Abstract
The Toll-like receptor (TLR) family of pathogen recognition molecules has an important role in recognizing microbial pathogens and microbial breakdown products. Activation of TLRs in the corneal epithelium induces CXC chemokine production and recruitment of neutrophils to the corneal stroma. Although essential for pathogen killing, neutrophils can cause extensive tissue damage, leading to visual impairment and blindness. In this review, we examine the role of TLRs in microbial keratitis and in noninfectious corneal inflammation, most commonly associated with contact lens wear. We present recent findings on TLR signaling pathways in the cornea, including MyD88- and TRIF-dependent responses and discuss the role of resident macrophages and dendritic cells. Finally, we examine the potential for targeting the TLR pathway as a potential therapeutic intervention for microbial keratitis and contact lens-associated corneal inflammation.
Keywords: cornea, inflammation, keratitis, lipopolysaccharide (LPS), MyD88, Toll-like receptor
I. INTRODUCTION
Microbial keratitis is a major cause of visual impairment and blindness worldwide. Microbial pathogens and their products activate resident cells within the tissue and initiate production of proinflammatory and chemotactic cytokines that induce a cellular infiltration. Polymorphonuclear leukocytes/neutrophils are generally the first cells recruited to the site, as their primary role is to kill invading organisms and prevent dissemination; however, these cells also secrete cytotoxic mediators, including proteinases and reactive oxygen species, which can cause extensive tissue damage.1 In the skin or other tissues, this response is generally not life-threatening; however, at the ocular surface, exposure of the corneal epithelium to bacteria, fungi, or parasites results in neutrophil infiltration and loss of corneal function, visual impairment, and blindness.
Many of the steps involved in neutrophil recruitment to the cornea and activation have been elucidated using animal models of corneal infection caused by Herpes simplex virus, Pseudomonas aeruginosa, Staphylococcus aureus, Fusarium species, and Onchocerca volvulus (river blindness).2-5 Although specific differences have been reported, many of the stages leading to neutrophil infiltration and inflammation have common features, including trauma-induced exposure of the corneal epithelium or stroma to microbial pathogens, production of proinflammatory cytokines and CXC chemokines by resident cells in the cornea, and rapid mobilization of neutrophils, primarily from peripheral limbal vessels.
One of the initial steps in the inflammatory process in relation to microbes is activation of the Toll-like receptor (TLR) family of pathogen recognition molecules. The TLR family is a class of single membrane-spanning non-catalytic receptors that recognize structurally conserved molecules derived from microbes once they have breached physical barriers, such as the skin, intestinal tract mucosa, or cornea, and activate immune cell responses. Thirteen TLRs have been identified in humans and mice, which respond to lipids, proteins or nucleic acids. TLRs that respond to lipid agonists include TLR4, which is activated by the lipid A portion of a lipopolysaccharide (LPS), and TLR2, which forms a heterodimer with TLR1 or TLR6 to recognize triacylated and diacylated lipopeptides, respectively. Protein-binding TLRs include TLR5, which is the receptor for bacterial flagellin, and TLR11, which binds uropathogenic E coli and Toxoplasma gondii profilin.6-8 Finally, nucleic acid-binding TLRs include TLR3, which is activated by double-stranded RNA, TLR7 and TLR8, which bind single-stranded RNA, and TLR9, which binds CpG-rich DNA more commonly found in microbes than in mammals.8 TLRs that recognize proteins and lipids are primarily located on the cell surface, whereas nucleic acid-binding TLRs are located in endosomal compartments. TLR4 is the most complex member of this receptor family, having accessory molecules LPS binding protein (LBP), CD14, and MD-2, which confer sensitivity to picomolar levels of LPS.9
OUTLINE
| I. Introduction |
| II. TIR domain containing adaptor molecules |
| III. Role of TLRs in microbial keratitis |
| IV. Role of TLRs in sterile corneal inflammation |
| V. TLR3 and TRIF-dependent responses in the cornea |
| VI. Role of dendritic cells and macrophages in TLR responses in the cornea |
| VII. Summary and conclusions:TLRs as potential targets of therapy |
II. TIR DOMAIN CONTAINING ADAPTOR MOLECULES
TLRs also initiate intracellular signaling through two major pathways that are dependent on members of the adaptor molecule family of proteins that bind to the intracellular Toll/IL-1 receptor (TIR) region of TLRs and recruit downstream kinases essential afor TLR signaling. Adaptor molecules contain a TIR domain, a death domain, and an intermediary domain. Five members of this family have been identified and are described in a recent review.10
The best-characterized adaptor molecule is MyD88, which mediates signaling by all TLRs except TLR3, and also mediates signaling by interleukin (IL)-1R and IL-18R. MyD88 recruits IL-1 receptor-associated kinase (IRAK)4 and IRAK1, and leads to activation of transforming growth factor (TGF)-β-associated kinase (TAK)1, and the mitogen-activated protein (MAP) kinases p38MAPK and JNK8 activation. These MAP kinases mediate several cell functions, including phosphorylation of transcription factors NFκB and AP-1, and transcription of proinflammatory and chemotactic cytokines.8,11-13 In contrast, the TRIF-dependent pathway is activated by TLR3 and TLR4, and leads to production of type I interferons associated with viral infection. TLR4 therefore activates cells through two independent signaling pathways, leading to a diverse and amplified response.
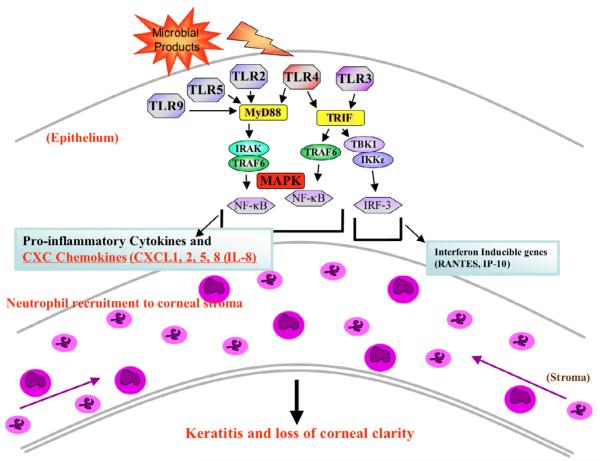
Other adaptor molecules associated with specific TLRs include Mal/TIRAP, which is membrane-bound and recruits MyD88 to TLR2 and TLR4 (though not TLR5), and TRAM, which recruits TRIF to TLR4 and facilitates activation of IRF3. Figure 1 illustrates TLR signaling in the corneal epithelium, focusing on the TLRs that will be discussed in this review.
