Abstract
Background
The association between CD209 promoter polymorphisms (-336A/G, -871A/G) and tuberculosis (TB) risk has been widely reported, but results of previous studies remain controversial and ambiguous. To assess the association between CD209 polymorphisms and TB risk, a meta-analysis was performed.
Methods
Based on comprehensive searches of the PubMed, Embase, Web of Science, Weipu, and CBM databases, we identified outcome data from all articles estimating the association between CD209 polymorphisms and TB risk. The pooled odds ratio (OR) with 95% confidence intervals (CIs) were calculated.
Results
A total of 14 studies with 3,610 cases and 3,539 controls were identified. There was no significant association between CD209 -336A/G polymorphism and TB risk (OR = 1.04, 95% CI = 0.91–1.19 for G vs. A; OR = 1.13, 95% CI = 0.84–1.53 for GG vs. AA; OR = 1.04, 95% CI = 0.87–1.24 for GG+AG vs. AA; OR = 1.11, 95% CI = 0.88–1.39 for GG vs. AG+AA). However, the significant association was revealed for Asians in GG vs. AA (OR = 2.48, 95% CI = 1.46–4.22, P = 0.0008) and GG vs. AG+AA (OR = 2.10, 95% CI = 1.33–3.32, P = 0.001). For the CD209 -871A/G polymorphism, lack of an association was also found (OR = 0.81, 95% CI = 0.70–0.95 for G vs. A; OR = 1.00, 95% CI = 0.52–1.93 for GG vs. AA; OR = 0.73, 95% CI = 0.60–0.89 for GG+AG vs. AA; OR = 1.09, 95% CI = 0.57–2.10 for GG vs. AG+AA).
Conclusion
The present meta-analysis suggested that CD209 promoter polymorphisms (-336A/G, -871A/G) were unlikely to substantially contribute to TB susceptibility. However, the GG genotype of CD209 -336A/G polymorphism might be a genetic risk factor that increases TB susceptibility for Asians in GG vs. AA and GG vs. AG+AA.
Introduction
Tuberculosis (TB) constitutes a serious threat to public health throughout the world, particularly in developing countries [1]. Recent data from the World Health Organization (WHO) show that nearly 10 million new cases arise and 1.7 million deaths die of TB annually [2]. It has been reported that one-third of population is infected with Mycobacterium tuberculosis; however, only one-tenth of infected individuals will ever develop active TB [3]. Thus, host genetic susceptibility, combined with environmental factors, may play a crucial role in exploring the infection mechanism of Mycobacterium tuberculosis [4]–[5].
The CD209 gene, located on human chromosome 19p13.2–3, is composed of 7 exons and 6 introns, and is about 13 kb in length [6]–[7]. This gene encoded Dendritic Cell-Specific ICAM3-Grabbing Non-integrin (DC-SIGN), which is one of the major receptor of Mycobacterium tuberculosis on human dendritic cells [8]. In addition, the Mycobacterium tuberculosis interacts with DC-SIGN to activate the Raf-1-acetylation-dependent signaling pathway that is involved in the regulation of adaptive immune response to tuberculosis. Furthermore, the DC-SIGN could suppress Toll-like receptor signaling leading to cytokine secretion. This effect may be a part of immune evasion to TB progression [9]–[10]. Therefore, the CD209 gene might play a crucial role in host immunity to TB and might be one of the candidate genes for susceptibility of TB.
A relatively large number of studies evaluated the association between CD209 polymorphisms (-336A/G, -871A/G) and TB risk, but the results have been inconsistent due to limited sample sizes and different study populations. To derive a more comprehensive and precise estimation of the relationship, we carried out a meta-analysis on all eligible case-control studies to estimate the effect of CD209 polymorphisms on the risk of TB and to quantify the potential between-study heterogeneity.
Results
Study Characteristics
Eleven publications, including 3,610 cases and 3,539 controls, met the inclusion criteria [6], [11]–[20]. A flowchart detailing the process for study identification and selection was shown in Fig. S1. The publication of Vannberg et al. presented four independent case-control studies, each study was considered separately for analysis. Therefore, 11 publications including 14 studies were involved in this meta-analysis. The main characteristics of the studies were shown in Table 1. The sample sizes ranged from 273 to 1093 patients (median 390, IQR 324–669). Nine of the 14 included studies reported the proportion of male patients, which ranged from 14.5% to 81.1% (median 62.25%, IQR 53.225%–68.5%). Thirteen of the 14 included studies clearly described the diagnostic criteria. One study didn’t provide the genotype number [18]. Gene frequencies of all the individual studies were shown in Table S1. The NOS scores ranged from 7 to 9, which indicated that the methodological quality was generally good. The quality assessment of included studies was shown in Table S2. The genotype distribution in the controls of all studies was in agreement with HWE.
