summary
The epidemiology of microbial keratitis has been investigated in several studies by analysis of organisms cultured from corneal scrapes. However, a comparison of the frequency of different organisms causing keratitis in different parts of the world is lacking. We present a review incorporating an analysis of data from studies worldwide. The data provide a comparison of the frequency of culture-positive organisms found in different parts of the world.
The highest proportion of bacterial corneal ulcers was reported in studies from North America, Australia, the Netherlands and Singapore. The highest proportion of staphylococcal ulcers was found in a study from Paraguay whilst the highest proportion of pseudomonas ulcers was reported in a study from Bangkok. The highest proportions of fungal infections were found in studies from India and Nepal. Possible explanations for these observed geographic variations are discussed.
Keywords: cornea, keratitis, eye, infection, epidemiology
INTRODUCTION
Microbial keratitis is a potentially serious corneal infection and a major cause of visual impairment worldwide. A conservative estimate of the number of corneal ulcers occurring annually in the developing world alone is 1.5-2 million.[1] Permanent visual dysfunction has been reported in a significant proportion of patients in both developing [2] and developed [3] countries. Srinivasan et al [4] comment that ulceration of the cornea in south India ‘is a blinding disease of epidemic proportions’.
Various micro-organisms can cause microbial keratitis and predisposing risk factors vary from one geographic region to another. They include pre-existing corneal disease as well as other risk factors such as contact lens wear, surgical or non-surgical trauma and ocular surface disease.[5-7]
There is limited comparative information on international patterns of causative organisms in microbial keratitis. With increasing rates of migration and international travel, an awareness of these geographical variations is relevant for clinicians treating microbial keratitis, and especially for those planning to work in regions where they have not previously practised. The aim of this review is to summarise the published literature that provides information on the worldwide variation in organisms causing microbial keratitis.
METHODS
Search Strategy
A systematic review of the current literature pertaining to the prevalence of causative organisms responsible for microbial keratitis was conducted. Pubmed searches were performed and verified in April 2009 by two independent investigators. The terms ‘microbial keratitis’, ‘bacterial keratitis’ and ‘infectious keratitis’ were entered into Pubmed. Only papers presenting data that were collected after 1st January 1990 were examined, and the search was restricted to English Language and human studies. Only studies that cultured at least 50 organisms in total were included. Titles and abstracts were read and a judgement was made as to whether the paper provided culture results for microbial keratitis in a specified geographical location. If this was felt to be the case then a full text request was made to access the original published data.
Studies looking only at infections related to use of contact lenses were excluded, as were studies looking only at limited age groups.
Extraction and Recording of Data
Papers were read and information was abstracted on the following variables: number of patients in the study, time period of reporting, region, method by which organisms were isolated, method of culture, rate of positive cultures, and number of contact lens wearers in the study sample. These data were then entered then into a Microsoft Excel spread sheet.
With regard to the microorganisms cultured, the total numbers in each of the following categories were abstracted and recorded: gram positive organisms, staphylococcal species, streptococcal species, gram negative organisms, pseudomonal species, protozoa, fungi/yeasts, aspergillus species and candida species.
Classification of income levels and GNI subheading
The prevalence of different causative organisms was compared according to countries’ gross national incomes (GNIs) (source = http://web.worldbank.org). Income groups were defined by 2007 GNI per capita, calculated using the World Bank Atlas method.[8] The groups distinguished were: low income, $935 or less; lower middle income, $936 - $3,705; upper middle income, $3,706 - $11,455; and high income, $11,456 or more.
Statistical analysis
Statistical analysis was performed using ‘Analyse-it’ version 2.20 software. Spearman’s correlation coefficients were used to explore associations between:
prevalence of certain types of organism and GNI; and between
prevalence of contact lens wear and prevalence of pseudomonas.
RESULTS
3883 publications were identified through the preliminary Pubmed search. Of these, 37 papers met the inclusion criteria. One paper was excluded because it included a significant number of cases that the authors deemed to constitute an outbreak of suture-related infections.[9] Twelve of the included papers were from the Indian subcontinent, 7 from North America and Canada, 6 from the Far East, 5 from Australasia, 4 from Europe, 2 from Africa (both from Ghana) and 1 from South America. The mean GNI of the countries studied was $20834 (range $470 – $59880). The number of patients ranged from 73 to 3183. The time periods of study ranged from 3 – 192 months, although three studies did not specify the study period. The proportion of keratitis patients with a recent history of contact lens wear was reported in only 22 studies and ranged from 0.33% (West Bengal [10]) to 50.3% (Paris[11]). Three studies reported on only culture-positive cases and so appear to have 100% culture-positive rates in Table 1. In the remainder of the studies culture-positive rates ranged from 35% - 86%.