Figure 1.
A model for TLR-induced corneal inflammation. Physical disruption of the corneal epithelium and exposure to microbial products, including lipoproteins (Pam3Cys), dsRNA (Poly[I:C]), LPS or flagellin, initiates MyD88-dependent or TRIF-dependent signaling. The MyD88 pathway activates MAP kinase (p42/p44, JNK, and p38) and NF-κB, resulting in expression of CXC chemokines. In contrast, the TRIF pathway activates IRF-3, MAP kinases JNK and p38, and NF-κB, resulting in production of CCL5/RANTES and type I interferons. Chemokine production stimulates macrophage and neutrophil infiltration into the cornea and subsequent loss of corneal clarity. (Adapted from Johnson A, Pearlman E73 and Johnson AC, Li X, Pearlman E.20)
III. ROLE OF TLRs IN MICROBIAL KERATITIS (TABLE 16,7,14-31)
Table 1.
Toll-like receptors and their role in corneal disease
| Toll Like Receptor | Ligand | Microbe | Role in corneal inflammation* |
|---|---|---|---|
| TLR2 | Lipoproteins | Gram −ve, +ve bacteria | HCEC** responses, killed S. aureus14-17 |
| TLR2/1§ | Tri-acylated lipoproteins | Mycoplasma | Unknown |
| TLR2/6 | Di-acylated lipoproteins | Gram −ve, +ve bacteria |
Wolbachia/river blindness, synthetic ligand (Pam3Cys)18,19 |
| TLR3¶ | dsRNA | dsRNA viruses | Synthetic ligand Poly(I:C) activates HCEC, mouse corneal epithelium 20-23 |
| TLR4 | Lipid A | Gram −ve bacteria | LPS, Pseudomonas keratitis 17,24-26 |
| TLR5 | Flagellin | Gram −ve bacteria | Flagellin activates HCEC, inhibitory activity in mice27,28 |
| TLR7, 8¶ | ssRNA | ssRNA viruses | Herpes simplex virus22 |
| TLR9¶ | CpG-rich DNA | dsDNA viruses, Gram −ve, +ve bacteria |
Synthetic CpG-rich oligonucleotides, Herpes simplex keratitis19,29 |
| TLR10 | Unknown, but may dimerize with TLR230 |
Unknown | Unknown |
| TLR11 | Profilin, unknown bacterial ligand |
Toxoplasma Uropathogenic E coli 6,7 |
Unknown |
TLR RNA expression has been shown in a number of studies,31 but only TLR activity is indicated in this table.
HCEC: Human Corneal Epithelial Cells.
All TLRs are thought to form either heterodimers or homodimers to initiate the signaling cascade.
Intracellular TLRs (endosomal).
TLRs are important in regulating the response to infectious agents in the cornea. Sarangi and colleagues recently showed that Herpes simplex keratitis (HSK) lesions are reduced in TLR2–/– TLR9–/– and MyD88–/– mice, and that MyD88–/– mice also succumbed to lethal encephalitis.32 Consistent with this finding, Wuest et al showed that TLR9 is important in HSK-associated chemokine and Type I interferon (IFN) production in the cornea.29 In Pseudomonas aeruginosa keratitis, TLR4-deficient mice on a BALB/c background were susceptible to infection with this organism and showed increased corneal disease compared with BALB/c mice.24 Staphylococcus aureus also causes severe keratitis in animal models4,33; however, the role of TLR2 versus IL-1R1 in systemic S. aureus infection is controversial. Although an essential role for MyD88 was found consistently, Takeuchi and coworkers showed that TLR2 has an essential role in bacterial virulence and clearance,34 whereas Miller and colleagues showed that IL-1R1 and not TLR2 is important.35 We showed that the TLR2/MyD88 pathway is essential for development of corneal inflammation induced by killed S. aureus,16 but their role in S. aureus corneal infection with live organisms has yet to be determined.
The TLR2/MyD88 pathway also mediates the host inflammatory response to endosymbiotic Wolbachia bacteria in a murine model of Onchocerca keratitis (river blindness).18,36-38 In this infection, it appears that O. volvulus microfilariae harboring Wolbachia cause no inflammatory response while present in the cornea; however, when the larvae die and Wolbachia are exposed to cells in the corneal stroma, a TLR2/TLR6 inflammatory response is induced.36 Recent studies identified Wolbachia proteoglycan-associated diacylated lipopeptides as a novel TLR2/TLR6 ligand that induces corneal inflammation (Taylor, Pearlman, unpublished observations). We also demonstrated recently that fungal keratitis caused by Fusarium is dependent on MyD88 and IL-R1, that fungal killing is dependent on TLR4, and that there is no apparent role for TLR2.5
IV. ROLE OF TLRS IN STERILE CORNEAL INFLAMMATION (TABLE 1)
Although microbial keratitis causes severe disease, clinical manifestations occur in the absence of active infection, mostly associated with contact lens wear. These include contact lens-associated red eye (CLARE), contact lens peripheral ulcers (CLPU), and contact lens-associated corneal infiltrates, which, although less severe than infectious keratitis, cause pain, redness, blurred vision, and severe discomfort.39-44 Given the number of contact lens wearers in the USA (34 million) and worldwide (~140 million), even a low percentage of contact lens wearers with side effects translates into a large number of affected individuals. Although the initial inflammatory stimulus is not known, it is reasonable to hypothesize that microbial breakdown products that activate TLRs would induce contact lens-associated corneal inflammation, based on the premises that: 1) the ocular surface is normally exposed to microbes and microbial products, often from contact lenses or lens cases43,45; 2) corneal biopsies of CLPU show that neutrophils have infiltrated the corneal stroma46; 3) topical exposure of injured rabbit or mouse corneal epithelium to LPS stimulates an inflammatory response25,47,48; and 4) human corneal epithelial cells express TLRs4 and TLRs5, and respond to bacteria and bacterial products.26-28
To examine whether direct activation of TLRs induces corneal inflammation, we developed a murine model in which we scarify the corneal epithelium of TLR and MyD88 gene knockout mice and added highly purified or synthetic TLR ligands. We found that activation of TLR2, TLR4, or TLR9 induced TLR-dependent secretion of CXC chemokines in the corneal epithelium, recruited neutrophils to the corneal stroma, and induced corneal haze/increased reflectivity, as measured by in vivo confocal microscopy; that these responses were dependent on MyD88 (Figure 2A).19,25 More recently, we demonstrated that TLR2 activation of human corneal epithelial cells in vitro or mouse corneal epithelium in vivo induced MAP kinase and IkB phosphorylation. We also used antagonists and gene knockout mice to demonstrate that c-Jun kinase (JNK) phosphorylation is an essential step in TLR2 corneal inflammation (Figure 3).14 Together, these findings lead us to conclude that specific activation of TLRs in the corneal epithelium induces corneal inflammation.