Table 1. Association between individual study characteristics and CD209 gene polymorphisms.
| Study | Origin | Ethnicity | Male patients (%) | Mean age(years) | Sample types | Sample size | Polymorphisms investigated | Clinical diagnoses performed | Control source | Genotypingmethod | Score | ||
| Cases | Controls | Cases | Controls | ||||||||||
| Kobayashi et al. [12] | Indonesian | Asian | 53.7 | 41.6±15.4 | 39.3±12.7 | PTB | 532 | 561 | -336A/G, -871A/G | Smear, radiologic,clinical symptoms | Healthy individuals | Sequencing | 8 |
| Ogarkov et al. [17] | Russian | Caucasian | 76.3 | 42.3±12.1 | 41.9±9.2 | PTB | 101 | 177 | -336A/G | NR | Healthy individuals | Taq Man LNA technology | 7 |
| EPTB | 90 | ||||||||||||
| Zheng et al. [19] | Chinese | Asian | 65.4 | 44.6±17.7 | NR | PTB | 237 | 244 | -336A/G, -871A/G | Culture, radiologic | Healthy individuals | Sequencing | 7 |
| Sadki et al. [15] | Moroccan | Mixed | 81.1 | 33.7±13.2 | NR | PTB | 122 | 151 | -336A/G | Smear, culture,histology, radiologic,clinical symptoms | Healthy unrelateddonors | Taq Man SNP genotyping assays | 8 |
| Selvaraj et al. [13] | Indian | Caucasian | 61.2 | 34.0±8.2 | 30.6±8.3 | PTB | 183 | 157 | -336A/G | Smear, culture,radiologic, clinicalsymptoms | Healthy individuals | PCR-RFLP | 7 |
| EPTB | 31 | ||||||||||||
| Zhuang et al. [20] | Chinese | Asian | 65.9 | 43(16–77) | 30(15–78) | PTB | 167 | 167 | -336A/G | Smear, culture,histology, radiologic,clinical symptoms | Healthy unrelateddonors with nohistory ofautoimmunedisease | SSP-PCR | 7 |
| Vannberg et al. (a) [14] | Gambian | African | NR | NR | NR | PTB | 676 | 327 | -336A/G | Smear, culture,histology | Healthy unrelateddonors | MALDI-TOF | 8 |
| Vannberg et al. (b) [14] | Guinean | African | NR | NR | NR | PTB | 151 | 180 | -336A/G | Smear, culture,histology | Healthy unrelated donors | MALDI-TOF | 8 |
| Vannberg et al. (c) [14] | Guinea-Bissau | African | NR | NR | NR | PTB | 162 | 141 | -336A/G | Smear, culture,histologyconfirmed TB | Healthy unrelateddonors | MALDI-TOF | 8 |
| Vannberg et al. (d) [14] | Malawian | African | NR | NR | NR | PTB | 244 | 295 | -336A/G | Smear, culture,histologyconfirmed TB | Healthy unrelated donors | MALDI-TOF | 8 |
| Ben-Ali et al. [18] | Tunisian | Mixed | NR | NR(18–65) | NR(25–60) | NR | 138 | 140 | -336A/G, -871A/G | Smear, culture,radiologic, clinicalsymptoms confirmedTB | Healthy unrelateddonors | Sequencing | 8 |
| Olesen et al. [16] | Guinea-Bissau | African | 60.4 | 37.3 | 38.1 | PTB | 315 | 340 | -336A/G | Smear, culture,histology, radiologic,clinical symptomsconfirmed TB | Healthy unrelateddonors | Taq Man SNP genotyping assays | 9 |
| Barreiro et al. [6] | SouthAfrican | African | 51.8 | 36.7±10.9 | 34.6±12.5 | PTB | 351 | 360 | -336A/G, -871A/G | Smear, cultureconfirmed TB | Healthy unrelateddonors | Taq Man or fluorescence polarization | 8 |
| Gómez et al. [11] | Colombian | Mixed | 14.5 | 40.0±15.0 | NR | NR | 110 | 299 | -336A/G | Smear, cultureconfirmed TB | Healthy unrelateddonors with nohistory ofautoimmunedisease | Sequencing | 8 |
