Abstract
All phenotypes result from interactions between Nature, Nurture and Chance. The constitutional genome is clearly the dominant factor in explaining the striking differences in the pace and patterns of ageing among species. We are now in a position to reveal salient features underlying these differential modulations, which are likely to be dominated by regulatory domains. By contrast, I shall argue that stochastic events are the major players underlying the surprisingly large intra-specific variations in lifespan and healthspan. I shall review well established as well as more speculative categories of chance events – somatic mutations, protein synthesis error catastrophe and variegations of gene expression (epigenetic drift), with special emphasis upon the latter. I shall argue that stochastic drifts in variegated gene expression are the major contributors to intra-specific differences in the pace and patterns of ageing within members of the same species. They may be responsible for the quasi-stochastic distributions of major types of geriatric pathologies, including the “big three” of Alzheimer's disease, atherosclerosis and, via the induction of hyperplasis, cancer. They may be responsible for altered stoichiometries of heteromultimeric mitochondrial complexes, potentially leading to such disorders as sarcopenia, nonischemic cardiomyopathy and Parkinson's disease.
Keywords: Somatic mutation, Epigenetic drift, Stochastic events, Evolutionary biology, Mitochondria, Geriatric pathology
1. The relative contributions of Nature, Nurture and Chance to inter-specific differences in the pace and patterns of ageing
The dramatic differences in maximum lifespan potentials among species clearly points to a dominant role for the constitutional genome. It is likely that the keys to understanding these differences lies in the “dark matter” of the non-coding regions of DNA, those that determine the regulation of gene expression; the proteins of closely related organisms with different healthspans and lifespans are extremely similar and unlikely to be the principle determinants of most phenotypic differences (King and Wilson, 1975). Given the spectacular advances in genomics, there are now great opportunities for comparative gerontologic studies aimed at identifying relevant DNA domains that help explain differences in healthspans and lifespans among closely related species. For a model of what can be done by comparative gerontologists, see (Pollard, 2009; Pollard et al., 2006).
The best studied environmental intervention for the extension of lifespan is dietary restriction, but these gains are modest in comparison with what evolution has achieved. Moreover, it is now clear that, even for the case of dietary restriction, the background genome is profoundly important (Liao et al., 2010). This is not to denigrate the role of environmental factors in the modulations of both inter-specific and intra-specific variations in the pace and patterns of ageing. For example, the role of environmental neurotoxins in the pathogenesis of neurodegenerative disorders deserves much more study. The thesis of this essay, however, is that stochastic events may be the major factors in the determination of intra-specific variations in healthspan and lifespan. In other words, while Nature (the constitutional genome) and Nurture (the environment) play important roles in determining one's health, they may be trumped, in many instances, by Chance. This hypothesis is summarized in Figures 1 and 2. The reader is also directed to early pioneering research on this subject(Finch and Kirkwood, 2000) (Rea et al., 2005).
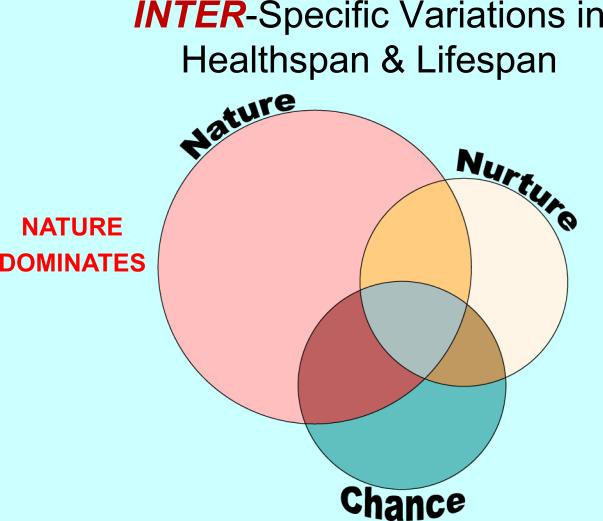
Figure1.
A Venn diagram illustrating the relative contributions of Nature (the constitutional genome), Nurture (the environment) and Chance (stochastic events) in the determination of healthspans and lifespans as they impact upon differences among species exhibiting contrasting healthspans and lifespans. The dominant contributor is Nature.
