Abstract
Context
Inhaled nitric oxide (NO) has shown evidence of efficacy in mouse models of sickle cell disease (SCD), case series of patients with acute chest syndrome, and 2 small placebo-controlled trials for treatment of vaso-occlusive pain crisis (VOC).
Objective
To determine whether inhaled NO gas reduces the duration of painful crisis in patients with SCD who present to the emergency room or hospital for care.
Design, Setting and Participants
Prospective, multicenter, double-blind, randomized, placebo-controlled clinical trial for up to 72 hours of inhaled NO gas versus inhaled nitrogen placebo in 150 participants presenting with VOC of SCD at 11 centers between October 5, 2004 and December 22, 2008. The primary endpoint was the time to resolution of painful crisis, defined by: 1) freedom from parenteral opioid use for 5 hours; 2) pain relief as assessed by visual analog pain scale scores ≤ 6 cm; 3) ability to walk; and 4) patient and family’s decision, with physician consensus, that the remaining pain could be managed at home.
Intervention
Inhaled NO gas versus inhaled nitrogen placebo.
Results
There was no significant change in the primary endpoint between the NO and the placebo groups, with a median time to resolution of crisis of 73.0 hours (95% CI: 46.0–91.0) and 65.5 hours (95% CI: 48.1–84.0), respectively (P=.87). There were no significant differences in secondary outcome measures, including length of hospitalization, VAS scores, cumulative opioid usage and the rate of acute chest syndrome. Inhaled NO was well tolerated with no increase in serious adverse events. Increases in venous methemoglobin concentration confirmed compliance and randomization, but did not exceed 5% in any study participant. Significant increases in plasma nitrate occurred in the treatment group, but there were no observed increases in plasma or whole blood nitrite.
Conclusions
Among patients with SCD hospitalized with VOC, the use of inhaled NO compared with placebo did not improve time to crisis resolution.
Keywords: Nitric oxide, sickle cell disease, vaso-occlusive pain crisis, acute chest syndrome
Introduction
Sickle cell disease (SCD) is an autosomal recessive disorder of the β globin gene. Mutant hemoglobin S polymerizes in erythrocytes causing occlusion of the small blood vessels, manifesting clinically as episodes of severe pain (vaso-occlusive crisis, or VOCs), damage to vital organs, and early death.1–7 VOC is common among patients with SCD, occurring at a rate of approximately 2 episodes per person year in the absence of treatment,7 with a mean length of hospitalization during VOC of 4.5 days for children 10–14 yrs of age.8, 9 As many as 20% of patients hospitalized for VOC develop the acute chest syndrome (ACS), a life threatening acute lung injury that prolongs the length of stay to a mean of 14 days.10, 11 The national expenditure for inpatient sickle cell medical care in 2004 is estimated at $571,000,000 in 2010 dollars.8 Given the suffering, high rate of morbidity and cost of care for VOC in SCD, and the absence of a current treatment option, there is an imperative to identify and evaluate new treatments.
0In recent years there has been much interest in understanding the possible pathophysiological and therapeutic roles of nitric oxide (NO) in SCD.12–23 Nitric oxide is the critical effector of endothelial-dependent vasodilation and exerts pleiotropic effects on vascular and circulating blood cells, including the inhibition of platelet aggregation, down-regulation of cellular adhesion molecules, and modulation of ischemia-reperfusion injury, all pathways adversely affected during VOC.24–30 Inhaled NO is a relatively safe agent, already approved by the Food and Drug Administration for hypoxic respiratory failure in newborn infants. Pre-clinical studies in transgenic mouse models have consistently demonstrated effects on inhibition of Gardos channels, reduction in red cell density, improved perfusion, or reductions in lung injury, microvascular vaso-occlusion and mortality.31–36 Early clinical trials suggested inhaled NO could improve hemoglobin oxygen affinity,14 although this result was not reproduced by other investigators.37 Case reports have suggested beneficial effects of NO inhalation in patients with ACS.38–40 A single institution placebo-controlled study of inhaled NO in children with SCD in VOC suggested decreased pain severity and reduced opioid analgesic usage, with trends toward reductions in length of hospitalization.9 Recently, an 18 patient multicenter placebo-controlled study of inhaled NO in adults demonstrated a significantly greater reduction in pain and a trend toward lower narcotic use.41
To further evaluate the efficacy of inhaled NO, we undertook a phase II, randomized, double-blind, placebo-controlled, multi-center study of NO inhalation for up to 72 hours in 150 participants with SCD presenting with VOC.
Methods
This was a prospective, multicenter, double-blind, placebo-controlled, randomized, phase II study of participants with SCD presenting with VOC. The study was approved by the National Heart, Lung and Blood Institutes (NHLBI) Institutional Review Board and by each participating center’s Institutional Review Boards. An NHLBI Data Safety and Monitoring Board (DSMB) monitored the study conduct and safety and an unblinded independent biostatistician (W. Blackwelder) reported interim methemoglobin safety results for children and adults to the DSMB. This was requested by the DSMB to ensure that children on inhaled NO in this trial were not subjected to increased risk of methemoglobinemia. Eleven sites (National Institutes of Health, Johns Hopkins University, Boston Children’s Hospital, University of Alabama at Birmingham, Howard University Hospital, St. Christopher’s Hospital for Children, Children’s Hospital Oakland/Alta Bates Medical Center, University of Colorado Health Science Center, Brigham and Women’s Hospital, Cleveland Case Western University, Children’s Hospital of Pittsburgh) participated between October 5, 2004 and December 22, 2008.
Participants with known SCD age 10 years and older were identified during presentation with VOC to the Emergency Department/Emergency Clinic (ED/EC) or other appropriate unit. Written informed consent was obtained, with patients under age 18 providing assent along with parental consent. Participants were also recruited in the outpatient setting while not in pain, signing a final consent form at the time of VOC presentation. Exclusion criteria included: HbSC disease; exposure to therapeutic NO within the previous 12 hours; use of phosphodiesterase 5 inhibitors, L-arginine, nitroprusside or nitroglycerine within the previous 12 hours; previous ED/EC or other appropriate unit treatment for a VOC within 48 hours, or hospitalization within14 days of presentation with VOC; ED/EC visits or hospitalizations for VOC >10 times in the preceding year; clinically diagnosed bacterial infection at presentation; current enrollment in any other investigational drug study, except for hydroxyurea studies; current pregnancy or nursing; chronic transfusion or exchange transfusion in the preceding 30 days; suspected splenic sequestration; new pulmonary infiltrate at presentation; or previous participation in the study.
Participants were randomized using block randomization by site and age at entry (age 10–15 and >15 years), in blocks of 4, in a 1:1 ratio of placebo to inhaled NO. Randomization was defined at the time a set of study placebo or NO gas cylinders was assigned and a cylinder was opened.
