Summary
Background:
Dolichoectasia of intracranial arteries is a rare arteriopathy characterized by elongation and widening of the arteries and disturbance of the laminar blood flow. It involves mostly vertebral and basilar arteries. In advanced cases, formation of a fusiform aneurysm is possible.
Case Report:
A sixty-four-year-old female with hypertension was admitted to the hospital with severe non-systemic vertigo and dysarthria, which had lasted for a couple of weeks. Imaging of the brain revealed dolichoectasia of arteries of the circle of Willis coexisting with a fusiform aneurysm of the basilar artery.
Conclusions:
Intracranial arterial dolichoectasia may be asymptomatic for a long time. However, in many cases it leads to neurological symptoms associated with haemodynamic disturbance (due to unstable wall clots) and mass effect caused by the widened vessel.
Keywords: dolichoectasia, fusiform aneurysm, cerebral arteries
Background
Dolichoectasia of intracranial arteries is a rare arteriopathy characterized by elongation, widening and tortuosity of the arteries. Its prevalence is estimated at 0.06–5.8% of the adult population. It concerns most frequently basilar and vertebral arteries, followed by the internal carotid artery and the middle cerebral artery [1].
The most common symptoms of dolichoectasia are complications caused by haemodynamic disturbance, especially cerebral ischemia, mainly in the area supplied by the basilar artery. Other symptoms are associated with compression of nerve structures. Clinically, dolichoectasia can manifest with headache, vertigo, vision disturbance and signs of cranial nerves’ impairment. However, part of the cases remains asymptomatic [2].
There are several stages of artery distension depending on its advancement. In the first stage the artery is widened and its diameter increases, in the next stage a fusiform aneurysm is formed and in the final stage – a serpentine one, which is defined as a vessel ectasia connected with a focal aneurysmal bulge [3]. The serpentine aneurysm is an uncommon fusiform arterial dilation in which the lumen extends longitudinally along the axis and curves of the original artery, creating a serpentine pathway with a separate entrance and outflow [4].
In our article we describe a case of advanced dolichoectasia of the anterior and posterior cerebral circulation arteries coexisting with a fusiform basilar artery aneurysm and we present a literature review of this topic.
Case Report
A 64-year-old woman with hypertension was admitted to the neurological ward with non-systemic vertigo, which intensified on the day of presentation, and dysarthric speech. Similar symptoms had lasted a couple of weeks. The patient had undergone a myocardial infarction fifteen years before, she was also diagnosed with hypertension and family hyperlipidemia.
The neurological examination showed horizontal-rotatory nystagmus while looking aside, dysarthric speech, mouth asymmetry on the right. Strength and tension of the upper extremities were symmetric. Excessive deep reflexes without pyramidal symptoms and ataxia in the heel-knee test bilaterally, more expressed on the left, were found.
Laboratory tests revealed lipid abnormalities: total cholesterol – 366 mg/dl (normal level up to 200 mg/dl), LDL-fraction 281 mg/dl (normally up to 135 mg/dl), triglycerides – 240 mg/dl (normally up to 150 mg/dl) and HDL-fraction level – 37 mg/dl (normally over 40 mg/dl). The CRP level was insignificantly increased – 5.4 mg/l (normally up to 5 mg/l).
On the presentation day computed tomography (CT) examination and computed tomography angiography (CTA) of the head with injection of the contrast bolus, with the range from the base of the skull to the cranial vault, with the layer thickness 0.6 mm were performed. The examinations showed an aneurysmal distention of the basilar artery displacing the brain stem to the right and compressing the other adjacent structures, including the fourth ventricle (Figure 1). In the CTA examination there were observed elongated, widened and tortuous arteries of the Willis’s circle: internal carotid artery – ICA (11 mm), vertebral artery – VA (7 mm), basilar artery – BA (beyond the aneurysm 12 mm), middle cerebral arteries – MCA (7 mm). The dilated vessels were accompanied by heavily calcified atherosclerotic plaques. Apart from that, there was a visible fusiform aneurysm of the middle section of the basilar artery of up to 23 mm in diameter (with width of the opacified lumen of up to 11 mm) with the evidence of a thick wall clot and small peripheral calcifications situated at the vessel wall (Figures 2–4).
