Abstract
Background
Dorstenia mannii (Moraceae) is a medicinal herb used traditionally for the treatment of many diseases. In the present study, the methanol extract of D. mannii and nine of its isolated compounds, namely dorsmanin A (1), B (2), C (3), D (4), E (6), F (7), G (8) dorsmanin I (9) and 6,8-diprenyleriodictyol (5), were tested for their antimicrobial activities against yeast, Mycobacteria and Gram-negative bacteria.
Methods
The microplate alamar blue assay (MABA) and the broth microdilution method were used to determine the minimal inhibitory concentration (MIC) and minimal microbicidal concentration (MMC) of the above extract and compounds on a panel of bacterial species.
Results
The results of the MIC determinations demonstrated that the methanol extract as well as compounds 3 and 8 were able to prevent the growth of all the fourteen studied microorganisms within the concentration range of 4 to 1024 μg/ml. The lowest MIC value for the methanol extract (64 μg/ml) was obtained on Candida albicans. The lowest value for individual compounds (4 μg/ml) was recorded with compounds 3 on Pseudomonas aeruginosa PA01 and 7 on Eschericia coli ATCC strain. The MIC values recorded with compounds 3 on P. aeruginosa PA01, 6 on C. albicans,7 on P. aeruginosa PA01 and K. pneumoniae ATCC strain and C. albicans,and 8 on P. aeruginosa PA01, PA124, P. stuartii, M. tuberculosis MTCS1 were lower than or equal to those of the reference drugs. MMC values not greater than 1024 μg/ml were recorded on all studied microorganisms with compounds 3 and 8.
Conclusion
The overall results of the present investigation provided evidence that the crude extract of D. mannii as well as some of its compounds such compounds 3 and 8 could be a potential source of natural antimicrobial products.
Background
Many plant species of the genus Dorstenia (Moraceae) are used for medicinal purposes in Africa, Middle East, Central and South America. African Dorstenia species has yielded a variety of mono-, di-, and triprenylated and also mono- and digeranylated flavonoids with interesting pharmacological properties [1-4]. Dorstenia mannii Hook f. (Moraceae) is a perennial herb growing in the tropical rain forest of West Africa [5]. A decoction of the leaves is used for the treatment of many diseases, but mainly for rheumatism and stomach disorders [6]. There are few pharmacological studies reported on D. mannii. However, prenylated flavonoids isolated from D. mannii such as 6,8-diprenyleriodictyol (5), dorsmanin C (3) and dorsmanin F (7) were found to be potent scavengers of the stable free radical 1,1-diphenyl-2-picrylhydrazyl [7]. Compounds 3, 5 and 7 also inhibited Cu2+-mediated oxidation of human low density lipoprotein [7]. In our continuous search of bioactive compounds from the genus Dorstenia, the present work was designed to evaluate the antimicrobial potency of the methanol extract and compounds isolated from D. mannii.
Methods
Plant material and extraction
The twigs of Dorstenia mannii Hook. f. were collected at Nkoljobe mountain, Yaounde, Center region of Cameroon in March 2008. The plant was identified by Mr. Victor Nana of the National herbarium (Yaoundé, Cameroon) where a voucher specimen was deposited under the reference number 2135/HNC.
The air dried and powdered twigs (1 kg) were extracted with methanol (MeOH) for 48 h at room temperature. The extract was then concentrated under reduced pressure to give 185 g of a brown residue that constituted the crude extract (DMT).
Chemicals for antimicrobial assay
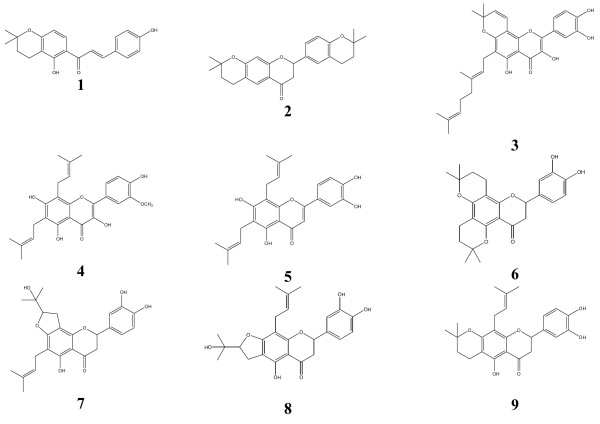
Chloramphenicol (Sigma-Aldrich, St. Quentin Fallavier, France) and Nystatin (Sigma-Aldrich) were used as reference antibiotics (RA) respectively against bacteria and Candida albicans. p-Iodonitrotetrazolium chloride (INT, Sigma-Aldrich) was used as microbial growth indicator [8,9]. Ciprofloxacin and isoniazid (INH) (Sigma) were used as reference antibiotics (RA) for M. smegmatis and M. tuberculosis respectively. The isolation and identification of compounds 1 to 9 from DMT were conducted as previously described [10-12]. The chemical structures of the isolated compounds are illustrated in Figure 1.
