Abstract
Growth hormone receptor gene–disrupted (GHR−/−) mice are dwarf, insulin sensitive, and long lived despite being obese. In order to identify characteristics associated with their increased longevity, we studied age-related plasma proteomic changes in these mice. Male and female GHR−/− mice and their littermate controls were followed longitudinally at 8, 16, and 24 months of ages for plasma proteomic analysis. Relative to control littermates, GHR−/− mice had increased levels of apolipoprotein A-4 and retinol-binding protein-4 and decreased levels of apolipoprotein E, haptoglobin, and mannose-binding protein-C. Female GHR−/− mice showed decreased inflammatory cytokines including interleukin-1β and monocyte chemotactic protein-1. Additionally, sex differences were found in specific isoforms of apolipoprotein E, RBP-4, haptoglobin, albumin, and hemoglobin subunit beta. In conclusion, we find plasma proteomic changes in GHR−/− mice that favor a longer life span as well as sex differences indicative of an improved health span in female mice.
Keywords: Growth hormone receptor, Plasma, Proteomics, Sex, Aging
Growth hormone (GH) is a multifunctional endocrine hormone important in metabolism and growth. GH induces the expression of insulin-like growth factor-1 (IGF-1), which mediates some actions of GH. Numerous studies have shown that an attenuated GH/IGF-1 axis delays aging in various organisms (1,2). In this regard, GH receptor gene–disrupted (GHR−/−) mice are dwarf and have markedly reduced serum IGF-1 levels. They are obese, yet insulin sensitive, and long lived (3,4,5,6). In addition, they show increased health span (7) including less age-associated memory loss (8), decreased morbidity as evidenced by resistance to streptozotocin-induced kidney damage (9), and reduced incidence and delayed occurrence of fatal neoplastic diseases (10). The mechanism of increased longevity seen in these mice is partly overlapping with that of caloric restricted animals (11,12,13).
Hints concerning the effects of GH on not only life span but also health span, defined as “the period of life characterized by freedom from disability and disease and the ability to enjoy an independent life without functional limitations” (7), are emerging in humans. For example, humans possessing mutations in the GHR gene or GH-induced signaling genes have reduced levels of IGF-1, increased levels of GH, and are dwarf. These GH-insensitive individuals possess a condition termed Laron Syndrome (LS), named after Dr. Zvi Laron, who first made the observation (14). Recently, LS individuals have been shown to have a reduced incidence of diabetes and cancer (15,16).
GHR−/− mice, also known as Laron mice, have been extensively studied with various physiological and endocrine parameters assessed. A review of these results has been recently reported (17). However, proteomic data are limited. To date, there is only one study examining the retina proteome of newborn GHR−/− mice (18). Also, although most studies have been carried out using male mice, a few reports have included females and indicate that significant sex differences exist, for example, female mice have a distinctive pattern of fat accumulation as they age compared with males (5). Thus, although it has never been reported, there are likely differences between sexes in proteomic profiles.
Proteomics explores the entire set of proteins in a given tissue. A proteomic approach allows for the identification of novel proteins differentially regulated in GHR−/− mice versus wild-type (WT) controls and may provide insight into the long-lived phenotype of these mice. Specifically, we hope to identify plasma proteins that serve as potential markers of the lack of GH action and, hopefully, extend the results to markers of increased longevity.
In the current longitudinal study, plasma proteins were analyzed in male and female GHR−/− mice of young adult (8 months), middle age (16 months), and old age (24 months) relative to WT littermates. Proteins were separated using 2-dimensional gel electrophoresis (2-DE) and subsequently identified by mass spectrometry (MS) and tandem MS (MS/MS). In addition, several physiological parameters were measured. We have discovered significant differences in the levels of several proteins in GHR−/− versus WT mice, which may provide clues to their extended life span. In addition, sex differences in the plasma proteome indicate a healthier state in female mice at advanced age.
Materials and Methods
Animals
The GHR−/− mouse line was described previously (19). These mice are in the C57BL/6J background. Two groups (male and female) of GHR−/− mice with their littermate control WT mice (n = 5 for WT and n = 6 for GHR−/−) were followed longitudinally at 8, 16, and 24 months of ages. Initially, we included six WT mice but one WT mouse for both sexes died before 24 months; therefore, an n = 5 for the WT controls was used for the final analyses. The ages examined represent young adult (8 months), middle (16 months), and old (24 months) age in mice (20). GHR−/− mice typically live up to ∼36 months of age (3), well beyond the 24-month “old age” time point for WT mice. Thus, as most WT mice die before GHR−/− mice, we chose 24 months to represent the old age group, meaning we matched WT and GHR−/− mice based on chronological age. Due to relatively small blood volumes that can be collected from the dwarf GHR−/− mice, plasma samples from an additional cohort of mice at 24 months were used for cytokine measurements (n = 10 for males and n = 5 for females). Because tissue weight data required the mice be sacrificed, separate cohorts of mice at 6 and 24 months of ages were used for collection of liver and adipose tissues (n = 7–10). Liver samples from these 24-month-old mice were used for messenger RNA (mRNA) expression analysis. All mice were housed at room temperature (22°C) in a 14-hour light, 10-hour dark cycle. Mice were fed a chow diet ad libitum (LabDiet 5P00 Prolab RMH3000). Animal protocols were approved by Ohio University’s Institutional Animal Care and Use Committee.
Blood and Plasma Collection
Blood was drawn via tail tip into heparinized capillary tubes following heat lamp exposure. Plasma was collected after centrifugation of whole blood at 7,000 g for 10 min. Plasma for proteomic, Western blotting, and cytokine analyses was from nonfasted mice. Four-hour fasted blood was collected for glucose and insulin measurements from the mice used in the longitudinal study 2– 4 weeks after each collection for the proteomic analyses.
Fasting Glucose and Insulin Measurements
Blood glucose was measured by ONE TOUCH glucometer from Lifescan (Milpitis, CA). Plasma was collected as described earlier, and insulin levels were measured by an ultrasensitive rat/mouse insulin Enzyme-linked immunosorbent assay kit (ALPCO, Windham, NH).
2-DE and Quantification of Proteins
Plasma total protein concentration was determined by the Bradford method (21) using a protein assay reagent from Bio-Rad (Hercules, CA). Seven hundred fifty micrograms of protein was loaded for each 2-D gel. A detailed description of 2-DE can be found in previous reports (22,23). The gels were stained using SYPRO Orange (1:5,000, Molecular Probes, Eugene, OR) and gel images captured using a Pharos FX plus laser-scanner (Bio-Rad). For quantification of plasma proteins, PDQuest software (Bio-Rad) was used. The intensity of each protein spot was determined according to the fluorescence signal strength and normalized to the total intensity of each gel.
Protein Identification by MS and MS/MS
Proteins of interest were excised manually from gels and sent to Protea Biosciences, Inc. (Morgantown, WV) for MS and MS/MS analyses using matrix-assisted laser desorption ionization-time of flight and matrix-assisted laser desorption ionization-time of flight-time of flight as described previously (22).
For protein identification, MS and MS/MS data were manually submitted to MASCOT at www.matrixscience.com as described previously (22). The searching criteria used were as follows: Swiss-Prot as the database; mouse as the species; trypsin digestion; maximum one missed cleavage; fixed carbamidomethylation of Cys; variable modifications of oxidation-M (methionine), phosphorylation of S, T, and Y; monoisotopic; and 0.5 Da of peptide mass or parent tolerance. For MS/MS ion searches, in addition to the previous conditions, a peptide charge of +1 and a fragment mass tolerance of 0.5 Da were used.