Figure 1.
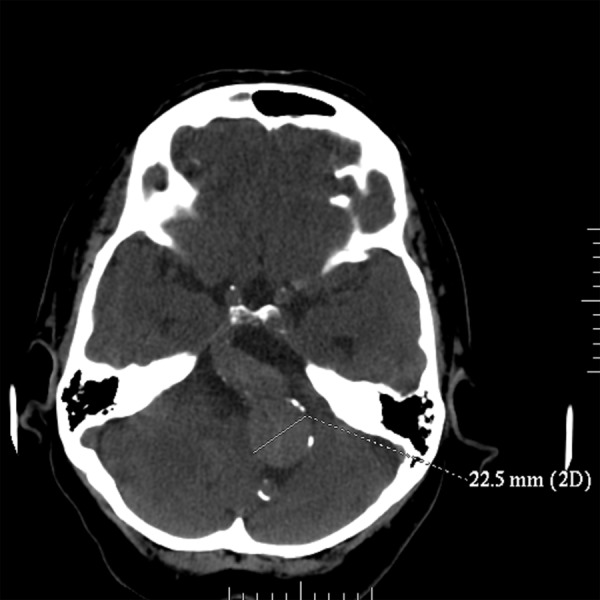
Non-contrast CT. Fusiform aneurysm of the basilar artery.
Figure 2.
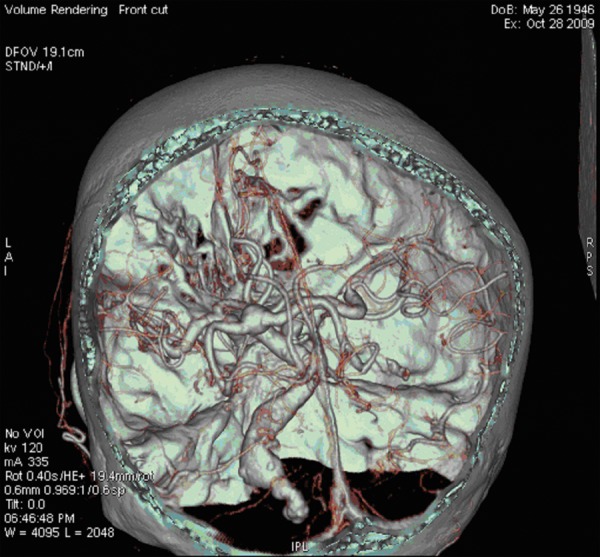
CTA, VR reconstruction. Dolichoectasia of the circle of Willis arteries and fusiform aneurysm of the basilar artery.
Figure 4.
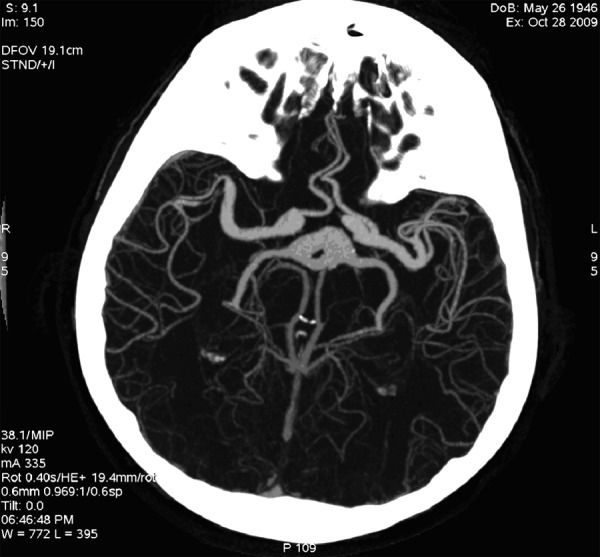
CTA, MIP reconstruction. Dolichoectasia of the circle of Willis arteries.
