Abstract
The redox-sensitive transcription factor NF-E2–related factor 2 (Nrf2) plays a key role in preserving a healthy endothelial phenotype and maintaining the functional integrity of the vasculature. Previous studies demonstrated that aging is associated with Nrf2 dysfunction in endothelial cells, which alters redox signaling and likely promotes the development of large vessel disease. Much less is known about the consequences of Nrf2 dysfunction at the level of the microcirculation. To test the hypothesis that Nrf2 regulates angiogenic capacity of endothelial cells, we determined whether disruption of Nrf2 signaling (by siRNA knockdown of Nrf2 and overexpression of Keap1, the cytosolic repressor of Nrf2) impairs angiogenic processes in cultured human coronary arterial endothelial cells stimulated with vascular endothelial growth factor and insulin-like growth factor-1. In the absence of functional Nrf2, coronary arterial endothelial cells exhibited impaired proliferation and adhesion to vitronectin and collagen. Disruption of Nrf2 signaling also reduced cellular migration (measured by a wound-healing assay using electric cell-substrate impedance sensing technology) and impaired the ability of coronary arterial endothelial cells to form capillary-like structures. Collectively, we find that Nrf2 is essential for normal endothelial angiogenic processes, suggesting that Nrf2 dysfunction may be a potential mechanism underlying impaired angiogenesis and microvascular rarefaction in aging.
Keywords: Vascular aging, Microcirculation, Capillary density, Angiogenesis, Heart
There is increasing evidence to suggest that the “cap’n’collar” transcription factor NF-E2–related factor 2 (Nrf2) has a key role in preserving a healthy endothelial phenotype and maintaining the functional integrity of the vasculature (1–8). Recent studies by our laboratories (9,10) and others (11) demonstrate that aging in blood vessels of laboratory rodents and nonhuman primates is associated with severe impairment of Nrf2 activity and dysregulation of cellular redox signaling. There is growing evidence suggesting that Nrf2 dysfunction contributes to the functional impairment of conduit arteries, increasing susceptibility of blood vessels to injury in metabolic diseases (5) and exacerbating the progress of atherosclerosis in aged animals (9,11).
It is well known that aging significantly impairs angiogenic capacity of microvascular endothelial cells (12–21), diminishing their responsiveness to angiogenic stimulation. It is thought that the age-related decline in the ability to form new blood vessels in the myocardium impairs both cardiac repair processes and adaptation to changes in myocardial oxygen supply and demand in the elderly (eg, in response to exercise or regional ischemia resulting from atherosclerotic narrowing of coronary arteries). However, the link between Nrf2 dysfunction and impaired angiogenic capacity of endothelial cells has not been elucidated.
The present study was designed to test the hypothesis that disruption of Nrf2 signaling impairs angiogenic capacity of coronary arterial endothelial cells (CAECs), mimicking the vascular aging phenotype. To test our hypotheses, we determined whether disruption of Nrf2 signaling (by small interfering RNA [siRNA] knockdown and overexpression of Keap1, the cytosolic repressor of Nrf2) impairs angiogenic processes in cultured human CAECs, including proliferation, adhesion, migration, and ability to form capillary-like structures.
MATERIALS AND METHODS
Cell Culture, Nrf2 Knockdown, and Keap1 Overexpression
Primary human CAECs (purchased from Cell Applications, Inc., San Diego, CA) were cultured in MesoEndo Endothelial Cell Growth Medium (Cell Applications, Inc.) as described (4,5,22). To disrupt Nrf2 signaling, Nrf2 was downregulated by RNA interference using proprietary siRNA sequences (Origen) and the electroporation-based Amaxa Nucleofector technology (Amaxa, Gaithersburg, MD), as we have previously reported (4,5,23,24). Experiments were performed on day 2 after the transfection when gene silencing was optimal. Keap1 overexpression was achieved in CAECs by transfection with a Keap1 full-length complementary DNA (cDNA) encoding plasmid (Origen) as described (5,25). To induce angiogenic processes, CAECs were treated with recombinant human vascular endothelial growth factor (VEGF, 100 ng/mL; R&D systems, Minneapolis, MN) and insulin-like growth factor-1 (IGF-1, 500 ng/mL). All reagents used in this study were purchased from Sigma-Aldrich (St Louis, MO) unless otherwise indicated.
Cell Adhesion Assays
Angiogenesis is a multistep process involving cell adhesion, proliferation, migration, and morphogenesis (26). To determine the effects of Nrf2 signaling in regulation of the adhesion capacity of CAECs, cells were transfected with control plasmid, Nrf2 siRNA, or Keap1 cDNA. After 24 hours, they were collected, washed, counted, and labeled with the fluorescent CyQuant dye (Invitrogen, Carlsbad, CA; incubation time: 60 minutes at 37°C). Equal amounts of cells, stimulated with VEGF (100 ng/mL) or IGF-1 (500 ng/mL), were seeded in 96-well plates previously coated with 50 μL of vitronectin (1.6 μg/mL), collagen (50 μg/mL), fibronectin (50 μg/mL), laminin (50 μg/mL), extracellular matrix compound (50 μg/mL; Cell Applications, Inc.), or bovine serum albumin (BSA) (12 μg/mL), which was used as the negative control. After 3 hours incubation at 37°C, unattached cells were removed by rinsing the wells three times with warm phosphate-buffered saline. The ratio of adhering cells was quantified by assessing the background-corrected fluorescence (excitation/emission: 508/527 nm, respectively) using an Infinite M200 plate reader (Tecan, Research Triangle Park, NC).
As an additional measurement, we used electric cell-substrate impedance sensing (ECIS) technology (Applied Biophysics, Troy, NY) to monitor adhesion of CAECs to collagen. Briefly, VEGF (100 ng/mL)-stimulated cells were seeded in collagen-coated 8-well array culture dishes containing gold film surface electrodes (ECIS 8W1E; in each well, one active electrode [d: 250 μm] and a large counter electrode). The same numbers of cells were added to each well in complete cell culture medium (2.5 × 105 cells per well). The arrays were placed in an incubator, and the time course for changes of capacitance (at 60 kHz) due to the adhesion of cells to the active electrode was obtained. Time to reach 50% cell adhesion (t 50 Adhesion) was used as an index of adhesiveness (100% change corresponds to the maximum level of cell coverage reached on the active electrode).
Cell Proliferation Assay
Cell proliferation capacity was assessed in CAEC transfected with either Nrf2 siRNA or scrambled control plasmid using the flow cytometry–based Guava CellGrowth assay (Guava Technologies, Inc., Hayward, CA). Briefly, cells were collected, resuspended in phosphate-buffered saline containing 0.1% BSA, and stained with 16 μmol/L carboxyfluorescein diacetate succinimidyl ester for 15 minutes at 37°C. This dye diffuses into cells and is cleaved by intracellular esterases to form an amine-reactive product that produces a detectable fluorescence and binds covalently to intracellular lysine residues and other amine sources. Upon cell division, carboxyfluorescein diacetate succinimidyl ester divides equally into the daughter cells halving the carboxyfluorescein diacetate succinimidyl ester concentration of the mother cell; therefore, there is an inverse correlation between the fluorescence intensity and the proliferation capacity of the cells. After incubation, unbound dye was quenched with serum-containing medium. Then, cells were washed three times and incubated for 24 hours with VEGF or IGF-1. Then, cells were collected, washed, stained with propidium iodide (to gate out dead cells), and analyzed with a flow cytometer (Guava EasyCyte 8HT; Millipore, Billerica, MA).