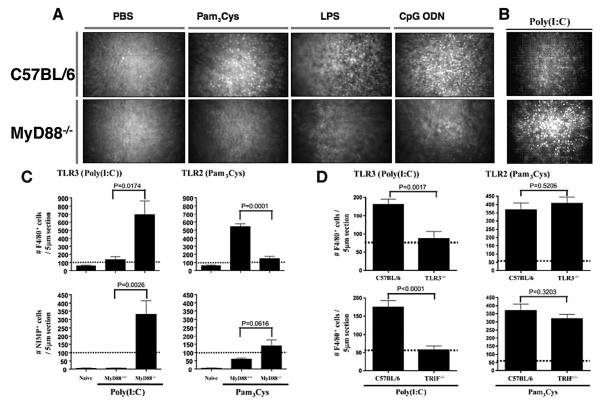
Figure 2.
A. TLR2-, TLR4, and TLR9-induced corneal inflammation is MyD88-dependent. Corneas of control and MyD88–/– mice were abraded and treated with PBS, Pam3Cys, LPS, or CpG ODN. After 24 h, cellular infiltration to the corneal stroma and stromal thickness and haze were determined by in vivo confocal microscopy (Confoscan™). Representative images of the central corneal stroma show a cellular infiltrate in wild type mice (upper panels) but not in MyD88–/– mice (lower panels). (Reprinted from Johnson AC, Heinzel FP, Diaconu E, et al19 with permission from Invest Ophthalmol Vis Sci.)
B and C: Exacerbated TLR3/TRIF responses in MyD88–/– mice. C57BL/6 or MyD88–/– corneas were abraded and treated with Poly(I:C) for 72 h, then examined by confocal microscopy or after immunohistochemical analysis for macrophages and neutrophils. Representative Confoscan™ images of the central corneal stroma demonstrate the severity of Poly(I:C)-induced inflammation in MyD88–/– mice (B), and increased neutrophils and macrophages (C). Note that TLR3/TRIF-induced responses were also enhanced in human corneal epithelial cells treated with siRNA to knockdown MyD88, but not in MyD88–/– bone marrow-derived macrophages (see Section v).
D. TLR3/TRIF-induced corneal inflammation. Poly(I:C) was added topically to the abraded corneal epithelium of C57BL/6, TLR3–/–, and TRIF–/– mice. After 72 h, corneas were processed for immunohistochemistry, and macrophages were detected using Ab to F4/80. (B-D reprinted from Johnson AC, Li X, Pearlman E.20 In accordance with policy of the Rockefeller University Press, the authors retain copyright to material published in J Biol Chem.)
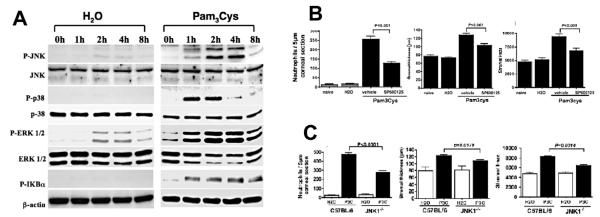
Figure 3.
A. Role of MAP kinases in TLR2-induced corneal inflammation. C57BL/6 corneas were gently scarified by three parallel scratches and treated with either H2O (trauma control) or with the TLR2 ligand Pam3Cys. At indicated times, corneas were dissected and processed for SDS-PAGE and Western blot analysis. Each sample represents a pool of two corneas and is representative of two repeat experiments.
B. SP600125 inhibits TLR2-induced corneal inflammation. C57BL/6 mice were treated topically with either SP600125 or with vehicle alone 1 h prior to, at the same time as, and 6 h after stimulation with Pam3Cys. After 24 h, neutrophils in the corneal stroma were detected by immunohistochemistry (A), and corneal thickness and haze were measured by in vivo confocal microscopy (B, C).
C. TLR2-induced corneal inflammation in JNK1–/– mice. Corneas of JNK-1–/– and C57BL/6 mice were scarified as described above and stimulated by topical application of Pam3Cys. (Reprinted from Adhikary G, Sun Y, Pearlman E14 with permission from J Leukoc Biol.)
While we and others have shown that TLR activation of corneal epithelium in animal models induced an inflammatory response, the ability of human corneal epithelial cells to respond to direct stimulation has been controversial. Whereas Kumar et al showed that TLR2 is expressed on the corneal surface and responds to TLR2 ligands and S. aureus,15 Ueta et al reported that TLR2 and TLR4 are intracellular and do not respond to ligands.17 Although these discrepancies have yet to be explained, and may be related to differences in the source of cells, we also showed that human corneal epithelial cells respond to Pam3Cys and to killed S. aureus.14 Intestinal epithelial cells can only respond to endotoxin if LBP, sCD14, and soluble MD-2 are added exogenously, and each of these proteins is likely to be present in biological fluids at the concentrations needed to drive endotoxin-dependent TLR4 activation.49
However, the ability of corneal epithelial cells to respond directly to LPS is less clear. Blais and colleagues showed that human lacrimal glands express TLR4 and MD-2, in addition to LBP and CD14, indicating that tears may be a source of exogenous MD-2.50 In addition, autologous MD-2 expression can also be induced by tumor necrosis factor (TNF)-α or IFN-γ in intestinal epithelial cells,51 or by IFN-γ in conjunctival epithelial cells.52 However, Visintin demonstrated conclusively that LPS responses are completely dependent on MD-2,53 and data from our laboratory support this finding (Pearlman, unpublished observations).