Table 1. Studies meeting criteria for inclusion in review.
| Location of study (reference) |
GNI per capita ($) |
Time period (months) |
Number of patients |
Proportion (%) of patients using contact lenses |
Method of culture | Proportion (%) of patients with positive cultures |
|---|---|---|---|---|---|---|
| Africa | ||||||
| Ghana(16) | 590 | 24 | 290 | unspecified | 2 | 50 |
| Accra, Ghana(18) |
590 | unspecified | 199 | unspecified | Chocolate, Sabouraud | 64 |
|
Indian Subcontinent |
||||||
| Nepal, India(19) | 340 | 36 | 447 | unspecified | Sheep’s blood agar, Chocolate, brain- heart infusion, Sabouraud |
68 |
| Chittagong, Bangladesh(20) |
470 | unspecified | 151 | unspecified | Blood, Chocolate,thiogylcollate,Sarbarouds |
63 |
| Tamil Nadu(16) | 950 | 24 | 800 | unspecified | 1 | 69 |
| East Bengal(10) | 950 | 36 | 1198 | 0.33 | Blood, chocoloate, potatoe dextrose agar, sabarouds dextrose |
68 |
| New Delhi(2) | 950 | unspecified | 100 | 2.0 | Blood, chocolate, thioglycollate, sabarouds |
65 |
| Madurai,S. India(4) |
950 | 3 | 434 | unspecified | Sheep’s blood agar, potato dextrose agar, chocolate, brain-heart infusion |
68 |
| Hyderabad, India(21) |
950 | 95 | 1092 | unspecified | Sheep’s blood agar, Chocolate, Nonnutrient, Sarbarouds, brain-heart infusion, potato dextrose agar |
35 |
| Tamil Nadu, India (22) |
950 | 36 | 3183 | 1.04 | unspecified | 71 |
| Hyderabad, India (17) |
950 | 15 | 170 | Unspecified | Unspecified | 69.4 |
| Delhi, India (23) | 950 | 12 | 1000 | 8.2 | Unspecified | 56.8 |
| Riyadh, Saudi Arabia** (24) |
15440 | 12 | 103 | 17.48 | blood, chocolate, Sabouraud’s and thioglycolate |
unspecified for 2005** |
| Baghdad, Iraq†(25) |
2320 | 36 | 394 | 6.09 | blood, chocolate, Sabouraud’s and brainheart infusion |
58.6 |
| South America | ||||||
| Asuncion, Paraquay(14) |
1670 | 162 | 660 | unspecified | Blood,Chocolate thiogylcollate, sabarouds | 79 |
| Far East | ||||||
| Taipei, Taiwan(26) | 16590 | 120 | 453 | 44.3* | Chocolate,sheeps, sabarouds | 56 |
| Taipai, Taiwan(27) |
16590 | 12 | 314 | 9.9 | Blood,Chocolate, thiogylcollate, brainheart infusion |
43 |
| Singapore(28) | 32470 | 60 | 80 | 22.5 | Blood, chocolate,thioglycollate, sabarouds, brain heart infusion |
100 |
| Singapore(29) | 32470 | 22 | 103 | 34 | Blood,Chocolate, thiogylcollate, Sabarouds |
50 |
| Hong Kong(5) | 31610 | 17 | 223 | 26.5 | Blood,Chocolate, thiogylcollate, Sabarouds ,non-nutrient Page’s saline agar |
35 |
| Bangkok, Thailand (15) |
34000 | 47 | 127 | 24.4 | sterile kimura spatula, blood, chocolate agar, thoiglycolate |
100 |
| Australasia | ||||||
| Auckland, NZ(3) | 28780 | 24 | 98 | 26 | Blood, sabarouds, thiogylcollate, brain- heart infusion, Page’s amoebic saline |
71 |
| Christchurch, New Zealand(30) |
28780 | 60 | 78 | unspecified | Sheep’s blood agar, Chocolate | 59 |
| Adelaide(14) | 35960 | 61 | 211 | unspecified | Blood, chocolate, sabarouds | 64 |
| Victoria, Australia(7) |
35960 | 24 | 291 | 33.7 | Chocolate, Sabarouds | 49 |
| Brisbane, Australia(6) |
35960 | 60 | 231 | 22.9 | blood, MacConkey, Chocolate, sabarouds |
65 |
|
North America and Canada |
||||||
| Toronto(31) | 39420 | 25 | 95 | 11.6 | Blood, chocolate, inhibitory mould agar, thioglycollate |
63 |
| Miami(32) | 46040 | 108 | 2920 | 10.4* | Chocolate,sheep, sabouraud, thioglycollate |
50 |
