Abstract
In the present study, we established an in vitro culture system suitable for generating fertilizable oocytes from premeiotic mouse female germ cells. These results were achieved after first establishing an in vitro culture system allowing immature oocytes from 12–14 day- old mice to reach meiotic maturation through culture onto preantral granulosa cell (PAGC) monolayers in the presence of Activin A (ActA). To generate mature oocytes from premeiotic germ cells, pieces of ovaries from 12.5 days post coitum (dpc) embryos were cultured in medium supplemented with ActA for 28 days and the oocytes formed within the explants were isolated and cocultured onto PAGC monolayers in the presence of ActA for 6–7 days. The oocytes were then subjected to a final meiotic maturation assay to evaluate their capability to undergo germinal vesicle break down (GVBD) and reach the metaphase II (MII) stage. We found that during the first 28 days of culture, a significant number of oocytes within the ovarian explants reached nearly full growth and formed preantral follicle-like structures with the surrounding somatic cells. GSH level and Cx37 expression in the oocytes within the explants were indicative of proper developmental conditions. Moreover, the imprinting of Igf2r and Peg3 genes in these oocytes was correctly established. Further culture onto PAGCs in the presence of ActA allowed about 16% of the oocytes to undergo GVBD, among which 17% reached the MII stage during the final 16–18 hr maturation culture. These MII oocytes showed normal spindle and chromosome assembly and a correct ERK1/2 activity. About 35% of the in vitro matured oocytes were fertilized and 53.44% of them were able to reach the 2-cell stage. Finally, around 7% of the 2-cell embryos developed to the morula/blastocyst stage.
Introduction
In mammalian females, oocytes originate from embryonic precursors termed the primordial germ cells (PGCs) [1]–[3]. In mice, PGC precursors arise in the proximal epiblast at around 6.25 days post coitum (dpc), and then move into the extraembryonic mesoderm at the base of the allantois where by 7.25 dpc they are specified as PGCs [4]–[5]. Between 7.5 to 8.5 dpc, PGCs move caudally from the extraembryonic mesoderm into the hindgut endoderm from where they migrate through the dorsal mesentery of the hindgut into the gonadal ridges from 9.5 to 11.5 dpc. Within the developing ovaries, PGCs continue to proliferate and finally around 13.5 dpc enter meiosis becoming primary oocytes [6]–[9]. Most oocytes have entered meiosis by 14.5 dpc, and become arrested at the diplotene stage of the first meiosis around birth (19.5 dpc -1-2 days post partum, dpp) [7], [10]. At this stage, oocytes are individually surrounded by pregranulosa cells to form primordial follicles [2], [11], [12].
Oocyte growth and maturation are strictly dependent on establishing functional communications with the surrounding granulosa cells through gap junctions and reciprocal interactions mediated by paracrine and endocrine signals. These processes are accurately regulated by numerous growth factors and hormones (for a review, see [13], [14]).The complex regulatory mechanisms controlling the early stages of oogenesis in mammals are only partially understood because of the difficulty to differentiate oocytes from PGCs in vitro [15]. A real challenge is to establish an in vitro culture system for studying oogenesis starting from PGCs and ending in producing mature oocytes [16]. Moreover, such a system could potentially provide an unlimited source of oocytes for biomedical application and invaluable for establishing the conditions to generate oocytes from stem cells, one of the major challenges of the present biology of reproduction [15], [17]. Several attempts have been made to reproduce early mouse oogenesis in vitro [18]–[30]. Oocytes derived from newborn mouse ovaries can undergo an apparent normal development in vitro, and the matured oocytes support the development to term to some extent [7], [18], [22]. In contrast, oocytes generated from PGCs or premeiotic germ cells in vitro remained arrested at the prophase of the first meiotic division and failed to complete maturation [7], [8], [19], [20], [23], [31]. Only combining in vivo ectopic transplantation of embryonic ovaries and in vitro culture of follicles, mature oocytes have been obtained from premeiotic 12.5 dpc germ cells by others and us [25], [26], [32], [33].
Activin A (ActA), a member of the transforming growth factor beta (TGFβ) super-family produced in the ovary as well in a variety of other organs (for a review, see [34]), is an important modulator of preantral follicle development in various species including humans [35]–[44]. Beside as local regulator of folliculogenesis, ActA is able to directly stimulate FSH synthesis and secretion, and to promote the release of the gonadotrophin-releasing hormone (GnRH) [43]. ActA can also stimulate the increase of FSH and LH receptors in granulosa cells, and plays roles in progesterone production and aromatase induction [41], [43]. Thus, granulosa cells are likely to be the main source of the paracrine factors, and are crucial for oocyte maturation.
The purpose of this study was to establish a simple method for obtaining mature oocytes from premeiotic female germ cells entirely in vitro. We found that the presence of ActA during the culture of explants of embryonic ovaries and the subsequent coculture of the growing oocytes generated within the explants onto granulosa cell monolayers were crucial for obtaining significant number of fertilizable oocytes that are able to develop to morula/blastocyst stages.
Materials and Methods
Animals
All procedures described in the present study were reviewed and approved by the Ethical Committee of Qingdao Agricultural University (No. 20090617). If not otherwise indicated, for easier recognition of the exogenous oocytes in the oocyte-preantral granulosa cells (PAGCs) cocultured experiments, transgenic mice carrying the Enhanced Green Fluorescent Protein driven by chicken beta-actin promoter and CMV intermediate early enhancer (EGFP transgenic mice [45] and CD-1 mice (Vital River, Beijing, China) were used (female: 6–8 weeks; male: 10–12 weeks) and maintained on a 12:12-h light/dark cycle (lights off at 20:00). The appearance of copulation plug was designated as 0.5 dpc.
Isolation of Oocytes from Adult and Prepubertal Mice and in vitro Culture of Immature Oocytes and PAGCs
Immature oocytes were isolated from 12–14-day-old EGFP transgenic mice, and PAGCs were isolated from 12–14-day-old prepubertal wild type mice as follows (Fig. 1A). Briefly, follicles were released by puncturing the ovaries in M2 medium [46]. They were then incubated for 15 minutes at 37°C in 0.25% trypsin (Gibco-BRL, Carlsbad, CA), 0.2% collagenase IV (Gibco-BRL) plus 0.02% DNase-I (Sigma) solution, and finally repeatedly pipetted with a drawn pipette. After adding culture medium containing α-minimal essential medium (α-MEM) and 10% heating-inactivated fetal calf serum (FCS) (Gibco-BRL), the cell suspension was resuspended in 100 µl of pre-warmed culture medium. Denuded oocytes (DO) and PAGCs were separated and used for subsequent experiments as indicated.