Assessment of Cell Migration by ECIS-Based Wound-Healing Assay
The ECIS technology was used to monitor migration of CAECs transfected with siRNA targeting Nrf2 (siNrf2) or a scrambled control vector in a wound-healing assay. Briefly, CAECs (2.5 × 105 cells per well) were seeded in 8-well array culture dishes (ECIS 8W1E), placed in an incubator (37°C), and changes in resistance and impedance were continuously monitored. When impedance reached a plateau, the medium was changed to a serum-free medium. After 1 hour stabilization, cells in each well were subjected to an elevated field pulse (wounding) of 5 mA applied for 20 seconds at 60 kHz, which killed the cells present on the small active electrode due to severe electroporation. The detachment of the dead cells was immediately evident as a sudden drop in resistance (monitored at 4000 Hz). VEGF (100 ng/mL) was immediately added to each well. CAECs surrounding the active electrode that had not been subjected to the wounding then migrated inward to replace the detached dead cells resulting in resistance recovery (continuously monitored at 4000 Hz for up to 24 hours). Time to reach 50% resistance recovery (corresponding to 50% confluence on the active electrode) was determined for control and siNrf2-treated cells, and this parameter and the known physical dimensions of the electrode were used to calculate the migration rate (expressed as microns per hour).
Tube Formation Assay
To investigate the influence of Nrf2 knockdown on tube formation ability, 24 hours after transfection with control plasmid, Nrf2 siRNA, or Keap1 cDNA, CAECs were plated on Geltrex Reduced Growth Factor Basement Membrane Matrix (Invitrogen) in Medium 200PRF (Invitrogen). Briefly, 150 μL per well of Geltrex was distributed in ice-cold 24-well plates. The gel was allowed to solidify while incubating the plates for 30 minutes at 37°C. CAECs were then seeded at a density of 5 × 104 cells per well and placed in the incubator for 24 hours. Microscopic images were captured using a Nikon Eclipse Ti microscope equipped with a ×10 phase contrast objective (Nikon Instruments, Inc., Melville, NY). The extent of tube formation was quantified by measuring total tube length in five random fields per well using NIS-Elements microscope imaging software (Nikon Instruments, Inc.). The mean of the total tube length per total area imaged (micron tube per square millimeter) was calculated for each well. Experiments were run in quadruplicates. The experimenter was blinded to the groups throughout the period of analysis.
Caspase-3/7 Activity Assay
To determine changes in cellular viability and sensitivity to oxidative stressors, caspase-3/7 activity (a useful measure of apoptotic cell death) was assessed in CAECs with or without Nrf2 knockdown using the Caspase-Glo 3/7 assay kit (Promega, Madison, WI) as previously reported (27–30). CAECs were transfected with control scrambled plasmid, Nrf2 siRNA, or Keap1 cDNA, seeded in white-walled 96-well plates, and treated (for 18 hours) with the pro-apoptotic stimuli hydrogen peroxide (H2O2, 3 and 10 μmol/L), oxidized low-density lipoprotein (5 μg/mL), or high glucose (30 mmol/L) as previously described (31,32). Then, Caspase-Glo 3/7 reagent was added to each well and mixed for 30 seconds, and peak luminescence signal intensities were determined using an Infinite M200 plate reader (Tecan).
Data Analysis
Statistical analyses were performed using one-way analysis of variance. p < .05 was considered statistically significant. Data are expressed as means ± SEMs.
RESULTS
Disruption of Nrf2 Signaling Inhibits Adhesion of CAECs to Extracellular Matrix Proteins
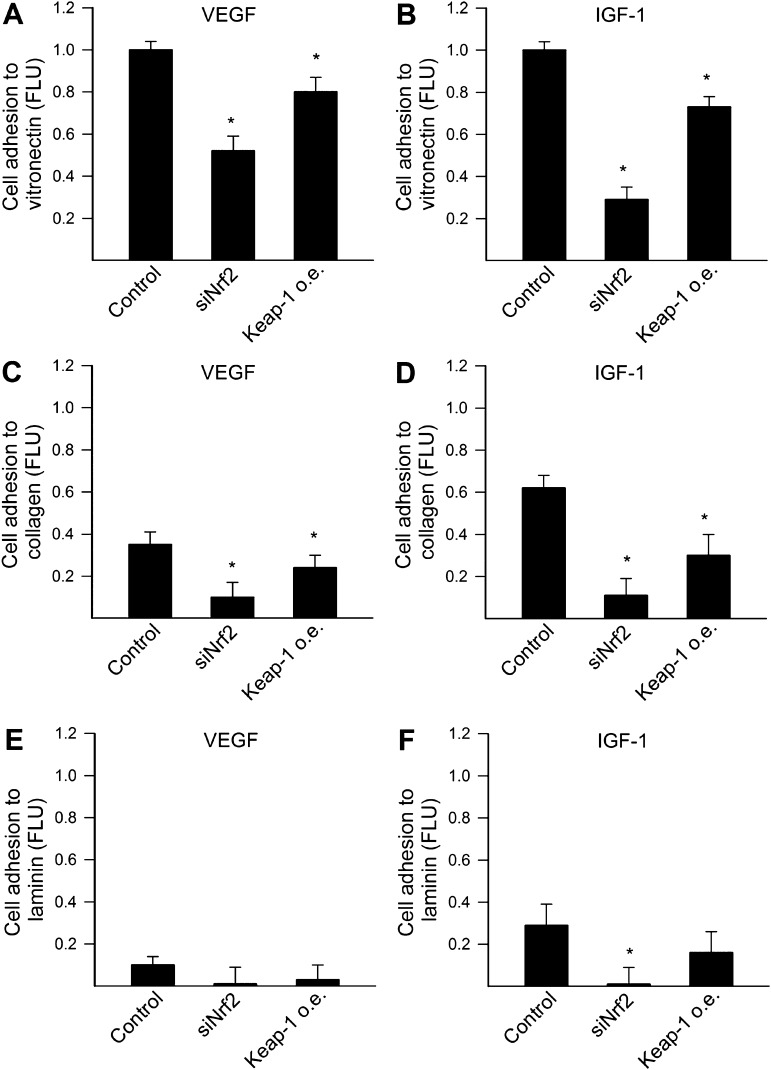
To determine the role of Nrf2 on angiogenic capacity, we knocked down Nrf2 using siRNA or overexpressed Keap1, the cytosolic repressor of Nrf2 in CAECs. Cell adhesion experiments were performed to investigate whether disruption of Nrf2 signaling affects VEGF- and IGF-1–induced adhesion of CAECs to different components of the extracellular matrix. We found that disruption of Nrf2 signaling by both Nrf2 siRNA treatment and overexpression of Keap1 impaired the ability of CAECs to adhere to vitronectin (Figure 1A and B) and collagen (Figure 1C and D). The ability of cells to adhere to laminin was significantly reduced (Figure 1E and F), but only the effect of Nrf2 siRNA treatment to inhibit adhesion of IGF-1–treated CAECs to laminin reached statistical significance (Figure 1F). The magnitude of the effects of knockdown of Nrf2 and overexpression of Keap1 appears to differ in some of the assays; however, the cause for this difference remains unknown.