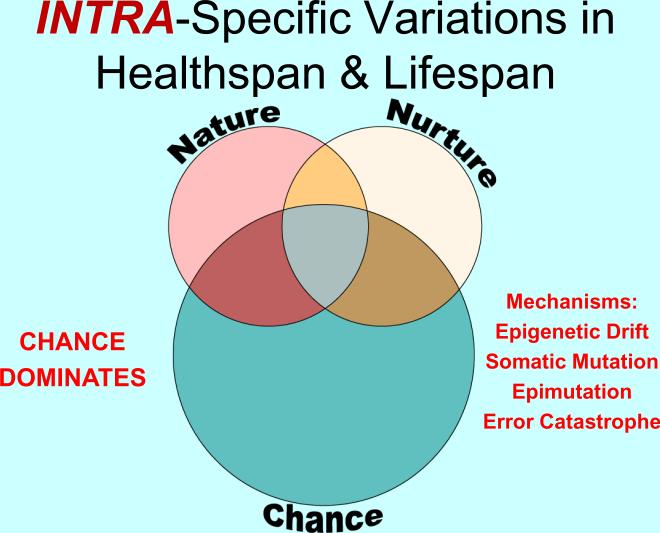
Figure 2.
A Venn diagram illustrating the relative contributions of Nature (the constitutional genome), Nurture (the environment) and Chance (stochastic events) in the determination of healthspans and lifespans as they impact upon differences among individual members of a species exhibiting contrasting healthspans and lifespans. The author proposes that the dominant contributor is Chance.
2. Somatic mutation, epimutation, protein synthesis error catastrophe and epigenetic drift: categories of stochastic enhancements of biological ageing and geriatric disease
Genomic instability, leading to chromosomal and point mutations, is increasingly accepted as a major mechanism of biological ageing and most certainly is a key to the origins of the many cancers whose rates of development appear to track with the lifespan potentials among mammalian species (see, e.g., (Albert et al., 1994). Constitutional epimutations are also of significance in the determination of healthspan and lifespan, but have only recently begun to be explored. Leslie Orgel's protein synthesis error catastrophe invoked random transcriptional or translational errors in the synthesis of proteins that were themselves involved in the synthesis of proteins (Orgel, 1963). That theory has gone out of favor, but I shall argue that it may have been given a premature death certificate. Epigenetic drift is a relatively new idea and will be examined in somewhat more detail. In particular, it will be argued that such drifts in gene expression may be responsible for what can be termed the quasi-stochastic distributions of lesions in a wide range of geriatric pathologies, including Alzheimer's disease, atherosclerosis, benign prostatic hyperplasia, benign and malignant neoplasms, osteoarthritis, sarcopenia, non-ischemic myocardiopathy and Parkinson's disease.
3. Somatic mutation
A concise review of the early history of research on somatic mutation and ageing has been published (Martin, 1996). Sir McFarlane Burnet summarized the evidence for the somatic mutational theory of ageing in an influential monograph (Burnet, 1974). Our lab contributed to the support of this theory via both clinical and experimental studies. A systematic screening of known genetic mutations for senescent phenotypes revealed strong associations with disorders characterized by genomic instability (Martin, 1978, 2005b). Particularly striking was the Werner syndrome. Somatic cells from such patients were shown to have very high rates of somatic mutations, particularly large deletions (Fukuchi et al., 1989). The Werner syndrome was later shown to be caused by homozygous null mutations at a member (WRN) of the RecQ family of helicases (Yu et al., 1996). Wil Bohr, the co-organizer of our symposium, has published extensively on the biochemistry of this helicase, most recently having shown the independent role of deficiencies of this gene produce upon the frequencies of base substitutions (Bacolla et al., 2011).
A series of important contributions has also been provided by the laboratory of Jan Vijg, most recently summarized, in part, by the following quotation from a 2009 review: “Hence, it is conceivable that DNA damage is at the very beginning in the chain of events leading to ageing and eventually death. In organisms with renewable tissues, a plausible scenario is that mutations/epimutations accumulate in stem cells over time thereby exhausting regenerative capacity.”
4. The protein synthesis error catastrophe theory of ageing
In 1970, Orgel provided a correction to his 1963 paper, in which he pointed out that a protein synthesis error catastrophe was not inevitable (Orgel, 1970). Subsequent assessments arguing against the idea were published (Baird et al., 1975). These and other criticisms led to the theory having been “pronounced dead” at a meeting of gerontologists (cited in (Martin and Bressler, 2000). Much of the experimental evidence against the theory, however, has come from cell culture studies and experiments with bacteria. In vivo studies have been rarely attempted. One from our lab, while negative, did not disprove the theory (Rabinovitch and Martin, 1982). We have argued elsewhere that a re-examination of this idea should be considered, particularly from the point of view of transcriptional infidelity (Martin and Bressler, 2000). It should also be apparent that these synthetic infidelities can lead to somatic mutations.