Western Blotting
Plasma samples containing 50 μg of proteins were subjected to Western blotting as described previously (22, 23). Antibodies used included rabbit anti-mouse apolipoprotein (APO) A4, goat anti-mouse APOE, and rabbit anti-mouse haptoglobin (HP) α-chain (all diluted 1:1,000) from Santa Cruz Biotechnology Inc. (Santa Cruz, CA).
Cytokine Multiplex Panel
As mentioned previously, nonfasted plasma of a separate cohort of mice at 24 months of age was used to determine the levels of cytokines related to inflammation because plasma proteomic findings suggested reduced inflammation in GHR−/− mice. A customized mouse cytokine panel containing selected pro- and anti-inflammatory cytokines including granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, interferon-γ, interleukin (IL)-10, IL-17, IL-1α, IL-1β, IL-2, IL-6, monocyte chemotactic protein-1, Regulated on Activation, Normal T Cell Expressed and Secreted, and tumor necrosis factor was used following the manufacturer’s instructions (Millipore, Billerica, MA) with detection limits of 0.9, 5.6, 0.9, 3.3, 0.5, 5.1, 2.0, 0.8, 1.8, 5.3, 2.5, and 1.0 (picograms per milliliter), respectively.
Quantitative Real-Time Polymerase Chain Reaction Analysis
Liver samples from 24-month-old male mice were used to assess the mRNA expression of selected proteins that demonstrated significant differences in the proteomic analysis. Liver samples were dissected and flash-frozen in liquid nitrogen and stored at −80°C until processing. Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA), followed by RNA quantity and purity evaluation using a NanoDrop Spectrophotometer 2000c (Thermo Scientific, Wilmington, DE) as well as quantity and quality assessment using the Agilent 2100 Bioanalyzer (Agilent Technologies, Foster City, CA) at the Genomics Facility (Ohio University). Samples showing an RNA integrity number >7.0 were used for analyses (n = 6 for WT and n = 4 for GHR−/− mice). For each sample, 400 μg RNA was reverse transcribed into complementary DNA using QuantiTect Reverse Transcription kit (Qiagen). Polymerase chain reaction (PCR) amplification efficiencies were evaluated for all genes (described later), with efficiencies ranging from 92% to 107% and linear dynamic ranges of at least five orders of magnitude. Quantitative real-time (qRT) PCR was performed using the QuantiTect SYBR Green PCR kit (Qiagen) in an iCycler 3.0.6070 (Bio-Rad). Gene expression values were normalized to reference gene expression using qBase plus 2.0 (Biogazelle, Zwijnaarde, Belgium) and then exported for statistical analysis.
Primer sequences for Apoa4 are from the RT Primer database at http://www.rtprimerdb.org/ (RTPrimerDB ID: 3852): forward primer “GCCAATGTGGTGTGGGATTAC” and reverse primer “TCCTGGAAGAGGGTACTGAGCT.” Retinol-binding protein-4 (Rbp4): forward primer “AGCCTCCTTTCTCCAGCGAGGA” and reverse primer “AGCTGTCTGCACAGGTGCCA.” Mannose-binding protein-C: forward primer “CCGGGGTTAAAAGGAGCAGTGGGA” and reverse primer “TCAGCTCTGATCGTAGGGCTGCAA.” Five reference genes were considered including albumin, beta-2 microglobulin (B2m), actin beta (Actb), hydroxymethylbilane synthase (Hmbs), and hypoxanthine guanine phosphoribosyl transferase (Hprt). Of these, Actb, Hmbs, and Hprt were selected using the GeNorm module of qBase as the three most stable reference genes and were subsequently used for normalization. Primers for these reference genes included are as follows: Actb, forward primer “CAGCTTCTTTGCAGCTCCTT” and reverse primer “CACGATGGAGGGGAATACAG”; Hmbs, forward primer “TCCCTGAAGGATGTGCCTAC” and reverse primer “GAATTCCAGGTGGGGGAACT”; and Hprt, forward primer “ATCAGTCAACGGGGGACATA” and reverse primer “AGAGGTCCTTTTCACCAGCA.”
Statistical Analysis
All statistical analyses were performed using SPSS 14.0 software (Chicago, IL). Data for body weight were subjected to 2-way analysis of variance with repeated measures (sex and genotype as fixed factors). Data for fasting glucose and insulin as well as adipose depot weights were subjected to 2-way analysis of variance test with sex and genotype as fixed factors. qRT-PCR and cytokine data were subjected to Student’s t test. For protein intensity, the log-transformed data were subjected to 2-way analysis of variance with repeated measures with genotype and sex as fixed factors. Values of p < .05 were considered statistically significant. All data are presented as mean ± standard error of the mean.
Results
Body Weight, Fasting Glucose, and Insulin Levels
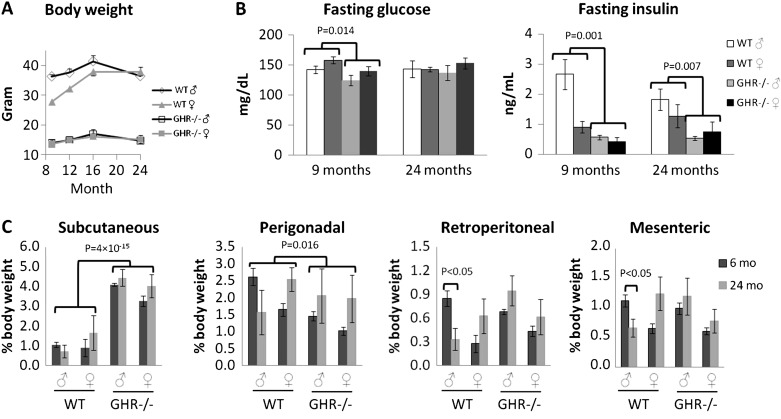
Similar to a previous study (5), we found decreased body weight in GHR−/− mice as compared with WT mice at all ages as well as the initiation of body weight loss at an advanced age for all mice. However, there was a less drastic weight loss at older ages in GHR−/− as compared with WT mice and in females as compared with males (Figure 1A; see the figure legend for statistical values). GHR−/− mice had lower fasting glucose levels at young adult ages but similar levels as compared with WT mice at old age. Insulin levels were lower in the young adult and old GHR−/− mice as compared with the controls (Figure 1B). In addition, females had lower insulin levels than males at young adult age, but the difference was diminished by 24 months (Figure 1B).
Figure 1.
Body weight, body composition, fasting glucose, and insulin levels. (A) Body weight. Two-way analysis of variance (ANOVA) with repeated measures showed a significant effect of age, F(3,54) = 24, p = 5 × 10− 10; genotype, F(1,18) = 297, p = 1 × 10− 12; an interaction between age and genotype, F(3,54) = 6, p = .003; and an interaction between age and sex, F(3,54) = 6.5, p = .004. (B) Fasting glucose and insulin levels. Two-way ANOVA showed a significant effect of genotype, F(1,18) = 7.4, p = .014, at 9 months for glucose; a significant effect of genotype, F(1,18) = 16.6, p = .001, and sex, F(1,18) = 18.7, p = .0004, at 9 months; and a significant effect of genotype, F(1,18) = 9.2, p = .007, at 24 months for insulin. (C) Normalized adipose depot weights. Subcutaneous depot shows significant effect for genotype, F(1,59) = 109.4, p = 4 × 10− 15; perigonadal depot shows significant effect for genotype, F(1,59) = 6.2, p = .016; retroperitoneal depot shows significant effect for sex, F(1,59) = 8.2, p = .006; interaction between sex and age, F(1,59) = 4.2, p = .045; and interaction between genotype, sex, and age, F(1,59) = 7.7, p = .007; and mesenteric depot shows significant effect for interaction between genotype and sex, F(1,59) = 4.6, p = .036; between sex and age, F(1,59) = 4.9, p = .031; and between genotype, sex, and age, F(1,59) = 6.5, p = .013.