| Pittsburgh(33) | 46040 | 60 | 825 | Unspecified | 4 | 100 |
| Los Angeles(12) | 46040 | 31 | 81 | Unspecified | Blood, chocolate, thioglycollate, sabarouds |
76 |
| Texas (34) | 46040 | 60 | 131 | 28.8 | scalpel blade, calcium alginate swabs or cotton tipped applicator. Chocolate, blood, thiglycollate, sabourauds dextrose |
52.5 |
| Durham, USA (35) |
46040 | 84 | 453 | unspecified | calcium alginate swabs, blood, chocolate,saborauds, thioglycolate |
68 |
| Europe | ||||||
| Paris(11) | 38500 | 21 | 291 | 50.3* | 3 | 68 |
| Laussanne, Switzerland(36) |
59880 | 21 | 85 | 36 | Blood, sarborauds, Chocolate, brain-heart infusion |
86 |
| Anatolia, Turkey (37) |
8020 | 192 | 620 | 3.2 | cotton-tipped swabs, chocolate, blood agar, sarborauds |
48.4 |
| Amsterdam and Rotterdam (38) |
45820 | 36 | 156 | 39.74 | blood agar, chocolate agar, cooked meat broth, Sabouraud agar |
58 |
sheeps blood agar, Sabarouds broth and sabarouds glucose agar and at tertiary centre brain heart infusion broth, chocolate agar and cysteine tryptone agar
sheeps blood agar, Sabarouds broth and inhibitory mould agar and at tertiary centre brain heart infusion broth, chocolate agar and cysteine tryptone agar
chocolate polyvitex agar, schaedler broth with globular extract, portagerm amies agar swab and sabouraud-chloramphencolgentamicin medium
sheep blood, chocolate, mannitol salt agar, sabourauds dextrose agar supplemented with gentamicin
paper did not specify the number of contact lens wearers. Instead they report on the number of isolates from contact lens wearers. The figure given in the table therefore represents the percentage of isolates retrieved from cases where CL wear was a risk factor
Estimated to be lower middle income ($936 to $3,705), value given in table is midpoint of this range
This paper presented paper for 1995 and 2005, only 2005 data have been extracted in our study
Among studies which looked at non-bacterial as well as bacterial organisms, Los Angeles [12] and Adelaide [13] had the highest percentages of bacterial cases (95% in both), with Paraguay [14] having the highest percentage of staphylococcal species (79%), and Bangkok [15] the highest proportion of pseudomonal infections (55%). Tamil Nadu [16] had the highest percentage of streptococcal infections (47%). The highest percentage of protozoal infections (7%) was found in a study from Hong Kong.[5]
East India [10] had the highest proportion of corneal infections attributable to fungi (67%). When considering those countries with a significant proportion of fungal ulcers (we have arbitrarily chosen a cut-off of 10% or more), East India also had the highest percentage of aspergillus (60% of all fungal cultures) whereas the highest percentage of fusarium (73% of all fungal cultures) was found in a study from Hyderabad [17].
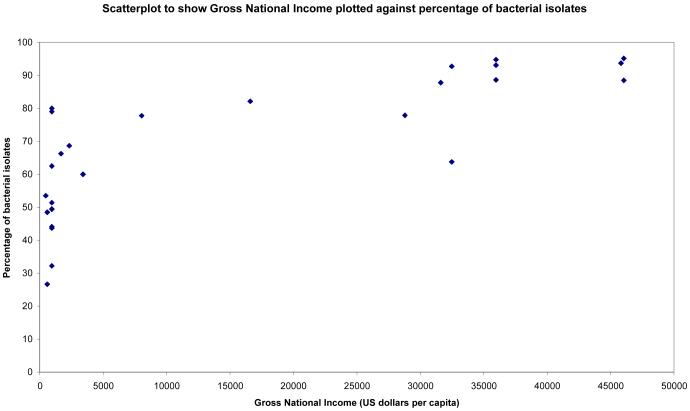
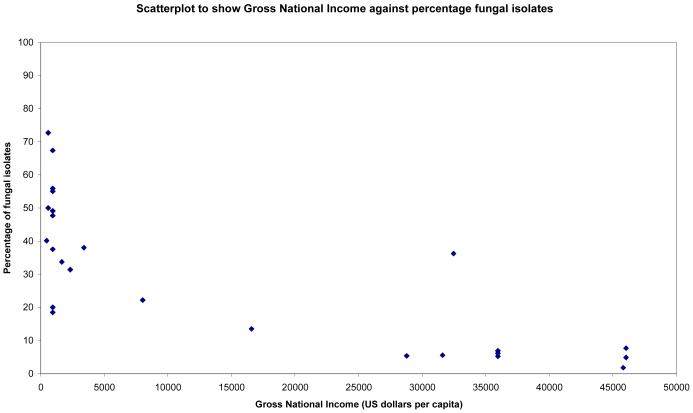
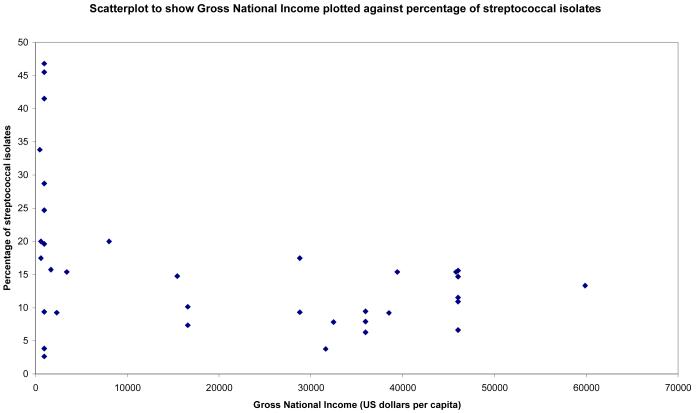
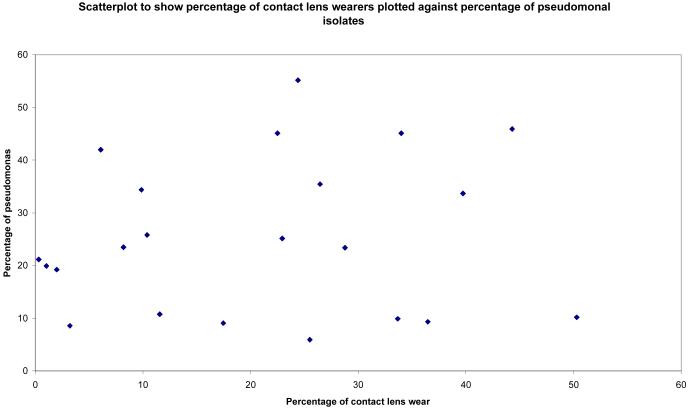
Statistically significant correlations were found between Gross National Income and percentages of bacterial, fungal and streptococcal isolates (see Figures 1-3). Surprisingly there was no statistically significant correlation between percentage of pseudomonal isolates and percentage of contact lens wearers (see Figure 4). 95% confidence intervals and p values for these analyses are provided in table 4.