NO for inhalation (Ikaria - formerly INO Therapeutics, Port Allen, LA) was supplied at a concentration of 800 ppm balanced with nitrogen (99.92% grade 5 nitrogen, 0.08% pharmaceutical grade NO). Placebo study gas was 100% Grade 5 nitrogen gas. Either NO or placebo was delivered with air and mixed with oxygen to achieve a constant FiO2 of 24%. Participants were treated via facemask using a continuous flow delivery system. Those randomized to inhaled NO received 80 ppm for 4 hours, followed by 4 hours of 40 ppm. For participants remaining in the hospital after the initial 8 hour dose, study gas was administered through a pulsed flow delivery system with 1L continuous oxygen via nasal cannula at a dose of 6 mL/pulse/breath of 800 ppm NO for body weight ≥27 kg, or 3 mL/pulse/breath if <27 kg, up to a maximum of 72 hours total study gas administration. This is the first clinical trial to use a pulse delivery system, which delivers a lower dose of NO gas to the circulation, equivalent to approximately 5 ppm depending on minute ventilation. The use of pulse nasal canula delivery was considered necessary to facilitate the practical use of prolonged gas therapy for inpatients. The oxygen flow was increased as required to maintain SaO2 ≥ 85%, with a maximum of 4L permissible to continue in the study. If study gas was interrupted for more than 1 hour, it was not restarted. If the gas was stopped or a patient withdrew from treatment, the time to resolution of the VOC was still collected.
Coded labels were applied to the study cylinders at the manufacturing site. A “blinded” version of the face mask NO delivery systems blanked out and covered the NO and NO2 monitor displays. The placebo gas was administered in the same way and over the same time to ensure that participants and investigators were blinded.
Pain associated with the VOC was measured on a scale of 0–10 using a visual analogue scale (VAS), which consisted of a 10 cm horizontal line with the ends representing the extreme limits of “no pain” (0) and “worst pain” (10). The participant was asked to make a mark along the line to indicate the intensity of pain at baseline and at hours 2, 4, 6 and 8 after the start of the study drug and then at 4 hour intervals. Each participant’s score was the measurement in cm from 0 of his mark, to the nearest 0.1 cm. Starting with hour 12, sleeping participants were not woken to complete the VAS and a missing value was assigned. Participants were not shown their previous response. Demographic, clinical and laboratory variables were collected by the site coordinators from source documents and recorded on study case report forms.
Primary Efficacy Variable
The primary efficacy variable was the time to VOC resolution, modified from the poloxomer-188trial,42 defined by all of the following criteria being met: freedom from parenteral opioid use for at least 5 hours; pain relief [VAS scores ≤6 cm maintained during 2 consecutive readings obtained at least 2 hours apart, each at least 3 hours after the last dose of parenteral opioids]; ability to walk (except for chronically non-ambulatory participants); and agreement of the physician, and patient and parent or guardian that residual pain was low enough to be manageable at home. Time to VOC resolution for participants who were discharged with missing endpoint data or with incomplete criteria for VOC resolution was censored at the actual time of discharge from the hospital. Death before discharge without meeting the definition for VOC resolution was censored at a time later than the latest time of censoring or VOC resolution in participants who did not die before discharge. For participants in the hospital longer than 30 days without crisis resolution, the duration of crisis was determined by the time of the discharge order.
Secondary Efficacy Variables
The following secondary efficacy variables were evaluated: length of hospitalization from admission to discharge (time of discharge order); VAS score over time; total dose of opioids in the first 8 hours after enrollment into the study and during the entire hospitalization; rate of ACS or pneumonia requiring blood transfusion; proportion discharged in the first 24 hours; proportion returned to ED or hospital within 30 days;; change in nitrate/nitrite levels and methemoglobin levels as measures of NO metabolism and reactions in the blood (Table 2). Secondary evaluation of possible pain relapse was determined by the proportion of participants treated again for pain in the ED/EC, hospital or other appropriate unit within 24 hours and within 30 days after hospital discharge.
Table 2.
Effect of Inhaled NO Gas on Secondary Outcomes
| Secondary Outcome | Inhaled NO (N=75) | Placebo (N=75) | P Valuea | |
|---|---|---|---|---|
| Length of Hospitalization, days | Median (IQR) | 4.1 (2.0–6.0) | 3.1 (1.7–6.4) | .30 |
| VAS at 24 hours, cm | Mean (95% CI) | 6.1 (5.3–6.8) | 6.0 (5.4–6.6) | .90 |
| VAS at 2, 4, 6, 8 hours (change from baseline, cm) | Mean (95% CI) | −0.4 [−0.8- (−0.1)] | −0.7 [−1.1- (−0.3)] | |
| −0.6 [−0.8- (−0.1)] | −0.8 [−1.3- (−0.3)] | .90 | ||
| −1.2 [−1.7- (−0.7)] | −1.1 [−1.6- (−0.6)] | |||
| −1.3 [−1.8- (−0.8)] | −1.2 [−1.8- (−0.7)] | |||
| Opioids in 1st 8 hours, mg/kg | Median (IQR) | 0.28 (0.09–0.54) | 0.23 (0.07–0.70) | .74 |
| Total Opioids, mg/kg | Median (IQR) | 2.8 (1.4–6.1) | 2.9 (1.1–9.9) | .73 |
| ACS Requiring Transfusion | N [% (95% CI)b] | 8 [10.7 (4.7–19.9)] | 7 [9.3 (3.8–18.3)] | .79 |
| Discharged within 24 hours | N [% (95% CI)b] | 5 [6.7 (2.2–14.9)] | 7 [9.3 (3.8–18.3)] | .55 |
| Returned to ED within 30 days | N [% (95% CI)b] | 8 [10.7 (4.7–19.9)] | 11 [15.1 (7.8–25.4)] | .44 |
| Rehospitalized within 30 days | N [% (95% CI)b] | 9 [12.2 (5.7–21.8)] | 17 [23.0 (14.0–34.2)] | .08 |
| Methemoglobin, %, at 0, 4, 24 hours | Mean (95% CI) | 0.73 (0.59–0.86) | 0.81 (0.65–0.96) | |
| 2.29 (2.05–2.52) | 0.82 (0.66–0.98) | <.001 | ||
| 1.32 (1.07–1.57) | 0.88 (1.06–0.58) | |||
| Plasma Nitratec at 0, 2, 4 days μmol/L | Mean (95% CI) | 24.5 (18.3–32.6) | 24.3 (18.0–32.9) | |
| 60.9 (48.5–76.3) | 22.2 (16.1–30.5) | .03 | ||
| 36.2 (22.3–58.8) | 20.9 (13.8–31.8) | |||
| Plasma Nitritec at 0, 2, 4 days μmol/L | Mean (95% CI) | 0.22 (0.18–0.26) | 0.21 (0.18–0.24) | |
| 0.30 (0.25–0.36) | 0.24 (0.19–0.30) | .77 | ||
| 0.23 (0.16–0.34) | 0.27 (0.22–0.32) | |||
| Whole Blood Nitrited at 0, 4, 8 hours μmol/L | Mean (95% CI) | 0.28 (0.14–0.56) | 0.23 (0.14–0.37) | |
| 0.40 (0.24–0.67) | 0.27 (0.17–0.41) | .31 | ||
| 0.45 (0.23–0.85) | 0.37 (0.22–0.62) |
From Wilcoxon 2-sample test for comparison of medians; unpaired t-test for comparison of means at specific time points; Pearson’s chi-square for comparison of proportions; Repeated Measures ANOVA for comparison of means over time
Clopper-Pearson (Exact) 95% confidence limits
From 83 samples from participants at sites with plasma storage
From 34 samples from participants at sites with plasma storage
Safety Monitoring
Since the primary known toxicity of inhaled NO is methemoglobinemia, venous methemoglobin was monitored at baseline and every 2 hours for the first 8 hours, then every 24 hours for the rest of the study. Bedside personnel and site investigators were not allowed access to methemoglobin levels, which were reported by designated laboratory personnel through an interactive voice response system at each site to a central safety monitor, who notified site investigators if dose change was indicated. Methemoglobin values were only accessible to the DSMB and an unblinded monitoring statistician reporting to the DSMB. If any value was ≥ 5%, the treatment dose was to be decreased by 50%. If any value was >7.5%, the investigational therapy was to be discontinued. Other stopping rules included: assessment of the investigator, treating physician or participant that discontinuation of the inhalation therapy was in the patient’s best interest; any serious adverse event thought to be related to the investigational therapy; clinically significant hypotension; sepsis or septic shock; or sustained pulse oxygen saturation below 85% for >15 minutes while on supplemental oxygen up to 4L by nasal cannula or 35% oxygen by mask. Participants who were discontinued from therapy remained in the study and continued with all data collection, unless consent to do so was withdrawn.
