We investigated the effectiveness and cost-effectiveness of screening for acute hepatitis C virus (HCV) infection in human immunodeficiency virus-infected men who have sex with men. One-time screening at enrollment in care was never optimal. Depending on HCV infection incidence, regular screening with liver function tests was cost-effective.
Abstract
Background. We used a Monte Carlo computer simulation to estimate the effectiveness and cost-effectiveness of screening for acute hepatitis C virus (HCV) infection in human immunodeficiency virus (HIV)–infected men who have sex with men.
Methods. One-time screening for prevalent HCV infection was performed at the time of enrollment in care, followed by either symptom-based screening, screening with liver function tests (LFTs), HCV antibody (Ab) screening, or HCV RNA screening in various combinations and intervals. We considered both treatment with pegylated interferon and ribavirin (PEG/RBV) alone and with an HCV protease inhibitor. Outcome measures were life expectancy, quality-adjusted life expectancy, direct medical costs, and cost-effectiveness, assuming a societal willingness to pay $100 000 per quality-adjusted life-year (QALY) gained.
Results. All strategies increased life expectancy (from 0.49 to 0.94 life-months), quality-adjusted life expectancy (from 0.47 to 1.00 quality-adjusted life-months), and costs (from $1900 to $7600), compared with symptom-based screening. The incremental cost-effectiveness ratio of screening with 6-month LFTs and a 12-month HCV Ab test, compared with symptom-based screening, was $43 700/QALY (for PEG/RBV alone) and $57 800/QALY (for PEG/RBV plus HCV protease inhibitor). The incremental cost-effectiveness ratio of screening with 3-month LFTs, compared with 6-month LFTs plus a 12-month HCV Ab test, was $129 700/QALY (for PEG/RBV alone) and $229 900/QALY (for PEG/RBV plus HCV protease inhibitor). With HCV protease inhibitor–based therapy, screening with 6-month LFTs and a 12-month HCV Ab test was the optimal strategy when the HCV infection incidence was ≤1.25 cases/100 person-years. The 3-month LFT strategy was optimal when the incidence was >1.25 cases/100 person-years.
Conclusions. Screening for acute HCV infection in HIV-infected MSM prolongs life expectancy and is cost-effective. Depending on incidence, regular screening with LFTs, with or without an HCV Ab test, is the optimal strategy.
Since 2000, outbreaks of acute hepatitis C virus (HCV) infection among human immunodeficiency virus (HIV)–infected men who have sex with men (MSM) have been reported in Europe, the United States, and Australia [1–13]. HCV infection in HIV-infected MSM is generally sexually transmitted and is associated with substantial morbidity and mortality [14, 15]. Because therapy for acute HCV infection is more effective than that for chronic HCV infection, identifying and treating acute HCV infection represents an opportunity to improve outcomes [16]. Guidelines for screening are emerging. In 2010, the European AIDS Treatment Network (NEAT) recommended HCV antibody (HCV Ab) screening for all HIV-infected persons at enrollment in care, followed by performance of liver function tests (LFTs) every 6 months and HCV Ab screening every 12 months [17]. The US Centers for Disease Control and Prevention Sexually Transmitted Disease guidelines recommend serial monitoring of LFTs and screening for HCV Ab or RNA in HIV-infected MSM with high-risk sexual behaviors [18].
Uncertainty remains concerning the most appropriate screening test for acute HCV infection, as well as the optimal interval for screening. A randomized trial comparing screening strategies is not feasible, as it would require many comparator arms and long follow-up to ensure adequate power to detect mortality differences [19]. When randomized trials are not possible, model-based analysis provides a method to integrate the costs and benefits of healthcare strategies [20]. We constructed a Monte Carlo simulation model to estimate the effectiveness and cost-effectiveness of strategies for identifying acute HCV infection in HIV-infected MSM.
METHODS
We developed the HEP-CE (HCV cost-effectiveness) model, a Monte Carlo simulation of screening for and treating HCV infection, to simulate the clinical progression of a cohort of HIV-infected MSM enrolling in US guideline-concordant HIV care [21]. We used Treeage Pro (Williamstown, MA) software to build the model. The cohort included both prevalent and incident HCV coinfection. The model simulated strategies for screening for HCV infection, including symptom-based screening, as well as for screening with LFTs, an HCV Ab test, and an HCV RNA test in various combinations and intervals. Individuals with identified HCV infection were eligible for treatment, while those with undetected infection progressed through the natural history of HCV infection. The model projected life expectancy, quality-adjusted life expectancy (QALE), and lifetime costs. The analysis assumed a societal perspective. Costs and QALE were discounted at 3% annually [22].
Model Structure
Cohort
We simulated a cohort of HIV-infected MSM enrolling in US guideline–concordant HIV care [21]. Cohort characteristics were from published reports [23, 24]
HCV Infection Incidence
HCV infection incidence was informed by reports from cohorts of HIV-infected MSM [1–13]. In the base case, we assumed that on average individuals remained at risk for new HCV infection until the age of 60 years. Those with spontaneous resolution or who were successfully treated for HCV infection had an elevated risk of reinfection [25].
Screening Strategies
At enrollment in care, all patients had 1 HCV Ab screen performed to identify prevalent cases of HCV infection. A percentage with incident infection (13%) developed symptoms and received a diagnosis of HCV infection on the basis of their clinical presentation [10]. We considered 10 HCV screening strategies: (1) symptom-based screening, (2) LFTs every 3 months, (3) LFTs every 6 months, (4) LFTs every 12 months, (5) LFTs every 6 months and an HCV Ab test every 12 months (the NEAT-recommended strategy) [17], (6) LFTs and an HCV Ab test every 3 months, (7) LFTs and an HCV Ab test every 6 months, (8) LFTs every 6 months and an HCV RNA test every 12 months, (9) LFTs and an HCV RNA test every 3 months, and (10) LFTs and an HCV RNA test every 6 months.
Test characteristics were from the literature [26–30]. We defined an elevated LFT result as an alanine aminotransferase level of >40 IU/mL [27]. The sensitivities of LFTs and HCV Ab screening were a function of time since infection. LFTs were most sensitive in the months following initial infection and decreased over time [27, 29]. Conversely, HCV Ab test sensitivity increased with time since infection [27]. The HCV RNA test was assumed to be highly sensitive and specific from the time of infection (Table 1). Positive results of screening led to confirmatory testing, including repeat LFTs, an acute hepatitis panel (tests for HCV Ab, hepatitis A virus immunoglobulin M, and hepatitis B virus core immunoglobulin M and surface antigen), and an HCV RNA test. In the base case, we assumed that clinicians and patients followed up 75% of screening tests that had positive results [31]. In sensitivity analyses, we considered a lower follow-up percentage for mildly elevated results of LFTs than for a newly converted HCV Ab or RNA test result.
Table 1.