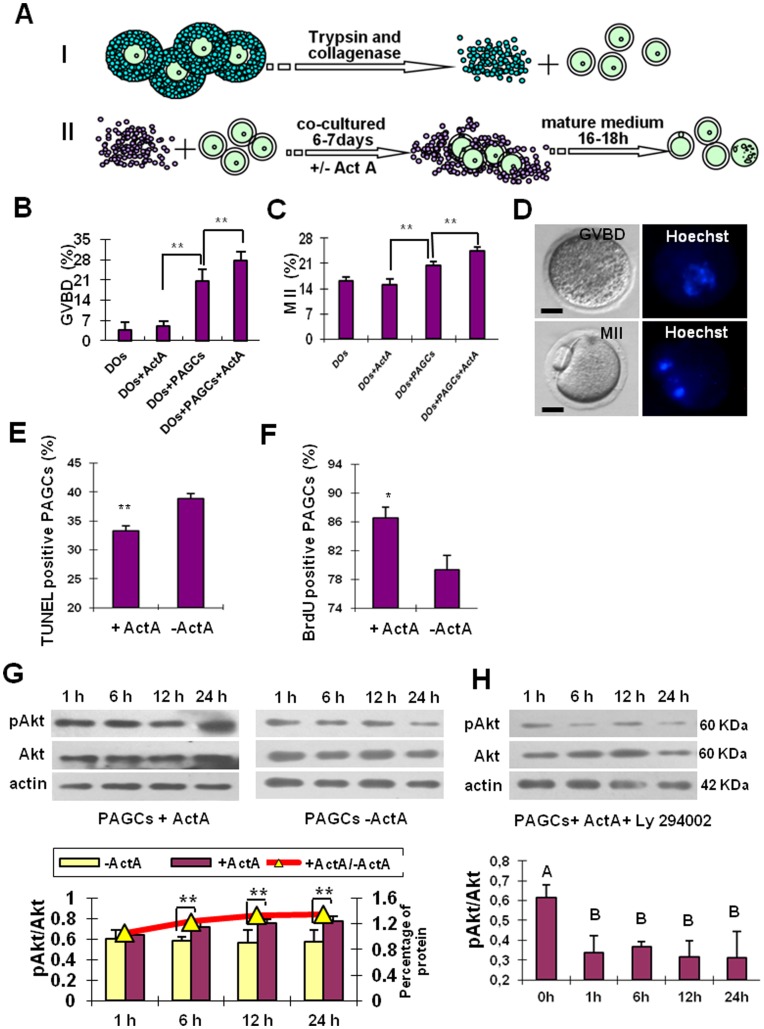
Figure 1. In vitro culture system of immature oocytes from preantral follicles.
(A) Schematic drawing of the main isolation and culture procedures. Oocytes (diameter 55–65 µm) were collected from 12–14-day-old EGFP transgenic mouse and preantral granulosa cells (PAGCs) were collected from 12–14-day-old mouse. PAGCs were cultured as described in M&M, denuded oocytes were divided into four groups: oocytes cocultured with PAGCs in the presence (DOs+PAGCs+ActA) or absence of 100 ng/ml ActA (DOs+PAGCs) for 6–7 days, and oocytes cultured alone in the presence (DOs+ActA) or absence of ActA (DOs) for the same period. Finally, GV oocytes were collected and matured in the in vitro maturation medium (IVM) for 16–18 h. (B–C) In vitro maturation of the oocytes of the four experimental groups as indicated by percents of GVBD and MII oocytes. Culture onto PAGCs results in a significant increase in the number of both GVBD and MII oocytes; ActA significantly improves both maturation capabilities. (D) An example of GVBD and MII oocytes observed under Nikon optics (left) and after DNA staining with Hoechst (right). (E) ActA decreases the number of apoptotic PAGCs cultured in vitro for 7 days as evaluated by the TUNEL staining. (F) Incubation for two days in the presence of ActA increases the number of BrdU positive PAGCs in vitro. (G) ActA increases phospho-Akt in PAGCs cultured in vitro for 1, 6, 12 and 24 h (immunoblotting and densitometric analysis). (H) The PI3K-specific inhibitor, LY294002, inhibits Akt phosphorylation at a concentration of 25 µM (immunoblotting and densitometric analysis). *P<0.05; **P<0.01.
PAGCs obtained from 20–30 follicles were transferred to 100 µl of culture medium (IVC) in a 6 cm culture dish (BD Biosciences, Bedford, MA, USA) covered with 6 ml of mineral oil (Sigma). The culture medium was composed of α-MEM supplemented with 10% FCS, 1% insulin-transferrin-selenium mix (ITS-mix; 1 mg/ml, 0.55 mg/ml and 5 ng/ml) (Gibco-BRL), 10 mIU/ml follicle-stimulating hormone (FSH; Sigma), 5 ng/ml recombinant epidermal growth factor (rEGF; Sigma), 0.23 mM pyruvic acid, 100 IU/ml penicillin G and 100 mg/ml streptomycin sulfate. Cell culture was carried out at 37°C in a humidified atmosphere supplemented with 5% CO2. Within 24 h, the granulosa cells spread out to form a complete monolayer. At this time 20 oocytes were seeded in each well (see below). Half of the medium was changed on the third day of culture.
Denuded oocytes were divided into four groups: oocytes alone (denuded oocytes, DOs), oocytes alone with 100 ng/ml ActA [45] (DOs+ActA), oocytes cocultured onto PAGCs (DOs+PAGCs) and oocytes cocultured onto PAGCs in the presence of ActA (DOs+ PAGCs+ ActA). All cultures were carried in drops of 100 µl of medium in 6 cm dishes covered with 6 ml of mineral oil. The cultures were carried out for 6–7 days at 37°C in a humidified atmosphere supplemented with 5% CO2.
Histology and Measurement of Follicles
Phosphate buffered solution (PBS) or ActA in PBS at the dose of 60 µg/kg (Sigma, St. Louis, MO, USA) was delivered to mice after 10 dpp by daily intraperitoneal injection. Ovaries were collected after the injection for 2, 4 and 6 days, and then fixed in Bouin’s fluid for 24 h and embedded in paraffin. Serial sections of 5 µm in thickness were stained with haematoxylin and eosin (Sigma, St. Louis, MO, USA). In order to quantitatively evaluate the number of primordial, primary and secondary follicles, slides from each ovary were arranged according to the order and every fifth section was marked for analysis. Follicles containing an intact oocyte with a nucleus and a single layer of flat granulosa cells were classified as primordial follicles. Follicles containing of an intact oocyte with a nucleus and a single layer of cuboidal granulosa cells were termed as primary follicles. Follicles containing an intact oocyte with a nucleus and two layers of granulosa cells were scored as early secondary follicles. Follicles containing an intact oocyte with a nucleus and more than two layers of granulosa cells were scored as secondary follicles. Similarly, in order to estimate the total number of follicles in each ovary, the total number of primordial, primary, secondary and antral follicles in the marked sections was multiplied by five, taking into account the fact that every fifth section was used in the analysis.
Isolation and Culture of 12.5 dpc Female Mouse Gonads
Fetal mouse gonads obtained from 12.5 dpc EGFP transgenic fetuses without mesonephros were divided into two pieces and cultured in 600 µl of medium composed of α-MEM supplemented with 10% FCS, 0.23 mM pyruvic acid, 10 mIU/ml FSH, 100 mIU/ml penicillin G and 100 mg/ml streptomycin sulfate in the presence and absence of 100 ng/ml ActA [24], [25]. In a 24-well plate (BD Biosciences) at 37°C in a modular incubation chamber (Billups Rothenberg, Del Mar, CA, USA) infused with a gas mixture of 5% CO2 and air. On the next day, the medium was increased to 900 µl, and the depleted medium was refreshed by exchanging 300 µl fresh medium every other day [25]. Unless indicated the culture was carried out for 28 days. When indicated, the oocytes formed within the ovarian tissue explants were isolated by incubating the tissues for 10–15 minutes in EDTA/trypsin and picked up with a drawn pipette for analysis or for further in vitro culture as described in the Results.
Maturation culture of the oocytes
Oocytes of all experimental groups were finally isolated and matured in 25 µl drops under mineral oil of maturation medium composed of α-MEM supplemented with 10% FCS, 100 mIU/ml FSH, 1 ng/ml rEGF, 0.23 mM pyruvic acid, 100 U/ml penicillin G and 100 mg/ml streptomycin sulfate. Cultures were carried out for 16–18 h at 37°C in a humidified atmosphere supplemented with 5% CO2. At the end of the culture, the oocytes were examined under a stereomicroscope to determine the percentage that had undergone germinal vesicle breakdown (GVBD) and produced a polar body (MII stage). As a control cumulus-enclosed oocytes (COCs) were isolated, from the ovaries of 21–22-day-old CD-1 mice by puncturing the largest antral follicles with a needle, and these GV oocytes were matured and fertilized in vitro (see below).