Doppler sonography of the carotid arteries showed small calcified atherosclerotic plaques in both carotid arteries bulbs, up to 0.5 mm thick, without haemodynamic significance. Blood flow in both carotid and vertebral arteries was normal. Functional tests were negative.
On the basis of the examinations mentioned above, dolichoectasia of the cerebral arteries was diagnosed with coexisting partially thrombosed aneurysm of the basilar cerebral artery. These disorders were considered as the cause of the patient’s symptoms.
Medical treatment was applied, using drugs to improve cerebral circulation, as well as antiaggregation, hypotensive and hypolipemic drugs. The decrease of headache was obtained, although the neurological symptoms persisted. After neurosurgical and neuroradiological consultation the patient was not qualified to an invasive procedure. It was recommended to follow up the disease with the magnetic resonance imaging (MRI).
In the follow-up MRI study the presence of the partially thrombosed fusiform aneurysm was confirmed. MR angiography (MRA) with time-of-flight (TOF) sequence, performed with the use of dedicated 16 channel HNS coil (GE), showed signal flow void of the patent part of the basilar artery. The aneurysm size did not change (Figure 5). The T2-weighted images showed multiple small hyperintensive foci in the periventricular white matter consistent with chronic ischaemia (leukoaraiosis).
Figure 5.
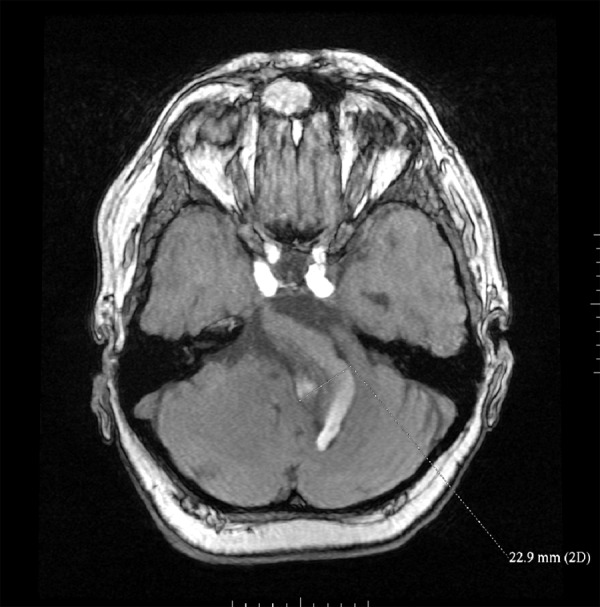
MR – follow up after 6 months, TOF sequence, source image. Fusiform aneurysm of basilar artery with flow void and thrombus on the right.
Discussion
Dolichoectasia is a name suggested in 1969 by Sacks and Lindenburg for pathologically distended, elongated, tortuous, intracranial arteries [5]. Pico et al. proposed in 2003 criteria of diagnosis of the basilar artery by means of the computed tomography [6].
Elongation criteria – “dolicho”:
the basilar artery is regarded as elongated, if it runs at any level laterally to the clivus or dorsum sellae margins,
the basilar artery bifurcation is located above the plane of the suprasellar cistern.
Distention criteria – “ectasia”:
the basilar artery exterior diameter exceeds 4,5 mm.
In our case all of the criteria mentioned above were met.
Among patients in whom imaging examinations of the intracranial arteries are performed approximately one out of eight has an increased diameter or length of these vessels. The incidence of the dolichoectasia of the intracranial vessels ranges between 0.06 and 5.8% [1].
The mechanisms responsible for the symptoms observed in dolichoectasia are: formation of the aneurysm with slowed down and multidirectional flow, compression of the brain stem and nerves by the widened and tortuous artery, recurrent thrombosis caused by turbulent flow, local or distal micro-embolisation, transient hypotension in a maximally compensated and autoregulated posterior circulation, occlusion of the pontine penetrator arteries [7,8]. Dolichoectasia can progress asymptomatically [9].