Abbreviations and definitions: PTB, pulmonary tuberculosis; EPTB, extra-pulmonary tuberculosis; NR, not report; PCR-RFLP, Polymerase Chain Reaction-Restriction Fragment Length Polymorphism; SSP-PCR, sequence specific primer-Polymerase Chain Reaction; MALDI-TOF, Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry.
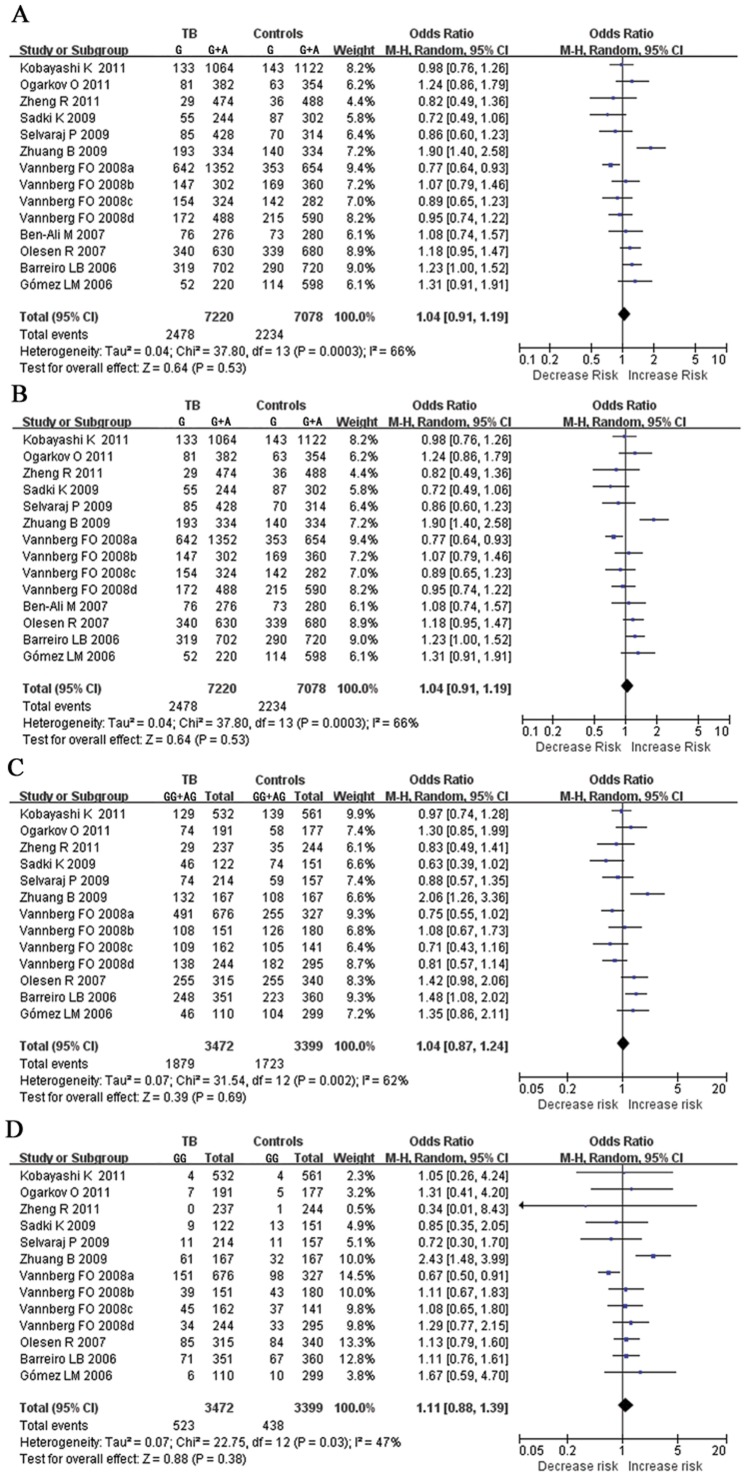
The CD209 -336A/G Alleles and Tuberculosis Susceptibility
Random effects models were used to calculate the pooled OR in all genetic models. Overall, the combined results showed that no significant association was found in all genetic models (OR = 1.04, 95% CI = 0.91–1.19 for G vs. A, OR = 1.13, 95% CI = 0.84–1.53 for GG vs. AA, OR = 1.04, 95% CI = 0.87–1.24 for GG+AG vs. AA, and OR = 1.11, 95% CI = 0.88–1.39 for GG vs. AG+AA). Forest plots on the basis of all studies were shown in Fig. 1A, B, C, D. When stratified by ethnicity, we observed a wide variation of G allele frequencies between the controls across different ethnicities. The G allele frequencies were significant difference in Africans, Asians, Caucasians and Mixed populations (P = 0.007). Forest plots were shown in Fig. S2. When meta-analysis was performed to assess association between CD209 -336A/G polymorphism and TB risk in different ethnicities, significant association was revealed for Asians in GG vs. AA (OR = 2.48, 95% CI = 1.46–4.22, P = 0.0008) and GG vs. AG+AA (OR = 2.10, 95% CI = 1.33–3.32, P = 0.001) (Fig. S3, Fig. S4, Fig. S5, and Fig. S6). On subgroup analysis by sample types, no evidence of association was found in all genetic models for pulmonary tuberculosis (PTB) and extra-pulmonary tuberculosis (EPTB). The results were shown in Table 2.
Figure 1. Forest plot of the overall risk of TB associated with the CD209 -336A/G promoter polymorphism.
No significant association was found between the CD209 -336A/G polymorphism and TB risk in all genetic models. A, G vs. A; B, GG vs. AA; C, dominant genetic model; D, recessive genetic model. Error bars indicate 95% CI. Solid squares represent each study in the meta-analysis. Solid diamonds represent pooled OR.
Table 2. Meta-analyses of CD209 -336A/G promoter polymorphism and risk of TB in each subgroup.
| Category | G vs. A | GG vs.AA | Dominant model | Recessive model |
| OR (95%CI) I 2 (%) | OR (95%CI) I 2 (%) | OR (95%CI) I 2 (%) | OR (95%CI) I 2 (%) | |
| Ethnicity | ||||
| African | 1.00 (0.85–1.19) 65 | 1.03 (0.75–1.42) 61 | 1.00 (0.76–1.32) 69 | 0.97 (0.83–1.15) 42 |
| Asian | 1.17 (0.71–1.94) 85 | 2.48 (1.46–4.22) 46 | 1.17 (0.71–1.93) 75 | 2.10 (1.33–3.32) 20 |
| Caucasian | 1.03 (0.80–1.33) 49 | 0.91 (0.45–1.82) 0 | 1.07 (0.79–1.45) 37 | 0.89 (0.45–1.78) 0 |
| Mixed | 1.01 (0.72–1.43) 60 | 1.03 (0.52–2.05) 45 | 0.93 (0.44–1.95) 80 | 1.11 (0.56–2.18) 0 |
| Sample types | ||||
| PTB | 1.02 (0.88–1.18) 69 | 1.08 (0.79–1.48) 62 | 1.01 (0.83–1.22) 62 | 1.08 (0.85–1.37) 51 |
| EPTB | 1.21 (0.84–1.74) 0 | 1.51 (0.58–3.92) 0 | 1.20 (0.77–1.86) 30 | 1.51 (0.59–3.89) 0 |
Abbreviations and definitions: PTB, pulmonary tuberculosis; EPTB, extra-pulmonary tuberculosis; CI, 95% confidence intervals; OR, odds ratio.