MD-2 is a bifunctional, 25-kD protein that is absolutely essential for TLR4 signaling by coupling endotoxin recognition to TLR4 activation. The sensitivity of TLR4-dependent cell activation by the lipid A moiety of LPS is due to sequen-tial interactions with these accessory proteins. Gioannini and colleagues showed that LBP and CD14 combine to extract single endotoxin molecules from endotoxin aggregates or from bacterial outer membrane and form monomeric endotoxin: CD14 complexes.47 LPS is then transferred from CD14 to MD-2, which together with the other accessory molecules, facilitate recognition of endotoxin at very low (picomolar) concentrations.9,54
LBP and CD14 gene knockout mice have reduced LPS binding and activation, whereas MD-2–/– mice have the same nonresponsive endotoxemia phenotype as TLR4–/– mice.55 The essential role for MD-2 in LPS signaling has been extensively demonstrated by functional studies,55,56 and by crystal structure analysis of the resting form of MD-2 alone and in association with antagonist lipid A.57 Given these combined findings, MD-2 can now be considered the recognition component of the LPS receptor complex, whereas TLR4 is the signal transduction element.
V. TLR3- AND TRIF-DEPENDENT RESPONSES IN THE CORNEA
The studies described above focused primarily on the TLR responses associated with the MyD88-dependent pathway; overall, in vitro and in vivo evidence support a role for TLR2, TLR4, TLR5 and TLR9, which all signal through MyD88. With the exception of TLR4, no MyD88-independent pathway is involved. As shown in Figure 1, TLR3 signals exclusively through TRIF, which activates transcription factors NFkB, AP-1, and also IRF-3, which induces type I interferon production and antiviral activity.
Ueta et al23 and Kumar et al21 reported that TLR3 mRNA is expressed in corneal epithelial cells and showed that activation with the synthetic TLR3 agonist Poly(I:C) induces production of IL-6, IL-8, IFN-β and expression of other antiviral genes. These findings support the notion that corneal epithelial cells have a functional TRIF pathway in addition to the MyD88 pathway, even though this was not directly shown.
To address directly the role of TLR3 and TRIF in corneal inflammation, we used gene knockout mice to demonstrate that Poly(I:C) activation of the corneal epithelium induces a predominant macrophage infiltrate in the corneal stroma of C57BL/6 mice, but not TLR3–/– or TRIF–/– mice (Figure 2C).20 In the same study, we showed that siRNA knockdown of TRIF in human corneal epithelial cells ablated Poly(I:C)-induced cytokine production, thereby demonstrating that the TRIF pathway is functional in corneal epithelial cells and that activation through TLR3/TRIF induces corneal inflammation.
Of particular interest, we found that Poly(I:C) stimulation of MyD88-/- corneas induced an exacerbated inflammatory response, with increased neutrophil and macrophage infiltration of the corneal stroma, resulting in increased reflectivity, as measured by in vivo confocal microscopy (Figure 2B, D).20 In support of this observation, MyD88 knockdown (by siRNA) in corneal epithelial cells had elevated Poly(I:C)-induced cytokine production that was dependent on JNK.20
Taken together, these findings reveal a novel regulatory mechanism in epithelial cells, as Poly(I:C)-induced corneal disease is exacerbated in the absence of functional MyD88 protein. Activity that is normally tightly controlled by MyD88 likely includes antiviral TLR3/TRIF responses and possibly TLR4/TRIF responses, although this has yet to be examined. We found a similar regulation role of MyD88 in lung epithelial cells (unpublished observations), but not in macrophages,20 indicating that this novel regulatory mechanism may be specific for epithelial cells. These observations increase our general understanding of the role of MyD88 in TLR signaling by demonstrating that this adaptor molecule functions as a negative regulator of the TLR3/TRIF-induced JNK activation in corneal epithelial cells.
VI. ROLE OF DENDRITIC CELLS AND MACROPHAGES IN TLR RESPONSES IN THE CORNEA
Although most studies have focused on the role of corneal epithelial cells in responding to microbial products, the presence of Langerhans-like dendritic cells in the peripheral cornea has long been known.58-60 Furthermore, time lapse, 2-photon microscopy analysis showed that these cells exhibit lateral movement in resting stage, which is increased after injury and is inhibited by IL-1 receptor antagonist.61 In addition, we recently demonstrated that CX3CR1 (Fractalkine receptor) is important for homing of Langerhans-like dendritic cells to the corneal epithelium, but not in homing of dendritic cells or macrophages to the corneal stroma.62 Resident macrophages and dendritic cells are also present in the corneal stroma of normal mice,63-66 and we recently showed that dendritic cells in the corneal stroma extend membrane nanotubes that are likely important for cell-cell communication and are increased after LPS stimulation (Figure 4).67 Our first consideration was that these cells contribute to development of corneal inflammation, and subsequent experiments using chimeric mice confirmed this notion. In particular, as macrophages and dendritic cells likely express TLRs at higher levels than epithelial cells, the role of bone marrow-derived cells in TLR-induced corneal inflammation has yet to be fully determined; however, our preliminary data indicates that bone marrow-derived cells are important in TLR4 but not TLR2 responses,68 which is consistent with reports (discussed in section IV) that corneal epithelial cells respond to TLR2, but not to TLR4.14-17,19,31 However, we found that LPS responses in corneas of TLR4–/– mice could be reconstituted by transfer of TLR4+ bone marrow cells, indicating that epithelial dendritic cells are important in initiating LPS-induced corneal inflammation.68
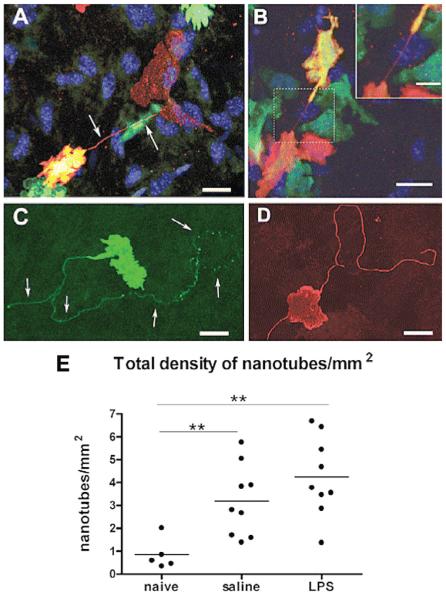
Figure 4.