Figure 1.
Disruption of Nrf2 signaling by siRNA knockdown of Nrf2 (siRNA targeting Nrf2) or by overexpression (o.e.) of Keap1 significantly impairs adhesion capacity of human coronary arterial endothelial cells (CAECs). CAECs, loaded with the fluorescent dye CyQuant and stimulated with vascular endothelial growth factor (VEGF; 100 ng/mL; A, C, and E, respectively) or insulin-like growth factor-1 (500 ng/mL; B, D, and F, respectively), were seeded in vitronectin- (A and B), collagen- (C and D), or laminin (E and F)-coated plates (see Materials and Methods section). After 3 hours incubation, nonadherent cells were washed away and the ratio of adhering cells was quantified by assessing the background-corrected fluorescence at 508/527 nm. Data are expressed as normalized FLU (fluorescence light units; means ± SEMs; n = 4–6 in each group), *p < .05 vs control.
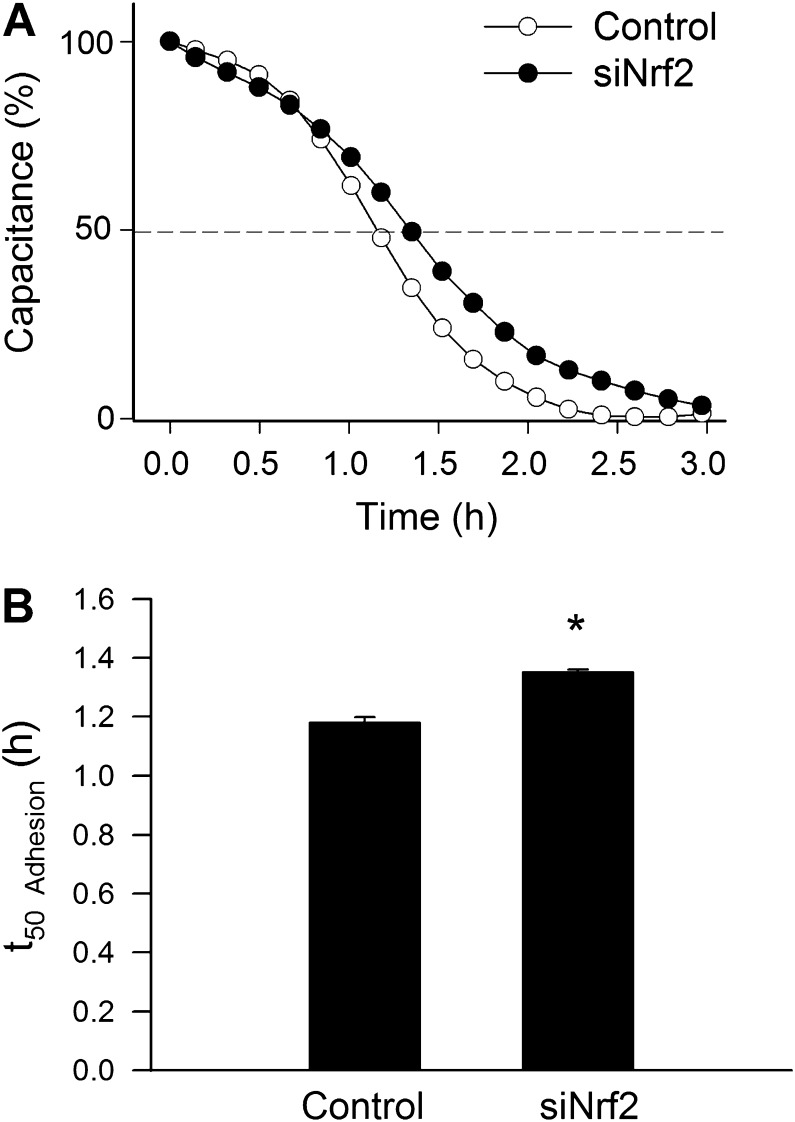
In other experiments, we used ECIS technology to monitor changes of capacitance (at 60 kHz) due to the adhesion of VEGF (100 ng/mL)-stimulated cells to the collagen-coated active electrode (Figure 2A). Time to reach 50% cell adhesion (t 50 Adhesion) was used as an index of adhesiveness. We found that siRNA knockdown of Nrf2 resulted in a significant increase in t 50 Adhesion (Figure 2B), indicating that downregulation of Nrf2 impairs the ability of VEGF-treated CAECs to adhere to collagen.
Figure 2.
Disruption of Nrf2 signaling by siRNA knockdown of Nrf2 (siRNA targeting Nrf2 [siNrf2]) significantly inhibits vascular endothelial growth factor (VEGF)–induced adhesion of human coronary arterial endothelial cells (CAECs). VEGF (100 ng/mL)-stimulated cell adhesion was monitored by electric cell-substrate impedance sensing technology (see Materials and Methods section). (A) Time course of changes of capacitance (at 60 kHz) after addition of CAECs to collagen-coated wells. One hundred percent change corresponds to the maximum level of cell coverage reached on the active electrode. Data are mean ± SEMs (n = 6 in each group). Time to reach 50% cell adhesion (t 50 Adhesion) was used as an index of adhesiveness. (B) Depicts the summary data for t 50 Adhesion in control and siNrf2-treated CAECs. Results indicate that siRNA knockdown of Nrf2 significantly increases t 50 Adhesion indicating an impaired adhesiveness. Data are means ± SEMs (n = 6 in each group), *p < .05 vs control. The longer t 50 Adhesion in siNrf2-treated cells indicate that Nrf2 dysfunction impairs adhesiveness of CAECs.
Disruption of Nrf2 Signaling Impairs Proliferative Capacity of CAECs
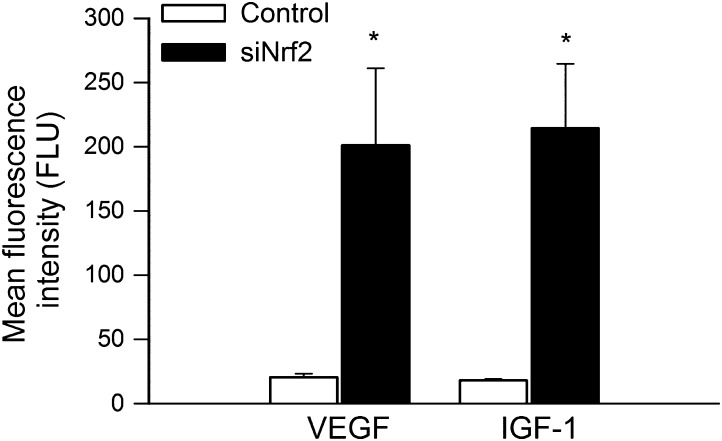
Proliferation represents a key step in angiogenesis. Proliferative capacity of CAECs transfected with siNrf2 or a scrambled control vector was compared after incubation with VEGF or IGF-1 for 24 hours. We found that Nrf2 knockdown significantly increased carboxyfluorescein diacetate succinimidyl ester fluorescence in CAECs, indicating that proliferation capacity is significantly impaired by Nrf2 dysfunction (Figure 3).
Figure 3.
