Abstract
Lipoproteins are an important class of surface associated proteins that have diverse roles and frequently are involved in the virulence of bacterial pathogens. As prolipoproteins are attached to the cell membrane by a single enzyme, prolipoprotein diacylglyceryl transferase (Lgt), deletion of the corresponding gene potentially allows the characterisation of the overall importance of lipoproteins for specific bacterial functions. We have used a Δlgt mutant strain of Streptococcus pneumoniae to investigate the effects of loss of lipoprotein attachment on cation acquisition, growth in media containing specific carbon sources, and virulence in different infection models. Immunoblots of triton X-114 extracts, flow cytometry and immuno-fluorescence microscopy confirmed the Δlgt mutant had markedly reduced lipoprotein expression on the cell surface. The Δlgt mutant had reduced growth in cation depleted medium, increased sensitivity to oxidative stress, reduced zinc uptake, and reduced intracellular levels of several cations. Doubling time of the Δlgt mutant was also increased slightly when grown in medium with glucose, raffinose and maltotriose as sole carbon sources. These multiple defects in cation and sugar ABC transporter function for the Δlgt mutant were associated with only slightly delayed growth in complete medium. However the Δlgt mutant had significantly reduced growth in blood or bronchoalveolar lavage fluid and a marked impairment in virulence in mouse models of nasopharyngeal colonisation, sepsis and pneumonia. These data suggest that for S. pneumoniae loss of surface localisation of lipoproteins has widespread effects on ABC transporter functions that collectively prevent the Δlgt mutant from establishing invasive infection.
Introduction
Lipoproteins are a major category of bacterial surface proteins that have diverse functions, and often have important effects on pathogen/host interactions during the development of infection. The majority of bacterial lipoproteins are substrate-binding proteins for ABC transporters involved in the transport of a wide range of substrates including cations, sugars, aminoacids, oligopeptides, polyamines, and minerals and which individually can be vital for full virulence [1]–[6]. As well as their important role for bacterial physiology, lipoproteins are also key mediators of the inflammatory response to Gram positive pathogens through recognition by toll-like receptor 2 (TLR2) [7]–[9]. The mechanism of lipoprotein attachment to the bacterial cell membrane and processing is conserved amongst bacteria. After initial extracellular secretion of prolipoproteins by the general secretory pathway (directed by an N terminal signal peptide sequence), lipoproteins are covalently linked to the cell membrane by the enzyme diacylglyceryl transferase (Lgt) [10]–[12]. A type II lipoprotein signal peptidase (Lsp) then cleaves the N terminal signal peptide adjacent to the ‘lipobox’ cysteine residue to form the mature lipoprotein [12]–[14]. Loss of Lgt reduces the quantity of lipoproteins attached to the bacterial cell membrane and usually but not always prevents Lsp function [10], [15], [16].
The importance of individual lipoprotein components of ABC transporters for bacterial physiology would suggest that deletion of lgt should have profound effects on bacterial growth and survival. For Gram negative bacteria this seems to be the case, as mutation of lgt is fatal [17], [18]. In contrast, for a variety of Gram positive bacteria mutation of lgt does not prevent viability and often has surprisingly little effects on growth. For example the lgt mutants of Streptococcus pneumoniae, Staphylococcus aureus, Streptococcus agalactiae, Streptococcus mutans, Streptococcus equi, Streptococcus suis, Streptococcus sanguinis, and Listeria monocytogenes have similar or only mildly impaired growth compared to the parental wild-type strain in complete or rich media [9], [16], [19]–[26]. Growth of lgt mutants is more often impaired in restrictive media, with for example, reduced growth in tissue culture or iron deficient media for a S. aureus lgt mutant [16], [27] and poor growth of a S. mutans lgt mutant in medium containing only meliobiose as a carbon source [24]. Mutation of individual lipoproteins can also have effects on bacterial sensitivity to environmental stress, adhesion to host tissues, and interactions with host phagocytes [28]–[30]. Phenotypes that might reflect these lipoprotein dependent functions have been described for some lgt mutants, including reduced intracellular replication and increased sensitivity to cationic peptides for the L. monocytogenes lgt mutant [25], and reduced adhesion and resistance to oxidative stress for the S. agalactiae lgt mutant [19].
The effects of lgt mutation on virulence are also often surprisingly weak and variable between different bacterial pathogens. For example, Petit et al. have described a S. pneumoniae lgt mutant that has greatly reduced virulence in a mouse model of pneumonia, whereas other streptococcal lgt mutants have either only mildly impaired, normal or even in the case of S. agalactiae increased virulence (attributed to reduced TLR2 dependent inflammatory responses) [21]–[23]. At present there has only been limited characterization of the physiological consequences of loss of Lgt for streptococci, and so there is no explanation for why effects on virulence are so variable between species. The S. pneumoniae genome contains approximately 40 genes predicted to encode lipoproteins [31], [32], many of which are involved in virulence as part of nutrient uptake ABC transporters [1]–[3], [33]–[42]. In particular, cation ABC transporters have major effects on the ability of S. pneumoniae to cause infection, with loss of the PspA manganese transporter lipoprotein or combined loss of the AdcA and AdcAII zinc or the PiaA and PiuA iron ABC transporter lipoproteins all resulting in strains that are greatly reduced in virulence [2], [3], [36], [39], [40], [42]. Hence if loss of lipoprotein anchoring to the cell membrane impairs cation uptake this could readily explain the reduced virulence of the S. pneumoniae lgt mutant, but at present there are no data on the effects of loss of Lgt on ABC transporter functions for S. pneumoniae. In addition, the S. pneumoniae genome contains seven ABC transporters annotated as involved in sugar uptake, including probable raffinose, galactose, and maltose/maltodextrin transporters as well as transporters of uncharacterised sugar substrates [31]. Several publications suggest that ABC sugar transporters are also required for full virulence in mouse models of infection [1], [33], [43]. However their importance might be offset by the considerable potential for redundancy in sugar acquisition due to the presence of multiple phosphoenolpyruvate (PEP)-dependent phosphotransferase system (PTS) sugar transporters in the S. pneumoniae genome. Assessing the effects of the lgt mutation on growth in different sugars could identify whether ABC transporters are necessary for S. pneumoniae sugar uptake despite the presence of numerous putative PTS sugar transporters.
We have therefore investigated whether a S. pneumoniae Δlgt mutant has phenotypes related to impaired cation and/or carbohydrate acquisition and the consequences of the lgt mutation for growth in physiological fluids. S. pneumoniae commonly causes infections in the blood as well as the lung, and colonises the nasopharynx. The physiological conditions at these varied sites vary substantially and this could affect the relative importance of lipoprotein function for bacterial survival. Hence, we have also investigated the effects of the lgt mutation on S. pneumoniae infection at these different anatomical sites.
Results
The lgt Operon and Construction of a Δlgt S. pneumoniae Strain
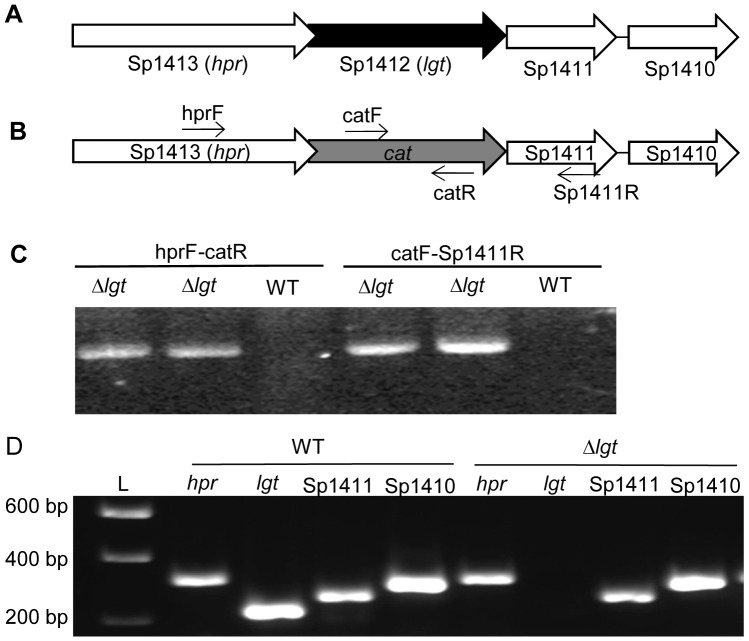
In the S. pneumoniae TIGR4 genome, the gene encoding Lgt is Sp_1412, the second gene in a putative four gene operon with either overlapping or very closely spaced open reading frames (Fig. 1A). The predicted product of lgt has a high degree of identity and similarity to the Lgt of other bacteria (Table 1). The other genes in this operon encode an Hpr (ser) kinase/phosphatase (Sp_1413) and two hypothetical proteins with unknown function (Sp_1411 and Sp_1410) (Fig. 1). BLAST searches show that homologs of Sp_1413 are associated with lgt in several other Gram positive bacteria, including S. suis, Streptococcus pyogenes, S. aureus, and Lactococcus lactis. To study the role of Lgt in S. pneumoniae, a non-polar deletion mutant (Δlgt) was created in which the Sp_1412 gene was replaced in frame by a chloramphenicol resistance cassette (cat) (Fig. 1B). Non-polar deletion of lgt was confirmed by PCR (Fig. 1C) and reverse transcriptase PCR (RT-PCR), which demonstrated the continued transcription of the remaining genes in this putative operon (Fig. 1D). The stability of the Δlgt mutant was confirmed by growth in THY without added chloramphenicol for two consecutive growth cycles and then plating on to blood agar plates with and without chloramphenicol, which resulted in 100% recovery of chloramphenicol resistant bacteria. Despite multiple attempts including insertion of an intact copy of lgt within the Sp_1413-10 operon or ectopically (data not shown) we have been unable to create a genetically complemented Δlgt strain.
Figure 1. Construction of the Δlgt strain.
(A) Schematic of the Sp_1410–1413 locus, with the TIGR4 genome gene number and the assigned gene names in parentheses when available. Arrows indicate transcriptional direction and lgt is shaded black. (B) Structure of the Sp_1410–1413 locus in the Δlgt mutant strain, showing replacement of lgt with an in-frame copy of cat which is shaded grey and position of primers used in (C). (C) Gel red stained agarose gels showing PCR analysis of two separately obtained Δlgt strains confirming replacement of lgt with cat. Primer pairs (Table 4) and the strain used for each reaction are given above each lane. (D) Gel red stained agarose gels of RT-PCR reactions using internal primers (Table 4) for each gene within the putative Sp_1410–1413 operon, confirming the non-polar deletion of lgt. Reactions not containing reverse transcriptase gave no products (not shown), and L marks the DNA ladder size marker with sizes listed on the left.
Table 1. Blast alignments of the Sp1412 (Lgt) amino acid sequence to other organisms.
| Organism | Gene number | Size (No of amino acids) | % identity/similaritya |
| S. sanguinis | HMPREF8578_1725 | 262 | 89/94 (202) |
| S. suis | SSU05_1605 | 267 | 68/83 (266) |
| S. agalactiae | SAL_0792 | 257 | 65/83 (192) |
| S. equi | Sez_1357 | 259 | 63/89 (199) |
| S. mutans | SmuNN2025_1248 | 263 | 65/80 (197) |
| L. monocytogenes | LMFG_00890 | 277 | 56/70 (205) |
| Bacillus subtilus | BSU6633_04292 | 269 | 53/69 (179) |
| S. aureus | SALG_00828 | 279 | 49/65 (176) |
| Escherichia coli | ECO157_010100032601 | 291 | 28/48 (185) |
Length of the amino acids compared.
