What is the basis of concern that humans can alter Earth's climate? One might think that discussions of global warming arise from the growing human discharge of energy into the air and waters. Worldwide annual usage rates of energy from fossil fuels (approximately 75% of global energy use) were about 3.7 × 1020 J in 1995, and have increased about 20-fold since 1850, and 4.5 times since 1950 (1). Yet this amount of energy is far less than the energy received from the sun and absorbed in the Earth-atmosphere system (237 W/m2, or 3.5 × 1024 J per year), approximately 9,000 times as large as yearly human usage. Instead, it is the greenhouse effect that causes human impact to be significant. It has been shown that, through the greenhouse effect enhanced by various human-produced trace gases now in the atmosphere, the Earth receives and is heated by an additional 2.6 W/m2 in the lower atmosphere. This heating is equivalent to an additional 1% of the solar input and it is growing.
The physics of the greenhouse effect has been investigated since the early nineteenth century by several scientists (2) including Fourier, Pouillet, Tyndall, Langley, and Arrhenius; the latter author performed quantitative calculations of the possible warming of the Earth due to human-caused increases of atmospheric carbon dioxide (2). Briefly, the sun emits energy (visible light, UV and infrared) nearly as a blackbody at 5,800 K; most of the visible light incident on Earth's upper atmosphere reaches the surface. The energy absorbed from this insolation is balanced by outgoing infrared radiation (IR). One can attempt to calculate a planet's temperature, which is governed by the energy-balance equation in the absence of net heat input:
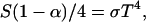 |
where S = the solar power per unit at the mean sun–Earth distance (1,372 W/m2, α is Earth's albedo, σ is the Stefan–Boltzmann constant, and T is temperature. For Earth, one calculates 255 K, which is of course, 33 K below the measured average temperature. For Mars, with its very thin atmosphere, this calculation matches observations within 6 K, but it grossly underestimates surface temperatures of Venus, which has a very thick atmosphere (3), demonstrating the reality of the greenhouse effect.
Coming back to Earth, the infrared energy emitted by the planetary surface (global average temperature of 288 K) partly is absorbed by the air above: The atmosphere is opaque or partly so at certain IR wavelengths because of certain polyatomic molecules in air. The emissions of the Earth-atmosphere system viewed from space originate largely from an atmospheric altitude where the temperature is 255 K. Without an atmosphere, the Earth's surface would emit as a blackbody at 255 K (see ref. 4, for example). Because pressure and temperature decrease with altitude (in the troposphere), the Earth's surface is warmer than it would be without this “greenhouse effect”; indeed, each atmospheric layer receives more IR energy from below than it sends to overlying layers. Although the atmosphere emits in specific wavelength bands and not like an ideal blackbody, the effective temperature Te = 255 K is still a useful measure of the planet's effective radiating temperature. The two most powerful natural greenhouse gases are water vapor and carbon dioxide. Vibrational-rotational bands of atmospheric water vapor intercept wavelengths <8 μm, whereas its rotational bands intercept radiation of wavelength >18 μm, and carbon dioxide dominates atmospheric absorption ≈15 μm (5).
In a previous issue of PNAS, Hansen et al. (6) presented an optimistic but plausible scenario in which future anthropogenic forcing of climate change is less than previous and currently conventional estimates. A standard measure of this forcing, called “radiative forcing,” is the change ΔQ in net radiation at the tropopause attributable to the increase in concentration of the gas in question. This measure allows direct comparison of forcing agents whereas before the mid-1980s, nearly all estimates of the greenhouse effect were given as temperature changes, that is, responses of the system (7). As introduced above, the human-induced increases in global atmospheric concentrations of CO2, CH4, several chlorofluorocarbon (CFC) gases, and N2O have led to an enhancement of 2.6 W/m2 in the greenhouse effect, or the equivalent of more than 1% of the incoming absorbed solar radiation. Continued increases in CO2 amounts, for example from preindustrial levels of 280 ppm to 560 ppm, would lead to ΔQ = 4.3 W/m2 [Intergovernmental Panel on Climate Change (8)]. Estimates of future forcing that included future growth of atmospheric CFCs and rapid CH4 increases led to greenhouse-gas-induced forcings of 4–9 W/m2 by the year 2050 (5). A dramatic example was the demonstration (9) that if CFC usage had continued to grow at 1970s' rates, their radiative forcing alone would have exceeded that caused by CO2 by the early 1990s.
International agreements that have greatly reduced CFC growth rates, along with lower than projected growth rates of CH4 concentrations, imply smaller future ΔQ values than had been estimated earlier, as has been shown by Hansen et al. (10), who also noted that observed losses of stratospheric ozone imply a negative radiative forcing component, as do sulfate aerosols from ground-level pollution (from combustion of fossil fuels that contain sulfur). The negative forcing by pollution-derived sulfate aerosol particles is real but difficult to quantify, especially as they might change cloud formation (11). Also, the forcing is very patchy spatially so that regional differences are likely and cancellation of greenhouse-gas forcing is, at best, a crude approximation.
