Abstract
The cross-linking with reducing sugars, known as glycation, is used to increase stiffness and strength of tissues and artificial collagen-based scaffolds. Non-destructive characterization methods that report on the structures within these materials could clarify the effects of glycation. For doing this non-destructive evaluation, we employed an in-situ one-photon fluorescence as well as multiphoton microscopy method that combined two-photon fluorescence and second harmonic generation signals. We incubated collagen hydrogels with glyceraldehyde, ribose and glucose and observed an increase in the in situ fluorescence and structural alterations within the materials during the course of glycation. The two-photon fluorescence emission maximum was observed at about 460 nm. The fluorescence emission in the one-photon excitation experiment (λex=360 nm) was broad with peaks centered at 445 nm and 460 nm. The 460 nm emission component subsequently became dominant during the course of glycation with glyceraldehyde. For the ribose, in addition to the 460 nm peak, the 445 nm component persisted. The glucose glycated hydrogels, exhibited broad fluorescence that did not increase significantly even after six weeks. As determined from measuring the fluorescence intensity at 460 nm maximum, glycation with glyceraldehyde was faster compared to ribose and generated stronger fluorescence signals. Upon excitation of glycated samples with 330 nm light different emission peaks were observed.
Introduction
In spite of the adverse ageing effects of glycation1-4 in vivo, in vitro this process is widely employed to increase stiffness and strength of tissues’ and artificial scaffolds’.2, 3, 5-7 Most present studies, however, are focused on following changes in the mechanical strengths of the glycated samples after the exposures to reducing sugars with little reference to the structures within. Characterization of the microstructures is necessary to clarify the effects of non-enzymatic crosslinking on tissues and biologically derived scaffolds.
We now report the effects of non-enzymatic crosslinking with glyceraldehyde, ribose and glucose on three-dimensional (3D) collagen-based hydrogels characterized by in-situ multi-photon microscopy (MPM) and fluorescence spectroscopy. The data and methods developed provide an outline to follow the mechanisms of glycation of protein-based materials by reducing sugars in situ.
MPM is a non-destructive optical method that utilizes femto-second pulses of near-infrared (NIR) laser light and performs high resolution and three-dimension imaging of biological samples. Its advantages include reduced scattering because NIR wavelengths are employed, no out-of focus absorption, very small sample volumes and deeper tissue penetration compared to confocal microscopy.8 The interaction between fibrillar collagen and NIR pulsed, femto-second laser light of MPM has been identified to result in second harmonic generation (SHG) and two-photon excited fluorescence (TPF)8, 9 signals. SHG is produced when photons interacting with fibrillar collagen are combined to form new photons with exactly twice the energy.10 SHG gained recognition in tissue imaging8-17 because this source of contrast resists photo-bleaching and had been used to successfully image structural proteins in various non-animal and animal sources18 with strong enhancement suitable for biomedical assessment of tissue structure.17 It is believed that the interaction between laser pulses and collagen’s non-centrosymmetric, triple helix structure in addition to molecular packing within collagen materials leads to scattering from the tertiary (fibrils),17 and quaternary (fibers)10 level of organization thus producing SHG.19 The intrinsic fluorescence is generated by UV/VIS absorbing molecules and proteins. In collagen protein, the non-enzymatic glycation products collectively known as advanced glycation endproducts (AGEs)20 absorb in the near-UV (320-370 nm) and fluoresce in the 380 to 460 nm range.
EXPERIMENTAL SECTION
Collagen materials formation and cross-linking
Soluble rat-tail type I collagen, 9.58 mg/ml (BD Biosciences) was in 0.02N acetic acid. The purity of this stock solution was verified with 4%-20% Tris-HCl gels (Bio-Rad) following a standard protocol. The stock solution was diluted with 0.02N acetic acid to obtain the 2X collagen aliquots. The reverse pipetting technique was used to pipette collagen stock solution. 2X initiation buffer was prepared from NaCl and phosphate buffer. The concentration of mono-and dibasic phosphate in the buffer at pH=7.4 was calculated with Henderson-Hasselbalch equation. The pH was adjusted drop-wise with 1N NaOH or HCl. Ionic strength was adjusted with NaCl. The initiation buffer had the following components: 6.40 g/l K2HPO4; 3.16 g/l KH2PO4; 38.55 g/l NaCl (ionic strength = 0.6 M). After the pH was adjusted to a desired value, the initiation buffer was filtered with 0.22 μm, 25 mm syringe filter (Fisher) and stored at 4°C. Prior to the beginning of material formation, both 2X collagen aliquots and initiation buffer were de-airated by placing in a 1.5 L desiccator (Fisher) and applying house vacuum for 2 hrs. Material formation was initiated by mixing 2X collagen aliquot with 2X initiation buffer on ice at 1:1 ratio, verifying the pH to be 7.4±0.1, and then incubating at 37 °C.