Disruption of Nrf2 signaling by siRNA knockdown of Nrf2 (siRNA targeting Nrf2) significantly impairs proliferation capacity of human coronary arterial endothelial cells (CAECs). Cell proliferation capacity was assessed in CAEC stimulated with vascular endothelial growth factor (VEGF; 100 ng/mL) or insulin-like growth factor-1 (500 ng/mL) using the flow cytometry–based Guava CellGrowth assay (see Materials and Methods section). There is an inverse correlation between the mean fluorescence intensity of the indicator dye carboxyfluorescein diacetate succinimidyl ester and the proliferation capacity of the cells. Higher values represent slower proliferative capacity. Data are means ± SEMs (n = 6 in each group), *p < .05 vs control.
Disruption of Nrf2 Signaling Impairs the Migratory Capability of CAECs
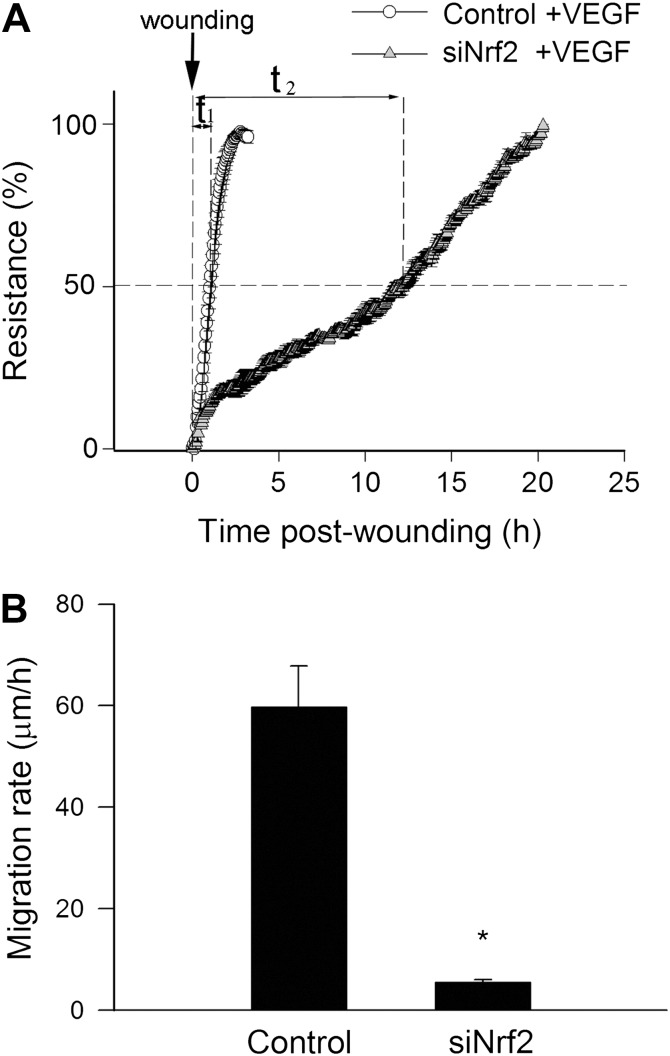
The migratory capability of vascular endothelial cells has a pivotal role in the maintenance of microvascular integrity and angiogenesis. An ECIS-based wound-healing assay was used to assess the effect of disruption of Nrf2 signaling on migratory capability of VEGF-treated CAECs. We found that siRNA knockdown of Nrf2 significantly increased the time for the cells to reach 50% of the maximum confluence (Figure 4A). Figure 4B indicates that the decline in the calculated migration rate in CAECs with Nrf2 knockdown was highly significant (Figure 4B).
Figure 4.
Disruption of Nrf2 signaling by siRNA knockdown of Nrf2 (siRNA targeting Nrf2 [siNrf2]) significantly impairs migration capacity of human coronary arterial endothelial cells (CAECs). vascular endothelial growth factor (VEGF; 100 ng/mL)-stimulated cell migration was monitored by electric cell-substrate impedance sensing technology in a wound-healing assay (see Materials and Methods section). (A) Time course of resistance recovery after wounding (electric pulse of 5 mA for 20 seconds at 60 kHz; 100% represents prewounding levels). Resistance (at 4000 Hz) was monitored every 160 seconds. Data are mean ± SEM (n = 6 in each group). Time to reach 50% resistance recovery (corresponding to 50% confluence on the active electrode) was determined for control and siNrf2-treated cells (t 1 and t 2, respectively), and this parameter and the known physical dimensions of the electrode were used to calculate the migration rate (expressed as microns per hour). (B) Depicts the summary data for migration rate in control and siNrf2-treated CAECs. Data are means ± SEMs (n = 6 in each group), *p < .05 vs control.
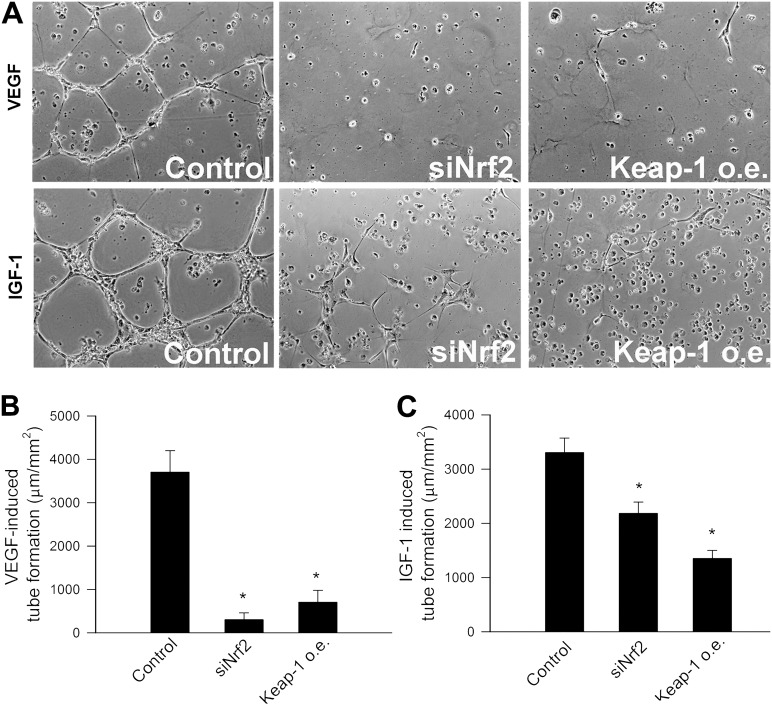
Disruption of Nrf2 Signaling Impairs Formation of Capillary-Like Structures by CAECs
When seeded onto Matrigel matrices, control cells formed elaborated capillary networks in the presence of both VEGF and IGF-1 (Figure 5A). We found that siRNA knockdown of Nrf2 or overexpression of Keap1 significantly inhibited the formation of capillary-like structures by CAECs (Figure 5A–C).
Figure 5.
A) Disruption of Nrf2 signaling in human coronary arterial endothelial cells (CAECs) by siRNA knockdown of Nrf2 (siRNA targeting Nrf2) or by overexpression (o.e.) of Keap1 significantly inhibits the formation of capillary-like structures. CAECs were plated on Matrigel-coated wells, and tube formation was induced by treating CAECs with vascular endothelial growth factor (VEGF; 100 ng/mL, for 24 hours) or insulin-like growth factor-1 (IGF-1; 500 ng/mL, for 24 hours). Representative examples of capillary-like structures are shown on (A). Summary data, expressed as total tube length per total area scanned (micron tube per square millimeter), are shown in (B; VEGF) and (C; IGF-1). Data are means ± SEMs, n = 4, *p < .05 vs control.
