Abstract
Dickkopf (DKK) family proteins are secreted modulators of the Wnt signaling pathway and are capable of regulating the development of many organs and tissues. We previously identified Dkk3 to be a molecule predominantly expressed in the mouse embryonic retina. However, which cell expresses Dkk3 in the developing and mature mouse retina remains to be elucidated. To examine the precise expression of the Dkk3 protein, we generated BAC-Dkk3-EGFP transgenic mice that express EGFP integrated into the Dkk3 gene in a BAC plasmid. Expression analysis using the BAC-Dkk3-EGFP transgenic mice revealed that Dkk3 is expressed in retinal progenitor cells (RPCs) at embryonic stages and in Müller glial cells in the adult retina. Since Müller glial cells may play a potential role in retinal regeneration, BAC-Dkk3-EGFP mice could be useful for retinal regeneration studies.
1. Introduction
In vertebrates, Dickkopf (Dkk) family genes encode four secreted proteins (Dkk1—4) and a unique Dkk3-related protein, Soggy or Dkk-like1 (Dkkl1) [1]. Dkks are potent inhibitors of β-catenin stabilization in the Wnt signaling pathway and suppress the expression of a variety of canonical Wnt/β-catenin signaling target genes [2]. Dkk1, Dkk2, and Dkk4 inhibit the Wnt signaling pathway through both their high-affinity binding to the Wnt coreceptors Lrp-5/6 [3–5] and blockade of the signaling cascade downstream of Wnt receptor Frizzled. In contrast, Dkk3 appears to inhibit the Wnt signaling pathway by directly binding to β-catenin through a complex with a β-transducin repeat-containing protein, βTrCP [6].
We previously reported identifying the Dkk3 gene as a molecule that is predominantly expressed in mouse retinal progenitor cells [7]. Dkk3 expression is observed during vertebrate development in various organs [1, 8–11]. In our previous report, we used in situ hybridization to show that mouse Dkk3 mRNAs were detectable in the entire neuroblastic layer (NBL) of the retina at embryonic stages, and then in the inner nuclear layer (INL) at postnatal stages [7]. Moreover, we generated a BAC-Dkk3-Cre transgenic mouse, which utilizes a bacterial artificial chromosome (BAC) expressing Cre recombinase inserted into the Dkk3 gene locus [7]. This transgenic mouse line is a powerful tool for ablating a gene of interest in mitotic retinal progenitor cells (RPCs) [12–14]. The expression pattern and the possible function of Dkk3 suggest that this molecule exerts a role in proliferation, cell fate determination, and/or maintenance of RPCs during mouse development. In addition, Dkk3 expression continues into the adult stage. However, it is still unclear what types of differentiated cells express Dkk3 in the developing retina.
Here, we report the generation of a BAC-Dkk3-EGFP mouse line, which expresses the EGFP gene under the control of Dkk3 regulatory elements. This mouse enables us to investigate the expression pattern of Dkk3 in the developing retina in detail. In the current study, we found that EGFP is specifically and strongly expressed in RPCs at embryonic stages, and in Müller glial cells and a subset of amacrine cells at mature stages.