For multi-photon microscopy measurements collagen materials were prepared in the 8-well chambered coverglass (MP Biomedicals). For one photon experiments collagen materials were prepared in 96-well plates (BD Falcon). In both cases, after 24 hour incubation at 37 °C, the materials were incubated in 0.1 M concentration of D,L-Glyceraldehyde, D-Ribose and D-Glucose (Sigma) at same temperature for the specified amount of times. The control reagents for the reducing sugars were 0.1 M sugar alcohols with the same number of carbons as respective reducing sugars. Glycerol, xylitol and sorbitol (Sigma) are the controls for glyceraldehyde, ribose and glucose respectively. When the samples incubated with ribose, glucose and sugar alcohols were stored for 6 weeks at 37° C, amphotercin B (Sigma) at 5.6 μg/ml final concentration and gentamicin sulphate salt (Sigma) at 50 μg/ml final concentration were added. The antibiotics were refreshed every couple of days.
One photon in-situ fluorescence measurements
Increase in fluorescence within the materials that was due to glycation was measured in situ, in a high-throughput format using a FlexStation microplate reader in the backscattering mode (Molecular Devices). The excitation wavelength was 360 nm and emission spectrum was collected from 420 to 530 nm. Additional excitation wavelength at 330 nm was used and the emission spectrum was collected from 360 nm to 500 nm. The spectra were collected with a 5 nm step.
Measurements for glyceraldehyde were taken every 1 h during the first 9 hrs and subsequently 2 measurements every 24 hrs; measurements for ribose and glucose were taken once every week. Per one experimental run, a spectrum was independently acquired from two wells. The background fluorescence was subtracted from each spectrum and the spectra were averaged. All experimental runs were repeated on different days and up to three times using identical settings for the measurements. The error bars are standard deviations from the mean.
After the fluorescence intensity reached the plateau, glyceraldehyde-glycated samples were shaken thoroughly in a warm (~30°C) 0.1 M Tris-HCl (pH 4.5) buffer as previously described2 to inactivate and remove adsorbed reducing sugars and/or other reactive carbonyl compounds.
In-situ multiphoton photon microscopy (MPM)
The inverted Zeiss LSM 510 NLO Meta laser scanning microscopy system (Carl Zeiss MicroImaging, Incorporated, Thornwood, New York) was used in this work. It was based on the Axiovert 200M inverted microscope equipped with standard illumination systems for transmitted light and epi-fluorescence detection and equipped with an NLO interface for a femto-second Titanium:Sapphire laser excitation source (Chameleon-Ultra, Coherent, Incorporated, Santa Clara, California) for multi-photon excitation. The Chameleon laser provided femto-second pulses at a repetition rate of about 80 MHz, with the center frequency tunable from 690 to 1040 nm. Long working distance objective (Zeiss, 40X water, N.A. 0.8) was used to acquire images shown in this work. The two-photon signals from the sample are epi-collected and discriminated by the short pass 650 nm dichroic beamsplitter. The SHG images were collected with the 390-465 nm band pass filter (excitation wavelength = 800 nm). The TPF images were collected using META detection module with emission sampled in a 405-501 nm detection range (excitation wavelength = 720 nm). Each image presented in this work is 12 bit, 512×512 pixels representing 225 um × 225 um field of view. To obtain the spectra we averaged about twenty-five regions of interest (ROIs) in the areas of the image that displayed glycation-induced fluorescence (sample) and subtracted about twenty averaged ROIs obtained from the image in the regions that did not have induced fluorescence (background). All the imaging and spectra were successfully reproduced on separate days, using up to eight independent samples on each day (about five field of views per sample).
