Abstract
Background
All the relevant risk factors contributing to breast cancer etiology are not fully known. Exposure to organochlorine pesticides has been linked to an increased incidence of the disease, although not all data have been consistent. Most published studies evaluated the exposure to organochlorines individually, ignoring the potential effects exerted by the mixtures of chemicals.
Methods
This population-based study was designed to evaluate the profile of mixtures of organochlorines detected in 103 healthy women and 121 women diagnosed with breast cancer from Gran Canaria Island, and the relation between the exposure to these compounds and breast cancer risk.
Results
The most prevalent mixture of organochlorines among healthy women was the combination of lindane and endrin, and this mixture was not detected in any affected women. Breast cancer patients presented more frequently a combination of aldrin, dichlorodiphenyldichloroethylene (DDE) and dichlorodiphenyldichloroethane (DDD), and this mixture was not found in any healthy woman. After adjusting for covariables, the risk of breast cancer was moderately associated with DDD (OR = 1.008, confidence interval 95% 1.001-1.015, p = 0.024).
Conclusions
This study indicates that healthy women show a very different profile of organochlorine pesticide mixtures than breast cancer patients, suggesting that organochlorine pesticide mixtures could play a relevant role in breast cancer risk.
Keywords: Breast cancer risk, Organochlorine pesticides mixtures, Dichlorodiphenyldichloroethane (DDD)
Background
Breast cancer (BC) is the most common cancer diagnosed in women worldwide [1]. In Spain, BC is currently the most significant cause of death from malignancies in women [2]. Whereas BC mortality and incidence are lower in mainland Spain than in other European countries, there are alarming rates of mortality because of this type of cancer in the archipelago of the Canary Islands, specifically in Gran Canaria Island (Figure 1) [3,4].
Figure 1.
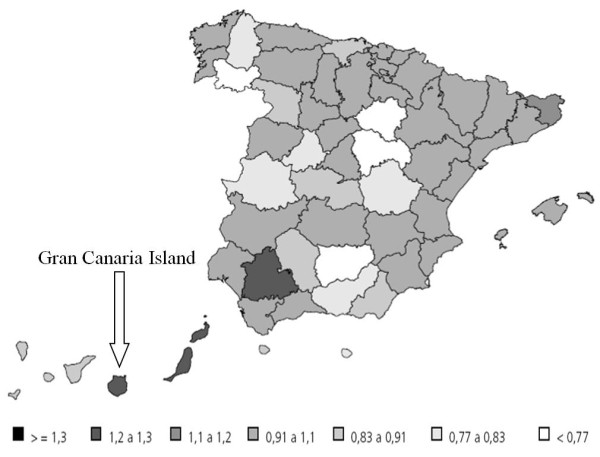
Geographic distribution of mortality for breast cancer among women in Spain during the period between the years 1996 to 2000. Numbers are referred to deaths per 100.000 inhabitants. (http://www.isciii.es/htdocs/centros/epidemiologia/libros/cancermsc.pdf).
The etiology of BC is complex, with genetic, epigenetic and environmental factors contributing to the development of the disease. BC risk is significantly influenced by genetics, but over 70% of the women that are diagnosed have sporadic cancer or tumours not associated with inheritance of any major identified high risk genes. It is thought that the risk of BC can be modified by lifestyle and environment. Besides the genetic influence, the most established factors contributing to BC are related to cumulative exposure of the breast tissue to endogenous estrogens [5]. Thus, early menarche, late age at first pregnancy, nulliparity, lactation, years of reproductive life, hormonal contraception and hormone replacement therapy have been associated to an estrogenic environment and implicated as BC risk factors. Because all the relevant risk factors contributing to breast cancer etiology are not fully known, other environmental factors have been studied during the past decade, including smoking habits [6], alcohol intake [7], obesity, diet [8,9], and exposure to environmental contaminants [10,11]. Specifically, exposure to organohalogenated contaminants has been linked to BC etiology. Among environmental contaminants linked to BC, most studies have focused in organochlorine pesticides (OCPs) due to their endocrine-disrupting properties (estrogenic or antiandrogenic effects) observed both in in vivo and in vitro studies [12,13], and to their biochemical characteristics (high lipophilic and resistant to biotransformation) that result in their accumulation in body fat. In fact, their persistence in the environment leads to bioaccumulation in animals and also to biomagnification in the food chain [14], resulting in the bioaccumulation of these chemicals in the human body, including adipose tissue, fatty tissue, breast milk, or serum [15-18]. Their long half-lives in human tissues make them a problem for up to several decades [19-21]. Due to such circumstances, OCPs were banned in the 1970s in most Western countries [22,23]. A number of OCPs such as dichlorodiphenyltrichloroethane (DDT), aldrin or dieldrin, as well as its metabolites, are considered as xenoestrogens [16,24] and have been linked to environment-induced breast cancer [16,18,20]. Because dichlorodiphenyldichloroethylene (DDE, the major metabolite of DDT) is the most prevalent organochlorine residue found in human tissues [16,18,25], most studies have focused in the potential role played by DDE as a risk factor for BC. However, epidemiologic studies linking organochlorine pesticides (mainly DDT-derivative pesticides) to BC risk have been controversial. Although several earlier studies suggested a positive association [20,26-29], other studies showed no increased risk [30-32]. In any case, it has to be highlighted that most studies focused in the study of only one compound, and they did not take into account that human beings are exposed simultaneously to multiple OCPs, and that the biological effects exerted by the mixture of OCPs vary considerably from those exerted by any OCP individually [33,34].