Figure 1.
Scatterplot showing percentage of bacterial isolates in studies not looking exclusively at bacterial causes of microbial keratitis plotted against gross national income (US Dollars per capita)
Figure 2.
Scatterplot to show percentage of fungal isolates plotted against gross national income (US Dollars per capita)
Figure 3.
Scatterplot to show streptococcal isolates (expressed as a percentage of total bacterial isolates) plotted against gross national income (US Dollars per capita)
Figure 4.
Scatterplot to show pseudomonas isolates (expressed as a percentage of total bacterial isolates) plotted against contact lens wearers (as a percentage of total number of patients included in study)
Table 4. 95% confidence intervals and p values for Spearman’s Correlation analysis.
| Variables analysed | Correlation coefficient | 95% confidence limit | 2 tailed p value |
|---|---|---|---|
| Prevalence of bacteria, GNI | 0.83 | 0.68 to 0.91 | <0.0001 |
| Prevalence of fungi, GNI | −0.81 | −0.90 to −0.66 | <0.0001 |
| Prevalence of Streptococci, GNI | −0.43 | −0.66 to −0.12 | 0.009 |
| Prevalence of pseudomonas, prevalence of contact lens wearers |
0.13 | −0.31 to 0.52 | 0.6 |
DISCUSSION
We have found a wide variation in the causative organisms for microbial keratitis in different parts of the world. To some degree this variation is explained by economic factors as well as contact lens wear. A high proportion of bacterial ulcers were reported from centres in developed countries (North America, Australia, and Western Europe). In these countries, patients are far less likely to be agricultural workers, and so have a reduced risk of trauma from organic matter, which is known to be a risk factor for fungal infection.[28]
A high percentage of staphylococcus species (79%) was recorded in the study from Paraguay [14] although the reason for this is not clear. Of note, the authors comment that their patients have to make long journeys to their hospital. Thus, their data may reflect more severe cases of microbial keratitis.
The study from Tamil Nadu [16] found the highest proportion of streptococcus species (46.8%). The authors noted that this figure was only 18.5% in 1986 and suggest that the trend might represent a genuine change in the bacterial flora due to changes in the climate and environment.
The study from Bangkok [15] had the highest proportion of pseudomonas infections (55%). Interestingly, this study did not have the highest proportion of contact lens wearers (only 24%). Other studies reported far higher proportions of contact lens wearers, for example 44% in a study from Taiwan [26] and 50% in the study from Paris [11]. When we compared the percentage of contact lens wearers with the percentage of pseudomonal infections (figure 4), the Spearman correlation coefficient was not statistically significant. Interestingly, Cohen et al. [39] at Wills Eye Hospital reported a decline in contact lens-related ulcers: during 1998 to 1991, contact lens wear accounted for 44% of all ulcers, but during 1992 to 1995, it accounted for only 30%. The authors speculated that their figures might reflect a reduction in the number of referrals to their unit due to the increased availability of fluoroquinolones in the community.
Trauma was a major risk factor for corneal infection in certain countries. In Paraguay [14], the percentage of cases with preceding trauma was 48%, in Eastern Nepal[19], 53%, in Madurai, South India[4], 65% and 83% in Eastern India[11] (most commonly from injury by the paddy or its stalk). The authors of this last study noted an increase in keratitis during harvesting season.
The above studies also addressed the frequency of self-medication prior to presentation at a tertiary referral unit. In the Madurai study, 20% of patients had been to a village healer and 87% had been started on topical medication, of whom 8% were on topical corticosteroids. In the study from Eastern India, 18% of patients had used medication before coming to clinic, and in the Paraguay study the proportion was 83%.
Jeng and McLeod[40] commented on the emerging resistance of bacterial infections to fluoroquinolones. In addition to changes in resistance patterns, studies have also demonstrated changing patterns of causative organisms over time in a given geographical location. Varaprasathan et al.[41] reported that the proportion of S. pneumoniae and P. aeruginosa ulcers in Northern California had decreased over a 50 year period whilst that of S. marcescens had increased over the same period. Sun et al.[24] reported a rise in the percentage of gram positive cocci in North China from 25% in 1991 to 70.8% in 1997, as well as a decrease in gram negative bacilli from 69% to 23.4% over a similar period.
