Summary
Background:
Pneumatosis cystoides intestinalis (PCI) is a rare disorder characterized by the presence of multiple gas collections in the subserosal or submucosal intestinal wall of the large or small intestine. We report two cases of PCI in the course of chronic graft-versus-host disease.
Case Report:
A 5-year-old girl was treated for acute lymphoblastic leukemia. Twenty-four months after the hematopoietic stem cell transplantation, in the course of graft-versus-host disease, she developed subcutaneous emphysema of the right inguinal and pudendal region. PCI was diagnosed based on a CT examination. A 3-year-old boy was treated for juvenile myelomonocytic leukemia. Fourteen months after the hematopoietic stem cell transplantation he presented with an increased severity of intestinal symptoms, including intermittent bleeding from large intestine. PCI was diagnosed based on a CT exam and was confirmed by a colonoscopy.
Conclusions:
Pneumatosis cystoides interstitialis in the course of chronic graft-versus-host disease has a heterogeneous clinical presentation that does not correlate with results of imaging. CT is a method of choice to diagnose PCI. In patients with PCI, the presence of free air in the peritoneal cavity does not confirm an intestinal perforation.
Keywords: pneumatosis cystoides intestinalis, graft-versus-host disease, computed tomography
Background
Pneumatosis cystoides intestinalis (PCI) is a relatively rare disease characterized by numerous gas collections in the intestinal wall [1]. In some cases, gas may be also observed in the intra-and extraperitoneal spaces [2], and in more severe cases, it is accompanied by an inflammatory reaction [3].
PCI occurs in patients with various disorders, including chronic obstructive pulmonary disease, gastrointestinal obstruction, bowel ischemia, neonatal nectrotizing enterocolitis, immunodeficiency syndromes (AIDS), in the course of bacterial or viral infections and after surgical procedures as well as colonoscopies [2, 4–6]. PCI may be also a complication of treatment in cancer patients [3].
Pathogenesis of this disorder is unclear and probably multi-factorial. Four most likely pathomechanisms of PCI were proposed. According to the mechanical theory, coexisting mechanical obstruction and mucosal damage leads to air movement along lymphatic vessels [7]. According to the pulmonary theory, the air from destroyed alveoli (e.g. in emphysema) travels within lung interstitium, mediastinum and along the mesentery into the intestinal wall [8]. Bacterial cause seems also probable in some cases, i.e. through production of gas by submucosal bacteriae of the Clostridium and Escherichia species [6]. On the other hand, according to the immunosuppression theory, cancer treatment, and steroid administration in particular, causes rapid constriction of lymphatic nodules with subsequent mucosal damage and aspiration of air from the bowel lumen [9].
The goal of this work is to discuss a variety of clinical presentations of PCI in the course of chronic graft-versus-host disease (cGVHD) and a role of computed tomography in diagnostics and treatment planning.
Case Studies
Case 1
A 5-year-old girl was treated for acute lymphoblastic leukemia with allogenic transplantation of hematopoetic stem cells from peripheral blood of an unrelated donor. The post-transplantation period was complicated by a cutaneous and intestinal cGVHR followed by a pulmonary form of this disease as well as adenovirus, cytomegalovirus and Polyoma BK infections with hemorrhagic cystitis. Immunosuppression with methylprednisolone and mycophenolate mophetil (Cell-Cept) was used in the course of treatment.
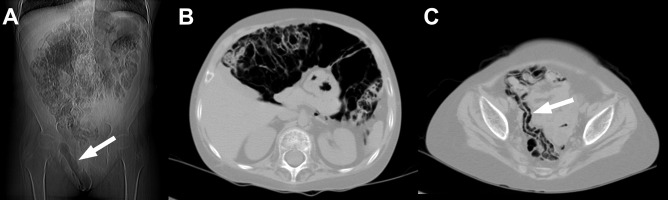
After 24 months from transplantation, the patient presented with isolated thickening of the right pudendal labium persisting for several days. Moreover, soft tissue crepitus was noted on physical examination in the right inguinal region. An X-ray image showed numerous, moderately distended intestinal loops and air in the right inguinal region without radiological signs of obstruction or perforation of the gastrointestinal tract. Computed tomography (CT) examination was performed, revealing air in the intestinal wall, multicystic air spaces within the mesentery and peritoneum as well as extraperitoneal air collections in the inguinal region and within the thickened right labium (Figure 1A). As there was no pathological contrast enhancement in the involved intestinal fragments, a mild form of PCI was diagnosed. Considering child’s good clinical condition and absence of inflammatory reaction in CT, laparoscopy was not performed. Treatment with wide-spectrum antibiotics and parenteral alimentation was implemented. Control abdominal CT scan performed after 2 weeks was unremarkable.