The level of contamination by OCPs showed by the general population of the Canary Islands has been extensively studied. Despite the fact that most OCPs pesticides were banned in Spain in the late 1970’s, our results have already shown that the people living in the Canary Islands presented a relatively high degree of contamination by OCPs (including DDT and its derivatives, aldrin dieldrin, endrin, and lindane). Furthermore, such results seemed to indicate the existence of chronic exposure to OCPs that persisted in the late 1990’s [23,35].
Bearing in mind the alarming rates of mortality by BC among women from the Canary Islands (Spain) and more specifically in women from Gran Canaria Island [2,4], the aim of our study was to compare the profile of mixtures of OCPs detected in women diagnosed with BC living in Gran Canaria Island with that detected in healthy women selected among participants from our previous population-based studies, and secondarily to evaluate whether OCPs´ exposure could be considered as a risk factor for BC in such population.
Methods
Study group
Adult healthy women from Gran Canaria Island (n = 103) were selected among the representative population-sample obtained in the Canary Islands Nutrition Survey (ENCA 1998) previously evaluated regarding their OCPs serum levels. The characteristics and methodology of this Nutritional Survey and all the data about the level of contamination by OCPs showed by the population of this Archipelago have been previously published [23,35,36].
On the other hand, women with a histological confirmed first diagnosis of breast cancer (n = 121) were recruited between April 1999 and June 2001 from the two University Hospitals of Gran Canaria Island [37]. Cases and controls were selected according to place of residence (Gran Canaria Island). The characteristics of the studied population are shown in Table 1. The local ethics committee approved the design of this study, and informed consent was obtained from all the participants.
Table 1.
Characteristics of the study population
| |
Healthy women (n = 103) |
Patients (n = 121) |
P |
|---|---|---|---|
| Age (years) Mean ± SD | 45.3 ± 13.8 | 58.0 ± 11.7 | <0.001 |
| < 35 |
31 (30.1%) |
2 (1.7%) |
<0.001 |
| 35-44.9 |
23 (22.3%) |
14 (11.6%) |
|
| 45-54.9 |
23 (22.3%) |
35 (28.9%) |
|
| 55-64.9 |
17 (16.6%) |
34 (28.1%) |
|
| >65 |
9 (8.7%) |
36 (29.7%) |
|
| BMI (kg/m2) Mean ± SD |
26.3 ± 4.3 |
27.7 ± 4.8 |
0.031 |
| Lactation |
|
|
|
| ≤ 8 months |
64 (84.2%) |
63 (76.8%) |
n. s. |
| >9 months |
12 (15.8%) |
19 (23.2%) |
|
| Smoking |
|
|
|
| Non-smokers |
70 (70.7%) |
95 (79.8%) |
n. s. |
| Smokers/Exsmoker |
29 (29.3%) |
24 (20.2%) |
|
| Menopause |
|
|
|
| Yes |
6 (5.8%) |
22 (18.2%) |
0.024 |
| No |
77 (74.8%) |
98 (81%) |
|
| ND | 20 (19.4) | 1 (0.8%) |
Abbreviations: BMI, body mass index; SD, standard deviation; ND, not determined;
n. s., non-significant.
Sample collection
Approximately 40 ml of blood was collected from each individual by venipuncture in a vacuum system tube, refrigerated, and centrifuged at 4°C (15 min x 3,000 rpm) within 4 hours to obtain serum. Serum was distributed in aliquots of 2–3 ml and immediately frozen at −80°C. The serum aliquots were used to determine biochemical (including lipid profile) and nutritional parameters. Once these initial analyses were completed, the remaining serum was kept frozen for further chemical analyses. All collection and handling equipment in contact with serum specimens were tested for possible OCP contamination. No contaminating material was identified.
Analytical methods
The OCPs residues measured in serum were: DDT isomers 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane (p,p´-DDT), 1,1,1-trichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl)ethane (o,p´-DDT); DDT metabolites 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (p,p´-DDE), 1,1-dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl)ethylene (o,p´-DDE), 1,1-dichloro-2,2-bis(p-chlorophenyl)ethane (p,p´-DDD), and 1,1-dichloro-2,(o-chlorophenyl)-2-(p-chlorophenyl)ethane (o,p´-DDD); the cyclodienes aldrin, dieldrin, and endrin; and the γ-isomer of hexachlorocyclohexane (lindane).
Chemical analysis procedure has been previously reported [23,35]. Briefly, quantitative analyses of the OCPs were undertaken on a gas-chromatography (GC)-electron capture detector (63Ni) (HP6890 with HP2397A μECD, Agilent Technologies Inc., CA, USA). A 5% phenyl methyl siloxane capillary column of 30 m x 0.22 mm i.d. x 0.25 μm (HP5 Supelco Co., Sigma-Aldrich Co., PA, USA) was used throughout the analyses. The GC operating conditions were as follows: carrier gas helium and argon-methane (95 + 5) as make-up gas at a flow rate of 1.5 ml/minute; pulsed splitless injection; an initial temperature of 50°C was held for 2 minutes, then the temperature was increased to 200°C at a rate of 30°C/minute, and finally to 280°C at a rate of 5°C/minute. The analytical limit of detection was 1 ppb (ng/g fat) for all the analytes. The accuracy of the analytical procedure was checked by using reference solutions of pure analytical grade.