Leck et al.[16] have previously compared corneal ulcers in Ghana and South India, whilst Lam et al.[5] have discussed differences between Hong Kong, Europe and North America. However, the present study is the first to present a worldwide comparison of corneal infections.
In interpreting this comparison, a number of limitations must be considered. Variations existed in the definition of microbial keratitis between studies. Lam et al, reporting on cases from Hong Kong [5], included patients with ‘the clinical presentation of a corneal stromal infiltrate >1 mm2’. This differs from Srinivasan et al [4] who included patients with ‘loss of the corneal epithelium with underlying stromal infiltration and suppuration associated with signs of inflammation with or without hypopyon’. There were variations in methods of culture. For example, one study [21] used Sheep’s blood agar, Chocolate, Non-nutrient, Sarbarouds, brain-heart infusion and potato dextrose agar, whilst another [18] used only Chocolate and Sabourauds media. Some studies did not specify the media used [17, 22, 23]. All studies included bacterial infections, but not all included fungal, protozoal and yeast organisms. The majority of studies looked at all cases of microbial keratitis whilst some looked only at patients requiring hospital admission (Wong et al. and Cheung et al. [3,31]). It is likely that in these studies, particularly virulent organisms will be over-represented. Finally, data are only available from centres that have conducted studies on microbial keratitis, limiting the coverage of certain regions of the world.
Despite these limitations, we have presented to our knowledge, for the first time, a worldwide overview of causative organisms in microbial keratitis demonstrating associations between specific types of microbial keratitis and national income.
Table 2. Proportion of culture-positive patients who tested positively for bacteria by location.
| Location of study | Number of isolates |
||||||
|---|---|---|---|---|---|---|---|
| Gram +ve bacteria |
Gram −ve bacteria |
||||||
| Any | Staphylococci | Streptococci | Other | Any | Pseudomonas | Other | |
| Africa | |||||||
| Ghana | 17 | 4 | 8 | 5 | 21 | 21 | 0 |
| Accra, Ghana | 34 | 18 | 11 | 5 | 29 | 17 | 12 |
|
Indian Subcontinent Nepal, India |
136 | 102 | 31 | 3 | 21 | 18 | 3 |
| Chittagong, Bangladesh | 27 | 2 | 23 | 2 | 39 | 34 | 5 |
| Tamil Nadu | 178 | 63 | 110 | 5 | 57 | 35 | 22 |
| East Bengal | 214 | 174 | 28 | 12 | 84 | 63 | 21 |
| New Delhi | 35 | 28 | 2 | 5 | 17 | 10 | 7 |
| Madurai, S. India | 132 | 26 | 76 | 30 | 35 | 24 | 11 |
| Hyderabad, India | 198 | 92 | 60 | 46 | 45 | 27 | 18 |
| Tamil Nadu, India | 814 | 259 | 492 | 63 | 325 | 236 | 89 |
| Hyderabad, India | 80 | 43 | 27 | 0 | 13 | 6 | 7 |
| Delhi, India | 223 | 200 | 10 | 13 | 152 | 88 | 64 |
| Riyadh, Saudi Arabia | 130 | 75 | 26 | 29 | 45 | 16 | 29 |
| Baghdad, Iraq | 88 | 70 | 15 | 3 | 74 | 68 | 6 |
| South America | |||||||
| Asuncion, Paraquay | 278 | 210 | 42 | 226 | 132 | 46 | 96 |
| Far East | |||||||
| Taiwan | 67 | 21 | 21 | 25 | 120 | 95 | 25 |
| Taipai, Taiwan | 57 | 39 | 12 | 6 | 106 | 56 | 50 |
| Singapore | unspecified | 4 | 4 | unspecified | 41 | 23 | 18 |
| Singapore | 9 | 5 | 4 | 0 | 42 | 23 | 19 |
| Hong Kong | 37 | 9 | 3 | 25 | 42 | 28 | 14 |
| Bangkok | 23 | 11 | 12 | 0 | unspecified | 43 | unspecified |
| Australasia | |||||||
| Auckland, NZ | 75 | 41 | 11 | 13 | 13 | 7 | 6 |
| Christchurch, New Zealand | 45 | 19 | 11 | 15 | 18 | 2 | 16 |
| Adelaide | 89 | 65 | 12 | 12 | 38 | 17 | 21 |
| Victoria, Australia | 72 | 56 | 8 | 8 | 29 | 10 | 19 |
| Brisbane, Australia | 75 | 41 | 11 | 23 | 56 | 44 | 12 |
| North America and Canada | |||||||
| Toronto | 43 | 32 | 10 | 1 | 20 | 7 | 13 |
| Miami | 637 | 278 | 89 | 270 | 664 | 345 | 319 |
| Pittsburgh | 797 | 638 | 115 | 44 | 256 | 71 | 185 |
| LA | 48 | 34 | 9 | 5 | 30 | 13 | 17 |
| Texas | 45 | 25 | 12 | 8 | 32 | 18 | 14 |
| Durham | 314 | 197 | 57 | 78 | 74 | 40 | 34 |
| Europe | |||||||
| Paris | 172 | 116 | 19 | 37 | 35 | 21 | 14 |
| Laussanne, Switzerland | 57 | 45 | 10 | 2 | 18 | 7 | 11 |
| Anatolia, Turkey | 155 | 115 | 35 | 5 | 20 | 15 | 5 |
| Amsterdam and Rotterdam | 46 | 25 | 16 | 5 | 58 | 35 | 23 |
Table 3. Proportion of culture-positive patients who tested positively for protozoa or fungi and yeasts by location.