5. Epigenetic drift
The first discussion of what is now known as epigenetic drift may have appeared in a 1984 paper implicating an epigenetic mechanism, via altered DNA methylation, in tumor progression; the term “phenotypic drift” was employed (Kerbel et al., 1984). Epigenetic drifts of gene expression for certain human loci were later well documented by Stephen Baylin, Jean-Pierre Issa and colleagues (e.g., (Issa, 2003; Issa et al., 1994; Issa et al., 1996; Kwabi-Addo et al., 2007; Post et al., 1999). This was followed by studies of ageing monozygotic twins by Manel Esteller, Mario Fraga and their colleagues (Fraga et al., 2005; Martin, 2005a). In 2009, I proposed (Martin, 2009) that epigenetic drift during ageing might be regarded as an antagonistic pleiotropic mechanism of ageing (Williams, 1957). The basic hypothesis was that evolution selected for random variations in gene expression long before meiosis was invented as a means to produce adaptive variegation in gene expression among cells, tissues and organisms and that the degree of such variegation might vary as a function of the degree of uncertainty in the environments in which a species evolves. Consider, for example, a population of organisms subjected to a powerful neurotoxin or hepatotoxin. A genetic scheme of regulation that ensured that all of the susceptible neurons or hepatocytes were “in lock step” with regards to their gene expressions would seem dangerous. A better scheme might be an appropriate degree of “bet-hedging” or “epigenetic gambling” in gene expression, thus ensuring that the right combination of polygenic expressions would be available to ensure the survival of a critical number of cells and a critical number of organisms in order to continue reproduction and the survival of the species. It was further envisioned that, once such a process is activated, it can lead to an increasing degree of epigenetic drift, causing multiple types of pathophysiology and contributing to intrinsic biological ageing. This would be consistent with the classical evolutionary theory of ageing, as the force of natural selection declines post-reproductively (Hamilton, 1966).
6. The implications of epigenetic drift for geriatric pathologies
Since the founding of cellular pathology in the 19th century by Robert Remak and Rudolph Virchow (Lagunoff, 2002), pathologists have looked for aberrations in individual cells as the basis of many types of pathologies. Except for the case of neoplasms, it is not generally recognized that, among families of genetically identical homologous cell types, only subsets of these create the diagnostic lesions. While it is the case that individual pathologies have a propensity to involve specific anatomic domains, there appear to be random involvements of individual sister cells within those domains. One can therefore refer to this process as being “quasi-stochastic”. Ageing humans (and other mammals) exhibit a large variety of diseases that can be characterized as quasi-stochastic in their distributions. In this brief essay, I shall review three of the most important of these, Alzheimer's disease, atherosclerosis, and neoplasia, but will also briefly allude to other examples worthy of research in this context.
7. Alzheimer's disease
Like so many neurodegenerative diseases of ageing, Alzheimer's disease targets particular neuroanatomical domains. In this case, they are primarily structures within the medial temporal cortex, particularly the hippocampus and entorhinal cortex (see, e.g., (Kerchner et al., 2010; Stranahan and Mattson, 2010). As can be seen in every textbook of neuropathology, the diagnostic neuritic plaques and neruofibrillary tangles, however, do not involve every neuron within those structures. Many regions within those domains can be spared of these diagnostic pathologies. Similar observations can be made for the vascular depositions of beta amyloid (see, e.g., (Love et al., 2009). Structures like the cerebellar cortex may also be impacted by the disease, usually late in its course. Our lab, for example, has noted apparently random distributions of diffuse deposits of beta amyloid in the cerebellar cortex (Figures 4 B and C) (Hu et al., 2000). More recent immunocytochemical studies have documented apparently random distributions of altered expressions of cell cycle markers with the cerebellar dentate nuclei (Chen et al., 2010a). Taken together, these various observations suggest the possibility that epigenetic drifts of gene products of relevance to the pathogenesis of Alzheimer's disease underlie the quasi-stochastic distributions of the lesions. Those relevant gene products are likely to include the several secretases and associated proteins that determine how APP is processed (Lichtenthaler et al., 2011; Thathiah and De Strooper, 2011) and enzymes that degrade beta amyloid (Chen et al., 2010b). We remain ignorant, however, of the relevant gene products whose actions are likely to precede those responsible for the differential metabolism of APP. APP metabolism may be a reaction to a variety of different types of injury. As such, a more heuristic nomenclature may be Dementias of the Alzheimer Type (DAT) (Martin, 2005b).