Figure 1.
Chemical structures of the compounds isolated from the twigs ofDortenia mannii. Dorsmanins A(1), B(2), C(3), D(4), E (6), F (7), G (8), I (9) and 6,8 diprenyleriodictyol (5).
Antimicrobial assays
Microbial strains and culture media
The studied microorganisms included strains of Providencia stuartii, Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterobacter aerogenes, Escherichia coli, Candida albicans four Mycobacteria namely M. smegmatis, drug-susceptible strain of M. tuberculosis H37Rv obtained from the American Type Culture Collection, and two clinical strains of M. tuberculosis MTCS1, MTCS2. M. smegmatis was cultured on Middlebrook 7 H11 agar and allowed to grow for 24 h. M. tuberculosis was plated on Löwenstein–Jensen medium and allowed to grow for 3–4 weeks at 37°C. Middlebrook 7 H9 broth was used to determine the MIC and MMC values of the test samples on M. smegmatis and M. tuberculosis. Nutrient Agar and Sabouraud Glucose Agar were used for the activation of Gram-negative bacteria and fungi respectively [13]. The clinical strains used in this work are our Laboratory collection previously obtained from Yaoundé General Hospital (Cameroon), and from the Mediterranean University (Marseille, France).
INT colorimetric assay for MIC and MMC determinations
The MIC determinations on M. smegmatis, fungi, and Gram-negative bacteria were conducted using rapid INT colorimetric assay according to previously described methods [8,9] with some modifications. The test samples and RA were first of all dissolved in DMSO/MHB or DMSO/7 H9 broth. The final concentration of DMSO was lower than 2.5% and does not affect the microbial growth [14]. The solution obtained was then added to 7 H9 broth (M. smegmatis) or MHB (other organisms), and serially diluted two fold (in a 96- wells microplate). 100 μl of inoculum 1.5 x 106 CFU/ml prepared in appropriate broth was then added [15]. The plates were covered with a sterile plate sealer, then agitated to mix the contents of the wells using a plate shaker and incubated at 37°C for 18 h. The assay was repeated thrice. Wells containing adequate broth, 100 μl of inoculum and DMSO to a final concentration of 2.5% served as negative control. The MIC of samples was detected after 18 h incubation at 37°C, following addition (40 μl) of 0.2 mg/ml p-iodonitrotetrazolium chloride (INT) and incubation at 37°C for 30 minutes. Viable bacteria reduced the yellow dye to a pink. MIC was defined as the sample concentration that prevented this change and exhibited complete inhibition of microbial growth. The MMC was determined by adding 50 μl aliquots of the preparations, which did not show any growth after incubation during MIC assays, to 150 μl of adequate broth. These preparations were incubated at 37°C for 48 h. The MMC was regarded as the lowest concentration of extract, which did not produce a color change after addition of INT as mentioned above [14,16].
Microplate Alamar Blue assay against M. tuberculosis
The activity of all samples against M. tuberculosis strains was tested using the MABA [17]. Briefly, each of the above M. tuberculosis strains was cultured at 37°C in Middlebrook 7 H9 broth supplemented with 0.2% glycerol and 10% Oleic Acid–Albumin–Dextrose–Catalase (Sigma) until logarithmic growth was reached. About 6x106 CFU/ml inoculum of M. tuberculosis was then added to the two fold serially diluted samples. The final concentration of DMSO in all assays was 2.5% or less and this dilution also served as solvent control. The samples were assayed in triplicate. All tests were carried out in sterile flat-bottomed 96-well microplates. Each microplate was incubated for 5 days at 37°C in a 5% CO2 atmosphere in a sealed plastic CO2-permeable bag. After 5 days of incubation, 32 μl of a mixture of freshly prepared Alamar Blue solution and 20% sterile Tween-80 (Sigma) 1:1 v/v were added to one growth-control well. The microplates were incubated again at 37°C for 24 h. If a color shift from blue to pink was observed in the growth-control sample, 32 μl of alamar blue solution was added to each of the remaining wells, and the microplate was further incubated for 24 h. A well-defined pink color was interpreted as positive bacterial growth, whereas a blue color indicated an absence of growth. The MIC corresponded to the greatest dilution of sample extract in which the color shift from blue to pink was not observed.