RNA Extraction, cDNA Synthesis and Quantitative PCR in Oocytes and PAGCs
Total RNA from 400 oocytes or 104 PAGCs was extracted by using an RNAprep pure Micro Kit (Tiangen, Beijing, China) according to the manufacturer’s instructions. The RNA was resuspended in 11 µl of nuclease-free water. Preantral granulosa cell cDNA was synthesized by using Superscript II reverse transcriptase (Invitrogen). Briefly, 20 µl of reaction mixture containing 11 µl of total RNA, 4 µl of 5× RT buffer, 1 µl of Oligo (dT) 20 primer, 2 µl of dNTPs, 1 µl of RNase inhibitor and 1 µl of Rever TraAce was allowed to react as specifically designed by the manufacturer. The real-time PCR was carried out using the SYBR® Premix Ex Taq™ kit (TaKaRa, Dalian, China) and performed on a Roche LightCycler 480 II real-time PCR instrument (F. Hoffmann-La Roche, Ltd) by using the standard curve method with β-actin as the reference gene. The primers used to amplify Cx37, Bcl-2 and Bax and β-actin were listed in Table S1. Amplification reactions were performed in 25 µl of reaction mixture containing 2 µl of cDNA, 12.5 µl of SYBR® Premix Ex Taq™ (2×) (TaKaRa), 9.5 µl of RNase-free water and 1 µl of forward and reverse specific primers (5 µM) for each gene according to manufacturer’s protocol. The average value and SEM were calculated from triplicate measurements, and the relative amount of gene expression for each sample was plotted. All data were expressed as Mean ± SEM [24], [25], [31], [47]–[48].
DNA Isolation, Bisulfite Sequencing and DNA Methylation Analysis in Oocytes
DNA was isolated by using a Proteinase K/SDS method, as described previously [49]. Briefly, 300–500 oocytes in each sample were resuspended in 18 µl of lysis solution containing 2 µg E. coli tRNA, 1 mM SDS, and 280 µg/ml proteinase K. The samples were sequentially incubated at 37°C for 30–90 min, and at 98°C for 15 min. The isolated DNA was treated with sodium bisulfite from a Methylamp™ DNA modification kit (Epigentek, USA) according to the manufacturer’s instructions. PCR reactions for Igf2r (insulin like growth factor 2 receptor), Peg3 (paternal expressed gene 3) and H19 genes were carried out by using bisulfite-treated DNA template and the primers specific to the converted DNA. The bisulfite converted DNA was amplified by nested PCR. The primers for the amplification of Igf2r, Peg3 and H19 were shown in Table S2. The DNA fragments separated by electrophoresis in 1% agarose gel were excised and purified with the Wizard SV gel and PCR clean-up system (Promega). The purified DNA was cloned into a pDM18-T vector (TaKaRa, Dalian, China) according to the manufacturer’s instructions. The positive clones were screened by aminobenzylpenicillin selection and the insert was sequenced at Union Gene (Shanghai, China)[48], [50]–[52].
Immunoblotting Blot Analysis
The proteins from 100 oocytes or 105 granulosa cell were extracted by vortexing the samples in RIPA lysis solution (Beyotime) for 30 min before adding appropriate volume of SDS and boiling for 5 min. Total protein was separated by SDS-PAGE for 50 min at 100 V followed by 1.5 h at 120 V, respectively. The separated proteins were transferred onto nitrocellulose membrane and then blocked in TBST buffer containing 10% BSA (Sigma) for 4 h at room temperature. The blot was incubated with rabbit anti-phospho-Akt (Ser 473) antibody (Abcam, Hong Kong) at the dilution ratio of 1∶500 in TBST buffer at 4°C for 2 h. After washing with TBST buffer for 3 times with 5 min each time, the membrane was incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Beyotime) at the dilution ratio of 1∶1000 in TBST at 37°C for 1 h. The membrane was washed with TBST buffer for three times and then processed by using the enhanced chemiluminescence (ECL) detection system. The membrane was washed to strip off bound antibody, and re-probed with polyclonal rabbit anti-Akt antibody (Beyotime) at the dilution ratio of 1∶800, and then incubated with HRP-labeled goat anti-rabbit IgG at the dilution ratio of 1∶1000. Similarly, the blot was cut according to 68-kDa molecular weight marker and then incubated with murine anti-p-ERK1/2 antibody (Santa Cruz, USA) at the dilution of 1∶500, and re-probed with polyclonal rabbit anti-ERK2 antibody (Santa Cruz) at the dilution of 1∶300. The band intensity was quantified by using β-actin (Abcam) as an internal control and IPWIN software was used for measuring the intensity of the bands. All experiments were repeated at least 3 times [53]–[55].
Analysis of the Oocyte Spindle
Oocyte zona pellucida (ZP) was removed with acidic M2 and zona-free oocytes were fixed in 4% paraformaldehyde for 30 min. and incubated with α-tubulin antibody (Sigma, USA) for 2 h. Nucleus was stained with propidium iodide (PI) for 15 min. Finally, oocytes were mounted on the slides with the DABCO antifade mounting agent and covered by a coverslip [48]. The oocytes were examined with a confocal laser-scanning microscope (Zeiss LSM 710 META, Germany).
Fertilization and in vitro Culture of Embryos
The caudal epididymis was removed from 10- to 12-week-old CD1 male mice and placed in 1 ml of a mutant potassium simplex optimized medium (mKSOM) supplemented with 0.4% (w/v) BSA in a sperm dispersion dish. The dish was placed in an incubator supplied with 5% CO2 for 20 min to allow the sperm to disperse. Totally 10 µl of sperm suspension was added to 90 µl of mixture of mKSOM with BSA in a fertilization dish. Capacitation was allowed to proceed for 45–60 min at 37°C in the incubator.
Oocytes were transferred to the fertilization droplet and incubated at 37°C in a modular incubation infused with a gas mixture of 5% CO2 and air for 4 h. Ten inseminated oocytes were incubated for 48 h in a 20 µl drop of mixture of KSOM- BSA to detect pronuclear formation and the capability to develop to 2-cell stage. Finally, the medium was replaced with 20 µl of mixture of mKSOM with BSA for an additional 48 h culture for morula/blastocyst development [24], [25].
Statistical Analysis
Statistical analysis was performed by using SAS 9.1 (SAS Institute Inc, Cary, NC) and Prism 4 (GraphPad Software Inc., San Diego, CA) softwares. The results were analyzed by variance analysis (ANOVA), and the difference was subjected to Tukey’s test. Normalized cycle threshold (Ct) values from real-time PCR were analyzed by Kruskal-Wallis test, and Dunn multiple comparison tests were performed among different groups. A significant difference was considered at P<0.05 for all tests.
Results
Establishment of in vitro Maturation Culture System for Immature Oocytes from Prepubertal Ovaries
In a recent study, we found that it was possible to obtain growing mouse oocytes in vitro from 12.5 dpc premeiotic female germ cells, but these oocytes were unable to complete the growing phase and thus fail to reach maturation [16]. The maximum diameter and the general morphological features of these oocytes after 21 days of culture were similar to those of 55–65 µm oocytes from 12–14 dpp ovaries. We reasoned that in vitro conditions suitable for promoting maturation of in vivo immature oocytes could also be proper for supporting maturation of in vitro generated oocytes. We decided to use preantral granulosa cell (PAGC) monolayers and activin A (ActA) as potential inducers of oocyte maturation (see Introduction). Oocytes were isolated from 12–14 dpp ovaries and cultured for 6–7 days under four different conditions: DOs, DOs+ActA, DOs+PAGCs and DOs+PAGCs+ActA (Fig. 1A, Fig. S1 and Supporting Information S1).