8. Atherosclerosis
Multifocal atheromas or atheromatous plaques are the canonical lesions of atherosclerosis. These raised, lipid-rich, fibrotic and discrete lesions are found in middle sized and large arteries. They have been shown to steadily increase in numbers as functions of age in all population groups so far evaluated, although with different kinetics (Eggen and Solberg, 1968). Their impacts upon geriatric mortality are particularly profound within the coronary arteries (leading to myocardial infarctions), the cerebral arteries (leading to strokes) and the aorta (leading to ruptured aneurysms). As for the case of the neuritic plaques of DAT, there are regional propensities even within one anatomical domain. For example, there are more atheromas in the abdominal aorta than in the thoracic aorta (Eggen and Solberg, 1968). Hemodyamic stress is thought to increase the likelihood that an atheroma will develop at a particular site (Pyle and Young, 2010), but the distribution within the aorta otherwise appears to be random. The late Earl P. Benditt took literally the nomenclature of atheroma (“oma” means “tumor”) and sought evidence that these were indeed benign tumors, arising from either a somatic mutation or a viral transformational event. Evidence that they had a monoclonal origin came from the findings that, in females who were heterozygous for a polymorphism at the X-linked G6PD locus, the individual atheromas were either type A or B (Benditt and Benditt, 1973). A monoclonal outcome for the proliferation of normal diploid somatic cells, however, could be the consequence of a process of clonal attenuation and selection rather than mutation (Martin et al., 1974). Progressive age-related drifts in gene expression remains an alternative hypothesis for the stochastic distributions of the lesions, at least for one or more steps in what is clearly a complex set of pathogenetic mechanisms, certainly including inflammatory and proliferative components (Woollard and Geissmann, 2010). An epigenetic process has recently been suggested for atherogenesis in the context of late effects of ionizing radiation (Baverstock and Karotki, 2011).
9. Neoplasia
The age-specific incidences of a wide variety of benign and malignant neoplasms increase as functions of age. Particularly robust evidence that cancers are strongly coupled to the biology of ageing comes from comparative gerontological studies showing kinetics that are proportional to lifespan (see, e.g., (Albert et al. 1994). Malignant neoplasms are characterized by large numbers of somatic mutations (http://www.sanger.ac.uk/genetics/CGP/cosmic/). As Larry Loeb has pointed out, key events in the pathogenesis appear to be mutations at loci that result in a great acceleration of the flux of somatic mutations – i.e., the emergence of mutator strains (Loeb, 2010; Loeb et al., 1974). Epimutations, including constitutional epimutations (Hitchins, 2010) also play important roles in the pathogenesis of cancer. Many neutral mutations have been recently documented in the non-cancerous tissues surrounding a neoplasm (Salk et al., 2009). I suggest that there is an even earlier stage in the somatic evolution of neoplasia, one that may in fact be the very first step in the pathogenesis of the common carcinomas of ageing. This first step may be related to epigenetic drifts in the gene expressions of loci that determine whether or not a cell exits the G0 stage of the cell cycle. The loss of proliferative homeostasis is a canonical phenotype of ageing mammalian tissues (Martin, 1979, 2007). This results in both atrophy and hyperplasia, often seen side by side. Physiological homeostasis presumably regulates the cell cycle behavior of various subsets of stem cells. The genesis of senescent atrophies and hyperplasias can be presumed to be related to aberrant stem cell behavior, perhaps driven by epigenetic drifts of relevant control loci. Such a scenario can explain the quasi-stochastic distributions of neoplasms. This is a testable hypothesis in that one would predict enhanced degrees of variegated gene expressions within a “field” of tissue surrounding the emerging or emerged neoplasm. A good example of a wide range of molecular markers that have been shown to be altered in hyperplasias associated with ongogenesis is given in a review of endometrial hyperplasias (Steinbakk et al., 2011). Many of these markers could be utilized for the determination of the degrees of variegation of gene expressions in normal tissues and tissues that juxtapose a range of neoplasms. Such neighboring tissues, according to the hypothesis of epigenetic drift, are predicted to exhibit enhanced variegation associated with markers of hyperplasia. Suitable methods would include quantitative immunofluorescent analysis of proteins or in-situ hybridizations and quantitative PCR for the quantitation of RNA species for single cell; the latter has been successfully used to demonstrate enhanced cell to cell variations of many RNA species among isolated myocardial cells from old mice (Bahar et al., 2006).
10. A few other geriatric pathologies that may be driven by epigenetic drifts of gene expression
The arguments above could readily be used for the case of benign prostatic hyperplasia (BPH), a disorder of proliferative homeostasis that involves both glandular and stromal tissue. Both types of lesions are quasi-stochastic in their distributions within the prostate. There are a number of pathogenetic factors, including inflammatory factors and hormonal growth factors, plus their inhibitors, their receptors and downstream signal transduction pathways (Rick et al., 2011). There are therefore many opportunities for mischief related to increasing degrees of variegated gene expression. Another extremely common geriatric disorder with quasi-stochasic distributions of its lesions is osteoarthritis, the pathology of which is characterized by atrophy of joint cartilage, but hyperplasia of nearby osteoblasts, resulting in variable sizes and distributions of Heberden's nodes (Irlenbusch and Dominick, 2006).