Lipoprotein Localisation in the S. pneumoniae Δlgt Strain
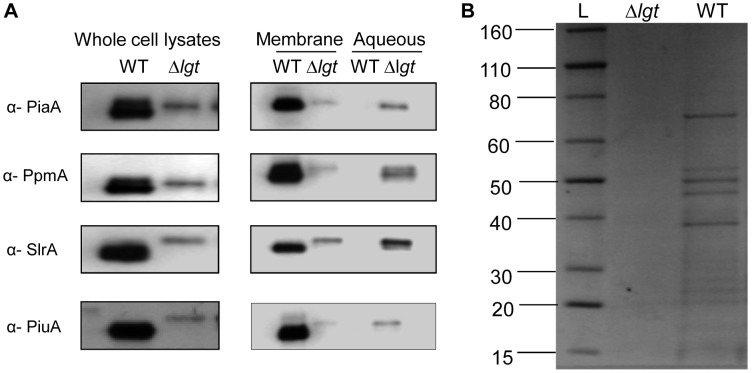
The effect of lgt deletion on S. pneumoniae lipoproteins was assessed by immunoblots of whole cell lysates using polyclonal mouse or rabbit antibodies to four S. pneumoniae lipoproteins, the iron ABC transporter lipoproteins PiuA and PiaA [2], and the non-ABC transporter associated lipoproteins PpmA and SlrA (kind gift from Peter Hermans, Radboud University) [44]. Although equal amounts of protein were loaded for both strains, the signal for all the lipoproteins investigated was stronger in the wild-type strain (Fig. 2A, lane 1) compared to the Δlgt strain, indicating reduced abundance of lipoproteins in the Δlgt strain. Membrane-associated proteins from the wild-type and the Δlgt strains were extracted using triton X-114, a non-ionic detergent which solubilises and extracts lipidated membrane proteins into the detergent phase with hydrophilic proteins remaining in the aqueous phase [45]. Immunoblots for the lipoproteins in the triton X-114 and aqueous extracts revealed a strong signal in the triton X-114 fraction for the wild-type strain with no detectable signal in the aqueous fraction (Fig. 2A), confirming that the lipoproteins in the wild-type are localised to the cell membrane. In contrast, for the Δlgt strain the signal for all the lipoproteins investigated was much weaker in the triton X-114 fraction and significant quantities of the lipoproteins were found in the aqueous fraction (Fig. 2A). Coomassie brilliant blue staining of the SDS-PAGE gel of the triton X-114 extracted proteins from the wild type strain demonstrated a large number of protein bands ranging between 15 and 80 KDa which previously we have shown to represent a range of lipoproteins including the cation transporters PiaA, AdcA and PsaA, and potential sugar transporters MalX and Sp_1683 [44]. However, these bands were largely absent for the triton X-114 extract from the Δlgt strain (Fig. 2B). These data indicate that, as expected, deletion of lgt resulted in loss of a number of lipoproteins from the membrane in the Δlgt strain including cation and sugar transporters.
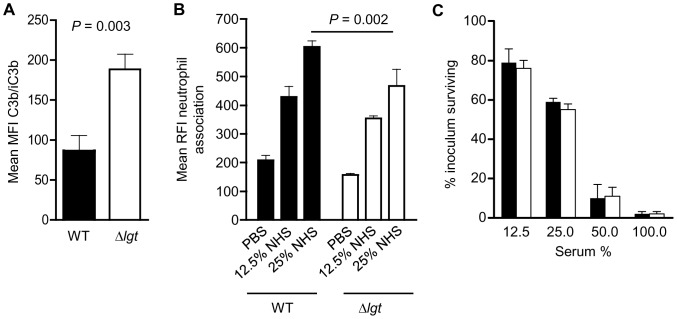
Figure 2. Effects of the Δlgt mutation on the localisation of S. pneumoniae lipoproteins.
(A) Immunoblots of whole cell lysates and the membrane and aqueous phases of triton X-114 extracts of wild-type (WT), Δlgt strains using antibodies to the S. pneumoniae lipoproteins PiaA, PpmA, SlrA and PiuA. (B) Coomasie blue staining of triton X-114 extracted membrane lipoproteins Δlgt and wild-type (WT) strains when separated on SDS–PAGE. Lane L, a standard protein ladder with molecular weights ranging from 15 KDa To 80 KDa.
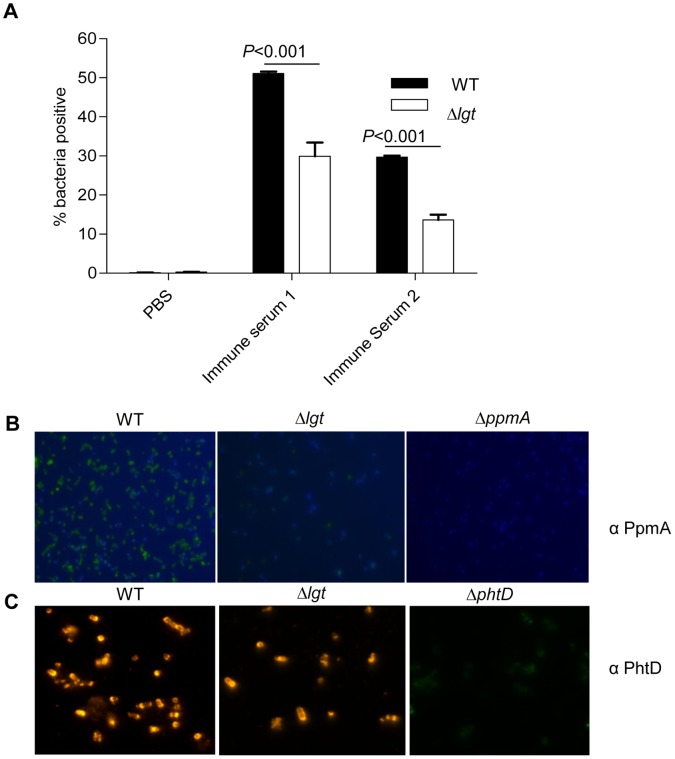
To further confirm the reduced cell surface location of lipoproteins in the Δlgt strain, IgG binding to live S. pneumoniae wild-type and Δlgt strain bacteria after incubation in polyclonal mouse sera from mice vaccinated with a S. pneumoniae Δpab strain [46] was assessed using flow cytometry. This sera contains high IgG antibody titres to the lipoproteins PsaA and PpmA as well several non-lipoprotein antigens [46]. IgG binding to the Δlgt strain was significantly reduced compared to IgG binding to the wild-type strain, compatible with reduced IgG recognition of lipoproteins in the Δlgt strain due to their loss from the bacterial surface (Fig. 3A). Furthermore, immuno-fluorescence microscopy using polyclonal antibodies to PpmA identified significant fluorescence with wild-type S. pneumoniae but much reduced fluorescence for the Δlgt strain and no fluorescence for the negative control ΔppmA strain (Fig. 3B). In contrast, immunoflorescence microscopy using polyclonal antibodies to the cell wall protein PhtD was not affected by in the Δlgt strain (Fig. 3C). Taken together, the immunoblots of triton X-114 extracts, flow cytometry and immuno-fluorescence microscopy demonstrate that the quantity of lipoproteins localised to the cell membrane and available for interactions with external agents is greatly reduced in the Δlgt strain.
Figure 3. Effect of the Δlgt mutation on surface accessability of S. pneumoniae lipoproteins.
(A) Flow cytometry analysis of the mean proportion of bacteria positive for IgG binding after incubation with immune sera containing high antibody titres towards lipoproteins of S. pneumoniae. Black columns represent wild-type strain and clear columns represent the Δlgt strain. Error bars represent SDs and P values were obtained using multiple ANOVA test with post-hoc analysis. (B) Immunofluorescence of the S. pneumoniae wild-type (WT), Δlgt and ΔPpmA strains using anti- PpmA antibody and FITC conjugated secondary antibody. (C) Immunofluorescence of the S. pneumoniae wild-type (WT), Δlgt and ΔPhtD strains using anti-PhtD antibody and Cy2 conjugated secondary antibody.
Cation ABC Transporter Function in the Δlgt Strain
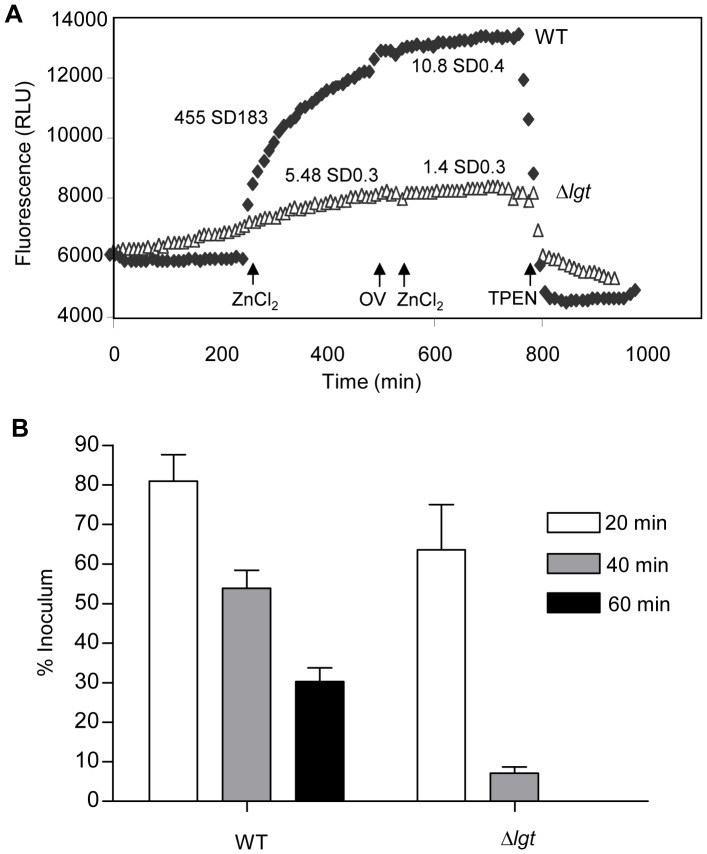
The ABC transporters Adc and AdcAII are required for zinc uptake by S. pneumoniae [39], [40]. Hence to directly assess the effects of the lgt mutation on a cation ABC transporter, zinc uptake was quantified using the fluorescent probe FluoZin-3 which fluoresces with an excitation/emission wavelength of 495/516 nm respectively when intracellular concentrations of zinc increase [42], [47]. After the addition of 10 µM ZnSO4, the wild-type strain showed a steady increase in fluorescence with time whereas there was only a minimal increase in fluorescence of the Δlgt strain (Fig. 4A). The rate of Zn+2 uptake, calculated from the slope of the curve was markedly lower in the Δlgt strain compared to that of the wild-type (Fig. 4A). After addition of a further 10 µM ZnSO4 preceded by 1 mM orthovanadate, an ATPase inhibitor [48], there was no further Zn+2 uptake even in the wild-type strain, confirming that the uptake of Zn+2 was ABC transporter mediated. The specificity of the FluoZin-3 assay for Zn2+ was confirmed by the addition of TPEN, a high affinity, membrane permeable Zn2+ chelator, which resulted in quenching of the fluorescence response in wild-type bacteria (Fig. 4A). These data demonstrate that the reduced membrane localisation of lipoproteins in the Δlgt strain was associated with markedly reduced function of zinc uptake ABC transporters.