The concentration of serum OCPs obtained in the chromatographic analysis was lipid-adjusted because lipid-adjusted serum concentrations of lipophilic compounds give a better estimation of the burden [38]. Total cholesterol and triglycerides were measured with an automatic Hitachi Analyzer 717 (Boehringer Manheim, IN, USA). Estimation of total serum lipids were calculated as previously reported [39,40]:
TL (total lipids) = 2.27 TC (total cholesterol) + TG (triglycerides) + 62.3
Due to the fact that o,p’-DDT, o,p’-DDE, and o,p’-DDD were detected in a small percentage of samples, they were not considered throughout this work. Thus, in this study we refer to p,p’- isomers of DDT, DDE and DDD as DDT, DDE and DDD, respectively. Similarly, we express the total DDT body burden as the sum of the three OCP-DDTs isomers more frequently measured (p,p´-DDT, p,p´-DDE, and p,p´-DDD); the total cyclodienes body burden (total Cyclodienes) as the sum of the three cyclodienes pesticides measured (aldrin, dieldrin and endrin); and the total OCPs body burden as the sum of the most frequently detected OCPs and metabolites measured (p,p’-DDT, p,p’-DDE, p,p’-DDD, aldrin, dieldrin, endrin, and lindane).
Statistical analysis
In our sample, the distribution of OCPs deviates significantly from normality. For this reason, non-parametric tests were employed. Thus, Mann–Whitney U-test was used to explore differences in OCPs levels between groups. Chi square test was used to compare differences in categorical variables. Correlation between OCP levels and continuous variables was analyzed with the Spearman’s correlation test. In order to explore the possibility that other factors could be determinants to OCPs serum levels, multivariate analysis (binary logistic regression) test was used, adjusting for the well-known confounding variables, such as age, body mass index (BMI), menopausal status, lactation and smoking habits. All test were two sided and considered as significant if P < 0.05.
Results
The studied population included 103 healthy women and 121 women diagnosed of BC, all of them from Gran Canaria Island. Healthy women were younger than BC patients (p < 0.001). Additionally, BMI values were higher in BC patients than in healthy women (p = 0.031). Related to menopausal status, most women from both groups were premenopausal women (74.8 and 81% in healthy and affected women, respectively). We observed statistical differences regarding to the distribution of menopausal status in the population (p = 0.024). We did not observe statistical differences between groups regarding to other demographical variables (Table 1).
A clear correlation between age and OCPs serum levels was evident in the group of healthy women. Thus, a positive relation between age and p,p´-DDE serum values was evident (r = 0.320; p = 0.001), while serum values of aldrin (r = −0.345; p < 0.001) and lindane (r = −0.242; p = 0.014) were negatively related to age. On the contrary, we did not observe these associations in the group of women affected of BC.
All analyzed samples had detectable residues of any OCP; samples from BC patients had a higher number of residues (an average of 3.66 different OCP residues per sample; range: 1–6; median: 4) than samples from healthy women (mean 3.25 OCPs per sample; range 2–7; median 3) (p = 0.008).
As shown in Table 2, DDE (the main DDT-metabolite) was detected in most samples from healthy women and BC patients (89 and 97%, respectively). The other DDT-metabolite, DDD, was also found in a high number of serum samples (72%) from women suffering BC, but it was detected in a small number of healthy subjects (6%) (p < 0.001). Interestingly, the main component of technical DDT (p,p´-DDT), used in commercial formulations of DDT and banned in Spain in last 1970’s, was present in more than 70% of the samples from both groups of women. With respect to non-DDT-derivatives, we observed discrepant results. Thus, despite the fact that the presence of residues of lindane and dieldrin was similar in both groups of women (more than 20% in both cases), the percentage of samples showing detectable levels of aldrin was clearly higher in BC patients than in healthy women (74 vs. 38%; p < 0.001). On the contrary, samples from healthy women showed a high presence of residues of endrin compared with samples from affected women (58 vs. 0%, respectively) (Table 2). Interestingly, these differences were observed also in the subgroup of subjects older than 45 years old (Table 3). The relative prevalence of OCPs in both groups of women is summarized in Figure 2.
Table 2.
Levels of organochloride residues (ng/g lipid) detected in serum samples in healthy women and in women diagnosed from breast cancer
| |
Healthy women (n =103) |
Breast cancer women (n =121) |
|
||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (p5-p95) | D (%) | Mean ± SD | Median (p5-p95) | D (%) | P | |
| Lindane |
24.7 ± 43.0 |
0.0 (0.0-111.4) |
28 (27.2%) |
53.2 ± 88.8 |
0.0 (0.0-220.0) |
33 (27.3%) |
n.s.a n.s.b |
| Aldrin |
27.1 ± 37.9 |
0.0 (0.0-100.1) |
39 (37.9%) |
72.5 ± 107.2 |
75.8 (0.0-116.4) |
90 (74.4%) |
<0.001a <0.001b |
| Dieldrin |
9.5 ± 16.4 |
0.0 (0.0-46.2) |
33 (32.0%) |
12.6 ± 29.5 |
0.0 (0.0-72.0) |
27 (22.3%) |
n.s.a n.s.b |
| Endrin |
231.4 ± 444.4 |
29.1 (0.0-1279.0) |
60 (58.3%) |
- |
- |
0 (0%) |
n.a.a n.a.b |
| Total Cyclodienes |
268.1 ± 443.3 |
91.4 (0.0-1297.8) |
81 (78.6%) |
85.2 ± 117.7 |
79.9 (0.0-156.1) |
95 (78.5%) |
n.sa 0.027b |
| p,p-DDE |
198.0 ± 207.5 |
167.7 (45.0-706.0) |
92(89.3%) |
357.4 ± 326.0 |
300.1 (106.1-653.3) |
118 (97.5%) |
0.012a <0.001b |
| p,p-DDD |
21.8 ± 131.2 |
0.0 (0.0-129.2) |
6 (5.8%) |
440.3 ± 412.7 |
551.1 (0.0-1108.2) |
87 (71.9%) |
<0.001a <0.001b |
| p,p-DDT |
319.5 ± 417.4 |
217.0 (0.0-1428.6) |
77 (74.8%) |
149.3 ± 111.7 |
153.0 (0.0-327.9) |
88 (72.7%) |
n.s.a <0.001b |
| Total DDTs |
880.8 ± 971.9 |
665.0 (69.1-3622.0) |
103 (100%) |
1049.6 ± 679.5 |
979.3 (159.2-2228.7) |
121 (100%) |
n.s.a 0.001b |
| Total OCPs | 1173.6 ±1339.9 | 706.0 (242.7-5263.6) |
103 (100%) | 1188.1 ± 657.2 | 1112.8 (184.2-2375.9) |
121 (100%) | n.s.a 0.001b |
Abbreviations: SD, standard deviation; D, detected compound; p5-p95, percentiles 5 and 95 of the distribution; n.s., non-significant; n.a., not applicable.
aχ2 test.
b Mann–Whitney U-test.
