819. [DOI] [PubMed] [Google Scholar]
- 34.Harnett W, Kusel JR, Barrowman MM. The use of aldehydes to show a relationship between host and parasite antigens at the surface of adult male Schistosoma mansoni. Parasite Immunol. 1985;7:415–428. doi: 10.1111/j.1365-3024.1985.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 35.Saunders N, Wilson RA, Coulson PS. The outer bilayer of the adult schistosome tegument surface has a low turnover rate in vitro and in vivo. Mol Biochem Parasitol. 1987;25:123–131. doi: 10.1016/0166-6851(87)90001-6. [DOI] [PubMed] [Google Scholar]
- 36.Snary D, Smith MA, Clegg JA. Surface proteins of Schistosoma mansoni and their expression during morphogenesis. Eur J Immunol. 1980;10:573–575. doi: 10.1002/eji.1830100716. [DOI] [PubMed] [Google Scholar]
- 37.Deelder AM, Klappe HT, van den Aardweg GJ, van Meerbeke EH. Schistosoma mansoni: demonstration of two circulating antigens in infected hamsters. Exp Parasitol. 1976;40:189–197. doi: 10.1016/0014-4894(76)90081-3. [DOI] [PubMed] [Google Scholar]
- 38.el-Raziky EH, Shaker ZA, Abbassy AF, Aboul-Ezz FM, Naguib YA. A preliminary report on materno-foetal immunological changes in schistosomiasis. II. Circulating antigens and antibodies. Egypt J Bilharz. 1978;5:77–84. [PubMed] [Google Scholar]
- 39.Dunne DW, Bickle QD. Identification and characterization of a polysaccharide-containing antigen from Schistosoma mansoni eggs which cross-reacts with the surface of schistosomula. Parasitology. 1987;94:255–268. doi: 10.1017/s0031182000053932. [DOI] [PubMed] [Google Scholar]
- 40.Harn DA, Mitsuyama M, David JR. Schistosoma mansoni. . 1984;159:1371–1387. doi: 10.1084/jem.159.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capron A, Dessaint JP. Immunologic aspects of schistosomiasis. Annu Rev Med. 1992;43:209–218. doi: 10.1146/annurev.me.43.020192.001233. [DOI] [PubMed] [Google Scholar]
- 42.Knopf PM, Coghlan RL. Maternal transfer of resistance to Schistosoma mansoni. J Parasitol. 1989;75:398–404. [PubMed] [Google Scholar]
- 43.Ford MJ, Dissous C, Pierce RJ, Taylor MG, Bickle QD, Capron A. The isotypes of antibody responsible for the ‘late’ passive transfer of immunity in rats vaccinated with highly irradiated cercariae. Parasitology. 1987;94:509–522. doi: 10.1017/s0031182000055852. [DOI] [PubMed] [Google Scholar]
- 44.Hagan P. IgE and protective immunity to helminth infections. Parasite Immunol. 1993;15:1–4. doi: 10.1111/j.1365-3024.1993.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 45.Berry SM, Fine N, Bichalski JA, Cotton DB, Dombrowski MP, Kaplan J. Circulating lymphocyte subsets in second- and third-trimester fetuses: comparison with newborns and adults. Am J Obstet Gynecol. 1992;167:895–900. doi: 10.1016/s0002-9378(12)80008-1. [DOI] [PubMed] [Google Scholar]
- 46.Bhat NM, Kantor AB, Bieber MM, Stall AM, Herzenberg LA, Teng NN. The ontogeny and functional characteristics of human B-1 (CD5+ B) cells. Int Immunol. 1992;4:243–252. doi: 10.1093/intimm/4.2.243. [DOI] [PubMed] [Google Scholar]
- 47.de Vries E, de Bruin-Versteeg S, Comans-Bitter WM, de Groot R, Hop WC, Boerma GJ, Lotgering FK, van Dongen JJ. Longitudinal survey of lymphocyte subpopulations in the first year of life. Pediatr Res. 2000;47:528–537. doi: 10.1203/00006450-200004000-00019. [DOI] [PubMed] [Google Scholar]
- 48.Casali P, Burastero SE, Nakamura M, Inghirami G, Notkins AL. Human lymphocytes making rheumatoid factor and antibody to ssDNA belong to Leu-1+ B-cell subset. Science. 1987;236:77–81. doi: 10.1126/science.3105056. [DOI] [PubMed] [Google Scholar]
- 49.Casali P, Notkins AL. CD5+ B lymphocytes, polyreactive antibodies and the human B-cell repertoire. Immunol Today. 1989;10:364–368. doi: 10.1016/0167-5699(89)90268-5. [DOI] [PubMed] [Google Scholar]
- 50.Hardy RR, Hayakawa K, Shimizu M, Yamasaki K, Kishimoto T. Rheumatoid factor secretion from human Leu-1+ B cells. Science. 1987;236:81–83. doi: 10.1126/science.3105057. [DOI] [PubMed] [Google Scholar]
- 51.Herzenberg LA, Kantor AB. Layered evolution in the immune system. A model for the ontogeny and development of multiple lymphocyte lineages. Ann N Y Acad Sci. 1992;651:1–9. doi: 10.1111/j.1749-6632.1992.tb24588.x. [DOI] [PubMed] [Google Scholar]
- 52.Erkeller-Yuksel FM, Deneys V, Yuksel B, Hannet I, Hulstaert F, Hamilton C, Mackinnon H, Stokes LT, Munhyeshuli V, Vanlangendonck F. Age-related changes in human blood lymphocyte subpopulations. J Pediatr. 1992;120:216–222. doi: 10.1016/s0022-3476(05)80430-5. [DOI] [PubMed] [Google Scholar]
- 53.Kotiranta-Ainamo A, Apajasalo M, Pohjavuori M, Rautonen N, Rautonen J. Mononuclear cell subpopulations in preterm and full-term neonates: independent effects of gestational age, neonatal infection, maternal pre-eclampsia, maternal betamethason therapy, and mode of delivery. Clin Exp Immunol. 1999;115:309–314. doi: 10.1046/j.1365-2249.1999.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Gorman MR, Millard DD, Lowder JN, Yogev R. Lymphocyte subpopulations in healthy 1–3-day-old infants. Cytometry. 1998;34:235–241. doi: 10.1002/(sici)1097-0320(19981015)34:5<235::aid-cyto5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 55.Rabian-Herzog C, Lesage S, Gluckman E. Characterization of lymphocyte subpopulations in cord blood. Bone Marrow Transplant. 1992;9((Suppl 1)):64–67. [PubMed] [Google Scholar]
- 56.Lalor MK, Floyd S, Gorak-Stolinska P, Ben-Smith A, Weir RE, Smith SG, Newport MJ, Blitz R, Mvula H, Branson K, McGrath N, Crampin AC, Fine PE, Dockrell HM. BCG vaccination induces different cytokine profiles following infant BCG vaccination in the UK and Malawi. J Infect Dis. 2011;204:1075–1085. doi: 10.1093/infdis/jir515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lalor MK, Smith SG, Floyd S, Gorak-Stolinska P, Weir RE, Blitz R, Branson K, Fine PE, Dockrell HM. Complex cytokine profiles induced by BCG vaccination in UK infants. Vaccine. 2010;28:1635–1641. doi: 10.1016/j.vaccine.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]