RESULTS AND DISCUSSION
In-situ characterization of glycated collagen materials with one photon fluorescence
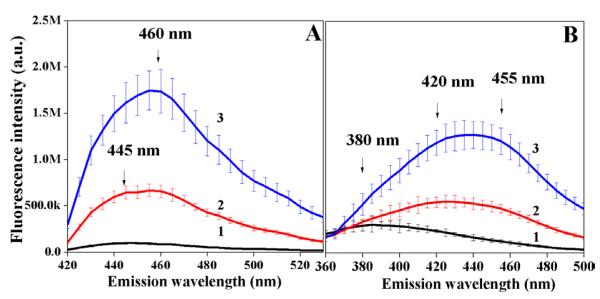
The incubation of acellular collagen hydrogels in 0.1 M solutions of reducing sugars caused the non-fluorescent samples to emit in-situ fluorescence. When the collagen hydrogels were exposed to glyceraldehydes and subsequently excited with 360 nm light, the detected fluorescence emission maximum observed was a broad band with peaks centered at 445 nm and 460 nm (Figure 1A). The 460 nm emission component subsequently became dominant as reaction progressed. The shift, however, didn’t occur for the materials incubated with ribose. In addition to a broad fluorescence maximum centered at 460 nm, the 445 nm component persisted (Supporting Information Figure S1A). The glucose glycated hydrogels, exhibited broad fluorescence that did not increase significantly even after 6 weeks (Supporting Information Figure S2A).
Figure 1.
The effect of glycation with 0.1 M glyceraldehyde on the production of advanced glycation endproducts. (A) Fluorescence emission spectra (λex = 360 nm) of the sample that was incubated with glyceraldehyde for 0 hr (1), 6 hr (2), 28 hr (3). (B) Fluorescence emission spectra (λex = 330 nm) of the sample that was incubated with glyceraldehyde for 0 hr (1), 6 hr (2), 29 hr (3). All samples were measured in triplicate, and error bars represent standard deviations from the mean.
The excitation of samples with a different wavelength of light (λex = 330 nm) produced a different fluorescence emission spectrum. For glyceraldehyde modified hydrogels, the 330 nm light excited fluorescence appeared in a form of a shoulder at 380 nm and very broad maximum with at least two peaks centered at 420 nm and 455 nm (Figure 1B). For the ribose and glucose glycated materials the 330 nm light excited fluorescence maximum was observed at about 385 nm until week 3 with a subsequent shift to about 410 nm by week 6 (Supporting Information Figure S1B and Figure S2B). For glucose an increase was minimal.
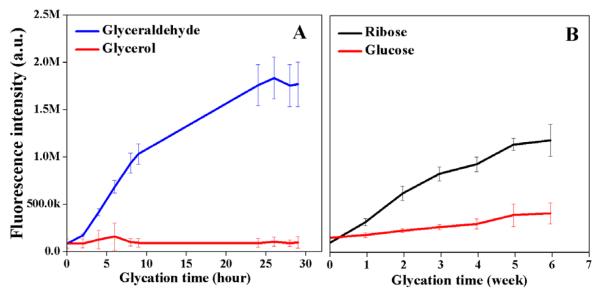
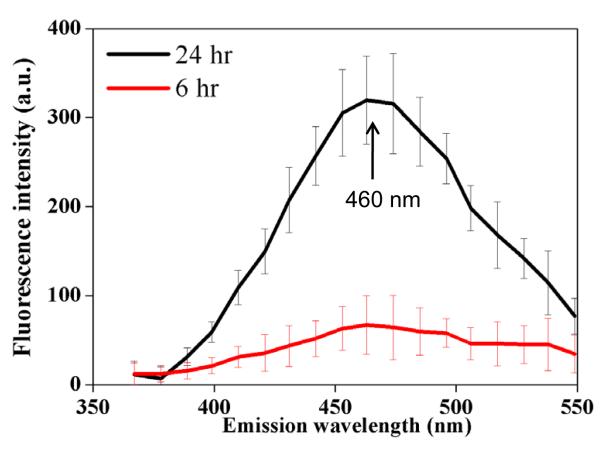
Overall, glycation of collagen hydrogels with glyceraldehyde produced significantly higher final fluorescence intensity at 460 nm (λex = 360 nm) compared to glycation with ribose or glucose and occurred with faster rate (Figure 2). The collagen hydrogels exposed to glyceraldehyde showed an increase in fluorescence intensity until reaching a plateau at twenty-five hours (Figure 2A). The materials exposed to ribose showed a significant increase in fluorescence intensity at six weeks (Figure 2B). There was virtually no increase in fluorescence intensity in the samples incubated with glucose. The controls consisting of collagen hydrogels incubated in solutions of sugar alcohols didn’t generate any detectable fluorescence.