2. Materials and Methods
2.1. Construction and Generation of the BAC-Dkk3-EGFP Transgenic Mouse
A BAC clone (clone ID: RP23-12M6) containing the entire Dkk3 gene was purchased from Children's Hospital Oakland Research Institute. According to the NCBI database (available at http://www.ncbi.nlm.nih.gov/), this BAC clone also contained a fragment of ubiquitin-specific peptidase 47 (Usp47), a microtubule-associated monooxygenase called calponin, and a LIM domain-containing gene (Mical2). However, neither the transcription initiation site nor the start codon of Usp47 is included in the BAC clone, but it does contain the 5′ untranslated region of Mical2. Two homology arms (5′ arm, 486 bp; 3′ arm, 453 bp) from exon 1 of the Dkk3 gene were amplified by PCR from the BAC clone DNA and cloned into a T-easy vector (Promega). The PCR primers used were 5′-GGCGCGCCTATGTCGCCTGTCTAG GGACTT-3′ and 5′-CCCGGGGCAAGCTGGATCTGGTCACGACCGG-3′ for the 5′ homology arm and 5′-TTAATTAATTGGGTGAGCGGTGGTCATCGTC-3′ and 5′-GG CCGGCCCAGACTCAATCCCTGCTGGAAACA-3′ for the 3′ homology arm. The two homologous arms were inserted into both sides of the enhanced green fluorescent protein-polyA (EGFP-pA) cassette in the modified pLD53 SCA-Cre-B shuttle vector [15]. The EGFP-pA cassette was introduced into the 5′UTR of the first exon of the Dkk3 gene by homologous recombination (Figure 1(a)) [15]. PCR and sequencing were used to confirm the correct insertion of the EGFP gene into the Dkk3 locus. The entire Dkk3-EGFP transgene was purified by dialysis on a filter (Millipore, VSWP02500) floating in an injection buffer (10 mM Tris, pH 7.5; 0.1 mM EDTA; 100 mM NaCl). The purified construct was injected as circular DNA into the pronuclei of fertilized one-cell eggs of B6C3F1 mice then implanted into pseudopregnant foster mothers (ICR, Japan SLC Inc). All procedures conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the procedures were approved by the Institutional Safety Committee on Recombinant DNA Experiments and the Animal Research Committee of Osaka Bioscience Institute.
Figure 1.
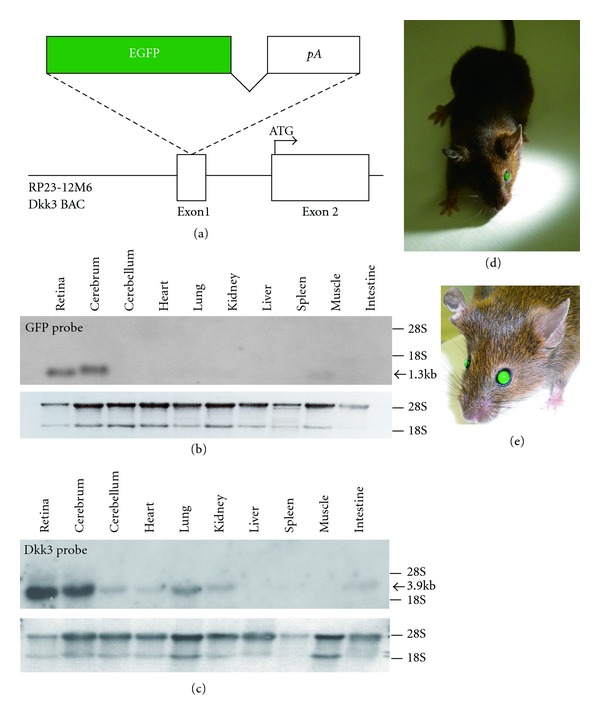
Strategy for preparing the Dkk3-EGFP transgene and Northern hybridization analysis of EGFP expression in adult mouse organs. (a) A schema of the modified BAC integrated with the EGFP-pA cassette into the first exon of the mouse Dkk3 gene. (b) Northern hybridization analysis of total RNA (retina, 10 μg; other tissues, 20 μg) isolated from adult organs in the BAC-Dkk3-EGFP mouse. The hybridization signal was obtained with the EGFP probe. The lower panel shows ethidium bromide staining of the RNA. (c) Northern blot analysis using a Dkk3 probe for wild-type mouse tissues. (d, e) Photographs of the adult BAC-Dkk3-EGFP mouse, which displays “green eyes” even under the room light because of the intense expression of EGFP in the retina.