Adipose Depot Weights
Given the reports of preferential subcutaneous fat deposition and its possible effect on insulin sensitivity in GHR−/− mice (5,24), we further analyzed adipose depot weights at 6 and 24 months of ages for both sexes in separate cohorts of mice. Depot weights were normalized to body weight (Figure 1C). GHR−/− mice had a significantly larger subcutaneous depot, as reported previously (4,24). Although GHR−/− mice are obese, their normalized perigonadal depot weight was significantly less than that of WT mice (Figure 1C). Retroperitoneal and mesenteric depot weights exhibited significant interactions between age, sex, and genotype, that is, only male WT mice lost fat at old compared with young age (Figure 1C), while all other mice maintained similar masses of retroperitoneal and mesenteric white adipose tissue (WAT) at old age. Finally, the retroperitoneal depot was significantly smaller in females than males (Figure 1C).
Plasma Proteomic Differences by Genotype and Sex
Plasma was sampled from mice at 8, 16, and 24 months of age and subjected to 2-DE analysis. Two-way analysis of variance with repeated measures revealed proteins that were significantly different by genotype, sex, age, and their interactions with each other (p < .05). These proteins included APOA4, APOE, mannose-binding protein C (MBP-C), APOA1, RBP-4, HP, hemoglobin subunit beta (HBB), and albumin (Figure 2). These proteins all existed as multiple “isoforms” on the 2-D gel, many exhibiting similar molecular weights but different isoelectric points. This has been reported previously (22,23) and is probably due to posttranslational modifications that change the protein’s charges but have little effect on molecular weights. We attempted to identify the posttranslational modifications of these isoforms using both MASCOT (freely available at www.matrixscience.com) and “PEAKS” (Bioinformatics Solutions, Inc.), but neither detected them. The chemical nature of the posttranslational modifications is an important subject for future studies.
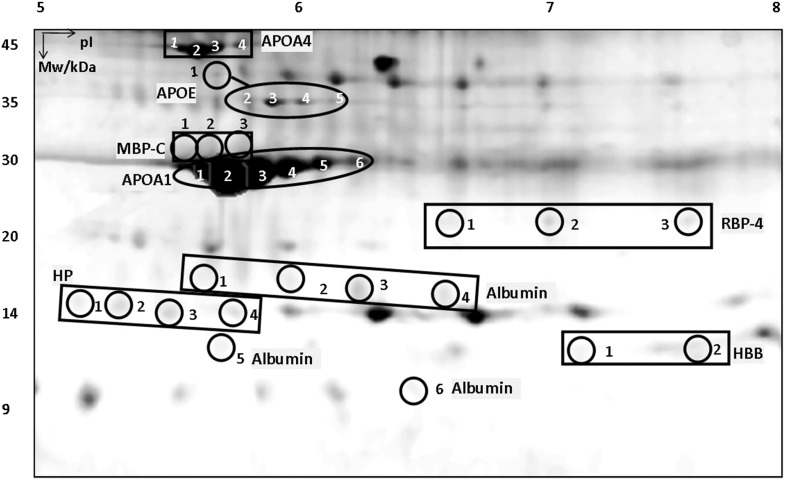
Figure 2.
2-D gel image with mouse plasma proteins. All proteins have been identified with multiple isoforms numbered 1, 2, etc. Mw = molecular weight; pI = isoelectric point; APOA4 = apolipoprotein A-4; APOA1 = apolipoprotein A-1; APOE = apolipoprotein E; HBB = hemoglobin subunit beta; HP = haptoglobin; MBP-C = mannose-binding protein-C; RBP-4 = retinol-binding protein-4.
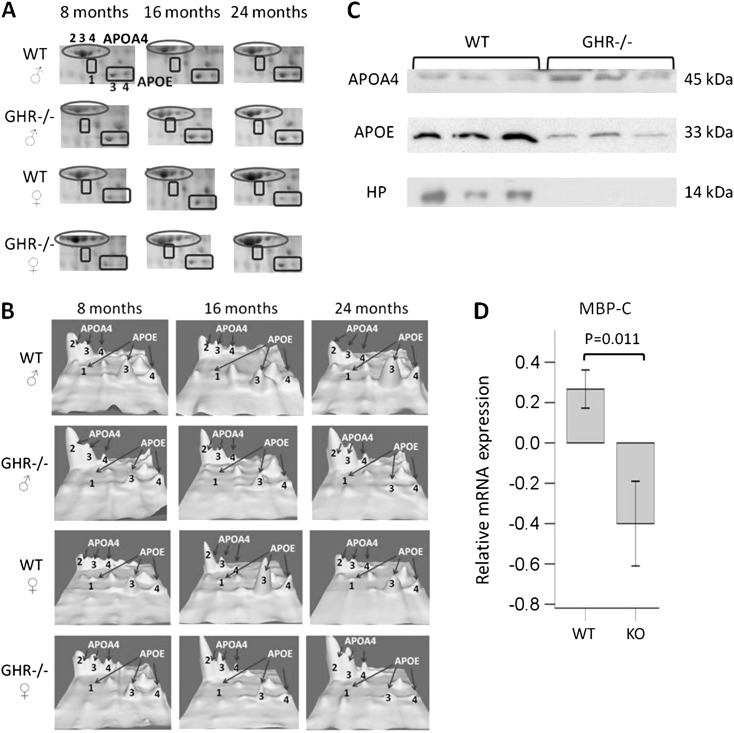
The MS and MS/MS identification of APOA4, APOE, MBP-C, APOA1, HP, and albumin and their respective isoforms using this 2-DE system has been reported previously both with aging in WT mice and in GH transgenic mice (22,23). The MS and MS/MS identification of the RBP-4 and HBB isoforms is listed in Supplementary Table 1. Selected protein changes were also confirmed by Western blotting of plasma proteins (Figure 3C) and qRT-PCR (Figure 3D) in the liver, the tissue that secretes the majority of these plasma proteins. Later we describe these proteins based on differences by genotype and sex.
Figure 3.
Differential plasma proteins in GHR−/− mice compared with WT mice. (A) Cropped 2-D gel images showing APOA4 isoforms 2, 3, and 4 and APOE isoforms 1, 3, and 4. (B) 3-D view of intensities of APOA4 isoforms 2, 3, and 4 and APOE isoforms 1, 3, and 4. (C) Western blotting on APOA4, APOE, and HP in 24-month-old male mice. Equal protein loading was verified by Commassie blue staining of sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels (not shown). (D) Quantitative real-time polymerase chain reaction analysis of MBP-C messenger RNA expression in the liver of 24-month-old male mice. Data were normalized relative to expression of three reference genes Actb, Hmbs, and Hprt using qBase Plus 2.0 program. APOE = apolipoprotein E; HP = haptoglobin; MBP-C = mannose-binding protein-C; WT = wild-type.