| Location of study | Number of isolates |
||||
|---|---|---|---|---|---|
| Protozoa |
Fungi and yeasts |
||||
| Any | Any | Aspergillus | Candida | Other | |
| Africa | |||||
| Ghana | 1 | 82 | 19 | 1 | 62 |
| Accra, Ghana | unspecified | 65 | 10 | 1 | 45 |
| Indian Subcontinent | |||||
| Nepal, India | unspecified | 200 | 75 | 9 | 116 |
| Chittagong, Bangladesh | unspecified | 48 | 24 | 1 | 23 |
| Tamil Nadu | 7 | 296 | 76 | 0 | 220 |
| East Bengal | 4 | 623 | 373 | 7 | 243 |
| New Delhi | unspecified | 13 | 6 | 0 | 7 |
| Madurai, S. India | 3 | 155 | 25 | unspecified | 116 |
| Hyderabad, India | unspecified | 146 | 43 | 2 | 101 |
| Tamil Nadu, India | 33 | 1176 | 294 | unspecified | 882 |
| Hyderabad, India | 3 | 22 | 5 | 1 | 16 |
| Delhi, India | 11 | 358 | 149 | 30 | 179 |
| Riyadh, Saudi Arabia | unspecified | unspecified | unspecified | unspecified | unspecified |
| Baghdad, Iraq | unspecified | 74 | 42 | 4 | 28 |
| South America | |||||
| Asuncion, Paraquay | unspecified | 209 | 37 | 4 | 168 |
| Far East | |||||
| Taiwan | 11 | 34 | 5 | 10 | 19 |
| Taipai, Taiwan | unspecified | unspecified | unspecified | unspecified | unspecified |
| Singapore | unspecified | 29 | 5 | 3 | 21 |
| Singapore | unspecified | unspecified | unspecified | unspecified | unspecified |
| Hong Kong | 6 | 5 | unspecified | 1 | 4 |
| Bangkok | 3 | 46 | 9 | 2 | 37 |
| Australasia | |||||
| Auckland, NZ | unspecified | 7 | unspecified | unspecified | unspecified |
| Christchurch,New Zealand | unspecified | unspecified | unspecified | unspecified | Unspecified |
| Adelaide | unspecified | 7 | 3 | 2 | 2 |
| Victoria, Australia | 4 | 7 | unspecified | 1 | 6 |
| Brisbane, Australia | unspecified | 13 | unspecified | unspecified | unspecified |
| North America and Canada | |||||
| Toronto | unspecified | unspecified | unspecified | unspecified | unspecified |
| Miami | unspecified | unspecified | unspecified | unspecified | unspecified |
| Pittsburgh | unspecified | Unspecified | unspecified | unspecified | |
| LA | unspecified | 4 | unspecified | unspecified | 4 |
| Texas | 4 | 8 | unspecified | 3 | unspecified |
| Durham | unspecified | unspecified | unspecified | unspecified | unspecified |
| Europe | |||||
| Paris | unspecified | unspecified | unspecified | unspecified | unspecified |
| Laussanne, Switzerland | unspecified | unspecified | unspecified | unspecified | unspecified |
| Anatolia, Turkey | unspecified | 50 | 10 | 15 | 25 |
| Amsterdam and Rotterdam | unspecified | 2 | 0 | 2 | 0 |
References
- 1.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: A global perspective. Bull World Health Organ. 2001;79:214–21. [PMC free article] [PubMed] [Google Scholar]
- 2.Vajpayee RB, Dada T, Saxena R, et al. Study of the First Contact Management Profile of Cases of Infectious Keratitis: A Hospital-Based Study. Cornea. 2000;19(1):52–56. doi: 10.1097/00003226-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Wong T, Ormonde S, Gamble G, et al. Severe infective keratitis leading to hospital admission in New Zealand. Br J Ophthalmol. 2003;87(9):1103–8. doi: 10.1136/bjo.87.9.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srinivasan M, Gonzales CA, George C, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, south India. Br J Ophthalmol. 1997;81(11):965–71. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam DS, Houang E, Fan DS, et al. Hong Kong Microbial Keratitis Study Group Incidence and risk factors for microbial keratitis in Hong Kong: comparison with Europe and North America. Eye. 2002;16(5):608–18. doi: 10.1038/sj.eye.6700151. [DOI] [PubMed] [Google Scholar]
- 6.Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27(1):22–7. doi: 10.1097/ICO.0b013e318156caf2. [DOI] [PubMed] [Google Scholar]
- 7.Keay L, Edwards K, Naduvilath T, et al. Microbial keratitis predisposing factors and morbidity. Ophthalmology. 2006;113(1):109–16. doi: 10.1016/j.ophtha.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 8. [accessed 21st June 2009]; http://web.worldbank.org/WBSITE/EXTERNAL/DATASTATISTICS/0,,contentMDK:20452009~isCURL:Y~menuPK:64133156~pagePK:64133150~piPK:64133175~theSitePK:239419,00.html.