2.2. Genotyping
PCR genotyping for the Dkk3-EGFP transgene was performed under the following conditions: 94°C for 2 min, then 94°C, 1 min 60°C, 1 min; 72°C, 1 min for a total of 35 cycles and followed by 72°C for 10 min (primers: 5′-CAGACCATACTAGTTTGGCAGTAC-3′ and 5′-GCAGCTTGCCGGTGGTGCAGATGAACT-3′). The primers amplify a fragment of the Dkk3-EGFP transgene.
2.3. Northern Blot Analysis
We extracted total RNAs (retina: 10 μg, other tissues: 20 μg) from adult BAC-Dkk3-EGFP transgenic mice using the TRIzol reagent (Invitrogen). Northern blot hybridization was performed as described previously [7]. A full-length cDNA of EGFP was used as a radiolabeled probe for hybridization.
2.4. Immunostaining
Mouse eye cups were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for immunohistochemistry. The samples were cryoprotected, embedded, frozen, and sectioned into 20 μm thick sections. We preincubated them with blocking solution (4% normal donkey serum and 0.1% Triton X-100 in PBS) for 30 min. Then, we incubated the sections with primary antibodies at 4°C overnight, rinsed them with blocking solution, and incubated them with secondary antibodies for 30 min. The sections were coverslipped with gelvatol after rinsing with 0.1% Triton X-100 in PBS. We used the following primary antibodies: rhodopsin (LSL, LB-5597; rabbit polyclonal; 1 : 10000), S-opsin (Chemicon, AB5407; rabbit polyclonal; 1 : 1000), M-opsin (Oriental Bioservice; rabbit polyclonal; 1 : 1000), S100β (Sigma, S-2532; mouse monoclonal; 1 : 1000), SOX9 (Chemicon, AB5535; rabbit polyclonal; 1 : 750), PAX6 (DSHB, P3U1; mouse monoclonal; 1 : 200), and CHX10 (MBL; rabbit polyclonal; 1 : 100) [16]. We used Cy3-conjugated anti-rabbit IgG (Jackson ImmunoResearch Laboratories, no. 711-165-152; 1 : 400) and anti-mouse IgG (Jackson ImmunoResearch Laboratories, no. 711-165-150; 1 : 400) secondary antibodies. DAPI was applied to stain nuclei (Sigma). We also used rhodamine-labeled Peanut Agglutinin (PNA, Vector Laboratories, RL-1072; 1 : 200). Confocal images were acquired with a Zeiss LSM 700 inverted confocal microscope.
3. Results
3.1. Generation of BAC-Dkk3-EGFP Mouse
To generate a Dkk3 regulatory elements-driven, EGFP-expressing mouse, we took advantage of a bacterial artificial chromosome (BAC) transgenic mouse, carrying BACs modified using homologous recombination in bacteria [15, 17, 18]. We designed a BAC construct with the EGFP cDNA placed in the 5′UTR of the Dkk3 gene by homologous recombination (Figure 1(a)). To verify the tissue-specific EGFP expression, total RNA from the BAC-Dkk3-EGFP mouse retina and other tissues at the adult stage was analyzed by Northern blotting. A single transcript was detected in the retina and cerebrum, but no significant signal was observed in other tissues examined (Figure 1(b)). Dkk3 expression was strongly detected in the retina and cerebrum by Northern blot analysis using wild-type adult mouse tissues (Figure 1(c)). Since the EGFP expression level was extremely high in the retina, we even observed the EGFP expression as “green eyes” under the room light at the adult stage after Mydrin-M ophthalmic solution 0.4% (Santen, no. 084162243) was administrated (Figures 1(d) and 1(e)).