Input Parameters for a Monte Carlo Simulation of Screening for Acute Hepatitis C Virus Infection in Human Immunodeficiency Virus–Infected US Men Who Have Sex With Men
| Variable | Base Case Value |
Source | |
|---|---|---|---|
| Cohort characteristics | |||
| Age, years (SD) | 43 (7) | [23] | |
| CD4 cell count, cells/µL (SD) | 374 (263) | [23] | |
| Prevalence of HCV infection, % | 9.8 (5–15) | [24] | |
| HCV genotype, % | [2, 6, 10] | ||
| Genotype 1/4 | 85 (70–95) | ||
| Genotype 2/3a | 15 (5–30) | ||
| Incidence of HCV infection, cases/100 person-years | 0.51 (0.01–1.0) | [1–13] | |
| Mean age at which HCV infection risk ends, years | 60 (50 until death) | Assumption | |
| Incidence of HCV reinfection, cases/100 person-years | 10.2 (0.51–40.0) | [25] | |
| Acute HCV with symptomatic disease, % | 0.13 (0.005–0.25) | [10] | |
| Screening test characteristics | |||
| LFTs | |||
| Sensitivity, months since infection [27, 29] | |||
| 0 | 0.76 (0.61–0.91) | ||
| 3 | 0.74 (0.59–0.89) | ||
| ≥6 | 0.71 (0.57–0.85) | ||
| Specificityb | 0.75 (0.65–0.85) | [30] | |
| HCV Ab test | |||
| Sensitivity, months since infection [27] | |||
| 0 | 0.25 (0.20–0.30) | ||
| 3 | 0.63 (0.50–0.76) | ||
| 6 | 0.87 (0.70–0.99) | ||
| 9 | 0.89 (0.71–0.99) | ||
| 12 | 0.95 (0.76–0.99) | ||
| Specificity | 0.98 (0.85–0.99) | [28] | |
| HCV RNA test | |||
| Sensitivity, from time of infection | 0.96 (0.90–0.99) | [26] | |
| Specificity | 0.99 (0.95–1.0) | ||
| Follow-up of positive screening tests, % | 75 (50–100) | [31] | |
| HCV disease progression | |||
| Rate of spontaneous resolution of acute HCV infection, % | 13 (5–20) | [32] | |
| Duration of acute HCV infection, months | 6 (3–12) | [32] | |
| Median time to cirrhosis, years since infection | 15 (5–25) | [8, 54, 55] | |
| Rate of liver-related death with cirrhosis, deaths/100 person-years | 2.44 (1.2–4.4) | [38] | |
| HIV disease progression | |||
| Rate of CD4 cell count decrease, cells/µL/mo | 3.7–6.4, depending on HIV RNA load | [56] | |
| Incidence AIDS events, events/100 person-years | 0.12–550, depending on CD4 cell count, OI history, and event type | [48, 57] | |
| Acute HCV therapy efficacy | Genotype 1/4 | Genotype 2/3a | |
| PEG/RBV | [40, 45, 47, 50–52] | ||
| Probability of RVR | 0.50 | 0.84 | |
| Probability of SVR given RVR | 0.88 | 0.90 | |
| Probability of SVR given no RVR | 0.67 | 0.57 | |
| Total probability of SVR | 0.78 (0.65–0.85) | 0.85 (0.75–0.95) | |
| PEG/RBV + HCV PI | [43, 44] and assumption | ||
| Probability of RVR | 0.85 | 0.90 | |
| Probability of SVR given RVR | 0.90 | 0.95 | |
| Probability of SVR given no RVR | 0.67 | 0.57 | |
| Total probability of SVR | 0.87 (0.60–0.90) | 0.91 (0.60–0.90) | |
| Chronic HCV therapy efficacy | Genotype 1/4 | Genotype 2/3a | |
| PEG/RBV | [47, 50–52] | ||
| Probability of RVR | 0.32 | 0.84 | |
| Probability of SVR given RVR | 0.69 | 0.90 | |
| Total probability of SVR | 0.22 (0.15–0.70) | 0.76 (0.70–0.85) | |
| PEG/RBV + HCV PI | [43, 44], assumption | ||
| Probability of RVR | 0.68 | 0.90 | |
| Probability of SVR given RVR | 0.90 | 0.95 | |
| Total probability of SVR | 0.63 (0.50–0.80) | 0.86 (0.7–0.90) | |
| HIV therapy | |||
| ART efficacy, % with an HIV RNA load <50 copies/mL | 15–86, depending on regimen | [58–62] | |
| CD4 cell count increase during suppressive ART, cells/µL/mo | 45–190, depending on regimen | [58–62] | |
| Quality of lifec | |||
| HCV infection | [35–37] | ||
| No fibrosis to moderate fibrosis | 0.89 (0.75–0.95) | ||
| Cirrhosis | 0.62 (0.55–0.75) | ||
| Decompensated cirrhosis | 0.48 (0.40–0.60) | ||
| HIV infection, CD4 cells/µL | [48, 63] | ||
| >500 | 0.87 (0.78–0.96) | ||
| 351–500 | 0.86 (0.77–0.95) | ||
| 251–350 | 0.86 (0.77–0.95) | ||
| 101–250 | 0.85 (0.76–0.94) | ||
| 51–100 | 0.85 (0.76–0.94) | ||
| ≤50 | 0.83 (0.74–0.92) | ||
| With acute AIDS-related event | 0.69–0.78, depending on event | ||
| Cost of screening testing, 2011 $US [64, 65] | |||
| LFTs | 12 (5–15) | ||
| HCV Ab test | 22 (15–25) | ||
| HCV RNA test (qualitative) | 53 (30–100) | ||
| Cost of confirmatory testing, 2011 $US [64, 65] | |||
| Hepatitis panel (HAV IgM, HBV core IgM, HBV sAg, HCV Ab) | 71 | ||
| HCV RNA test (quantitative) | 65 | ||
| LFTs | 12 | ||
| Total | 148 (100–200) | ||
| Cost of HCV therapy/month, 2011 $US [47, 65, 66] | |||
| PEG | 610 | ||
| RBV | 950 | ||
| Neupogen, for 12% of those receiving therapy | 14 200 | ||
| Epogen, for 10% of those receiving therapy | 2600 | ||
| Total | 3500 (2000–4000) | ||
| Cost of HCV PI/mo, 2011 $US | 4500 (4500–9000) | [46, 65, 67, 68] | |
Abbreviations: Ab, antibody; ART, antiretroviral therapy; HAV, hepatitis A virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IgM, immunoglobulin M; LFT, liver function test; OI, opportunistic infection; PEG, pegylated interferon alpha 2a; PI, protease inhibitor; RBV, ribavirin; RVR, rapid virologic response; sAg, surface antigen; SD, standard deviation; SVR, sustained virologic response.
a For scenarios that included an HCV PI, we did not consider HCV genotype 2 infection (against which current PIs have no efficacy). We simplified by assuming that all genotype 2/3 infections were genotype 3 (see Methods).
b Assuming an incidence of acute HCV infection of 0.51 cases/100 person-years and the sensitivity and specificity values above, the probability that a patient with a positive LFT result has true underlying acute HCV infection (positive predictive value) is approximately 0.7%.
c We used multiplicative assumptions to estimate the utility of health states during HIV/HCV coinfection [69].