Samples with recorded MIC values following MABA were assayed for their mycobactericidal effect [17]. Briefly, 5 μl of the undeveloped mycobacterial suspensions were transferred from the former to a new microplate that contained 195 μl of fresh culture medium per well. Three wells were inoculated with 100 μl of fresh inoculum as for MABA and three more wells were incubated with 200 μl of culture medium only, as negative controls. The microplates were incubated and developed with alamar blue as for MABA. The MMC corresponded to the minimum sample concentration that did not cause a color shift in cultures that were re-incubated in fresh medium.
Results and discussion
The tested compounds were isolated from DMT and identified as previously described as dorsmanin A (1), B (2), C (3), D (4) and 6,8-diprenyleriodictyol (5) [10], dorsmanin E (6), F (7), G (8) [11] and dorsmanin I (9) [12]. These compounds together with the crude methanol extract were tested for their antimicrobial activities against bacteria and yeasts and the results are reported in Tables 1 and 2.
Table 1.
Minimal inhibitory concentrations (MIC in μg/ml) of the studied samples and reference antibiotics against the tested microorganisms
|
Tested samples* |
Microorganisms, strains and MIC (μg/ml) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
E. coli |
P. aeruginosa |
K. pneumoniae |
E.aerogenes |
P. stuartii |
C. albicans |
M. smegmatis |
M. tuberculosis |
||||||
| ATCC10536 | AG100 | PA01 | PA124 | ATCC11296 | KP55 | ATCC13048 | CM64 | NAE16 | ATCC 9002 | ATCC 700084 | ATCC 27294 | MTCS1 | MTCS2 | |
| DMT |
256 |
512 |
512 |
1024 |
128 |
512 |
128 |
512 |
128 |
64 |
128 |
128 |
1024 |
512 |
|
1 |
512 |
- |
- |
- |
- |
- |
128 |
- |
128 |
- |
- |
NT |
NT |
NT |
|
2 |
128 |
128 |
- |
- |
- |
- |
- |
- |
1024 |
- |
512 |
512 |
- |
- |
|
3 |
64 |
64 |
4 |
64 |
128 |
64 |
32 |
64 |
16 |
64 |
64 |
32 |
128 |
32 |
|
4 |
- |
- |
128 |
- |
512 |
- |
- |
- |
1024 |
- |
- |
NT |
NT |
NT |
|
5 |
512 |
- |
- |
- |
1024 |
- |
32 |
- |
128 |
32 |
- |
NT |
NT |
NT |
|
6 |
512 |
128 |
512 |
- |
128 |
- |
16 |
- |
256 |
8 |
- |
NT |
NT |
NT |
|
7 |
4 |
256 |
32 |
- |
8 |
64 |
16 |
- |
64 |
16 |
128 |
128 |
256 |
128 |
|
8 |
16 |
128 |
8 |
32 |
128 |
32 |
64 |
32 |
32 |
128 |
64 |
64 |
64 |
64 |
|
9 |
- |
- |
- |
- |
- |
- |
256 |
- |
256 |
32 |
128 |
256 |
- |
512 |
| RAb | 2 | 8 | 64 | 32 | 8 | 4 | 8 | 4 | 32 | 16 | 0.5 | 0.5 | 64 | 2 |
aTested samples [DMT: methanol extract from the twigs of Dorstenia mannii; dorsmanins A(1), B(2), C(3), D(4) E(6), F(7), G(8), I (9) and 6,8 diprenyleriodictyol (5); bRA : reference antibiotics were chloramphenicol for bacteria, nystatin for C. albicans, ciprofloxacin for M. smegmatis, isoniazid for M.tuberculosis; (−): MIC > 1024 μg/ml
Table 2.