Plasma proteins that exhibited significant genotype differences.—
Several proteins showed significant changes between GHR−/− and WT mice regardless of sex or age. These proteins are summarized in Table 1. Because no sex or age differences were observed for these proteins, only a single sex and age group (24-month-old male samples) were used as representatives for subsequent analyses of select proteins. Western blotting on plasma samples showed that the total plasma protein levels were consistent with the isoform changes for APOA4, APOE, and HP. In addition, qRT-PCR on liver samples from a single sex and age group (24-month-old male) was performed for genes of APOA4, RBP-4, and MBP-C. Only mRNA data but not protein data in liver were shown because all the proteins examined are secreted into blood. Most likely, the protein levels in the tissue will be minimal or may be contamination from blood as the liver is highly vascularized. Thus, the mRNA level is a better indicator than protein level for tissue expression. Our results show that APOA4 (Table 1, Figure 3A, B, and C) and RBP-4 (Table 1) were upregulated, while APOE (Table 1, Figure 3A, B, and C) and MBP-C (Table 1) were downregulated in GHR−/− mice versus controls. MBP-C mRNA levels were also decreased in GHR−/− mouse liver (Figure 3D), indicating that the plasma MBP-C reduction in GHR−/− mice was most likely due to downregulated mRNA expression of this gene in the liver.
Table 1.
Proteins Significantly Different in GHR−/− Compared with Wild-Type (WT) Mice (p < .01)
| Protein ID | Isoform Numbers | Fold Difference | Significance Level |
| APOA4 | 3 | ↑ 1.7 | F(1,18) = 70.3, p = 1 × 10−7 |
| 4 | ↑ 1.9 | F(1,18) = 19.4, p = .0003 | |
| Total | ↑ 1.6 | F(1,18) = 88.8, p = 2 × 10−8 | |
| RBP-4 | 1 | ↑ 1.8 | F(1,18) = 18.2, p = .0005 |
| 2 | ↑ 3.0 | F(1,18) = 17.6, p = .001 | |
| 3 | ↑ 4.2 | F(1,18) = 15.8, p = .0001 | |
| APOE | 1 | ↓ 8.3 | F(1,18) = 10.2, p = .005 |
| 3 | ↓ 2.5 | F(1,18) = 46.7, p = 2 × 10−6 | |
| 4 | ↓ 1.7 | F(1,18) = 12.9, p = .002 | |
| Total | ↓ 1.6 | F(1,18) = 19.7, p = .0003 | |
| MBP-C | 1 | ↓ 11.5 | F(1,18) = 11, p = .004 |
| 2 | ↓ 11.1 | F(1,18) = 8.3, p = .01 | |
| HP | 1 | ↓ 2.6 | F(1,18) = 4.7, p = .044 |
Notes: Protein isoform Numbers correspond to those shown in Figure 3. The fold difference was calculated as the ratio of the mean values of GHR−/− mice and WT mice (pooled samples regardless of sex or age). “↑” Means upregulation and “↓” means downregulation. Two-way analysis of variance with repeated measures revealed a significant genotype difference. APOE = apolipoprotein E; HP = haptoglobin; MBP-C = mannose-binding protein-C; RBP-4 = retinol-binding protein-4.
Plasma proteins that exhibited significant sex differences.—
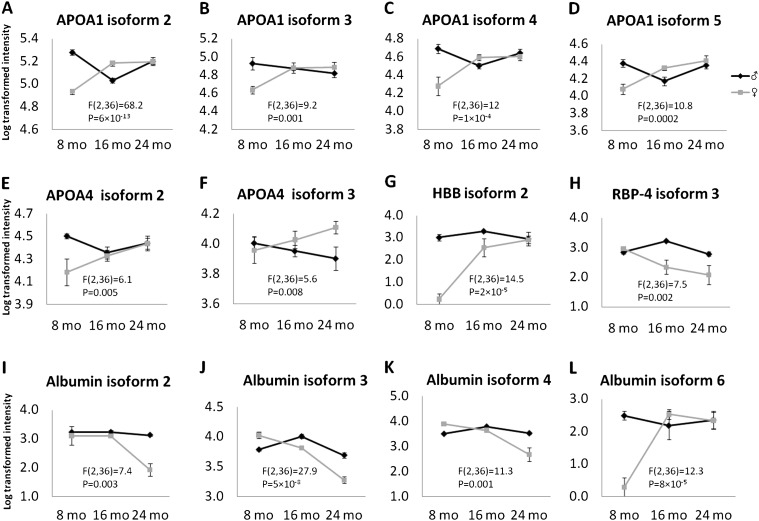
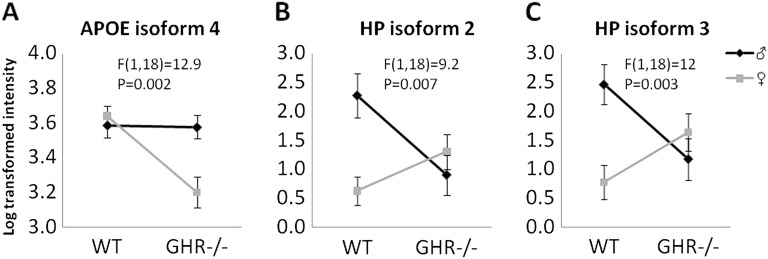
We found four types of sex differences: (i) Sex difference regardless of genotype or age. These proteins are summarized in Table 2. APOE isoform 1 was increased in the females, whereas RBP-4 isoform 2, HP isoform 1, albumin isoforms 1 and 5, and HBB isoform 1 were decreased in females compared with males. (ii) Sex difference in the context of age regardless of genotype (ie, significant interaction between sex and age). The levels of these proteins showed little change as a function of age in males but changed significantly in aging females (Figure 4). For example, several APOA1, APOA4 isoforms, and one HBB isoform (Figure 4A–G) increased as female mice aged but were not changed in males. Also, one isoform of RBP-4 and three isoforms of albumin decreased (Figure 4H–K), and one isoform of albumin increased (Figure 4L) in females but not in males during aging. (iii) Sex difference in the context of genotype regardless of age (Figure 5). For example, APOE isoform 4 showed no sex difference in WT mice but was lower in the GHR−/− females compared with males, and HP isoforms 2 and 3 showed no sex difference in GHR−/− mice but was lower in WT females compared with males. (iv) Sex difference in the context of both genotype and age (Figure 6), where RBP-4 isoform 1 showed an age-dependent decline in WT female mice but no change in all other groups.
Table 2.
Proteins Significantly Different in Males and Females (p < .05)
| Protein ID | Isoform Numbers | Fold Difference | Significance Level |
| APOE | 1 | ↑ 3.2 | F(1,18) = 30, p = 3 × 10−5 |
| RBP-4 | 2 | ↓ 2 | F(1,18) = 8.7, p = .009 |
| HP | 1 | ↓ 12.5 | F(1,18) = 22.2, p = .0002 |
| Albumin | 1 | ↓ 2.7 | F(1,18) = 11.1, p = .004 |
| 5 | ↓ 4 | F(1,18) = 15.1, p = .001 | |
| HBB | 1 | ↓ 9.1 | F(1,18) = 18.2, p = .0005 |
Notes: Protein isoform numbers correspond to those shown in Figure 3. The fold difference of male vs female was calculated as the mean value of female/mean value of male (pooled samples regardless of genotype or age). “↑” Means upregulation and “↓” means downregulation. Two-way analysis of variance with repeated measures revealed a significant sex difference. APOE = apolipoprotein E; HBB = hemoglobin subunit beta; HP = haptoglobin; RBP-4 = retinol-binding protein-4.