Figure 1.
Patient 1. Numerous air collections and air in the inguinal region and soft tissues of the right pudendal labium visible on a CT scan (arrow) (A). A CT image at the level of mid-abdomen shows multi-cystic air collections in intestinal walls, free air in peritoneal cavity and single air collections in the extraperitoneal space (B). A CT scan of the lesser pelvis reveals air in the wall of ileum (arrow) (C).
Case 2
A 3-year-old boy had been treated for one year due to juvenile myelomonocytic leukemia with transplantation of stem cells from an unrelated donor. Treatment was complicated with acute and then chronic cutaneous and intestinal graft-versus-host disease and cytomegalovirus infection. Systemic steroid therapy was applied, but every attempt at tampering the steroid dose resulted in deterioration of boy’s clinical status. Several endoscopic examinations of the upper and lower gastrointestinal tract revealed lesions corresponding to the diagnosis of cGVHD.
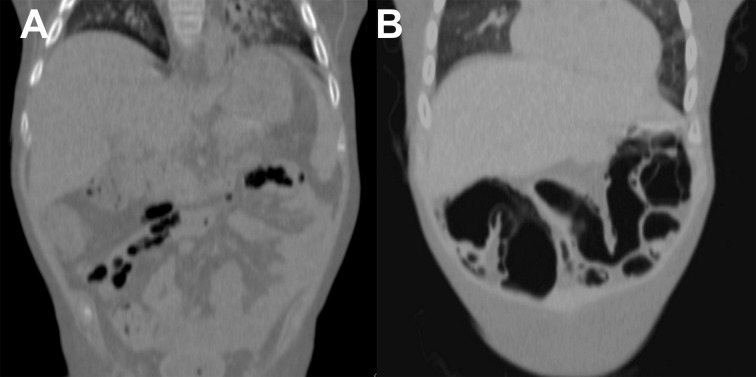
After 14 months from transplantation, the child’s clinical state significantly deteriorated: intestinal symptoms exacerbated and there were episodes of gastrointestinal bleeding. Abdominal CT examination showed air in the intestinal wall and single extraintestinal air collections present within fat tissue and mesentery (Figure 2). Individual intestinal loops were dilated to 3 cm with contrast-enhanced walls. Presence of air in the large bowel wall was confirmed with endoscopy (Figure 3). Conservative combination treatment was applied and the entire time of therapy was complicated by episodes of massive lower gastrointestinal bleeding. In a control abdominal CT scan performed 3 weeks later there was no air in the intestinal walls or outside the bowel, but contrast enhancement persisted in the walls of some intestinal loops. Endoscopy did not show any previously visible air collections either.
Figure 2.
Patient 2. In computed tomography air collections are visible in the periintestinal fat (A) and gastrointestinal wall (B).
Figure 3.
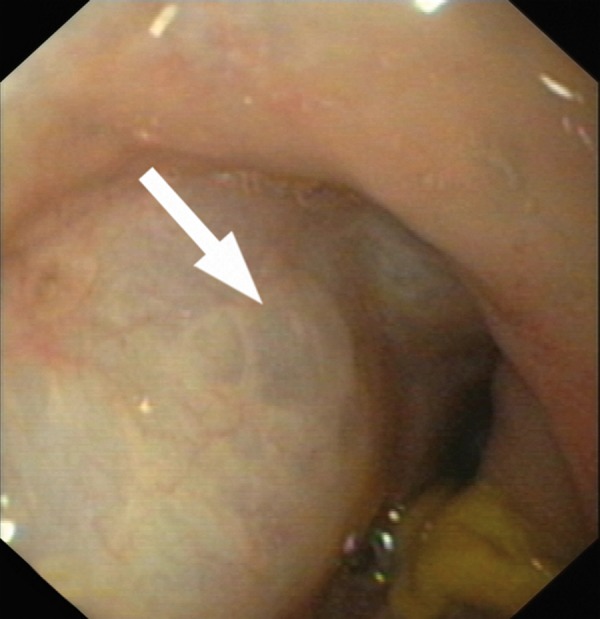
Patient 2. Endoscopic Picture of PCI with a submucosal air collection (arrow).