Individuals with positive results of confirmatory testing were considered HCV infected and referred for therapy. Individuals with positive HCV Ab and negative HCV RNA test results were considered to have resolved infection. Such individuals continued to undergo HCV surveillance with either LFTs or HCV RNA tests but not with additional HCV Ab tests. For strategies that included >1 screening test, we assumed conditional independence of results [20].
HCV Disease Progression
Newly acquired HCV infection remained in the acute phase with a 13% probability of spontaneous resolution for 6 months following infection [32]. HCV infection that did not spontaneously resolve entered 3 stages of liver disease: mild to moderate fibrosis, cirrhosis, and decompensated cirrhosis [33]. At all stages, HCV infection was associated with increased resource use and decreased quality of life [34–37]. When infection reached the cirrhosis stage, individuals experienced increased mortality attributable to liver disease [38]. With spontaneous resolution or successful HCV therapy, disease progression halted, and mortality, resource use, and quality of life returned to that of HIV monoinfected individuals [39]. If, however, a patient's HCV infection reached the cirrhosis stage, his mortality and resource use remained elevated, even if he achieved sustained virologic response. If an individual was reinfected with HCV, he resumed the course of fibrosis progression at the stage reached during prior infection.
Acute HCV Infection Therapy
Individuals treated for acute infection were observed for 3 months, at which time, if HCV RNA remained positive, the patient was eligible to initiate therapy [40, 41]. In the base case, 81% of eligible patients elected to start treatment [42].
We modeled treatment with pegylated interferon and ribavirin (PEG/RBV) alone, as well as with an HCV protease inhibitor [43, 44]. For both regimens, individuals with undetectable HCV RNA at treatment week 4 (rapid virologic response) continued treatment for a planned 24-week course. Those who did not achieve rapid virologic response continued for a planned 48-week course [45, 46]. The HCV protease inhibitor scenario assumed therapy with a protease in combination with PEG/RBV for 3 months, followed by PEG/RBV alone to complete the planned treatment course.
The probability of achieving rapid virologic response and sustained virologic response were functions of time since infection and HCV genotype [45]. Treatment efficacy was constant throughout the acute infection phase [45]. The probabilities of rapid virologic response and sustained virologic response decreased linearly over the subsequent 6 months to reach the lower probabilities associated with chronic infection.
Chronic HCV Infection Therapy
Individuals with prevalent HCV infection at enrollment in care were classified as having chronic infection. For both the PEG/RBV and protease inhibitor scenarios, we modeled response-guided therapy. We assumed that in HIV/HCV coinfected persons with HCV genotype 1/4 infection, the intended treatment duration would be 48 weeks. Individuals who achieved rapid virologic response continued to receive either a planned 48-week course of therapy (genotype 1/4 infection) or 24-week course (genotype 3 infection). Those who did not achieve rapid virologic response stopped treatment. Throughout the course of treatment for both acute and chronic HCV infection, individuals faced a risk of treatment-ending toxicity [47].
HIV Disease Progression
We used the CEPAC (cost-effectiveness of preventing AIDS complications) model to project HIV-related outcomes [48, 49]. The HEP-CE model used the CEPAC projections as model inputs. Details of the CEPAC model and the method of linking HEP-CE and CEPAC are in the Supplementary Materials. The incidence of AIDS-related events increased with a lower CD4 cell count. Antiretroviral therapy initiation criteria included a CD4 cell count ≤500/μL or development of an AIDS-related opportunistic infection [21]. Patients receiving suppressive antiretroviral therapy experienced a rising CD4 cell count and a decreasing incidence of AIDS-related events. The rate of increase was a function of time, with larger gains in the CD4 cell count in the first 12 months. With long-term suppressive antiretroviral therapy, the CD4 cell count did not rise to >1200 cells/μL.
HIV infection increased the HCV-related fibrosis progression rate, decreased the likelihood of spontaneous clearance of HCV infection, and lowered the efficacy of therapy. We excluded antiretroviral agents that are not preferred for HCV coinfected individuals (Supplementary Table A-1) [21]. Antiretroviral therapy efficacy was the same for HIV monoinfected and HIV/HCV coinfected individuals.
Base Case Parameters
The mean cohort age was 43 years, and the mean CD4 cell count at enrollment in care was 374 cells/μL (Table 1) [23]. HCV infection prevalence was 9.8%, with 85% of those infections being HCV genotype 1/4 [2, 6, 10, 24]. The incidence of acute HCV infection was 0.51 cases/100 person-years [11]. The sensitivity of LFTs ranged from 71% to 76%, depending on time since infection, and LFTs were 75% specific [27, 29, 30]. The sensitivity of HCV Ab testing ranged from 25% to 95%, depending on the time since infection, and HCV Ab testing was 98% specific [27, 28]. HCV RNA testing was 96% sensitive and 99% specific from the time of infection [26]. The total probability of sustained virologic response for genotype 1/4 infections with PEG/RBV treatment ranged from 22% to 78%, depending on the time since infection [40, 45, 47, 50–52], and from 63% to 87% for therapy with protease inhibitors [43, 44]. Base case model parameters are provided in Table 1.
Analyses
For stable estimates, we simulated the progression of a cohort of 10 million HIV-infected MSM enrolling in clinical care and compared outcomes for each of the 10 screening strategies. For cost-effectiveness interpretation, we assumed a societal willingness to pay of $100 000/QALY gained [53]. We performed a series of deterministic and probabilistic sensitivity analyses to identify parameters that had the greatest impact on cost-effectiveness.
RESULTS
Base Case
Symptom-based screening was associated with a projected life expectancy from the time of enrollment in HIV care of 343.83 months (28.65 years undiscounted); a projected QALE of 182.84 discounted, quality-adjusted life-months (QALMs) (15.24 QALYs); and a mean discounted lifetime cost of $479 600 (Table 2). All screening strategies increased life expectancy, compared with symptom-based screening, from 0.49 to 0.59 additional undiscounted life-months, improved QALE from 0.47 to 0.62 additional quality-adjusted life-months, and increased average lifetime cost from $1800 to $5000. These small individual-level benefits are expected because the majority of individuals were never HCV infected. In comparison, routine screening for HIV-infection among high-risk individuals is associated with an average per person life expectancy gain of 0.9 QALMs [49]. Among individuals who contracted HCV infection (mean age at infection, 43 years), NEAT-recommended screening resulted in a gain of 8.5 discounted QALMs, compared with symptom-based screening, at an average increased cost of $24 600/person.
Table 2.