Figure 4.
Proteins that showed significant interactions between sex and age. Two-way analysis of variance with repeated measures revealed a significant interaction between age and sex. Statistical values for this interaction are labeled in each graph.
Figure 5.
Proteins that showed significant interactions between sex and genotype. Two-way analysis of variance with repeated measures revealed a significant interaction between sex and genotype. Statistical values for this interaction are labeled in each graph. The data were pooled from 8, 16, and 24 months as no age difference was found for these proteins.
Figure 6.
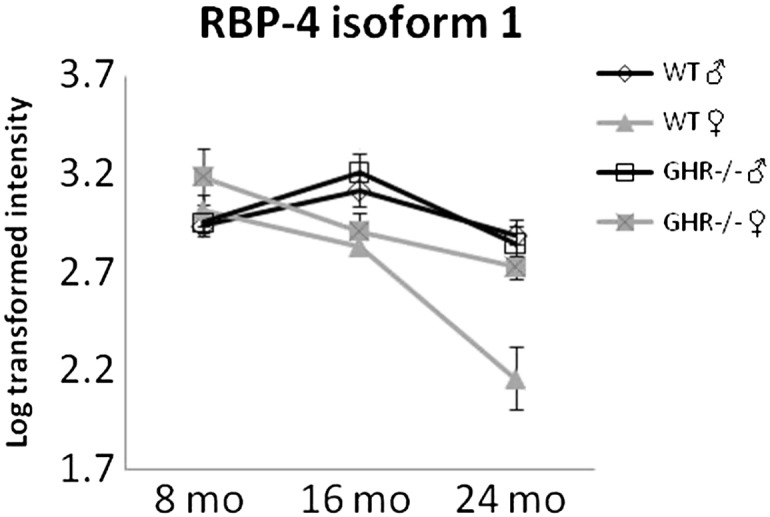
RBP-4 isoform 1 is decreased as a function of age only in WT female mice. Two-way analysis of variance with repeated measures revealed a significant interaction between age and sex: F(2,36) = 8.5, p = .001, as well as a significant interaction between genotype and sex: F(1,18) = 8.3, p = .01. RBP-4 = retinol-binding protein-4; WT = wild-type.
While genotype differences discussed in the previous section were usually different both at the isoform and at the total protein levels, sex differences tend to occur only at the level of specific protein isoforms, for example, increased APOE isoform 1, decreased albumin isoforms 1 and 5, and HBB isoform 1 in females when compared with males. However, for RBP-4 and HP, total levels are decreased in females when compared with males (Figure 4H, Figure 5B and C, Figure 6, Table 2).
Cytokine Measurements
A few of the proteomic changes observed (such as MBP-C and HP) are acute-phase proteins indicative of inflammation. Downregulation of these proteins in GHR−/− mice might indicate that these animals have decreased inflammation compared with WT mice. To determine the levels of various inflammatory cytokines, plasma samples from 24-month-old mice (a different cohort) were analyzed using a mouse cytokine multiplex panel containing 12 cytokines. The mean values of these cytokines in each group of mice are given in Supplementary Table 2. Although no significant differences were observed between male GHR−/− and WT mice, IL-6 showed a trend of downregulation in GHR−/− mice (Table S2). On the other hand, female GHR−/− mice revealed a significant decrease in monocyte chemotactic protein-1 and IL-1β (Figure 7).
Figure 7.
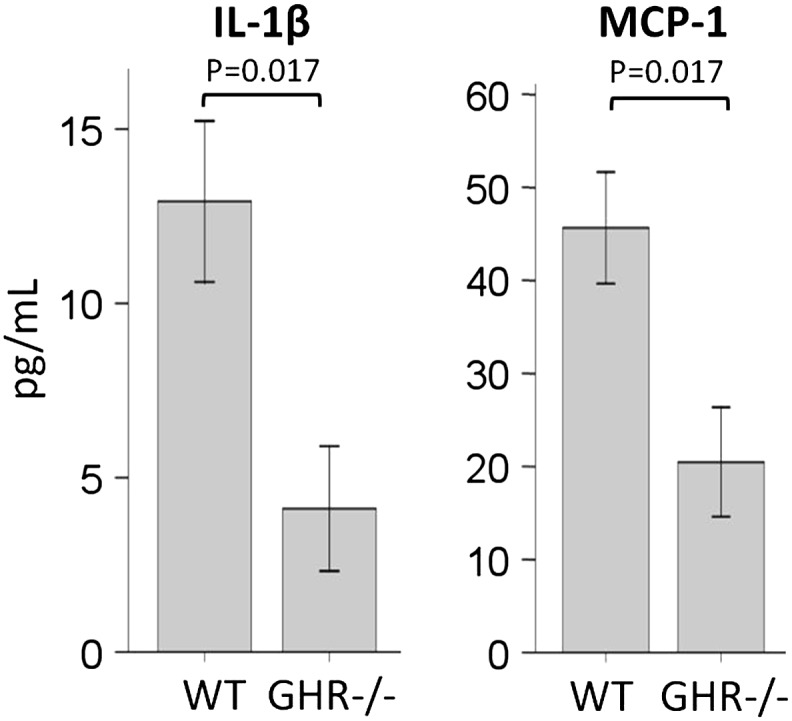
Inflammatory cytokines in 24-month-old female mice.
Discussion
GHR−/− mice have been used as a mouse model to study human LS due to various physiological and biological similarities, including lower IGF-1 levels, dwarfism, and obesity. GHR−/− mice also are known to have prolonged life span. A review of the physiological characteristics of these mice has recently been reported (17). However, LS individuals do not appear to have increased life span. This is a major difference between LS humans and GHR−/− mice. One possibility is the species difference. Another possibility is environmental factors, as mice are kept in pathogen-free laboratories, with precisely controlled diet, lighting, temperature, humidity, and restricted movements. A carefully controlled environment is obviously not possible for human studies. However, our GHR−/− mice have a reduced incidence of cancer and diabetes. In this regard, LS patients also have a decreased incidence of cancer and diabetes as recently reported by Guevara-Aguirre and colleagues (15). The authors of this paper also comment that “the lack of life-span extension in LS subjects may be explained in large part by the high proportion of deaths (70%) caused by convulsive disorders, alcohol toxicity, accidents, liver cirrhosis, and other non–age-related causes.” Thus, the GHR−/− mice are still particularly useful to study the biological mechanisms involved in the aging process as several prominent disease states are reduced in both mice and men with GH insensitivity.
The present study characterized the plasma proteomes of GHR−/− mice and control WT littermates in a longitudinal study of both sexes as they aged. Several physiological measurements were also determined. Plasma proteins that are differentially regulated in GHR−/− versus control mice were identified. Notable sex differences were also found in the proteomic profiles. Our results suggest that the GH status and sex may affect life span and health span. Later we discuss the significance of these differences as a function of genotype and sex.
GHR−/− Mice Show Beneficial Apolipoprotein Changes Despite Overall Obesity
GHR−/− mice had higher APOA4 but lower APOE than WT mice in both sexes. APOA4 is a major component of high-density lipoprotein, whereas APOE is associated with all classes of cholesterol but mainly with very low-density lipoprotein and low-density lipoprotein. Although overly simplified, a disproportion of APOA4 over APOE might indicate a better APO profile in GHR−/− mice. In this regard, GHR−/− mice have reduced plasma cholesterol levels (both high- and low-density lipoproteins) compared with WT mice (25,26).