Figure 4. Cation dependent phenotypes of the Δlgt strain.
(A) Uptake of Zn+2 by the Δlgt (triangles) and wild-type (filled diamonds) strains measured using a using FluoZin-3 fluorescence. The arrows numbered 1, 2 and 3 indicate the time points at which ZnCl2, 1 mM ortho-vanadate and TPEN were added to the strains respectively. Mean (SD) Zn2+ uptake in RFLU sec−1 before and after addition of orthovanadate and 10 µm ZnCl2 are stated next to the corresponding line. For comparison of zinc uptake by wild-type and Δlgt strains, P = 0.01 using Student’s t-test. (B) Proportion of wild-type and Δlgt strain bacteria surviving after exposure to 60 mM paraquat for 20 min (clear columns), 40 min (grey columns) and 60 min (black columns). No Δlgt strain bacteria survived after 60 minutes incubation. For the comparison between wild-type and Δlgt strains, at 40 and 60 min time points P values were <0.01 and <0.05 (2 way ANOVA with post-hoc tests).
To investigate whether the Δlgt strain had significant difficulties in obtaining other cations imported using ABC transporters, intracellular cations concentrations were measured using ICP-MS (Table 2). For the Δlgt strain intracellular concentrations of Fe2+, Zn2+, Mn2+, Ni2+ and Cu2+ were all significantly reduced, with values ranging from less than 1/100 (Fe2+ and Ni2+) to 1/9 (Mn2+) of the values obtained for the wild-type strain. Intracellular Mn2+ imported by the lipoprotein PsaA is required by S. pneumoniae to protect against oxidative stress [30], [49]. Hence, to help confirm a reduced cation content of the Δlgt strain, the sensitivity of the wild-type and the Δlgt strains to oxidative stress was assessed using 60 mM paraquat. Only 7% (SD 2.3) of the Δlgt strain inoculum remained viable after 20 min incubation with paraquat compared to the 53.9% (SD 6.37) of the wild-type strain, and after 60 minutes no Δlgt strain bacteria were recovered compared to 30.3% (SD 4.8) of the wild-type strains (Fig. 4B). Overall, the results of these assays demonstrate that the Δlgt strain has a phenotype compatible with the defective function of several cation ABC transporters.
Table 2. Quantification of intracellular S. pneumoniae and media cation contents using ICP-MS and expressed in Ppb (+/− SD).
| Cation | S. pneumoniae strain | Media | |||
| Wild-type | Δlgt | THY | THY Chelex | C+Y | |
| Fe2+ | 3800+/−38 | 32+/−2 | NA | NA | NA |
| Mn2+ | 18+/−1 | 1.9+/−0.1 | 670+/−4 | 1.31+/−0.02 | 5.2+/−0.2 |
| Zn2+ | 1020+/−22 | 37+/−4.3 | 2237+/−45 | 17.52+/−3.14 | 241+/−34 |
| Ni2+ | 110+/−1 | 0.8+/−0.02 | 520+/−50 | 0.45+/−0.03 | 6.2+/−0.3 |
| Cu2+ | 380+/−4 | 16+/−0.5 | 920+/−11 | 1.02+/−0.17 | 11.4+/−4 |
NA – not available.
Effects of Limited Cation Availability on Growth of the S. pneumoniae Δlgt Strain
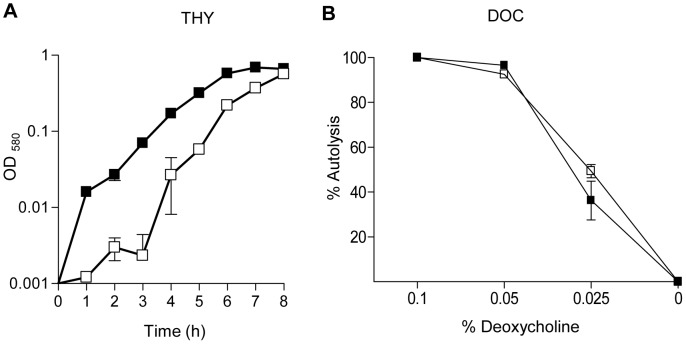
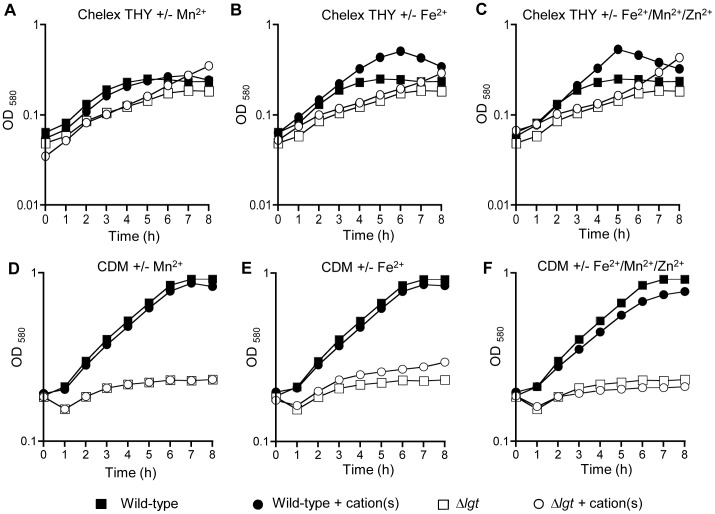
To investigate the physiological consequences of impaired cation transport, growth of the Δlgt and wild-type strains was compared in the complete medium THY, in THY treated with chelex to deplete cation availability, and in chemically defined media with known concentrations of cations. Although there were no significant difference in the doubling times between the wild-type and the Δlgt strain in THY (Table 3), the Δlgt strain did have a longer lag phase (Fig. 5A) demonstrating that the Δlgt strain had some growth defect even in this undefined complete medium. The Δlgt strain was also very slightly more susceptible to lysis in response to increasing concentrations of deoxycholate (DOC) (Fig. 5B). In chelex-THY the Δlgt strain had a markedly increased doubling time and reduced maximum OD580 compared to the wild-type strain (Table 3, Fig. 6A). Supplementation of chelex-THY with Zn2+ impaired growth of both the wild-type and Δlgt strains (Table 3), compatible with the known toxicity of excess zinc to S. pneumoniae [50]. Supplementation of chelex-THY with Mn2+ had little effect on growth of the wild–type strain but decreased the doubling time of the Δlgt strain and allowed it to eventually reach a maximum OD580 similar to the wild-type strain, suggesting a reduced ability to acquire Mn2+ is one cause of the reduced growth of the Δlgt strain in chelex-THY (Table 3, Fig. 6A). Supplementation of chelex-THY with Fe2+ markedly enhanced the maximum OD580 reached by the wild-type strain, indicating as previously demonstrated that lack of iron is the major limiting factor for the growth of this strain in chelex-THY [51] (Table 3, Fig. 6B). For the Δlgt strain supplementation with Fe2+ had a small effect on the maximum OD580 but no effect on the doubling time, suggesting the Δlgt strain was unable to fully utilise exogenous iron to overcome the growth defect caused by treating THY with chelex. Supplementation with all three cations enhanced growth of the wild-type strain no more than supplementation with Fe2+ alone, but for the Δlgt strain increased the maximum OD580 to a greater extent than supplementation with Fe2+ or Mn2+ alone (Table 3, Fig. 6C). These data suggest an impaired ability to obtain Mn2+ and Fe2+ by the S. pneumoniae Δlgt strain could cause growth defects in cation restricted conditions. Growth of the Δlgt strain was very poor in CDM media even when supplemented with all three cations (Fig. 6D to F) preventing the assessment of the effects of specific nutrient deficiencies using this media.
Table 3. Doubling times (mins) (SD)a for the wild-type and Δlgt strains in different media.
| Broth medium | Wild-type | Δlgt | Ratio Δlgt/wild-type |
| THY | 45.0 (3.6) | 44.7 (2.82) | 0.99 |
| C+Y+ glucosea/sucroseb | 49.3 (2.44) | 59.4 (1.56) | 1.20 |
| C+Y+ glucosea | 47.8 (3.79) | 63.0 (1.14) | 1.32 |
| C+Y+ raffinosec | 63.0 (4.85) | 77.0 (1.35) | 1.22 |
| C+Y+ maltotrioseb | 57.3 (4.56) | 83.5 (2.55) | 1.46 |
| Chelex THY | 92.4 (5.24) | 244.6 (8.23) | 2.65 |
| Chelex THY +Fe2+ | 96.7 (4.25) | 259.9 (6.58) | 2.69 |
| Chelex THY +Mn2+ | 94.5 (3.45) | 173.2 (8.65) | 1.83 |
| Chelex THY +Zn2+ | 106.6 (5.68) | n/c | – |
| Chelex THY +Fe2++Mn2++Zn2+ | 96.7 (3.68) | 166.3 (4.88) | 1.72 |
n/c = not calculated as the slope of increase of OD580 was too shallow for an accurate assessment of the doubling time.
= uptake PTS system dependent.
= uptake ABC transporter and PTS system dependent.
= uptake ABC transporter dependent only.
Figure 5. Growth of the wild-type and Δlgt strains in complete medium and susceptibility to DOC-induced lysis.
(A) Growth of the wild-type and Δlgt strains in THY assessed by measuring broth culture log10 OD580 over time. (B) Proportion of bacteria surviving after incubation with increasing concentrations of DOC. Squares represent the wild-type strain, triangles the Δlgt strain. Error bars represent SDs, and when not visible are within the symbol.
Figure 6. Growth of the wild-type and Δlgt strains in cation depleted media.
Assessed by measuring broth culture log10 OD580 over time. (A) to (C) Growth in cation depleted chelex-THY and (D) to (E) in CDM with and without cation supplementation: (A) and (D) with and without Mn2+ supplementation; (C) and (E) with and without Fe2+ supplementation; and (D) and (F) with and without combined Mn2+, Fe2+ and Zn2+ supplementation. Filled symbols represent growth of the wild-type strain, empty symbols growth of the Δlgt strain. Squares represent growth in unsupplemented media, inverted triangles in media supplemented with 50 µM Mn2+, diamonds in media supplemented with 50 µM Fe+2 and circles in media supplemented with 50 µM Mn+2, Fe+2 and Zn+2.