In the GENIC study concerning 510 patients, the authors have proved that “small-vessel disease”– multilacunar infarction (>1 lacunar infarct), leukoaraiosis, état criblé, is significantly more frequent in the patients with intracranial dolichoectasia [9]. It was confirmed in our patient as well in the control MR as in the initial neurological exam.
In the prospective clinical and imaging follow-up study conducted by Passero et al. of 156 patients with intracranial dolichoectasia followed for an average of 11.7 years, 93 patients (60%) experienced at least one event possibly related to dolichoectasia. 75 patients (48%) had at least one vascular event (39 TIA, 57 ischemic stroke, 21 hemorrhagic stroke), 31 patients (20%) had new compressive symptoms involving the cranial nerves that course in the cerebellopontine angle cistern (n 24), the bulbar nerves (n 6), or the third cranial nerve (n 1), and 2 patients (1%) had hydrocephalus. During the observation period, intracranial dolichoectasia progressed in 67 patients (43%). 62 patients (40%) died – 40% of patients from stroke, 37% from unrelated causes other than cardiac, 19% from cardiac causes, and 3% from hydrocephalus [10].
Basing on the analysis of cases of dolichoectasia conducted by Levine et al. the following was proved: out of 128 cases of patients with dolichoectasia of basilar artery, 74 (58%) presented the symptoms of cranial nerves compression. The most common were: facial nerve paralysis observed in 29 patients (39%) and trigeminal neuralgia observed in 20 patients (27%) [11].
In the cohort study comparing 45 patients with dolichoectasia to 45 patients of the same age and sex without dolichoectasia the authors have proved 36% mortality in the group of patients with dolichoectasia, with 50% occurring within 34 months of the initial diagnosis. On the contrary, in the control group 6 patients died (13%) [7].
Pathogenesis of dolichoectasia is not completely known. Two types are distinguished: juvenile and senile – the latter associated with visible advanced atherosclerotic changes [3].
The juvenile type is not accompanied by either atherosclerosis or hypertension, in the histopathological examination a loss of internal elastic membrane and tunica media in the vessel walls was observed [12]. Therefore it is speculated, that the cause of this condition is related to inborn biochemical-histopathological abnormalities of a similar origin as in the Marfan or Ehlers-Danlos diseases [1,3,13]. Dolichoectasia, due to not fully explained reasons, may coexist also with the following diseases: autosomal dominant inherited policystic kidney disease, sickle cell anaemia, Fabry’s disease, AIDS [1].
In the literature it is speculated, that dolichoectasia can be a result of quantitative disturbance concerning extracellular matrix fibers in the artery wall. This disturbance can result from imbalance between proteases and antiproteases of the matrix [6]. This imbalance leads to vessel architecture changes, especially to destruction of the artery elastic membrane, which is characteristic for the dolichoectasia in the histopathological examination, in which the arteries show increased diameter and thinned wall [9].
Matrix metalloproteinases (MMP) are a group of enzymes, which degradate extra-cell matrix proteins, hence the increased activity of these enzymes in the vessel wall may explain its widening. In the study carried out by Pico et al. patients with the dolichoectasia of intracranial vessels presented a considerably decreased level of the third metaloproteinase in serum in comparison to patients without dolichoectasia. Homozygotes 5A are characterized by a lower concentration of this enzyme in serum, with a simultaneous higher enzyme concentration in the aorta wall than homozygotes 6A and heterozygotes 5A6A. Till now the concentration of this enzyme in the cerebral arteries wall was not measured, however it could be speculated that carriers of the 5A allel of the third metaloproteinase gene may also be in an increased risk of the cerebral arteries’ dolichoectasia [14].
Among factors of the senile, acquired type of dolichoectasia there are: age (over forty), male gender, arterial hypertension, previous myocardial infarction [6]. At the moment of dolichoectasia diagnosis our patient was 64 years old, had a diagnosed arterial hypertension, and had undergone a myocardial infarction at the age of 49, therefore the senile form of dolichoectasia could be diagnosed.