Table 3.
Levels of organochloride residues (ng/g lipid) detected in serum samples in healthy women and in women diagnosed from breast cancer older than 45 years old
| |
Healthy women (n = 49) |
Breast cancer women (n =105) |
|
||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (p5-p95) | D (%) | Mean ± SD | Median (p5-p95) | D (%) | P | |
| Lindane |
16.6 ± 34.6 |
0.0 (0.0-104.5) |
10 (20.4%) |
49.5 ± 85.4 |
0.0 (0.0-215.2) |
27 (25.7%) |
n.s.a n.s.b |
| Aldrin |
15.0 ± 27.5 |
0.0 (0.0-73.2) |
12 (24.5%) |
71.7 ± 114.3 |
74.8 (0.0-115.7) |
76 (72.4%) |
<0.001 a <0.001 b |
| Dieldrin |
7.0 ± 16.0 |
0.0 (0.0-49.7) |
10 (20.4%) |
12.2 ± 30.0 |
0.0 (0.0-71.7) |
22 (21.0%) |
n.s.a n.s.b |
| Endrin |
234.2 ± 466.0 |
0.0 (0.0-1553.1) |
24 (49.0%) |
- |
- |
0 (0%) |
n.a.a n.a.b |
| Total Cyclodienes |
256.1 ± 463.6 |
46.5 (0.0-1553.1) |
33 (67.3%) |
83.9 ± 125.2 |
77.5 (0.0-156.7) |
80 (76.2%) |
n.s.a n.s.b |
| p,p-DDE |
275.6 ± 266.2 |
220.8.7 (0.0-653.1) |
46 (93.9%) |
354.5 ± 327.3 |
303.8 (106.1-648.8) |
102 (97.1%) |
n.s.a 0.003b |
| p,p-DDD |
8.6 ± 38.3 |
0.0 (0.0-94.3) |
3 (6.1%) |
412.4 ± 412.1 |
126.5.1 (0.0-1094.7) |
72 (68.6%) |
<0.001a <0.001b |
| p,p-DDT |
324.8 ± 395.5 |
205.4 (0.0-1334.6) |
40 (81.6%) |
153.2 ± 114.0 |
158.7 (0.0-327.3) |
76 (72.4%) |
n.s.a 0.008b |
| Total DDTs |
937.3 ± 824.2 |
706.0 (133.2-3017.4) |
49 (100%) |
1022.7 ± 684.7 |
892.7 (143.0-2225.8) |
105 (100%) |
n.s.a n.s.b |
| Total OCPs | 1210.1 ± 1243.6 | 729.7 (267.9-4570.5) |
49 (100%) | 1156.0 ± 661.1 | 1057.3 (181.7-2364.8) |
105 (100%) | n.s.a 0.043b |
Abbreviations: SD, standard deviation; D, detected compound; p5-p95, percentiles 5 and 95 of the distribution; n.s., non-significant; n.a., not applicable.
aχ2 test.
b Mann–Whitney U-test.
Figure 2.
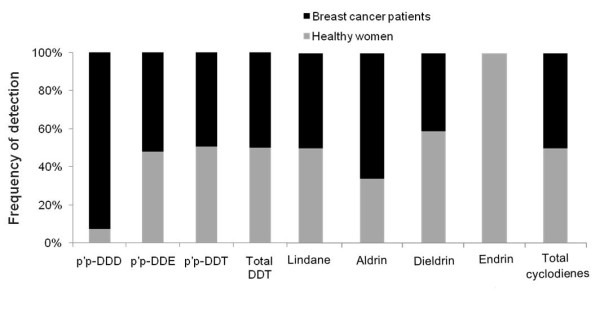
Frequency of detection of organochlorine pesticides among breast cancer patients and healthy women.
As shown in Table 2 median value of DDT was higher among healthy women than in affected women (217 vs. 153 ng/g lipid, respectively; p < 0.001). However, regarding to DDE and DDD the residue levels were higher among BC patients (p < 0.001 for both cases). As a consequence, serum samples from affected women showed higher median values of total DDT body burden (around 980 ng/g fat) than those values found in healthy women (665 ng/g fat) (p = 0.001). On the contrary, total cyclodienes body burden was higher in healthy women than in serum samples from BC patients (p = 0.027).
Nevertheless, if we consider the total OCPs residues as the sum of the three DDT-derivatives measured and the sum of the three cyclodienes measured plus lindane, we could observe that median levels of total OCP compounds were higher in BC subjects than in healthy women (p = 0.001) (Table 2). Moreover, this difference was also observed in the subgroup of healthy women and BC patients older than 45 years old (p = 0.043) (Table 3).
As stated previously, among BC patients, 30 subjects (24.8%) presented a combination of aldrin, DDE, and DDD, followed by DDD alone and DDD and DDE (10 subjects). Interestingly, we did not observe overlaps between these OCP-mixtures among both groups of women, that is: no healthy subject had combination of residues of aldrin plus DDE plus DDD, and no BC patient had combination of residues of lindane plus endrin.