Serious adverse events were recorded during study gas inhalation and defined as any event that at any dose required hospitalization or resulted in disability or death (Table 3). As is standard in clinical trials in the SCD field, ACS was considered a serious adverse event during active treatment with NO because it is one of the major complications that occurs during hospitalizations for pain crisis. ACS requiring blood transfusion was also captured as a secondary outcome measure both on and off NO or placebo treatment (Table 2).
Table 3.
Number and Percentage of Participants with Serious Adverse Events
| Inhaled NO (N=75) | Placebo (N=75) | ||
|---|---|---|---|
| System Organ Class | N [% (95% CI)a] | N [% (95% CI)a] | P Valueb |
| Preferred Term | |||
| Blood and Lymphatic System Disorders | |||
| Acute Chest Syndrome | 5 [6.7 (2.2–14.9)] | 5 [6.7 (2.2–14.9)] | >0.99 |
| Gastrointestinal Disorders | |||
| Dysphagia | 1 [1.3 (0.03–7.2)] | 0 [0.0 (0.00–4.8)] | >0.99 |
| General Disorders and Administration | |||
| Site Conditions | |||
| Pyrexia | 1 [1.3 (0.03–7.2)] | 1 [1.3 (0.03–7.2)] | >0.99 |
| Sensation of Foreign Body | 1 [1.3 (0.03–7.2)] | 0 [0.0 (0.00–4.8)] | >0.99 |
| Investigations | |||
| Hemoglobin Decreased | 1 [1.3 (0.03–7.2)] | 0 [0.0 (0.00–4.8)] | >0.99 |
Clopper-Pearson (Exact) 95% confidence limits
From Fisher’s exact test
Statistical and Analytic Plans
At the end of the study, the dataset was analyzed by the steering committee, independently of the sponsor, using pre-specified analyses, endpoints and subgroups. Interim analysis of efficacy was not planned or performed.
Sample size was estimated based on data from a previous study of NO therapy in children9 and AHRQ,8 predicting a mean length of stay approximating 106 hrs. For the purposes of the calculation, time to crisis resolution, which was selected to minimize effects of factors unrelated to medical condition that impact discharge time, was assumed to approximate length of stay. The study was designed to have 80% power to find a significant decrease in duration of crisis if the true decrease was 24 hours. Sample size was based on a difference in log10 (duration), since the logarithm of duration was more normally distributed than untransformed values. Assuming a 2-sided Wilcoxon rank-sum test at the 5% significance level, normal distributions for log10 (duration) with difference 0.11 (log10 (106) − log10 (82)) and common standard deviation 0.22, we obtained a sample size of 68 per group, or a total of 136. Allowing for 9% of participants withdrawing or censored, the sample size became 75 participants per group, or a total of 150. Sample size and power calculations were done using PASS 2005 software (Number Cruncher Statistical Systems, Kaysville, Utah).
All analyses were intention to treat and 2-sided P-values less than 0.05 were considered significant. Clinical and laboratory characteristics were compared between the inhaled NO treatment group and the placebo group by the nonparametric Wilcoxon rank sum test for continuous variables and Pearson’s chi-square statistic for categorical variables. Treatment efficacy was evaluated by calculating Kaplan-Meier survival curves for duration of crisis over time, using the log-rank test to determine significant differences in time to resolution between the 2 groups. The effect of inhaled NO vs. placebo was also examined in pre-defined subgroups by age (10–15 years and >15 years) and hydroxyurea use, as suggested by the poloxomer-188 trial in which young patients and those on hydroxyrea therapy appeared to respond better to treatment.42 The effect of treatment on length of hospital stay and opioid use, both total and cumulative, were examined using the Wilcoxon 2-sample test. Treatment related changes in pain score and NO metabolites were evaluated using an unpaired 2-tailed t-test to examine differences at specific points in time and repeated measures ANOVA to examine changes over time. The frequency of dichotomous secondary outcomes and serious adverse events was examined using either the Pearson’s chi-square or the Fisher’s exact test.
Cox Proportional Hazards regression models were used to examine the association of treatment with duration of crisis while adjusting for significant covariates. The likelihood ratio test was used to determine the significance of individual regression coefficients. As a post-hoc analysis, associations of potential confounders, including study site, gender, baseline VAS score, age and hydroxyurea use, with time to crisis resolution were examined using Kaplan-Meier analysis. The effect of study site on length of hospitalization, total opioids and baseline VAS score was performed using either the Kruskal-Wallis test or ANOVA.
In a post-hoc analysis, comparisons of time to resolution with other measures of disease severity were examined using the Spearman rank order correlation coefficient and the Wilcoxon 2-sample test. All analyses were performed using SAS version 9.1.3 (SAS Institute Inc, Cary, NC) and Stata version 9.0 (StatCorp LP, College Station, TX).
Results
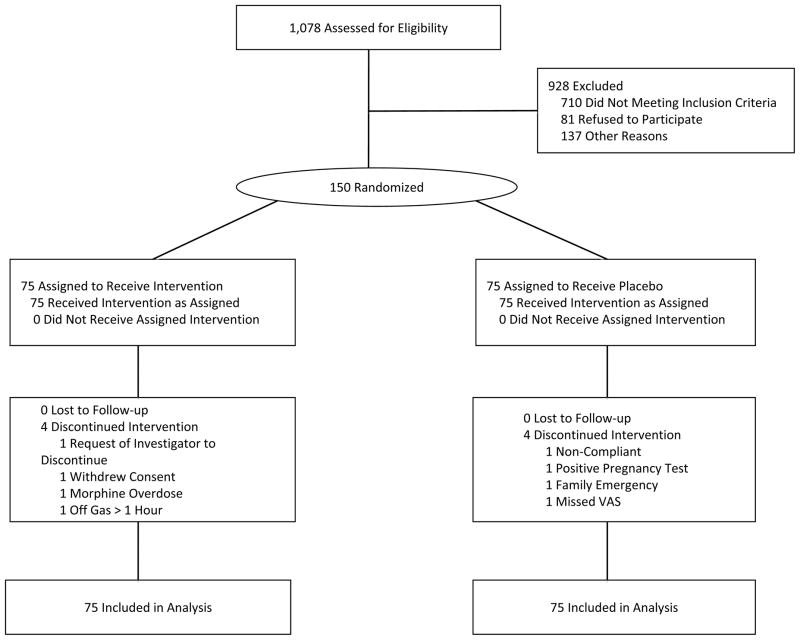
Of 1,078 patients with SCD who were assessed for eligibility or pre-enrolled in steady state, 150 presenting during VOC were enrolled and randomized. Four participants withdrew in each group but were included in the intention to treat analysis (Figure 1). There were no deaths. Table 1 describes the characteristics of the NO-treated and-placebo treated groups, which were balanced in terms of age, gender, genotype, hydroxyurea use, vital signs, pain scores, and laboratory values.