Discussion
Pneumatosis cystoides intestinalis is not associated with a homogeneous clinical picture. In some cases it is asymptomatic [2], but in most patients it presents with nausea, vomiting, diarrhea and abdominal pain. In cases complicated by infections, the mortality in the course of PCI may reach 75% [10].
It appears that a direct cause of PCI in the described patients was intestinal cGVHD and prolonged steroid therapy. GVHD is a quite common complication of allogenic stem cell or bone marrow transplantation, in which immunocompetent donor cells recognize recipient’s cells as foreign. In its chronic form, the disease appears after 100 days from transplantation and is associated with release of autoreactive T cells and stimulation of antibody production by autoreactive B cells. In a gastrointestinal form cGVHD leads to atrophic mucositis with ulcer formation, bacterial and fungal superinfection, fibrosis and development of malabsorption syndromes. Damage to intestinal mucosa, coexisting infection and inflammatory infiltration with concomitant steroid therapy predisposes to PCI according to the previously mentioned immunosupression theory [9]. Cases of pneumatosis in the course of cGVHD described in the literature occurred 2–8 months after bone marrow transplantation and were usually mild [3,11,12]. In our patients they appeared relatively late – in the 14th and 24th month after transplantation. In our first patient the only clinical sign of pneumatosis was subcutaneous emphysema in the inguinal and pudendal regions. In the second case, lower gastrointestinal bleeding and presence of inflammatory reaction in the bowel wall reflected great severity of the process.
Diagnosis of PCI is sometimes possible on the basis of an abdominal X-ray [3,13], but the methods of choice are computed tomography with assessment of air spaces in the pulmonary window and endoscopy. Endoscopic examination of the lower part of gastrointestinal tract revealed numerous cystic protuberances in the hyperemic intestinal mucosa, which collapsed upon a biopsy attempt [13]. Such a picture was obtained during the first colonoscopy performed in Patient 2. Air-filled spaces disappeared after treatment. Computed tomography seems to be the method of choice for diagnosing PCI and coexisting pathologies. In CT scans gaseous collections in the intestinal wall take an appearance ranging from a linear intramural emphysema (Figure 1C) to large cystic formations (Figures 1B, 2B). Based on CT pictures of the described subjects one may say that in the course of PCI, the size and number of gas collections does not correlate with the severity of clinical symptoms. It is important, as gastrointestinal symptoms in patients on steroid therapy with primary intestinal cGVHD are not always unequivocal [3].
An important advantage of CT is the possibility of finding additional signs reflecting the severity of the pathology, i.e. intestinal wall thickening, pathological contrast enhancement, bowel lumen dilatation, which may indicate obstruction, soft tissue infiltrations, fluid in peritoneal cavity and gas in the portal vein [14]. Therefore, performing a CT scan of the abdomen and lesser pelvis before and after contrast application is indicated upon suspicion of PCI. However, additional filling of the intestinal lumen is controversial. Neither one of our patients received contrast or water orally or rectally before CT examination due to boy’s severe with contrast medium or water in CT examination does not significantly influence the quality of assessment of air in the intestinal walls, but helps to identify the signs of obstruction or ischemia.
In case of PCI in patients with cGVHD, the radiologist’s main objective, apart from stating the diagnosis of pneumatosis, is to detect possible gastrointestinal perforation or ischemic mucosal lesions with threatening perforation [10,15]. As it was described in Patient 1, occurrence of free air in peritoneal cavity is not a proof of perforation. Only the overall clinical picture and presence of fluid in the peritoneal cavity may suggest occurrence of such a complication of PCI. Pathological contrast enhancement does not explicitly indicate bowel wall necrosis either, especially in patients with cGVHD [3]. Therefore, strict cooperation with clinicians and knowledge of the disease process is crucial.