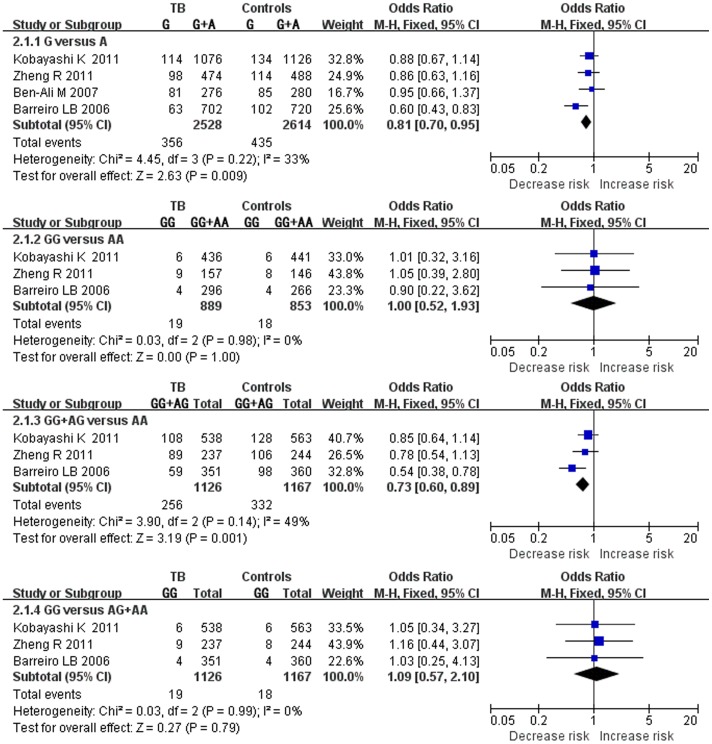
The CD209 -871A/G Alleles and Tuberculosis Susceptibility
The results on the CD209 -871A/G polymorphism indicated that the G allele had no significant association to TB susceptibility as compared to the A allele under the fixed effects models (Fig. 2). The results were as followed: G vs. A (OR = 0.81, 95% CI = 0.70–0.95), GG vs. AA (OR = 1.00, 95% CI = 0.52–1.93), GG+AG vs. AA (OR = 0.73, 95% CI = 0.60–0.89), GG vs. AG+AA (OR = 1.09, 95% CI = 0.57–2.10).
Figure 2. Forest plot of the overall risk of TB associated with the CD209 -871A/G promoter polymorphism.
No significant association was found between the CD209 -871A/G polymorphism and TB risk in all genetic models. Error bars indicate 95% CI. Solid squares represent each study in the meta-analysis. Solid diamonds represent pooled OR.
Heterogeneity Analysis
For CD209 -336A/G polymorphism, there were statistically significant heterogeneity in G vs. A (I 2 = 66%, P heterogeneity = 0.0003), GG vs. AA (I 2 = 60%, P heterogeneity = 0.003), dominant genetic model (I 2 = 62%, P heterogeneity = 0.002), and recessive genetic model (I 2 = 47%, P heterogeneity = 0.03). To explain the heterogeneity, Galbraith plots were performed in all genetic models. The two studies of Zhuang et al. and Vannberg FO(a) et al. were outliers in the G vs. A, GG vs. AA, and recessive genetic model (Fig. 3A, B, D). The three studies of Zhuang et al., Vannberg FO(a) et al. and Barreiro LB et al. were outliers in the dominant genetic model (Fig. 3C). When the studies of Zhuang et al., Vannberg FO(a) et al. and Barreiro LB et al. were excluded respectively, all I 2 values were less than 50% and P heterogeneity were greater than 0.1 (Table 3). The significant of pooled OR in all genetic models was not influenced after excluding the studies. By meta-regression analysis, the heterogeneity sources were attributed to the sample size, control source, NOS scores, and the frequency of G allele. However, the four above-mentioned factors had no significant impact on pooled OR in all genetic models (Table S3).
Figure 3. Galbraith plot of CD209 -336A/G promoter polymorphism and TB risk.
A, The two studies of Zhuang et al. and Vannberg FO(a) et al. were outliers in G vs. A; B, The two studies of Zhuang et al. and Vannberg FO(a) et al. were outliers in GG vs. AA; C, The three studies of Zhuang et al., Vannberg FO(a) et al. and Barreiro LB et al. were outliers in dominant genetic model; D, The two studies of Zhuang et al. and Vannberg FO(a) et al. were outliers in recessive genetic model.
Table 3. Meta-analyses of CD209 -336A/G promoter polymorphism and risk of TB after omitting the studies.
| Polymorphism | OR(95% CI) | Z | P OR | I 2 (%) | P heterogeneity | Effectmodel |
| G vs. Aa | 1.05(0.96, 1.14) | 1.12 | 0.26 | 17 | 0.28 | F |
| GG vs. AAa | 1.16(0.95, 1.42) | 1.46 | 0.15 | 0 | 0.74 | F |
| GG+AG vs. AAb | 0.98(0.86, 1.11) | 0.29 | 0.77 | 36 | 0.12 | F |
| GG vs. AG+AAa | 1.11(0.93, 1.32) | 1.14 | 0.26 | 0 | 0.98 | F |
Abbreviations and definitions: TB, tuberculosis; CI, 95% confidence intervals; OR, odds ratio; P heterogeneity, P value of Q test for heterogeneity; F, fixed-effect models.