In order to detect the in vitro meiotic maturation ability of the oocytes cultured under different conditions described above, germinal vesicle breakdown (GVBD) and polar body emission (the metaphase II, MII) were evaluated (see Materials and Methods) (Fig. 1B–D). In the DOs+PAGCs+ActA group, the percents of GVBD and MII oocytes were 27.60±1.20% and 24.50±1.09% (n = 1120), respectively, significantly higher than those in the DO+ PAGCs group (20.5±1.01% and 20.5±0.95% (n = 1233), respectively) and in the groups without PAGCs (around 7% and 14%, respectively) (P<0.05 or P<0.01). As note, in these latter groups the capability of oocytes to undergo GVBD and reach MII was not influenced by the presence of ActA (Fig. 1B–C). Under the same culture conditions, 95.7% (111/116) of the oocytes from 21–22-day-old females underwent GVBD and 86.5% (96/111) of them progressed to the MII stage.
In order to clarify the action of ActA on the granulosa cells, we investigated the effect of the growth factor on the proliferation and apoptosis of the PAGCs (Supporting Information S2 and S3). Using the BrdU incorporation assay, we found that ActA significantly promoted the proliferation of PAGCs after 2 days of stimulation in culture (86.47±1.55% vs 79.33±2.07%; P<0.05) (Fig. 1E and Fig. S2A). Furthermore, ActA caused a significant reduction of PAGC apoptosis after 7 days of culture as evaluated by the TUNEL assay (38.86±0.92% vs 33.34±0.87%; P<0.01) (Fig. 1F and Fig. S2B).
To clarify the molecular pathways stimulated by ActA in PAGCs, the phosphorylation of Akt in these cells was assessed. The levels of Akt and phospho-Akt in PAGCs cultured in vitro for 1, 6, 12 and 24 h were detected (Fig. 1G). The results showed that the ratio of pAkt/Akt was increased in PAGCs cultured with ActA during 1–24 h. The phosphorylation of Akt was significantly inhibited by pre-treatment with the PI3K-specific inhibitor LY294002 (Fig. 1H). Thus these results indicate that ActA promotes the phosphorylation of Akt in PAGCs via the canonical PI3K signaling pathway.
ActA Promotes Follicle Development in vivo
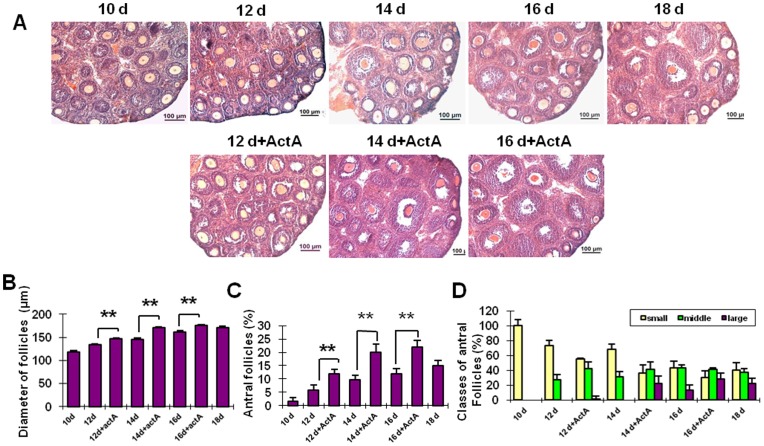
In order to explore the impact of ActA on follicular development in vivo, ActA was injected into 10 dpp female mice at the dose of 60 µg/kg/day for 6 consecutive days (Fig. 2A and Fig. S3). After ActA injection for 2, 4 and 6 days, the follicular diameters were 147.60±1.31, 170.22±2.41 and 175.39±2.15 µm, respectively, significantly higher than those in the control group (133.36±2.17, 145.31±2.85 and 161.48±3.22 µm) (Fig. 2 B) (P<0.01). In addition, in order to evaluate the effect of ActA on the follicular dynamics, the percent of antral follicles was also evaluated. As shown in Fig. 2C, the percentages of small antral follicles were 1.50±1.38%, 5.80±1.82%, 9.80±1.61% and 12.03±1.77% at the postnatal days of 10, 12, 14 and 16, respectively in control groups. However, the populations of small antral follicles in the presence of ActA were 11.70±1.59%, 20.40±3.14% and 22.00±2.62% at the postnatal days 12, 14 and 16, significantly higher than those of the control groups (P < 0.01). In order to further analyze the follicular development, the antral follicles were divided into three groups: small follicles (<150 µm), medium follicles (150–180 µm) and large follicles (>180 µm). As shown in Fig. 2D, the degree of follicular growth was significantly increased following ActA administration.
Figure 2. Injection of ActA promotes follicologenesis.
Injection of ActA for 2, 4 and 6 days results in a significant increase in the diameter of follicles (A, B) and the percent of antral follicles (C) when compared to the control group. Antral follicles were divided into three groups: small (<140 µm), medium (140–180 µm) and large (>180 µm). The proportion of antral follicles in each group is shown in D. Scale bars: 100 µm. *P<0.05; **P<0.01.
ActA Promotes the Survival and Growth of Oocytes Generated from Premeiotic Germ Cells in vitro
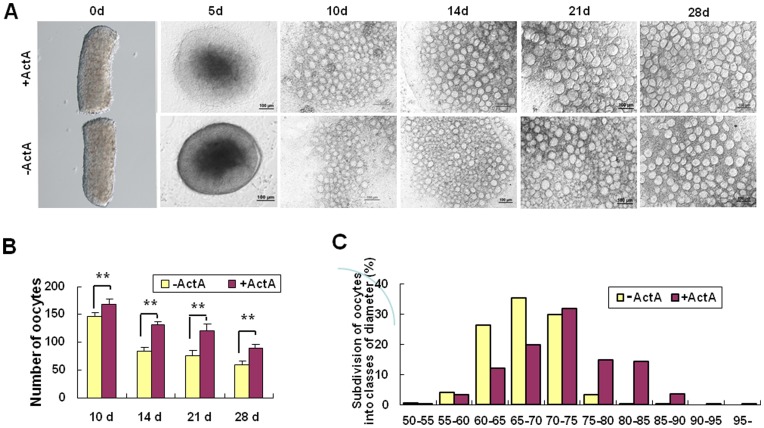
Fetal ovaries were isolated from 12.5 dpc fetuses. Each ovary without mesonephros was then divided in two pieces and cultured in the presence or absence of 100 ng/ml ActA. Morphological examination of the explants revealed that the oocytes in the ovarian tissues cultured with ActA were more numerous and exhibited a higher growth rate than the group without ActA. In the presence of ActA, structures resembling primordial/primary and preantral secondary follicle were also observed from 12 days of culture onwards (Fig. S4).
As shown in Fig. 3A and Fig. S5, at the day 10 of culture the number of oocytes in the explants cultured in the presence of ActA was 168.52±7.13, significantly higher than that in the explants without ActA (146.08±8.77) (P<0.01). Similarly, at the day 28 of culture, the number of oocytes in the experimental group with ActA was 89.63±6.38 and 59.52±7.14 in the group without ActA (P<0.01). After 28 days of culture, the percent of the oocytes with a diameter between 75–80 µm was significantly higher in the explants with ActA. Moreover, oocytes with diameter greater than 85 µm were only observed in the presence of ActA (Fig. 3B–C).
Figure 3. In vitro culture of embryonic ovary explants.
(A) Pieces of ovaries from 12.5 dpc mouse embryos were cultured in vitro in the presence or absence of ActA for up to 28 days. (B) Number of oocytes scored during the explant culture in vitro in the presence or absence of ActA at 10, 14, 21 and 28 days. (C) Diameters of oocytes in the presence or absence of ActA for 28 days. IVC = In vitro culture medium; IVM = In vitro maturation medium. *P<0.05; **P<0.01.
