Abstract
Aging is a complex phenomenon that has been shown to affect many organ systems including the innate and adaptive immune systems. The current study was designed to examine the potential effect of immunosenescence on the pulmonary immune response using a Francisella tularensis live vaccine strain (LVS) inhalation infection model. F. tularensis is a gram-negative intracellular pathogen that can cause a severe pneumonia.In this study both young (8-12 week old) and aged (20-24 month old) mice were infected intranasally with LVS. Lung tissues from young and aged mice were used to assess pathology, recruitment of immune cell types and cytokine expression levels at various times post infection. Bacterial burdens were also assessed. Interestingly, the lungs of aged animals harbored fewer organisms at early time points of infection (day 1, day 3) compared with their younger counterparts. In addition, only aged animals displayed small perivascular aggregates at these early time points that appeared mostly mononuclear in nature. However, the kinetics of infiltrating polymorphonuclear neutrophils (PMNs) and increased cytokine levels measured in the bronchial alveolar lavage fluid (BALF) were delayed in infected aged animals relative to young infected animals with neutrophils appearing at day 5 post infection (PI) in the aged animals as opposed to day 3 PI in the young infected animals. Also evident were alterations in the ratios of mononuclear to PMNs at distinct post infection times. The above evidence indicates that aged mice elicit an altered immune response in the lung to respiratory Francisella tularensis LVS infections compared to their younger counterparts.
Keywords: Lung, Bacterial Infection, Host response, Aging
Introduction
Pneumonia, bacteremia and influenza are among of the top ten causes of morbidity and mortality among the elderly (Gavazzi and Kruse, 2002). As age progresses, the immune system undergoes numerous changes that may affect our susceptibility to infection. For example, CD4+:CD8+ T cell ratios, relative numbers of naïve T cells, and NK cell numbers and function have all been reported to fluctuate with respect to age (Ginaldi et al. 2000, Pawelec et al. 2004, Opal et al. 2005). Recently, components of the innate immune system have also been reported to undergo modifications with regard to age. Variations in function of phagocytic cells of the innate immune system have been described in aged individuals (Butcher et al. 2000, Ginald et al. 2001, Gomez et al. 2005, Plowden et al. 2004, Solana et al. 2006). Collectively, the changes in both the adaptive and innate immune system is known as immunosenescence. However, in vivo studies looking at age related changes in organ specific immunity to infectious organisms are less common in the literature (Meyer 2005).
Francisella tularensis subsp. tularensis and subsp. holarctica are extremely virulent pathogens capable of causing disease with very low infectious doses (Sjostedt 2006). Low infectious doses coupled with ease of dissemination and history as a bioweapon have led to the inclusion of Francisella on the CDC's Category A list of potential biowarfare agents (Oyston et al. 2004). Delivery of this pathogen through the pulmonary route can lead to the development of flu-like symptoms and subsequent pneumonia, sepsis, and eventually death if left untreated. F. tularensis LVS has been used as a model strain in the laboratory as it is highly virulent in mice and can be studied with less risk to the investigator.
The elderly population is among the fastest growing segments of society with increases in longevity and quality of life. As lung infections are one of the leading causes of disease among the elderly, understanding the potential role of aging in pulmonary diseases is critical. Recently, many studies have been initiated to elucidate the host response to Francisella, but none have focused on how immune responses may change with respect to age. Therefore, we infected mice intranasally with F. tularensis LVS to study potential differences in the pulmonary immune response between young and aged mice in vivo. Initially we examined the associated pathology in lung tissue sections and noted several differences between the cell types involved in the immune responses in young versus aged mice. We also studied leukocytes present in the bronchoalveolar lavage fluid (BALF) as well as concentrations of key cytokines important for their emigration into the air spaces. Our results indicate a delay in aged animals in the arrival of polymorphonuclear neutrophils (PMN) to the site of infection, a similar delay in the kinetics of increased cytokine production as well as alterations in the ratio of mononuclear to PMNs at distinct times post infection.
Materials and Methods
Mice
C57BL/6 male mice 6-8 weeks old along with 20-24 month old male mice were purchased from Harlan (Indianapolis, IN). Intranasal infection was performed by initially anesthetizing mice with an intramuscular injection of 100 μl ketamine-xylazine mixture (30 mg/ml ketamine, 4mg/ml xylazine in phosphate-buffered saline [PBS], diluted 1 to 5 in PBS) followed by inoculation of 10μl of bacterial suspension in each nostril drop by drop (20μl total volume) and allowing the mice to slowly inhale the inoculum. All the experimental procedures were in compliance with Federal guidelines and the guidelines of the institutional animal care and use committee at both UTSA and UTHSCSA.