CD209 -336A/G promoter polymorphism and risk of TB after excluding the two studies of Zhuang et al. and Vannberg FO(a) et al.
CD209 -336A/G promoter polymorphism and risk of TB after excluding the three studies of Zhuang et al., Vannberg FO(a) et al. and Barreiro LB et al.
Sensitivity Analysis
Sensitivity analysis was performed though sequential excluding individual studies. Statistically similar results were obtained in all genetic models after sequentially excluding each study, indicated that our dates were stability and liability in this meta-analysis.
Publication Bias
Publication bias was estimated by the funnel plots. As shown in Fig. S7, the shape of the funnel plots revealed asymmetry in some degree due to the limited number of literatures. Then, Egger’s linear regression test was used to provide statistical evidence of funnel plots asymmetry. For CD209 -336A/G polymorphism, the P values of Egger’s linear regression test were 0.789, 0.880, 0.951, and 0.606 for G vs. A, GG vs. AA, dominant genetic model, and recessive genetic model, respectively. For CD209 -871A/G polymorphism, the P values were 0.779, 0.117, 0.516, and 0.302, respectively. The result still did not suggest any evidence of publication bias.
Discussion
According to WHO, The estimates of TB global burden were 9.4 million incident cases, 14 million prevalent cases, and 1.68 million deaths (with 0.38 million in HIV-positive individuals) in 2009 [21]. So, it is necessary to explore the infection mechanism. Clearly, the genetic contribution of the host plays a significant role in determining susceptibility to TB [22]. The CD209 gene, encoding DC-SIGN, might play a crucial role in the pathogenesis of TB. The association between CD209 gene polymorphisms and TB risk was first reported in a South African populations by Barreiro et al [6]; however, as discussed above, conflicting data regarding the role of CD209 in TB susceptibility and presentation have been reported [12]–[15], [19]. Against this backdrop, we performed a meta-analysis to clarify the relationship between CD209 polymorphisms and TB risk.
In this meta-analysis, 14 studies (14 subgroups for -336A/G polymorphism, 4 subgroups for -871A/G polymorphism) on CD209 gene were performed to provide the most comprehensive assessment of the relationship between polymorphisms and TB. For CD209 -336A/G polymorphism, the G allele of -336A/G polymorphism had no association with the TB susceptibility for the G vs. A, GG vs. AA, dominant genetic model, and recessive genetic model in overall populations. For the CD209 -871A/G polymorphism, lack of an association was also found. Currently, many meta-analyses have reported the association between gene polymorphisms and TB susceptibility. Compared with the CD209 gene polymorphisms, the interferon-γ (IFN-γ), natural resistance-associated macrophage protein 1(NRAMP1), and Vitamin D receptor (VDR) gene polymorphisms contribute to susceptibility to TB [23]–[25]. The P2X7, Mannose-binding lectin 2 (MBL2), tumor necrosis factor-α (TNF-α), and Interleukin-10 (IL-10) gene polymorphisms are not associated with TB risk [26]–[29].
In view of the complex effect of genetic polymorphisms on disease progression, the lack of an association between CD209 polymorphisms and TB susceptibility may attribute to other polymorphisms in CD209 gene promoter which could affect the expression of DC-SIGN. In current study, we also performed meta-analysis to identify the association between CD209 -939G/A polymorphism and TB risk. There was no association between CD209 -939G/A polymorphism and TB risk (Fig. S8). de Wit E et al. [30] found that two interactions (DC-SIGN -871 A/G: NRAMP1(GT)n repeat and DC-SIGN -871 A/G:MBL) influenced the risk of developing TB. Thus, the interaction between gene and gene might play a crucial role in the association of CD209 polymorphisms with TB risk.
Due to different racial or ethnic populations with different frequencies of alleles, different genetic backgrounds may affect TB susceptibilities. Therefore, subgroup analyses were performed according to ethnicity. First, we detected whether there was G allele frequency of variation in different ethnicities. For CD209 -336A/G polymorphism, the G allele frequency has significant differences in different populations. Next, the association between CD209 -336A/G polymorphism and different ethnicities was explored. The significant association was revealed for Asians in GG vs. AA (OR = 2.48, 95% CI = 1.46–4.22, P = 0.0008) and GG vs. AG+AA (OR = 2.10, 95% CI = 1.33–3.32, P = 0.001). The ethic-dependent association may attribute to interplay among different human alleles, locally predominant, and endemic mycobacterial lineages [31]. Ogarkov et al. [17] demonstrated that the G allele of CD209 -336A/G polymorphism could increase the risk of infection with Mycobacterium tuberculosis Beijing but not non-Beijing strain in Russian male population. Thus, the TB in different host infected with the same genotype may manifest as different outcome in clinic.
In our meta-analysis, obvious heterogeneity was observed for CD209 -336A/G polymorphism. Then, we used the Galbraith plots to explore the sources of heterogeneity. We found all of the I 2 values were less than 50% and P heterogeneity were greater than 0.1 after excluding the studies of Zhuang et al., Vannberg FO(a) et al. and Barreiro LB et al, respectively. The results indicated that the three studies might be the major source of the heterogeneity for the CD209 -336A/G polymorphism. The results of subgroup analyses revealed that the ethnicity and sample type might contribute to the potential heterogeneity. Owing to the limited number of studies in this meta-analysis, we restricted meta-regression analysis to four factors (sample size, control source, NOS scores, and the frequency of G allele), which are the most likely to cause the heterogeneity between studies. However, the four above-mentioned factors had no significant impact on the heterogeneity.