Effects of Limited Carbohydrate Sources on Growth of the S. pneumoniae Δlgt Strain
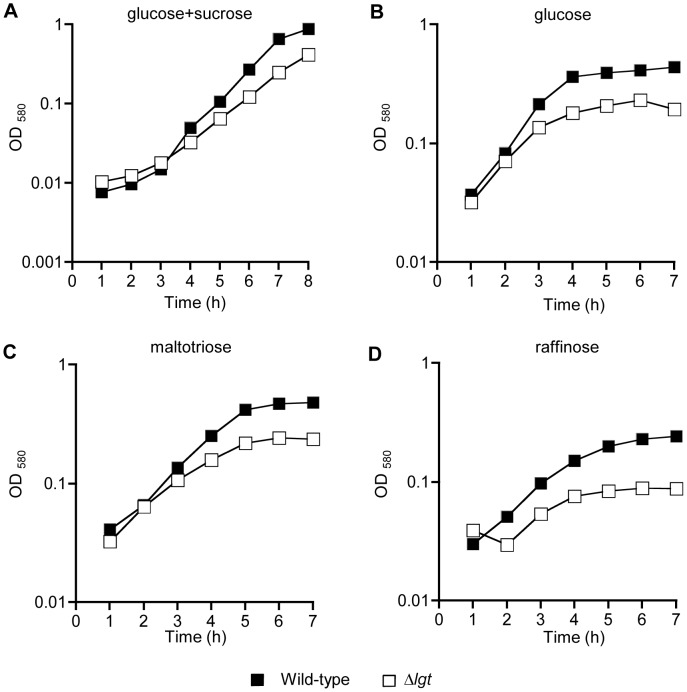
In the S. pneumoniae genome seven ABC transporters are annotated as involved in sugar uptake, including probable raffinose, galactose, and maltose/maltodextrin transporters but excluding a glucose transporter [31], [52]. Of these only raffinose is imported by an ABC transporter system alone, with import of the other sugars also occurring via by at least one PTS system [52]. The global reduction of lipoproteins in the Δlgt strain allowed the investigation of whether sugar ABC transporters are vital for growth in conditions with restricted carbon sources or whether PTS transporters provide adequate sugar uptake. The growth of the Δlgt strain was compared to the wild-type strain in the partially defined cation supplemented medium C+Y containing specific sugars as the sole carbohydrate source. Compared to the wild-type strain growth of the Δlgt strain was slightly delayed when sucrose and glucose in combination were the sole carbohydrate source similar to the growth results for THY (Table 3 and Fig. 7A). When glucose, raffinose, or maltotriose were the sole carbohydrate sources the impaired growth of the Δlgt strain compared to the wild-type was increased and a lower maximum OD580 achieved, with the most marked affect seen when raffinose was the sole carbohydrate source (Fig. 7B–D). There were also slight increases in the ratio of the doubling times for the wild-type and Δlgt strains in C+Y with glucose or maltotriose (Table 3). These data indicate that loss of lipoproteins significantly impaired growth of S. pneumoniae in restricted carbohydrate sources, despite the potential for PTS systems to compensate for reduced ABC transporter function.
Figure 7. Growth of the wild-type and Δlgt strains in restricted carbohydrate sources.
Assessed by measuring broth culture log10 OD580 over time. (A) C+Y medium supplemented with 10 mM sucrose and glucose each; (B) C+Y medium supplemented with 10 mM glucose; (C) C+Y medium supplemented with 10 mM maltotriose; and (D) C+Y medium supplemented with 10 mM raffinose. Error bars represent SDs, and when not visible are within the symbol. Filled symbols represent growth of the wild-type strain, empty symbols growth of the Δlgt strain.
Effects of lgt Deletion on Replication of S. pneumoniae in Physiological Fluids and Interactions with Neutrophils
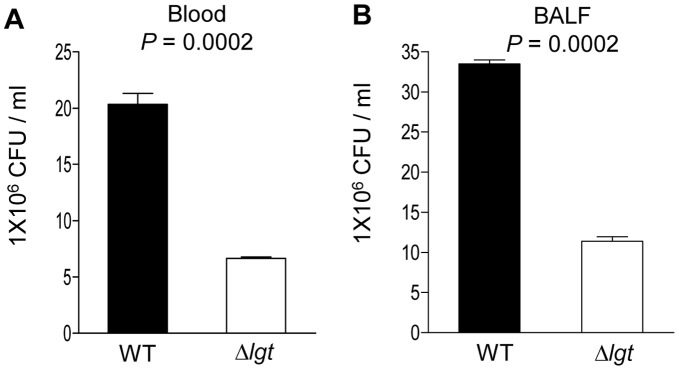
To investigate whether the effects of the lgt mutation on growth in restricted media results in impaired S. pneumoniae replication in physiologically relevant conditions, the replication rates of the wild-type and Δlgt strains in human blood and mouse bronchoalveolar lavage fluid (BALF) were compared. In blood, after 4 hours incubation CFU of the wild-type strain had increased 5.1-fold whereas CFU of the Δlgt strain had increased only 1.5-fold (Fig. 8A). The reduced increase in Δlgt strain CFU could be caused by poor replication of this strain in blood or by increased sensitivity to neutrophil killing. Flow cytometry assays showed that complement deposition was increased on the Δlgt strain compared to the wild-type, yet association with neutrophils (mainly due to phagocytosis) [53] was slightly lower (Fig. 9A and B). Overall, there were no differences seen between the wild-type and the Δlgt strain in a neutrophil-killing assay (Fig. 9C). Furthermore the Δlgt strain also replicated poorly in cell free BALF, with an increase in CFU of only 1.2-fold after 4 hours compared to 3.3-fold for the wild-type strain (Fig. 8B). These data suggest that the lgt mutant strain replicates poorly under physiological conditions and that the mutation has some effects on interactions with phagocytes without leading to major changes in bacterial susceptibility to neutrophil killing.
Figure 8. Growth of the wild-type and Δlgt strains in blood (A), or BALF (B).
(A) and (B) Bacterial CFU after 4 hours replication in blood (B) or BALF (B). Data is presented as the mean (SD) bacterial CFU per ml for the wild type (black columns) and the Δlgt strain (clear columns). P values were obtained using two-tailed Student’s t-tests.
Figure 9. Effects of the Δlgt mutation on interactions with neutrophils.
(A) C3b/iC3b deposition on the wild-type and Δlgt strains after incubation in 20% serum as measured by geometric mean fluorescent intensity using a flow cytometry assay. (B) Neutrophil association of the wild-type and Δlgt strains after incubation in 20% serum and human neutrophils as measured by mean relative fluorescent intensity using a flow cytometry assay. (C) Neutrophil killing assays of the wild-type (black columns) and Δlgt strains (clear columns) after incubation in different concentrations of human serum with fresh human neutrophils (MOI 1 bacteria to 800 neutrophils). There were no statistically significant differences between the wild-type and Δlgt strains. Data are presented as the percentage of the inoculum surviving after 30 mins incubation. For all panels, data are for mean values with error bars representing SDs. Statistical comparisons were made using unpaired two tailed T tests, and P values inserted for selected data showing significant differences.
Effect of lgt Deletion on Virulence of S. pneumoniae
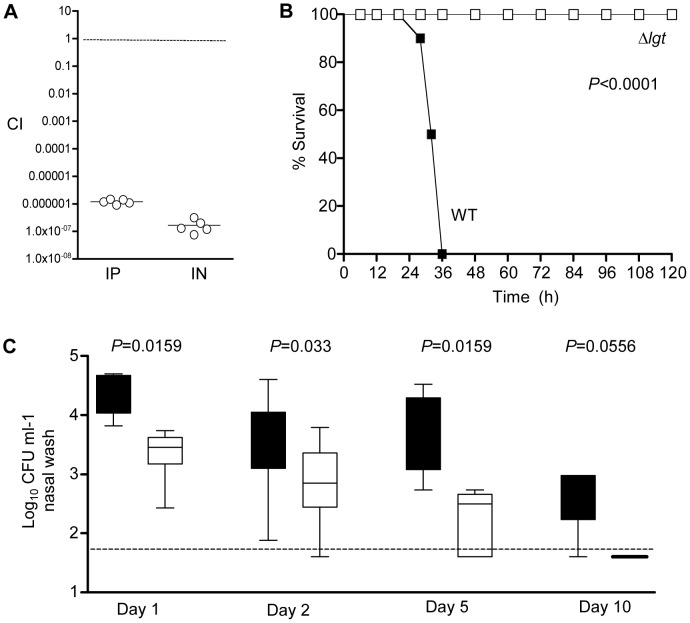
Previously the S. pneumoniae Δlgt strain has been shown to be impaired in virulence in a mouse model of pneumonia [23]. To investigate whether the virulence of the Δlgt strain is also impaired during sepsis we initially used competitive infections. For both septicaemia and pneumonia models, after inoculation in a 50/50 ratio with the wild-type 0100993 strain no Δlgt CFU were found in the spleen or lungs respectively despite recovery of >5 log wild-type CFU ml−1, giving CIs of less than 0.0001 (Fig. 10A). These data demonstrate that the Δlgt strain had a severe competitive disadvantage during infection, but even very low CIs sometimes do not reflect an inability to cause infection when the mutant strain is given as a pure inoculum [1], [44]. Hence, to further investigate the degree of attenuation in virulence of the Δlgt strain we used a mouse model of sepsis in which inoculation of 100 wild-type CFU is fatal. Groups of 10 mice were inoculated IP with 3×103 CFU of the wild-type or Δlgt 0100993 strain and the development of disease monitored over time. All mice inoculated with the wild-type strain developed fatal infection within 50 hours, whereas no mice infected with the Δlgt strain showed signs of disease and all survived beyond 14 days (Fig. 10B). To assess the ability of the Δlgt strain to establish infection in the lung, mice were inoculated IN with 5×106 CFU of the wild-type or Δlgt 0100993 strain and bacterial CFU calculated by serial plating of BALF recovered 4 hours later. For mice inoculated with the wild-type strain 4.3 log10 (SD 0.75) CFU ml−1 of BALF were recovered, whereas for the Δlgt strain no CFU were recovered from any mice. These data confirm that the lgt mutant is avirulent during systemic infection and is very rapidly cleared from the lungs in the pneumonia model, compatible with the in vitro growth defects for the Δlgt strain when cultured in blood or BALF. The physiological conditions in the nasopharynx are significantly different to those within the lung and the blood, and could potentially support growth of the lgt strain. Hence whether loss of lipoproteins prevented S. pneumoniae colonisation of the nasopharynx was investigated by transferring the Δlgt mutation to the capsular serotype 2 D39 strain which (unlike the serotype 3 0100993 strain) can colonise the mouse nasopharynx for at least 11 days [54]–[56]. The D39 Δlgt strain was able to establish colonisation of the nasopharynx for up to 5 days, demonstrating that this strain was still able to replicate at this anatomical site. However, the D39 Δlgt strain was entirely cleared from the nasopharynx by day 10, at which time point the majority of mice were still colonised with wild-type D39 (Fig. 10C). Furthermore, there were approximately half a log10 CFU fewer present per ml of nasal wash compared to the results for the wild-type D39 strain at days 1, 2, and 5 (Fig. 10C). Hence loss of surface lipoproteins strongly impaired nasopharyngeal colonisation by S. pneumoniae as well as preventing systemic infection.
Figure 10. Virulence of the Δlgt mutant strain.
(A) CIs for the Δlgt strain versus the wild-type strain in mouse models of septicaemia at 24 hours (IP inoculation, bacteria recovered from the spleen) and pneumonia at 48 hours (IN inoculation, bacteria recovered from the lungs). Each point represents the CI for a single animal. (B) Time course of the development of fatal infection for groups of 10 mice inoculated IP with 3×103 CFU of the wild-type (WT) and Δlgt strains (P<0.0001, log rank test). (C) Log10 bacterial CFU per ml of nasal wash recovered 1, 2, 5 and 10 days after nasopharyngeal colonisation of mice with 5×106 CFU of the wild-type (black columns) and Δlgt D39 (clear columns) strains. P values were calculated using Mann Whitney U tests for each time point.