Minimal microbicidal concentrations (MMC in μg/ml) of the studied samples and reference antibiotics against the tested microorganisms
|
Tested samples* |
Microorganisms, strains and MMC (μg/ml) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
E. coli |
P. aeruginosa |
K. pneumoniae |
E.aerogenes |
P. stuartii |
C. albicans |
M. smegmatis |
M. tuberculosis |
||||||
| ATCC10536 | AG100 | PA01 | PA124 | ATCC11296 | KP55 | ATCC13048 | CM64 | NAE16 | ATCC 9002 | ATCC 700084 | ATCC 27294 | MTCS1 | MTCS2 | |
| DMT |
512 |
>1024 |
1024 |
>1024 |
512 |
1024 |
512 |
>1024 |
256 |
128 |
256 |
256 |
>1024 |
>1024 |
|
1 |
>1024 |
ND |
ND |
ND |
ND |
ND |
1024 |
ND |
1024 |
ND |
ND |
NT |
NT |
NT |
|
2 |
1024 |
1024 |
ND |
ND |
ND |
ND |
ND |
ND |
>1024 |
ND |
512 |
>1024 |
ND |
ND |
|
3 |
128 |
128 |
8 |
128 |
256 |
128 |
64 |
128 |
64 |
128 |
128 |
64 |
256 |
64 |
|
4 |
ND |
ND |
1024 |
ND |
>1024 |
ND |
ND |
ND |
>1024 |
ND |
ND |
NT |
NT |
NT |
|
5 |
>1024 |
ND |
ND |
ND |
>1024 |
ND |
128 |
ND |
1024 |
64 |
ND |
NT |
NT |
NT |
|
6 |
>1024 |
>1024 |
>1024 |
ND |
256 |
ND |
64 |
ND |
>1024 |
16 |
ND |
NT |
NT |
NT |
|
7 |
8 |
512 |
64 |
ND |
16 |
256 |
32 |
ND |
256 |
32 |
256 |
256 |
1024 |
512 |
|
8 |
32 |
512 |
16 |
64 |
256 |
128 |
128 |
128 |
64 |
256 |
128 |
128 |
128 |
128 |
|
9 |
ND |
ND |
ND |
ND |
ND |
ND |
1024 |
ND |
>1024 |
64 |
1024 |
512 |
ND |
>1024 |
| RAb | 4 | 32 | 128 | 128 | 32 | 16 | 16 | 16 | 128 | 32 | 1 | 1 | 128 | 4 |
aTested samples [DMT: methanol extract from the twigs of Dorstenia mannii; dorsmanins A(1), B(2), C(3), D(4) E(6), F(7), G(8), I (9) and 6,8 diprenyleriodictyol (5); bRA : reference antibiotics were chloramphenicol for bacteria, nystatin for C. albicans, ciprofloxacin for M. smegmatis, isoniazid for M.tuberculosis; (−): MIC > 1024 μg/ml; (ND): not determined as MIC was >1024 μg/ml
The results of the MIC determinations (Table 1) demonstrated that the methanol extract as well as compounds 3 and 8 were able to prevent the growth of all the fourteen studied microorganisms, including mycobacteria, yeast and Gram-negative bacteria, within the concentration range of 4 to 1024 μg/ml. Other compounds showed selective activities, their inhibitory effects being noted on 12/14 (85.7%) studied pathogens for compound 7, 7/14 (50%) for compound 6, 6/14 (42.9%) for compounds 9, 5/14 (35.7%) for compounds 2 and 5, 3/14 (21.4%) for compounds 1 and 4. The lowest MIC value for the methanol extract (64 μg/ml) was obtained on C. albicans. The lowest value for individual compounds (4 μg/ml) was recorded with compounds 3 on P. aeruginosa PA01 and 7 on E. coli ATCC strain. The corresponding values for the RA ranged from 0.5 to 64 μg/ml, P. aeruginosa PA01 and M. tubercolosis MTCS1 being the least sensitive. Results of MMC determinations (Table 2) also showed good activities for some of the tested samples such as compounds 3 and 8. MMC values not greater than 1024 μg/ml were recorded on all studied microorganisms with compounds 3 and 8, on 12/14 (85.7%) studied organisms for compound 7, 9/14 (64.3%) for the crude extract, 4/14 (28.6%) for compound 9, 3/14 (21.4%) for compounds 5 and 6, 2/14 (14.3%) for compounds 1 and 2, 1/14 (7.1%) for compound 4.