One can imagine that the maintenance of mitochondrial homeostasis is particularly vulnerable to epigenetic drifts in gene expression, as various electron transport complexes are heteromultimers consisting of both nuclear and mitochondrial gene products, thus perhaps posing special difficulties for assembly for proper stoichiometries. In order to assemble a properly functioning cytochrome oxidase complex, for example, a mitochondrial organelle needs to put together three sub-units coded by the mitochondrial genome with thirteen sub-units coded by the nuclear genome (Tsukihara et al., 1996). It is conceivable that at least three quite different and important geriatric disorders are driven by such a molecular mitochondrial dyshomeostasis. There has long been evidence that Parkinson's disease is driven by aberrant mitochondrial function (for a recent review, see (Swerdlow, 2011). Only a particular domain of the substantia nigra is impacted by this disease and only sub-sets of neurons are involved, thus qualifying as being quasi-stochastic in the distribution of its lesions. It takes many years of ageing to finally reach a phenotypic threshold. Probably the best evidence of a multifocal involvement of skeletal muscle atrophy and mitochondrial aberrations in age-associated sarcopenia comes from studies in mice (Wanagat et al., 2001). It is a leading theory for the pathogenesis of human sarcopenia (reviewed by (Parise and De Lisio, 2010). Human nonischemic cardiomyopathy is less well studied from the point of view of mitochondrial dysfunction, but there are publications consistent with an important role for such dysfunction in its pathogenesis (see, e.g., (Quigley et al., 2000). In my opinion, given demographic projections for substantial increases in the proportion of older people in the developed and developing societies, coupled with improved interventions for ischemic heart disease, nonischemic congestive heart failure of the elderly will become among the major causes for hospital admissions. An important mouse model of this disorder has been described in ageing cohorts of mice; these studies strongly implicate mitochondrial dysfunction in the pathogenesis (Dai et al., 2009; Schriner et al., 2005). While mutations in mitochondria occur in all of the conditions described above, it is quite possible that, as for the case suggested for cancer, they are secondary to epigenetic drifts in gene expression, perhaps driven both by reactive oxygen species and by compensatory mitochondrial DNA replication.
11. Perspectives and Conclusions
Figures 1 and 2 provide concise summaries of the author's general views on the relative impacts of Nature, Nature and Chance on the pace and patterns of ageing as they occur among a group of species or among a group of individuals within a species. Medical science and practice is of course mainly devoted to the latter. Almost all clinicians and pathologists who see old patients would agree that no two patients age in exactly the same way, including identical twins. Most would likely assume, however, that the differences they see between twins are likely attributable to variations in environmental factors (Fraga et al., 2005). We have suggested, however, that while such perturbations are undoubtedly important, perhaps chance factors are even more important (Martin, 2005a, 2009). These chance factors include somatic mutations and epimutations and, more controversially, stochastic variegations in gene expression that undergo wider and wider excursions during ageing, eventually setting the stage for a diverse set of geriatric disorders, including major causes of death and disability. While highly speculative, these are testable hypotheses, particularly given advances in single cell analysis such as qPCR and semi-quantitative immunocytochemistry. Finally, I have suggested that our community should consider re-examining the corpse of yet another stochastic theory of ageing–Leslie Orgel's protein synthesis catastrophe theory.
Highlights
The constitutional genome (Nature) dominates over the environment (Nurture) and Chance (stochastic events) in the determination of the striking differences in healthspans and lifespans observed among different species. The key changes are likely to involve loci that regulate gene expression.
Stochastic events (Chance events) are thought to be more important than Nature or Nurture in the determination of the differences in healthspans and lifespans observed among members of the same species
Somatic mutations are well documented as one type of stochastic event that makes a difference in the pace and patterns of ageing among individuals within a species
Errors in the synthesis of proteins that are themselves utilized for the synthesis of other proteins is another potentially important type of chance event. This mechanism (the Orgel hypothesis) has been widely thought not to be a significant contributor to ageing, but the author suggests that additional research in this area is warranted
The author proposes that the most significant contributors to the differences in the pace and patterns of ageing among individuals within a species are random fluctuations in gene expression (epigenetic drifts). Different degrees of such fluctuations are thought to have evolved to reflect different ecologies during speciation. Ecologies characterized by particularly uncertain environmental fluctuations are thought to have selected for greater initial degrees of epigenetic drift as adaptive gene actions.
During ageing, variegations in gene expression are thought to become more extreme, leading to pathophysiological features of senescence.
The increasing degrees of epigenetic drifts during ageing are thought to be the initial pathogenetic events in the genesis of many different geriatric pathologies characterized by quasi-stochastic distributions of lesions.
Geriatric disorders having quasi-stochastic distributions of lesions and thought to be initiated by epigenetic drifts include Alzheimer's disease, atherosclerosis, cancer, benign prostatic hyperplasia, and osteoarthritis.
Appropriate stoichiometries of mitochondrial heteromultimeric complexes may be particularly susceptible to epigenetic drift and could explain at least three additional important geriatric disorders – sarcopenia, nonischemic cardiomyopathy and Parkinson's disease.