Figure 1. Patient Enrollment and Randomization.
The patient population consisted of 150 patients presenting with VOC who were enrolled and randomized in a prospective, placebo-controlled, double-blind clinical trial of inhaled NO versus placebo at sites in the United States. Four patients withdrew in each group, and the numbers resolving pain crisis were 65 and 64 for the iNO and placebo groups, respectively. All 150 participants were evaluated by intention to treat. The large number of patients assessed for eligibility (1,078) reflects the pre-enrollment of out-patients at many of the sites.
Table 1.
Demographics and Baseline Characteristics of Study Participants by Treatment
| Inhaled NO | Placebo | Overall | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Characteristic | N | Median (IQR)1 | N | Median (IQR) 1 | P Value2 | N | Median (IQR) 1 |
| Age, y | 75 | 22.9 (17.8–33.9) | 75 | 24.5 (17.1–31.8) | 0.81 | 150 | 24.2 (17.4–33.7) |
| Men, N (%) | 75 | 37 (49.3) | 75 | 38 (50.7) | 0.87 | 150 | 75 (50.0) |
| Sickle Cell Genotype (SS/Sβ+), N (%) | 75 | 70(93.3)/5(6.7) | 75 | 66(88.0)/9(12.0) | 0.26 | 150 | 136 (90.7)/14(9.3) |
| Hydroxyurea use, N (%) | 75 | 43 (57.3) | 75 | 46 (61.3) | 0.62 | 150 | 89 (59.3) |
| Systolic blood pressure, mm Hg | 75 | 118 (108–128) | 75 | 117 (108–131) | 0.90 | 150 | 118 (108–129) |
| Diastolic blood pressure, mm Hg | 75 | 64 (59–71) | 75 | 65 (57–74) | 0.91 | 150 | 65 (58–72) |
| Visual analogue pain score (0–10), cm | 75 | 7.7 (6.0–9.1) | 75 | 7.6 (6.5–9.1) | 0.83 | 150 | 7.7 (6.3–9.1) |
| Hemoglobin, g/dL | 75 | 8.8 (7.7–9.8) | 75 | 8.8 (7.9–10.2) | 0.21 | 150 | 8.8 (7.9–10.1) |
| White blood cell count, × 10−3/3L | 73 | 12.9 (9.1–16.8) | 75 | 12.4 (9.4–16.3) | 0.68 | 148 | 12.8 (9.3–16.4) |
| Red blood cell count, × 10−6/3L | 75 | 2.7 (2.3–3.0) | 75 | 2.8 (2.4–3.3) | 0.12 | 150 | 2.7 (2.3–3.2) |
| Platelet count, × 10−3/3L | 73 | 383 (290–493) | 75 | 348 (227–475) | 0.07 | 148 | 367 (254–483) |
| Reticulocyte count, × 10−3/3L | 71 | 240 (91–306) | 72 | 154 (17–293) | 0.11 | 143 | 200 (23–300) |
| MCV, μm3 | 75 | 95.3 (86.3–103) | 75 | 91.0 (82.0–103.0) | 0.21 | 150 | 93.7 (84.1–103.0) |
| MCH, μ μg | 75 | 33.1 (29.9–36.4) | 75 | 31.4 (27.9–35.9) | 0.09 | 150 | 32.4 (28.7–36.2) |
| MCHC, gm/dL | 75 | 34.7 (34.0–35.6) | 75 | 34.5 (33.5–35.6) | 0.35 | 150 | 34.7 (33.8–35.6) |
| Creatinine, mg/dL | 75 | 0.6 (0.5–0.7) | 75 | 0.6 (0.5–0.8) | 0.98 | 150 | 0.6 (0.5–0.7) |
| Blood urea nitrogen, mg/dL | 75 | 7 (5–9) | 74 | 7 (5–10) | 0.92 | 149 | 7 (5–9) |
| GGT, U/L | 68 | 27.0 (17.5–56.0) | 68 | 33.5 (19.5–66.5) | 0.40 | 136 | 31.0 (19.0–64.5) |
| Lactate dehydrogenase, U/L | 68 | 373 (295–509) | 68 | 414 (297–566) | 0.32 | 136 | 389 (297–553) |
| Alkaline phosphatase, U/L | 73 | 95 (70–148) | 75 | 103 (79–147) | 0.37 | 148 | 99 (73–148) |
| Total bilirubin, mg/dL | 72 | 2.8 (1.7–4.3) | 75 | 2.3 (1.7–4.0) | 0.37 | 147 | 2.6 (1.7–4.1) |
| Direct bilirubin, mg/dL | 70 | 0.4 (0.3–0.5) | 69 | 0.4 (0.4–0.6) | 0.88 | 139 | 0.4 (0.3–0.6) |
| Indirect bilirubin, mg/dL | 70 | 2.2 (1.4–4.1) | 69 | 2.0 (1.4–3.3) | 0.50 | 139 | 2.1 (1.4–3.6) |
| Alanine aminotransferase, U/L | 73 | 24 (18–36) | 75 | 26 (17–35) | 0.60 | 148 | 25 (17–35) |
| Aspartate aminotransferase, U/L | 69 | 36 (28–55) | 72 | 41 (30–56) | 0.48 | 141 | 39 (30–56) |
Except where otherwise noted
From Wilcoxon two-sample test or chi-square test
Efficacy of inhaled NO gas versus placebo on VOC
Time to VOC resolution did not differ significantly according to treatment (p=.87; Figure 2), with an estimated median time to resolution of crisis of 73.0 hours (95% CI: 46.0–91.0) for the inhaled NO group and 65.5 hours (95% CI: 48.1–84.0) in the placebo group. Additionally, the planned secondary analyses did not differ significantly according to experimental treatment (Table 2), including median length of hospitalization [4.1 (IQR 2.0–6.0) vs. 3.1 (IQR 1.7–6.4) days, inhaled NO vs. placebo; p=.30], mean VAS scores [6.1 (95%CI: 5.3–6.8) vs 6.0 (95% CI: 5.4–6.6) cm at 24 hours, p=.90; eFigure 1A], and median total opioid use [2.8 (IQR: 1.4–6.1) vs. 2.9 (IQR: 1.1–9.9) mg of morphine equivalents/kg, p=.73]. Decreases in mean VAS scores over 2 hour intervals in the first 8 hours of treatment were also not different by treatment group, with reductions in pain score in the inhaled NO group ranging from 0.4 (95% CI 0.1–0.8) to 1.3 (95% CI 0.8–1.8) compared with reductions from 0.7 (95% CI 0.3–1.1) to 1.2 (95% CI 0.7–1.8) in the placebo group (p=.90). Cumulative opioid use up to 72 hours after presentation also yielded no effect of inhaled NO vs. placebo [0.44 (IQR: 0.3–0.7) vs. 0.47 (IQR: 0.3–0.6) mg/kg over 4 hours, p=.47; 0.71 (IQR: 0.6–0.9) vs. 0.70 (IQR: 0.3–0.9) mg/kg over 8 hours, p=.19; and 0.95 (IQR: 0.8–1.3) vs. 0.92 (IQR: 0.7–1.2) mg/kg over 12 hours, p=.35; eFigure 1B]. There were no differences between the groups in the percentage of participants that developed ACS requiring a transfusion over the entire study period (Table 2) or in those with ACS as a reported serious adverse event during study gas inhalation (Table 3). The rare event of being rehospitalized within 30 days was almost twice as frequent in the placebo group, but the difference was not statistically significant (Table 2).