Finally, since age, BMI and menopausal status seem to play an important role in BC etiology [41], and having into account that these parameters and others (such as lactation, and smoking habits) seem to be important determinants of the levels of contamination by OCPs [23,35,42], we carried out a multivariate analysis, including all the pesticides simultaneously in the model and adjusting by age, BMI, menopause status, lactation and smoking habits; in order to evaluate the existence of any association between OCPs´ exposure and a BC risk. As shown in Table 4, among the OCPs evaluated, only p,p’-DDD showed a moderate increment in the risk of developing BC (odds ratio 1.008 [95%CI, 1.001-1.015], p = 0.024).
Table 4.
Binary logistic regression of pesticide in multivariate analysis adjusted by age, BMI, menopausal status, lactation and smoking habits
| Pesticide |
Odds ratio, 95% CI |
P |
|---|---|---|
| Healthy women | (Ref. category) | |
| Lindane |
1.097 (0.420-28.412) |
0.988 |
| Aldrin |
1.027 (0.991-1.065) |
0.147 |
| Dieldrin |
1.002 (0.956-1.050) |
0.927 |
| Endrin |
N.A. |
|
|
p,p’-DDD |
1.008 (1.001-1.015) |
0.024 |
|
p,p’-DDE |
0.999 (0.996-1.001) |
0.284 |
| p,p’-DDT | 0.994 (0.987-1.002) | 0.139 |
Abbreviations: 95% CI, 95% confidence interval; N.A., not applicable.
Discussion
Although the vast majority of epidemiologic studies do not support the association between BC and OCPs [15,20,32,43,44], the well-known association between BC and prolonged exposure to estrogens suggests that environmental estrogens, may play a critical role in the cellular and molecular changes that occur during breast carcinogenesis [45]. In this sense, most OCPs are considered as xenoestrogens and may modulate steroid sex hormones homeostasis, such as estrogen or testosterone, as agonists or antagonists or as mixed effects [46]. In fact, there are a number of studies that seem to indicate that the association between BC and OCPs could exist [27,47].
The best known and studied OCP with respect to its role in breast cancer is the main DDT-metabolite, DDE. DDT is converted to DDE, that apparently does not undergo further biotransformation, and it is stored for an indefinite period of time in adipose tissues. Due to this fact, this compound is the highest prevalent DDT-derivative found in human beings [18]. On the other hand, the major detoxification pathway of DDT is via dechlorination to DDD. The population of the Canary Islands presents similar serum levels of DDT and DDE to those found in other European countries [23]. In the present study, serum levels of DDE were higher in BC patients than in healthy women, although, after a multivariate analysis, DDE do not seem to be a risk factor for BC. These results agree with those reported previously [32]. However, there are other highly prevalent OCPs (DDT- and non-DDT-derivatives) in female populations at concentrations highly enough to exert biological effects on breast cells [34]. In this sense, our findings demonstrating that serum levels of DDD were higher among BC patients than in non-affected women and that exposure to DDD could represent, at least, a moderately risk factor for developing BC (OR = 1.008), point to the possibility that other less evaluated OCPs, such as DDD, could play a more relevant role as risk factor for BC.
We reported that background exposure to non-DDT-derivative-OCPs was higher in non-affected women than in BC patients. This result was mainly due to the fact that endrin was not detected in any sample from BC patients, while residues of endrin were present in more than 58% of the samples from healthy women at median concentrations higher than 29 ng/g fat. On the contrary, the presence of residues of aldrin was higher in serum samples from BC patients than in healthy women. Thus, depending on the specific subclass of OCP, the environmental exposure to these contaminants might be related or not with the etiology of BC. Our results showing that DDD could be considered as risk factors for BC must be taken with caution due to the several limitations of our study. Firstly, controls were originally selected for a different purpose (ENCA survey) than analyzed in this study. The use of controls from a nutritional survey may introduce bias if the probability of selection into the study is associated with factors that influence both the prevalence of exposure and the probability of disease status. One such factor is age, which was statistically different among groups of subjects (47.6% of healthy women were older than 45 years old while 86.8% of BC patients were in this age subgroup); together with BMI or menopause status (both variables also considered as BC risk factors and important for the prevalence of exposure to OCPs). Secondly, several established risk factors for breast cancer (mainly family history of breast cancer, reproductive factors (such as number of children or duration of lactation), or alcohol intake) were not available nor in controls nor in BC patients, and, as a consequence, unmeasured or residual confounding factors have not been included in the analyses. Thirdly, because these chemicals are stored in fat, it is believed that adipose tissue loss could result in increased \organ and blood concentrations of these compounds [48]. Thus, if changes in body weight occur as a consequence of the development of cancers, the levels of OCPs measured in this study may not reflect the background exposure to these chemicals for both groups of women. In addition, body fat is an important risk factor for breast cancer [49]. Although we adjusted for BMI in our analyses, BMI as a measure of body fat has certain limitations [50]. To make matters worse, because serum concentrations of OCPs are highly correlated with each other and can be correlated with unmeasured substances, the associations observed here might not always have represented the direct effect of the OCPs measured.