At 28 days of culture, the increased number of oocytes generated in the presence of ActA was associated to reduced apoptosis (235/826, 28.45±0.55% TUNEL positive oocytes vs 34.41±1.33%, 96/279, P<0.01, Fig. S6A). In line with the apoptosis evaluation, we found that Bcl-2 and Bax transcripts were increased and decreased, respectively, in oocytes generated in the presence of ActA in comparison to that formed in the absence of the growth factor (Fig. S6B). Finally, the levels of GSH and of the Cx37 transcripts measured in oocytes >50 µm in diameter were much higher in the presence of ActA (GSH, 17.42±3.75 µM vs 5.53±0.76 µM, P<0.01, Fig. D6C) (Cx37, Fig. S6D).
ActA Promotes the Methylation of Oocytes Derived from Premeiotic Germ Cells in vitro
To determine whether DNA methylation of imprinted genes in the oocytes from premeiotic germ cells was completed during in vitro culture, the oocytes cultured within the ovarian explants for 21 and 28 days with or without ActA were collected, and the differentially methylated regions (DMRs) of the maternal imprinted genes Igf2r and Peg3 were examined. As shown in Fig. 4, the percentage of methylated CpG sites in the Igf2r and Peg3 DMRs were 61.71% and 20.02% respectively in the oocytes in the presence of ActA in vitro for 21 days, and increased to 81.32% and 46.10% after culture for 28 days, respectively. These values were much, higher than those in oocytes cultured in the absence of ActA (Igf2r, 9.31 to 42.72%; Peg3, 4.44 to 21.72%, at 21 and 28 days, respectively). H19, a paternal imprinted gene, was also analyzed as a control. We found that H19 gene maintained its correct unmethylated status during 21 and 28 days of culture with or without ActA.
Figure 4. Establishment of the gene imprinting in the oocytes generated in vitro.
Bisulfite sequencing analysis of DMR in maternally imprinted Igf2r, Peg3 and H19 in the oocytes derived from 12.5 dpc ovaries cultured in vitro for 21 and 28 days in the presence or absence of ActA, as well as the oocytes from 14 and 21 dpp ovaries. Individual lines, clones sequenced; circles, CpG sites within the analyzed regions; filled circles, methylated cytosines; open circles, unmethylated cytosines.
ActA Promotes Meiotic Maturation of Oocytes Generated from Premeiotic Germ Cells in vitro
The oocytes isolated from the explants after 28 days of culture with or without ActA and soon subjected to the maturation assay were unable to undergo GVBD regardless of their diameter (GVBD 0/551).
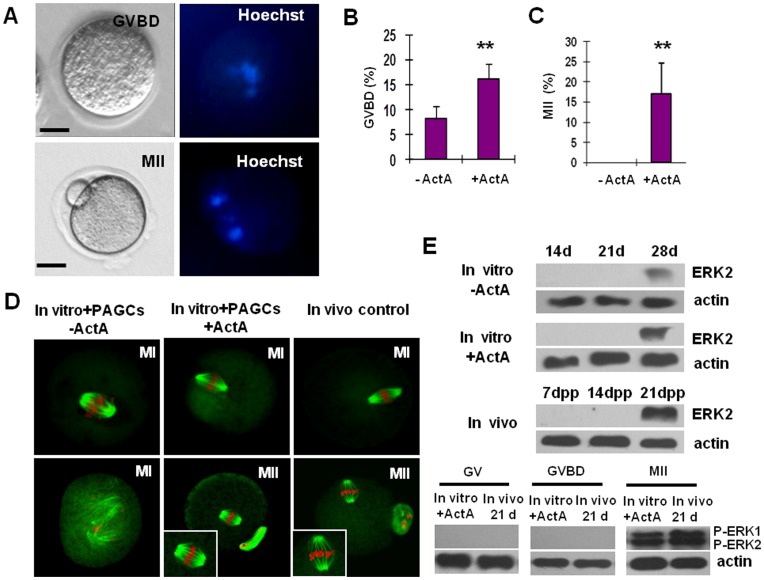
In order to promote the meiotic maturation, oocytes with a diameter >50 µm after culture in the presence of ActA for 28 days were isolated and further cocultured with PAGCs for 6–7 days in the presence or absence of ActA (Fig. S5). During this period, no significant increase in the oocyte diameters was observed (data no shown). At the end of this period, the capability of the oocytes to undergo GVBD and reach MII in IVM was evaluated. The results showed that 95/581 (16.35%) of the oocytes cocultured onto PAGCs with ActA underwent GVBD, amont which 17/95 (17.89%) reached MII stages. In contrast, only 15/183 (8.20%) of the oocytes cocultured onto PAGCs without ActA underwent GVBD and none of them (0/15) were able to reach MII (P<0.01) (Fig. 5A–C). Oocytes cultured with PAGCs in the presence of ActA showed normal MI and MII spindles. In contrast, although part of the oocytes cocultured with PAGCs in the absence of ActA were able to undergo GVBD, they failed to form typical spindle structure and the chromosomes were misaligned (Fig. 5D).
Figure 5. Characterization of the maturation status of the oocytes generated in vitro from 12.5 dpc embryonic ovaries.
(A) An example of GVDB and MII oocytes observed under Nikon optics (left) and after DNA staining with Hoechst (right). (B, C) Percents of GVDB and MII oocytes generated within the ovarian explants after 28 days of culture in the presence of ActA and cocultured onto PAGCs for 7 days in the presence or absence of ActA. (D) MI and MII spindle morphology in oocytes. Oocytes generated in vitro from premeiotic germ cells (In vitro+PAGCs+ActA) show normal MI spindle, MII spindle and chromosome assembly as compared to those of control oocytes isolated from ovaries of 21–22-day-old mice (In vivo control). In contrast, oocyte generated in vitro as described above but cocultured onto PAGCs in the absence of ActA (In vitro+PAGCs-ActA) show anomalous MI spindle and chromosome misalignment. (E) Phosphorylation (activation) of ERK1/2 in oocytes as detected by immunoblotting. Expression of ERK2 protein was analyzed in oocytes obtained from ovarian explants at 14, 21 and 28 days of culture + or – ActA and from 7, 14 and 21 dpp ovaries; only 28 day oocytes in vitro and 21 dpp oocytes in vivo showed detectable ERK2 expression. Phosphorylation of ERK1/2 (pERK1/ERK2) was analyzed in GV, GVDB and MII oocytes generated in vitro (28 days within ovary explants+ActA and 7 days onto PAGCs+ActA) or isolated from ovaries of adult females (21 d); only in vitro and in vivo MII oocytes showed pERK1/ERK2. *P<0.05; **P<0.01.
The meiotic maturation of oocytes is regulated by a cascade of protein phosphorylation/de-phosporylation events. Two isoforms of MAP kinases, ERK1 (p44) and ERK2 (p42), appear to play a pivotal role in this orchestration [56], [57]. For this reason, ERK1/2 phosphorylation during the final in vitro maturation of the in vitro generated oocytes was also investigated by immunoblotting. The results showed that after 16–18 hr in IVM, ERKs were activated in MII oocytes derived from ovarian explants and PAGC cocultures in the presence of ActA and in control MII oocytes obtained from adult ovaries (21–22 dpp) as well. On the contrary, ERKs remained inactive in GV and GVBD oocytes generated in the presence of ActA and subcultured onto PAGCs without ActA (Fig. 5E).
In vitro Generated Oocytes from Premeiotic Germ Cells Support Fertilization and Morula-blastocyst Development
In order to assess the developmental capacity of the oocytes produced and matured as reported above in the presence of ActA, they were subjected to in vitro fertilization (Fig. 6). 189 of 533 (35.46%) oocytes (GVBD and MII stage oocytes) were fertilized as shown by the presence of two pronuclei, among which 53.44% (101/189) reached 2-cell stage. Finally, 6.93% 2-cell embryos (7/101) developed to the morula/blastocyst stage (Table 1). As a control 90.6% (87/96) MII oocytes obtained from 21–22 days old mice were fertilized, and 95.4% (83/87) of fertilized eggs developed to 2-cell embryos, among which 95.2% (79/83) reached morula/blastocysts.