Figure 2. Efficacy of Inhaled NO Gas Versus Placebo on VOC.
There was no significant effect of inhaled NO on time to VOC resolution by Kaplan-Meier analysis (A), p=.87, log-rank test.
Effect of inhaled NO gas on methemoglobin, nitrate and nitrite
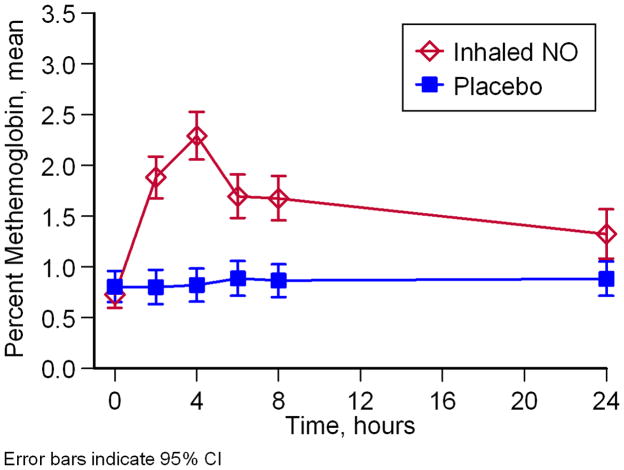
We measured the reaction products of NO in blood and plasma, methemoglobin, nitrate and nitrite. Participants on NO gas inhalation had significantly higher levels of methemoglobin in the venous blood (p<.001; Figure 3) consistent with NO gas exposure, but no participant’s methemoglobin values exceeded 5%, considered a toxic level. Participants randomized to inhaled NO showed an increase in mean venous methemoglobin at 4 hours [2.3 (95% CI 2.1–2.5) vs. 0.8 (95% CI 0.7–1.0), p<.001] and 8 hours [1.7 (95% CI 1.5–1.9 vs. 0.9 (95% CI 0.7–1.0), p<.001]. Participants receiving NO had higher nitrate levels in plasma (p=.03) but did not demonstrate significant increases in nitrite in plasma or whole blood (p=.77 and .31) (eFigures 2A, B and C).
Figure 3. Effect of Inhaled NO Gas on methemoglobin.
Methemoglobin concentrations were significantly higher for participants on inhaled NO gas than for participants in the placebo group (A; p < .0001). Blood samples for nitrate and nitrite testing were taken before crisis when possible (labeled baseline), on Day 1 before treatment started (labeled Pre-Tx), and on each subsequent day until discharge.
Potential confounding factors
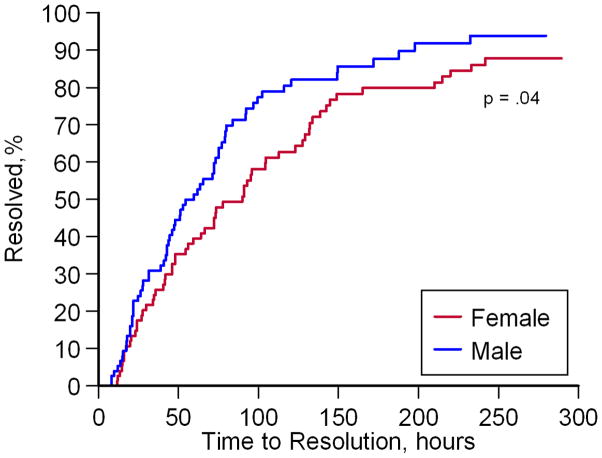
Study site, gender, pain score, alkaline phosphatase and total bilirubin were independently associated with time to VOC resolution (eTable 1). The estimated median time to VOC resolution (Figure 4A–C) was significantly shorter at selected study sites [41.5 (95% CI 28.0–59.3) hours at NIH and JHU vs. 91.0 (95% CI 73.7–123.0) hours at other sites; p<.001], with shorter time to VOC resolution in males [59.5 (95% CI 43.8–75.0) vs. 90.0 (95% CI 59.3–112.7) hours, males vs. females; p=.04], and longer time to VOC resolution in those presenting with higher pain scores [91.0 (95% CI 73.7–123.0) vs. 47.3 (95% CI 31.4–64.0) hours, VAS ≥7.7 vs. <7.7 cm; p<.001]. Patients on hydroxyurea therapy did not differ significantly from those who were not on therapy [73.7 (95% CI 59.3–93.0) vs. 56.0 (95% CI 42.7–75.3) hours, HU vs. no HU, p=.4; Figure 4D]. Median time to VOC resolution also did not differ significantly by age [76.3 (95% CI 46.1–132.0) vs. 72.0 (95% CI 52.8–78.0) hours, ≤15 vs. >15 years; p=.3]. The study site effect was significant and consistent across many variables. The NIH and JHU sites enrolled participants who were reporting less pain, had shorter hospitalization times, and received significantly less cumulative opioid (eTable 2). Adjustment for these potentially confounding effects did not affect the responses to NO gas inhalation vs. placebo.
Figure 4. Kaplan-Meier Analysis of VOC Resolution by Study Site, Gender and Pain at Baseline.
Shorter times to VOC resolution were observed for participants at NIH and JHU (A; p<.001, log-rank test), males (B; p=.04, log-rank test), and participants with baseline VAS scores less than 7.7 cm (C; p<.001, log-rank test). No significant differences in time to resolution were observed in participants on hydroxyurea therapy (D; p=.4, log-rank test).
Validation of time to VOC resolution and endpoint analysis
The time to VOC resolution as defined in this study appeared to correlate well with other markers of disease severity. Spearman rank order correlation coefficients of time to resolution of VOC with various other secondary endpoints were 0.92 (p<.001) for duration of hospitalization, 0.84 (p<.001) for total opioid use, and 0.40 (p<.001) for pain score at baseline. Time to resolution of VOC was also greater in participants with ACS compared to participants without ACS [142.4 (IQR 91.0–219.8) vs. 56.0 (IQR 24.8–99.6) hours; Wilcoxon p<.001] and in participants reporting one or more SAEs, compared with participants reporting no serious adverse events [95.7 (IQR 78.0–172.0) vs. 59.5 (IQR 26.5–104.4) hours; Wilcoxon p=.01].
Discussion
Inhaled NO gas had no effect on the time to VOC resolution or on any of the planned secondary analyses, including length of hospitalization, change in VAS scores, and total opioid use. Sustained inhaled NO delivered for 8 hours by face mask and for up to 72 hours by a nasal cannula pulse system was well tolerated. No value of methemoglobin exceeded 5% and no increase in serious adverse events were observed.