Acknowledgements
Our studies of progeroid syndromes have been supported for many years by an NIH grant, R24CA07088. The studies on Alzheimer's disease were supported by R35AG010917 and R01AG019711. The studies on nonischemic heart disease were supported by P01AG001751 (PI: Peter S. Rabinovitch). This paper is dedicated to Professor Kalluri Suba Rao, who enriched our lives during his postdoctoral studies in Seattle.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert RE, Benjamin SA, Shukla R. Life span and cancer mortality in the beagle dog and humans. Mech Ageing Dev. 1994;74:149–159. doi: 10.1016/0047-6374(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Bacolla A, Wang G, Jain A, Chuzhanova NA, Cer RZ, Collins JR, Cooper DN, Bohr VA, Vasquez KM. Non-B DNA-forming sequences and WRN deficiency independently increase the frequency of base substitution in human cells. J Biol Chem. 2011 doi: 10.1074/jbc.M110.176636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar R, Hartmann CH, Rodriguez KA, Denny AD, Busuttil RA, Dolle ME, Calder RB, Chisholm GB, Pollock BH, Klein CA, Vijg J. Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature. 2006;441:1011–1014. doi: 10.1038/nature04844. [DOI] [PubMed] [Google Scholar]
- Baird MG, Samis HV, Massie HR, Zimmerman JA. A brief argument in opposition to the Orgel hypothesis. Gerontologia. 1975;21:57–63. doi: 10.1159/000212031. [DOI] [PubMed] [Google Scholar]
- Baverstock K, Karotki AV. Towards a unifying theory of late stochastic effects of ionizing radiation. Mutat Res. 2011;718:1–9. doi: 10.1016/j.mrgentox.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Benditt EP, Benditt JM. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973;70:1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet FM. Intrinsic mutagenesis, a genetic approach to ageing. J. Wiley; New York: 1974. [DOI] [PubMed] [Google Scholar]
- Chen J, Cohen ML, Lerner AJ, Yang Y, Herrup K. DNA damage and cell cycle events implicate cerebellar dentate nucleus neurons as targets of Alzheimer's disease. Mol Neurodegener. 2010a;5:60. doi: 10.1186/1750-1326-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PT, Liao TY, Hu CJ, Wu ST, Wang SS, Chen RP. A highly sensitive peptide substrate for detecting two Ass-degrading enzymes: neprilysin and insulin-degrading enzyme. J Neurosci Methods. 2010b;190:57–62. doi: 10.1016/j.jneumeth.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggen DA, Solberg LA. Variation of atherosclerosis with age. Lab Invest. 1968;18:571–579. [PubMed] [Google Scholar]
- Finch CE, Kirkwood TBL. Chance, development, and aging. Oxford University Press; New York: 2000. [Google Scholar]
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suner D, Cigudosa JC, Urioste M, Benitez J, Boix-Chornet M, Sanchez-Aguilera A, Ling C, Carlsson E, Poulsen P, Vaag A, Stephan Z, Spector TD, Wu YZ, Plass C, Esteller M. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102:10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi K, Martin GM, Monnat RJ., Jr. Mutator phenotype of Werner syndrome is characterized by extensive deletions. Proc Natl Acad Sci U S A. 1989;86:5893–5897. doi: 10.1073/pnas.86.15.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WD. The moulding of senescence by natural selection. J Theor Biol. 1966;12:12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- Hitchins MP. Inheritance of epigenetic aberrations (constitutional epimutations) in cancer susceptibility. Adv Genet. 2010;70:201–243. doi: 10.1016/B978-0-12-380866-0.60008-3. [DOI] [PubMed] [Google Scholar]
- Hu Q, Jin LW, Starbuck MY, Martin GM. Broadly altered expression of the mRNA isoforms of FE65, a facilitator of beta amyloidogenesis, in Alzheimer cerebellum and other brain regions. J Neurosci Res. 2000;60:73–86. doi: 10.1002/(SICI)1097-4547(20000401)60:1<73::AID-JNR8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Irlenbusch U, Dominick G. Investigations in generalized osteoarthritis. Part 2: special histological features in generalized osteoarthritis (histological investigations in Heberden's nodes using a histological score) Osteoarthritis Cartilage. 2006;14:428–434. doi: 10.1016/j.joca.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Issa JP. Age-related epigenetic changes and the immune system. Clin Immunol. 2003;109:103–108. doi: 10.1016/s1521-6616(03)00203-1. [DOI] [PubMed] [Google Scholar]