Disruption of Nrf2 Signaling Renders CAECs Susceptible to Oxidative Stress–Induced Apoptosis
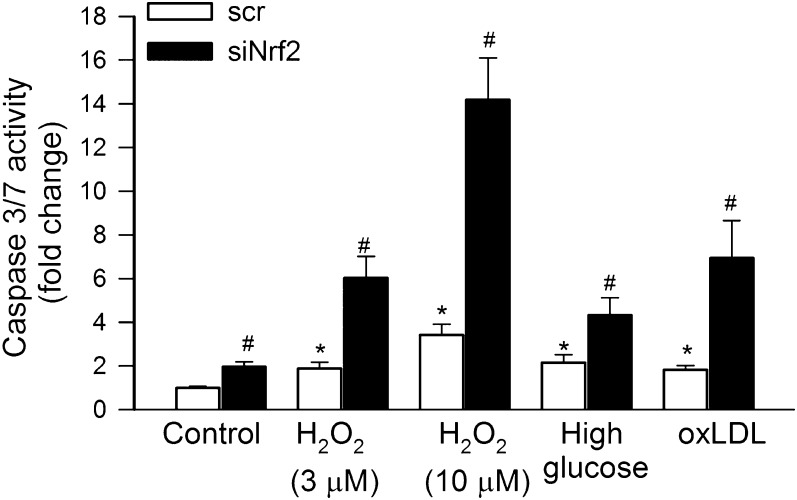
An increased susceptibility to apoptotic cell death is thought to contribute to impaired angiogenesis and microvascular rarefaction in various pathophysiological conditions (21) and is considered an important mechanism of aging (29,30,33–39). To test whether siRNA knockdown of Nrf2 negatively affects cell survival, we treated CAECs with various pro-apoptotic stimuli. We found that knockdown of Nrf2 rendered CAECs significantly more susceptible to the pro-apoptotic effects of H2O2, high glucose, or oxidized low-density lipoprotein as compared with the control group (Figure 6).
Figure 6.
In primary human coronary arterial endothelial cells, H2O2 (3 and 10 μmol/L), high glucose (30 mmol/L), and oxidized low-density lipoprotein (oxLDL; 5 μg/mL) significantly increased apoptotic cell death as shown by the increased caspase-3/7 activity (see Materials and Methods section; *p < .05 vs untreated control). Control cells were transfected with a scrambled nonsense DNA (scr). siRNA knockdown of Nrf2 (siRNA targeting Nrf2) significantly augmented endothelial apoptosis induced by H2O2, high glucose, and oxLDL (#p < .05 vs respective treated control). Data are mean ± SEM (n = 6 in each group).
DISCUSSION
The principal new finding of this study is that a functional Nrf2 pathway is essential for a healthy endothelial angiogenic response because all the major steps of the angiogenic process, including adhesion, proliferation, migration, and formation of capillary-like structures, are compromised by disruption of Nrf2 signaling in endothelial cells.
Increased production of reactive oxygen species (ROS) is known to activate Nrf2 via facilitating its dissociation from the inhibitory protein Keap1. The findings showing that Nrf2 regulates angiogenesis are consistent with a central role of redox signaling pathways in multiple angiogenic processes in endothelial cells and the role of ROS in signal transduction by angiogenic growth factors (eg, VEGF, IGF-1, and angiopoetin-1; 40–44). Accordingly, VEGF stimulation was shown to increase ROS production via activation of Rac-dependent NAD(P)H oxidase in endothelial cells (43–48), which mediates Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) autophosphorylation (47). Recent studies demonstrate that NAD(P)H oxidase–dependent ROS production is involved in IGF-1 signaling as well (42). It is logical to assume that local increases in ROS levels induced by activation of the VEGF and IGF-1 receptors activate Nrf2, which modulates the pro-angiogenic effects of VEGF and IGF-1. In addition, VEGF and IGF-1 may also directly activate Nrf2 in endothelial cells via phosphorylation mediated by the phosphatidylinositol 3-kinase–Akt pathway (8,49). It is possible that Nrf2-driven induction of antioxidant enzymes has an important role in localizing ROS production and integration of specific redox signaling events, which are involved in angiogenesis. Indeed, recent studies demonstrate that Nrf2-driven pathways have an important role in angiogenic growth factor signaling by controlling the activity of the serine/threonine kinase domain of growth factor receptors (50) and, or, modulating the effects of HIF-1α (51). This hypothesis is supported by the observations that genetic depletion of Nrf2 significantly impairs the development of the secondary capillary network in the retina, which is known to form by angiogenesis in most species (52). Moreover, a recent study reports that Nrf2 blockade suppresses colon tumor angiogenesis (53). Furthermore, the Nrf2 targets, heme oxygenase-1 (54,55), and thioredoxin (56) were shown to confer pro-angiogenic effects in animal disease models.
In addition to regulating angiogenic processes, Nrf2 also confers antiapoptotic effects, which are likely to have important roles in preserving the structural integrity of newly formed capillaries. Importantly, the present findings and results of previous studies demonstrate that Nrf2 dysfunction significantly impairs oxidative stress resistance in endothelial cells exacerbating apoptotic cell death under conditions associated with increased production of ROS (9,10). These findings also extend the results obtained in Nrf2−/− murine embryonic fibroblasts showing decreased growth and downregulation of SIRT1 associated with a significantly shorter cell survival (57). There is good reason to believe that mitochondrial protective effects of Nrf2 activation (which likely prevent induction of the mitochondrial pathway of programmed cell death) significantly contribute to its antiapoptotic effects (58). In addition, there is also evidence that Nrf2 may modulate cellular sensitivity to apoptosis mediated via death receptor activation (59).
The pathophysiological consequences of impaired angiogenesis and increased endothelial vulnerability to apoptosis associated with Nrf2 dysfunction are likely multiple. We posit that Nrf2 depletion may decrease capillary density in the heart and negatively affect cardiac angiogenesis and, or, collateral formation induced by physiological (eg, exercise) or pathological (eg, pressure overload) stimuli. Recent studies demonstrate that aging results in severe impairment of Nrf2 expression and activity in the vasculature (9,10), which is associated with an increased rate of endothelial apoptosis (10,27,60) and microvascular rarefaction (61–63). Laboratory studies also show that the ability of angiogenic cytokines to induce angiogenesis is significantly blunted in aged endothelial cells (64). Age-related Nrf2 dysfunction is associated with significant increases in the production of ROS in endothelial cells (9,10). Although ROS signaling is necessary for physiological angiogenesis (43,44,46), there is evidence that increased oxidative stress has detrimental effects on endothelial angiogenic processes (65).