Cost-effectiveness of Screening for Acute Hepatitis C Virus in Human Immunodeficiency Virus–Infected Men Who Have Sex With Men
| Strategy | Mean Discounted Lifetime Costs, 2011 $US | Undiscounted Life Expectancy, Mo | Discounted QALMs | ICER, 2011 $US/QALYa |
|---|---|---|---|---|
| PEG/RBV | ||||
| Symptom based | 479 600 | 343.83 | 182.84 | … |
| 12-mo LFTs | 481 500 | 344.32 | 183.31 | Dominated |
| 6-mo LFTs/12-mo HCV Ab test | 481 600 | 344.34 | 183.36 | 43 700 |
| 6-mo LFTs | 481 700 | 344.35 | 183.36 | Dominated |
| 6-mo HCV Ab test and LFTs | 481 900 | 344.36 | 183.37 | Dominated |
| 6-mo LFTs/12-mo HCV RNA test | 482 100 | 344.36 | 183.38 | Dominated |
| 3-mo LFTs | 482 300 | 344.41 | 183.43 | 129 700 |
| 3-mo HCV Ab test and LFTs | 482 700 | 344.43 | 183.44 | 401 000 |
| 6-mo HCV RNA test and LFTs | 482 900 | 344.35 | 183.38 | Dominated |
| 3-mo HCV RNA test and LFTs | 484 600 | 344.42 | 183.45 | 1 700 000 |
| PEG/RBV + HCV PI | ||||
| Symptom based | 483 700 | 345.43 | 184.81 | … |
| 12-mo LFTs | 488 000 | 346.25 | 185.69 | Dominated |
| 6-mo LFTs/12-mo HCV Ab test | 488 300 | 346.34 | 185.74 | 57 800 |
| 6-mo LFTs | 488 500 | 346.29 | 185.73 | Dominated |
| 6-mo HCV Ab test and LFTs | 488 700 | 346.35 | 185.75 | Dominated |
| 6-mo LFTs/12-mo HCV RNA test | 488 900 | 346.37 | 185.75 | Dominated |
| 3-mo LFTs | 489 100 | 346.37 | 185.78 | 229 900 |
| 3-mo HCV Ab test and LFTs | 489 500 | 346.37 | 185.80 | 367 100 |
| 6-mo HCV RNA test and LFTs | 489 700 | 346.33 | 185.77 | Dominated |
| 3-mo HCV RNA test and LFTs | 491 500 | 346.34 | 185.81 | 1 400 000 |
Abbreviations: Ab, antibody; HCV, hepatitis C virus; ICER, incremental cost-effectiveness ratio; LFT, liver function test; pegylated interferon alpha 2a; PEG/RBV, pegylated interferon and ribavirin; PI, protease inhibitor; QALY, quality-adjusted life-year; QALM, quality-adjusted life-month; RBV, ribavirin.
a The term “dominated” refers to a strategy that provides lower life expectancy than another option at a higher cost or improves life expectancy at a greater cost/QALY gained.
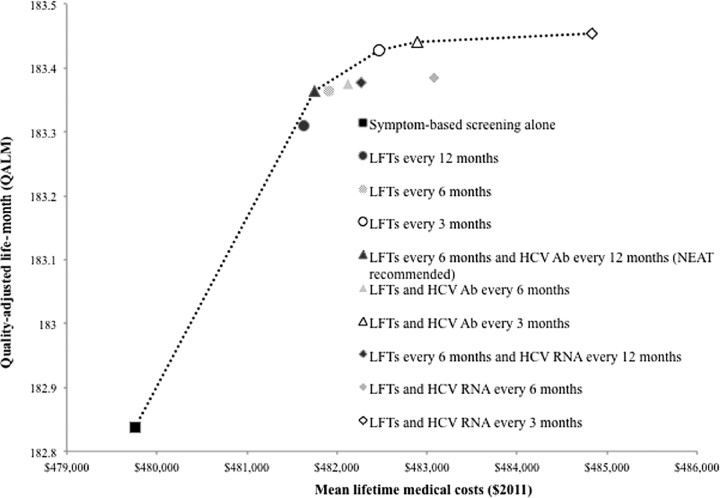
Screening with LFTs every 12 months was the least costly screening strategy, compared with symptom-based screening (incremental cost $1900), but NEAT-recommended screening resulted in a larger gain in QALE at a lower cost/QALY gained (Figure 1). The incremental cost-effectiveness ratio (ICER) for NEAT-recommended screening, compared with symptom-based screening, was $43 700/QALY gained (Table 2).
Figure 1.
Efficiency frontier of a cost-effectiveness analysis of screening for acute hepatitis C virus (HCV) infection in human immunodeficiency virus (HIV)–infected men who have sex with men. The figure illustrates the effectiveness, cost, and incremental cost-effectiveness of all 10 strategies for screening. The black square in the lower left corner represents symptom-based screening alone, the comparator for the analysis. Circles represent strategies that use liver function tests (LFTs) alone. Triangles represent strategies employing LFTs and HCV antibody tests. Diamonds represent strategies that use LFTs and HCV RNA tests. The color of each shape represents the frequency of screening. The dotted line represents the efficiency frontier. Strategies that lie to the right of the efficiency frontier result in either lower quality-adjusted life expectancy at higher cost than an alternative strategy or a higher cost per quality-adjusted life-year gained. All costs and quality-adjusted life expectancy were discounted at a 3% annual rate. Abbreviations: Ab, antibody; HCV, hepatitis C virus; LFT, liver function test; RNA, ribonucleic acid.
Compared with NEAT guidelines, 3-month LFTs provided an additional 0.06 discounted QALMs at an additional discounted lifetime cost of $700, corresponding to an ICER of $129 700/QALY gained. All other strategies were dominated or had an ICER >$400 000/QALY gained (Figure 1).
Scenario With HCV Protease Inhibitor
When we repeated the analysis and assumed higher treatment efficacy and costs associated with HCV protease inhibitors, the ordering of strategies did not change, nor did their relative dominance. Because the relative benefit of early compared with late HCV treatment was smaller, however, the ICER for all strategies increased. Assuming the availability of an HCV protease inhibitor, the ICER of NEAT-recommended screening, compared with symptom-based screening, was $57 800/QALY gained. The ICER of 3-month LFTs, compared with NEAT-recommended screening, was $229 900/QALY-gained.