Membrane nanotubes in the corneal stroma. C57BL/6 mice were irradiated and given a bone marrow transplant from eGFP mice as described,66 and were either untreated or stimulated by topical application of LPS. Corneal whole mounts were immunostained and examined by confocal microscopy.
A. Donor-derived (GFP+/green) MHC class II+ (red) and double positive (yellow) cell connecting via a fine membrane nanotube (arrows) to a resident MHC class II+ GFP negative cell (red).
B. GFP+ donor-derived cells expressing MHC class II+ cells appear to connect by a membrane nanotube (inset shows higher magnification).
C and D. Long, nonbridged membrane nanotubes on MHC class II+ cells in the naive (C) and inflamed (D) mouse corneal stroma. Connected neighboring cells were not identifiable. Scale bars = 20 μm; inset scale bar = 10 μm. E. Frequency of membrane nanotubes. Pooled data from all regions of the cornea reveal a higher density of nanotubes in inflamed corneas (both saline- and LPS-treated eyes) compared with naive corneas. (Reprinted from Chinnery HR, Pearlman E, McMenamin PG67 with permission from J Immunol).
VII. SUMMARY AND CONCLUSIONS: TLRs AS POTENTIAL TARGETS OF THERAPY
Studies presented in this review highlight the role of TLRs in microbial infection and in development of corneal inflammation in which live bacteria are not involved. Conversely, blockade of this pathway has the potential to inhibit corneal inflammation. To this end, several approaches have been taken to target the TLR responses in the cornea to reduce inflammation and regulate bacterial replication, silencing TLR expression and identifying inhibitory pathways in TLR signaling.
Huang et al showed that targeting TLR9 using siRNA reduced the inflammatory response and decreased bacterial clearance in P. aeruginosa keratitis.69 Conversely, P. aeruginosa keratitis was exacerbated in mice in which the inhibitory adaptor molecule SIGIRR was deleted.70 Furthermore, Kumar et al showed that the TLR5 ligand flagellin not only has a suppressive effect on corneal disease caused by P. aeruginosa, but also enhances bacterial clearance by upregulating expression of antimicrobial peptides by corneal epithelial cells.27,71
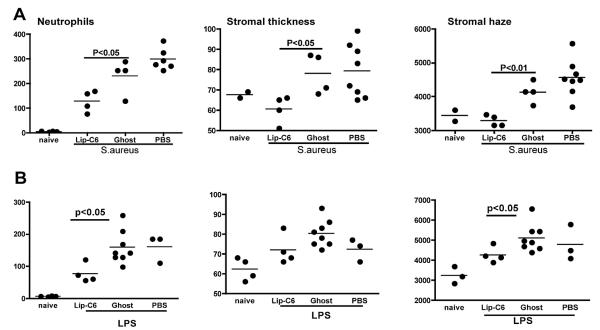
A critical stage in TLR-induced corneal inflammation is regulation of neutrophil recruitment to the corneal stroma. We recently demonstrated that short-chain ceramide (C6) in nanoparticle formulation inhibits TLR-induced CXC chemokine production by corneal epithelial cells via a JNK-dependent mechanism. It also inhibits neutrophil activation, and topical application of C6 nanoparticles blocks TLR2 and TLR4-induced corneal inflammation72 (Figure 5). In summary, it has become clear that an increased understanding of the role of TLRs at the ocular surface has set the stage for translational research on TLRs and TLR signaling pathways as potential targets for immune or therapeutic intervention.
Figure 5.
Effect of topical C6 ceramide in nanoparticle formulation (Lip-C6) on S. aureus- and LPS-induced corneal inflammation. The central corneal stroma of C57BL/6 mice was abraded as described,72 and 2nMoles (811ng) topical Lip-C6 was given 1 h before and 6 h after exposure to heat-killed S. aureus. After 24 h, neutrophils were detected by immunohistochemistry, and corneal thickness and haze were calculated as described in references 38, 39, and 69. Data points represent individual corneas from groups of 4-8 mice. (Reprinted from Sun Y, Fox T, Adhikary G, et al72 with permission from J Leukoc Biol.)
Acknowledgments
This research was funded by NIH grants RO1EY14362 (EP) and P30EY11373 (EP), and with support from The Research to Prevent Blindness Foundation and the Ohio Lions Eye Research Foundation.
ABBREVIATIONS AND GLOSSARY OF TERMS
- Chemokine
One of a large group of proteins that attract white blood cells and are involved in a wide variety of processes, including acute and chronic types of inflammation, infectious diseases, and cancer. Most chemokines belong to one of two major subfamilies based on organization of N-terminal cysteines: CXC and CC subfamilies.
- CC chemokine
Primarily a chemoattractant for monocytes, lymphocytes, basophils, and eosinophils.
- CXC chemokine
Subgroup with N terminal elr motif, including CXCL1, 2, 5, 8 (IL-8), are chemoattractant for neutrophils; others, including CXCl9 and CXCL10, are chemotactic for lymphocytes.
- CX3CR1
A third family of chemokines that to date has only one member. This chemokine (also called fracktalkine) is important in dendritic cell homing to tissues, including the corneal epithelium (see Section VI).
- IFN (interferon)
Type I IFNs include IFN-α and IFN-β which are produced by most nucleated cells and are important in antiviral responses. Type II IFNs include IFN-γ, which is produced by CD4 and CD8 T cells and natural killer cells, and which activates macrophages.
- IRAK
IL-1 receptor-associated kinase involved in signaling of Tlrs and IL-1R.
- LBP (Lipopolysaccharide-binding protein)
Serum protein that binds LPS.
- Lipoprotein / Lipopeptide
Cell wall components of Gram negative and Gram positive bacteria comprising acylated (lipid attached) proteins or peptides and which activate TLR2 on host cells.
- LPS (Lipopolysaccharide)
Major outer membrane component of Gram negative bacteria comprising a lipid a core (endotoxin) and polysaccharide (carbohydrate) of varying length and composition. TLR4/MD-2 bind and respond to the lipid a moeity.
- MD-2 (myeloid differentiation-2)
A small secreted glycoprotein that binds to lipid a of LPS and the extracellular domain of TLR4. Now considered to be the recognition component of the LPS receptor complex.