- 9.Simcock PR, Butcher JM, Armstrong M, Lloyd IC, Tullo AB. Investigation of microbial keratitis: an audit from 1988-1992. Acta Ophthalmol Scand. 1996;74(2):183–6. doi: 10.1111/j.1600-0420.1996.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 10.Basak SK, Basak S, Mohanta A, Bhowmick A. Epidemiological and microbiological diagnosis of suppurative keratitis in Gangetic West Bengal, eastern India. Indian J Ophthalmol. 2005;53(1):17–22. doi: 10.4103/0301-4738.15280. [DOI] [PubMed] [Google Scholar]
- 11.Bourcier T, Thomas F, Borderie V, Chaumeil C, Laroche L. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87(7):834–8. doi: 10.1136/bjo.87.7.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLeod SD, Kolahdouz-Isfahani A, Rostamian K, Flowers CW, Lee PP, McDonnell PJ. The role of smears, cultures, and antibiotic sensitivity testing in the management of suspected infectious keratitis. Ophthalmology. 1996;103(1):23–8. doi: 10.1016/s0161-6420(96)30738-0. [DOI] [PubMed] [Google Scholar]
- 13.Leibovitch I, Lai TF, Senarath L, Hsuan J, Selva D. Eur J Ophthalmol. 2005;15(1):23–6. Infectious keratitis in South Australia: emerging resistance to cephazolin. [PubMed] [Google Scholar]
- 14.Laspina F, Samudio M, Cibils D, Ta CN, Fariña N, Sanabria R, Klauss V, Miño de Kaspar H. Epidemiological characteristics of microbiological results on patients with infectious corneal ulcers: a 13-year survey in Paraguay. Graefes Arch Clin Exp Ophthalmol. 2004;242(3):204–9. doi: 10.1007/s00417-003-0808-4. [DOI] [PubMed] [Google Scholar]
- 15.Sirikul T, Prabriputaloong T, Smathivat A, Chuck RS, Vongthongsri A. Predisposing factors and etiologic diagnosis of ulcerative keratitis. Cornea. 2008;27(3):283–7. doi: 10.1097/ICO.0b013e31815ca0bb. [DOI] [PubMed] [Google Scholar]
- 16.Leck AK, Thomas PA, Hagan M, Kaliamurthy J, Ackuaku E, John M, Newman MJ, Codjoe FS, Opintan JA, Kalavathy CM, Essuman V, Jesudasan CA, Johnson GJ. Aetiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of fungal keratitis. Br J Ophthalmol. 2002;86(11):1211–5. doi: 10.1136/bjo.86.11.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma S, Taneja M, Gupta R, Upponi A, Gopinathan U, Nutheti R, Garg P. Comparison of clinical and microbiological profiles in smear-positive and smear-negative cases of suspected microbial keratitis. Indian J Ophthalmol. 2007;55(1):21–5. doi: 10.4103/0301-4738.29490. [DOI] [PubMed] [Google Scholar]
- 18.Hagan M, Wright E, Newman M, Dolin P, Johnson G. Causes of suppurative keratitis in Ghana. Br J Ophthalmol. 1995;79(11):1024–8. doi: 10.1136/bjo.79.11.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khanal B, Deb M, Panda A, Sethi HS. Laboratory diagnosis in ulcerative keratitis. Ophthalmic Res. 2005;37(3):123–7. doi: 10.1159/000084273. [DOI] [PubMed] [Google Scholar]
- 20.Dunlop AA, Wright ED, Howlader SA, Nazrul I, Husain R, McClellan K, Billson FA. Suppurative corneal ulceration in Bangladesh. A study of 142 cases examining the microbiological diagnosis, clinical and epidemiological features of bacterial and fungal keratitis. Aust N Z J Ophthalmol. 1994;22(2):105–10. doi: 10.1111/j.1442-9071.1994.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 21.Sharma S, Kunimoto DY, Gopinathan U, Athmanathan S, Garg P, Rao GN. Evaluation of corneal scraping smear examination methods in the diagnosis of bacterial and fungal keratitis: a survey of eight years of laboratory experience. Cornea. 2002;21(7):643–7. doi: 10.1097/00003226-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Bharathi MJ, Ramakrishnan R, Meenakshi R, Padmavathy S, Shivakumar C, Srinivasan M. Microbial keratitis in South India: influence of risk factors, climate, and geographical variation. Ophthalmic Epidemiol. 2007;14(2):61–9. doi: 10.1080/09286580601001347. [DOI] [PubMed] [Google Scholar]
- 23.Panda A, Satpathy G, Nayak N, Kumar S, Kumar A. Demographic pattern, predisposing factors and management of ulcerative keratitis: evaluation of one thousand unilateral cases at a tertiary care centre. Clin Experiment Ophthalmol. 2007;35(1):44–50. doi: 10.1111/j.1442-9071.2007.01417.x. [DOI] [PubMed] [Google Scholar]