Deterministic Sensitivity Analyses
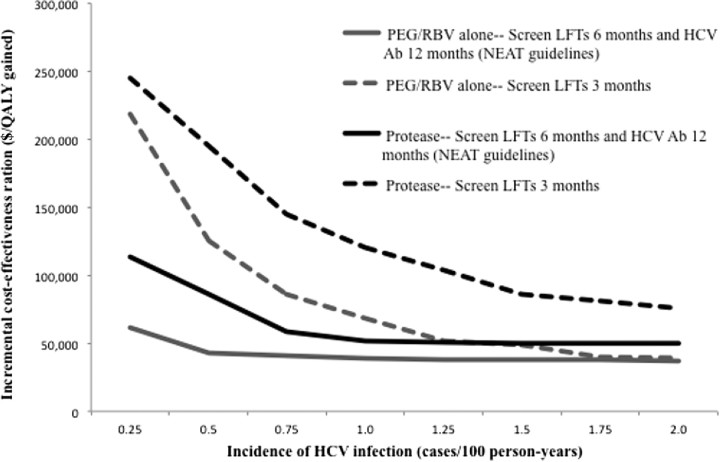
The optimal interval for HCV screening was sensitive to incidence. At all incidences between 0.25 and 2.0 cases/100 person-years, assuming treatment with PEG/RBV alone as well as with an HCV protease inhibitor, the ICER of NEAT guidelines, compared with the next best alternative (which varied depending on incidence), was approximately ≤$100 000/QALY gained (Figure 2). The cost-effectiveness of 3-month LFTs, however, was more sensitive to HCV infection incidence. Assuming a willingness-to-pay of $100 000/QALY gained and acute HCV therapy with PEG/RBV alone, 3-month LFTs was cost-effective when the HCV infection incidence was >0.5 cases/100 person-years (Figure 2). With the addition of an HCV protease inhibitor, performance of 3-month LFTs was cost-effective only when HCV infection incidence was >1.25 cases/100 person-years.
Figure 2.
Sensitivity analysis on the cost-effectiveness of screening for acute hepatitis C virus (HCV) infection as a function of the incidence of HCV infection. Black lines represent screening followed by treatment with pegylated interferon and ribavirin (PEG/RBV) alone. Grey lines illustrate therapy with an HCV protease inhibitor in combination with PEG/RBV. Solid lines illustrate the incremental cost-effectiveness of current European AIDS Treatment Network (NEAT) guidelines for HCV screening (liver function tests [LFTs] every 6 months plus an HCV antibody test every 12 months), compared with the next best alternative, which varied depending on the incidence of HCV infection. Dashed lines represent the incremental cost-effectiveness of screening with LFTs every 3 months, compared with NEAT guidelines. Abbreviations: Ab, antibody; HCV, hepatitis C virus; LFT, liver function test; NEAT, European AIDS Treatment Network; PEG, pegylated interferon; RBV, ribavirin; $/QALY, cost/quality-adjusted life-year.
The rate of HCV reinfection after spontaneous clearance or successful treatment affected the relationship between the cost-effectiveness of screening and the HCV infection incidence. In the analysis presented in Figure 2, we assumed a high incidence of reinfection (10.2 cases/100 person-years), but that incidence was independent of the initial HCV infection incidence. When we allowed the incidence of reinfection to vary as a function of the initial HCV infection incidence, ICERs generally rose with increasing incidence. Assuming therapy with PEG/RBV alone, this did not change cost-effectiveness conclusions. With the availability of HCV protease inhibitors, however, when the HCV infection incidence >1.75 cases/100 person-years (corresponding to a reinfection rate of 35 cases/100 person-years), only performance of 12-month LFTs was cost-effective.
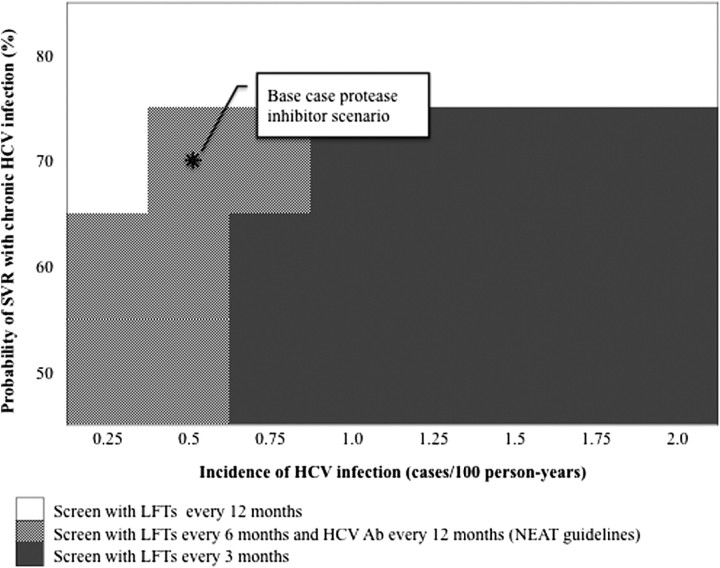
Also important was the efficacy of therapy for chronic HCV infection. More frequent screening was cost-effective with lower chronic HCV infection treatment efficacy and higher incidence (Figure 3). At the base case incidence of 0.51 cases/100 person-years, assuming use of protease inhibitor–based therapy that is 86% effective for treatment of acute HCV and a societal willingness to pay of $100 000/QALY gained, NEAT-recommended screening was the optimal strategy when therapy for chronic HCV infection was <80% effective. If, however, the incidence of HCV infection was ≥1.25 cases/100 person-years, as reported in some sites in Europe and the United States, performance of 3-month LFTs was the optimal strategy unless therapy for chronic HCV infection was ≥80% effective [6, 10].
Figure 3.
A 2-way sensitivity analysis of the cost-effectiveness of screening for acute hepatitis C virus (HCV) infection as a function of HCV incidence and the probability of attaining sustained virologic response (SVR) with treatment for chronic HCV. The figure illustrates the optimal strategy for screening for acute HCV infection, with various efficacies of new agents for treating HCV infection and different HCV infection incidence rates, assuming that 86% of those treated for acute HCV infection attain SVR. The shade of the graph at the point of intersection corresponding to a given HCV incidence and chronic HCV therapeutic efficacy illustrates the optimal screening strategy, assuming a societal willingness to pay of $100 000 per quality-adjusted life-year gained. Abbreviations: Ab, antibody; HCV, hepatitis C virus; LFT, liver function test; NEAT, European AIDS Treatment Network; SVR, sustained virologic response.
On the other hand, when the efficacy of therapy for chronic HCV infection was fixed at that of PEG/RBV and the efficacy of therapy for acute infection was varied, NEAT-recommended screening remained cost-effective, even when the efficacy of treatment for acute HCV infection was reduced to 65%.
The fibrosis progression rate in patients with HCV infection also impacted the cost-effectiveness of screening. When we assumed rapid progression to cirrhosis (median duration, 5 years), as reported in some articles, performance of 3-month LFTs became more economically appealing, with an ICER of $90 500/QALY gained, compared with NEAT-recommended screening [8]. When we extended the median time to cirrhosis to 25 years, the ICER of 3-month LFTs was $164 000/QALY gained, but NEAT-recommended screening remained cost-effective ($55 300/QALY gained).
Screening with LFTs, however, was critically dependent on clinician response to elevated LFT results. In the base case, 3-month LFTs remained cost-effective, even when the specificity of LFTs for acute infection was decreased to 65%, as well as when we increased the cost of confirmatory testing of an initial positive LFT result to $200. Alternatively, if providers and patients followed-up only 50% of positive LFT results, use of 3-month LFTs was a dominated strategy, and NEAT-recommended screening became the optimal approach.