Discussion
Lipoproteins are an important class of surface associated proteins that have diverse roles and frequently are involved in the virulence of bacterial pathogens. As lipoproteins are attached to the cell membrane by a single enzyme, Lgt, with additional processing by Lsp, deletion of the corresponding genes potentially allows the investigation of the global function of lipoproteins for an individual bacterial species. Several lgt mutants of Gram positive bacteria have been described, but the published data have shown that the phenotypes of lgt mutant strains vary with species. In particular, the lgt mutation has a strikingly pleiotropic effect on bacterial virulence, causing markedly reduced virulence for some species, no effect on virulence for other species, and even in some publications increasing the virulence of S. aureus and S. agalactiae [9], [22]. In contrast, the consequences of loss of Lgt for lipoprotein attachment are very similar between species, resulting in a greatly reduced lipoprotein content of the cell membrane for the lgt mutant strains [15], [16], [57]. Why lgt mutations vary in their associated phenotypes between species probably therefore reflect differences between bacterial species in the functional consequences of reduced lipoprotein content.
Previously, Petit et al. have demonstrated that in contrast to other streptococci a S. pneumoniae Δlgt strain was greatly reduced in virulence in a mouse model of pneumonia [23]. The reasons for the loss of virulence of the S. pneumoniae lgt mutant were not characterised. We have confirmed the loss of virulence of the S. pneumoniae Δlgt strain and demonstrated that this strain is also avirulent during systemic infection and is cleared from the lungs within 4 hours of inoculation. Multiple S. pneumoniae ABC transporters have significant roles during disease pathogenesis [33]–[35], including the manganese transporter Psa [36], the iron transporters Piu, Pia and Pit [3], [51], amino acid transporters [1], [37], the polyamine transporter Pot [38], the zinc transporters AdcA and AdcAII [39], [40], [42], and the phosphate transporter Pst [41]. We have therefore investigated the effects of the lgt mutation on ABC transporter related functions that might affect virulence, specifically concentrating on cation transport due to the profound effects of impaired manganese, iron or zinc uptake on S. pneumoniae virulence [36], [42], [51]. As expected, immunoblots, flow cytometry and immunofluorescence all showed a marked reduction in surface-associated lipoproteins for the S. pneumoniae Δlgt strain and retention of the N terminal signal peptide, a similar phenotype to the Δlgt mutants for most other bacteria [15], [16], [57]. The phenotype of the S. pneumoniae Δlgt strain suggested this strain has impaired ability to acquire a range of cations, with markedly reduced Zn2+ uptake, an increased sensitivity to oxidative stress compatible with low Mn2+ levels, and an accentuated growth defect in cation-depleted medium. In addition the Δlgt strain had greatly reduced intracellular levels of Fe2+, Mn2+, Zn2+, Cu2+ and Ni2+, cations that are either known to be or are predicted to be acquired by S. pneumoniae or other bacterial pathogens using ABC transporters [2], [3], [5], [36], [39], [58]. Hence, the Δlgt strain has defects in acquisition of several cations that are known to affect virulence. Although we have been unable to complement the Δlgt strain, RT-PCR confirmed continued transcription of the downstream genes in the lgt operon, and the multiple phenotypes of this strain compatible with impaired ABC transporter function are unlikely to be caused by an unidentified secondary mutation that occurred during the transformation process.
Reduced iron uptake is thought to partially explain reduced virulence of a S. aureus lgt mutant [27], and similarly reduced uptake of cations could readily explain why the S. pneumoniae Δlgt strain cannot cause invasive infection. In addition, the effects of the lgt mutation on other ABC transporters could also be relevant. For example, growth curves also suggested the S. pneumoniae Δlgt strain had impaired utilisation sugar sources. The largest difference in OD580 compared to the wild-type strain was seen when raffinose was the sole carbohydrate source, supporting recent data suggesting raffinose is the only sugar transported only by an ABC transporter system [52]. However the primary sugar available in blood is glucose, which is transported by a PTS systems alone [52]. Impaired uptake of other ABC transporter substrates such as phosphate, polyamines and amino acids could also cause reduced virulence, as might loss of function of non-ABC transporter lipoproteins such as PpmA and SlrA [1], [29], [38], [41], [59]. The main mechanisms of bacterial clearance during S. pneumoniae infection is neutrophil phagocytosis [60]. Although the increased sensitivity of the Δlgt strain to oxidative stress might be assumed to result in increased susceptibility to neutrophil oxidative killing mechanisms, S. pneumoniae killing is independent of oxidative killing mechanisms [61], and mice with defects in oxidative killing are actually more resistant to S. pneumoniae infection [62]. Furthermore we have previously demonstrated that the effects of defects in resistance to oxidative killing on virulence were independent of oxidative killing mechanisms [63]. In vitro assays gave conflicting results about the susceptibility of the Δlgt strain to opsonophagocytosis. This strain had some increased sensitivity to complement activity yet reduced uptake by neutrophils when incubated in human sera, possibly due to reduced lipoprotein targets for specific serum antibody [46]. Overall the Δlgt strain did not have an increased susceptibility to neutrophil killing. Other important immune mechanisms such as anti-bacterial peptides or effects of the Δlgt mutation on adhesion could potentially contribute to the reduced virulence of this strain. However, these immune mechanisms are mainly thought to be important during mucosal infection [60], [64] and are unlikely to cause such a severe virulence defect after intraperitoneal inoculation. Although increased susceptibility to host immunity may account for some of the loss of virulence, the data suggest loss of virulence of the S. pneumoniae Δlgt strain was largely due to its major growth defects under physiological conditions as a consequence of impaired ABC transporter function. This was confirmed by demonstrating that the Δlgt strain had a greatly reduced replication rate in blood or BALF compared to the wild-type parental strain.
Why does loss of Lgt has such a strong effect on the virulence of S. pneumoniae compared to the same mutation in other streptococci? Similar numbers of lipoproteins are expressed by S. pneumoniae as other streptococci (eg S. agalactiae), so it is not simply that there are more lipoproteins in S. pneumoniae. However, unlike the S. pneumoniae Δlgt strain mutant the S. agalactiae lgt mutant had no growth defect in cation-depleted medium [19] and the S. sanguinis lgt mutant only had a growth defect in complete medium during competitive infection with the wild-type strain [20]. In addition, reduced zinc uptake has a more profound effect on S. pneumoniae virulence than for other bacterial pathogens [42], [65]–[67]. These data suggest that lipoprotein-dependent functions are generally of greater importance during S. pneumoniae infection than they are for other streptococci, resulting in a stronger phenotype for the Δlgt mutant in animal models. Despite the profound effects on virulence during lung and systemic infection, the S. pneumoniae Δlgt strain could colonise the nasopharynx for up to 5 days demonstrating lipoprotein functions are of lesser importance for bacterial replication in the nasopharyngeal environment compared to the lung or in the blood. This observation perhaps suggests that the acquisition of lipoprotein-dependent functions is one factor that allows S. pneumoniae to be an invasive pathogen.
Previously we have shown that deletion of the zinc uptake lipoproteins adcA and adcAII prevented nasopharyngeal colonisation by S. pneumoniae [42], a more profound defect than observed with the S. pneumoniae Δlgt strain. In addition, despite the range of functions associated with ABC transporters and lipoproteins that together might be predicted to essential for bacterial viability, the S. pneumoniae Δlgt strain still grew in complete and some restricted media as well as the mouse nasopharynx. This suggests that the partial retention of prolipoproteins on the surface of the lgt mutant shown by the immunoblots and immunofluorescence results in some functional activity. Alternatively uptake ABC transporters functions may have a residual level of function even without their lipoprotein component, but this seems unlikely given the profound phenotype of the adcA and adcAII double mutant [42]. Similarly for some phenotypes in S. sanguinis, S. equi, and B. subtilus deletion of a single lipoprotein had stronger effects than mutation of lgt, and this was thought to be due to partial lipid anchoring of prolipoproteins via the retained N terminal signal peptide [15], [20], [21]. For the S. pneumoniae Δlgt strain some retention of prolipoprotein in the cell membrane may allow adequate ABC transporter function for growth under conditions with limited stress such as in complete medium or the nasopharynx. However, blood or the lungs are likely to be more stringent environments that require a greater level of lipoprotein function for sufficient S. pneumoniae replication to cause infection, resulting in loss of virulence of the Δlgt strain. For many Gram positive pathogens lipoproteins are major ligands for TLR2-dependent inflammatory responses [8], [68], but their role during inflammatory responses to S. pneumoniae has not been evaluated as yet. The effects of lipoproteins on inflammatory responses need investigating as potentially compensatory TLR4 and TLR-independent mechanisms of inflammation during S. pneumoniae infection have been described, and data from animal models questions the overall importance of TLR2 during S. pneumoniae infection [69]–[73]. Even if lipoproteins are important pro-inflammatory signals during infection with S. pneumoniae and the lgt strain was able to avoid immune recognition, an inability to replicate during invasive infection would still prevent this strain from causing significant infection.
In conclusion, we have presented data demonstrating that deletion of the S. pneumoniae lgt results in a mutant strain with reduced cation uptake, increased sensitivity to cation and sugar restriction, and with poor growth in physiological media resulting in an inability to cause invasive infection. This striking contrast with the infection phenotypes of lgt mutants for some other bacterial pathogens suggest lipoprotein and ABC transporters have a corresponding greater importance during the development of infections caused by S. pneumoniae than they do for at least some other streptococci.
Methods and Materials
Ethics Statement
Experiments were approved by the UCL Biological Services Ethical Committee and the UK Home Office (Project Licence PPL70/6510). Experiments were performed according to UK national guidelines for animal use and care, under UK Home Office licence.
Bacterial Strains and Culture Conditions
S. pneumoniae strains used in this work are listed in Table 4. The mutant strains used for this work were constructed in the 0100993 capsular serotype 3 clinical isolate [34]. S. pneumoniae strains were cultured at 37°C and 5% CO2 on Columbia agar supplemented with 5% horse blood, in Todd–Hewitt broth supplemented with 0.5% yeast extract (THY). chloramphenicol (10 µg ml−1) and erythromycin (0.2 µg ml−1) were added to blood agar plates where appropriate. Cations were depleted from the THY medium by treating it with 2% chelex-100 (Bio-Rad) overnight under continuous agitation and filtering the medium with 0.45 µ filters [44], [51]. Growth of strains was compared in broth culture by measuring OD580 in THY, THY-chelex, Chemically Defined Medium (CDM) [74] and a semi synthetic medium, C+Y [75] media at regular intervals. Working stocks of bacterial cultures in THY (OD580 0.3–0.4) were stored at −80°C with 10% glycerol.