Figure 2.
The effect of glycation with 0.1 M reducing sugars on the production of fluorescent advanced glycation endproducts. (A) The fluorescence intensity (λex/λem = 360 nm/460 nm) as a function of cross-linking time for glyceraldehyde and glycerol used as control. (B) The fluorescence intensity (λex/λem= 360 nm/460 nm) as a function of cross-linking time for ribose and glucose. All samples were measured in triplicate, and error bars represent standard deviations from the mean.
One photon spectroscopy is a common technique for the identification of fluorescence generating advanced glycation endproducts (AGEs). For the collagen samples glycated with glyceraldehyde we detected the fluorescence emission maximum around 460 nm (λex = 360 nm). This maximum is similar to fluorescence emission detected from biochemically identified lysine-OH-triosidine (LHT).2,4 Tessier et al.2,4 excited this product of non-enzymatic cross-linking formed by reacting glyceraldehyde and Nα - acetyl - L -lysine with 354 nm and observed fluorescence at 440 nm. Danilov et al.2, 4 treated rabbit scleral tissue with glyceraldehyde and observed glycation products in papain-digest solutions to fluoresce at 453 nm upon excitation with 370 nm light. Treating a bovine kidney tubular basement membrane with glucose21 lead to induction of 460 nm centered fluorescence emission (λex = 360 nm). All previous studies, therefore, suggest that three reducing sugars employed in our work induce the formation of the LHT fluorophore. Additional peaks, however, persisted when we excited collagen hydrogels treated with ribose and glucose with 360 nm light.
The excitation of the glycated collagen materials with 330 nm light showed a different fluorescence emission spectrum compared to one obtained upon excitation of samples with 360 nm light. This observed spectrum also differs from that measured in solution by other investigators.4 The 380 nm fluorescence emission that in our work appeared as a shoulder upon excitation of samples with 330 nm light had been previously attributed to fluorescence from arginine-OH- triosidine (AHT).2, 4,2, 4
Our observations imply that 1) the in-situ fluorescence of LHT and AHT fluorophores is more complex compared to that observed in solution digests. In situ, either the AHT and LHT fluorophores are localized in different physico-chemical microenvironments or more extensive diversity of fluorophores is formed upon glycation of collagen hydrogels. Although not previously observed in solution, the different fluorophores or different states of the same fluorophore could be detected when we carry out fluorescence measurements in-situ. 2) overall, the fluorescence emission peaks were different for glyceraldehyde treated hydrogels compared to those treated with ribose and glucose. This difference is due to either the different advanced glycation endproducts (AGEs) formed as a result of treatment by the three sugars or to the difference in the overall time scales of forming AGEs. The rate of glycation is thought to depend on the amount of sugar present in the open chain form.6 The glyceraldehyde exists only in the open-chain form and therefore exhibits the fastest glycation rate. Ribose is about 17 times more likely than glucose to be present in the open form6 and modifies collagen materials at the faster rate compared to glucose.
In-situ characterization of glycated collagen materials with multiphoton microscopy and spectroscopy using second harmonic generation (SHG) and two photon fluorescence (TPF) signals
Multi-photon microscopy provides an opportunity to monitor the microscopic structures in situ during glycation of collagen-based materials with reducing sugars.