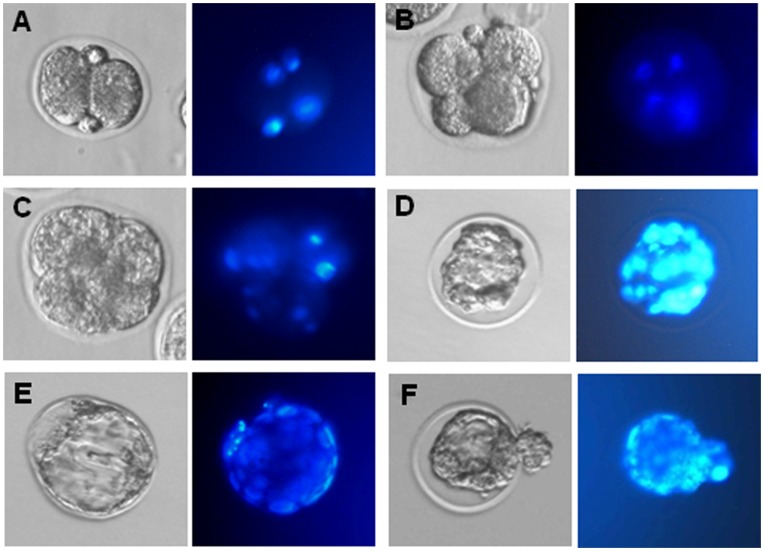
Figure 6. Oocytes generated in vitro in the presence of ActA can be fertilized and fertilized eggs develop to morula and blastocyst stages.
(A–E) Example of 2-cell embryo, 4-cell embryo, morula and blastocyst developed from oocytes generated in vitro in the presence of ActA. Left, phase contrast microscopy observations; right, Hoechst staining of the cell nuclei.
Table 1. Development of embryos produced by the oocytes from pre-meiotic germ cells in vitro.
| oocytes | Fertilized | 2-cell | 4-cell | 8-cell | Morula/Blastocyst | |
| Number | 533 | 189 | 101 | 42 | 26 | 7 |
| Percentage | – | 35.46 | 53.44 | 41.58 | 25.74 | 6.93 |
Discussion
In the present paper, we report for the first time the production of significant numbers of mature and fertilizable mouse oocytes exclusively through in vitro culture. We achieved this result essentially on the basis of two key findings. First, previous observations by others and us have shown that immature mouse GV oocytes can be obtained from premeiotic germ cells after a prolonged period of in vitro culture from explants of embryonic ovarian tissues [8], [15], [58]–[61]. Second, the results obtained in the present paper that immature mouse GV oocytes can be matured in vitro following a 7 day coculture onto PAGCs in the presence of 100 ng/ml ActA. In particular, in the first series of experiments, we found that PAGCs and ActA cooperated to ensure the growth and maturation of immature oocytes in vitro. Granulosa cells can provide nutrients and signal molecules crucial for oogenesis [62]. ActA belongs to the TGF-β superfamily including inhibins, mullerian inhibitor substance (MIS) and bone morphogenetic proteins (BMP), which are autocrine/paracrine intraovarian regulators (see Introduction) [63]. In line with our present data, previous results showed that ActA was able to increase the developmental competence of bovine and human oocytes [40], [64], [65].
In the present paper, we obtained also evidence that while PAGCs favored mainly the in vitro survival of the immature oocytes by reducing apoptosis, ActA exerted a more complex effect on the maturation of such oocytes. In fact, we found that ActA favored both the accumulation of GSH and presumably oocyte-granulosa cell communications (increasing level of Cx37 transcripts). It is well known that high level of GSH is considered a reliable marker of oocyte cytoplasmic maturation [64] and that gap junctions between the oocyte and the surrounding granulosa cells are required for proper oocyte growth and maturation. Cx37 is present in gap junctions between the oocyte and surrounding cumulus cells and has been localized at the surface of the oocytes. The gap protein Cx32, Cx43, and Cx45 have been localized between granulosa cells in mouse ovarian follicles. Cx43 and Cx37 are thought to play more critical roles in ovarian function because the absence of either connexins causes a loss of oocyte-granulosa cell coupling and disruption of folliculogenesis [66]–[71].
Our data also showed that the ActA effects on the immature oocytes were dependent on the presence of PAGCs. The proliferation promotion effect and anti-apoptotic effect of ActA in the presence of the PAGCs that we demonstrated here are in line with its complex role as local regulator of folliculogenesis reported in several species [65], [70]. It must be pointed out, however, that the growth and the capability to resume meiosis and reach the MII stage of the immature oocytes cultured onto PAGCs plus ActA are still significantly lower than those of oocytes of equivalent in vivo chronological age (21–22 dpp). Thus, these data indicate that co-culture with ActA and onto PAGCs can only partly reproduce the complex ovarian environment necessary for complete oocyte maturation.
Most importantly, in the second series of experiments, we were able to produce significant number of mature and fertilizable oocytes by adding ActA to the medium used for the embryonic ovarian explants culture and maintaining this growth factor during the subsequent coculture onto PAGCs. ActA was able to markedly increase the number and the growing rate of oocytes within the explants. Moreover, most of the oocytes cultured in the presence of ActA became surrounded by one or more rings of flatted or cuboidal granulosa cells resembling primordial and primary/early secondary follicles, respectively (Fig. S4A–B). This latter result is in line with previous results showing that ActA is able to organize two dimensional rat follicles from monolayers cultures of granulosa cells and oocytes in vitro [42]. The observations that apoptosis evaluated by TUNEL and Bax mRNA was reduced in oocytes in the presence of ActA while Bcl2 mRNA level was increased, suggest that the increased number of oocytes was mainly due to a reduction of their apoptotic rate. Stimulation of mitosis by ActA in both the granulosa cells which can encapsulate individual oocyte, and germ cells which still do not enter meiosis, is an alternative possibility [72]. Interestingly, ActA has been shown to promote germ cell survival and proliferation in the developing human ovary before primordial follicle formation [73]. Like in immature oocytes cocultured onto PAGCs, ActA appeared able to increase the level of GSH in the oocytes developing within the explants of the embryonic ovaries and to improve the communication between granulosa cells and oocyte by increasing the mRNA level of the Cx37.
The oocyte growing phase is of particular importance for subsequent embryogenesis, since at this stage gene transcription occurs. The mRNAs synthesized are required for oocyte metabolism and development or as maternal factor used in the early development of the embryo. Oocyte growth is also a critical period for establishing epigenetic marks on the female genome. In the female germ cells, many imprinted genes appear to be controlled by methylation of the DNA on their imprint control regions (ICR) [74]. In this regard, it is particularly important we find here that ActA is required to sustain de novo methylation occurring during oocyte growth at least for the imprinted genes analyzed (Igf2r and Peg3). At the same time, we found that H19 gene maintained its correct unmethylated status during the culture period.
Probably the most interesting results were that about 35% of the oocytes produced in the cultures in the presence of ActA were fertilizable and about 7% of them were able to reach the morula/blastocyst stage. These last results represent a further significant step towards the development of in vitro system for production of mature oocytes. Further investigations on in vitro oogenesis and improvement of this technology will be crucial for a more comprehensive understanding of germ cell biology in general, as well as for the advancement of reproductive technologies and medicine [75].