The compounds isolated from D. mannii and tested herein were all flavonoids. This class of compounds is very common in the genus Dorstenia[10-12] and their antimicrobial activities were also reported [2-4]. In the present work, broad spectrum of antimicrobial activities was recorded with the crude extract and compounds from D. mannii. Phytochemicals are routinely classified as antimicrobials on the basis of susceptibility tests that produce MIC in the range of 100 to 1000 μg/ml [18]. Activity is considered to be significant if MIC values are below 100 μg/ml for crude extract and moderate when the MIC values vary from 100 to 625 μg/ml [19,20]. Therefore, the activity recorded with the crude extract on C. albicans can be considered as important. Also, compounds with significant activities (MIC < 10 μg/ml) on at least one of the studied organisms include 3, 6, 7 and 8. The MIC values recorded with compounds 3 on P. aeruginosa PA01, 6 on C. albicans,7 on P. aeruginosa PA01 and K. pneumoniae ATCC strain, and C. albicans,8 on P. aeruginosa PA01, PA124, P. stuartiiM. tuberculosis MTCS1 were lower than or equal to those of the reference drugs, highlighting their interesting activities. This observation is in consistence with previous work on flavonoids isolated from the genus Dorstenia. In fact, isobavachalcone, 4-hydroxylolonchocarpin, kanzonol C, stipulin, and many other flavonoids isolated from this genus were reported for their good antimicrobial potencies, with MIC values below 10 μg/ml on several tested microorganisms [2-4,21]. A Keen look at the MMC values indicates that most of them are not more than fourfold their corresponding MICs. This proves that the killing effects of many tested samples could be expected on the sensitive strains [22]. The continuous emergence of multidrug-resistant (MDR) bacteria drastically reduces the efficacy of our antibiotic armory and, consequently, increases the frequency of therapeutic failure [23]. MDR Enterobacteriaceae, including K. pneumoniae, E. aerogenes and E. coli have also been classified as antimicrobial-resistant organisms of concern in healthcare facilities [24]. Besides, K. pneumoniae KP55 tested herein was reported to be resistant to most of the commonly used antibiotics, showing high levels of resistance to ampicillin, ceftazidime, and aztreonam with MIC values up to 512 μg/ml [25]. In addition Pseudomonas aeruginosa has emerged as one of the most problematic Gram-negative pathogens, with the alarmingly high antibiotics resistance rates [26]. The good activities of compounds 3 and 8 on most of the tested strains belonging to MDR phenotypes such as E. coli AG100, P. aeruginosa PA124, E. aerogenes CM64, K. pneumoniae KP55 as observed herein reinforce the hypothesis that these compounds are natural products with interesting antimicrobial potencies.
Tuberculosis (TB) is widely expanded in poor countries with the highest incidence (more than 80% of cases) occurring in Asia and Africa [27]. Annual incidence of TB (over 600 cases per 100 000) has been reported in many sub-Saharan African countries [28]. In this work, only compounds with inhibitory activity on M. smegmatis were tested on M. tuberculosis. However, it has been demonstrated that the sensitivity of M. tuberculosis is closer to that of M. smegmatis, a non pathogenic microorganism [29]. Therefore, this microorganism can be used for a preliminary study to select samples with potential activity against M. tuberculosis[29]. Hence, the results obtained herein are in accordance with such recommendation.
To the best of our knowledge, the antimicrobial activity of D. mannii as well as that of the isolated compounds is being reported for the first time. However 6,8-diprenyleridictyol (5) an,dorsmanin F (7) were reported for their antitrichomonal activities [30].
Conclusion
The data reported herein are very important, taking into account the medical importance of the studied microorganisms. Hence, the overall results of the present investigation provide evidence that the crude extract of D. mannii as well as some of its compounds such as compounds 3 and 8 could be considered as interesting natural antimicrobial products.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ATM, VK and BN carried out the study; ATM and VK wrote the manuscript; VK, BTN, VPB, JJMM and NL supervised the work. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Armelle T Mbaveng, Email: armkuete@yahoo.fr.
Victor Kuete, Email: kuetevictor@yahoo.fr.
Bathelemy Ngameni, Email: bath_ngameni@yahoo.fr.
Veronique P Beng, Email: v.penlap@yahoo.fr.
Bonaventure T Ngadjui, Email: ngadjuibt@yahoo.fr.
Jacobus J Marion Meyer, Email: marion.meyer@up.ac.za.