Now Hansen et al. (6) suggest that strong attention to non-CO2 greenhouse gases could halt or even reverse the growth in their atmospheric concentrations. Moreover, if CH4 emissions can be reduced by approximately 30%, and if tropospheric ozone amounts can be stabilized through cleaner combustion (decreased nitrogen oxide releases), then the radiative forcing caused by non-CO2 greenhouse gases could be the same in 2050 as now, as opposed to being much larger. Further, if the growth rate of atmospheric CO2 could be held to those of the past 20 years, the added forcing by 2050 would be only about 1 W/m2. Limiting atmospheric CO2 increases would require increased energy efficiency and continued terrestrial sequestration of carbon (12). This scenario further assumes that black carbon from dirty combustion be limited, that CFCs remain under control, and that N2O growth rates do not increase.
Methane is an attractive target for manipulation because its atmospheric residence time is only about 10 years (13), although perturbations to its sources and sinks excite other modes with longer time constants (14). A great deal is known about the major sources of atmospheric methane (15) such as rice paddies, ruminant animals, landfills, and natural gas handling, and there is evidence that methane emissions can be suppressed. For example, Sass et al. (16) have shown that water management such as mid-year draining can reduce methane emissions from rice fields while not reducing crop yields, and McCrabb et al. (17) have suppressed methane production in Brahman cattle without affecting animal growth; indeed, the animals required slightly less food to achieve the same weight gains as control animals. The technique incorporates a novel antimethanogenic compound into the feed, a technique that could be used while animals reside in feedlots (mostly in advanced countries). Before actually trying to manipulate rice fields or animal feeds, however, we must estimate the potential quantitative benefits and convince farmers and ranchers to permit the interventions. There are also confounding factors. For example, methane emissions from rice are generally largest when fresh organic matter is added to the soil before planting (18), yet there are large differences between fields and some preconditioning of the organic matter changes the results, such as with green manure (19). Similarly, although CH4 emissions from landfills can be reduced, they are difficult to quantify (20), and long-closed landfills may emit as much methane as open landfills (21), thus making it difficult for us to identify and manipulate them. Overall, one must realize that to reduce methane emmissions by 30% would require decreasing anthropogenic sources by nearly 50% (15), a difficult task.
Tropospheric ozone, especially in the cold upper troposphere (6- to 10-km altitude), is an effective greenhouse gas largely because of pressure broadening of its 9.6-μm absorption band. Measured amounts of ozone at the surface and throughout the troposphere showed significant increases throughout the twentieth century, but these trends slowed and/or vanished in North America and Western Europe during the 1980s and 1990s, as shown by Logan (22), and for Europe, as shown by Simmonds et al. (23). By contrast, Lee et al. (24) have shown that ozone amounts continue to increase over Japan and that the likely cause is increasing emissions of ozone precursors (principally nitrogen oxides and carbon monoxide from combustion) from China, Taiwan, Korea, and Japan. Lee et al. (24) also estimate that emissions of NOx compounds from these four countries increased by approximately 60% between 1987 and 1998, a much larger increase than occurred in North America or Western Europe where pollution-control technology has been deployed relatively well. Thus, if pollution-control devices such as NOx reducers are emplaced in developing countries, tropospheric ozone amounts can be limited or even reduced while energy usage increases, as Hansen et al. have suggested (6).
Human ability to limit air pollution also is illustrated by recent experience with atmospheric sulfur compounds and related emissions. In at least one advanced country, data show that reduced sulfur dioxide emissions have led to decreased amounts of sulfate in atmospheric aerosols (and presumably to decreased precipitation of sulfuric acid). Measured aerosol sulfate amounts declined approximately 45% and 30% from 1979 to 1996 at Whiteface Mountain and Mayville (New York), respectively, from corresponding amounts averaged over the period 1981–1991, and during this time, upwind emissions of SO2 from the U.S. Midwest declined by approximately 35% (25). Also, measurements of CO in surface air show decreases in the early 1990s, and globally averaged amounts have decreased by 2–5% per year (23, 26, 27). Because many natural and anthropogenic sources contribute CO to the atmosphere, the cause of this decrease is not clear but neither did CO amounts simply increase with fossil-fuel consumption.
For these reasons, the Hansen et al. (6) scenario is quite interesting. And it is encouraging in that most attention has been focused on the “business-as-usual” scenarios developed by Intergovernmental Panel on Climate Change that assume larger future radiative forcing. To implement the actions needed to decrease atmospheric methane and tropospheric ozone amounts, however, we must sharpen our knowledge and perhaps mount some prototype, demonstration experiments. Effective international cooperation will be essential.
Are there other ways to counter human-caused climatic change? A variety of interventions have been suggested that could alter the reflectivity of the Earth's surface or otherwise deflecting sunlight, or by selectively fertilizing the world's oceans to draw in CO2, such as with iron (see ref. 28, for example). Generally, such solutions are not yet quantified, their side effects are not yet studied, or they are very distant for other reasons.