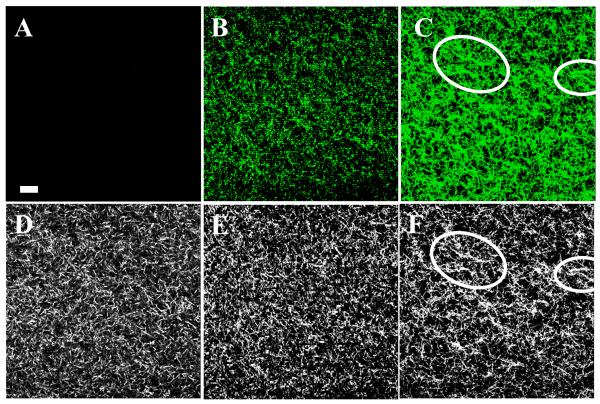
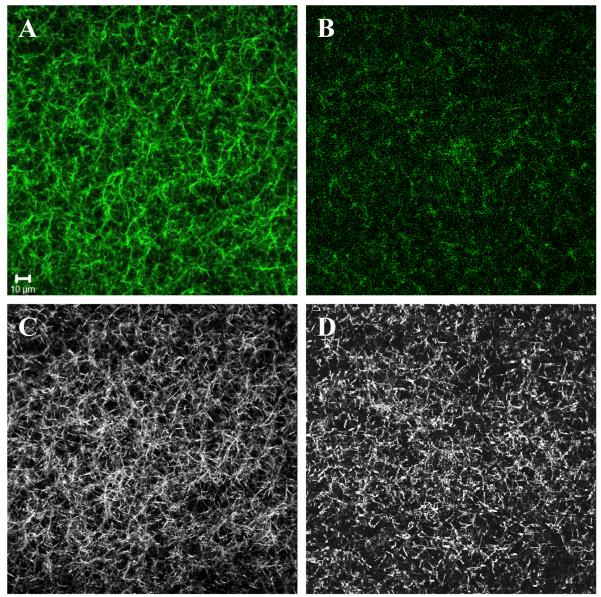
To follow the extent of glycation we excited the collagen hydrogels with 720 nm light and monitored the evolution of two-photon fluorescence intensity in the X-Y plane through the 405-501 nm filter (Figure 3A-C). At 8 hours of glyceraldehyde glycation time, the bright fluorescence spots were observed (Figure 3B). When the glycation time reached 48 hours, the accumulated fluorescence spots exhibited higher fluorescence intensity, grew in diameter and merged into larger structures (Figure 3C, white outline). The TPF images taken in X-Z plane showed that upon extended glycation (24 hrs and more) an increase of fluorescence occurred throughout the entire depth of collagen hydrogels (Figure 4D). After 6 weeks, the ribose-glycated samples produced strong fluorescence signals (Figure 5A). The glucose-glycated samples didn’t generate significant fluorescence even after 6 weeks (Figure 5B). The control group that had collagen samples exposed to sugar alcohols also did not exhibit detectable fluorescence (Figure 4C and Supporting Information Figure S3C-S5C).
Figure 3.
Typical two photon fluorescence (TPF) and second harmonic generation (SHG) images of a sample incubated with 0.1 M glyceraldehyde for different times. The images are taken in X-Y plane. TPF: (A) 0 hr. (B) 8 hr. (C) 48 hr. SHG: (D) 0 hr. (E) 8 hr. (F) 48 hr. The ‘fiber-like’ structures modified to long, aggregated fibers with length longer than 20 μm can be seen within the areas circled with the white outline. The collagen concentration of the sample was 2.0 g/l and the incubation temperature was 37°C. The scale bar is 20 μm.
Figure 4.
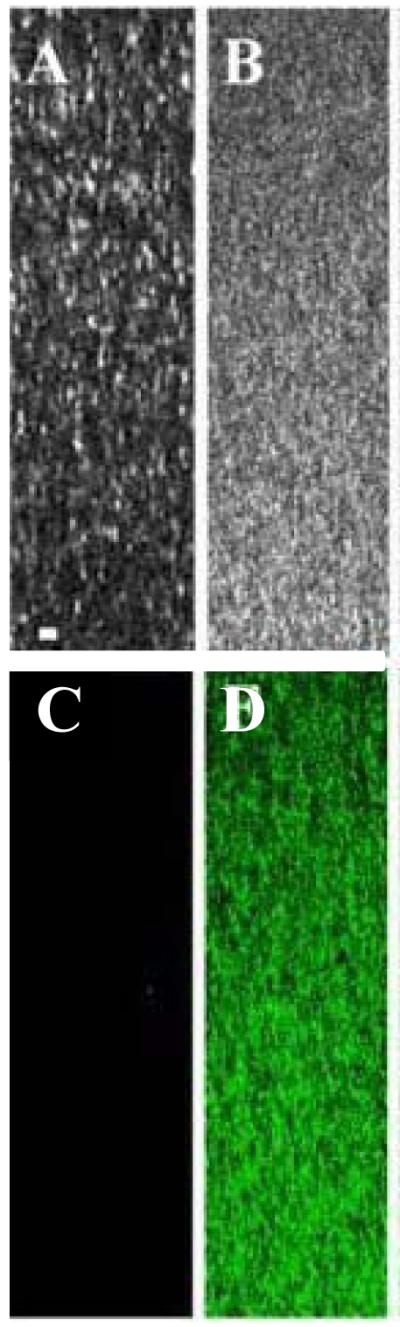
Typical second harmonic generation (SHG) and two photon fluorescence (TPF) images of a sample incubated with 0.1 M glyceraldehyde. The images were taken in X-Z plane. The collagen concentration was 4.68 g/l. Horizontal is X- axis, and vertical is Z-axis. SHG: (A) and (B), TPF: (C) and (D). Glycerol control: (A),(C). Glyceraldehyde: (B),(D). The scale is 20 μm. The top of each image represents the actual top of the imaged gel.