It is assumed that the biological effects exerted by environmental contaminants on human tissues, taken individually, clearly differ from the effects exerted by their combinations. However, most studies about environmental contaminants as risk factors for BC have focused on single-chemicals. Currently, it seems clear the importance of the exposure to chemical mixtures and their contribution to the disease by causing cellular-level dysfunction along key pathways. In this sense, we have recently reported that DDD, DDE, aldrin, and dieldrin, sharply upregulated the expression of a number of protein kinases genes that could be involved in the etiology of BC, such as ACVRL1, ALK-1, KIT, ERBB3, and ALK-1, at concentrations close to those detected in human populations [34]. Additionally, it has to be taken into account that the potential effects exerted on breast cells by environmental contaminants could be due not only to the organochlorine pesticides measured in the present study, even more so other environmental pollutants not measured could be implicated. Complex interactions between chemicals, endogenous or exogenous hormones, and their natural ligands and receptors may alter the internal homeostasis of the estrogenic environment of mammary tissue, leading to malignant transformation and cancer. Our findings agree with those observed in vitro by Aubé (2011) suggesting that aldrin and DDD, together with other OCP compounds, increased the proliferation of MCF-7 cells [51]. However, to our knowledge, population-based studies evaluating the role of OCPs mixtures in relation to breast cancer are scarce [52]. Our findings showing the lack of overlaps between the profiles of mixtures of OCPs among healthy and affected women suggest a relevant role of these chemical mixtures in relation to the disease. Future studies achieving the association between environmental contaminants and BC should analyze the combined effect of these compounds and the interactions with endogenous hormones and other substances that affect endocrine function.
Conclusions
In conclusion, our results indicate that serum concentrations of OCPs were higher in BC patients than in healthy women in this specific sample of the Spanish population, and suggest that the combination of certain OCPs could be highly relevant in the potential role played by these chemicals as risk factors for BC. More specifically, our results seem to indicate that the mixture of aldrin plus DDE plus DDD, may play a relevant role of as a potential environmental factor related to BC. In this context, despite the controversial and complex issues involved with continued exposure to residues of OCPs through the environment and food, their potential role as risk factor for BC, should be considered by Public Health Authorities in countries where BC alarming rates of BC incidence or mortality exists.
Abbreviations
BC, Breast cancer; BMI, Body mass index; DDT, Dichlorodiphenyltrichloroethane; DDE, Dichlorodiphenyldichloroethylene; DDD, Dichlorodiphenyldichloroethane; OCPs, Organochlorine pesticides.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Authors’ contribution
LDB has made substantial contributions to conception and design, has participate in analysis and interpretation of data, has been involved in drafting the manuscript and has given final approval of the version to be published; MZ has made substantial contributions to conception and design and has been involved in acquisition of data; LAHH has participate in analysis and interpretation of data and has been involved in drafting the manuscript; MAG has been involved in acquisition of data; EEAL has participate in analysis and interpretation of data; LSM has revised the manuscript critically for important intellectual content; OPL has made substantial contributions to conception and design and has been involved in acquisition of data and has revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Contributor Information
Luis D Boada, Email: ldominguez@dcc.ulpgc.es.
Manuel Zumbado, Email: mzumbado@dcc.ulpgc.es.
Luis Alberto Henríquez-Hernández, Email: lhenriquez@dcc.ulpgc.es.
Maira Almeida-González, Email: maira.almeida.gonzalez@gmail.com.
Eva E Álvarez-León, Email: ealvleo@gobiernodecanarias.org.
Lluis Serra-Majem, Email: lserra@dcc.ulpgc.es.
Octavio P Luzardo, Email: operez@dcc.ulpgc.es.
Acknowledgments
The authors acknowledge the working group of the Nutrition Survey of the Canary Islands (ENCA), for the collaboration in the collection of information as well as the sampling and storage of the serum samples.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Cabanes A, Vidal E, Aragones N, Perez-Gomez B, Pollan M, Lope V, Lopez-Abente G. Cancer mortality trends in Spain: 1980–2007. Ann Oncol. 2010;21(Suppl 3:iii):14–20. doi: 10.1093/annonc/mdq089. [DOI] [PubMed] [Google Scholar]
- Cabanes A, Vidal E, Perez-Gomez B, Aragones N, Lopez-Abente G, Pollan M. Age-specific breast, uterine and ovarian cancer mortality trends in Spain: changes from 1980 to 2006. Canc Epidemiol. 2009;33:169–175. doi: 10.1016/j.canep.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Lopez-Abente G, Pollan M, Aragones N, Perez Gomez B, Hernandez Barrera V, Lope V, Suarez B. [State of cancer in Spain: incidence] An Sist Sanit Navar. 2004;27:165–173. doi: 10.4321/s1137-66272004000300001. [DOI] [PubMed] [Google Scholar]