Bacterial strains and culture media
Francisella tularensis subsp. holarctica strain LVS was obtained from Dr. Bernard Arulanandam (UTSA) through Dr. Fran Nano (University of Victoria). LVS was grown in Tripticase Soy Broth (Becton Dickinson, Franklin Lakes, NJ) supplemented with 0.1% cysteine (TSAcys) and plated on Chocolate II Agar (BD). LVS was then resuspended and taken to a titer of 2-3 × 103 cfu/20μl. This dose was used to approximate the LD50 of LVS. Actual numbers of viable organisms inoculated were confirmed at the time of infection by re-plating on Chocolate II Agar.
Lung section preparation
Lungs were harvested as previously described (Coalson, 1983) with some modification (Mares et al. 2008). Briefly, mice were anesthetized at serial time points with a mixture of ketamine and xylazine (100 μl of undiluted ketamine-xylazine) as described above. Pericardium and trachea were exposed by dissection and the lungs were subsequently perfused with sterile PBS. An incision was made in the trachea and a sterile-flexible cannula attached to a 3ml syringe was inserted. Lungs of mice were inflated slowly with 0.5-1.0 ml of Z-Fix (zinc formalin) (Anatech LTD., Battle Creek, MI) solution. The trachea as well as the right and left bronchus of each lung was tied off. Inflated lungs were then removed, and placed in 5 ml of Z-Fix compound and stored at room temperature overnight. After the lungs were removed the mice were humanely euthanized by cervical dislocation. Lungs were embedded in paraffin followed by cutting and staining with a Brown & Hopps stain by the Pathology Department at UTHSCSA.
Examination of Lung Pathology
Lung sections were scored for pathology in four different categories (Clark et al. 1998, Dormans et al. 2004, Harrod et al. 2005, Malik et al. 2006). Bronchioles and blood vessels were observed and given scores based on the presence and extent of severity of infiltrating cells surrounding the aforementioned structures. Alveoli (45 per section) were selected randomly and scored on the basis of the integrity of their structure (alveolar wall thickening and breakage) along with the presence of infiltrating cells in the alveolar septa. Alveolar air spaces were also given a score based on their clarity in terms of the presence of alveolar exudates and/or infiltrating cells in the air spaces. Number and/or size of lesions were also recorded and assigned a score. Scoring was performed on 3 different animals per time point. Scores for each time point were averaged and the SEM was calculated.
Bronchoalveolar Lavage
At serial time points a tracheotomy was performed after mice were anesthetized with a mixture of ketamine-xylazine; a sterile-flexible cannula attached to a 3ml syringe was inserted into the trachea. The lungs were lavaged with 3.0 ml of lavage solution (1× PBS, 3 mM EDTA and 100 μM isoproterenol) in 0.5 ml aliquots. Bronchoalveolar lavage fluid (BALF) was centrifuged at 1000 rpm for 7 min, and the supernatant was stored at -80°C for subsequent cytokine and chemokine analyses which were performed by using the Rodent Multi-Analyte Profile (Luminex) available from Rules Based Medicine (Austin, TX). The remaining cell pellet was resuspended in sterile PBS and taken to a concentration of 1 × 105 cells/ml. Cytocentrifugation was performed at 1000 rpm for 7 min followed by Diff-Quik staining (Dade Behring Inc., Newark, DE) for differential cell count.
Statistical Analysis
Statistics were calculated between young and aged mice at their respective time points using the Student's t-test through SigmaPlot 8.0. Values of p<.05 were considered significant.
Results
Age related differences in LVS pulmonary infections
Initially, we infected young (8-12 wk, n=16) and aged (20-24 mo, n=19) mice by the intranasal route with LVS (2 × 103 cfu/20 μl) and monitored them for disease severity. Although a couple of the aged animals died a day or two earlier than young animals, our data indicate that there was no significant difference with regard to survival between the two age groups (p=0.99). We also determined the bacterial burden in the lungs as well as the number of organisms (cfu) in the BALF of young and aged mice. Interestingly, comparatively fewer organisms were detected in both lungs and BALF at 3 DPI in aged mice when compared to young mice. However, the numbers of organisms were similar in young and aged animals at 5 DPI and 8 DPI (Fig. 1A, 1B).
Figure 1.