We posit that microvascular rarefaction and impairment of compensatory proliferation of the coronary resistance vessels and the capillary network (61) associated with age-related Nrf2 dysfunction may play a prominent role in the occurrence of cardiac dysfunction and failure with age. Importantly, although pro-angiogenic strategies work well in healthy, young laboratory animals, a number of large randomized placebo-controlled phase II/III clinical trials aimed at improving collateral circulation with angiogenic growth factors in patients with ischemic heart disease yielded disappointing results (66–71). It is thought that the negative results of the aforementioned clinical studies are due to the unresponsiveness of the vasculature to angiogenic stimulation in elderly patients (66). In order to design more effective therapeutic approaches, it will be essential to determine whether pharmacological activation of Nrf2 renders aged endothelial cells responsive to angiogenic stimulation and, or, protects newly formed blood vessels from apoptosis. In that regard, it is significant that in endothelial cells, Nrf2 can be activated pharmacologically by the polyphenol resveratrol (4). Although resveratrol in supraphysiological concentrations (30 μmol/L) was shown to inhibit angiogenic processes in cultured endothelial cells (likely by blocking cell proliferation and inhibiting endothelial αvβ3 integrin function, 72, and, or, by causing dysregulation of microRNA signaling; Ungvari, MD, PhD and Csiszar, MD, PhD, unpublished data, 2011), treatment with physiological doses of resveratrol in vivo was shown to promote angiogenesis in rodent models of myocardial infarction (73). Resveratrol treatment was also shown to increase the number of capillaries in the brain of aged mice (74) and protect endothelial cells from apoptosis (25,75,76), in addition to its documented diverse antiaging actions (75,77–83). Further studies are warranted to determine whether resveratrol and other activators of Nrf2 confer similar pro-angiogenic and endothelial protective effects in nonhuman primates and elderly patients as well.
FUNDING
This work was supported by grants from the American Diabetes Association (to Z.U.), American Federation for Aging Research (to A.C.), the Oklahoma Center for the Advancement of Science and Technology (to A.C. and Z.U.), the University of Oklahoma College of Medicine Alumni Association (to A.C.), the American Heart Association (A.C.), the N ational I nstitutes of H ealth (AG031085 to A.C., AT006526 to Z.U., AG038747, NS056218, and P01 AG11370 to W.E.S.), and the Intramural Research Program of National Institutes of Health (to R.D.C.).
Acknowledgments
The authors would like to express their gratitude for the support of the Donald W. Reynolds Foundation, which funds aging research at the University of Oklahoma Health Sciences Center under its Aging and Quality of Life Program.
References
- 1.Afonyushkin T, Oskolkova OV, Philippova M, et al. Oxidized phospholipids regulate expression of ATF4 and VEGF in endothelial cells via NRF2-dependent mechanism: novel point of convergence between electrophilic and unfolded protein stress pathways. Arterioscler Thromb Vasc Biol. 2010;30:1007–1013. doi: 10.1161/ATVBAHA.110.204354. [DOI] [PubMed] [Google Scholar]
- 2.Hosoya T, Maruyama A, Kang MI, et al. Differential responses of the Nrf2-Keap1 system to laminar and oscillatory shear stresses in endothelial cells. J Biol Chem. 2005;280:27244–27250. doi: 10.1074/jbc.M502551200. [DOI] [PubMed] [Google Scholar]
- 3.Jyrkkanen HK, Kansanen E, Inkala M, et al. Nrf2 regulates antioxidant gene expression evoked by oxidized phospholipids in endothelial cells and murine arteries in vivo. Circ Res. 2008;103:e1–e9. doi: 10.1161/CIRCRESAHA.108.176883. [DOI] [PubMed] [Google Scholar]
- 4.Ungvari Z, Bagi Z, Feher A, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–H24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ungvari ZI, Bailey-Downs L, Gautam T, et al. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol. 2011;300:H1133–H1140. doi: 10.1152/ajpheart.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zakkar M, Van der Heiden K, Luong LA, et al. Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler Thromb Vasc Biol. 2009;29(11):1851–1857. doi: 10.1161/ATVBAHA.109.193375. [DOI] [PubMed] [Google Scholar]
- 7.Dai G, Vaughn S, Zhang Y, Wang ET, Garcia-Cardena G, Gimbrone MA., Jr. Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circ Res. 2007;101:723–733. doi: 10.1161/CIRCRESAHA.107.152942. [DOI] [PubMed] [Google Scholar]
- 8.Bailey-Downs LC, Mitschelen M, Sosnowska D, et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci. 2011 doi: 10.1093/gerona/glr164. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ungvari Z, Bailey-Downs L, Gautam T, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction and NF-kB activation in the non-human primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–875. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ungvari Z, Bailey-Downs L, Sosnowska D, et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of Nrf2-mediated antioxidant response. Am J Physiol. 2011;301:H363–H372. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins AR, Lyon CJ, Xia X, et al. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104:e42–e54. doi: 10.1161/CIRCRESAHA.108.188771. [DOI] [PubMed] [Google Scholar]
- 12.Gao P, Shen F, Gabriel RA, et al. Attenuation of brain response to vascular endothelial growth factor-mediated angiogenesis and neurogenesis in aged mice. Stroke. 2009;40:3596–3600. doi: 10.1161/STROKEAHA.109.561050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitlinska J, Lee EW, Movafagh S, Pons J, Zukowska Z. Neuropeptide Y-induced angiogenesis in aging. Peptides. 2002;23:71–77. doi: 10.1016/s0196-9781(01)00581-2. [DOI] [PubMed] [Google Scholar]
- 14.Bach MH, Sadoun E, Reed MJ. Defects in activation of nitric oxide synthases occur during delayed angiogenesis in aging. Mech Ageing Dev. 2005;126:467–473. doi: 10.1016/j.mad.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Facchetti F, Monzani E, Cavallini G, Bergamini E, La Porta CA. Effect of a caloric restriction regimen on the angiogenic capacity of aorta and on the expression of endothelin-1 during ageing. Exp Gerontol. 2007;42:662–667. doi: 10.1016/j.exger.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Iemitsu M, Maeda S, Jesmin S, Otsuki T, Miyauchi T. Exercise training improves aging-induced downregulation of VEGF angiogenic signaling cascade in hearts. Am J Physiol Heart Circ Physiol. 2006;291:H1290–H1298. doi: 10.1152/ajpheart.00820.2005. [DOI] [PubMed] [Google Scholar]
- 17.Jozsi AC, Dupont-Versteegden EE, Taylor-Jones JM, et al. Aged human muscle demonstrates an altered gene expression profile consistent with an impaired response to exercise. Mech Ageing Dev. 2000;120:45–56. doi: 10.1016/s0047-6374(00)00178-0. [DOI] [PubMed] [Google Scholar]
- 18.Rivard A, Berthou-Soulie L, Principe N, et al. Age-dependent defect in vascular endothelial growth factor expression is associated with reduced hypoxia-inducible factor 1 activity. J Biol Chem. 2000;275:29643–29647. doi: 10.1074/jbc.M001029200. [DOI] [PubMed] [Google Scholar]