In addition to cholesterol transportation and metabolism, APOA4 is also an antioxidant protein (27). It has been shown to have anti-atherosclerosis and anti-inflammation effects (28). Over expression of APOA4 in APOE−/− mice (a mouse model of atherosclerosis) protect these mice from diet-induced atherosclerosis (29). Interestingly, fasting induces expression of APOA4 in mice, and this response is diminished in old mice (30). In humans, APOA4 is a marker for coronary heart disease, with lower levels indicating higher risk (31). Conversely, people with higher APOA4 have a lower risk of atherosclerosis and cardiovascular diseases (32). Although the mechanism of the beneficial effect of APOA4 in cardiovascular disease risks is still unknown, it is possible that increased APOA4 levels confer protection against heart problems in the obese GHR−/− mice. The increased APOA4 in GHR−/− mice is consistent with a previous study where the GH-injected WT mice show decreased APOA4 (33).
Previous reports have shown that GHR−/− mice are obese, and male GHR−/− mice have enlarged subcutaneous WAT both at young and old ages (4,5,24). Our study further showed that this is true for females as well. It has been reported that subcutaneous fat is the “healthy” fat, whereas visceral fat is the “culprit” for insulin resistance (34,35). In rodents, transplant studies have repeatedly demonstrated that the subcutaneous depot has a positive role in insulin sensitivity (36). Together, the specific enlargement of subcutaneous fat in GHR−/− mice is consistent with their increased insulin sensitivity.
In addition, our proteomic result indicates that GHR−/− mice have elevated RBP-4, a protein associated with obesity (37), and is known to be expressed more in subcutaneous than visceral WAT depots (38). However, RBP-4 is also associated with insulin resistance (39); yet, GHR−/− mice are relatively insulin sensitive (17). Typically, obesity and insulin resistance are positively correlated. However, GHR−/− mice are obese but insulin sensitive. GH is known to possess an anti-insulin effect (40). In this regard, one possibility could be that GH signaling may be a mediator of RBP-4–dependent effects on insulin resistance, thereby uncoupling the association between RBP-4 and insulin resistance in GHR−/− mice. More work is obviously needed to confirm this hypothesis.
Lastly, GHR −/−mice of both sexes showed decreased insulin levels relative to WT mice at both young adult and old ages, indicating increased insulin sensitivity in GHR−/− mice even at the advanced age. This is a very significant factor especially in light of the recent report of lower diabetes incidence in LS humans (15). Other GH deficient mouse strains, such as Ames dwarf mice, Snell dwarf mice, and “little” mice, are also insulin sensitive and obese (41). Therefore, it does appear consistent that GH is an important link between obesity and insulin resistance. We are unaware of GH- or GHR-deficient mutants that do not display increased insulin sensitivity.
GHR−/− Mice Show Reduced Inflammatory Proteins
MBP-C and HP are both acute-phase proteins secreted in large amounts by the liver under conditions of acute infection, injury, or infection. Their high levels generally are a sign of inflammation (42). Interestingly, GHR−/− mice had lower levels of these proteins in both sexes, perhaps indicating reduced inflammation in these long-lived mice. Furthermore, downregulation of MBP-C at the mRNA level in GHR−/− liver compared with WT controls suggests that reduction in MBP-C levels may be due to altered expression in the liver.
Inflammation is associated with aging (43). Previously, we have found HP to be increased in WT mice as they age (22). In addition, bovine GH transgenic mice, which have an increase in GH action and are short lived, show increased HP as compared with WT mice at old ages and increased MBP-C throughout their life span (23). Thus, the current study is consistent with previous studies and indicates reduced inflammation in GHR−/− mice. In order to confirm this, we measured 12 inflammation-related cytokines. For many of the cytokines, nondetectable levels were found, perhaps due to the relative rapid clearance of cytokines (44) and the detection limit of the assay. Also, all animals appeared relatively healthy; hence, inflammatory cytokines may have been in the low range despite advancing age. As a result, values for many of the cytokines in the mice were zero (nondetectable), leading to nonsignificant difference in GHR−/− mice compared with WT mice. A recent study has found that healthy old women (70–80 year) have similar levels of inflammatory cytokines compared with young people in their 20s (45). Therefore, it is possible that healthy mice show similar levels of cytokines regardless of genotype. However, female GHR−/− mice did show significantly lower monocyte chemotactic protein-1 and IL-1β than female WT mice, suggesting there might be a reduced inflammatory status in GHR−/− mice at old ages in females. Future studies are needed to confirm the inflammatory status in other GH-related mouse strains.
Notable Sex Differences Indicate Female Mice Are “ Healthier” at Advanced Age
Most of the studies involving GHR−/− mice focus on males. In this study, we have identified several significant differences related to sex. RBP-4 and HP showed reduced levels in female mice compared with the males. As mentioned earlier, RBP-4 is associated with obesity and insulin resistance. Thus, lower levels of RBP-4 indicate reduced fat and/or increased insulin sensitivity in females. In agreement with this, we find that females had a smaller retroperitoneal adipose depot and lower insulin levels than males, also reported previously (46). Interestingly, in humans, females also have lower serum RBP-4 than males (47). In addition, lower HP levels may indicate reduced inflammation in females than males. In the present study, we did not observe significant differences in the inflammatory cytokines between sexes, although a nonsignificant trend of a decrease in several of the cytokines including granulocyte-macrophage colony-stimulating factor, IL-1β, IL-6, monocyte chemotactic protein-1, Regulated on Activation, Normal T Cell Expressed and Secreted, and tumor necrosis factor-α was found (Supplementary Table 2). Future studies with more animals are needed to clarify any sex difference in inflammation.
Twelve plasma protein spots are significantly different as a function of age in females compared with males regardless of genotype (Figure 5). None of these proteins showed significant differences in a previous study investigating biomarkers of aging in male WT mice (22). Therefore, this suggests that female mice age differently from males, at least relative to their plasma proteomic profile. In particular, APOA1 and APOA4 increased during aging in females but not in males. Because both APOA1 and APOA4 transport high-density lipoprotein, this may imply a beneficial effect on the cardiovascular health of females as they age.
In humans and many other mammals, females live longer than males (48). Recently, a large-scale cross-sectional study of sex differences in 38,000 individuals show significant sex differences and sex by age interaction effects in age-related physiological markers, including decreased age trajectory slope of inflammation burden and decreased overall metabolic syndrome in females than males (49). However, it is not clear if the same is true in mice; that is, data are lacking regarding whether the female mice live longer than the males and whether the females show less age-related physiological deterioration. In the original longevity study of GHR−/− mice in both C57BL/6J and Ola-BALB/cJ backgrounds, WT females showed longer life span than WT males (821 ± 49 vs 756 ± 68 days), although statistical significance was not reached (3). Another study showed a trend of longer maximum life span in WT females compared with WT males; however, statistical significance was not reported (50). Although it is not known whether female mice live significantly longer than males, a recent study shows that short-term calorie restriction shifts liver gene expression in male mice toward that typical of females (51). Consistent with this, we show that weight loss that is common at old age (52) was much less dramatic in females compared with males at 24 months. Overall, the plasma proteomic and physiological differences in female mice found in this study indicate that female mice may be healthier than male mice at older ages although their life span may not be significantly increased. However, despite a less advantageous health span in male mice, the male GHR−/− mice still have a longer life span compared with both sexes of WT mice, demonstrating that disruption of GH signaling in mice is “strong enough” to convey increased longevity in both sexes.