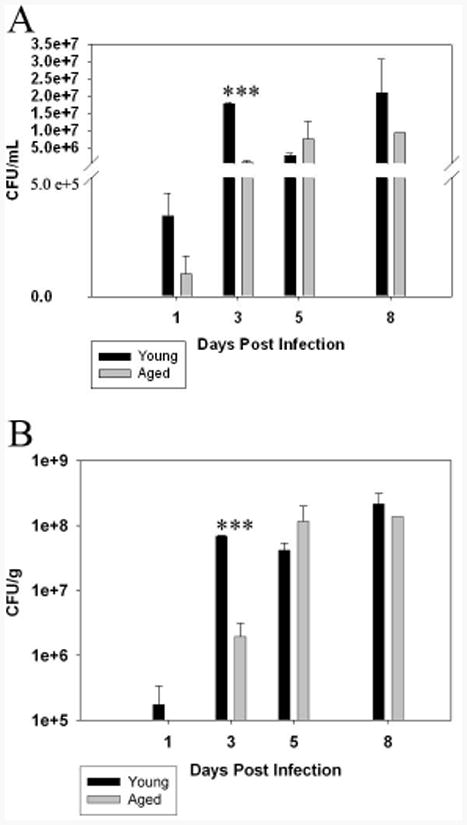
Bacterial burden in lungs of young and aged mice infected with LVS. Young and aged mice were icted with LVS (n=3 per group; results shown are representative of two independent experiments) and sacrificed at 1, 3, 5 and 8 days post infection (DPI). Bronchoalveolar lavage fluid was harvested and an aliquot was taken to plate on TSA plates to enumerate the number of viable CFU's. Figure 1 A depicts the number of CFU's recovered from the BALF. Significantly more CFU's were detected in the BALF of young mice at 3 DPI vs. there aged counterparts at the same time point (***p<.005). Figure1 B shows the number of CFU's isolated from the lungs. Briefly, after BALF was recovered, the lungs were removed and homogenized. Serial dilutions were made and then plated as described above. Similar to the data acquired from the BALF, the number of CFU's recovered from the lungs was significantly less (***p<.005) in the aged mice at 3 DPI and recovered to similar levels in both young and aged mice at later time points.
We also analyzed the pathology associated with pneumonic LVS infections in both infected young and aged mice in addition to uninfected controls. Noticeable differences were observed between the two age groups in mock infected control animals. For example, more cells were present around the bronchial epithelium as well as the endothelium in aged controls (Figure 2 A, F, K, P). After infection, aged animals at 2 DPI displayed a similar degree of hypercellularity in the alveoli (especially peribronchial alveoli) which increased at 3 DPI (Figure 2 L, arrow and Figure 2M, arrow). Such evidence of hypercellularity was not evident until 3 DPI in the young mice (Figure 2 C & H arrows), and to a lesser extent compared with the aged (Figure 2B, 2C). In addition, the hypercellularity in the alveoli of young mice appeared to be mostly PMNs whereas in aged animals most of the cells were mononuclear. Also of distinction was the presence of aggregates of small perivascular mononuclear infiltration at 3 DPI distributed throughout the lung tissue in aged animals (Figure 2 M arrowhead, R *) that was absent in young mice at this time point. Some of the more striking differences in pathology between the young and aged groups appeared by day 5 PI. In both cases there was evidence of edema, vasculitits as well as the establishment of a condensed inflammatory response circumventing the bronchovascular dyads. However, in infected young mice at 5 DPI, the response was a mixed PMN-mononuclear response. Several cells with pyknotic nuclei were also present in and around the lesions at 5 DPI in the young infected mice. This is in contrast to the response observed in aged animals which was mononuclear dominant at 3 DPI and 5 DPI with only rare foci of neutrophilic infiltrates observed at the latter time points (Fig. 2 C, D, R, S (* indicates perivascular/peribronchial infiltrates). Interestingly, by 8 DPI, the dominant cell type observed in the young response switched from a mixed PMN-mononuclear response to a mononuclear dominant population. At 8 DPI the aged response remained a mixed PMN-mononuclear response with several cells appearing pyknotic as well as what appeared to be the initiation of a granulomatous response (evidence of epithelioid cells) (Figure 2 O). The aged animals seemed to exhibit a more extensive confluent bronchopneumonia than their young counterparts at 8 DPI. We also observed that more microcolonies of LVS were readily observed in the aged tissues at 8 DPI when compared to the infected young mice at the same time point. Another interesting observation was the consistent presence and relative abundance of alveolar exudate found in the air spaces throughout the aged tissues when compared to their young counterparts at 8 DPI (Figure 2 O, T). Table I summarizes pathology scores obtained for both mock young and aged mice as well as infected young and aged mice in various areas of the lung.
Figure 2.
Pathology associated with LVS infections is altered in the lungs of aged mice. Young and aged mice (n=3 per group; results shown are representative of two independent experiments) were intranasally infected with LVS (2.9×103 CFU/20μL) and sacrificed at 2 DPI, 3 DPI, 5 DPI, and 8 DPI. Their lungs were inflated with zinc formalin and subsequently embedded in paraffin. The tissues were then cut and then mounted on slides and stained with Brown and Hopps. All pictures are shown at a magnification of 200× with the exception of Figures 2 E & J which are shown at 100×. Figures 2 A-J represent lungs taken from young mice. Figures 2 K-T represent lungs taken from aged mice. Mock lungs from young (Figure 2 A & F) and aged (Figure 2 K & P) are shown for comparison. LVS infected young mice exhibited little changes in their lungs through 3 DPI (B & C). In contrast, mononuclear perivascular infiltrates were observed in aged mice at both 2 DPI and 3 DPI (arrows in Figure 2 L & M indicate hypercellularity in alveoli; * in Figure 2 Q & R indicate perivascular infiltrates). A mixed monocyte and PMN infiltrate was observed in young mice at 3 DPI while this was not detectable until 5 DPI in the aged (Figures 2 I & S, respectively). Figures 2 E and O show young and aged lungs at 8 DPI. In both groups the lung pathology was dominated in large part by a confluent bronchopneumonia. Although, the aged lungs did appear to be slightly more confluent as well as contain substantially more exudate occluding the alveolar airspaces throughout the entire tissue (arrows in Figures 2 J & T).