The design of this study overcame many of the challenges that have affected previous therapeutic trials in SCD VOC. The placebo and treatment groups were well balanced. While many of the endpoints were necessarily subjective, we found that these correlated well with objective measures of disease severity. We designed our trial to minimize missing and censored data at discharge. The time to VOC resolution was chosen as an endpoint, rather than the actual duration of hospitalization, to capture more precisely the time at which the crisis functionally ended, independent of non-medical factors that impact discharge time, such as the availability of family members or transportation and the timing of physician and nursing shift cycles. Our adoption of a discharge VAS pain score ≤ 6 cm, rather than ≤ 4 cm, appears to have resulted in fewer censored participants than the prior study using this endpoint. 42
It is notable that the median duration of crisis and length of hospitalization were much shorter than predicted based on data obtained from AHRQ and prior studies, perhaps partly due to our entry criteria, which required ED presentation but not necessarily a decision to hospitalize.8, 9 The time to resolution in patients with ACS of 142.4 hours was much shorter than reported in prior studies and the 10% incidence of ACS during hospitalization lower than reported in prior studies. A requirement for mechanical ventilation was a rare event in this trial and none of the patients died. The lower duration of hospitalization for ACS is potentially related to the rapid evaluation and treatment of pre-enrolled patients, a lower threshold for hospitalization in the context of this trial, as well as aggressive transfusion therapy for complications, a practice that is now routine in the participating centers.
The lack of any observable effect of inhaled NO in this trial may be the result of a lack of systemic generation of nitrite, which has been shown to exhibit therapeutic effects in models of ischemia and reperfusion injury.43, 44 In pre-clinical studies of inhaled NO for ischemia and reperfusion injury, the levels of nitrite in the circulation and target organs increased significantly.45–47 The absence of increase in nitrite could be due to our mode of NO administration. The pulse delivery of NO provides a pulse of pure NO in nitrogen at the “front” of the tidal volume. This reduces mixing of NO with oxygen in the airways, which can form nitrogen dioxide, dinitrogen trioxide and nitrite. Failure to see an effect of NO could also be due to the fact that tissue injury in VOC is not reversible by the time it is recognized clinically.
We observed major effects of study site. The NIH and JHU sites admitted more participants to the study with less pain, who used less opioids and exhibited shorter durations of crisis. The reasons for these site variations are not clear, but could represent an increased capability to identify and enroll patients early in their crises at these sites, which was an expressed goal of this trial, before a decision about admission to the hospital was made. While the NIH site pre-enrolled patients and directly admitted patients with VOC, the JHU site enrolled patients out of the emergency room, suggesting that care delivery structure did not determine this effect.
The overall median crisis duration of 65.5 hours in the placebo arm is of potential concern, as the study was designed to have adequate statistical power to observe a reduction of approximately 1 day from an expected 4 day duration of crisis in placebo controls. However, given that there was no evident effect of NO, even when adjusted for sites with longer durations of VOC, it seems unlikely that this accounts for the lack of apparent efficacy. A lack of effect on secondary outcomes such as pain scores and narcotic use also supports a primary lack of efficacy. An unexpected observation was that female participants had longer durations of crisis and hospitalization, which has been reported in retrospective studies, but this finding deserves further study.48 Increasing VAS at admission was associated with a longer length of stay, consistent with prior studies.48 The characteristics of VOC and its management should inform the design of future VOC trials.
Limitations of this study include the relatively small sample size (150 patients) and the broad, but overlapping confidence intervals for the medians of the primary outcome variable, leaving open the possibility that a small treatment effect may have been missed. If such a treatment effect exists, it is likely to be less that our predetermined minimal clinical significance of a 25 % reduction in duration of crisis. Finally, this study used a subjective endpoint, as there are no true objective indicators of cessation of crisis.
In summary, the results of this study indicate that inhaled NO in the doses and methods of administration used in this study does not reduce VOC severity in SCD. These results underscore the need for new agents and a sustained clinical trials apparatus for studying VOC, with sufficient numbers of patients to provide adequate power to rapidly test promising therapeutics in patients with SCD.
Supplementary Material
Acknowledgments
This project was collaboratively supported and funded by Ikaria® and the Intramural Research Division of the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services. The DSMB was assembled and supported by the Intramural Research Division of the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services. Statistical analysis was supported using funds from the Vascular Medicine Institute, Hemophilia Center of Western Pennsylvania and the Institute for Transfusion Medicine (MTG).
Role of the Sponsors:
Ikaria and the Intramural Research Division of the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services. The DSMB was assembled and supported by the Intramural Research Division of the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services. Statistical analysis was supported and independently conducted by the Investigators using funds from the Vascular Medicine Institute, Hemophilia Center of Western Pennsylvania and the Institute for Transfusion Medicine (MTG).
Role of Ikaria in the “design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript": Study drug, placebo and delivery devices were provided by Ikaria®. Ikaria, formerly INO Therapeutics, provided the Data Coordinating Center so were involved in the conduct of the study, collection of data, and management of the site data collection. Dr. Baldassarre is an employee of Ikaria and contributed to the analysis of data as an author. Ikaria and Dr. Baldassarre reviewed the manuscript but did not have rights to approve the manuscript. The study design and statistical analysis was performed independently by study Investigators and was performed under the auspices of a National Institutes of Health Government Collaborative Research and Development Agreement. The blinded statistical reviewer was employed by the NIH and not Ikaria.
DeNOVO clinical site support teams (sub-investigators, study coordinators, and respiratory therapists): National Institutes of Health: Catarina Minniti, MD, Kevin Coles, RRT; Howard University Hospital: Abiodun Akintilo, MS, Alice Ukaegbu, MSN, FNP, George Tandongfuet, RRT; John’s Hopkins Univ. of School of Medicine: Phillip Seaman, PA; Gary Oldenburg, RRT; St. Christopher’s Hospital for Children: Maureen Meier (Rockstein), RN, CCRC, Marylou MacDermott, MSN, CRNP, Steven Johnson CRTT, MEd, Annette Horton, RRT; Alta Bates Medical Center: Vickie Nolan, Terri Nielsen, RN, BSN, Lou Schroel; Children’s Hospital Boston: Eric Fleegler, MD, Matthew Heeney, MD, Pam Boardman, MPH, Nancy Craig, RTT; Univ. of Colorado Health Science Center: Julie McAfee, BA, Laura Cole, Donna Parker, RRT; Brigham and Women’s Hospital: Ronald McCaffrey, MD, Ainlsey Ross, BA; Case Western University: Philip Toltzis, MD; University of Alabama at Birmingham: Lindley Webb, CPNP, Roy McDonald, Lindley Webb, CPNP, Mary Jones, Craig Wooten, RT; Children’s Hospital of Pittsburgh: Maria Schmucker, Al Saville, RRT, Mike Simpson, RRT
Footnotes
Trial Registration: clinicaltrials.gov Identifier: NCT00094887
Conflict of Interest and Financial Disclosure:
Mark T. Gladwin has received research support in the form of a Collaborative Research and Development Agreement between the US Government and Ikaria/INO Therapeutics and is listed as a co-inventor on a US Government Patent for the use of nitrite salts for cardiovascular indications. Dr. Gladwin receives grant support from the Institute of Transfusion Medicine, the Hemophilia Center of Western Pennsylvania and Federal funding by the NHLBI, NIDDK and NIAMS of the National Institutes of health (NIH grants R01HL098032, RO1HL096973, RC1DK085852, P30AR058910).
Gregory J. Kato has received research grant support from a cooperative research and development agreement between the National Institutes of Health and Ikaria INO Therapeutics and from the Division of Intramural Research of the National Institutes of Health. Victor R. Gordeuk has received research support from Biomarin and TRF-Pharma, and Emmaus Pharmaceuticals.
Lakshmanan Krishnamurti has received research support from the National Heart Lung and Blood Institute (NHLBI HB-06-06).
James F. Casella has received research support from the National Institutes of Health, NHLBI, NINDS, NCRR, MCHB, Maryland DHMH. Dr. Casella received past research support from Cytrex Corporation for the investigation of a drug (Poloxamer 188) for use in vaso-occlusive crisis. The results of these studies have been reported (ref). No ongoing relationship exists.