The Interaction Between GH and Sex
There were several notable physiological changes that showed sex-dependent genotype differences. For adipose tissue, an age-dependent fat (retroperitoneal and mesenteric depots) loss specific to WT male mice was observed. Thus, it appears that loss of GH signaling or being female preserves fat mass at old age in mice. Our study also identified specific isoforms of HP to be affected by sex in a genotype-specific manner, such that being female or loss of GH signaling both resulted in reduced levels of HP, an indicator of inflammation. These suggest that an intact GH signaling system as seen in WT mice (but not in GHR−/− mice) has a sexually dimorphic effect on fat distribution and specific plasma proteins.
Numerous sex differences relative to GH action have been reported previously. GH itself is secreted in a sexually dimorphic manner, that is, GH secretion is pulsatile in males but less so in females (53). In addition, estrogen inhibits GH action in females (54). GH also affects the liver in a sexually dimorphic manner (55,56,57), for example, on drug-metabolizing genes, hepatic nuclear factor-4α, and phosphorylation of Stat5b. Because the plasma proteins described in this paper are secreted mainly from the liver, it is possible that their sex-specific differences are in part due to the sexually dimorphic effect of GH on liver.
In summary, the present study has identified physiological and plasma proteomic differences between male and female GHR−/− mice and WT littermates. These include beneficial fat preservation at old age, redistribution of WAT, favorable insulin/glucose profiles, improved apolipoprotein levels, and reduced inflammatory proteins, all of which may contribute to the longer life span of GHR−/− mice. Furthermore, female mice exhibit significant differences in aging compared with males with improved apolipoprotein levels, lower insulin levels, and favorable WAT redistribution. This implies that female mice may be healthier than their male counterparts at advanced ages and thus better able to mitigate the deleterious effects of the aging process. Together, our data imply that lack of GH signaling or being female may be beneficial for life span and/or health span in mice.
Funding
This work was supported by funds from the National Institute of Aging (AG19899, AG031736, and AG031736); the National Institute of Diabetes and Digestive and Kidney Diseases (DK075436); the State of Ohio's Eminent Scholar Program that includes a gift from Milton and Lawrence Goll; a grant from DiAthegen LLC; the Diabetes Research Initiative at Ohio University; and by a grant from AMVETS.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/.
Conflict of Interest
None of the authors have any conflict of interest.
Acknowledgments
We would like to thank Elahu Gosney and Dr. Lucila Sackmann-Sala for assistance with the qRT-PCR experiments.
References
- 1.Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimokawa I, Chiba T, Yamaza H, Komatsu T. Longevity genes: insights from calorie restriction and genetic longevity models. Mol Cells. 2008;26:427–435. [PubMed] [Google Scholar]
- 3.Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- 4.Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004;14:309–318. doi: 10.1016/j.ghir.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Berryman DE, List EO, Palmer AJ, et al. Two-year body composition analyses of long-lived GHR null mice. J Gerontol A Biol Sci Med Sci. 2010;65:31–40. doi: 10.1093/gerona/glp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu JL, Coschigano KT, Robertson K, et al. Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. Am J Physiol Endocrinol Metab. 2004;287:E405–E413. doi: 10.1152/ajpendo.00423.2003. [DOI] [PubMed] [Google Scholar]
- 7.Thorner MO. Statement by the Growth Hormone Research Society on the GH/IGF-I axis in extending health span. J Gerontol A Biol Sci Med Sci. 2009;64:1039–1044. doi: 10.1093/gerona/glp091. [DOI] [PubMed] [Google Scholar]
- 8.Kinney BA, Coschigano KT, Kopchick JJ, Steger RW, Bartke A. Evidence that age-induced decline in memory retention is delayed in growth hormone resistant GH-R-KO (Laron) mice. Physiol Behav. 2001;72:653–660. doi: 10.1016/s0031-9384(01)00423-1. [DOI] [PubMed] [Google Scholar]
- 9.Bellush LL, Doublier S, Holland AN, Striker LJ, Striker GE, Kopchick JJ. Protection against diabetes-induced nephropathy in growth hormone receptor/binding protein gene-disrupted mice. Endocrinology. 2000;141:163–168. doi: 10.1210/endo.141.1.7284. [DOI] [PubMed] [Google Scholar]
- 10.Ikeno Y, Hubbard GB, Lee S, et al. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2009;64:522–529. doi: 10.1093/gerona/glp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller RA, Chang Y, Galecki AT, Al-Regaiey K, Kopchick JJ, Bartke A. Gene expression patterns in calorically restricted mice: partial overlap with long-lived mutant mice. Mol Endocrinol. 2002;16:2657–2666. doi: 10.1210/me.2002-0142. [DOI] [PubMed] [Google Scholar]
- 12.Bonkowski MS, Dominici FP, Arum O, et al. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS One. 2009;4:e4567. doi: 10.1371/journal.pone.0004567. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Gesing A, Masternak MM, Wang F, et al. Expression of key regulators of mitochondrial biogenesis in growth hormone receptor knockout (GHRKO) mice is enhanced but is not further improved by other potential life-extending interventions. J Gerontol A Biol Sci Med Sci. 2011;66:1062–1076. doi: 10.1093/gerona/glr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laron Z, Kowadlo-Silbergeld A, Eshet R, Pertzelan A. Growth hormone resistance. Ann Clin Res. 1980;12:269–277. [PubMed] [Google Scholar]
- 15.Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol. 2011;164:485–489. doi: 10.1530/EJE-10-0859. [DOI] [PubMed] [Google Scholar]
- 17.List EO, Sackmann-Sala L, Berryman DE, et al. Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR-/-) mouse. Endocr Rev. 2011;32:356–386. doi: 10.1210/er.2010-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baudet ML, Hassanali Z, Sawicki G, List EO, Kopchick JJ, Harvey S. Growth hormone action in the developing neural retina: a proteomic analysis. Proteomics. 2008;8:389–401. doi: 10.1002/pmic.200700952. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Xu BC, Maheshwari HG, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Natl Acad Sci U S A. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller RA, Nadon NL. Principles of animal use for gerontological research. J Gerontol A Biol Sci Med Sci. 2000;55:B117–B123. doi: 10.1093/gerona/55.3.B117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 22.Ding J, Kopchick JJ. Plasma biomarkers of mouse aging. Age (Dordr) 2010;33:291–307. doi: 10.1007/s11357-010-9179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding J, Berryman DE, Kopchick JJ. Plasma proteomic profiles of bovine growth hormone transgenic mice as they age. Transgenic Res. 2011 doi: 10.1007/s11248-011-9499-5. 20:1305–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berryman DE, List EO, Kohn DT, Coschigano KT, Seeley RJ, Kopchick JJ. Effect of growth hormone on susceptibility to diet-induced obesity. Endocrinology. 2006;147:2801–2808. doi: 10.1210/en.2006-0086. [DOI] [PubMed] [Google Scholar]