Table I. Pathology scores for young and aged mice intranasally infected with LVS1.
| Time Post Infection | |||||
|---|---|---|---|---|---|
| Mock | 2 DPI | 3 DPI | 5 DPI | 8 DPI | |
| Alveoli | |||||
| Young | 2.00±.00 | 1.67±.33 | 2.16±.00 | 4.00±.00 | 5.00±.58 |
| Aged | 2.33±.33 | 3.00±.00* | 3.33±.67* | 4.67±.88 | 8.67±.33*** |
| Peribronchial | |||||
| Young | .33 ±.07 | .33 ±.09 | .57 ±.12 | 1.53±.33 | 2.36±.34 |
| Aged | .73 ±.30 | 1.00±.12* | 1.13±.29 | 1.13±.19 | 3.03±.22 |
| Perivascular | |||||
| Young | .00±.00 | .13±.07 | .33±.15 | 1.53±.12 | 2.80±.59 |
| Aged | .57±.07 | .97±.03** | 1.27±.32*** | 1.90±.12 | 3.43±.29 |
| Total2 | |||||
| Young | 2.33±.07 | 3.13±.47 | 5.73±.53 | 9.73±.12 | 13.83±1.48 |
| Aged | 3.63±.58 | 6.63±.47** | 7.07±1.23 | 9.03±1.22 | 18.80±.29** |
Pathology scores for different areas were calculated as described in the Materials and Methods section.
p<05.
p<01.
p<005.
The total score is the sum of the scores for the alveoli, peribronchial, perivascular and includes the score for the number and/or the nature of the lesions.
Appearance of neutrophils in the BALF of young and aged mice infected with LVS
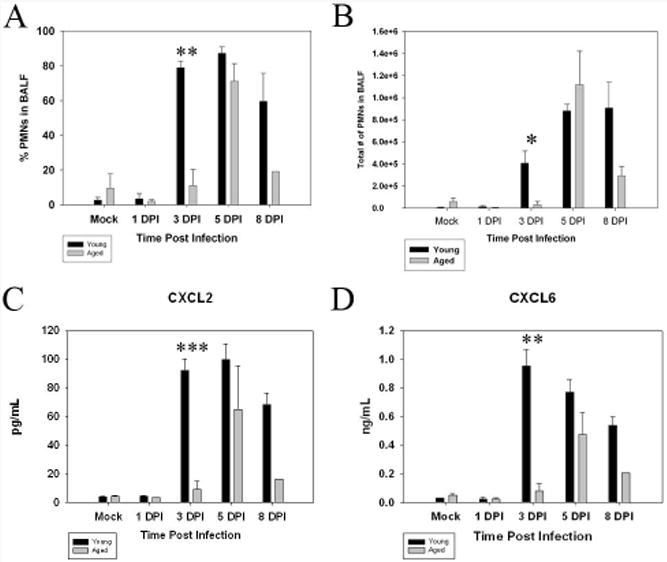
Upon observing the difference in the kinetics of the PMN response between aged and young animals in situ, we investigated the nature of cells present in the bronchoalveolar lavage (BALF). BALF was harvested, cytocentrifuged unto slides which were then stained with Diff-Quik to allow differentiation between neutrophils and mononuclear cells. The BALF collected from young infected animals showed a significant increase (p<.005) in the percentage of neutrophils in the BALF as early as 3 DPI when compared to aged animals at the same time point. Neutrophils in young mice continued to be present in the BALF throughout the infection (Figure 3 A). An increased percentage as well as total number of neutrophils in the BALF of aged subjects was not found until 5 DPI with the cells mostly mononuclear at 3 DPI (Fig 3B). The percentage and total number of neutrophils remained elevated through 8 DPI in the BALF of aged infected mice, albeit to a lesser extent than their young counterparts.
Figure 3.