- Chen WY. Exogenous and endogenous hormones and breast cancer. Best Pract Res Clin Endocrinol Metab. 2008;22:573–585. doi: 10.1016/j.beem.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KC, Miller AB, Collishaw NE, Palmer JR, Hammond SK, Salmon AG, Cantor KP, Miller MD, Boyd NF, Millar J, Turcotte F. Active smoking and secondhand smoke increase breast cancer risk: the report of the Canadian Expert Panel on Tobacco Smoke and Breast Cancer Risk (2009) Tob Control. 2011;20:e2. doi: 10.1136/tc.2010.035931. [DOI] [PubMed] [Google Scholar]
- Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006;7:149–156. doi: 10.1016/S1470-2045(06)70577-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez CA, Riboli E. Diet and cancer prevention: Contributions from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Cancer. 2010;46:2555–2562. doi: 10.1016/j.ejca.2010.07.025. [DOI] [PubMed] [Google Scholar]
- Jevtic M, Velicki R, Popovic M, Cemerlic-Adjic N, Babovic SS, Velicki L. Dietary influence on breast cancer. J BUON. 2010;15:455–461. [PubMed] [Google Scholar]
- Laden F, Hunter DJ. Environmental risk factors and female breast cancer. Annu Rev Public Health. 1998;19:101–123. doi: 10.1146/annurev.publhealth.19.1.101. [DOI] [PubMed] [Google Scholar]
- Salehi F, Turner MC, Phillips KP, Wigle DT, Krewski D, Aronson KJ. Review of the etiology of breast cancer with special attention to organochlorines as potential endocrine disruptors. J Toxicol Environ Health B Crit Rev. 2008;11:276–300. doi: 10.1080/10937400701875923. [DOI] [PubMed] [Google Scholar]
- Gellert RJ, Heinrichs WL, Swerdloff RS. DDT homologues: estrogen-like effects on the vagina, uterus and pituitary of the rat. Endocrinology. 1972;91:1095–1100. doi: 10.1210/endo-91-4-1095. [DOI] [PubMed] [Google Scholar]
- Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Serrano FO. The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ Health Perspect. 1995;103(Suppl 7):113–122. doi: 10.1289/ehp.95103s7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe SH. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol. 1994;24:87–149. doi: 10.3109/10408449409049308. [DOI] [PubMed] [Google Scholar]
- Zheng T, Holford TR, Mayne ST, Ward B, Carter D, Owens PH, Dubrow R, Zahm SH, Boyle P, Archibeque S, Tessari J. DDE and DDT in breast adipose tissue and risk of female breast cancer. Am J Epidemiol. 1999;150:453–458. doi: 10.1093/oxfordjournals.aje.a010033. [DOI] [PubMed] [Google Scholar]
- Snedeker SM. Pesticides and breast cancer risk: a review of DDT, DDE, and dieldrin. Environ Health Perspect. 2001;109(Suppl 1):35–47. doi: 10.1289/ehp.01109s135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covaci A, Jorens P, Jacquemyn Y, Schepens P. Distribution of PCBs and organochlorine pesticides in umbilical cord and maternal serum. Sci Total Environ. 2002;298:45–53. doi: 10.1016/S0048-9697(02)00167-5. [DOI] [PubMed] [Google Scholar]
- Jaga K, Dharmani C. Global surveillance of DDT and DDE levels in human tissues. Int J Occup Med Environ Health. 2003;16:7–20. [PubMed] [Google Scholar]
- Hunter DJ, Hankinson SE, Laden F, Colditz GA, Manson JE, Willett WC, Speizer FE, Wolff MS. Plasma organochlorine levels and the risk of breast cancer. N Engl J Med. 1997;337:1253–1258. doi: 10.1056/NEJM199710303371801. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Zeleniuch-Jacquotte A, Dubin N, Toniolo P. Risk of breast cancer and organochlorine exposure. Canc Epidemiol Biomarkers Prev. 2000;9:271–277. [PubMed] [Google Scholar]
- Seegal RF, Fitzgerald EF, Hills EA, Wolff MS, Haase RF, Todd AC, Parsons P, Molho ES, Higgins DS, Factor SA. et al. Estimating the half-lives of PCB congeners in former capacitor workers measured over a 28-year interval. J Expo Sci Environ Epidemiol. 2011;21:234–246. doi: 10.1038/jes.2010.3. [DOI] [PubMed] [Google Scholar]
- Bolt HM, Degen GH. Comparative assessment of endocrine modulators with oestrogenic activity. II. Persistent organochlorine pollutants. Arch Toxicol. 2002;76:187–193. doi: 10.1007/s00204-002-0336-8. [DOI] [PubMed] [Google Scholar]
- Zumbado M, Goethals M, Alvarez-Leon EE, Luzardo OP, Cabrera F, Serra-Majem L, Dominguez-Boada L. Inadvertent exposure to organochlorine pesticides DDT and derivatives in people from the Canary Islands (Spain) Sci Total Environ. 2005;339:49–62. doi: 10.1016/j.scitotenv.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker MP, Rogan WJ, Lucier G. The human health effects of DDT (dichlorodiphenyltrichloroethane) and PCBS (polychlorinated biphenyls) and an overview of organochlorines in public health. Annu Rev Publ Health. 1997;18:211–244. doi: 10.1146/annurev.publhealth.18.1.211. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Toniolo PG. Environmental organochlorine exposure as a potential etiologic factor in breast cancer. Environ Health Perspect. 1995;103(Suppl 7):141–145. doi: 10.1289/ehp.95103s7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Toniolo PG, Lee EW, Rivera M, Dubin N. Blood levels of organochlorine residues and risk of breast cancer. J Natl Canc Inst. 1993;85:648–652. doi: 10.1093/jnci/85.8.648. [DOI] [PubMed] [Google Scholar]
- Cassidy RA, Natarajan S, Vaughan GM. The link between the insecticide heptachlor epoxide, estradiol, and breast cancer. Breast Canc Res Treat. 2005;90:55–64. doi: 10.1007/s10549-004-2755-0. [DOI] [PubMed] [Google Scholar]
- Fenton SE. Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology. 2006;147:S18–S24. doi: 10.1210/en.2005-1131. [DOI] [PubMed] [Google Scholar]
- Stellman SD, Djordjevic MV, Britton JA, Muscat JE, Citron ML, Kemeny M, Busch E, Gong L. Breast cancer risk in relation to adipose concentrations of organochlorine pesticides and polychlorinated biphenyls in Long Island, New York. Cancer Epidemiol Biomarkers Prev. 2000;9:1241–1249. [PubMed] [Google Scholar]