Table 4. Strains and primers used in this study.
| Name | Description/sequence (source/reference)a |
| Strains | |
| 0100993 | S. pneumoniae capsular serotype 3 clinical isolate [34] |
| Δlgt ST3 | 0100993 with in-frame deletion of Sp1412: cmr (this study) |
| JSB3PpmA − | 0100993 with deletion of PpmA : eryr [44] |
| D39 | S. pneumoniae capsular serotype 2 strain (kind gift from James Paton, University of Adelaide) |
| Δlgt D39 | D39 with in-frame deletion of Sp1412: cmr (this study) |
| Primers | |
| Sp1411F | GAGTCATCAAGAGCTTCGG |
| Cm-1411R | GCCTAATGACTGGCTTTTATAAATGTTAGAAGTTGCATATATTC |
| Cm-1413F | ACATTATCCATTAAAAATCAAATCAAGCAT TTTGCACCTCATTT |
| Sp1413R | CATGCCTTCCAACAGCCG |
| CmF | TTATAAAAGCCAGTCATTAG |
| CmR | TTTGATTTTTAATGGATAATG |
| hprRTF | GGTGACCACGTTTGACAAG |
| hprRTR | CTGATCAGCATGCCTTCC |
| lgtRTF | GGCCGTGATATGACCTCG |
| lgtRTR | GTTTGGCCATTTACGGTGG |
| Sp1411RTF | GCTGACAGACTTGCACCAG |
| Sp1411RTR | GCTTGGTCGTGTCATCGATG |
| Sp1410RTF | GAGTCATCAAGAGCTTCGG |
| SP1410RTR | GGTGCAGCTCTTGCCTTG |
Construction of Δlgt Deletion Mutant Strain
For the in-frame deletion of lgt (Sp1412), a construct was created in which 703 bp of flanking DNA 5′ to the SP1412 ATG (primers Sp1413F and Cm-Sp1413R) and 750 bp of flanking DNA 3′ to the Sp1412 ORF (primers Cm-Sp1411F and Sp1411R) were amplified by PCR from S. pneumoniae 0100993 genomic DNA and fused with the chloramphenicol resistance marker (cat, amplified from pID701, a suicide vector containing cat gene, with primers CmF and CmR) by overlap extension PCR [76]. Primers used for the overlap extension PCRs are shown in Table 2. The constructs were transformed into S. pneumoniae by homologous recombination and allelic replacement using competence stimulating peptide (CSP-1) and standard protocols [34], [77].
DNA, RNA Extraction and RT PCR
Genomic DNA and total RNA were isolated from S. pneumoniae strains using the Wizard genomic DNA isolation kit and the SV total RNA isolation system (Promega) respectively, following the manufacturer’s instructions except that cells were incubated with 0.1% deoxycholicacid (Sigma) at 37°C for 10 min before extraction. 0.5% RNasin (Promega) was added to extracted RNA to prevent it from degradation. cDNA was derived and amplified from RNA using the Access RT-PCR system (Promega) and target specific primers. Primers used for the transcriptional analysis of the Sp1410-1413 operon are described in Table 2. The National Centre for Biotechnology Information website (http://blast.ncbi.nlm.nih.gov/Blast) was used for DNA and protein BLAST searches.
Protein Immunoblots and Triton X-114 Extraction
Protein samples from whole cell lysates and triton X-114 extracts were separated on SDS-PAGE 12% resolving gels, blotted onto nitrocellulose membranes and probed with specific antisera (1∶2500 dilution) according to standard protocols [78]. Membrane proteins were extracted by triton X-114 extraction as described previously [45], [79]. Briefly, exponentially growing S. pneumoniae cells were digested with 100 µl of 0.1% DOC (Sigma) in PBS for 30 min at 37°C and sonicated with 3 pulses of 15 sec with a 10 sec cooling time using a Soniprep 150 (Sanyo) ultrasonicator. 800 µl of PBS and 100 µl of triton X-114 (10% in PBS) were then added to the lysates, which were incubated at 4°C for 2 h followed by centrifugation to pellet insoluble debris. Supernatants were then incubated at 37°C for 30 min to allow phase separation, followed by centrifugation at room temperature to pellet the detergent phase proteins. The detergent phase proteins were washed and diluted 1∶2 in PBS prior to solubilization in Laemmli sample buffer for SDS-PAGE.
IgG Binding to Live S. pneumoniae TIGR4
Flow cytometry assays of IgG deposition on the surface of S. pneumoniae strains were performed using a previously described protocol of Jomaa et al. [80] and mouse sera obtained from surviving mice after systemic infection with an attenuated TIGR4 mutant strain (unpublished data). Bacterial pellets containing 5×106 CFU, pooled mouse serum (1∶5 dilution in PBS), and 1∶50 dilution of phycoerythrin conjugated goat anti-mouse IgG (Jackson ImmunoResearch) were used for the assay. Results are presented as the percentage of bacteria positive for IgG binding.
Immunofluorescence Microscopy
The immunofluorescence microscopy was performed according to a previously described method [4]. Briefly, bacteria grown to an OD580 of 0.3 in THY broth were washed in PBS prior to fixing in 3% paraformaldehyde for 15 min at room temperature followed by 45 min on ice. Cells were then deposited onto poly-L-lysine-coated slides and permeabilized in cold methanol for 5 min. Slides were blocked for 30 min at room temperature with 5% (w/v) skimmed dry milk in PBS (saturation buffer) and then incubated for 1 h with anti-PpmA antibody (1∶50 dilution) in saturation buffer. The slides were then washed twice in PBS and incubated in the dark with a 1∶200 dilution of FITC-conjugated goat anti-rabbit immunoglobulin G (Jackson immunoresearch) in saturation buffer for 1 h. After successive washes with PBS and water, cells were incubated with ProLong gold mounting agent (Invitrogen, UK) containing 6-diamidino-2-phenylindole (DAPI) and dried overnight. The slides were examined with an Zeiss Axioscope microscope equipped with Zeiss Acroplan 100x O-PH/3 objective and a QImaging Retiga- SRV 1394 cooled charge-coupled device camera.
C3b/iC3b Deposition and Neutrophil Phagocytosis Assays
To assess the effect of lgt deletion on the complement deposition on S. pneumoniae and on the interaction with phagocytes flow cytometry assays were performed according to previously described methods [46], [53]. For C3b/iC3b deposition 2×106 CFU of bacterial pellets, human serum (1∶4 dilution in PBS) and FITC-conjugated polyclonal goat anti-human C3 antibody (ICN Cappel, Aurora, OH, USA, 1∶300) were used. The proportion of bacteria positive for C3b/iC3b and mean fluorescence intensity (MFI) was obtained using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, USA), collecting data from at least 20,000 bacteria. For the opsonophagocytosis assay, the proportion of freshly extracted human neutrophils associated with 5, 6-carboxyfluorescein-succinimidyl ester (FAM-SE, Molecular Probes, Eugene, Oreg) labelled fluorescent bacteria (1×106 CFU) was measured by flow cytometry after opsonization with 1/8 and 1/4 dilutions of normal human serum (NHS) and at a multiplicity of infection of 10.
Neutrophil Killing Assays
For the killing assays, S. pneumoniae strains previously incubated in various concentrations of human sera obtained from healthy volunteers (diluted in PBS) at room temperature for 30 mins were added to fresh human neutrophils extracted from blood [81] in HBSS with divalent cations at an MOI of 1∶800. After 45 mins at 37°C, the numbers of surviving bacteria were calculated by plating serial dilutions, and the results expressed as a percentage of the inoculum CFU.
ABC Transporter Phenotype Analysis
Sensitivity to oxidative stress and cation transport were studied by exposure of S. pneumoniae strains (106 cfu) to 60 mM of paraquat (Sigma) [30] at 37°C for 20, 40 and 60 min. The proportion of survivors after the exposure was calculated by plating serial dilutions on blood agar plates. Zn+2 uptake was measured by a fluorescence assay as described by Bayle et al [42]. Bacteria (2×108 CFU) grown to mid log phase in CDM were washed in PBS and incubated with 5 µM FluoZin-3 AM, (acetoxy methyl ester) cell permeant (Molecular Probes) for 30 min at room temperature. The bacteria were washed three times in PBS and then incubated for a further 30 min to allow complete de-esterification of intracellular acetoxymethyl FluoZin-3 esters. All the experiments were performed at 37°C under stirring conditions using a Photon Technology International Quanta Master I spectrofluorimeter. Upon the addition of 10 µM of ZnSO4 and excitation of the sample at 494 nm, fluorescence emission was recorded at 516 nm and the rate of zinc uptake (arbitrary unit sec−1) was calculated from the slope of the curve.
ICP-MS Analysis
Total internal concentrations of metal ions was carried out by the highly sensitive ICP-MS analysis [82]. 5×108 CFU of mid log phase bacteria grown in THY–chelex were washed extensively with chelex treated PBS and resuspended in 5 ml of 2% nitric acid. The bacteria were further lysed by sonication (3 pulses of 20 sec with a 20 sec cooling time) using a Soniprep 150 (Sanyo) ultrasonicator and filtered through 0.45 µ millipore filters to discard cellular debris. MilliQ water and PBS needed for dilution and washes were treated over night with Chelex 100. The ICP-MS analysis was carried out with a Varian ICP-MS instrument.
Growth in Physiological Fluids
Replication of S. pneumoniae strains in freshly obtained human blood and frozen mouse bronchoalveolar lavage fluid (BALF) was determined by inoculating with 2×106 CFU ml−1. After 4 h of growth at 37°C under CO2, serial dilutions were plated on to blood agar plates to enumerate the CFU.
In vivo Studies
All animal experiments conformed to institutional and governmental guidelines and regulations. Outbred CD1 female white mice (Charles Rivers Breeders) weighing 18–22 g were used for animal infection experiments. For the pneumonia model mice were anaesthetized by inhalation of halothane (Zeneca) and inoculated IN (intra nasal) with an inoculum of 5×106 CFU/mouse in 50 µl volume, and for the septicaemia model by IP (intra peritoneal) inoculation of 7×103 CFU in 100 µl volume. Mixed infection experiments were used to calculate CIs (the ratio of mutant to wild-type strain recovered from the mice divided by the ratio of mutant to wild-type strain in the inoculum). Mice were sacrificed after 24°h (septicaemia model) or 48°h (pneumonia model), target organs recovered and homogenized in sterile PBS, before plating dilutions on non-selective and selective medium for calculation of the CI. For the nasopharygneal colonisation model, 107 CFU of bacteria in 10 µl were administered by intranasal inoculation under halothane general anaesthesia, and nasal washes were obtained after various time points. Serial dilutions of the samples were plated onto Columbia blood agar plates containing optochin (50 µg ml−1) and/or gentamycin (5 µg ml−1) to differentiate pneumococcus from other contaminating streptococci and to enumerate CFU. To compare the course of disease between the Δlgt and wild-type strains, groups of 10 mice were inoculated with 3×103 CFU IP of either strain and closely observed over the next 14 days. Mice were sacrificed when they exhibited the following signs of disease: hunched posture, poor mobility, weight loss, coughing and tachypnoea.