Infiltration of neutrophils into the BALF and kinetics of neutrophil chemoattractants are delayed in aged mice in response to LVS infections. Young and aged mice were intranasally infected with LVS and their BALF was harvested and cytocentrifuged onto slides. The slides were then stained with Diff-quik and the percentage of polymorphonuclear cells was determined by calculated in samples from 3 mice per time point (at least 3 slides per mouse; results shown are representative of two independent experiments). Significantly more PMNs (with regard to both percentage and total number of neutrophils, Figure 2 A and B, respectively) were observed in the BALF of young mice at 3 DPI (**p<.01, *p<.05) and remained elevated throughout the time points observed. LVS did not elicit a PMN response in aged animals with regard to percentage increase (Figure 3 A) or total number of neutrophils (Figure 3 B) until 5 DPI. BALF was harvested from young and aged infected mice and the concentration of cytokines present in the sample was analyzed by utilizing a Multianaylate profile assay (Luminex). CXCL2 (MIP-2, Figure 3 C) and CXCL6 (GCP-2, Figure 3 D) are potent chemoattractants for PMNs and are both significantly increased in young animals at 3 DPI when compared to aged mice at the same time PI (***p<.005, **p<.01).
Cytokines present in the BALF display delayed kinetics in aged mice infected with LVS
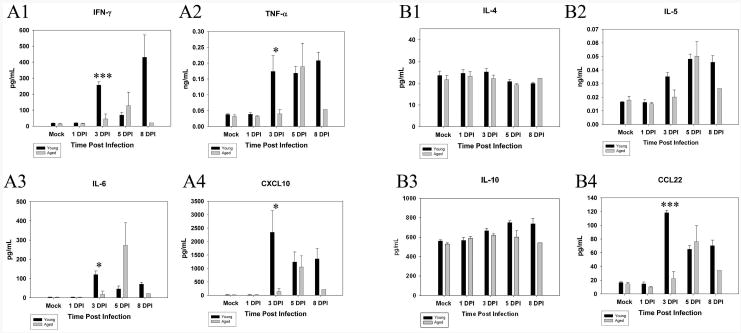
The levels of two important chemokines involved in neutrophil chemotaxis were then compared in the BALF between young and aged animals. We utilized Luminex technology to examine the kinetics of CXCL2 (MIP-2) and CXCL6 (GCP-2) levels in the BALF (Fig. 3 C, D). The concentrations of both chemokines were low in the BALF from mock-infected control animals as well as through 1 DPI in both age groups. However, the peak in production of these two chemokines in infected young mice occurred at 3 DPI (CXCL2 p<0.001, CXCL6 p<0.005) while levels in infected aged mice did not increase until day 5 PI (Figure 3 C, D). We also examined the levels of other pro-inflammatory cytokines including IFN-γ, TNF-α, IL-6 and CXCL10 (IP-10) in the BALF and noted a similar delay in all cases (Figure 4 A1-A4). IFN-γ, TNF-α, IL-6 and CXCL10 levels were increased at 3 DPI (IFN-γ p<.005, TNF-α p=.05, IL-6 p<05, CXCL10 p=.05) in the young whereas no significant increases in any of these cytokines were detected in infected aged animals until 5 DPI. Anti-inflammatory cytokines IL-4, IL-5, IL-10 and CCL22 were also measured in the BALF of infected young and aged mice (Figure 4 B1-B4). IL-4 and IL-10 showed very little change with time post infection. However, IL-5 and CCL22 were elevated at 3 DPI (CCL22 p<.005) in young infected mice while no appreciable increase was detected until 5 DPI in the aged mice.
Figure 4.
Kinetics of cytokines in the BALF is delayed in aged mice. BALF was harvested from young and aged infected mice (n=3 in each group; results shown are representative of two independent experiments) and the concentration of cytokines present in the sample was analyzed by utilizing a Multianaylate profile assay (Luminex). IFN-γ (A1), TNF-α (A2), IL-6 (A3), and CXCL10 (A4) were all monitored at 1 DPI, 3 DPI, 5 DPI, and 8 DPI. All four pro-inflammatory cytokines showed a significant increase in young animals when compared to their aged counterparts at 3 DPI (IFN-γ ***p<005, TNF-α *p=05, IL-6 *p<05, CXCL10 *p=05). Anti-inflammatory cytokines IL-4 (B1), IL-5 (B2), IL-10 (B3), CCL22 (B4) were also monitored in the BALF. IL-4 and IL-10 showed little change in their pattern of release into the BALF throughout the infection. IL-5 and CCL22 increased at 3 DPI (CCL22 ***p<005) in the young mice and did not increase until 5 DPI in the aged mice.