3.2. The EGFP Expression Patterns in the Developing Retina of the BAC-Dkk3-EGFP Mouse
In the BAC-Dkk3-EGFP mouse embryo, we observed an EGFP expression in the eye, heart, and spinal cord at embryonic day 9.5 (E9.5) (Figures 2(a) and 2(b)). An EGFP signal was weakly detected in the palate and heart at E16.5 (Figures 2(c) and 2(d)). In our previous study, Dkk3 mRNA expression was detected in the entire NBL of the embryonic retina and in the INL in the postnatal retina using in situ hybridization [7]. To confirm this result, we investigated EGFP expression in the developing retina of the BAC-Dkk3-EGFP mouse. From E15.5 to postnatal day 0 (P0), EGFP was detected in most mitotic and undifferentiated RPCs throughout the NBL (Figures 2(e)–2(g)). At P3, EGFP expression was limited to RPCs, and differentiated retinal cells were not labeled by EGFP (Figure 2(h)). At P6, EGFP was expressed in Müller glia-like cells, which exhibit long glial fibers and localize their cell bodies in the INL (Figure 2(i)). At P14, only Müller glial cells in the INL-expressed EGFP, and we observed a large number of glial fibers extending into the surrounding retinal layers (Figure 2(j)).
Figure 2.
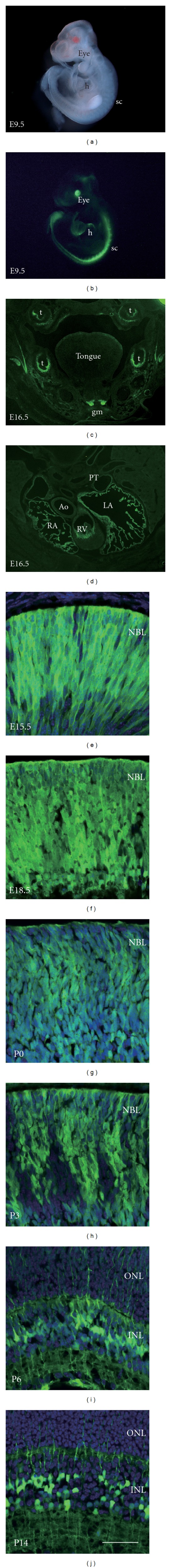
(a, b) Photographs of the BAC-DKK3-EGFP mouse embryo at E9.5. The embryo was visualized under bright-field (a) and fluorescence (b). h, heart; sc, spinal cord. (c) A coronal section through the palatal region of the BAC-Dkk3-EGFP mouse at E16.5. t, tooth; gm, genioglossus muscle. (d) A coronal section through the heart of the BAC-DKK3-EGFP mouse at E16.5. PT, pulmonary trunk; Ao, Aorta; LA, left atrium; RA, right atrium; RV, right ventricle. (e–j) EGFP expression in the BAC-Dkk3-EGFP mouse retina. EGFP expression was detected at E15.5 (e), E18.5 (f), P0 (g), P3 (h), P6 (i), and P14 (j). Nuclei were counterstained with DAPI. NBL, neuroblastic layer; ONL, outer nuclear layer; INL, inner nuclear layer. Scale bar represents 50 μm.
3.3. Cell Types Expressing EGFP in the Adult BAC-Dkk3-EGFP Mouse Retina
It remains to be clarified which cell types express Dkk3 in the adult mouse retina. To examine cell types expressing EGFP, we performed immunostaining using antibodies against several photoreceptor cell-specific markers (Figure 3). Using antibodies against rhodopsin (rod photoreceptors), PNA, S-opsin, and M-opsin (cone photoreceptors), we did not detect an EGFP signal significantly overlapping with any photoreceptor cell-specific markers (Figures 3(a)–3(l)). Then, we immunostained the adult retina with another set of retinal cell-type antibodies, including antibodies against S100β, SOX9 (Müller glial cells), PAX6 (amacrine cells), and CHX10 (bipolar cells) (Figure 4). We observed an EGFP signal overlapping with that of S100β in the Müller glial fibers extending into other retinal layers (Figures 4(a)–4(c)). Moreover, cell bodies of the Müller glial cells immunostained with SOX9 antibody were positive for EGFP (Figures 4(d)–4(f)). We also found that a small number of amacrine cells express EGFP in the INL (Figures 4(g)–4(i)). In contrast, bipolar cells were easily distinguished from EGFP-expressing cells (Figures 4(j)–4(l)).
Figure 3.