Additional parameters, including the cost of HCV protease inhibitors, the sensitivity and specificity of screening tests, the cost of screening tests, and quality-of-life with HCV infection, impacted the cost-effectiveness of 3-month LFT screening, but they did not have a major effect on the cost-effectiveness of NEAT-recommended screening when varied by plausible ranges.
Probabilistic Sensitivity Analyses
In 1000 simulations that assumed treatment with PEG/RBV alone and incorporated uncertainty in HCV incidence and disease progression parameters, symptom-based screening was never the optimal strategy at a societal willingness to pay of $100 000/QALY gained, NEAT-recommended screening was the optimal strategy in 48% of simulations, and performance of 3-month LFTs was optimal in 52%. With the availability of HCV protease inhibitor–based therapy, symptom-based screening was never optimal, but NEAT-recommended screening was optimal in 60% of simulations, while 3-month LFTs screening was optimal in 40%.
DISCUSSION
Evidence-based guidelines are needed to inform screening for acute HCV infection in HIV-infected MSM [15]. This analysis demonstrates that one-time screening for prevalent HCV infection at enrollment in HIV care is not adequate. Routine periodic screening for newly acquired HCV infection extends life expectancy and is cost-effective.
Two strategies emerged from the analysis as potentially cost-effective approaches to identifying acute infection: (1) NEAT-recommended screening (6-month LFTs and a 12-month HCV Ab screen), which had an ICER ≤$100 000/QALY gained, compared with symptom-based screening, across a broad range of assumptions about HCV infection incidence, disease progression, treatment efficacy, and costs; and (2) 3-month screening with LFTs, which was sensitive to assumptions about treatment efficacy and HCV infection incidence but was cost-effective in HCV protease inhibitor scenarios when the incidence was ≥1.25 cases/100 person-years.
As more effective therapies for chronic HCV infection become available, the optimal screening guidelines depend largely on HCV infection incidence. When the incidence is <1.25 cases/100 person-years, NEAT-recommended screening is a cost-effective policy. In areas with an HCV infection incidence ≥1.25 cases/100 person-years, 3-month LFTs are cost-effective.
The incidence of HCV reinfection impacts the cost-effectiveness of screening. Even with high reinfection rates (20%), we found that NEAT-recommended screening remained cost-effective. If, however, settings with high HCV infection incidence experience reinfection rates >20%, screening may only be cost-effective when coupled with interventions to reduce the incidence of reinfection.
In addition, for LFT screening to be effective, follow-up of mildly abnormal LFT results is important. When we assumed a 50% follow-up rate for abnormal LFTs, 3-month LFTs were no longer cost-effective and NEAT-recommended screening was preferred. Because HCV Ab testing is poorly sensitive for diagnosing acute infection, 3-month HCV Ab screening provided little new information and did not justify the increased cost of such frequent Ab screening.
There are several limitations to this analysis. First, the model does not include secondary HCV infection cases and therefore cannot capture the public health benefits of HCV screening. Our estimates of cost-effectiveness are therefore conservative, as they include only 1 component of the potential benefits of screening. Additionally, as with any modeling study, uncertainty in model parameters can affect results. We took multiple steps, however, to minimize the impact of uncertainty and to transparently demonstrate its importance to the conclusions. We chose conservative estimates of key model parameters and performed broad 1- and 2-way deterministic sensitivity analyses, as well as probabilistic sensitivity analyses, to estimate confidence in model-based outcomes. While uncertainty exists around model parameters, decisions must be made about screening for acute HCV infection. This analysis provides an evidence-based tool to integrate the best data and formulate well-informed policy.
Acute HCV infection is a common, sexually transmitted infection in HIV-infected MSM. This study demonstrates that in well-resourced settings, one-time screening for prevalent HCV infection in HIV-infected MSM is not adequate. When the incidence of HCV infection is <1.25%, routine screening for acute HCV infection with LFTs every 6 months and an HCV Ab test annually will increase life expectancy and be cost-effective. When the HCV infection incidence is ≥1.25%, screening with LFTs every 3 months is optimal.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Devra Barter, MS, for her help with performing final analyses and manuscript preparation.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Allergy And Infectious Diseases or the National Institutes of Health.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (K01AI073193, K24AI062476, R37AI42006, R01 DA027379, and U19 AI066345). The project described was supported by grants from the National Institute of Allergy and Infectious Diseases and the National Institute on Drug Abuse.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ghosn J, Deveau C, Goujard C, et al. Increase in hepatitis C virus incidence in HIV-1-infected patients followed up since primary infection. Sex Transm Infect. 2006;82:458–60. doi: 10.1136/sti.2006.021493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danta M, Brown D, Bhagani S, et al. Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. AIDS. 2007;21:983–91. doi: 10.1097/QAD.0b013e3281053a0c. [DOI] [PubMed] [Google Scholar]
- 3.Browne R, Asboe D, Gilleece Y, et al. Increased numbers of acute hepatitis C infections in HIV positive homosexual men; is sexual transmission feeding the increase? Sex Transm Infect. 2004;80:326–7. doi: 10.1136/sti.2003.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gotz HM, van Doornum G, Niesters HG, den Hollander JG, Thio HB, de Zwart O. A cluster of acute hepatitis C virus infection among men who have sex with men—results from contact tracing and public health implications. AIDS. 2005;19:969–74. doi: 10.1097/01.aids.0000171412.61360.f8. [DOI] [PubMed] [Google Scholar]
- 5.Gambotti L, Batisse D, Colin-de-Verdiere N, et al. Acute hepatitis C infection in HIV positive men who have sex with men in Paris, France, 2001–2004. Euro Surveill. 2005;10:115–7. [PubMed] [Google Scholar]
- 6.van de Laar TJ, van der Bij AK, Prins M, et al. Increase in HCV incidence among men who have sex with men in Amsterdam most likely caused by sexual transmission. J Infect Dis. 2007;196:230–8. doi: 10.1086/518796. [DOI] [PubMed] [Google Scholar]
- 7.Serpaggi J, Chaix ML, Batisse D, et al. Sexually transmitted acute infection with a clustered genotype 4 hepatitis C virus in HIV-1-infected men and inefficacy of early antiviral therapy. AIDS. 2006;20:233–40. doi: 10.1097/01.aids.0000200541.40633.56. [DOI] [PubMed] [Google Scholar]