Statistical Analysis
All in vitro data use three or more samples per strain tested, and are representative of experiments repeated at least twice that gave similar results. Results for phenotype assays were compared between strains using Student’s t test or ANOVA. Experiments comparing the course of disease between the Δlgt and wild-type strains were repeated twice, giving similar results, and the data analysed using the log rank method for survival.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was undertaken at UCLH/UCL, who received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centre’s funding scheme, and was supported by the UCL Charities funding and grants from the Wellcome Trust (grant reference 076442) and the Medical Research Council (grants G0700829 and G0600410). Financial support from the Région Rhône-Alpes through the “Explo’ra pro 2010” programme to CD is also acknowledged. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Basavanna S, Khandavilli S, Yuste J, Cohen JM, Hosie AH, et al. Screening of Streptococcus pneumoniae ABC transporter mutants demonstrates that LivJHMGF, a branched-chain amino acid ABC transporter, is necessary for disease pathogenesis. Infect Immun. 2009;77:3412–3423. doi: 10.1128/IAI.01543-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown JS, Ogunniyi AD, Woodrow MC, Holden DW, Paton JC. Immunization with components of two iron-uptake ABC transporters protects mice against systemic infection with Streptococcus pneumoniae. Infect Immun. 2001;69:6702–6706. doi: 10.1128/IAI.69.11.6702-6706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown JS, Gilliland SM, Ruiz-Albert J, Holden DW. Characterization of pit, a Streptococcus pneumoniae iron uptake ABC transporter. Infect Immun. 2002;70:4389–4398. doi: 10.1128/IAI.70.8.4389-4398.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loisel E, Chimalapati S, Bougault C, Imberty A, Gallet B, et al. Biochemical characterization of the histidine triad protein PhtD as a cell surface zinc-binding protein of pneumococcus. Biochem. 2011;50:3551–3558. doi: 10.1021/bi200012f. [DOI] [PubMed] [Google Scholar]
- 5.Hiron A, Posteraro B, Carriere M, Remy L, Delporte C, et al. A nickel ABC-transporter of Staphylococcus aureus is involved in urinary tract infection. Mol Microbiol. 2010;77:1246–1260. doi: 10.1111/j.1365-2958.2010.07287.x. [DOI] [PubMed] [Google Scholar]
- 6.Jonsson IM, Juuti JT, Francois P, AlMajidi R, Pietiainen M, et al. Inactivation of the Ecs ABC transporter of Staphylococcus aureus attenuates virulence by altering composition and function of bacterial wall. PLoS One. 2010;5:e14209. doi: 10.1371/journal.pone.0014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banaiee N, Kincaid EK, Buchwald U, Jacobs WR, Jr, Ernst JD. Potent inhibition of macrophage responses to IFN-gamma by live virulent Mycobacterium tuberculosis is independent of mature mycobacterial lipoproteins but dependent on TLR2. J Immunol. 2006;176:3019–3027. doi: 10.4049/jimmunol.176.5.3019. [DOI] [PubMed] [Google Scholar]
- 8.Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 9.Bubeck Wardenburg J, Williams WA, Missiakas D. Host defenses against Staphylococcus aureus infection require recognition of bacterial lipoproteins. Proc Natl Acad Sci USA. 2006;103:13831–13836. doi: 10.1073/pnas.0603072103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tokunaga M, Tokunaga H, Wu HC. Post-translational modification and processing of Escherichia coli prolipoprotein in vitro. Proc Natl Acad Sci USA. 1982;79:2255–2259. doi: 10.1073/pnas.79.7.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inouye S, Hsu CP, Itakura K, Inouye M. Requirement for signal peptide cleavage of Escherichia coli prolipoprotein. Science. 1983;221:59–61. doi: 10.1126/science.6344218. [DOI] [PubMed] [Google Scholar]
- 12.Sutcliffe IC, Harrington DJ. Pattern searches for the identification of putative lipoprotein genes in Gram-positive bacterial genomes. Microbiol. 2002;148:2065–2077. doi: 10.1099/00221287-148-7-2065. [DOI] [PubMed] [Google Scholar]
- 13.Sankaran K, Wu HC. Lipid modification of bacterial prolipoprotein. Transfer of diacylglyceryl moiety from phosphatidylglycerol. J Biol Chem. 1994;269:19701–19706. [PubMed] [Google Scholar]
- 14.Tjalsma H, Kontinen VP, Pragai Z, Wu H, Meima R, et al. The role of lipoprotein processing by signal peptidase II in the Gram-positive eubacterium Bacillus subtilis. Signal peptidase II is required for the efficient secretion of alpha-amylase, a non-lipoprotein. J Biol Chem. 1999;274:1698–1707. doi: 10.1074/jbc.274.3.1698. [DOI] [PubMed] [Google Scholar]
- 15.Leskela S, Wahlstrom E, Kontinen VP, Sarvas M. Lipid modification of prelipoproteins is dispensable for growth but essential for efficient protein secretion in Bacillus subtilis: characterization of the lgt gene. Mol Microbiol. 1999;31:1075–1085. doi: 10.1046/j.1365-2958.1999.01247.x. [DOI] [PubMed] [Google Scholar]
- 16.Stoll H, Dengjel J, Nerz C, Gotz F. Staphylococcus aureus deficient in lipidation of prelipoproteins is attenuated in growth and immune activation. Infect Immun. 2005;73:2411–2413. doi: 10.1128/IAI.73.4.2411-2423.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gan K, Gupta SD, Sankaran K, Schmid MB, Wu HC. Isolation and characterization of a temperature-sensitive mutant of Salmonella typhimurium defective in prolipoprotein modification. J Biol. 1993;Chem268:16544–16550. [PubMed] [Google Scholar]
- 18.Yamagata H, Taguchi N, Daishima K, Mizushima S. Genetic characterization of a gene for prolipoprotein signal peptidase in Escherichia coli. Mol Gen Genet : MGG. 1983;192:10–14. doi: 10.1007/BF00327640. [DOI] [PubMed] [Google Scholar]
- 19.Bray BA, Sutcliffe IC, Harrington DJ. Impact of lgt mutation on lipoprotein biosynthesis and in vitro phenotypes of Streptococcus agalactiae. Microbiol. 2009;155:1451–1458. doi: 10.1099/mic.0.025213-0. [DOI] [PubMed] [Google Scholar]
- 20.Das S, Kanamoto T, Ge X, Xu P, Unoki T, et al. Contribution of lipoproteins and lipoprotein processing to endocarditis virulence in Streptococcus sanguinis. J Bacteriol. 2009;191:4166–4179. doi: 10.1128/JB.01739-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton A, Robinson C, Sutcliffe IC, Slater J, Maskell DJ, et al. Mutation of the maturase lipoprotein attenuates the virulence of Streptococcus equi to a greater extent than does loss of general lipoprotein lipidation. Infect Immun. 2006;74:6907–6919. doi: 10.1128/IAI.01116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henneke P, Dramsi S, Mancuso G, Chraibi K, Pellegrini E, et al. Lipoproteins are critical TLR2 activating toxins in group B streptococcal sepsis. J Immunol. 2008;180:6149–6158. doi: 10.4049/jimmunol.180.9.6149. [DOI] [PubMed] [Google Scholar]
- 23.Petit CM, Brown JR, Ingraham K, Bryant AP, Holmes DJ. Lipid modification of prelipoproteins is dispensable for growth in vitro but essential for virulence in Streptococcus pneumoniae. FEMS Microbiol Lett. 2001;200:229–233. doi: 10.1111/j.1574-6968.2001.tb10720.x. [DOI] [PubMed] [Google Scholar]
- 24.Arimoto T, Igarashi T. Role of prolipoprotein diacylglyceryl transferase (Lgt) and lipoprotein-specific signal peptidase II (LspA) in localization and physiological function of lipoprotein MsmE in Streptococcus mutans. Oral Microbiol Immunol. 2008;23:515–519. doi: 10.1111/j.1399-302X.2008.00455.x. [DOI] [PubMed] [Google Scholar]
- 25.Machata S, Tchatalbachev S, Mohamed W, Jansch L, Hain T, et al. Lipoproteins of Listeria monocytogenes are critical for virulence and TLR2-mediated immune activation. J Immunol. 2008;181:2028–2035. doi: 10.4049/jimmunol.181.3.2028. [DOI] [PubMed] [Google Scholar]
- 26.Wichgers Schreur PJ, Rebel JM, Smits MA, van Putten JP, Smith HE. Lgt processing is an essential step in Streptococcus suis lipoprotein mediated innate immune activation. PLoS One. 2011;6:e22299. doi: 10.1371/journal.pone.0022299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmaler M, Jann NJ, Ferracin F, Landolt LZ, Biswas L, et al. Lipoproteins in Staphylococcus aureus mediate inflammation by TLR2 and iron-dependent growth in vivo. J Immunol. 2009;182:7110–7118. doi: 10.4049/jimmunol.0804292. [DOI] [PubMed] [Google Scholar]
- 28.Burnette-Curley D, Wells V, Viscount H, Munro CL, Fenno JC, et al. FimA, a major virulence factor associated with Streptococcus parasanguis endocarditis. Infect Immun. 1995;63:4669–4674. doi: 10.1128/iai.63.12.4669-4674.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hermans PW, Adrian PV, Albert C, Estevao S, Hoogenboezem T, et al. The streptococcal lipoprotein rotamase A (SlrA) is a functional peptidyl-prolyl isomerase involved in pneumococcal colonization. J Biol Chem. 2006;281:968–976. doi: 10.1074/jbc.M510014200. [DOI] [PubMed] [Google Scholar]
- 30.Tseng HJ, McEwan AG, Paton JC, Jennings MP. Virulence of Streptococcus pneumoniae: PsaA mutants are hypersensitive to oxidative stress. Infect Immun. 2002;70:1635–1639. doi: 10.1128/IAI.70.3.1635-1639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293:498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- 32.Bergmann S, Hammerschmidt S. Versatility of pneumococcal surface proteins. Microbiol. 2006;152:295–303. doi: 10.1099/mic.0.28610-0. [DOI] [PubMed] [Google Scholar]
- 33.Hava DL, Camilli A. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol Microbiol. 2002;45:1389–1406. [PMC free article] [PubMed] [Google Scholar]
- 34.Lau GW, Haataja S, Lonetto M, Kensit SE, Marra A, et al. A functional genomic analysis of type 3 Streptococcus pneumoniae virulence. Mol Microbiol. 2001;40:555–571. doi: 10.1046/j.1365-2958.2001.02335.x. [DOI] [PubMed] [Google Scholar]
- 35.Polissi A, Pontiggia A, Feger G, Altieri M, Mottl H, et al. Large-scale identification of virulence genes from Streptococcus pneumoniae. Infect Immun. 1998;66:5620–5629. doi: 10.1128/iai.66.12.5620-5629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marra A, Lawson S, Asundi JS, Brigham D, Hromockyj AE. In vivo characterization of the psa genes from Streptococcus pneumoniae in multiple models of infection. Microbiol. 2002;148:1483–1491. doi: 10.1099/00221287-148-5-1483. [DOI] [PubMed] [Google Scholar]
- 37.Molzen TE, Burghout P, Bootsma HJ, Brandt CT, van der Gaast-de Jongh CE, et al. Genome-wide identification of Streptococcus pneumoniae genes essential for bacterial replication during experimental meningitis. Infect Immun. 2011;79:288–297. doi: 10.1128/IAI.00631-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ware D, Jiang Y, Lin W, Swiatlo E. Involvement of potD in Streptococcus pneumoniae polyamine transport and pathogenesis. Infect Immun. 2006;74:352–361. doi: 10.1128/IAI.74.1.352-361.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dintilhac A, Alloing G, Granadel C, Claverys JP. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol Microbiol. 1997;25:727–739. doi: 10.1046/j.1365-2958.1997.5111879.x. [DOI] [PubMed] [Google Scholar]