There are some limitations to this meta-analysis. Firstly, the retrieved literature is potentially not comprehensive enough. We did not track the unpublished articles to obtain data for analysis. The potential effect of this publication bias is unknown. Secondly, the small sample sizes in some subgroup analyses may have limited statistical power to estimate the possible risk for CD209 polymorphisms. Thirdly, TB is a multifactorial disease and potential interactions among gene-gene and gene-environment should be considered. Moreover, as many other factors such as age or gender may participate in the progression of TB, we did not carry out subgroup analysis based on these factors due to limited data.
Conclusively, the CD209 promoter polymorphisms (-336A/G, -871A/G) may lack association with genetic susceptibility of TB. However, genotype GG of CD209 -336A/G polymorphism might play a role as risk factor for Asians in GG vs. AA and GG vs. AG+AA, which indicated the different genetic backgrounds may affect TB susceptibilities. Moreover, further studies with large sample size of different ethnic populations will be necessary to combine genetic factors together with poor economic conditions, malnutrition, stress and overcrowding.
Materials and Methods
Data Sources and Search Strategy
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) criteria [32]. Two investigators (K.C. and S.D.) independently performed a systematic electronic search of the PubMed, Embase, Web of Science, Weipu, and CBM databases for original articles published until 1 December, 2011 to identify potentially relevant articles and abstracts. Search terms used were “CD209 or DC-SIGN” and “tuberculosis or TB” and “polymorphism or mutation or variant”. There were no language restrictions. We reviewed the bibliographies of all selection articles to identify additional relevant studies.
Selection of Publications
Two reviewers (K.C. and W.L.) independently screened titles and abstracts of all studies for relevancy. Disagreements were resolved by a third opinion (M.C.). Full-text publications were retrieved for relevant articles. The strength of the individual studies was weighed for relevance, based on the following items: (1) evaluation of the CD209 -336A/G or -871A/G polymorphisms and TB risk, (2) case-control studied (family-based study design with linkage considerations was excluded), (2) sufficient data for estimating an odds ratio (OR) with 95% confidence intervals (CIs), (3) genotype distribution of control population in Hardy-Weinberg equilibrium (HWE), and (4) studies written in English or Chinese. For the studies with the same or overlapping data by the same authors, the most recent or largest population was selected.
Data Extraction
Data were extracted independently from each study by two reviewers (K.C. and S.D.) according to the inclusion criteria listed above. Agreement was reached after discussion for conflicting data. The following data were collected from each study: first author’s name, publication year, original country, ethnicity, sample size, sample types, TB definition, genotyping method, and genotype number in cases and controls.
Quality Assessment
The quality of included studies was assessed independently by the same two investigators using the Newcastle-Ottawa Scale (NOS) (Stang A., 2010). The NOS uses a ‘star’ rating system to judge quality based on 3 aspects of the study: selection, comparability, and exposure. Scores were ranged from 0 stars (worst) to 9 stars (best). Studies with a score of 7 stars or greater were considered to be of high quality. Disagreement was settled as described above.
Statistical Analysis
The strength of association between CD209 polymorphisms and TB risk was estimated by OR and corresponding 95% CIs. The pooled OR was calculated respectively for G vs. A, GG vs. AA, dominant genetic model (GG+AG vs. AA), and recessive genetic model (GG vs. AG+AA). Between-study heterogeneity was assessed by the Q-test and I 2 test, P heterogeneity<0.10 and I 2>50% indicated evidence of heterogeneity. Then, the random-effects model (the DerSimonian and Laird method) [33]–[34] was used to calculate the pooled OR. Otherwise, the fixed-effects model (Mantel-Haenszel) [35] was adopted. Subgroup analyses and meta-regression were used to analyze the sources of heterogeneity.
Sensitivity analysis was mainly performed to assess the stability of the results, namely, a single study in the meta-analysis was deleted each time to reflect the influence of the individual data set to the pooled OR. Asymmetry funnel plots were inspected to assess potential publication bias. The Egger’s linear regression test [36] was also used to assess publication bias statistically.
Data were analyzed by using STATA 11.0 (Stata Corporation, College Station, TX, USA) and Revman 5.0 (The Cochrane Collaboration).
Supporting Information
Flow diagram of the selection of eligible studies.
(TIF)
Frequencies of the minor allele (G allele) of the CD209 -336A/G polymorphism among controls subjects stratified by ethnicity. The G allele frequencies were significant difference in Africans, Asians, Caucasians and Mixed populations (P = 0.007)
(TIF)
Forest plot of CD209 -336A/G promoter polymorphism and risk of TB in G vs. A for each subgroup. No significant association was found between the CD209 -336A/G polymorphism and TB risk in G vs. A.
(TIF)
Forest plot of CD209 -336A/G promoter polymorphism and risk of TB in GG vs. AA for each subgroup. The significant association was revealed for Asians in GG vs. AA (OR = 2.48, 95% CI = 1.46–4.22, P = 0.0008).
(TIF)
Forest plot of CD209 -336A/G promoter polymorphism and risk of TB in dominant model for each subgroup. No significant association was found between the CD209 -336A/G polymorphism and TB risk in dominant model.
(TIF)
Forest plot of CD209 -336A/G promoter polymorphism and risk of TB in recessive model for each subgroup. The significant association was revealed for Asians in recessive model (OR = 2.10, 95% CI = 1.33–3.32, P = 0.001).