In the study by Pico et al. who assessed risk factors of dolichoectasia, no statistically significant differences concerning smoking, presence of diabetes, level of LDL and total cholesterol between the group of patients with dolichoectasia and the control group were found. However, the authors have proved a strong association between dolichoectasia of the intracranial arteries and an acute coronary syndrome in the past. There are two hypotheses explaining that correlation. According to one of them coexistence of dolichoectasia of intracranial arteries and coronary ectasia suggest the presence of the same pathological process causing dolichoectasia in both intracranial and coronary arteries. Another provides that both intracranial arterial dolichoectasia and acute coronary syndrome are markers of severe atherosclerosis [6].
Two theories have been proposed to explain the relationship between dolichoectasia of intracranial arteries and atherosclerosis. Some authors believe that dolichoectasia of intracranial arteries is an ectatic and severe form of atherosclerosis. Others argue that atherosclerotic process results from morphological and haemodynamic modifications in the dolichoectatic arteries, whereas the mechanism underlying dolichoectasia is different from atherosclerosis [4].
Krishnankutty et al. report higher prevalence of coronary ectasia in heterozygous patients with familial hypercholesterolemia. They suggest that the remodeling of the arterial wall due to atherosclerosis can lead either to stenosis of the vessel, or rarely to its ectasia. Imbalance between protease and antiprotease activities in the extracellular matrix of the arterial wall due to oxidative stress associated with inflammatory process accompanying atherosclerosis and increased expression of MMP3 lead to thinning of the elastic layer of the arterial wall causing ectasia of the vessel. Dolichoectasia of intracranial arteries in our patient may be a rare effect of atherosclerosis caused by long lasting complex hyperlipidemia [15].
However, Pico et al. proved there is no association between dolichoectasia of intracranial arteries and markers for carotid atherosclerosis [6]. Our case confirms this fact; in our patient Doppler ultrasound of extracranial carotid arteries showed the presence of mild atherosclerotic changes, adequate for the age. Thus, dolichoectatic process was more advanced in intracranial arteries than in extracranial carotid and vertebral arteries. In our patient coexistence of hyperlipidemia and hypertension contributed to the atherosclerotic process [12] in dolichoectatic intracranial arteries where hemodynamic conditions were modified, while there was no systemic atherosclerosis in our patient.
Patients with hyperlipidemia show usually significantly higher levels of C-reactive protein (CRP), as a marker of inflammatory process associated with atherosclerosis [16], in our patient CRP was slightly increased (5.4 mg/l; normal level up to 5 mg/l).
Pico et al. emphasized the association between dolichoectasia of intracranial arteries, coronary ectasia and abdominal aortic aneurysm [14]. They also proved a statistically significant correlation between the dolichoectasia of intracranial arteries and enlarged descending thoracic aorta [12]. Moreover, a study by Sacks and Lindenburg based on autopsy of 34 cases showed in 35% [12/34] of the patients coexistence of dolichoectasia of intracranial arteries and abdominal aortic aneurysm [5]. Thus, the mechanism underlying dolichoectasia of intracranial arteries may be systemic.
Due to frequent coexistence of dolichoectasia of intracranial arteries and coronary disease and abdominal aortic aneurysm, we suggest prophylactic cardiological examination and sonography of the abdomen in the patients with dolichoectasia of intracranial arteries.
For many years diagnosis of intracranial arterial dolichoectasia was based on angiography. Nowadays, computed tomography angiography is the primary method of evaluating vascular pathology. Modern multi-detector CT scanners allow for acquisition after contrast medium administration with isotropic resolution. Thus, the multi-plane and multi-volume imaging with resolution of less than 1 mm, is performed in just a few seconds. The possibility of a quick visualization of intracranial arteries has substantially improved the vascular diagnostics and the patients’ comfort, as well as reduced the number of examination failures resulting from motion artifacts. Other advantages of CTA examination include visualization of the vascular wall, lumen and adjacent tissues at the same time, and the possibility of presenting the anatomy of the vessels in any view, using data from a single acquisition only [17–21].