Although human-caused climatic change seems evident already with growing impacts in the future, there is no current belief that humans can control such changes once they are forced. Hansen et al. (6) demonstrate that minimizing future growth in radiative forcing is much more within our reach.
Footnotes
See companion article on page 9875 in issue 18 of volume 97.
References
- 1.The President's Committee of Advisors on Science and Technology Energy Research and Development Panel, Chair, Holdren, J. Report to the President on Federal Energy Research and Development Challenges for the Twenty-First Century. Washington, DC: Executive Office of the President; 1997. pp. 1-4–1-21. [Google Scholar]
- 2.Rodhe H, Charlson R, Crawford E. In: The Legacy of Svante Arrhenius Understanding the Greenhouse Effect. Rodhe H, Charlson R, editors. Stockholm: R. Swedish Acad. Sci. & Stockholm Univ.; 1998. pp. 13–20. [Google Scholar]
- 3.Wayne R. Chemistry of Atmospheres. 2nd Ed. Oxford: Clarendon; 1991. [Google Scholar]
- 4.Ramanathan V, Barkstrom B, Harrison E. Phys Today. 1989;42:22–32. [Google Scholar]
- 5.Dickinson R, Cicerone R. Nature (London) 1986;319:109–115. [Google Scholar]
- 6.Hansen J, Sato M, Ruedy R, Lacis A, Oinas V. Proc Natl Acad Sci USA. 2000;97:9875–9880. doi: 10.1073/pnas.170278997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charlson R. Nature (London) 1999;401:741–742. [Google Scholar]
- 8.Houghton J, Meira Filho L, Bruce J, Lee H, Callandar B, Haites E, Harris N, Maskell K, editors. Intergovernmental Panel on Climate Change. Climate Change 1994 (Radiative Forcing of Climate Change) Cambridge, U.K.: Cambridge Univ. Press; 1995. [Google Scholar]
- 9.Hansen J, Lacis A, Prather M. J Geophys Res. 1989;94:16417–16421. [Google Scholar]
- 10.Hansen J, Sato M, Lacis A, Ruedy R, Tegen I, Mathews E. Proc Natl Acad Sci USA. 1998;95:12753–12758. doi: 10.1073/pnas.95.22.12753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlson R, Langner J, Rodhe H, Leovy C, Warren S. Tellus. 1991;43AB:152–163. [Google Scholar]
- 12.Battle M, Bender M, Tans P, White J, Ellis J, Conway T, Francey R. Science. 2000;287:2467–2470. doi: 10.1126/science.287.5462.2467. [DOI] [PubMed] [Google Scholar]
- 13.Prinn R, Weiss R, Miller B, Huang J, Alyea F, Cunnold D, Fraser P, Hartley D, Simmonds P. Science. 1995;269:187–192. doi: 10.1126/science.269.5221.187. [DOI] [PubMed] [Google Scholar]
- 14.Prather M. Geophys Res Lett. 1994;21:801–804. [Google Scholar]
- 15.Cicerone R, Oremland R. Global Biogeochem Cycles. 1988;2:299–328. [Google Scholar]
- 16.Sass R, Fisher F, Wang F, Turner F, Jund M. Global Biogeochem Cycles. 1992;6:249–262. [Google Scholar]
- 17.McCrabb G, Berger K, Magner T, May C, Hunter R. Aust J Agric Res. 1997;48:323–329. [Google Scholar]
- 18.Yagi K, Minami K. Soil Sci Plant Nutr. 1990;36:599–610. [Google Scholar]
- 19.Cai Z, Tsuruta H, Minami K. J Geophys Res. 2000;105:17231–17242. [Google Scholar]
- 20.Reeburgh W. In: Microbial Growth on C1 Compounds. Lidstrom M, Tabita F R, editors. Dordrecht, The Netherlands: Kluwer; 1996. pp. 334–342. [Google Scholar]
- 21.Blaha D, Bartlett K, Czepiel P, Harriss R, Crill P. Atmos Env. 1999;33:243–255. [Google Scholar]
- 22.Logan J. J Geophys Res. 1994;99:25553–25585. [Google Scholar]
- 23.Simmonds P, Seuring S, Nickless G, Derwent R. J Atmos Chem. 1997;28:45–59. [Google Scholar]
- 24.Lee S, Akimoto H, Nakane H, Kurnosenko S, Kinjo Y. Geophys Res Lett. 1998;25:1637–1640. [Google Scholar]
- 25.Husain L, Dutkiewicz V, Das M. Geophys Res Lett. 1998;25:967–970. [Google Scholar]
- 26.Khalil M, Rasmussen R A. Nature (London) 1994;370:639–641. [Google Scholar]
- 27.Novelli P, Masarie K, Tans P, Lang P. Science. 1994;263:1587–1590. doi: 10.1126/science.263.5153.1587. [DOI] [PubMed] [Google Scholar]
- 28.Keith, D. (2000) Ann. Rev. Energy Environ.25, in press.