Discussion
Lung infections are collectively recognized as a leading cause of infections among all sectors of the population, regardless of affluence (Mizgerd 2006). Importantly, the prevalence and severity of pneumonia increases with advancing age (Janssens et al. 2004) suggesting changes in immune defense. Many alterations have been described for the adaptive immune system. However, relatively fewer studies have examined the role of the localized pulmonary innate immune response, and if it changes during infection with respect to age. We have utilized the respiratory pathogen Francisella in order to study potential differences in the pulmonary immune response between young and aged individuals. We noted an initial lower bacterial burden in both the BALF and lungs of aged mice. A few other studies in the literature also suggest an altered response in aged mice to intracellular infections. In particular, High et al. (High et al. 2007) have demonstrated that aged mice do at least as well if not better than their younger counterparts when infected with Brucella abortus (High et al. 2007). Aged mice infected with B. abortus were more capable of clearing the intracellular infection as were aged mice that were infected with Leishmania (Ehrchen et al. 2004). Another difference we observed was the presence of mononuclear perivascular infiltrates at early time points in the lungs of infected aged animals. These infiltrates were noticeably absent in young mice. Interestingly, similar studies by Turner et al. (Turner et al. 2002) have demonstrated an earlier immune response in aged mice to the bacterium Mycobacterium tuberculosis. Taken together, all of these studies including those described herein, suggest that aged mice are capable of mounting an equally effective, if not enhanced, initial immune response to intracellular pathogens. It is possible that the early perivascular infiltrates in aged LVS infected mice may ultimately have to do with the changes already documented to occur with regard to macrophages and aging (Plowden et al. 2004, Solana et al. 2006). It will also be interesting to determine the contribution of the initial proinflammatory environment known as inflammaging to the changes observed in this Francisella infection model (Ginald et al. 2001, Franceschi and Bonafe 2003).
One of the hallmarks of Francisella infections is a 2-3 day delay in characteristic pro-inflammatory cytokines, chemokines and infiltrating leukocytes compared with other pulmonary pathogens that induce such responses within hours after infection (Bosio et al. 2005, Andersson et al. 2006, Mares et al. 2008). This cannot be explained by the less stimulatory LPS of Francisella (Sjostedt 2006, Sandstrom et al. 1992), as the organism does cause early inflammatory responses with other routes of infection (Golovliov et al., 1996, Stenmark et al., 1999). We have proposed that the delay in innate responses to F. novicida, a highly pathogenic strain in mice, is a virulence mechanism of the organism that allows it to replicate out of control leading to sepsis and death within 4-6 days (Mares et al. 2008, Sharma et al., 2009). Consistent with delayed innate responses to Francisella, young animals infected with the less virulent LVS strain exhibited little increase in pro-inflammatory chemokines/cytokines or neutrophil influx until day 3 PI. This was followed by a mixed neutrophil-mononuclear response at 5 DPI in the young mice which returned to a mononuclear dominant response by 8 DPI in this age group. Young animals began to succumb at day 10. In contrast, in aged mice the response remained mononuclear dominant through 5 DPI with infrequent foci of neutrophilic infiltrates consistent with the altered, delayed kinetics of CXCL2 and CXCL6 and other proinflammatory cytokines compared with the younger mice. The first aged animal died at day 8, but there was no significant difference in survival rates between the two aged groups at this dose. Thus, aged animals appear to exhibit a 48 hour delay in the production of multiple cytokines, the influx of neutrophils and the subsequent shift to mononuclear cells. It is unclear if this represents an age related delay in induction of distinct innate responses or is the result of the enhanced ability of aged mice to initially control bacterial burdens.
Various aspects of neutrophil function have been reported to change with regard to age. For example, neutrophil chemotaxis has been reported to be defective in the elderly (Wenisch et al. 2000). Studies have also shown that phagocytosis as well cell signaling events are also altered in aged neutrophils (Fulop et al. 2004, Butcher et al. 2000). However, many of these studies have focused on the role of these cells ex vivo and few have reported on the response to infection in vivo with regard to neutrophils. Our observations suggest that a differential neutrophil response or age related defective/diminished neutrophil function may potentially be having a pronounced effect in the alveoli of aged mice. This may also help to explain the reduced cytokine responses at day 8 in aged animals. We consistently observed the alveoli of aged mice to be occluded with debris, inflammatory cells, and increased cell death which ultimately led to them receiving a higher pathology score than their younger counterparts at 8 DPI and an earlier death in isolated individuals. It should also be noted that Francisella itself appears to affect neutrophil function (McCaffrey and Allen, 2006).
Francisella's ability to subvert the innate immune response coupled with a delay in the kinetics of the immune response in aged mice may be important contributors to the altered pathology associated with LVS infected aged mice. We propose that the early inflammation, comprised of mononuclear cells in the lungs of aged mice, may be responsible for the early control of bacterial replication of LVS in the lungs of aged mice. We further speculate that this initial response is only capable of shifting or delaying the kinetics of the host response in aged mice by 24 to 48 hours. Eventually it appears that the highly virulent and evasive nature of this pathogen dominate and although the host response appears to initially control the infection at early time points, ultimately both young and aged mice succumb to the disease which is associated with an overwhelming pulmonary pathology and is characterized by an inability to clear apoptotic debris. We also observed that the pathology in aged mice seemed more severe at 8 DPI and was associated with occluded alveoli filled with exudates and immune cells. We surmise that the increased amount of pathology is due to existing damage in the aged lung in the absence of infection (mock-infected young vs. aged mice) coupled with a decreased ability of aged mice to clear exudates and apoptotic debris from the alveoli thus exacerbnfeating the disease process.