Despite many advantages, the CTA examination has also its disadvantages. The most significant is the exposure to X-rays. Allergic reaction to iodine or other components of the contrast medium is also possible (however, with quick multi-detector scanners there are alternative – gadolinium-based – contrast agents, which can be administered in patients with iodine allergy). Nephrotoxic effects of contrast agents should be remembered as well (contrast-induced nephropathy – CIN) [4].
Another diagnostic method used to confirm dolichoectasia is non-invasive time–of-flight magnetic resonance angiography (MRA). Along with plain MR it enables displaying vascular anatomy and the relationship of arteries to posterior fossa structures, to visualize mural clot or dissections of the wall as well as the pathological changes in the brain structures (e.g. in the brainstem) caused by compression. It is not necessary to administrate a contrast material to visualize vessels. However, TOF MRA is effective in evaluation of arteries with rapid blood flow, while arteries with low or non-laminar flow may be not well visualized. Therefore it may be a serious disadvantage while imaging dolichoectatic arteries, often characterized by slow and impaired flow (Figure 5). Other disadvantages of MR imaging include: lower spatial resolution as compared to CTA, long time of acquisition, during which the patient needs to remain motionless to avoid artifacts and degradation of image quality, which is often difficult because of symptoms presented by patients, e.g. dizziness. CTA, as compared to MR, requires shorter scanning time, allows to delineate small calcium deposits in mural thrombi, but it requires intravenous administration of contrast material, expose the patient to X-rays and doesn’t provide information about the hemodynamics of the flow [3]. It is considered that CTA allows to depict accurately arteries of low flow (poststenotic segments). MRI (T1-weighted scans) with intravenous administration of contrast material can also be used to evaluate vessels with low flow, but is not as accurate as CTA [22]. The steady state sequences (f.e. FIESTA), due to high resolution, allow to assess more accurately the compression of nerve structures. T2-weighted scans are helpful in the diagnostics of vascular cerebral oedema, whereas diffusion- weighted imaging (DWI) is valuable in early detection of recent ischemic changes and cytotoxic cerebral oedema.
Dolichoectasia of intracranial arteries, if not complicated by intracranial bleeding, is treated conservatively in the great majority of cases, especially in older patients. Rares are the cases when an endovascular or microchirurgical treatment is performed. As the risk of brain infarction associated with microthrombosis is increased, the conservative treatment includes anticoagulation, regardless whether there is a thrombus in the artery or not, also in symptomatic patients with no thrombus in dolichoectatic artery but with irregularities found in Doppler study of extracerebral carotid arteries. Moreover, thorough management of arterial hypertension is recommended [23].
Endovascular intervention of dolichoectasia as well as microsurgical treatment are difficult because of long segments of pathologically changed arteries with thinned walls and frequently concomitant advanced atherosclerosis. Therefore, attempts of neurosurgical procedures of grafting by-passes are performed. Recent developments of endovascular techniques include placement of flow diverted stents into the aneurysmal sack, especially in the basilar artery. The stent is placed in the artery before its widening, then across the vessel till the point where the artery starts to narrow. An appropriate porosity of the stent facilitates significantly maintaining of the patency of small arteries branching from the aneurysmal artery, thus decreasing the risk of ischaemic incidents. Besides, it is possible to implant coils into the wide sack of the aneurysm, based on the wall of the stent. Endovascular method is less risky than surgical intervention; however its effectiveness in urgent interventions after rupture of the aneurysm and subarachnoid bleeding has not been established yet [8,24].
Conclusions
Intracranial arterial dolichoectasia can occur at any age and stay asymptomatic for a long time.
In many cases of intracranial arterial dolichoectasia neurological symptoms are observed. They are associated with haemodynamic disturbance, caused by mural thrombi or with mass effect resulted from an ectatic vessel.