- 40.Loisel E, Jacquamet L, Serre L, Bauvois C, Ferrer JL, et al. AdcAII, a new pneumococcal zn-binding protein homologous with ABC transporters: biochemical and structural analysis. J Mol Biol. 2008;381:594–606. doi: 10.1016/j.jmb.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 41.Orihuela CJ, Mills J, Robb CW, Wilson CJ, Watson DA, et al. Streptococcus pneumoniae PstS production is phosphate responsive and enhanced during growth in the murine peritoneal cavity. Infect Immun. 2001;69:7565–7571. doi: 10.1128/IAI.69.12.7565-7571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bayle L, Chimalapati S, Schoehn G, Brown J, Vernet T, et al. Zinc uptake by Streptococcus pneumoniae depends on both AdcA and AdcAII and is essential for normal bacterial morphology and virulence. Mol Microbiol. 2011;82:904–916. doi: 10.1111/j.1365-2958.2011.07862.x. [DOI] [PubMed] [Google Scholar]
- 43.Marion C, Aten AE, Woodiga SA, King SJ. Identification of an ATPase, MsmK, which energizes multiple carbohydrate ABC transporters in Streptococcus pneumoniae. Infect Immun. 2011;79:4193–4200. doi: 10.1128/IAI.05290-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khandavilli S, Homer KA, Yuste J, Basavanna S, Mitchell T, et al. Maturation of Streptococcus pneumoniae lipoproteins by a type II signal peptidase is required for ABC transporter function and full virulence. Mol Microbiol. 2008;67:541–557. doi: 10.1111/j.1365-2958.2007.06065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- 46.Chimalapati S, Cohen J, Camberlein E, Durmort C, Baxendale H, et al. Infection with conditionally virulent Streptococcus pneumoniae Δpab bacteria induces antibody to conserved protein antigens but does not protect against systemic infection with heterologous strains. Infect. 2011;Immun79:4965–4976. doi: 10.1128/IAI.05923-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gee KR, Zhou ZL, Ton-That D, Sensi SL, Weiss JH. Measuring zinc in living cells.: A new generation of sensitive and selective fluorescent probes. Cell Calcium. 2002;31:245–251. doi: 10.1016/S0143-4160(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 48.Pezza RJ, Villarreal MA, Montich GG, Argarana CE. Vanadate inhibits the ATPase activity and DNA binding capability of bacterial MutS. A structural model for the vanadate-MutS interaction at the Walker A motif. Nucleic Acids Res. 2002;30:4700–4708. doi: 10.1093/nar/gkf606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCluskey J, Hinds J, Husain S, Witney A, Mitchell TJ. A two-component system that controls the expression of pneumococcal surface antigen A (PsaA) and regulates virulence and resistance to oxidative stress in Streptococcus pneumoniae. Mol Microbiol. 2004;51:1661–1675. doi: 10.1111/j.1365-2958.2003.03917.x. [DOI] [PubMed] [Google Scholar]
- 50.Jacobsen FE, Kazmierczak KM, Lisher JP, Winkler ME, Giedroc DP. Interplay between manganese and zinc homeostasis in the human pathogen Streptococcus pneumoniae. Metallomics. 2011;3:38–41. doi: 10.1039/c0mt00050g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown JS, Gilliland SM, Holden DW. A Streptococcus pneumoniae pathogenicity island encoding an ABC transporter involved in iron uptake and virulence. Mol Microbiol. 2001;40:572–585. doi: 10.1046/j.1365-2958.2001.02414.x. [DOI] [PubMed] [Google Scholar]
- 52.Bidossi A, Mulas L, Decorosi F, Colomba L, Ricci S, et al. A functional genomics approach to establish the complement of carbohydrate transporters in Streptococcus pneumoniae. PloS one. 2012;7:e33320. doi: 10.1371/journal.pone.0033320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hyams C, Camberlein E, Cohen JM, Bax K, Brown JS. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect Immun. 2010;78:704–715. doi: 10.1128/IAI.00881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richards L, Ferreira DM, Miyaji EN, Andrew PW, Kadioglu A. The immunising effect of pneumococcal nasopharyngeal colonisation; protection against future colonisation and fatal invasive disease. Immunobiol. 2010;215:251–263. doi: 10.1016/j.imbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 55.van Rossum AM, Lysenko ES, Weiser JN. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect Immun. 2005;73:7718–7726. doi: 10.1128/IAI.73.11.7718-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cohen JM, Khandavilli S, Camberlein E, Hyams C, Baxendale HE, et al. Protective contributions against invasive Streptococcus pneumoniae pneumonia of antibody and Th17-Cell responses to nasopharyngeal colonisation. PLoS One. 2011;6:e25558. doi: 10.1371/journal.pone.0025558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baumgartner M, Karst U, Gerstel B, Loessner M, Wehland J, et al. Inactivation of Lgt allows systematic characterization of lipoproteins from Listeria monocytogenes. . J Bacteriol. 2007;189:313–324. doi: 10.1128/JB.00976-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Janulczyk R, Pallon J, Bjorck L. Identification and characterization of a Streptococcus pyogenes ABC transporter with multiple specificity for metal cations. Mol Microbiol. 1999;34:596–606. doi: 10.1046/j.1365-2958.1999.01626.x. [DOI] [PubMed] [Google Scholar]
- 59.Overweg K, Kerr A, Sluijter M, Jackson MH, Mitchell TJ, et al. The putative proteinase maturation protein A of Streptococcus pneumoniae is a conserved surface protein with potential to elicit protective immune responses. Infect Immun. 2000;68:4180–4188. doi: 10.1128/iai.68.7.4180-4188.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008;6:288–301. doi: 10.1038/nrmicro1871. [DOI] [PubMed] [Google Scholar]
- 61.Standish AJ, Weiser JN. Human neutrophils kill Streptococcus pneumoniae via serine proteases. J Immunol. 2009;183:2602–2609. doi: 10.4049/jimmunol.0900688. [DOI] [PubMed] [Google Scholar]
- 62.Marriott HM, Jackson LE, Wilkinson TS, Simpson AJ, Mitchell TJ, et al. Reactive oxygen species regulate neutrophil recruitment and survival in pneumococcal pneumonia. Am J Resp Crit Care Med. 2008;177:887–895. doi: 10.1164/rccm.200707-990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown JS, Gilliland SM, Spratt BG, Holden DW. A locus contained within a variable region of pneumococcal pathogenicity island 1 contributes to virulence in mice. Infect Immun. 2004;72:1587–1593. doi: 10.1128/IAI.72.3.1587-1593.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dawid S, Roche AM, Weiser JN. The blp bacteriocins of Streptococcus pneumoniae mediate intraspecies competition both in vitro and in vivo. Infect Immun. 2007;75:443–451. doi: 10.1128/IAI.01775-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Desrosiers DC, Bearden SW, Mier I, Jr, Abney J, Paulley JT, et al. Znu is the predominant zinc importer in Yersinia pestis during in vitro growth but is not essential for virulence. Infect Immun. 2010;78:5163–5177. doi: 10.1128/IAI.00732-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gabbianelli R, Scotti R, Ammendola S, Petrarca P, Nicolini L, et al. Role of ZnuABC and ZinT in Escherichia coli O157:H7 zinc acquisition and interaction with epithelial cells. BMC Microbiol. 2011;11:36. doi: 10.1186/1471-2180-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petrarca P, Ammendola S, Pasquali P, Battistoni A. The Zur-regulated ZinT protein is an auxiliary component of the high-affinity ZnuABC zinc transporter that facilitates metal recruitment during severe zinc shortage. J Bacteriol. 2010;192:1553–1564. doi: 10.1128/JB.01310-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eriksson EM, Jackson DC. Recent advances with TLR2-targeting lipopeptide-based vaccines. Curr Protein Pept Sci. 2007;8:412–417. doi: 10.2174/138920307781369436. [DOI] [PubMed] [Google Scholar]
- 69.Echchannaoui H, Frei K, Schnell C, Leib SL, Zimmerli W, et al. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J Infect Dis. 2002;186:798–806. doi: 10.1086/342845. [DOI] [PubMed] [Google Scholar]
- 70.Knapp S, Wieland CW, van ‘t Veer C, Takeuchi O, Akira S, et al. Toll-like receptor 2 plays a role in the early inflammatory response to murine pneumococcal pneumonia but does not contribute to antibacterial defense. J Immunol. 2004;172:3132–3138. doi: 10.4049/jimmunol.172.5.3132. [DOI] [PubMed] [Google Scholar]
- 71.Koedel U, Angele B, Rupprecht T, Wagner H, Roggenkamp A, et al. Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J Immunol. 2003;170:438–444. doi: 10.4049/jimmunol.170.1.438. [DOI] [PubMed] [Google Scholar]
- 72.Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, et al. Recognition of pneumolysin by toll-like receptor 4 confers resistance to pneumococcal infection. Proc Natl Acad Sci USA. 2003;100:1966–1971. doi: 10.1073/pnas.0435928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, et al. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 74.Samen U, Gottschalk B, Eikmanns BJ, Reinscheid DJ. Relevance of peptide uptake systems to the physiology and virulence of Streptococcus agalactiae. J Bacteriol. 2004;186:1398–1408. doi: 10.1128/JB.186.5.1398-1408.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lacks SA, Ayalew S, de la Campa AG, Greenberg B. Regulation of competence for genetic transformation in Streptococcus pneumoniae: expression of dpnA, a late competence gene encoding a DNA methyltransferase of the DpnII restriction system. Mol Microbiol. 2000;35:1089–1098. doi: 10.1046/j.1365-2958.2000.01777.x. [DOI] [PubMed] [Google Scholar]
- 76.Link AJ, Phillips D, Church GM. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J Bacteriol. 1997;179:6228–6237. doi: 10.1128/jb.179.20.6228-6237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Havarstein LS, Coomaraswamy G, Morrison DA. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc Natl Acad Sci USA. 1995;92:11140–11144. doi: 10.1073/pnas.92.24.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning. A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. 1989.
- 79.Cockayne A, Hill PJ, Powell NB, Bishop K, Sims C, et al. Molecular cloning of a 32-kilodalton lipoprotein component of a novel iron-regulated Staphylococcus epidermidis ABC transporter. Infect Immun. 1998;66:3767–3774. doi: 10.1128/iai.66.8.3767-3774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jomaa M, Yuste J, Paton JC, Jones C, Dougan G, et al. Immune responses to immunisation with two iron uptake ABC transporter lipoproteins, PiaA and PiuA. Infect Immun. 2005;73:6852–6859. doi: 10.1128/IAI.73.10.6852-6859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Segal AW, Jones OT. Absence of cytochrome b reduction in stimulated neutrophils from both female and male patients with chronic granulomatous disease. FEBS Lett. 1980;110:111–114. doi: 10.1016/0014-5793(80)80035-4. [DOI] [PubMed] [Google Scholar]
- 82.McAllister LJ, Tseng HJ, Ogunniyi AD, Jennings MP, McEwan AG, et al. Molecular analysis of the psa permease complex of Streptococcus pneumoniae. Mol Microbiol. 2004;53:889–901. doi: 10.1111/j.1365-2958.2004.04164.x. [DOI] [PubMed] [Google Scholar]