- 19.Rivard A, Fabre JE, Silver M, et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 20.Sadoun E, Reed MJ. Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J Histochem Cytochem. 2003;51:1119–1130. doi: 10.1177/002215540305100902. [DOI] [PubMed] [Google Scholar]
- 21.Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65:1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ungvari Z, Labinskyy N, Mukhopadhyay P, et al. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–H1881. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Csiszar A, Ahmad M, Smith KE, et al. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am J Pathol. 2006;168:629–638. doi: 10.2353/ajpath.2006.050284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Csiszar A, Labinskyy N, Jimenez R, et al. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Csiszar A, Labinskyy N, Podlutsky A, et al. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294:H2721–H2735. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clapp C, Thebault S, Jeziorski MC, Martinez De La Escalera G. Peptide hormone regulation of angiogenesis. Physiol Rev. 2009;89:1177–1215. doi: 10.1152/physrev.00024.2009. [DOI] [PubMed] [Google Scholar]
- 27.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-TNFalfa treatment in aging. Am J Pathol. 2007;170:388–698. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pepe M, Mamdani M, Zentilin L, et al. Intramyocardial VEGF-B167 gene delivery delays the progression towards congestive failure in dogs with pacing-induced dilated cardiomyopathy. Circ Res. 2010;106:1893–1903. doi: 10.1161/CIRCRESAHA.110.220855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ungvari Z, Ridgway I, Philipp EE, et al. Extreme longevity is associated with increased resistance to oxidative stress in Arctica islandica, the longest-living non-colonial animal. J Gerontol A Biol Sci Med Sci. 2011;66(7):741–750. doi: 10.1093/gerona/glr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ungvari Z, Sosnowska D, Podlutsky A, Koncz P, Sonntag WE, Csiszar A. Free radical production, antioxidant capacity, and oxidative stress response signatures in fibroblasts from Lewis dwarf rats: effects of life span-extending peripubertal GH treatment. J Gerontol A Biol Sci Med Sci. 2011;66(5):501–510. doi: 10.1093/gerona/glr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Csiszar A, Labinskyy N, Zhao X, et al. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell. 2007;6:783–797. doi: 10.1111/j.1474-9726.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- 32.Labinskyy N, Mukhopadhyay P, Toth J, et al. Longevity is associated with increased vascular resistance to high glucose-induced oxidative stress and inflammatory gene expression in Peromyscus leucopus. Am J Physiol Heart Circ Physiol. 2009;296:H946–H956. doi: 10.1152/ajpheart.00693.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behrens MI, Silva M, Schmied A, et al. Age-dependent increases in apoptosis/necrosis ratios in human lymphocytes exposed to oxidative stress. J Gerontol A Biol Sci Med Sci. 2011;66:732–740. doi: 10.1093/gerona/glr039. [DOI] [PubMed] [Google Scholar]
- 34.Campisi J, Vijg J. Does damage to DNA and other macromolecules play a role in aging? If so, how? J Gerontol A Biol Sci Med Sci. 2009;64:175–178. doi: 10.1093/gerona/gln065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dekker P, de Lange MJ, Dirks RW, et al. Relation between maximum replicative capacity and oxidative stress-induced responses in human skin fibroblasts in vitro. J Gerontol A Biol Sci Med Sci. 2011;66:45–50. doi: 10.1093/gerona/glq159. [DOI] [PubMed] [Google Scholar]
- 36.Forman K, Vara E, Garcia C, et al. Effect of a combined treatment with growth hormone and melatonin in the cardiological aging on male SAMP8 mice. J Gerontol A Biol Sci Med Sci. 2011;66:823–834. doi: 10.1093/gerona/glr083. [DOI] [PubMed] [Google Scholar]
- 37.Kakarla SK, Rice KM, Katta A, et al. Possible molecular mechanisms underlying age-related cardiomyocyte apoptosis in the F344XBN rat heart. J Gerontol A Biol Sci Med Sci. 2010;65:147–155. doi: 10.1093/gerona/glp203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller RA. Cell stress and aging: new emphasis on multiplex resistance mechanisms. J Gerontol A Biol Sci Med Sci. 2009;64:179–182. doi: 10.1093/gerona/gln072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Csiszar A, Podlutsky A, Podlutskaya N, et al. Testing the oxidative stress hypothesis of aging in primate fibroblasts: is there a correlation between species longevity and cellular ROS production? J Gerontol A Biol Sci Med Sci. 2011 doi: 10.1093/gerona/glr216. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.West XZ, Malinin NL, Merkulova AA, et al. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature. 2010;467:972–976. doi: 10.1038/nature09421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ushio-Fukai M, Nakamura Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008;266:37–52. doi: 10.1016/j.canlet.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Handayaningsih AE, Iguchi G, Fukuoka H, et al. Reactive oxygen species play an essential role in IGF-I signaling and IGF-I-induced myocyte hypertrophy in C2C12 myocytes. Endocrinology. 2011;152:912–921. doi: 10.1210/en.2010-0981. [DOI] [PubMed] [Google Scholar]
- 43.Abid MR, Kachra Z, Spokes KC, Aird WC. NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett. 2000;486:252–256. doi: 10.1016/s0014-5793(00)02305-x. [DOI] [PubMed] [Google Scholar]
- 44.Abid MR, Spokes KC, Shih SC, Aird WC. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J Biol Chem. 2007;282:35373–35385. doi: 10.1074/jbc.M702175200. [DOI] [PubMed] [Google Scholar]
- 45.Yamaoka-Tojo M, Ushio-Fukai M, Hilenski L, et al. IQGAP1, a novel vascular endothelial growth factor receptor binding protein, is involved in reactive oxygen species—dependent endothelial migration and proliferation. Circ Res. 2004;95:276–283. doi: 10.1161/01.RES.0000136522.58649.60. [DOI] [PubMed] [Google Scholar]
- 46.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 47.Colavitti R, Pani G, Bedogni B, et al. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J Biol Chem. 2002;277:3101–3108. doi: 10.1074/jbc.M107711200. [DOI] [PubMed] [Google Scholar]
- 48.Lin MT, Yen ML, Lin CY, Kuo ML. Inhibition of vascular endothelial growth factor-induced angiogenesis by resveratrol through interruption of Src-dependent vascular endothelial cadherin tyrosine phosphorylation. Mol Pharmacol. 2003;64:1029–1036. doi: 10.1124/mol.64.5.1029. [DOI] [PubMed] [Google Scholar]
- 49.Papaiahgari S, Zhang Q, Kleeberger SR, Cho HY, Reddy SP. Hyperoxia stimulates an Nrf2-ARE transcriptional response via ROS-EGFR-PI3K-Akt/ERK MAP kinase signaling in pulmonary epithelial cells. Antioxid Redox Signal. 2006;8:43–52. doi: 10.1089/ars.2006.8.43. [DOI] [PubMed] [Google Scholar]
- 50.Beyer TA, Xu W, Teupser D. Impaired liver regeneration in Nrf2 knockout mice: role of ROS-mediated insulin/IGF-1 resistance. EMBO J. 2008;27:212–223. doi: 10.1038/sj.emboj.7601950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loboda A, Stachurska A, Florczyk U, et al. HIF-1 induction attenuates Nrf2-dependent IL-8 expression in human endothelial cells. Antioxid Redox Signal. 2009;11:1501–1517. doi: 10.1089/ars.2008.2211. [DOI] [PubMed] [Google Scholar]