Namrita Lall, Email: Namrita.Lall@up.ac.za.
Acknowledgements
Authors are thankful to the Cameroon National Herbarium (Yaounde) for the plant identification. Authors are also grateful to the International Foundation for Science (IFS-Grant F/4579-2 to VK). Authors are also thankful to UMR-MD1 (Mediterranean University, Marseille, France) for providing some clinical bacteria.
References
- Abegaz BM, Ngadjui BT, Dongo E, Bezabih M-T. Chemistry of the genus Dorstenia. Curr Org Chem. 2000;2000(4):107–109. [Google Scholar]
- Kuete V, Simo IK, Ngameni B, Bigoga JD, Watchueng J, Kapguep RN, Etoa FX, Tchaleu BN, Beng VP. Antimicrobial activity of the methanolic extract, fractions and four flavonoids from the twigs of Dorstenia angusticornis Engl. (Moraceae) J Ethnopharmacol. 2007;112:271–277. doi: 10.1016/j.jep.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Mbaveng AT, Ngameni B, Kuete V, Konga Simo I, Ambassa T, Roy R, Bezabih M, Etoa FX, Ngadjui BT, Abegaz BM, Meyer JJM, Lall N, Penlap BV. Antimicrobial activity of the crude extracts and five flavonoids from the twigs of Dorstenia barteri (Moraceae) J Ethnopharmacol. 2008;116:483–489. doi: 10.1016/j.jep.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Ngameni B, Kuete V, Konga Simo I, Mbaveng AT, Awoussong PK, Patnam R, Roy R, Ngadjui BT. Antibacterial and antifungal activities of the crude extract and compounds from Dorstenia turbinata (Moraceae) S Afr J Bot. 2009;75:256–261. doi: 10.1016/j.sajb.2008.11.006. [DOI] [Google Scholar]
- Hutchinson J, Dalziel JM. In: Flora of West Tropical Africa, 2nd ed. Keay RWJ, editor. , ; 1954. [Google Scholar]
- Bouquet A. Feticheurs et Medecines Traditionnelles du Congo Brazaville. ORSTOM, Paris; 1969. [Google Scholar]
- Dufall KG, Ngadjui BT, Simeon KF, Abegaz BM, Croft KD. Antioxidant activity of prenylated flavonoids from the West African medicinal plant Dorstenia mannii. J Ethnopharmaco. 2003;87:67–72. doi: 10.1016/S0378-8741(03)00108-9. [DOI] [PubMed] [Google Scholar]
- Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64:711–713. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- Mativandlela SPN, Lall N, Meyer JJM. Antibacterial, antifungal and antitubercular activity of (the roots of) Pelargonium reniforme (CURT) and Pelargonium sidoides (DC) (Geraniaceae) root. S Afr J Bot. 2006;72:232–237. doi: 10.1016/j.sajb.2005.08.002. [DOI] [Google Scholar]
- Ngadjui BT, Abegaz BM, Dongo E, Tanboue H, Fogue K. Geranylated and Prenylated flavonoids from the twigs of Dorstenia mannii. Phytochemistry. 1998;48:349–354. doi: 10.1016/S0031-9422(97)01120-5. [DOI] [Google Scholar]
- Ngadjui BT, Dongo E, Tanboue H, Fogue K, Abegaz BM. Prenylated flavanones from the twigs of Dorstenia mannii. Phytochemistry. 1998;50:1401–1406. [Google Scholar]
- Ngadjui BT, Kouam SF, Dongo E, Kapche GWF, Abegaz BM. Prenylated flavanoids from the aerial parts of Dorstenia mannii. Phytochemistry. 2000;55:915–919. doi: 10.1016/S0031-9422(00)00215-6. [DOI] [PubMed] [Google Scholar]
- Kuete V, Kamga J, Sandjo LP, Ngameni B, Poumale HM, Ambassa P, Ngadjui BT. Antimicrobial activities of the methanol extract, fractions and compounds from Ficus polita Vahl. (Moraceae) BMC Complement Altern Med. 2011;11:6. doi: 10.1186/1472-6882-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuete V, Ngameni B, Fotso Simo CC, Kengap Tankeu R, Tchaleu Ngadjui B, Meyer JJM, Lall N, Kuiate JR. Antimicrobial activity of the crude extracts and compounds from Ficus chlamydocarpa and Ficus cordata (Moraceae) J Ethnopharmacol. 2008;120:17–24. doi: 10.1016/j.jep.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Tereschuk ML, Riera MVQ, Castro GR, Abdala LR. Antimicrobial activity of flavonoid from leaves of Tagetes minuta. J Ethnopharmacol. 1997;56:227–232. doi: 10.1016/S0378-8741(97)00038-X. [DOI] [PubMed] [Google Scholar]