- Issa JP, Ottaviano YL, Celano P, Hamilton SR, Davidson NE, Baylin SB. Methylation of the oestrogen receptor CpG island links ageing and neoplasia in human colon. Nat Genet. 1994;7:536–540. doi: 10.1038/ng0894-536. [DOI] [PubMed] [Google Scholar]
- Issa JP, Vertino PM, Boehm CD, Newsham IF, Baylin SB. Switch from monoallelic to biallelic human IGF2 promoter methylation during aging and carcinogenesis. Proc Natl Acad Sci U S A. 1996;93:11757–11762. doi: 10.1073/pnas.93.21.11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel RS, Frost P, Liteplo R, Carlow DA, Elliott BE. Possible epigenetic mechanisms of tumor progression: induction of high-frequency heritable but phenotypically unstable changes in the tumorigenic and metastatic properties of tumor cell populations by 5-azacytidine treatment. J Cell Physiol Suppl. 1984;3:87–97. doi: 10.1002/jcp.1041210411. [DOI] [PubMed] [Google Scholar]
- Kerchner GA, Hess CP, Hammond-Rosenbluth KE, Xu D, Rabinovici GD, Kelley DA, Vigneron DB, Nelson SJ, Miller BL. Hippocampal CA1 apical neuropil atrophy in mild Alzheimer disease visualized with 7-T MRI. Neurology. 2010;75:1381–1387. doi: 10.1212/WNL.0b013e3181f736a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MC, Wilson AC. Evolution at two levels in humans and chimpanzees. Science. 1975;188:107–116. doi: 10.1126/science.1090005. [DOI] [PubMed] [Google Scholar]
- Kwabi-Addo B, Chung W, Shen L, Ittmann M, Wheeler T, Jelinek J, Issa JP. Age-related DNA methylation changes in normal human prostate tissues. Clin Cancer Res. 2007;13:3796–3802. doi: 10.1158/1078-0432.CCR-07-0085. [DOI] [PubMed] [Google Scholar]
- Lagunoff D. Portraits of science. A Polish, Jewish scientist in 19th-century Prussia. Science. 2002;298:2331. doi: 10.1126/science.1080726. [DOI] [PubMed] [Google Scholar]
- Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9:92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler SF, Haass C, Steiner H. Regulated Intramembrane Proteolysis - Lessons from Amyloid Precursor Protein Processing. J Neurochem. 2011 doi: 10.1111/j.1471-4159.2011.07248.x. [DOI] [PubMed] [Google Scholar]
- Loeb LA. Mutator phenotype in cancer: origin and consequences. Semin Cancer Biol. 2010;20:279–280. doi: 10.1016/j.semcancer.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb LA, Springgate CF, Battula N. Errors in DNA replication as a basis of malignant changes. Cancer Res. 1974;34:2311–2321. [PubMed] [Google Scholar]
- Love S, Miners S, Palmer J, Chalmers K, Kehoe P. Insights into the pathogenesis and pathogenicity of cerebral amyloid angiopathy. Front Biosci. 2009;14:4778–4792. doi: 10.2741/3567. [DOI] [PubMed] [Google Scholar]
- Martin GM. Genetic syndromes in man with potential relevance to the pathobiology of aging. Birth Defects Orig Artic Ser. 1978;14:5–39. [PubMed] [Google Scholar]
- Martin GM. Proliferative homeostasis and its age-related aberrations. Mech Ageing Dev. 1979;9:385–391. doi: 10.1016/0047-6374(79)90080-0. [DOI] [PubMed] [Google Scholar]
- Martin GM. Somatic mutagenesis and antimutagenesis in aging research. Mutat Res. 1996;350:35–41. doi: 10.1016/0027-5107(95)00088-7. [DOI] [PubMed] [Google Scholar]
- Martin GM. Epigenetic drift in aging identical twins. Proc Natl Acad Sci U S A. 2005a;102:10413–10414. doi: 10.1073/pnas.0504743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GM. Genetic modulation of senescent phenotypes in Homo sapiens. Cell. 2005b;120:523–532. doi: 10.1016/j.cell.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Martin GM. The genetics and epigenetics of altered proliferative homeostasis in ageing and cancer. Mech Ageing Dev. 2007;128:9–12. doi: 10.1016/j.mad.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GM. Epigenetic gambling and epigenetic drift as an antagonistic pleiotropic mechanism of aging. Aging Cell. 2009;8:761–764. doi: 10.1111/j.1474-9726.2009.00515.x. [DOI] [PubMed] [Google Scholar]
- Martin GM, Bressler SL. Transcriptional infidelity in aging cells and its relevance for the Orgel hypothesis. Neurobiol Aging. 2000;21:897–900. doi: 10.1016/s0197-4580(00)00193-7. discussion 903–894. [DOI] [PubMed] [Google Scholar]
- Martin GM, Sprague CA, Norwood TH, Pendergrass WR. Clonal selection, attenuation and differentiation in an in vitro model of hyperplasia. Am J Pathol. 1974;74:137–154. [PMC free article] [PubMed] [Google Scholar]