- 52.Uno K, Prow TW, Bhutto IA, et al. Role of Nrf2 in retinal vascular development and the vaso-obliterative phase of oxygen-induced retinopathy. Exp Eye Res. 2010;90:493–500. doi: 10.1016/j.exer.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim TH, Hur EG, Kang SJ, et al. NRF2 blockade suppresses colon tumor angiogenesis by inhibiting hypoxia-induced activation of HIF-1{alpha} Cancer Res. 2011;71(6):2260–2275. doi: 10.1158/0008-5472.CAN-10-3007. [DOI] [PubMed] [Google Scholar]
- 54.Deramaudt BM, Braunstein S, Remy P, Abraham NG. Gene transfer of human heme oxygenase into coronary endothelial cells potentially promotes angiogenesis. J Cell Biochem. 1998;68:121–127. doi: 10.1002/(sici)1097-4644(19980101)68:1<121::aid-jcb12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 55.Grochot-Przeczek A, Dulak J, Jozkowicz A. Heme oxygenase-1 in neovascularisation: a diabetic perspective. Thromb Haemost. 2010;104:424–431. doi: 10.1160/TH09-12-0825. [DOI] [PubMed] [Google Scholar]
- 56.Samuel SM, Thirunavukkarasu M, Penumathsa SV, et al. Thioredoxin-1 gene therapy enhances angiogenic signaling and reduces ventricular remodeling in infarcted myocardium of diabetic rats. Circulation. 2010;121:1244–1255. doi: 10.1161/CIRCULATIONAHA.109.872481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jodar L, Mercken EM, Ariza J, et al. Genetic deletion of nrf2 promotes immortalization and decreases life span of murine embryonic fibroblasts. J Gerontol A Biol Sci Med Sci. 2011;66:247–256. doi: 10.1093/gerona/glq181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okouchi M, Okayama N, Alexander JS, Aw TY. NRF2-dependent glutamate-L-cysteine ligase catalytic subunit expression mediates insulin protection against hyperglycemia- induced brain endothelial cell apoptosis. Curr Neurovasc Res. 2006;3:249–261. doi: 10.2174/156720206778792876. [DOI] [PubMed] [Google Scholar]
- 59.Morito N, Yoh K, Itoh K, et al. Nrf2 regulates the sensitivity of death receptor signals by affecting intracellular glutathione levels. Oncogene. 2003;22:9275–9281. doi: 10.1038/sj.onc.1207024. [DOI] [PubMed] [Google Scholar]
- 60.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- 61.Anversa P, Li P, Sonnenblick EH, Olivetti G. Effects of aging on quantitative structural properties of coronary vasculature and microvasculature in rats. Am J Physiol. 1994;267:H1062–H1073. doi: 10.1152/ajpheart.1994.267.3.H1062. [DOI] [PubMed] [Google Scholar]
- 62.Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Ageing Res Rev. 2003;2:149–168. doi: 10.1016/s1568-1637(02)00064-8. [DOI] [PubMed] [Google Scholar]
- 63.Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- 64.Phillips GD, Stone AM. PDGF-BB induced chemotaxis is impaired in aged capillary endothelial cells. Mech Ageing Dev. 1994;73:189–196. doi: 10.1016/0047-6374(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 65.Benndorf RA, Schwedhelm E, Gnann A, et al. Isoprostanes inhibit vascular endothelial growth factor-induced endothelial cell migration, tube formation, and cardiac vessel sprouting in vitro, as well as angiogenesis in vivo via activation of the thromboxane A(2) receptor: a potential link between oxidative stress and impaired angiogenesis. Circ Res. 2008;103:1037–1046. doi: 10.1161/CIRCRESAHA.108.184036. [DOI] [PubMed] [Google Scholar]
- 66.Khurana R, Simons M, Martin JF, Zachary IC. Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation. 2005;112:1813–1824. doi: 10.1161/CIRCULATIONAHA.105.535294. [DOI] [PubMed] [Google Scholar]
- 67.Pislaru SV, Simari RD. Gene transfer for ischemic cardiovascular disease: is this the end of the beginning or the beginning of the end? Nat Clin Pract Cardiovasc Med. 2005;2:138–144. doi: 10.1038/ncpcardio0136. [DOI] [PubMed] [Google Scholar]
- 68.Henry TD, Annex BH, McKendall GR, et al. The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 69.Simons M, Annex BH, Laham RJ, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation. 2002;105:788–793. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- 70.Grines CL, Watkins MW, Helmer G, et al. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002;105:1291–1297. doi: 10.1161/hc1102.105595. [DOI] [PubMed] [Google Scholar]
- 71.Kastrup J, Jorgensen E, Ruck A, et al. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris. A randomized double-blind placebo-controlled study: the Euroinject One trial. J Am Coll Cardiol. 2005;45:982–988. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 72.Belleri M, Ribatti D, Nicoli S, et al. Antiangiogenic and vascular-targeting activity of the microtubule-destabilizing trans-resveratrol derivative 3,5,4’-trimethoxystilbene. Mol Pharmacol. 2005;67AA:1451–1459. doi: 10.1124/mol.104.009043. [DOI] [PubMed] [Google Scholar]
- 73.Fukuda S, Kaga S, Zhan L, et al. Resveratrol ameliorates myocardial damage by inducing vascular endothelial growth factor-angiogenesis and tyrosine kinase receptor Flk-1. Cell Biochem Biophys. 2006;44:43–49. doi: 10.1385/CBB:44:1:043. [DOI] [PubMed] [Google Scholar]
- 74.Oomen CA, Farkas E, Roman V, van der Beek EM, Luiten PG, Meerlo P. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front Aging Neurosci. 2009;1:4. doi: 10.3389/neuro.24.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ungvari Z, Orosz Z, Rivera A, et al. Resveratrol increases vascular oxidative stress resistance. Am J Physiol. 2007;292:H2417–H2424. doi: 10.1152/ajpheart.01258.2006. [DOI] [PubMed] [Google Scholar]
- 77.Chandrashekara KT, Shakarad MN. Aloe vera or resveratrol supplementation in larval diet delays adult aging in the fruit fly, Drosophila melanogaster. J Gerontol A Biol Sci Med Sci. 2011;66:965–971. doi: 10.1093/gerona/glr103. [DOI] [PubMed] [Google Scholar]
- 78.Giovannelli L, Pitozzi V, Jacomelli M, et al. Protective effects of resveratrol against senescence-associated changes in cultured human fibroblasts. J Gerontol A Biol Sci Med Sci. 2011;66:9–18. doi: 10.1093/gerona/glq161. [DOI] [PubMed] [Google Scholar]
- 79.Jackson JR, Ryan MJ, Alway SE. Long-term supplementation with resveratrol alleviates oxidative stress but does not attenuate sarcopenia in aged mice. J Gerontol A Biol Sci Med Sci. 2011;66:751–764. doi: 10.1093/gerona/glr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Labbe A, Garand C, Cogger VC, et al. Resveratrol improves insulin resistance hyperglycemia and hepatosteatosis but not hypertriglyceridemia, inflammation, and life span in a mouse model for werner syndrome. J Gerontol A Biol Sci Med Sci. 2011;66:264–278. doi: 10.1093/gerona/glq184. [DOI] [PubMed] [Google Scholar]
- 81.Ryan MJ, Jackson JR, Hao Y, et al. Suppression of oxidative stress by resveratrol after isometric contractions in gastrocnemius muscles of aged mice. J Gerontol A Biol Sci Med Sci. 2010;65:815–831. doi: 10.1093/gerona/glq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smoliga JM, Vang O, Baur JA. Challenges of translating basic research into therapeutics: resveratrol as an example. J Gerontol A Biol Sci Med Sci. 2011 doi: 10.1093/gerona/glr062. doi:10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]