- MAPK
Mitogen-activated protein kinases. These include JNK (c-Jun N-terminal kinases), p38, and ERK (extracellular signal-regulated kinases).These are also known as stress-activated protein kinases (SAPKs). They are critical mediators in signaling pathways, including TLR signaling, where they activate transcription factors leading to gene transcription.
- MyD88
Adaptor molecule activated by all TLRs except TLR3, which activates the Tlr signaling pathway and transfers signal from Tlr receptor to downstream proteins (IRAK4), resulting in the NFκB activation. This adaptor molecule is also used by IL-1R and IL-18R, and provides a platform for recruitment of kinases, primarily IRAK-4 and IRAK-1.
- TAK (Transforming growth factor-beta (TGF-β) kinase)
Signaling pathway upstream of MAP kinases.
- TIR (Toll/IL-1R intracellular domain)
Region of the TLR that binds adaptor molecules MyD88 or TRIF. Death domain is a structure common to other receptors (including those involved in apoptosis; hence the name), but is the site on an adaptor molecule that interacts with the intracellular domains of Tlrs.
- TLR (Toll-like receptor)
See text.
- TRIF
TIR domain-containing adaptor inducing IFN-β.
- TRAM
TRIF-related adaptor molecule
Footnotes
Disclosures: Inhibitory studies using C6 ccramide (Figure 5, International Patent Application no. PCT/US2007/072905) were partially funded by Bausch and Lomb Inc. (EP). MK has licensed nanotechnologies with TRACON Pharmaceuticals and is Chief Medical Officer for KeystoneNano, Inc.
The other authors have no proprietary or commercial interest in any concept or product discussed in this article.
REFERENCES
- 1.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–82. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 2.Hazlett LD. Bacterial infections of the cornea (Pseudomonas aeruginosa) Chem Immunol Allergy. 2007;92:185–94. doi: 10.1159/000099269. [DOI] [PubMed] [Google Scholar]
- 3.Pearlman E, Gillette-ferguson I. Onchocerca volvulus, Wolbachia and river blindness. Chem Immunol Allergy. 2007;92:254–65. doi: 10.1159/000099276. [DOI] [PubMed] [Google Scholar]
- 4.Hume EB, Dajcs JJ, Moreau JM, et al. Staphylococcus corneal virulence in a new topical model of infection. Invest Ophthalmol Vis Sci. 2001;42:2904–8. [PubMed] [Google Scholar]
- 5.Tarabishy AB, Aldabagh B, Sun Y, et al. MyD88 regulation of fusarium solani keratitis is dependent on TLR4 and IL-1R1, but not TLR2. J Immunol. 2008;181:593–600. doi: 10.4049/jimmunol.181.1.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yarovinsky F, Zhang D, Andersen JF, et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–9. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 7.Zhang D, Zhang G, Hayden M, et al. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–6. doi: 10.1126/science.1094351. S. [DOI] [PubMed] [Google Scholar]
- 8.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Gioannini TL, Teghanemt A, Zhang D, et al. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci U S A. 2004;101:4186–91. doi: 10.1073/pnas.0306906101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–64. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 11.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–12. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 12.Pearson GF, Robinson F, Gibson T Beers, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–83. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 13.Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007;76:447–80. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- 14.Adhikary G, Sun Y, Pearlman E. C-Jun Nh2 terminal kinase (JNK) is an essential mediator of Toll-like receptor 2-induced corneal inflammation. J Leukoc Biol. 2008;83:991–7. doi: 10.1189/jlb.1107783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar A, Zhang J, Yu FS. Toll-like receptor 2-mediated expression of beta-defensin-2 in human corneal epithelial cells. Microbes Infect. 2006;8:380–9. doi: 10.1016/j.micinf.2005.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y, Hise AG, Kalsow CM, Pearlman E. Staphylococcus aureus-induced corneal inflammation is dependent on Toll-like receptor 2 and myeloid differentiation factor 88. Infect Immun. 2006;74:5325–32. doi: 10.1128/IAI.00645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueta M, Nochi T, Jang MH, et al. Intracellularly expressed TLR2s and TLR4s contribution to an immunosilent environment at the ocular mucosal epithelium. J Immunol. 2004;173:3337–47. doi: 10.4049/jimmunol.173.5.3337. [DOI] [PubMed] [Google Scholar]
- 18.Gillette-ferguson I, Daehnel K, Hise AG, et al. Toll-like receptor 2 regulates CXC chemokine production and neutrophil recruitment to the cornea in Onchocerca volvulus/Wolbachia-induced keratitis. Infect Immun. 2007;75:5908–15. doi: 10.1128/IAI.00991-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson AC, Heinzel FP, Diaconu E, et al. Activation of toll-like receptor (TLR)2, TLR4, and TLR9 in the mammalian cornea induces MyD88-dependent corneal inflammation. Invest Ophthalmol Vis Sci. 2005;46:589–95. doi: 10.1167/iovs.04-1077. [DOI] [PubMed] [Google Scholar]
- 20.Johnson AC, Li X, Pearlman E. MyD88 functions as a negative regulator of TLR3/TRIF-induced corneal inflammation by inhibiting activation of c-Jun N-terminal kinase. J Biol Chem. 2008;283:3988–96. doi: 10.1074/jbc.M707264200. [DOI] [PubMed] [Google Scholar]
- 21.Kumar A, Zhang J, Yu FS. Toll-like receptor 3 agonist poly(I: c)-induced antiviral response in human corneal epithelial cells. Immunology. 2006;117:11–21. doi: 10.1111/j.1365-2567.2005.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Zhang J, Kumar A, et al. Herpes simplex virus 1 infection induces the expression of proinflammatory cytokines. Biochem Biophys Res Commun. 2005;331:285–294. [Google Scholar]