(TIF)
Funnel plots of all genetic models in overall studies. A. G vs. A; B. GG vs. AA; C. dominant model (GG+AG vs. AA); D. recessive model (GG vs. AG+AA). Funnel plots of dominant model seemed asymmetry. Each point represents a separate study for the indicated association.
(TIF)
Forest plot of the overall risk of TB associated with the CD209 -939G/A promoter polymorphism. No significant association was found between the CD209 -939G/A polymorphism and TB risk in all genetic models. Error bars indicate 95% CI. Solid squares represent each study in the meta-analysis. Solid diamonds represent pooled OR.
(TIF)
Gene frequencies of all the individual studies used for the meta-analysis.
(DOC)
Quality assessment of included studies.
(DOC)
Meta-regression analysis of CD209 -336A/G promoter polymorphism and risk of TB after omitting the studies.
(DOC)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The Major Project Chinese National Programs for High Technology Research and Development (863 Program, 2007AA02Z416), the Chinese National Natural Science Foundation (No. 81071428, 30400107), and the National Infectious Disease Prevention and Cure Special Project of China (No. 2008ZX10003-012). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Connell DW, Berry M, Cooke G, Kon OM. Update on tuberculosis: TB in the early 21st century. Eur Respir Rev. 2011;20:71–84. doi: 10.1183/09059180.00000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization WH. Global Tuberculosis Control 2010.WHO/HTM/TB/2010.7. Geneva, World Health Organization. 2010.
- 3.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 4.Hill AV. Aspects of genetic susceptibility to human infectious diseases. Annu Rev Genet. 2006;40:469–486. doi: 10.1146/annurev.genet.40.110405.090546. [DOI] [PubMed] [Google Scholar]
- 5.Jepson A, Fowler A, Banya W, Singh M, Bennett S, et al. Genetic regulation of acquired immune responses to antigens of Mycobacterium tuberculosis: a study of twins in West Africa. Infect Immun. 2001;69:3989–3994. doi: 10.1128/IAI.69.6.3989-3994.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barreiro LB, Neyrolles O, Babb CL, Tailleux L, Quach H, et al. Promoter variation in the DC-SIGN-encoding gene CD209 is associated with tuberculosis. PLoS Med. 2006;3:e20. doi: 10.1371/journal.pmed.0030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soilleux EJ, Barten R, Trowsdale J. DC-SIGN; a related gene, DC-SIGNR; and CD23 form a cluster on 19p13. J Immunol. 2000;165:2937–2942. doi: 10.4049/jimmunol.165.6.2937. [DOI] [PubMed] [Google Scholar]
- 8.Tailleux L, Schwartz O, Herrmann JL, Pivert E, Jackson M, et al. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J Exp Med. 2003;197:121–127. doi: 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geijtenbeek TB, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geijtenbeek TB, Krooshoop DJ, Bleijs DA, van Vliet SJ, van Duijnhoven GC, et al. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat Immunol. 2000;1:353–357. doi: 10.1038/79815. [DOI] [PubMed] [Google Scholar]
- 11.Gomez LM, Anaya JM, Sierra-Filardi E, Cadena J, Corbi A, et al. Analysis of DC-SIGN (CD209) functional variants in patients with tuberculosis. Hum Immunol. 2006;67:808–811. doi: 10.1016/j.humimm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi K, Yuliwulandari R, Yanai H, Lien LT, Hang NTL, et al. Association of CD209 polymorphisms with tuberculosis in an Indonesian population. Hum Immunol. 2011;72:741–745. doi: 10.1016/j.humimm.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Selvaraj P, Alagarasu K, Swaminathan S, Harishankar M, Narendran G. CD209 gene polymorphisms in South Indian HIV and HIV-TB patients. Infect Genet Evol. 2009;9:256–262. doi: 10.1016/j.meegid.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Vannberg FO, Chapman SJ, Khor CC, Tosh K, Floyd S, et al. CD209 genetic polymorphism and tuberculosis disease. PLoS One. 2008;3:e1388. doi: 10.1371/journal.pone.0001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadki K, Lamsyah H, Rueda B, Lahlou O, El Aouad R, et al. CD209 Promoter Single Nucleotide Polymorphism-336A/G and the Risk of Susceptibility to Tuberculosis Disease in the Moroccan Population. Int J Hum Genet. 2009;9:239–243. [Google Scholar]
- 16.Olesen R, Wejse C, Velez DR, Bisseye C, Sodemann M, et al. DC-SIGN (CD209), pentraxin 3 and vitamin D receptor gene variants associate with pulmonary tuberculosis risk in West Africans. Genes Immun. 2007;8:456–467. doi: 10.1038/sj.gene.6364410. [DOI] [PubMed] [Google Scholar]
- 17.Ogarkov O, Mokrousov I, Sinkov V, Zhdanova S, Antipina S, et al. 'Lethal' combination of Mycobacterium tuberculosis Beijing genotype and human CD209–336G allele in Russian male population. Infect Genet Evol. 2012;12:732–736. doi: 10.1016/j.meegid.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Ali M, Barreiro LB, Chabbou A, Haltiti R, Braham E, et al. Promoter and neck region length variation of DC-SIGN is not associated with susceptibility to tuberculosis in Tunisian patients. Hum Immunol. 2007;68:908–912. doi: 10.1016/j.humimm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Zheng R, Zhou Y, Qin L, Jin R, Wang J, et al. Relationship between polymorphism of DC-SIGN (CD209) gene and the susceptibility to pulmonary tuberculosis in an eastern Chinese population. Hum Immunol. 2011;72:183–186. doi: 10.1016/j.humimm.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhuang B, Yang BF, Song HM, Li F, Kong LB. Study on correlation between CD209 polymorphisms and susceptibility to pulmonary tuberculosis. Clin J Public Health. 2009;25:517–519. [Google Scholar]