- Orgel LE. The maintenance of the accuracy of protein synthesis and its relevance to ageing. Proc Natl Acad Sci U S A. 1963;49:517–521. doi: 10.1073/pnas.49.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgel LE. The maintenance of the accuracy of protein synthesis and its relevance to ageing: a correction. Proc Natl Acad Sci U S A. 1970;67:1476. doi: 10.1073/pnas.67.3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parise G, De Lisio M. Mitochondrial theory of aging in human age-related sarcopenia. Interdiscip Top Gerontol. 2010;37:142–156. doi: 10.1159/000319999. [DOI] [PubMed] [Google Scholar]
- Pollard KS. What Makes Us Human? Sci Am. 2009;300:44–49. doi: 10.1038/scientificamerican0509-44. [DOI] [PubMed] [Google Scholar]
- Pollard KS, Salama SR, King B, Kern AD, Dreszer T, Katzman S, Siepel A, Pedersen JS, Bejerano G, Baertsch R, Rosenbloom KR, Kent J, Haussler D. Forces shaping the fastest evolving regions in the human genome. Plos Genet. 2006;2:1599–1611. doi: 10.1371/journal.pgen.0020168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post WS, Goldschmidt-Clermont PJ, Wilhide CC, Heldman AW, Sussman MS, Ouyang P, Milliken EE, Issa JP. Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovasc Res. 1999;43:985–991. doi: 10.1016/s0008-6363(99)00153-4. [DOI] [PubMed] [Google Scholar]
- Pyle AL, Young PP. Atheromas feel the pressure: biomechanical stress and atherosclerosis. Am J Pathol. 2010;177:4–9. doi: 10.2353/ajpath.2010.090615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley AF, Kapsa RM, Esmore D, Hale G, Byrne E. Mitochondrial respiratory chain activity in idiopathic dilated cardiomyopathy. J Card Fail. 2000;6:47–55. doi: 10.1016/s1071-9164(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Rabinovitch PS, Martin GM. Encephalomyocarditis virus as a probe of errors in macromolecular synthesis in aging mice. Mech Ageing Dev. 1982;20:155–163. doi: 10.1016/0047-6374(82)90066-5. [DOI] [PubMed] [Google Scholar]
- Rea SL, Wu D, Cypser JR, Vaupel JW, Johnson TE. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat Genet. 2005;37:894–898. doi: 10.1038/ng1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick FG, Schally AV, Block NL, Nadji M, Szepeshazi K, Zarandi M, Vidaurre I, Perez R, Halmos G, Szalontay L. Antagonists of growth hormone-releasing hormone (GHRH) reduce prostate size in experimental benign prostatic hyperplasia. Proc Natl Acad Sci U S A. 2011;108:3755–3760. doi: 10.1073/pnas.1018086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salk JJ, Salipante SJ, Risques RA, Crispin DA, Li L, Bronner MP, Brentnall TA, Rabinovitch PS, Horwitz MS, Loeb LA. Clonal expansions in ulcerative colitis identify patients with neoplasia. Proc Natl Acad Sci U S A. 2009;106:20871–20876. doi: 10.1073/pnas.0909428106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Steinbakk A, Gudlaugsson E, Aasprong OG, Skaland I, Malpica A, Feng W, Janssen EA, Baak JP. Molecular biomarkers in endometrial hyperplasias predict cancer progression. Am J Obstet Gynecol. 2011;204:357, e351–312. doi: 10.1016/j.ajog.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Mattson MP. Selective Vulnerability of Neurons in Layer II of the Entorhinal Cortex during Aging and Alzheimer's Disease. Neural Plast. 2010;2010:108190. doi: 10.1155/2010/108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. Does Mitochondrial DNA Play a Role in Parkinson's Disease? A Review of Cybrid and other Supportive Evidence. Antioxid Redox Signal. 2011 doi: 10.1089/ars.2011.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thathiah A, De Strooper B. The role of G protein-coupled receptors in the pathology of Alzheimer's disease. Nat Rev Neurosci. 2011;12:73–87. doi: 10.1038/nrn2977. [DOI] [PubMed] [Google Scholar]
- Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima R, Yaono R, Yoshikawa S. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science. 1996;272:1136–1144. doi: 10.1126/science.272.5265.1136. [DOI] [PubMed] [Google Scholar]
- Wanagat J, Cao Z, Pathare P, Aiken JM. Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fiber splitting, and oxidative damage in sarcopenia. FASEB J. 2001;15:322–332. doi: 10.1096/fj.00-0320com. [DOI] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, Natural-Selection, and the Evolution of Senescence. Evolution. 1957;11:398–411. [Google Scholar]
- Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CE, Oshima J, Fu YH, Wijsman EM, Hisama F, Alisch R, Matthews S, Nakura J, Miki T, Ouais S, Martin GM, Mulligan J, Schellenberg GD. Positional cloning of the Werner's syndrome gene. Science. 1996;272:258–262. doi: 10.1126/science.272.5259.258. [DOI] [PubMed] [Google Scholar]