- Aronson KJ, Miller AB, Woolcott CG, Sterns EE, McCready DR, Lickley LA, Fish EB, Hiraki GY, Holloway C, Ross T. et al. Breast adipose tissue concentrations of polychlorinated biphenyls and other organochlorines and breast cancer risk. Canc Epidemiol Biomarkers Prev. 2000;9:55–63. [PubMed] [Google Scholar]
- Lopez-Cervantes M, Torres-Sanchez L, Tobias A, Lopez-Carrillo L. Dichlorodiphenyldichloroethane burden and breast cancer risk: a meta-analysis of the epidemiologic evidence. Environ Health Perspect. 2004;112:207–214. doi: 10.1289/ehp.112-1241830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boada LD, Lara PC, Alvarez-Leon EE, Losada A, Zumbado ML, Liminana-Canal JM, Apolinario R, Serra-Majem L, Luzardo OP. Serum levels of insulin-like growth factor-I in relation to organochlorine pesticides exposure. Growth Horm IGF Res. 2007;17:506–511. doi: 10.1016/j.ghir.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Valeron PF, Pestano JJ, Luzardo OP, Zumbado ML, Almeida M, Boada LD. Differential effects exerted on human mammary epithelial cells by environmentally relevant organochlorine pesticides either individually or in combination. Chem Biol Interact. 2009;180:485–491. doi: 10.1016/j.cbi.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Luzardo OP, Goethals M, Zumbado M, Alvarez-Leon EE, Cabrera F, Serra-Majem L, Boada LD. Increasing serum levels of non-DDT-derivative organochlorine pesticides in the younger population of the Canary Islands (Spain) Sci Total Environ. 2006;367:129–138. doi: 10.1016/j.scitotenv.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Serra-Majem L, Santana-Armas JF, Salmona E. Dietary habits and nutritional status in Spain. World Rev Nutr Diet. 2000;87:127–159. doi: 10.1159/000059725. [DOI] [PubMed] [Google Scholar]
- Garcia-Segovia P, Sanchez-Villegas A, Doreste J, Santana F, Serra-Majem L. Olive oil consumption and risk of breast cancer in the Canary Islands: a population-based case–control study. Publ Health Nutr. 2006;9:163–167. doi: 10.1079/phn2005940. [DOI] [PubMed] [Google Scholar]
- Koppen G, Covaci A, Van Cleuvenbergen R, Schepens P, Winneke G, Nelen V, van Larebeke N, Vlietinck R, Schoeters G. Persistent organochlorine pollutants in human serum of 50–65 years old women in the Flanders Environmental and Health Study (FLEHS). Part 1: Concentrations and regional differences. Chemosphere. 2002;48:811–825. doi: 10.1016/S0045-6535(02)00111-X. [DOI] [PubMed] [Google Scholar]
- Kang JH, Park H, Chang YS, Choi JW. Distribution of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in human serum from urban areas in Korea. Chemosphere. 2008;73:1625–1631. doi: 10.1016/j.chemosphere.2008.07.087. [DOI] [PubMed] [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert JT, Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18:495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- Crouse DL, Ross NA, Goldberg MS. Double burden of deprivation and high concentrations of ambient air pollution at the neighbourhood scale in Montreal, Canada. Soc Sci Med. 2009;69:971–981. doi: 10.1016/j.socscimed.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Glynn AW, Granath F, Aune M, Atuma S, Darnerud PO, Bjerselius R, Vainio H, Weiderpass E. Organochlorines in Swedish women: determinants of serum concentrations. Environ Health Perspect. 2003;111:349–355. doi: 10.1289/ehp.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward EM, Schulte P, Grajewski B, Andersen A, Patterson DG, Turner W, Jellum E, Deddens JA, Friedland J, Roeleveld N. et al. Serum organochlorine levels and breast cancer: a nested case–control study of Norwegian women. Canc Epidemiol Biomarkers Prev. 2000;9:1357–1367. [PubMed] [Google Scholar]
- Calle EE, Frumkin H, Henley SJ, Savitz DA, Thun MJ. Organochlorines and breast cancer risk. CA Cancer J Clin. 2002;52:301–309. doi: 10.3322/canjclin.52.5.301. [DOI] [PubMed] [Google Scholar]
- Ejaz S, Akram W, Lim CW, Lee JJ, Hussain I. Endocrine disrupting pesticides: a leading cause of cancer among rural people in Pakistan. Exp Oncol. 2004;26:98–105. [PubMed] [Google Scholar]
- Sonnenschein C, Soto AM. An updated review of environmental estrogen and androgen mimics and antagonists. J Steroid Biochem Mol Biol. 1998;65:143–150. doi: 10.1016/S0960-0760(98)00027-2. [DOI] [PubMed] [Google Scholar]
- Charlier C, Albert A, Herman P, Hamoir E, Gaspard U, Meurisse M, Plomteux G. Breast cancer and serum organochlorine residues. Occup Environ Med. 2003;60:348–351. doi: 10.1136/oem.60.5.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue O, Marcotte J, Berrigan F, Simoneau M, Dore J, Marceau P, Marceau S, Tremblay A, Teasdale N. Plasma concentration of organochlorine compounds is associated with age and not obesity. Chemosphere. 2007;67:1463–1467. doi: 10.1016/j.chemosphere.2006.10.033. [DOI] [PubMed] [Google Scholar]
- Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau-Roberts G, Saudny-Unterberger H, Kuhnlein HV, Egeland GM. Body mass index may overestimate the prevalence of overweight and obesity among the Inuit. Int J Circumpolar Health. 2005;64:163–169. doi: 10.3402/ijch.v64i2.17969. [DOI] [PubMed] [Google Scholar]
- Aube M, Larochelle C, Ayotte P. Differential effects of a complex organochlorine mixture on the proliferation of breast cancer cell lines. Environ Res. 2011;111:337–347. doi: 10.1016/j.envres.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Ibarluzea Jm J, Fernandez MF, Santa-Marina L, Olea-Serrano MF, Rivas AM, Aurrekoetxea JJ, Exposito J, Lorenzo M, Torne P, Villalobos M. et al. Breast cancer risk and the combined effect of environmental estrogens. Canc Causes Contr. 2004;15:591–600. doi: 10.1023/B:CACO.0000036167.51236.86. [DOI] [PubMed] [Google Scholar]