- 24.Al-Shehri A, Jastaneiah S, Wagoner MD. Changing trends in the clinical course and outcome of bacterial keratitis at King Khaled Eye Specialist Hospital. Int Ophthalmol. 2008 Apr 3; doi: 10.1007/s10792-008-9206-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Al-Shakarchi F. Initial therapy for suppurative microbial keratitis in Iraq. Br J Ophthalmol. 2007 Dec;91(12):1583–7. doi: 10.1136/bjo.2007.123208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fong CF, Tseng CH, Hu FR, Wang IJ, Chen WL, Hou YC. Clinical characteristics of microbial keratitis in a university hospital in Taiwan. Am J Ophthalmol. 2004;137(2):329–36. doi: 10.1016/j.ajo.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Wang AG, Wu CC, Liu JH. Bacterial corneal ulcer: a multivariate study. Ophthalmologica. 1998;212(2):126–32. doi: 10.1159/000027291. [DOI] [PubMed] [Google Scholar]
- 28.Wong TY, Ng TP, Fong KS, Tan DT. Risk factors and clinical outcomes between fungal and bacterial keratitis: a comparative study. CLAO J. 1997;23(4):275–81. [PubMed] [Google Scholar]
- 29.Tan DT, Lee CP, Lim AS. Corneal ulcers in two institutions in Singapore: analysis of causative factors, organisms and antibiotic resistance. Ann Acad Med Singapore. 1995;24(6):823–9. [PubMed] [Google Scholar]
- 30.Hall RC, McKellar MJ. Bacterial keratitis in Christchurch, New Zealand, 1997-2001. Clin Experiment Ophthalmol. 2004;32(5):478–81. doi: 10.1111/j.1442-9071.2004.00867.x. [DOI] [PubMed] [Google Scholar]
- 31.Cheung J, Slomovic AR. Microbial etiology and predisposing factors among patients hospitalized for corneal ulceration. Can J Ophthalmol. 1995;30(5):251–5. [PubMed] [Google Scholar]
- 32.Alexandrakis G, Alfonso EC, Miller D. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology. 2000;107(8):1497–502. doi: 10.1016/s0161-6420(00)00179-2. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein MH, Kowalski RP, Gordon YJ. Emerging fluoroquinolone resistance in bacterial keratitis: a 5-year review. Ophthalmology. 1999;106(7):1313–8. [PubMed] [Google Scholar]
- 34.Pachigolla G, Blomquist P, Cavanagh HD. Microbial keratitis pathogens and antibiotic susceptibilities: a 5-year review of cases at an urban county hospital in north Texas. Eye Contact Lens. 2007;33(1):45–9. doi: 10.1097/01.icl.0000234002.88643.d0. [DOI] [PubMed] [Google Scholar]
- 35.Yeh DL, Stinnett SS, Afshari NA. Analysis of bacterial cultures in infectious keratitis, 1997 to 2004. Am J Ophthalmol. 2006;142(6):1066–8. doi: 10.1016/j.ajo.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 36.Schaefer F, Bruttin O, Zografos L, Guex-Crosier Y. Bacterial keratitis: a prospective clinical and microbiological study. Br J Ophthalmol. 2001;85(7):842–7. doi: 10.1136/bjo.85.7.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yilmaz S, Ozturk I, Maden A. Microbial keratitis in West Anatolia, Turkey: a retrospective review. Int Ophthalmol. 2007;27(4):261–8. doi: 10.1007/s10792-007-9069-2. [DOI] [PubMed] [Google Scholar]
- 38.van der Meulen IJ, van Rooij J, Nieuwendaal CP, Van Cleijnenbreugel H, Geerards AJ, Remeijer L. Age-related risk factors, culture outcomes, and prognosis in patients admitted with infectious keratitis to two Dutch tertiary referral centers. Cornea. 2008 Jun;27(5):539–44. doi: 10.1097/ICO.0b013e318165b200. [DOI] [PubMed] [Google Scholar]
- 39.Cohen EJ, Fulton JC, Hoffman CJ, Rapuano CJ, Laibson PR. Trends in contact lens-associated corneal ulcers. Cornea. 1996;15(6):566–70. [PubMed] [Google Scholar]
- 40.Jeng BH, McLeod SD. Microbial keratitis. Br J Ophthalmol. 2003;87(7):805–6. doi: 10.1136/bjo.87.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varaprasathan G, Miller K, Lietman T, Whitcher JP, Cevallos V, Okumoto M, Margolis TP, Yinghui M, Cunningham ET., Jr. Trends in the etiology of infectious corneal ulcers at the F. I. Proctor Foundation. Cornea. 2004;23(4):360–4. doi: 10.1097/00003226-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Sun X, Deng S, Li R, Wang Z, Luo S, Jin X, Zhang W. Distribution and shifting trends of bacterial keratitis in north China (1989-98) Br J Ophthalmol. 2004;88(2):165–6. doi: 10.1136/bjo.2002.011205. [DOI] [PMC free article] [PubMed] [Google Scholar]