- 21.Yew WW, Sotgiu G, Migliori GB. Update in tuberculosis and nontuberculous mycobacterial disease 2010. Am J Respir Crit Care Med. 2011;184:180–185. doi: 10.1164/rccm.201102-0325UP. [DOI] [PubMed] [Google Scholar]
- 22.Cooke GS, Hill AV. Genetics of susceptibility to human infectious disease. Nat Rev Genet. 2001;2:967–977. doi: 10.1038/35103577. [DOI] [PubMed] [Google Scholar]
- 23.Tian C, Zhang Y, Zhang J, Deng Y, Li X, et al. The +874T/A polymorphism in the interferon-gamma gene and tuberculosis risk: an update by meta-analysis. Hum Immunol. 2011;72:1137–1142. doi: 10.1016/j.humimm.2011.07.310. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Yang Y, Zhou F, Zhang Y, Lu H, et al. SLC11A1 (NRAMP1) polymorphisms and tuberculosis susceptibility: updated systematic review and meta-analysis. PLoS One. 2011;6:e15831. doi: 10.1371/journal.pone.0015831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao L, Tao Y, Zhang L, Jin Q. Vitamin D receptor genetic polymorphisms and tuberculosis: updated systematic review and meta-analysis. Int J Tuberc Lung Dis. 2010;14:15–23. [PubMed] [Google Scholar]
- 26.Zhang J, Chen Y, Nie XB, Wu WH, Zhang H, et al. Interleukin-10 polymorphisms and tuberculosis susceptibility: a meta-analysis. Int J Tuberc Lung Dis. 2011;15:594–601. doi: 10.5588/ijtld.09.0703. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Zhan P, Qiu LX, Qian Q, Yu LK. TNF-308 gene polymorphism and tuberculosis susceptibility: a meta-analysis involving 18 studies. Mol Biol Rep. 2012;39:3393–3400. doi: 10.1007/s11033-011-1110-x. [DOI] [PubMed] [Google Scholar]
- 28.Xiao J, Sun L, Yan H, Jiao W, Miao Q, et al. Metaanalysis of P2X7 gene polymorphisms and tuberculosis susceptibility. FEMS Immunol Med Microbiol. 2010;60:165–170. doi: 10.1111/j.1574-695X.2010.00735.x. [DOI] [PubMed] [Google Scholar]
- 29.Denholm JT, McBryde ES, Eisen DP. Mannose-binding lectin and susceptibility to tuberculosis: a meta-analysis. Clin Exp Immunol. 2010;162:84–90. doi: 10.1111/j.1365-2249.2010.04221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Wit E, van der Merwe L, van Helden PD, Hoal EG. Gene-gene interaction between tuberculosis candidate genes in a South African population.Mamm Genome. 2011;22:100–110. doi: 10.1007/s00335-010-9280-8. [DOI] [PubMed] [Google Scholar]
- 31.Caws M, Thwaites G, Dunstan S, Hawn TR, Lan NT, et al. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog. 2008;28:e1000034. doi: 10.1371/journal.ppat.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 34.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 36.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow diagram of the selection of eligible studies.
(TIF)
Frequencies of the minor allele (G allele) of the CD209 -336A/G polymorphism among controls subjects stratified by ethnicity. The G allele frequencies were significant difference in Africans, Asians, Caucasians and Mixed populations (P = 0.007)
(TIF)
Forest plot of CD209 -336A/G promoter polymorphism and risk of TB in G vs. A for each subgroup. No significant association was found between the CD209 -336A/G polymorphism and TB risk in G vs. A.
(TIF)
Forest plot of CD209 -336A/G promoter polymorphism and risk of TB in GG vs. AA for each subgroup. The significant association was revealed for Asians in GG vs. AA (OR = 2.48, 95% CI = 1.46–4.22, P = 0.0008).
(TIF)
Forest plot of CD209 -336A/G promoter polymorphism and risk of TB in dominant model for each subgroup. No significant association was found between the CD209 -336A/G polymorphism and TB risk in dominant model.
(TIF)
Forest plot of CD209 -336A/G promoter polymorphism and risk of TB in recessive model for each subgroup. The significant association was revealed for Asians in recessive model (OR = 2.10, 95% CI = 1.33–3.32, P = 0.001).
(TIF)
Funnel plots of all genetic models in overall studies. A. G vs. A; B. GG vs. AA; C. dominant model (GG+AG vs. AA); D. recessive model (GG vs. AG+AA). Funnel plots of dominant model seemed asymmetry. Each point represents a separate study for the indicated association.
(TIF)
Forest plot of the overall risk of TB associated with the CD209 -939G/A promoter polymorphism. No significant association was found between the CD209 -939G/A polymorphism and TB risk in all genetic models. Error bars indicate 95% CI. Solid squares represent each study in the meta-analysis. Solid diamonds represent pooled OR.
(TIF)
Gene frequencies of all the individual studies used for the meta-analysis.
(DOC)
Quality assessment of included studies.
(DOC)
Meta-regression analysis of CD209 -336A/G promoter polymorphism and risk of TB after omitting the studies.
(DOC)