- 8.Fierer DS, Uriel AJ, Carriero DC, et al. Liver fibrosis during an outbreak of acute hepatitis C virus infection in HIV-infected men: a prospective cohort study. J Infect Dis. 2008;198:683–6. doi: 10.1086/590430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luetkemeyer A, Hare CB, Stansell J, et al. Clinical presentation and course of acute hepatitis C infection in HIV-infected patients. J Acquir Immune Defic Syndr. 2006;41:31–6. doi: 10.1097/01.qai.0000191281.77954.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bottieau E, Apers L, Esbroeck MV, Vandenbruaene M, Florence E. Hepatitis C virus infection in HIV-infected men who have sex with men: sustained rising incidence in Antwerp, Belgium, 2001–2009. Euro Surveill. 2010;15:19673. [PubMed] [Google Scholar]
- 11.Taylor LE, Holubar M, Wu K, et al. Incident hepatitis C virus infection among US HIV-infected men enrolled in clinical trials. Clin Infect Dis. 2011;52:812–8. doi: 10.1093/cid/ciq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rauch A, Rickenbach M, Weber R, et al. Unsafe sex and increased incidence of hepatitis C virus infection among HIV-infected men who have sex with men: the Swiss HIV Cohort Study. Clin Infect Dis. 2005;41:395–402. doi: 10.1086/431486. [DOI] [PubMed] [Google Scholar]
- 13.Gamage DG, Read TR, Bradshaw CS, et al. Incidence of hepatitis-C among HIV infected men who have sex with men (MSM) attending a sexual health service: a cohort study. BMC Infect Dis. 2011;11:39. doi: 10.1186/1471-2334-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Backus LI, Phillips BR, Boothroyd DB, et al. Effects of hepatitis C virus coinfection on survival in veterans with HIV treated with highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2005;39:613–9. [PubMed] [Google Scholar]
- 15.Sexual transmission of hepatitis C virus among HIV-infected men who have sex with men –- New York City, 2005–2010. MMWR Morbidity and Mortality Weekly Report. 2011;60:945–50. [PubMed] [Google Scholar]
- 16.van de Laar TJ, Matthews GV, Prins M, Danta M. Acute hepatitis C in HIV-infected men who have sex with men: an emerging sexually transmitted infection. AIDS. 2010;24:1799–812. doi: 10.1097/QAD.0b013e32833c11a5. [DOI] [PubMed] [Google Scholar]
- 17.Acute hepatitis C in HIV-infected individuals: recommendations from the European AIDS Treatment Network (NEAT) consensus conference. AIDS. 2011;25:399–409. doi: 10.1097/QAD.0b013e328343443b. [DOI] [PubMed] [Google Scholar]
- 18.Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59:1–110. [PubMed] [Google Scholar]
- 19.Chou R, Clark EC, Helfand M. Screening for hepatitis C virus infection: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140:465–79. doi: 10.7326/0003-4819-140-6-200403160-00014. [DOI] [PubMed] [Google Scholar]
- 20.Gold MR, Siegel J, Russell LB, Weinstein MC, editors. Cost effectiveness in health and medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 21.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 10 January 2011. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 7 February 2011. [Google Scholar]
- 22.Weinstein MC, O'Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices–Modeling Studies. Value Health. 2003;6:9–17. doi: 10.1046/j.1524-4733.2003.00234.x. [DOI] [PubMed] [Google Scholar]
- 23.Althoff KN, Gange SJ, Klein MB, et al. Late presentation for human immunodeficiency virus care in the United States and Canada. Clin Infect Dis. 2010;50:1512–20. doi: 10.1086/652650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JH, Psevdos G, Suh J, Sharp VL. Co-infection of hepatitis B and hepatitis C virus in human immunodeficiency virus-infected patients in New York City, United States. World J Gastroenterol. 2008;14:6689–93. doi: 10.3748/wjg.14.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambers FA, Prins M, Thomas X, et al. Alarming incidence of hepatitis C virus re-infection after treatment of sexually acquired acute hepatitis C virus infection in HIV-infected MSM. AIDS. 2011;25:F21–7. doi: 10.1097/QAD.0b013e32834bac44. [DOI] [PubMed] [Google Scholar]
- 26.Albadalejo J, Alonso R, Antinozzi R, et al. Multicenter evaluation of the COBAS AMPLICOR HCV assay, an integrated PCR system for rapid detection of hepatitis C virus RNA in the diagnostic laboratory. J Clin Microbiol. 1998;36:862–5. doi: 10.1128/jcm.36.4.862-865.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomson EC, Nastouli E, Main J, et al. Delayed anti-HCV antibody response in HIV-positive men acutely infected with HCV. AIDS. 2009;23:89–93. doi: 10.1097/QAD.0b013e32831940a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao HY, Ren FR, Guan WL, et al. Evaluation of the performance of the EIAgen HCV test for detection of hepatitis C virus infection. J Virol Methods. 2009;162:203–7. doi: 10.1016/j.jviromet.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Pardo M, Lopez-Alcorocho JM, Rodriguez-Inigo E, Castillo I, Carreno V. Comparative study between occult hepatitis C virus infection and chronic hepatitis C. J Viral Hepat. 2007;14:36–40. doi: 10.1111/j.1365-2893.2006.00783.x. [DOI] [PubMed] [Google Scholar]
- 30.Crum-Cianflone N, Dilay A, Collins G, et al. Nonalcoholic fatty liver disease among HIV-infected persons. J Acquir Immune Defic Syndr. 2009;50:464–73. doi: 10.1097/QAI.0b013e318198a88a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekwueme DU, Pinkerton SD, Holtgrave DR, Branson BM. Cost comparison of three HIV counseling and testing technologies. Am J Prev Med. 2003;25:112–21. doi: 10.1016/s0749-3797(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 32.Schnuriger A, Dominguez S, Guiguet M, et al. Acute hepatitis C in HIV-infected patients: rare spontaneous clearance correlates with weak memory CD4 T-cell responses to hepatitis C virus. AIDS. 2009;23:2079–89. doi: 10.1097/QAD.0b013e328330ed24. [DOI] [PubMed] [Google Scholar]
- 33.Giron-Gonzalez JA, Brun F, Terron A, Vergara A, Arizcorreta A. Natural history of compensated and decompensated HCV-related cirrhosis in HIV-infected patients: a prospective multicentre study. Antivir Ther. 2007;12:899–907. doi: 10.1177/135965350701200605. [DOI] [PubMed] [Google Scholar]
- 34.Linas BP, Wang B, Smurzynski M, et al. The impact of HIV/HCV co-infection on healthcare utilization and disability: results of the ACTG Longitudinal Linked Randomized Trials (ALLRT) Cohort. J Viral Hepat. 2011;18:506–12. doi: 10.1111/j.1365-2893.2010.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chong CA, Gulamhussein A, Heathcote EJ, et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol. 2003;98:630–8. doi: 10.1111/j.1572-0241.2003.07332.x. [DOI] [PubMed] [Google Scholar]
- 36.Grieve R, Roberts J, Wright M, et al. Cost effectiveness of interferon alpha or peginterferon alpha with ribavirin for histologically mild chronic hepatitis C. Gut. 2006;55:1332–8. doi: 10.1136/gut.2005.064774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stein K, Dalziel K, Walker A, et al. Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice. Health Technol Assess. 2002;6:1–122. [PubMed] [Google Scholar]