Supporting Information
Development of immature oocytes in vitro. (A) Representative pictures of the oocytes and PAGCs of the four experimental groups. Arrows indicate degenerating oocytes. Green cells are EGFP transgenic ones, and gray cell are normal cells. Scale bars: 100 µm. (B) Growth of the immature oocytes from 12–14-day-old mice in the four experimental groups (see, Fig. 1). Oocytes cultured onto PAGCs (DOs+PAGCs) or onto PAGCs in the presence ActA show a significant increase of diameter. (C) Intracellular GSH levels in the oocytes of the four experimental groups as above. Oocytes of the DOs+PAGCs+ActA show a significant increase of GSH levels. For comparison the GSH levels of 14 and 21 dpp oocytes are also shown. (D) Evaluation of apoptosis in the oocytes of the four experimental groups. The culture onto PAGCs results in a significant reduction of the number of apoptotic oocytes. (E) Real-time quantitative PCR analysis of Bax and Bcl-2 transcripts in the oocytes of the four experimental groups. ActA causes a significant reduction of the Bax mRNA both in DOs and in DOs+PAGCs. (F) Real-time quantitative PCR analysis of Cx37 transcripts in the oocytes of the four experimental groups. Oocytes cultured onto PAGCs show a significant higher level of Cx37 mRNA; ActA causes a further increase of the transcript levels.
(TIF)
Effects of ActA on PAGCs in vitro . (A) Incubation for two days in the presence of ActA increases the number of BrdU positive PAGCs in vitro. (B) ActA decreases the number of apoptotic PAGCs cultured in vitro for 7 days as evaluated by the TUNEL staining.
(TIF)
The histology of 10 dpp mouse ovary injected with 60 µg/kg/day ActA for 6 days. (A, B) The quantity of the primordial follicles in 10 dpp mouse injected with 60 µg/kg/day, and the 10 and 18 dpp mice as the controls. (C) The “primitive follicle-rich region” in 10 dpp mouse injected with ActA or physiological saline for 4 days. *P<0.05; **P<0.01.
(TIF)
Morphologies of oocytes and follicles within the ovarian explants in the presence of ActA. (A, B) Primary and preantral secondary follicle-like structures around growing oocytes at different culture times (arrows). (C, D) Many oocytes grew together as ‘siamese twins’ and oocytes shared the zona pellucida (white arrows). The quantity of oocytes in the ovaries cultured in vitro in the presence of ActA revealed a reasonable density (E), not to be overmuch (F).
(TIF)
In vitro maturation of oocytes co-cultured with PAGCs. Examples of oocytes isolated from ovaries cultured for 28 days +ActA and transferred onto PAGCs + or – ActA in IVC at the beginning (Co-IVC 0d) and after 7 day of culture (Co-IVC 7d); oocytes onto PAGCs + or – ActA in IVC for 7 days matured in IVM at the beginning (IVM 0 h) and after 16–18 h (IVM 16–18 h).
(TIF)
Characterization of the oocytes generated in vitro from 12.5 dpc embryonic ovaries. (A) Percent of apoptotic oocytes (TUNEL positivity) isolated from the ovary explants after 28 days in the presence or absence of ActA. (B,D) Real-time quantitative PCR analysis of Bax, Bcl-2 and Cx37. Oocytes generated in vitro from explants of ovaries of 12.5 dpc embryos after 28 days of culture in the presence or absence of ActA. (C) GSH levels in the oocytes as above.
(TIF)
Assay of intracellular glutathione (GSH) in oocytes.
(DOC)
Proliferation and apoptosis assays of preantral granulosa cells.
(DOC)
Establishment of in vitro maturation culture system for immature oocytes from prepubertal ovaries.
(DOC)
Details of primers used for Real-time PCR.
(DOCX)
The primers used for amplification of imprint gene by nested PCR.
(DOCX)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work is supported by National Basic Research Program of China (973 Program, 2012CB944401, 2011CB944501 and 2007CB947401), National Transgenic Biology Program of China (2009ZX08008-006B), National Nature Science Foundation (31001010, 31171376 and 31101716), Foundation of Distinguished Young Scholars (JQ201109), Doctoral Foundation (BS2010NY010) and Taishan Scholar Foundation of Shandong Province. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Tam PP, Zhou SX. The allocation of epiblast cells to ectodermal and germ-line lineages is influenced by the position of the cells in the gastrulating mouse embryo. Dev Biol. 1996;178:124–132. doi: 10.1006/dbio.1996.0203. [DOI] [PubMed] [Google Scholar]
- 2.Pepling ME, Spradling AC. Female mouse germ cells form synchronously dividing cysts. Development. 1998;125:3323–3328. doi: 10.1242/dev.125.17.3323. [DOI] [PubMed] [Google Scholar]
- 3.Wylie C. Germ cells. Cell. 1999;96:165–174. doi: 10.1016/s0092-8674(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 4.Ohinata Y, Payer B, O’Carroll D, Ancelin K, Ono Y, et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature. 2005;436(7048):207–13. doi: 10.1038/nature03813. [DOI] [PubMed] [Google Scholar]
- 5.McLaren A, Lawson KA. How is the mouse germ-cell lineage established? Differentiation. 2005;73(9–10):435–437. doi: 10.1111/j.1432-0436.2005.00049.x. [DOI] [PubMed] [Google Scholar]
- 6.McLaren A, Buehr M. Development of mouse germ cells in cultures of fetal gonads. Cell Differ Dev. 1990;31:185–195. doi: 10.1016/0922-3371(90)90131-f. [DOI] [PubMed] [Google Scholar]
- 7.McLaren A. Germ and somatic cell lineages in the developing gonad. Mol Cell Endocrinol. 2000;163:3–9. doi: 10.1016/s0303-7207(99)00234-8. [DOI] [PubMed] [Google Scholar]
- 8.Byskov AG, Guoliang X, Andersen CY. The cortex-medulla oocyte growth pattern is organized during fetal life: an in-vitro study of the mouse ovary. Mol Hum Reprod. 1997;3:795–800. doi: 10.1093/molehr/3.9.795. [DOI] [PubMed] [Google Scholar]
- 9.Molyneaux KA, Stallock J, Schaible K, Wylie C. Time-lapse analysis of living mouse germ cell migration. Dev Biol. 2001;240:488–498. doi: 10.1006/dbio.2001.0436. [DOI] [PubMed] [Google Scholar]
- 10.Chuma S, Nakatsuji N. Autonomous transition into meiosis of mouse fetal germ cells in vitro and its inhibition by gp130-mediated signaling. Dev Biol. 2001;229:468–479. doi: 10.1006/dbio.2000.9989. [DOI] [PubMed] [Google Scholar]
- 11.Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234:339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- 12.Epifano O, Dean J. Genetic control of early folliculogenesis in mice. Trends Endocrinol Metab. 2002;13:169–173. doi: 10.1016/s1043-2760(02)00576-3. [DOI] [PubMed] [Google Scholar]
- 13.Cortvrindt RG, Smitz JE. Follicle culture in reproductive toxicology: a tool for in-vitro testing of ovarian function? Hum Reprod Update. 2002;8:243–254. doi: 10.1093/humupd/8.3.243. [DOI] [PubMed] [Google Scholar]