James Baldassarre is an employee of Ikaria.
Debra Weiner, MD, PhD has received research support from the FDA Orphan Product Development Grant Program.
Onyinye Onyekwere, MD, has received research support from Novartis Pharmaceuticals, Eli Lilly and Company, Icagen/McNeil, NIH/NHLBI and Anthera Pharmaceuticals, Inc
Carlton Dampier MD received research grant support during the period of this study from Icagen, Inc, BioMarin Pharmaceuticals, Inc, and the National Institutes of Health (U54HL070585, U10HL083705, R21HD049244, N01HB07159). Dr Dampier also had consulting relationships with Anthera Pharmaceuticals Inc, GlycoMimetics Inc, and Wyeth Pharmaceuticals Inc
Lewis Hsu, MD, R. Ward Hagar, MD, Thomas Howard, MD, Brigitta G. Mueller, MD, Rachelle Nuss, MD, Maureen Okam, MD, Lakshmanan Krishnamurti, MD; Brian Berman, M.D., Oswaldo Castro, MD, Victor R. Gordeuk, MD,; Wynona Coles, RT, Mariana Hildesheim, MS, Mary K. Hall, CIP, William C. Blackwelder, PhD, James Baldassarre, MD, Deborah Weiner, MD, James Casella, MD, Carlton Dampier, MD report no relevant conflict of interest related to the participant of this manuscript.
Author Contributions:
Dr. Gladwin had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design (Steering Committee): Gladwin, Casella, Baldassarre, Weiner.
Acquisition of data: Mark Gladwin, MD, Gregory Kato, MD, Onyinye Onyekwere, MD, Lewis Hsu, MD, R. Ward Hagar, MD, Thomas Howard, MD, Brigitta G. Mueller, MD, Rachelle Nuss, MD, Maureen Okam, MD, Lakshmanan Krishnamurti, MD; Brian Berman, M.D., Victor R. Gordeuk, MD,; Wynona Coles, RT, Mariana Hildesheim, MS, William C. Blackwelder, PhD, James Baldassarre, MD, Debra Weiner, MD, PhD, James Casella, MD
Analysis and interpretation of data: Gladwin, Kato, Casella, Baldassarre, Weiner, Hildesheim, Blackwelder.
Drafting of the manuscript: Gladwin, Kato, Casella, Baldassarre, Weiner, Hildesheim.
Critical revision of the manuscript for important intellectual content: Mark Gladwin, MD, Gregory Kato, MD, Debra Weiner, MD, PhD, Onyinye Onyekwere, MD, Lewis Hsu, MD, R. Ward Hagar, MD, Thomas Howard, MD, Brigitta G. Mueller, MD, Rachelle Nuss, MD, Maureen Okam, MD, MPH, Lakshmanan Krishnamurti, MD; Brian Berman, M.D., Victor R. Gordeuk, MD,; Wynona Coles, RT, Mariana Hildesheim, MS, William C. Blackwelder, PhD, James Baldassarre, MD, James Casella
Statistical analysis: Hildesheim, Blackwelder, Gladwin.
Obtained funding: Gladwin.
Study supervision: Mark Gladwin, MD, Gregory Kato, MD, Debra Weiner, MD, PhD, Onyinye Onyekwere, MD, Lewis Hsu, MD, R. Ward Hagar, MD, Thomas Howard, MD, Brigitta G. Mueller, MD, Rachelle Nuss, MD, Maureen Okam, MD, MPH, Lakshmanan Krishnamurti, MD; Brian Berman, M.D., Victor R. Gordeuk, MD,; Wynona Coles, RT, Mariana Hildesheim, MS, William C. Blackwelder, PhD, James Baldassarre, MD, James Casella
References
- 1.Kaul DK, Hebbel RP. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice. J Clin Invest. 2000;106(3):411–420. doi: 10.1172/JCI9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belcher JD, Bryant CJ, Nguyen J, et al. Transgenic sickle mice have vascular inflammation. Blood. 2003 May 15;101(10):3953–3959. doi: 10.1182/blood-2002-10-3313. [DOI] [PubMed] [Google Scholar]
- 3.Belcher JD, Marker PH, Weber JP, Hebbel RP, Vercellotti GM. Activated monocytes in sickle cell disease: potential role in the activation of vascular endothelium and vaso-occlusion. Blood. 2000;96(7):2451–2459. [PubMed] [Google Scholar]
- 4.Belcher JD, Mahaseth H, Welch TE, et al. Critical role of endothelial cell activation in hypoxia-induced vasoocclusion in transgenic sickle mice. Am J Physiol Heart Circ Physiol. 2005 Jun;288(6):H2715–2725. doi: 10.1152/ajpheart.00986.2004. [DOI] [PubMed] [Google Scholar]
- 5.Platt OS. Sickle cell anemia as an inflammatory disease [comment] J Clin Invest. 2000;106(3):337–338. doi: 10.1172/JCI10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frenette PS. Sickle cell vaso-occlusion: multistep and multicellular paradigm. Curr Opin Hematol. 2002 Mar;9(2):101–106. doi: 10.1097/00062752-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994 Jun 9;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 8.Steiner CAM JL. Quality AfHRa. Sickle cell disease patients in US hospitals. Vol. 2006. Rockville, Md: 2004. [PubMed] [Google Scholar]
- 9.Weiner DL, Hibberd PL, Betit P, Cooper AB, Botelho CA, Brugnara C. Preliminary assessment of inhaled nitric oxide for acute vaso-occlusive crisis in pediatric patients with sickle cell disease. Jama. 2003 Mar 5;289(9):1136–1142. doi: 10.1001/jama.289.9.1136. [DOI] [PubMed] [Google Scholar]
- 10.Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. N Engl J Med. 2008 Nov 20;359(21):2254–2265. doi: 10.1056/NEJMra0804411. [DOI] [PubMed] [Google Scholar]
- 11.Vichinsky EP, Neumayr LD, Earles AN, et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N Engl J Med. 2000 Jun 22;342(25):1855–1865. doi: 10.1056/NEJM200006223422502. [DOI] [PubMed] [Google Scholar]
- 12.Rees DC, Cervi P, Grimwade D, et al. The metabolites of nitric oxide in sickle-cell disease. Br J Haematol. 1995 Dec;91(4):834–837. doi: 10.1111/j.1365-2141.1995.tb05397.x. [DOI] [PubMed] [Google Scholar]
- 13.Lopez BL, Barnett J, Ballas SK, Christopher TA, Davis-Moon L, Ma X. Nitric oxide metabolite levels in acute vaso-occlusive sickle-cell crisis. Acad Emerg Med. 1996 Dec;3(12):1098–1103. doi: 10.1111/j.1553-2712.1996.tb03367.x. [DOI] [PubMed] [Google Scholar]
- 14.Head CA, Brugnara C, Martinez-Ruiz R, et al. Low concentrations of nitric oxide increase oxygen affinity of sickle erythrocytes in vitro and in vivo. Journal of Clinical Investigation. 1997 Sep 01;100(5):1193–1198. doi: 10.1172/JCI119631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gladwin MT, Schechter AN, Shelhamer JH, et al. Inhaled nitric oxide augments nitric oxide transport on sickle cell hemoglobin without affecting oxygen affinity. J Clin Invest. 1999 Oct;104(7):937–945. doi: 10.1172/JCI7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glover RE, Ivy ED, Orringer EP, Maeda H, Mason RP. Detection of nitrosyl hemoglobin in venous blood in the treatment of sickle cell anemia with hydroxyurea. Mol Pharmacol. 1999 Jun;55(6):1006–1010. doi: 10.1124/mol.55.6.1006. [DOI] [PubMed] [Google Scholar]
- 17.Morris CR, Kuypers FA, Larkin S, et al. Arginine therapy: a novel strategy to induce nitric oxide production in sickle cell disease. Br J Haematol. 2000 Nov;111(2):498–500. doi: 10.1046/j.1365-2141.2000.02403.x. [DOI] [PubMed] [Google Scholar]