Acknowledgments
This work was supported by awards 1P01A10157986, NS35974, AI 59703 from the National Institutes of Health to J.M.T. C.A.M. was also supported through departmental training grant T32AI7271 from the NIH and 1R36AG033400-01 from the National Institutes of Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andersson H, Hartmanova B, Kuolee R, Ryden P, Conlan W, Chen W, Sjostedt A. Transcriptional profiling of host responses in mouse lungs following aerosol infection with type A Francisella tularensis. J Med Microbiol. 2006;55:263–71. doi: 10.1099/jmm.0.46313-0. [DOI] [PubMed] [Google Scholar]
- 2.Bosio CM, Bielefeldt-Ohmann H, Belisle JT. Active suppression of the pulmonary immune response by Francisella tularensis Schu4. J Immunol. 2005;178:4538–47. doi: 10.4049/jimmunol.178.7.4538. [DOI] [PubMed] [Google Scholar]
- 3.Boukhvalova MS, Yim KC, Kuhn KH, Hemming JP, Prince GA, Porter DD, Blanco JC. Age-related differences in pulmonary cytokine response to respiratory syncytial virus infection: modulation by anti-inflammatory and antiviral treatment. J Infect Dis. 2007;195:511–8. doi: 10.1086/510628. [DOI] [PubMed] [Google Scholar]
- 4.Butcher S, Chahel H, Lord JM. Review article: ageing and the neutrophil: no appetite for killing? Immunology. 2000;100:411–6. doi: 10.1046/j.1365-2567.2000.00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiavolini D, Alroy J, King CA, Jorth P, Weir S, Madico G, Murphy JR, Wetzler LM. Identification of immunologic and pathologic parameters of death versus survival in respiratory tularemia. Infect Immun. 2008;76:486–96. doi: 10.1128/IAI.00862-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark JG, Madtes DK, Hackman RC, Chen W, Cheever MA, Martin PJ. Lung injury induced by alloreactive Th1 cells is characterized by host-derived mononuclear cell inflammation and activation of alveolar macrophages. J Immunol. 1998;161:1913–20. [PubMed] [Google Scholar]
- 7.Coalson JJ. A simple method of lung perfusion fixation. Anat Rec. 1983;205:233–8. doi: 10.1002/ar.1092050214. [DOI] [PubMed] [Google Scholar]
- 8.Dormans J, Burger M, Aguilar D, Hernandez-Pando R, Kremer K, Roholl P, Arend SM, van Soolingen D. Correlation of virulence, lung pathology, bacterial load and delayed type hypersensitivity responses after infection with different Mycobacterium tuberculosis genotypes in a BALB/c mouse model. Clin Exp Immunol. 2004;137:460–8. doi: 10.1111/j.1365-2249.2004.02551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrchen J, Sindrilaru A, Grabbe S, Schonlau F, Schlesiger C, Sorg C, Scharffetter-Kochanek K, Sunderkotter C. Senescent BALB/c mice are able to develop resistance to Leishmania major infection. Infect Immun. 2004;72:5106–14. doi: 10.1128/IAI.72.9.5106-5114.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franceschi C, Bonafe M. Centenarians as a model for healthy aging. Biochem Soc Trans. 2003;31:457–61. doi: 10.1042/bst0310457. [DOI] [PubMed] [Google Scholar]
- 11.Fulop T, Larbi A, Douziech N, Fortin C, Guerard KP, Lesur O, Khalil A, Dupuis G. Signal transduction and functional changes in neutrophils with aging. Aging Cell. 2004;3:217–26. doi: 10.1111/j.1474-9728.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 12.Gavazzi G, Krause KH. Ageing and infection. Lancet Infect Dis. 2002;2:659–66. doi: 10.1016/s1473-3099(02)00437-1. [DOI] [PubMed] [Google Scholar]
- 13.Ginaldi L, De Martinis M, Modesti M, Loreto F, Corsi MP, Quaglino D. Immunophenotypical changes of T lymphocytes in the elderly. Gerontology. 2000;46:242–8. doi: 10.1159/000022167. [DOI] [PubMed] [Google Scholar]
- 14.Ginaldi L, Loreto MF, Corsi MP, Modesti M, De Martinis M. Immunosenescence and infectious diseases. Microbes Infect. 2001;3:851–7. doi: 10.1016/s1286-4579(01)01443-5. [DOI] [PubMed] [Google Scholar]
- 15.Golovliov I, Kuoppa K, Sjostedt A, Tarnvik A, Sandstrom G. Cytokine expression in the liver of mice infected with a highly virulent strain of Francisella tularensis. FEMS Immunol Med Microbiol. 1996;13:239–44. doi: 10.1111/j.1574-695X.1996.tb00244.x. [DOI] [PubMed] [Google Scholar]
- 16.Gomez CR, Boehmer ED, Kovacs EJ. The aging innate immune system. Curr Opin Immunol. 2005;17:457–62. doi: 10.1016/j.coi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Gomez CR, Hirano S, Cutro BT, Birjandi S, Baila H, Nomellini V, Kovacs EJ. Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Crit Care Med. 2007;35:246–51. doi: 10.1097/01.CCM.0000251639.05135.E0. [DOI] [PubMed] [Google Scholar]