Figure 5.
Typical two photon fluorescence (TPF) and second harmonic generation (SHG) images of the sample incubated with 0.1 M ribose and 0.1 M glucose for 6 weeks. These images were taken in X-Y plane. (A) Ribose, TPF. (B) Glucose, TPF. (C) Ribose, SHG. (D) Glucose, SHG. The scale is 10 μm.
To determine if there are structural modifications of collagen hydrogels that are caused by glycation, we obtained second harmonic generation (SHG) images in addition to fluorescence. As seen in SHG images (Figure 3D-F), extended glycation (24 hrs and more) clearly alters the microstructure of collagen hydrogels. When the glyceraldehyde glycation time was 8 hours, the “fiber-like” structures within collagen hydrogels remained short with lengths around 5 to 10 μm (Figure 3D and 3E). The short “fiber-like” structures began to disappear at about 24 hours. When the glycation time reached 48 hours, the short ‘fiber-like’ structures became modified to longer, aggregated threads with length greater than 20 μm (Figure 3F). For ribose treated samples the collagen hydrogel remodeling began to occur after 1 week. After 6 weeks the short “fiber-like” structures began to transform to longer, aggregated fibers with length greater than 20 μm (Figure 5C). It took 2 weeks before any remodeling due to glucose could be detected and even after 6 weeks, the samples remain largely unchanged (Figure 5D). The control group, which had collagen samples exposed to sugar alcohols corresponding to glyceraldehydes, ribose and glucose did not have any structural changes that could be identified in the SHG images (Figure 4A and Supporting Information Figure S3A-S5A). In a separate set of imaging experiments we verified that there was no time dependent structural remodeling taking place within collagen hydrogels. When we employed the sterile technique, TPF and SHG images collected twenty-four hours after preparing samples looked identical to those acquired seven weeks later.
The SHG images show a clear glycation-induced remodeling of fibrils/fibers within the collagen materials. Our findings are in agreement with several previous studies that recognized the structural changes within various glycated collagen-based tissues and materials. For example, when tendons were exposed to reducing sugars, changes in collagen molecular packing were detected in the medium-angle X-ray diffraction studies.22 When rat tail tendons were incubated in 0.2 M ribose, electron micrographs of tendons cross-sections showed an increased fibril packing density, fusion of fibrils and irregular fibril diameters.23 Scanning force microscopy studies revealed structural alterations in the radius of fibrils gap depth when tendons were incubated with 0.5 M glucose for two weeks.24 Interestingly, cellular behavior was altered when cells were placed within the glycated collagen materials. For example, the fibroblasts underwent reorganization of actin cytoskeleton. Their morphology, attachment, proliferation, and migration were all altered.25,26 Glycated collagen materials suppressed the adhesive and migratory abilities of normal human keratinocytes from neonatal skin, therefore suggesting that modification diminished the binding capacity of type I collagen.25, 26
All the techniques employed in previous works that recognized the structural remodeling within glycated collagen tissues/materials have either imposed a non-reversible damage to the samples and/or provided only partial structural information. For example, the samples studied with X-ray and electron beams that are destructive in their nature, could not be used in any subsequent studies. The scanning force microscopy only provided information regarding collagen structures on the surface without penetrating deep into the tissues.
The near-infrared wavelength of light utilized in our two-photon fluorescence (TPF) and second harmonic generation (SHG) imaging is non-destructive to protein-based samples like collagen 3D hydrogels. It can provide three-dimensional structural information regarding the extent of glycation within them. At present there is only one study reported in literature that briefly mentions a 40% decrease in the SHG intensity of a glycated human tendon as compared to the normal tendon.27 That study did not provide data regarding structures and/or glycation-induced fluorescence.27
To relate the fluorescence observed in TPF images to the properties of the produced fluorophores, we obtained the two-photon emission spectra from the collagen hydrogels glycated with glyceraldehyde (Figure 6) and ribose (Supporting Information Figure S6). For the samples treated with glyceraldehydes, at six hours post initiation of glycation, the spectrum was a broad fluorescence band. At twenty-four hours, the band became centered at about 460 nm and the fluorescence intensity increased five-fold compared to six hours post initiation of glycation. For the ribose glycated hydrogels, the two-photon emission spectrum collected at six weeks looked similar to that collected at twenty four hours from the collagen hydrogels glycated with glyceraldehyde (Supporting Information Figure S6). The spectrum for the control group in which 0.1 M sugar alcohols were added to the collagen materials instead of reducing sugars indicated no increase of the TPF signals. The peak observed at about 460 nm possibly results from the fluorophore lysine-OH-triosidine (LHT). We believe it involves the same electronic transition excited with a 360 nm light (Figure 1).