- 18.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002 Dec;8(12):1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 19.Reiter CD, Gladwin MT. An emerging role for nitric oxide in sickle cell disease vascular homeostasis and therapy. Curr Opin Hematol. 2003 Mar;10(2):99–107. doi: 10.1097/00062752-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Nath KA, Shah V, Haggard JJ, et al. Mechanisms of vascular instability in a transgenic mouse model of sickle cell disease. Am J Physiol Regul Integr Comp Physiol. 2000 Dec;279(6):R1949–1955. doi: 10.1152/ajpregu.2000.279.6.R1949. [DOI] [PubMed] [Google Scholar]
- 21.Aslan M, Ryan TM, Adler B, et al. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci U S A. 2001 Dec 18;98(26):15215–15220. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gladwin MT, Schechter AN, Ognibene FP, et al. Divergent nitric oxide bioavailability in men and women with sickle cell disease. Circulation. 2003 Jan 21;107(2):271–278. doi: 10.1161/01.cir.0000044943.12533.a8. [DOI] [PubMed] [Google Scholar]
- 23.Kaul DK, Liu XD, Chang HY, Nagel RL, Fabry ME. Effect of fetal hemoglobin on microvascular regulation in sickle transgenic-knockout mice. J Clin Invest. 2004 Oct;114(8):1136–1145. doi: 10.1172/JCI21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 25.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 27.Panza JA, Casino PR, Kilcoyne CM, Quyyumi AA. Role of endothelium-derived nitric oxide in the abnormal endothelium- dependent vascular relaxation of patients with essential hypertension. Circulation. 1993;87(5):1468–1474. doi: 10.1161/01.cir.87.5.1468. [DOI] [PubMed] [Google Scholar]
- 28.De Caterina R, Libby P, Peng HB, et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96(1):60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cosby K, Partovi KS, Crawford JH, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003 Dec;9(12):1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 30.Villagra J, Shiva S, Hunter LA, Machado RF, Gladwin MT, Kato GJ. Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood. 2007 Sep 15;110(6):2166–2172. doi: 10.1182/blood-2006-12-061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez-Ruiz R, Montero-Huerta P, Hromi J, Head CA. Inhaled nitric oxide improves survival rates during hypoxia in a sickle cell (SAD) mouse model. Anesthesiology. 2001 Jun;94(6):1113–1118. doi: 10.1097/00000542-200106000-00028. [DOI] [PubMed] [Google Scholar]
- 32.Romero JR, Suzuka SM, Nagel RL, Fabry ME. Arginine supplementation of sickle transgenic mice reduces red cell density and Gardos channel activity. Blood. 2002 Feb 15;99(4):1103–1108. doi: 10.1182/blood.v99.4.1103. [DOI] [PubMed] [Google Scholar]
- 33.de Franceschi L, Baron A, Scarpa A, et al. Inhaled nitric oxide protects transgenic SAD mice from sickle cell disease-specific lung injury induced by hypoxia/reoxygenation. Blood. 2003 Aug 1;102(3):1087–1096. doi: 10.1182/blood-2002-07-2135. [DOI] [PubMed] [Google Scholar]
- 34.Fasipe FR, Ubawike AE, Eva R, Fabry ME. Arginine supplementation improves rotorod performance in sickle transgenic mice. Hematology. 2004 Aug;9(4):301–305. doi: 10.1080/10245330410001714185. [DOI] [PubMed] [Google Scholar]
- 35.Dasgupta T, Hebbel RP, Kaul DK. Protective effect of arginine on oxidative stress in transgenic sickle mouse models. Free Radic Biol Med. 2006 Dec 15;41(12):1771–1780. doi: 10.1016/j.freeradbiomed.2006.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Franceschi L, Malpeli G, Scarpa A, et al. Protective effects of S-nitrosoalbumin on lung injury induced by hypoxia-reoxygenation in mouse model of sickle cell disease. Am J Physiol Lung Cell Mol Physiol. 2006 Sep;291(3):L457–465. doi: 10.1152/ajplung.00462.2005. [DOI] [PubMed] [Google Scholar]
- 37.Gladwin MT, Schechter AN, Shelhamer JH, et al. Inhaled nitric oxide augments nitric oxide transport on sickle cell hemoglobin without affecting oxygen affinity. Journal of Clinical Investigation. 1999;104(7):937–945. doi: 10.1172/JCI7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oppert M, Jorres A, Barckow D, Eckardt KU, Frei U, Kaisers U. Inhaled nitric oxide for ARDS due to sickle cell disease. Swiss Med Wkly. 2004 Mar 20;134(11–12):165–167. doi: 10.4414/smw.2004.10521. [DOI] [PubMed] [Google Scholar]
- 39.Atz AM, Wessel DL. Inhaled nitric oxide in sickle cell disease with acute chest syndrome. Anesthesiology. 1997;87(4):988–990. doi: 10.1097/00000542-199710000-00037. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan KJ, Goodwin SR, Evangelist J, Moore RD, Mehta P. Nitric oxide successfully used to treat acute chest syndrome of sickle cell disease in a young adolescent. Crit Care Med. 1999;27(11):2563–2568. doi: 10.1097/00003246-199911000-00039. [DOI] [PubMed] [Google Scholar]
- 41.Head CA, Swerdlow P, McDade WA, et al. Beneficial effects of nitric oxide breathing in adult patients with sickle cell crisis. Am J Hematol. Oct;85(10):800–802. doi: 10.1002/ajh.21832. [DOI] [PubMed] [Google Scholar]
- 42.Orringer EP, Casella JF, Ataga KI, et al. Purified poloxamer 188 for treatment of acute vaso-occlusive crisis of sickle cell disease: A randomized controlled trial. Jama. 2001 Nov 7;286(17):2099–2106. doi: 10.1001/jama.286.17.2099. [DOI] [PubMed] [Google Scholar]
- 43.Shiva S, Sack MN, Greer JJ, et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007 Sep 3;204(9):2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duranski MR, Greer JJ, Dejam A, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005 May;115(5):1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lang JD, Jr, Teng X, Chumley P, et al. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007 Sep;117(9):2583–2591. doi: 10.1172/JCI31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathru M, Huda R, Solanki DR, Hays S, Lang JD. Inhaled nitric oxide attenuates reperfusion inflammatory responses in humans. Anesthesiology. 2007 Feb;106(2):275–282. doi: 10.1097/00000542-200702000-00015. [DOI] [PubMed] [Google Scholar]
- 47.Hataishi R, Rodrigues AC, Neilan TG, et al. Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006 Jul;291(1):H379–384. doi: 10.1152/ajpheart.01172.2005. [DOI] [PubMed] [Google Scholar]
- 48.Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol. 2005 May;79(1):17–25. doi: 10.1002/ajh.20336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.