- 18.Harrod KS, Jaramillo RJ, Berger JA, Gigliotti AP, Seilkop SK, Reed MD. Inhaled diesel engine emissions reduce bacterial clearance and exacerbate lung disease to Pseudomonas aeruginosa infection in vivo. Toxicol Sci. 2005;83:155–65. doi: 10.1093/toxsci/kfi007. [DOI] [PubMed] [Google Scholar]
- 19.High KP, Prasad R, Marion CR, Schurig GG, Boyle SM, Sriranganathan N. Outcome and immune responses after Brucella abortus infection in young adult and aged mice. Biogerontology. 2007;8:583–93. doi: 10.1007/s10522-007-9106-6. [DOI] [PubMed] [Google Scholar]
- 20.Janssens JP, Krause KH. Pneumonia in the very old. Lancet Infect Dis. 2004;4:112–24. doi: 10.1016/S1473-3099(04)00931-4. [DOI] [PubMed] [Google Scholar]
- 21.Malik M, Bakshi CS, Sahay B, Shah A, Lotz SA, Sellati TJ. Toll-like receptor 2 is required for control of pulmonary infection with Francisella tularensis. Infect Immun. 2006;74:3657–62. doi: 10.1128/IAI.02030-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mares CA, Ojeda SS, Morris EG, Li Q, Teale JM. Initial delay in the immune response to Francisella tularensis is followed by hypercytokinema characteristic of severe sepsis and correlating with upregulation and release of damage associated molecular patterns. Infect Immun. 2008;76:3001–10. doi: 10.1128/IAI.00215-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCaffrey RL, Allen LH. Francisella tularensis LVS evades killing by human neutrophils via inhibition of the respiratory burst and phagosome escape. J Leukoc Biol. 2006;80:1224–30. doi: 10.1189/jlb.0406287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer KC. Aging. Proc Am Thorac Soc. 2005;2:433–9. doi: 10.1513/pats.200508-081JS. [DOI] [PubMed] [Google Scholar]
- 25.Mizgerd JP. Lung infection--a public health priority. PLoS Med. 2006;3:e76. doi: 10.1371/journal.pmed.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Opal SM, Girard TD, Ely EW. The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis. 2005;41(7):S504–12. doi: 10.1086/432007. [DOI] [PubMed] [Google Scholar]
- 27.Oyston PC, Sjostedt A, Titball RW. Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat Rev Microbiol. 2004;2:967–78. doi: 10.1038/nrmicro1045. [DOI] [PubMed] [Google Scholar]
- 28.Pawelec G, Akbar A, Caruso C, Effros R, Grubeck-Loebenstein B, Wikby A. Is immunosenescence infectious? Trends Immunol. 2004;25:406–10. doi: 10.1016/j.it.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3:161–7. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- 30.Sandstrom G, Sjostedt A, Johansson T, Kuoppa K, Williams JC. Immunogenicity and toxicity of lipopolysaccharide from Francisella tularensis LVS. FEMS Microbiol Immunol. 1992;5:201–10. doi: 10.1111/j.1574-6968.1992.tb05902.x. [DOI] [PubMed] [Google Scholar]
- 31.Sharma J, Li Q, Mishra BB, Pena C, Teale JM. Letal pulmonary infection with Francisella novicida is associated with severe sepsis. J Leukoc Biol. 2009;86 doi: 10.1189/jlb1208728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sjostedt A. Intracellular survival mechanisms of Francisella tularensis, a stealth pathogen. Microbes Infect. 2006;8:561–7. doi: 10.1016/j.micinf.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Solana R, Pawelec G, Tarazona R. Aging and innate immunity. Immunity. 2006;24:491–4. doi: 10.1016/j.immuni.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Stenmark S, Sunnemark D, Bucht A, Sjostedt A. Rapid local expression of interleukin-12, tumor necrosis factor alpha, and gamma interferon after cutaneous Francisella tularensis infection in tularemia-immune mice. Infect Immun. 1999;67:1789–97. doi: 10.1128/iai.67.4.1789-1797.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turner J, Frank AA, Orme IM. Old mice express a transient early resistance to pulmonary tuberculosis that is mediated by CD8 T cells. Infect Immun. 2002;70:4628–37. doi: 10.1128/IAI.70.8.4628-4637.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wenisch C, Patruta S, Daxbock F, Krause R, Horl W. Effect of age on human neutrophil function. J Leukoc Biol. 2000;67:40–5. doi: 10.1002/jlb.67.1.40. [DOI] [PubMed] [Google Scholar]