Figure 6.
Two photon fluorescence emission spectra (λex = 720 nm) of the sample incubated with 0.1M glyceraldehyde for a different amount of time.
CONCLUSION
We probed the effects of non-enzymatic cross-linking with multi-photon microscopy (MPM) and one-photon fluorescence methods in situ. In this study, collagen hydrogels were modified with glyceraldehyde, ribose and glucose. The glyceraldehyde and ribose modifications led to an induction of strong auto-fluorescence and significant micro-structural changes in ‘fiber-like’ features within collagen hydrogels. The glucose glycated hydrogels exhibited broad, weak auto-fluorescence and after six weeks the samples remained largely unchanged.
The two-photon fluorescence (TPF) emission maximum was observed at about 460 nm for the samples glycated with glyceraldehyde. The emission maximum in the one-photon excitation experiment was a broad band with peaks centered at 445 nm and 460 nm (λex = 360 nm). For the glyceraldehyde, the 460 nm emission component subsequently became dominant as reaction progressed. For the ribose, in addition to the 460 nm peak, the 445 nm component persisted. With 330 nm excitation wavelength different emission peaks were observed. Glyceraldehyde-glycated collagen hydrogels generated stronger fluorescence signals and were modified at a faster rate compared to ribose and glucose-glycated samples.
The second harmonic generation (SHG) images were effective in demonstrating remodeling of ‘fiber-like’ structures throughout the collagen hydrogels. For glyceraldehyde, when the glycation time reached forty eight hours, the short 5 to 10 μm structures became modified to long, aggregated threads with length longer than 20 μm.
The potential in situ applications of the presented optical methods go beyond evaluating the effects of non-enzymatic cross-linking on tissues and biologically derived scaffolds. They range from diagnosing pathological complication to evaluating food processing strategies and skin products that target glycation.
Supplementary Material
ACKNOWLEDGMENT
The authors would like to thank Prof. Jiayu Liao for access to the FlexStation fluorescence microplate reader (Molecular Devices) at UC Riverside. We thank Yong Song for the technical expertise and initial assistance with FlexStation. We also thank Prof. Bruce J. Tromberg for access to a Zeiss LSM 510 NLO Meta microscopy system at UC Irvine and Dr. Tatiana Krasieva for the initial assistance. This research was performed with support from the Laser Microbeam and Medical Program (LAMMP), a NIH Biomedical Technology Resource, grant #P41-RR01192, at the University of California, Irvine. The work was supported by the UC Riverside startup research funds (J.G.L.), NSF CAREER Award CBET-0847070 (J.G.L).