Figure 3.
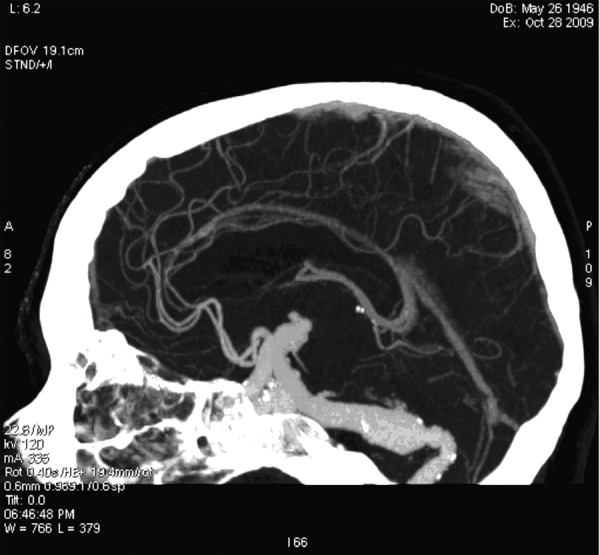
CTA, MIP reconstruction. Fusiform aneurysm of the basilar artery.
References:
- 1.Lou M, Caplan LR. Vertebrobasilar dilatative arteriopathy (dolichoectasia) Ann N Y Acad Sci. 2010;1184:121–33. doi: 10.1111/j.1749-6632.2009.05114.x. [DOI] [PubMed] [Google Scholar]
- 2.Harrigan MR, Deveikis JP. Dolichoectatic, fusiform and serpentine aneurysms. In: Harrigan MR, Deveikis JP, editors. Handbook of Cerebrovascular Disease and Neurointerventional Technique. Humana Press; 2009. p. 490. 13.5.7. [Google Scholar]
- 3.Osborn A. Diagnostic Imaging: Brain 2 ed. Subarachnoid hemorrhage and aneurysms. Amirsys. 2010:20–25. Section 3. [Google Scholar]
- 4.Van Rooij WJ, Sluzewski M, Beute GN. Endovascular treatment of giant serpentine aneurysms. AJNR. 2009;29:1418–19. doi: 10.3174/ajnr.A1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacks JG, Lindenburg R. Dolicho-ectatic intracranial arteries: symptomatology and pathogenesis of arterial elongation and distension. Johns Hopkins Med J. 1969;125:95–106. [PubMed] [Google Scholar]
- 6.Pico F, Labreuche J, Touboul PJ, et al. Inrtacranial arterial dolichoectasia and its relation with atherosclerosis and stroke subtype. Neurology. 2003;61:1736–42. doi: 10.1212/01.wnl.0000103168.14885.a8. [DOI] [PubMed] [Google Scholar]
- 7.Ubogu EE, Zaidat OO. Vertebrobasilar dolichoectasia diagnosed by magnetic resonance angiography and risk of stroke and death: a cohort study. J Neurol Neurosurg Psychiatry. 2004;75:22–26. [PMC free article] [PubMed] [Google Scholar]
- 8.Guarnieri G, Lavanga A, Granato F, et al. Endovascular Treatment of a Fusiform Cerebral Aneurysm by Stenting Alone: Two Case Reports and Literature Review. Neuroradiol J. 2010;23(3):368–75. doi: 10.1177/197140091002300320. [DOI] [PubMed] [Google Scholar]
- 9.Pico F, Labreuche J, Toubou PJ, et al. Intracranial arterial dolichoectasia and small-vessel disease in stroke patients. Ann Neurol. 2005;57:472–79. doi: 10.1002/ana.20423. [DOI] [PubMed] [Google Scholar]
- 10.Passero SG, Rossi S. Natural history of vertebrobasilar dolichoectasia. Neurology. 2008;70:66–72. doi: 10.1212/01.wnl.0000286947.89193.f3. [DOI] [PubMed] [Google Scholar]
- 11.Levine RL, Turski PA, Grist TM. Basilar artery dolichoectasia. Review of the literature and six patients studied with magnetic resonance angiography. J Neuroimaging. 1995;5(3):164–70. doi: 10.1111/jon199553164. [DOI] [PubMed] [Google Scholar]
- 12.Pico F, Labreuche J, Cohen A, et al. Intracranial arterial dolichoectasia is associated with enlarged descending thoracic aorta. Neurology. 2004;63:2016–21. doi: 10.1212/01.wnl.0000145845.12577.0f. [DOI] [PubMed] [Google Scholar]