(a–l) Immunostaining of the retina of the BAC-Dkk3-EGFP mouse with antibodies against photoreceptor cell-specific markers. Immunostaining with photoreceptor cell-specific markers was performed with primary antibodies against rhodopsin (rod photoreceptors; (a–c)), PNA (cone photoreceptors; (d–f)), S-opsin (cone photoreceptors; (g–i)), and M-opsin (cone photoreceptors; (j–l)). Nuclei were counterstained with DAPI. Scale bar represents 50 μm.
Figure 4.

(a–l) Immunostaining on the retina of the BAC-Dkk3-EGFP mouse with antibodies against retinal cell type-specific markers. Immunostaining of retinal cell-type-specific markers was performed with primary antibodies against S100β (Müller glia; (a–c)), SOX9 (Müller glia; (d–f)), PAX6 (amacrine cells; (g–i)), and CHX10 (bipolar cells; (j–l)). Nuclei were counterstained with DAPI. Scale bar represents 50 μm.
4. Discussion
Dkk3 was reported to be expressed in RPCs at embryonic stages and INL cells at postnatal stages, as detected by in situ hybridization [7, 10]. However, the precise identification of the cell types expressing Dkk3 remains to be elucidated. In the current study, we generated a BAC-Dkk3-EGFP transgenic mouse in which Dkk3-expressing cells were labeled by EGFP and easily distinguished from Dkk3-nonexpressing cells (Figures 3 and 4). We showed that almost all RPCs in embryonic retinas and the Müller glial cells in postnatal retinas were labeled by EGFP (Figures 2, 3, and 4), indicating that the BAC-Dkk3-EGFP mouse is a useful tool to specifically identify the Müller glial cells in vivo.
Recently, growing evidence suggests that the Müller glial cells appear to contain a similar potential to RPCs in cases of acute injury to the retina [19, 20]. In response to excitotoxic damage elicited by N-methyl-D-aspartate (NMDA), a large number of Müller glial cells undergo dedifferentiation, reentering the cell cycle, and upregulating transcription factors expressed in RPCs including Pax6, Chx10 [21], Six3 [22], Sox2 [23, 24], and Sox9 [23, 25]. These reports suggest that the Müller glial cells are a potential source of neurons in retinal regeneration in the adult retina [21]. Thus, further studies of the Müller glial cells may lead to development of new therapies for retinal damage. The BAC-Dkk3-EGFP mouse may give us clues to address the molecular and cellular nature of the Müller glial cells.
Abnormal overactivation of Wnt signaling is a major feature of various human cancers. A large number of studies have focused on the Wnt signaling pathway to develop a novel cancer therapy [26–28]. Notably, Dkk3 has been identified as a downregulated gene in many human cancer cell lines [29, 30]. Moreover, Dkk3 exhibits an ability to suppress cancer cell growth due to its inhibitory function in the Wnt signaling pathway, suggesting that Dkk3 can be a potential clinical target in cancer therapies [2, 30]. The BAC-Dkk3-EGFP mouse may thus be useful for the development of innovative cancer therapies as well as that in the Müller glial cell studies, because Dkk3 expression can be easily detected by the intense EGFP signals.
5. Conclusions
In the current study, we generated the BAC-Dkk3-EGFP transgenic mouse in which Dkk3-expressing cells were strongly labeled by EGFP. By analyzing the developing retina of this mouse, we showed that almost all RPCs express EGFP at embryonic stages and the Müller glial cells at postnatal stages. Because of the important function of the Müller glial cells in retinal regeneration, the BAC-Dkk3-EGFP mouse is a valuable tool for developing regenerative therapies of the retina. Furthermore, since Dkk3 is an attractive tool for anticancer drug development, the BAC-Dkk3-EGFP mouse may contribute to the advancement of novel cancer therapies.