- 38.Pineda JA, Aguilar-Guisado M, Rivero A, et al. Natural history of compensated hepatitis C virus-related cirrhosis in HIV-infected patients. Clin Infect Dis. 2009;49:1274–82. doi: 10.1086/605676. [DOI] [PubMed] [Google Scholar]
- 39.Berenguer J, Alvarez-Pellicer J, Martin PM, et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2009;50:407–13. doi: 10.1002/hep.23020. [DOI] [PubMed] [Google Scholar]
- 40.Matthews GV, Hellard M, Haber P, et al. Characteristics and treatment outcomes among HIV-infected individuals in the Australian Trial in Acute Hepatitis C. Clin Infect Dis. 2009;48:650–8. doi: 10.1086/596770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iorio A, Marchesini E, Awad T, Gluud LL. Antiviral treatment for chronic hepatitis C in patients with human immunodeficiency virus. Cochrane Database Syst Rev. 2010;1:CD004888. doi: 10.1002/14651858.CD004888.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Matthews G, Kronborg IJ, Dore GJ. Treatment for hepatitis C virus infection among current injection drug users in Australia. Clin Infect Dis. 2005;40(Suppl 5):S325–9. doi: 10.1086/427448. [DOI] [PubMed] [Google Scholar]
- 43.Sulkowski M, Dieterich D, Sherman KE, et al. Interim analysis of a phase 2a double-blind study of teleprevir in combination with pegylated int4erferon alpha-2a and ribavirin in HIV/HCV co-infected patients [abstract 146LB] 2011 Presented at: Conference on Retroviruses and Opportunistic Infections (Boston, MA) [Google Scholar]
- 44.Sherman KE, Rockstroh JK, Dieterich DT, et al. Telaprevir combination with peginterferon alfa-2a/ribavirin in HCV/HIV coinfected patients: 24-week treatment interim analysis [abstract LB-8] 2011 Presented at: 62nd Annual Meeting of the American Association for the Study of Liver Disease (San Francisco), 4–8 November. [Google Scholar]
- 45.Piroth L, Larsen C, Binquet C, et al. Treatment of acute hepatitis C in human immunodeficiency virus-infected patients: the HEPAIG study. Hepatology. 2010;52:1915–21. doi: 10.1002/hep.23959. [DOI] [PubMed] [Google Scholar]
- 46.Lambers FA, Brinkman K, Schinkel J, et al. Treatment of acute hepatitis C virus infection in HIV-infected MSM: the effect of treatment duration. AIDS. 2011;25:1333–6. doi: 10.1097/QAD.0b013e3283480144. [DOI] [PubMed] [Google Scholar]
- 47.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–50. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 48.Freedberg KA, Losina E, Weinstein MC, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001;344:824–31. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 49.Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States–an analysis of cost-effectiveness. N Engl J Med. 2005;352:586–95. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 50.Chung RT, Andersen J, Volberding P, et al. Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–9. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin-Carbonero L, Nunez M, Marino A, et al. Undetectable hepatitis C virus RNA at week 4 as predictor of sustained virological response in HIV patients with chronic hepatitis C. AIDS. 2008;22:15–21. doi: 10.1097/QAD.0b013e3282f1da99. [DOI] [PubMed] [Google Scholar]
- 52.Carrat F, Bani-Sadr F, Pol S, et al. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA. 2004;292:2839–48. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- 53.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn't it increase at the rate of inflation? Arch Intern Med. 2003;163:1637–41. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 54.Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008;22:1979–91. doi: 10.1097/QAD.0b013e32830e6d51. [DOI] [PubMed] [Google Scholar]
- 55.Vogel M, Page E, Boesecke C, et al. Liver fibrosis progression after acute hepatitis C virus infection in HIV-positive individuals. Clin Infect Dis. 2011;54:556–9. doi: 10.1093/cid/cir854. [DOI] [PubMed] [Google Scholar]
- 56.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 57.Linas BP, Losina E, Rockwell A, Walensky RP, Cranston K, Freedberg KA. Improving outcomes in state AIDS drug assistance programs. J Acquir Immune Defic Syndr. 2009;51:513–21. doi: 10.1097/QAI.0b013e3181b16d00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gallant JE, DeJesus E, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–60. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 59.Grinsztejn B, Nguyen BY, Katlama C, et al. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet. 2007;369:1261–9. doi: 10.1016/S0140-6736(07)60597-2. [DOI] [PubMed] [Google Scholar]
- 60.Johnson M, Grinsztejn B, Rodriguez C, et al. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS. 2005;19:685–94. doi: 10.1097/01.aids.0000166091.39317.99. [DOI] [PubMed] [Google Scholar]
- 61.Lalezari J, Goodrich J, DeJesus E. Efficacy and safety of maraviroc plus optimized background therapy in viremic ART experienced patients infected with CCR5-tropic HIV-1: 24-week results of a phase 2b/3 study in the US and Canada [abstract 104bLB] Presented at: 14th CROI (Los Angeles, CA), 25–28 February 2007. [Google Scholar]
- 62.Nelson M, Arasteh K, Clotet B, et al. Durable efficacy of enfuvirtide over 48 weeks in heavily treatment-experienced HIV-1-infected patients in the T-20 versus optimized background regimen only 1 and 2 clinical trials. J Acquir Immune Defic Syndr. 2005;40:404–12. doi: 10.1097/01.qai.0000185314.56556.c3. [DOI] [PubMed] [Google Scholar]
- 63.Schackman BR, Goldie SJ, Freedberg KA, Losina E, Brazier J, Weinstein MC. Comparison of health state utilities using community and patient preference weights derived from a survey of patients with HIV/AIDS. Med Decis Making. 2002;22:27–38. doi: 10.1177/0272989X0202200103. [DOI] [PubMed] [Google Scholar]
- 64.Center for Medicare Services. US Department of Health and Human Services. 2009 Clinical Diagnostic Laboratory Fee Schedule. Available at: http://www.cms.hhs.gov/ClinicalLabFeeSched/02_clinlab.asp-TopOfPage. Accessed 25 May 2010. [Google Scholar]
- 65.United States Department of Management and Budget. Budget of the United States government: historical tables fiscal year 2009. Table 10.1. Available at: http://www.gpoaccess.gov/usbudget/fy09/hist.html. Accessed 26 September 2011. [Google Scholar]
- 66.Physicians Desk Reference/Micromedex. 2009 Red book: Pharmacy's fundamental reference. Montvale, NJ: Thomson Reuters Healthcare; 2009. [Google Scholar]
- 67.Hepatitis C New Drug Research and Liver Health. Available at: http://hepatitiscnewdrugresearch.com/cost-of-treating-with-telaprevir.html. Accessed 14 June 2011. [Google Scholar]
- 68.Pollack A. Merck's hepatitis C drug wins FDA approval. New York Times. 14 May 2011:B6. [Google Scholar]
- 69.Bo H, Fu AZ. Predicting utility for joint health states: a general framework and a new nonparametric estimator. Med Decis Making. 2010;30:E29–39. doi: 10.1177/0272989X10374508. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.