- 25.Egecioglu E, Bjursell M, Ljungberg A, et al. Growth hormone receptor deficiency results in blunted ghrelin feeding response, obesity and hypolipidemia in mice. Am J Physiol Endocrinol Metab. 2005;292:E1418–E1425. doi: 10.1152/ajpendo.00181.2005. [DOI] [PubMed] [Google Scholar]
- 26.Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, et al. Caloric restriction results in decreased expression of peroxisome proliferator-activated receptor superfamily in muscle of normal and long-lived growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2005;60:1238–1245. doi: 10.1093/gerona/60.10.1238. [DOI] [PubMed] [Google Scholar]
- 27.Wong W-MR, Gerry AB, Putt W, et al. Common variants of apolipoprotein A-IV differ in their ability to inhibit low density lipoprotein oxidation. Atherosclerosis. 2007;192:266–274. doi: 10.1016/j.atherosclerosis.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Recalde D, Ostos MA, Badell E, et al. Human apolipoprotein A-IV reduces secretion of proinflammatory cytokines and atherosclerotic effects of a chronic infection mimicked by lipopolysaccharide. Arterioscler Thromb Vasc Biol. 2004;24:756–761. doi: 10.1161/01.ATV.0000119353.03690.22. [DOI] [PubMed] [Google Scholar]
- 29.Duverger N, Tremp G, Caillaud JM, et al. Protection against atherogenesis in mice mediated by human apolipoprotein A-IV. Science. 1996;273:966–968. doi: 10.1126/science.273.5277.966. [DOI] [PubMed] [Google Scholar]
- 30.Araki S, Okazaki M, Goto S. Impaired lipid metabolism in aged mice as revealed by fasting-induced expression of apolipoprotein mRNAs in the liver and changes in serum lipids. Gerontology. 2004;50:206–215. doi: 10.1159/000078349. [DOI] [PubMed] [Google Scholar]
- 31.Warner MM, Guo J, Zhao Y. The relationship between plasma apolipoprotein A-IV levels and coronary heart disease. Chin Med J (Engl) 2001;114:275–279. [PubMed] [Google Scholar]
- 32.Omori M, Watanabe M, Matsumoto K, Honda H, Hattori H, Akizawa T. Impact of serum apolipoprotein A-IV as a marker of cardiovascular disease in maintenance hemodialysis patients. Ther Apher Dial. 2010;14:341–348. doi: 10.1111/j.1744-9987.2010.00809.x. [DOI] [PubMed] [Google Scholar]
- 33.Ding J, List EO, Bower B, Kopchick J. Differential effects of growth hormone versus insulin-like growth factor-I on the mouse plasma proteome. Endocrinology. 2011;152:3791–3802. doi: 10.1210/en.2011-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicklas BJ, Cesari M, Penninx BW, et al. Abdominal obesity is an independent risk factor for chronic heart failure in older people. J Am Geriatr Soc. 2006;54:413–420. doi: 10.1111/j.1532-5415.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- 35.Tanko LB, Bagger YZ, Alexandersen P, Larsen PJ, Christiansen C. Peripheral adiposity exhibits an independent dominant antiatherogenic effect in elderly women. Circulation. 2003;107:1626–1631. doi: 10.1161/01.CIR.0000057974.74060.68. [DOI] [PubMed] [Google Scholar]
- 36.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kloting N, Graham TE, Berndt J, et al. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab. 2007;6:79–87. doi: 10.1016/j.cmet.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Bajzova M, Kovacikova M, Vitkova M, et al. Retinol-binding protein 4 expression in visceral and subcutaneous fat in human obesity. Physiol Res. 2008;57:927–934. doi: 10.33549/physiolres.931379. [DOI] [PubMed] [Google Scholar]
- 39.Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 40.Rabinowitz D, Klassen GA, Zierler KL. Effect of human growth hormone on muscle and adipose tissue metabolism in the forearm of man. J Clin Invest. 1965;44:51–61. doi: 10.1172/JCI105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- 42.Fleck A. Clinical and nutritional aspects of changes in acute-phase proteins during inflammation. Proc Nutr Soc. 1989;48:347–354. doi: 10.1079/pns19890050. [DOI] [PubMed] [Google Scholar]
- 43.Dowling DK, Simmons LW. Reactive oxygen species as universal constraints in life-history evolution. Proc Biol Sci. 2009;276:1737–1745. doi: 10.1098/rspb.2008.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bindon C, Czerniecki M, Ruell P, et al. Clearance rates and systemic effects of intravenously administered interleukin 2 (IL-2) containing preparations in human subjects. Br J Cancer. 1983;47:123–133. doi: 10.1038/bjc.1983.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goetzl EJ, Huang MC, Kon J, et al. Gender specificity of altered human immune cytokine profiles in aging. FASEB J. 2010;24:3580–3589. doi: 10.1096/fj.10-160911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo Y, Lu Y, Houle D, et al. Pancreatic islet-specific expression of an insulin-like growth factor-I transgene compensates islet cell growth in growth hormone receptor gene-deficient mice. Endocrinology. 2005;146:2602–2609. doi: 10.1210/en.2004-1203. [DOI] [PubMed] [Google Scholar]
- 47.Lee DC, Lee JW, Im JA. Association of serum retinol binding protein 4 and insulin resistance in apparently healthy adolescents. Metabolism. 2007;56:327–331. doi: 10.1016/j.metabol.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 48.Promislow DEL. Costs of sexual selection in natural populations of mammals. Proc Biol Sci. 1992;247:203–210. [Google Scholar]
- 49.Yang Y, Kozloski M. Sex differences in age trajectories of physiological dysregulation: inflammation, metabolic syndrome, and allostatic load. J Gerontol A Biol Sci Med Sci. 2011;66:493–500. doi: 10.1093/gerona/glr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Estep PW, 3rd, Warner JB, Bulyk ML. Short-term calorie restriction in male mice feminizes gene expression and alters key regulators of conserved aging regulatory pathways. PLoS One. 2009;4:e5242. doi: 10.1371/journal.pone.0005242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baumgartner RN, Stauber PM, McHugh D, Koehler KM, Garry PJ. Cross-sectional age differences in body composition in persons 60+ years of age. J Gerontol A Biol Sci Med Sci. 1995;50:M307–M316. doi: 10.1093/gerona/50a.6.m307. [DOI] [PubMed] [Google Scholar]
- 53.MacLeod JN, Pampori NA, Shapiro BH. Sex differences in the ultradian pattern of plasma growth hormone concentrations in mice. J Endocrinol. 1991;131:395–399. doi: 10.1677/joe.0.1310395. [DOI] [PubMed] [Google Scholar]
- 54.Meinhardt UJ, Ho KK. Regulation of growth hormone action by gonadal steroids. Endocrinol Metab Clin North Am. 2007;36:57–73. doi: 10.1016/j.ecl.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 55.Waxman DJ, O’Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol. 2006;20:2613–2629. doi: 10.1210/me.2006-0007. [DOI] [PubMed] [Google Scholar]
- 56.Wiwi CA, Waxman DJ. Role of hepatocyte nuclear factors in growth hormone-regulated, sexually dimorphic expression of liver cytochromes P450. Growth Factors. 2004;22:79–88. doi: 10.1080/08977190410001715172. [DOI] [PubMed] [Google Scholar]
- 57.Ling G, Sugathan A, Mazor T, Fraenkel E, Waxman DJ. Unbiased, genome-wide in vivo mapping of transcriptional regulatory elements reveals sex differences in chromatin structure associated with sex-specific liver gene expression. Mol Cell Biol. 2011;30:5531–5544. doi: 10.1128/MCB.00601-10. [DOI] [PMC free article] [PubMed] [Google Scholar]