- Zgoda JR, Porter JR. A convenient microdilution method screening natural products against bacteria and fungi. Pharmaceut Biol. 2001;39:221–225. doi: 10.1076/phbi.39.3.221.5934. [DOI] [Google Scholar]
- Jimenez-Arellanes A, Meckes M, Ramirez R, Torres J, Luna-Herrera J. Activity against multidrug-resistant Mycobacterium tuberculosis in Mexican plants used to treat respiratory diseases. Phytother Res. 2003;17:903–908. doi: 10.1002/ptr.1377. [DOI] [PubMed] [Google Scholar]
- Simões M, Bennett RN, Rosa EA. Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat Prod Rep. 2009;26:746–757. doi: 10.1039/b821648g. [DOI] [PubMed] [Google Scholar]
- Kuete V. Potential of Cameroonian plants and derived products against microbial infections: a review. Planta Med. 2010;76:1479–1491. doi: 10.1055/s-0030-1250027. [DOI] [PubMed] [Google Scholar]
- Kuete V, Efferth T. Cameroonian medicinal plants: pharmacology and derived natural products. Front Pharmacol. 2010;1:123. doi: 10.3389/fphar.2010.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuete V, Ngameni B, Mbaveng AT, Ngadjui B, Marion Meyer JJ, Lall N. Evaluation of flavonoids from Dorstenia barteri for their antimycobacterial, antigonorrheal and anti-reverse transcriptase activities. Acta Trop. 2010;116:100–104. doi: 10.1016/j.actatropica.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Mims CA, Playfair JHL, Roitt IM, Wakelin D, Williams R, In: Antimicrobials and chemotherapy. Mims CA, editor. 1993. pp. 1–34. Med Microbiol Rev 35. [Google Scholar]
- Rice LB. Unmet medical needs in antibacterial therapy. Biochem Pharmacol. 2006;71:991–995. doi: 10.1016/j.bcp.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nicolle LE. Infection control programmes to contain antimicrobial resistance. World Health Organization, Geneva; 2001. WHO/CDS/CSR/DRS/2001.7. http://whqlibdoc.who.int/hq/2001/WHO CDS CSR DRS 2001.7.pdf [accessed January 2009] [Google Scholar]
- Chevalier J, Pagès J-M, Eyraud A, Malléa M. Membrane permeability modifications are involved in antibiotic resistance in Klebsiella pneumoniae. Biochem Biophys Res Commun. 2000;274:496–499. doi: 10.1006/bbrc.2000.3159. [DOI] [PubMed] [Google Scholar]
- Savafi L, Duran N, Savafi N, Onlen Y, Ocak S. The prevalence and resistance patterns of Pseudomonas aeruginosa in intensive care units in a university hospital. Turk J Med Sci. 2005;35:317–322. [Google Scholar]
- Zager EM, McNerney R. Multidrug-resistant tuberculosis. BMC Infect Dis. 2008;8:10. doi: 10.1186/1471-2334-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett EL, Marston B, Churchyard GJ, De Cock KM. Tuberculosis in sub- Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet. 2006;367:926–927. doi: 10.1016/S0140-6736(06)68383-9. [DOI] [PubMed] [Google Scholar]
- Newton SM, Lau C, Gurcha SS, Besra GS, Wright CW. The evaluation of forty-three plant species for in vitro antimycobacterial activities; isolation of active constituents from Psoralea corylifolia and Sanguinaria Canadensis. J Ethnopharmacol. 2002;79:57–67. doi: 10.1016/S0378-8741(01)00350-6. [DOI] [PubMed] [Google Scholar]
- Omisore NOA, Adewunmi CO, Iwalewa EO, Ngadjui BT, Adenowo TK, Abegaz BM, Ojewole JA, Watchueng J. Antitrichomonal and antioxidant activities of Dorstenia barteri and Dorstenia convexa. Braz J Med Biol Res. 2005;38:1087–1094. doi: 10.1590/S0100-879X2005000700012. [DOI] [PubMed] [Google Scholar]