Acknowledgments
This work was supported by CREST and PRESTO from Japan Science and Technology Agency, a grant for Molecular Brain Science, Grants-in-Aid for Scientific Research on Priority Areas, Grant-in-Aid for Scientific Research (B), Young Scientists (B), Specially Designated Research Promotion and Scientific Research on Innovative Areas “Intracellular Logistics” from the Ministry of Education, Culture, Sports and Technology of Japan, The Takeda Science Foundation, The Uehara Memorial Foundation, Novartis Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, The Naito Foundation, Senri Life Science Foundation, Kato Memorial Bioscience Foundation, Daiichi-Sankyo Foundation of Life Science, Japanese Retinitis Pigmentosa Society Foundation, and Research Foundation for Opto-Science and Technology. The authors thank A. Tani, M. Kadowaki, T. Tsujii, A. Ishimaru, Y. Saioka, H. Abe, and S. Kennedy for technical assistance.
References
- 1.Krupnik VE, Sharp JD, Jiang C, et al. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999;238(2):301–313. doi: 10.1016/s0378-1119(99)00365-0. [DOI] [PubMed] [Google Scholar]
- 2.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25(57):7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 3.Semënov MV, Tamai K, Brott BK, Kühl M, Sokol S, He XX. Head inducer dickkopf-1 is a ligand for Wnt coreceptor LRP6. Current Biology. 2001;11(12):951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 4.Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nature Cell Biology. 2001;3(7):683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- 5.Mao B, Wu W, Li Y, et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411(6835):321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 6.Lee EJ, Jo M, Rho SB, et al. Dkk3, downregulated in cervical cancer, functions as a negative regulator of β-catenin. International Journal of Cancer. 2009;124(2):287–297. doi: 10.1002/ijc.23913. [DOI] [PubMed] [Google Scholar]
- 7.Sato S, Inoue T, Terada K, et al. Dkk3-Cre BAC transgenic mouse line: a tool for highly efficient gene deletion in retinal progenitor cells. Genesis. 2007;45(8):502–507. doi: 10.1002/dvg.20318. [DOI] [PubMed] [Google Scholar]
- 8.Ang SJ, Stump RJW, Lovicu FJ, McAvoy JW. Spatial and temporal expression of Wnt and Dickkopf genes during murine lens development. Gene Expression Patterns. 2004;4(3):289–295. doi: 10.1016/j.modgep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Diep DB, Hoen N, Backman M, MacHon O, Krauss S. Characterisation of the Wnt antagonists and their response to conditionally activated Wnt signalling in the developing mouse forebrain. Developmental Brain Research. 2004;153(2):261–270. doi: 10.1016/j.devbrainres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Hackam AS, Strom R, Liu D, et al. Identification of gene expression changes associated with the progression of retinal degeneration in the rd1 mouse. Investigative Ophthalmology and Visual Science. 2004;45(9):2929–2942. doi: 10.1167/iovs.03-1184. [DOI] [PubMed] [Google Scholar]
- 11.Monaghan AP, Kioschis P, Wu W, et al. Dickkopf genes are co-ordinately expressed in mesodermal lineages. Mechanisms of Development. 1999;87(1-2):45–56. doi: 10.1016/s0925-4773(99)00138-0. [DOI] [PubMed] [Google Scholar]
- 12.Katoh K, Omori Y, Onishi A, Sato S, Kondo M, Furukawa T. Blimp1 suppresses Chx10 expression in differentiating retinal photoreceptor precursors to ensure proper photoreceptor development. Journal of Neuroscience. 2010;30(19):6515–6526. doi: 10.1523/JNEUROSCI.0771-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iida A, Shinoe T, Baba Y, Mano H, Watanabe S. Dicer plays essential roles for retinal development by regulation of survival and differentiation. Investigative Ophthalmology and Visual Science. 2011;52(6):3008–3017. doi: 10.1167/iovs.10-6428. [DOI] [PubMed] [Google Scholar]