- 23.Ueta M, Hamuro J, Kiyono H, Kinoshita S. Triggering of TLR3 by polyI:c in human corneal epithelial cells to induce inflammatory cytokines. Biochem Biophys Res Commun. 2005;331:285–94. doi: 10.1016/j.bbrc.2005.02.196. [DOI] [PubMed] [Google Scholar]
- 24.Huang X, Du W, Mcclellan SA, et al. TLR4 is required for host resistance in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2006;47:4910–6. doi: 10.1167/iovs.06-0537. [DOI] [PubMed] [Google Scholar]
- 25.Khatri S, Lass JH, Heinzel FP, et al. Regulation of endotoxin-induced keratitis by PECAM-1, MIP-2, and toll-like receptor 4. Invest Ophthalmol Vis Sci. 2002;43:2278–84. [PubMed] [Google Scholar]
- 26.Song PI, Abraham TA, Park Y, et al. The expression of functional LPS receptor proteins CD14 and toll-like receptor 4 in human corneal cells. Invest Ophthalmol Vis Sci. 2001;42:2867–77. [PubMed] [Google Scholar]
- 27.Kumar A, Yin J, Zhang J, Yu FS. Modulation of corneal epithelial innate immune response to pseudomonas infection by flagellin pretreatment. Invest Ophthalmol Vis Sci. 2007;48:4664–70. doi: 10.1167/iovs.07-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Xu K, Ambati B, Yu FS. Toll-like receptor 5-mediated corneal epithelial inflammatory responses to Pseudomonas aeruginosa flagellin. Invest Ophthalmol Vis Sci. 2003;44:4247–54. doi: 10.1167/iovs.03-0219. [DOI] [PubMed] [Google Scholar]
- 29.Wuest T, Austin BA, Uematsu S, et al. Intact TRL 9 and type I interferon signaling pathways are required to augment HSV-1 induced corneal CXCL9 and CXCL10. J Neuroimmunol. 2006;179:46–52. doi: 10.1016/j.jneuroim.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasan U, Chaffois C, Gaillard C, et al. Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J Immunol. 2005;174:2942–50. doi: 10.4049/jimmunol.174.5.2942. [DOI] [PubMed] [Google Scholar]
- 31.Kumar A, Yu FS. Toll-like receptors and corneal innate immunity. Curr Mol Med. 2006;6:327–37. doi: 10.2174/156652406776894572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarangi PP, Kim B, Kurt-Jones E, Rouse BT. Innate recognition network driving herpes simplex virus-induced corneal immunopathology: role of the toll pathway in early inflammatory events in stromal keratitis. J Virol. 2007;81:11128–38. doi: 10.1128/JVI.01008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Girgis DO, Sloop GD, Reed JM, O’callaghan RJ. A new topical model of Staphylococcus corneal infection in the mouse. Invest Ophthalmol Vis Sci. 2003;44:1591–97. doi: 10.1167/iovs.02-0656. [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi O, Hoshino K, Akira S. Cutting edge: Tlr2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165:5392–6. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 35.Miller LS, O’connell RM, Gutierrez MA, et al. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Hise AG, Daehnel K, Gillette-ferguson I, et al. Innate immune re-sponses to endosymbiotic Wolbachia bacteria in Brugia malayi and Onchocerca volvulus are dependent on TLR2, TLR6, MyD88, and Mal, but not TLR4, TRIF, or TRAM. J Immunol. 2007;178:1068–76. doi: 10.4049/jimmunol.178.2.1068. [DOI] [PubMed] [Google Scholar]
- 37.Daehnel K, Gillette-ferguson I, Hise AG, et al. Filaria/Wolbachia activation of dendritic cells and development of Th1-associated responses is dependent on Toll-like receptor 2 in a mouse model of ocular onchocerciasis (river blindness) Parasite Immunol. 2007;29:455–65. doi: 10.1111/j.1365-3024.2007.00962.x. [DOI] [PubMed] [Google Scholar]
- 38.Gillette-Ferguson I, Hise AG, Sun Y, et al. Wolbachia- and Onchocerca volvulus-induced keratitis (river blindness) is dependent on myeloid differentiation factor 88. Infect Immun. 2006;74:2442–5. doi: 10.1128/IAI.74.4.2442-2445.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stapleton F, Keay L, Jalbert I, Cole N. The epidemiology of contact lens related infiltrates. Optom Vis Sci. 2007;84:257–72. doi: 10.1097/OPX.0b013e3180485d5f. [DOI] [PubMed] [Google Scholar]
- 40.Szczotka-Flynn L, Diaz M. Risk of corneal inflammatory events with silicone hydrogel and low dk hydrogel extended contact lens wear: a meta-analysis. Optom Vis Sci. 2007;84:247–56. doi: 10.1097/OPX.0b013e3180421c47. [DOI] [PubMed] [Google Scholar]
- 41.Szczotka-Flynn L, Debanne SM, Cheruvu VK, et al. Predictive factors for corneal infiltrates with continuous wear of silicone hydrogel contact lenses. Arch Ophthalmol. 2007;125:488–92. doi: 10.1001/archopht.125.4.488. [DOI] [PubMed] [Google Scholar]
- 42.Keay L, Edwards K, Naduvilath T, et al. Factors affecting the morbidity of contact lens-related microbial keratitis: a population study. Invest Ophthalmol Vis Sci. 2006;47:4302–8. doi: 10.1167/iovs.06-0564. [DOI] [PubMed] [Google Scholar]
- 43.Keay L, Edwards K, Naduvilath T, et al. Microbial keratitis predisposing factors and morbidity. Ophthalmology. 2006;113:109–16. doi: 10.1016/j.ophtha.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 44.Robboy MW, Comstock TL, Kalsow CM. Contact lens-associated corneal infiltrates. Eye Contact Lens. 2003;29:146–54. doi: 10.1097/01.ICL.0000072830.41886.1E. [DOI] [PubMed] [Google Scholar]
- 45.Bourcier T, Thomas F, Borderie V, et al. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87:834–8. doi: 10.1136/bjo.87.7.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holden BA, Reddy MK, Sankaridurg PR, et al. Contact lens-induced peripheral ulcers with extended wear of disposable hydrogel lenses: histopathologic observations on the nature and type of corneal infiltrate. Cornea. 1999;18:538–43. [PubMed] [Google Scholar]
- 47.Schultz CL, Morck DW, McKay SG, et al. Lipopolysaccharide induced acute red eye and corneal ulcers. Exp Eye Res. 1997;64:3–9. doi: 10.1006/exer.1996.0190. [DOI] [PubMed] [Google Scholar]