Conclusions
Pneumatosis cystoides intestinalis in the course of chronic graft-versus-host disease may present with a diversity of clinical manifestations, which do not correlate with the results of imaging studies. The method of choice for PCI diagnostics is computed tomography, in which one notes the presence of gas in the intestinal walls as well as, at times, intra- and extraperitoneally. Detecting free air in the peritoneal cavity in patients with PCI does not necessarily signify gastrointestinal perforation.
References:
- 1.Read NW, Al-Janabi MN, Cann PA. Is raised breath hydrogen related to the pathogenesis of pneumatosis coli? Gut. 1984;25(8):839–45. doi: 10.1136/gut.25.8.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai Y, Hikichi M, Isogaki J. Pneumatosis cystoides intestinalis associated with massive free air mimicking perforated diffuse peritonitis. World J Gastroenterol. 2008;14(43):6753–56. doi: 10.3748/wjg.14.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCarville MB, Whittle SB, Goodin GS, et al. Clinical and CT features of benign pneumatosis intestinalis in pediatric hematopoietic stem cell transplant and oncology patients. Pediatr Radiol. 2008;38(10):1074–83. doi: 10.1007/s00247-008-0944-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wandtke J, Skucas J, Spataro R, et al. Pneumatosis intestinalis as a complication of jejunoileal bypass. Am J Roentgenol. 1977;129(4):601–4. doi: 10.2214/ajr.129.4.601. [DOI] [PubMed] [Google Scholar]
- 5.McCollister DL, Hammerman HJ. Air, air, everywhere: pneumatosis cystoides coli after colonoscopy. Gastrointest Endosc. 1990;36(1):75–76. doi: 10.1016/s0016-5107(90)70936-4. [DOI] [PubMed] [Google Scholar]
- 6.Gagliardi G, Thompson IW, Hershman MJ, et al. Pneumatosis coli: a proposed pathogenesis based on study of 25 cases and review of the literature. Int J Colorectal Dis. 1996;11(3):111–18. doi: 10.1007/s003840050031. [DOI] [PubMed] [Google Scholar]
- 7.Stiennon OA. Pneumatosis intestinals in the newborn. Am J Dis Child. 1951;81(5):651–63. doi: 10.1001/archpedi.1951.02040030664004. [DOI] [PubMed] [Google Scholar]
- 8.Keyting WS, McCarver RR, Kovarik JL, et al. Pneumatosis intestinalis: a new concept. Radiology. 1961;76:733–41. doi: 10.1148/76.5.733. [DOI] [PubMed] [Google Scholar]
- 9.Borns PF, Johnston TA. Indolent pneumatosis of the bowel wall associated with immune suppresive therapy. Ann Radiol. 1973;16:163–66. [Google Scholar]
- 10.Knechtle SJ, Davidoff AM, Rice RP. Pneumatosis intestinalis. Surgical management and clinical outcome. Ann Surg. 1990;212(2):160–55. doi: 10.1097/00000658-199008000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulenburg A, Herold C, Eisenhuber E, et al. Pneumocystis cystoides intestinalis with pneumoperitoneum and pneumoretroperitoneum in a patient with extensive chronic graft-versus-host disease. Bone Marrow Transplantation. 1999;24(3):331–33. doi: 10.1038/sj.bmt.1701897. [DOI] [PubMed] [Google Scholar]
- 12.Navari RM, Sharma P, Deeg HL, et al. Pneumatosis cystoides intestinalis following allogeneic marrow transplantation. Transplant Proc. 1983;15(2):1720–24. [PubMed] [Google Scholar]
- 13.Azzaroli F, Turco L, Ceroni L, et al. Pneumatosis cystoides intestinalis. World J Gastroenterol. 2011;17(44):4932–36. doi: 10.3748/wjg.v17.i44.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olson DE, Kim YW, Ying J, et al. CT predictors for differentiating benign and clinically worrisome pneumatosis intestinalis in children beyond the neonatal period. Radiology. 2009;253(2):513–19. doi: 10.1148/radiol.2532090168. [DOI] [PubMed] [Google Scholar]
- 15.Day DL, Ramsay NK, Letourneau JG. Pneumatosis intestinalis after bone marrow transplantation. Am J Roentgenol. 1988;151(1):85–87. doi: 10.2214/ajr.151.1.85. [DOI] [PubMed] [Google Scholar]