- 14.Ogata-Iwao M, Inatani M, Iwao K, et al. Heparan sulfate regulates intraretinal axon pathfinding by retinal ganglion cells. Investigative Ophthalmology & Visual Science. 2011;52(9):6671–6679. doi: 10.1167/iovs.11-7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang XW, Model P, Heintz N. Homologous recombination based modification in Esherichia coli and germline transmission in transgenic mice of a bacterial artificial chromsome. Nature Biotechnology. 1997;15(9):859–865. doi: 10.1038/nbt0997-859. [DOI] [PubMed] [Google Scholar]
- 16.Nishida A, Furukawa A, Koike C, et al. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nature Neuroscience. 2003;6(12):1255–1263. doi: 10.1038/nn1155. [DOI] [PubMed] [Google Scholar]
- 17.Gong S, Zheng C, Doughty ML, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425(6961):917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 18.Heintz N. BAC to the future: the use of BAC Transgenic mice for neuroscience research. Nature Reviews Neuroscience. 2001;2(12):861–870. doi: 10.1038/35104049. [DOI] [PubMed] [Google Scholar]
- 19.Jadhav AP, Roesch K, Cepko CL. Development and neurogenic potential of Müller glial cells in the vertebrate retina. Progress in Retinal and Eye Research. 2009;28(4):249–262. doi: 10.1016/j.preteyeres.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer AJ, Bongini R. Turning Müller glia into neural progenitors in the retina. Molecular Neurobiology. 2010;42(3):199–209. doi: 10.1007/s12035-010-8152-2. [DOI] [PubMed] [Google Scholar]
- 21.Fischer AJ, Reh TA. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nature Neuroscience. 2001;4(3):247–252. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- 22.Fischer AJ. Neural regeneration in the chick retina. Progress in Retinal and Eye Research. 2005;24(2):161–182. doi: 10.1016/j.preteyeres.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Fischer AJ, Scott MA, Tuten W. Mitogen-activated protein kinase-signaling stimulates Müller glia to proliferate in acutely damaged chicken retina. Glia. 2009;57(2):166–181. doi: 10.1002/glia.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes S, Nelson BR, Buckingham B, Reh TA. Notch signaling regulates regeneration in the avian retina. Developmental Biology. 2007;312(1):300–311. doi: 10.1016/j.ydbio.2007.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer AJ, Scott MA, Ritchey ER, Sherwood P. Mitogen-activated protein kinase-signaling regulates the ability of Müller glia to proliferate and protect retinal neurons against excitotoxicity. Glia. 2009;57(14):1538–1552. doi: 10.1002/glia.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao H, Ashihara E, Maekawa T. Targeting the Wnt/β-catenin signaling pathway in human cancers. Expert Opinion on Therapeutic Targets. 2011;15(7):873–887. doi: 10.1517/14728222.2011.577418. [DOI] [PubMed] [Google Scholar]
- 27.Prosperi JR, Goss KH. A Wnt-ow of opportunity: targeting the Wnt/β-catenin pathway in breast cancer. Current Drug Targets. 2010;11(9):1074–1088. doi: 10.2174/138945010792006780. [DOI] [PubMed] [Google Scholar]
- 28.Herbst A, Kolligs FT. Wnt signaling as a therapeutic target for cancer. Methods in Molecular Biology. 2007;361:63–91. doi: 10.1385/1-59745-208-4:63. [DOI] [PubMed] [Google Scholar]
- 29.Tsuji T, Miyazaki M, Sakaguchi M, Inoue Y, Namba M. A REIC gene shows down-regulation in human immortalized cells and human tumor-derived cell lines. Biochemical and Biophysical Research Communications. 2000;268(1):20–24. doi: 10.1006/bbrc.1999.2067. [DOI] [PubMed] [Google Scholar]
- 30.Veeck J, Dahl E. Targeting the Wnt pathway in cancer: the emerging role of Dickkopf-3. Biochimica et Biophysica Acta. 2012;1825(1):18–28. doi: 10.1016/j.bbcan.2011.09.003. [DOI] [PubMed] [Google Scholar]