- 48.Schultz CL, Buret AG, Olson ME, et al. Lipopolysaccharide entry in the damaged cornea and specific uptake by polymorphonuclear neutrophils. Infect Immun. 2000;68:1731–4. doi: 10.1128/iai.68.3.1731-1734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gribar SC, Anand RJ, Sodhi CP, Hackam DJ. The role of epithelial Toll-like receptor signaling in the pathogenesis of intestinal inflammation. J Leukoc Biol. 2008;83:493–8. doi: 10.1189/jlb.0607358. [DOI] [PubMed] [Google Scholar]
- 50.Blais DR, Vascotto SG, Griffith M, Altosaar I. LBP and CD14 secreted in tears by the lacrimal glands modulate the LPS response of corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:4235–44. doi: 10.1167/iovs.05-0543. [DOI] [PubMed] [Google Scholar]
- 51.Abreu MT, Arnold ET, Thomas LS, et al. TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. J Biol Chem. 2002;277:20431–7. doi: 10.1074/jbc.M110333200. [DOI] [PubMed] [Google Scholar]
- 52.Talreja J, Dileepan K, Puri S, et al. Human conjunctival epithelial cells lack lipopolysaccharide responsiveness due to deficient expression of MD2 but respond after interferon-gamma priming or soluble MD2 supplementation. Inflammation. 2005;29:170–81. doi: 10.1007/s10753-006-9014-y. [DOI] [PubMed] [Google Scholar]
- 53.Visintin A, Halmen KA, Khan N, et al. MD-2 expression is not required for cell surface targeting of Toll-like receptor 4 (TLR4) J Leukoc Biol. 2006;80:1584–92. doi: 10.1189/jlb.0606388. [DOI] [PubMed] [Google Scholar]
- 54.Gioannini TL, Teghanemt A, Zhang D, et al. Monomeric endotoxin: protein complexes are essential for TLR4-dependent cell activation. J Endotoxin Res. 2005;11:117–23. doi: 10.1179/096805105X35198. [DOI] [PubMed] [Google Scholar]
- 55.Visintin A, Iliev DB, Monks BG, et al. Md-2. Immunobiology. 2006;211:437–47. doi: 10.1016/j.imbio.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 56.Shimazu R, Akashi S, Ogata H, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–82. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohto U, Fukase K, Miyake K, Satow Y. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science. 2007;316:1632–4. doi: 10.1126/science.1139111. [DOI] [PubMed] [Google Scholar]
- 58.Hazlett LD, Moon MM, Dawisha S, Berk RS. Age alters ADPase positive dendritic (langerhans) cell response to P. aeruginosa ocular challenge. Curr Eye Res. 1986;5:343–55. doi: 10.3109/02713688609025172. [DOI] [PubMed] [Google Scholar]
- 59.Gillette TE, Chandler JW, Greiner JV. Langerhans cells of the ocular surface. Ophthalmology. 1982;89:700–11. doi: 10.1016/s0161-6420(82)34737-5. [DOI] [PubMed] [Google Scholar]
- 60.Rodrigues MM, Rowden G, Hackett J, Bakos I. Langerhans cells in the normal conjunctiva and peripheral cornea of selected species. Invest Ophthalmol Vis Sci. 1981;21:759–65. [PubMed] [Google Scholar]
- 61.Ward BR, Jester JV, Nishibu A, et al. Local thermal injury elicits immediate dynamic behavioural responses by corneal langerhans cells. Immunology. 2007;120:556–72. doi: 10.1111/j.1365-2567.2006.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chinnery HR, Ruitenberg MJ, Plant GW, et al. The chemokine receptor CX3CR1 mediates homing of Mhc class II-positive cells to the normal mouse corneal epithelium. Invest Ophthalmol Vis Sci. 2007;48:1568–74. doi: 10.1167/iovs.06-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci. 2002;43:2264–71. [PMC free article] [PubMed] [Google Scholar]
- 64.Hamrah P, Liu Y, Zhang Q, Dana MR. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest Ophthalmol Vis Sci. 2003;44:581–9. doi: 10.1167/iovs.02-0838. [DOI] [PubMed] [Google Scholar]
- 65.Nakamura T, Ishikawa F, Sonoda KH, et al. Characterization and distribution of bone marrow-derived cells in mouse cornea. Invest Ophthalmol Vis Sci. 2005;46:497–503. doi: 10.1167/iovs.04-1154. [DOI] [PubMed] [Google Scholar]
- 66.Chinnery HR, Humphries T, Clare A, et al. Turnover of bone marrow-derived cells in the irradiated mouse cornea. Immunology. 2008 doi: 10.1111/j.1365-2567.2008.02868.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chinnery HR, Pearlman E, McMenamin PG. Cutting edge: Membrane nanotubes in vivo: a feature of Mhc class II+ cells in the mouse cornea. J Immunol. 2008;180:5779–83. doi: 10.4049/jimmunol.180.9.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chinnery HR, Sun Y, Carlson E, et al. TLR ligand-induced keratitis is partially reconstituted in TLR-/- chimeric mice by donor TLR+ bone marrow-derived cells in the corneal stroma. Invest Ophthalmol Vis Sci. 2008;(suppl) e-abstract 498. [Google Scholar]
- 69.Huang X, Barrett RP, Mcclellan SA, Hazlett LD. Silencing Toll-like receptor-9 in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2005;46:4209–16. doi: 10.1167/iovs.05-0185. [DOI] [PubMed] [Google Scholar]
- 70.Huang X, Hazlett LD, Du W, Barrett RP. SIGIrr promotes resistance against Pseudomonas aeruginosa keratitis by down-regulating type-1 immunity and IL-1R1 and TLR4 signaling. J Immunol. 2006;177:548–56. doi: 10.4049/jimmunol.177.1.548. [DOI] [PubMed] [Google Scholar]
- 71.Kumar A, Hazlett LD, Yu FS. Flagellin suppresses the inflammatory response and enhances bacterial clearance in a murine model of Pseudomonas aeruginosa keratitis. Infect Immun. 2008;76:89–96. doi: 10.1128/IAI.01232-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun Y, Fox T, Adhikary G, et al. Inhibition of corneal inflammation by liposomal delivery of short-chain, C-6 ceramide. J Leukoc Biol. 2008;83:1512–21. doi: 10.1189/jlb.0108076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson A, Pearlman E. Toll-like receptors in the cornea. Ocul Surf. 2005;3(4 suppl):S187–9. doi: 10.1016/s1542-0124(12)70252-5. 2005. [DOI] [PubMed] [Google Scholar]