- 13.Dereń-Wagermann I, Kuliszkiewicz-Janus M, Schiller J. Ehlers-Danlos syndrome. Adv Clin Exp Med. 2010;19(4):537–42. [Google Scholar]
- 14.Pico F, Jacob MP, Labreuche J, et al. Matrix metalloproteinase-3 and intracranial arterial dolichoectasia. Ann Neurol. 2010;67:508–15. doi: 10.1002/ana.21922. [DOI] [PubMed] [Google Scholar]
- 15.Krishnankutty S, Ports TA, Amidon TM, et al. Increased prevalence of coronary ectasia in heterozygous familial hypercholesterolemia. Circulation. 1995;9:1375–80. doi: 10.1161/01.cir.91.5.1375. [DOI] [PubMed] [Google Scholar]
- 16.Kraml P, Syrovátka P, Štípek S. Hyperlipoproteinemia impairs endothelium-dependent vasodilation. Physiol Res. 2004;53:471–80. [PubMed] [Google Scholar]
- 17.Kowalewski K, Zimny A, Sąsiadek M. CT angiography for the detection of cerebral aneurysms – an analysis of 436 verified cases. Pol J Radiol. 2008;73(3):25–36. [Google Scholar]
- 18.Jakubiak A, Waliszewska M, Guziński M, et al. The value of 64-detector computed tomography angiography as a diagnostic method during emergency service in acute lower limbs ischemia. Pol J Radiol. 2009;74(3):37–41. [Google Scholar]
- 19.Kornafel O, Baran B, Pawlikowska I, et al. Analysis of anatomical variations of the main arteries branching from the abdominal aorta, with 64-detector computed tomography. Pol J Radiol. 2010;75(2):38–45. [PMC free article] [PubMed] [Google Scholar]
- 20.Waliszewska M, Jakubiak A, Guziński M, et al. Application of the 64-slice computed tomography as a diagnostic method in acute posttraumatic ischaemia of the upper limbs – 3 case reports. Pol J Radiol. 2010;75(2):94–97. [PMC free article] [PubMed] [Google Scholar]
- 21.Miloševič Medenica S, Vučkovič V, Prstojevič B. 64-Slice CT Angiography in the Detection of Intracranial Aneurysms: Comparison with DSA and Surgical Findings. Neuradiol J. 2010;23(1):55–61. doi: 10.1177/197140091002300110. [DOI] [PubMed] [Google Scholar]
- 22.Bash SJ, Villablanca P, et al. Intracranial Vascular Stenosis and Occlusive Disease: Evaluation with CT Angiography, MR Angiography, and Digital Subtraction Angiography. AJNR Am J Neuroradiol. 2005;26:1012–21. [PMC free article] [PubMed] [Google Scholar]
- 23.Dziewas R, Freund M, Lademann P, et al. Treatment options in vertebrobasilar dolichoectasia – case report and review of the literature. Eur Neurol. 2003;49:245–47. doi: 10.1159/000070196. [DOI] [PubMed] [Google Scholar]
- 24.Hanel RA, Boulos AS, Sauvageau EG, et al. Stent Placement for the Treatment of Nonsaccular Aneurysms. Neurosurg Focus. 2005;18(2):E8. doi: 10.3171/foc.2005.18.2.9. [DOI] [PubMed] [Google Scholar]