REFERENCES
- (1).Bailey AJ, Paul RG, Knott L. Mech. Ageing Dev. 1998;106:1–56. doi: 10.1016/s0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- (2).Danilov NA, Ignatieva NY, Iomdina EN, Semenova SA, Rudenskaya GN, Grokhovskaya TE, Lunin VV. Biochim. Biophys. Acta. 2008;1780:764–772. doi: 10.1016/j.bbagen.2008.01.014. [DOI] [PubMed] [Google Scholar]
- (3).Francis-Sedlak ME, Uriel S, Larson JC, PGreisler HP, Venerus DC, Brey EM. Biomaterials. 2009;30:1851–1856. doi: 10.1016/j.biomaterials.2008.12.014. [DOI] [PubMed] [Google Scholar]
- (4).Tessier F, Monnier VM, Sayre LM, Kornfield JA. Biochem. J. 2003;369:705–719. doi: 10.1042/BJ20020668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Girton TS, Oegema TR, Grassl ED, Isenberg BC, Tranquillo RT. ASME. 2000;122:216–223. doi: 10.1115/1.429652. [DOI] [PubMed] [Google Scholar]
- (6).Girton TS, Oegema TR, Tranquillo RT. J. Biomed. Mater. Res. 1998;46:87–92. doi: 10.1002/(sici)1097-4636(199907)46:1<87::aid-jbm10>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- (7).Reddy GK. Exp. Diab. Res. 2004;5:143–153. doi: 10.1080/15438600490277860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Zipfel WR, Williams RM, Christie R, Nikitin AY, Hyman BT, Webb WW. Proc. Natl. Acad. Sci. USA. 2003;100:7075–7080. doi: 10.1073/pnas.0832308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Yeh AT, Nassif N, Zoumi A, Tromberg BJ. Optics Letters. 2002;27:2082–2084. doi: 10.1364/ol.27.002082. [DOI] [PubMed] [Google Scholar]
- (10).Raub CB, Suresh V, Krasieva T, Lyubovitsky J, Mih JD, Putnam AJ, Tormberg BJ, Geroge SC. Biophys. J. 2006;92:2212–2222. doi: 10.1529/biophysj.106.097998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Brown E, McKee T, DiTomaso E, Pluen A, Seed B, Boucher Y. Nat. Med. 2003;9:796–801. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- (12).Campagnola PJ, Millard AC, Terasaki M, Hoppe PE, Malone CJ, Mohler WA. Biophys. J. 2002;82:493–508. doi: 10.1016/S0006-3495(02)75414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Guo Y, Savage HE, Liu F, Schantz SP, Ho PP, Alfano RR. Proc. Natl. Acad. Sci. USA. 1999;96:10854–10856. doi: 10.1073/pnas.96.19.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Masters BR, So PTC. Microsc. Res. Techniq. 2004;63:3–11. doi: 10.1002/jemt.10418. [DOI] [PubMed] [Google Scholar]
- (15).Moreaux L, Sandre O, Charpack S, Blanchard-Desce M, Mertz J. Biophys. J. 2001;80:1568–1574. doi: 10.1016/S0006-3495(01)76129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Schenke-Layland K, Riemann I, Damour O, Stock UA, Konig K. Advance Drug Delivery Reviews. 2006;58:878–896. doi: 10.1016/j.addr.2006.07.004. [DOI] [PubMed] [Google Scholar]
- (17).Williams RM, Zipfel WR, Webb WW. Biophys. J. 2005;88:1377–1386. doi: 10.1529/biophysj.104.047308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Legare F, Pfeffer C, Olsen BR. Biophys. J. 2007;93:1312–1320. doi: 10.1529/biophysj.106.100586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Roth S, Freund I. J.Chem.Phys. 1979;70:1637–1643. [Google Scholar]
- (20).Baynes JW, Monnier VM, editors. Alan R. Liss, Inc.; New York: 1988. [Google Scholar]
- (21).Anderson SS, Tsilibary EC, Charonis AS. J. Clin. Invest. 1993;92:3045–3052. doi: 10.1172/JCI116929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Tanaka S, Avigad G, Brodsky B, Eikenberry EF. J. Mol. Biol. 1988;203:495–505. doi: 10.1016/0022-2836(88)90015-0. [DOI] [PubMed] [Google Scholar]
- (23).Bai P, Phua K, Hardt T, Cernadas M, Brodsky B. Connect. Tissue Research. 1991;28:1–12. doi: 10.3109/03008209209014224. [DOI] [PubMed] [Google Scholar]
- (24).Odetti P, Aragno I, Rolandi R, Garibaldi S, Valentini S, Cosso L, Traverso N, Cottalasso D, Pronzato MA, Marinari UM. Diabetes Metab. Res. Rev. 2000;16:74–81. doi: 10.1002/(sici)1520-7560(200003/04)16:2<74::aid-dmrr80>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- (25).Liao H, Zakhaleva J, Chen W. Biomaterials. 2009;30:1689–1696. doi: 10.1016/j.biomaterials.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Morita K, Urabe K, Moroi Y, Koga T, Nagai R, Horiuchi S, M. F. Wound Repair Regen. 2005;13:93–101. doi: 10.1111/j.1067-1927.2005.130112.x. [DOI] [PubMed] [Google Scholar]
- (27).Kim BM, Eichler J, Reiser KM, Rubenchik AM, Da Silva LB. Laser Surg. Med. 2000;27:329–335. doi: 10.1002/1096-9101(2000)27:4<329::aid-lsm5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.