- 14.Trombly DJ, Woodruff TK, Mayo KE. Roles for transforming growth factor beta superfamily proteins in early folliculogenesis. Semin Reprod Med. 2009;27(1):14–23. doi: 10.1055/s-0028-1108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong HS, Li L, Song ZH, Tang J, Xu B, et al. Premeiotic fetal murine germ cells cultured in vitro form typical oocyte-like cells but do not progress through meiosis. Theriogenology. 2009;72:219–231. doi: 10.1016/j.theriogenology.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Clark GF. Molecular models for mouse sperm-oocyte binding. Glycobiology. 2011 Jan. 2010;21(1):3–5. doi: 10.1093/glycob/cwq159. [DOI] [PubMed] [Google Scholar]
- 17.Eppig JJ, O’Brien M, Wigglesworth K. Mammalian oocyte growth and development in vitro. Mol Reprod Dev. 1996;44:260–273. doi: 10.1002/(SICI)1098-2795(199606)44:2<260::AID-MRD17>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- 19.Klinger FG, De Felici M. In vitro development of growing oocytes from fetal mouse oocytes: stage-specific regulation by stem cell factor and granulosa cells. Dev Biol. 2002;244:85–95. doi: 10.1006/dbio.2002.0592. [DOI] [PubMed] [Google Scholar]
- 20.Obata Y, Kono T. Maternal primary imprinting is established at a specific time for each gene throughout oocyte growth. J Biol Chem. 2002;277:5285–5289. doi: 10.1074/jbc.M108586200. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682–1686. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- 22.Lenie S, Cortvrindt R, Adriaenssens T, Smitz J. A reproducible two-step culture system for isolated primary mouse ovarian follicles as single functional units. Biol Reprod. 2004;71:1730–1738. doi: 10.1095/biolreprod.104.028415. [DOI] [PubMed] [Google Scholar]
- 23.Niwa K, Takano R, Obata Y, Hiura H, Komiyama J, et al. Nuclei of oocytes derived from mouse parthenogenetic embryos are competent to support development to term. Biol Reprod. 2004;71:1560–1567. doi: 10.1095/biolreprod.104.030908. [DOI] [PubMed] [Google Scholar]
- 24.Shen W, Li L, Zhang D, Pan Q, Ding M, et al. Mouse oocytes derived from fetal germ cells are competent to support the development of embryos by in vitro fertilization. Mol Reprod Dev. 2006;73:1312–1317. doi: 10.1002/mrd.20535. [DOI] [PubMed] [Google Scholar]
- 25.Shen W, Zhang D, Qing T, Cheng J, Bai Z, et al. Live offspring produced by mouse oocytes derived from premeiotic fetal germ cells. Biol Reprod. 2006;75:615–623. doi: 10.1095/biolreprod.106.051482. [DOI] [PubMed] [Google Scholar]
- 26.McLaren A, Southee D. Entry of mouse embryonic germ cells into meiosis. Dev Biol. 1997;187:107–113. doi: 10.1006/dbio.1997.8584. [DOI] [PubMed] [Google Scholar]
- 27.Thomas FH, Walters KA, Telfer EE. How to make a good oocyte: an update on in-vitro models to study follicle regulation. Hum Reprod Update. 2003;9(6):541–555. doi: 10.1093/humupd/dmg042. [DOI] [PubMed] [Google Scholar]
- 28.Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23(5):1151–1158. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- 29.Honda A, Hirose M, Inoue K, Hiura H, Miki H, et al. Large-scale production of growing oocytes in vitro from neonatal mouse ovaries. Int J Dev Biol. 2009;53:605–613. doi: 10.1387/ijdb.082607ah. [DOI] [PubMed] [Google Scholar]
- 30.Kono T, Obata Y, Wu Q, Niwa K, Ono Y, et al. Birth of parthenogenetic mice that can develop to adulthood. Nature. 2004;428:860–864. doi: 10.1038/nature02402. [DOI] [PubMed] [Google Scholar]
- 31.Shen W, Li L, Bai Z, Pan Q, Ding M, et al. In vitro development of mouse fetal germ cells into mature oocytes. Reproduction. 2007;134:223–231. doi: 10.1530/REP-06-0378. [DOI] [PubMed] [Google Scholar]
- 32.Obata Y, Kono T, Hatada I. Gene silencing: maturation of mouse fetal germ cells in vitro. Nature. 2002;418:497. doi: 10.1038/418497a. [DOI] [PubMed] [Google Scholar]
- 33.Rossi P, Dolci S, Farini D, De Felici M. Germ Cells. In: Cell Signaling and Growth Factors in Development : From Molecules to Organogenesis, vol. 1 (K. Unsicker and K. Krieglstein editors), Wiley-VCH, Weinheim, 39–72. 2005.
- 34.Chapman SC, Kenny HA, Woodruff TK. Activin, Inhibin and Follistatin in the ovary physiology. In: The Ovary, Leung PCK and Adashi EY (eds) second edition, Elsevier Academic Press. 2005;2005:273–288. [Google Scholar]
- 35.Ethier JF, Findlay JK. Roles of activin and its signal transduction mechanisms in reproductive tissues. Reproduction. 2001;121:667–675. doi: 10.1530/rep.0.1210667. [DOI] [PubMed] [Google Scholar]
- 36.Thomas FH, Armstrong DG, Telfer EE. Activin promotes oocyte development in ovine preantral follicles in vitro. Reprod Biol Endocrinol. 2003;1:76. doi: 10.1186/1477-7827-1-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva JR, Tharasanit T, Taverne MA, van der Weijden GC, Santos RR, et al. The activin-follistatin system and in vitro early follicle development in goats. J Endocrinol. 2006;189:113–125. doi: 10.1677/joe.1.06487. [DOI] [PubMed] [Google Scholar]
- 38.Itoh M, Igarashi M, Yamada K, Hasegawa Y, Seki M, et al. Activin A stimulates meiotic maturation of the rat oocyte in vitro. Biochem Biophys Res Commun. 1990;166:1479–1484. doi: 10.1016/0006-291x(90)91034-p. [DOI] [PubMed] [Google Scholar]
- 39.Alak BM, Smith GD, Woodruff TK, Stouffer RL, Wolf DP. Enhancement of primate oocyte maturation and fertilization in vitro by inhibin A and activin A. Fertil Steril. 1996;66:646–653. [PubMed] [Google Scholar]
- 40.Stock AE, Woodruff TK, Smith LC. Effects of inhibin A and activin A during in vitro maturation of bovine oocytes in hormone- and serum-free medium. Biol Reprod. 1997;56:1559–1564. doi: 10.1095/biolreprod56.6.1559. [DOI] [PubMed] [Google Scholar]
- 41.Alak BM, Coskun S, Friedman CI, Kennard EA, Kim MH, et al. Activin A stimulates meiotic maturation of human oocytes and modulates granulosa cell steroidogenesis in vitro. Fertil Steril. 1998;70:1126–1130. doi: 10.1016/s0015-0282(98)00386-0. [DOI] [PubMed] [Google Scholar]
- 42.Li RH, Phillips DM, Mather JP. Activin promotes ovarian follicle development in vitro. Endocrinology. 1995;136:849–856. doi: 10.1210/endo.136.3.7867593. [DOI] [PubMed] [Google Scholar]
- 43.Norwitz ER, Xu S, Jeong KH, Bedecarrats GY, Winebrenner LD, et al. Activin A augments GnRH-mediated transcriptional activation of the mouse GnRH receptor gene. Endocrinology. 2002;143:985–997. doi: 10.1210/endo.143.3.8663. [DOI] [PubMed] [Google Scholar]
- 44.Coutts SM, Childs AJ, Fulton N, Collins C, Bayne RAL, et al. Activin signals via SMAD2/3 between germ and somatic cells in the human fetal ovary and regulates kit ligand expression. Dev Biol. 2008;314:189–199. doi: 10.1016/j.ydbio.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 45.Shen W, Li L, Pan Q J, Min LJ, Dong HS, et al. Efficient and simple production of transgenic mice and rabbits using the new DMSO-sperm mediated exogenous DNA transfer method. Mol Reprod Dev. 2006;73:589–594. doi: 10.1002/mrd.20401. [DOI] [PubMed] [Google Scholar]
- 46.Kim CG, Yong H, Lee G, Cho J. Effect of the polyvinylpyrrolidone concentration of cryoprotectant on mouse embryo development and production of pups: 7.5% of PVP is beneficial for in vitro and in vivo development of frozen-thawed mouse embryos. J Reprod Dev. 2008;54(4):250–253. doi: 10.1262/jrd.19185. [DOI] [PubMed] [Google Scholar]
- 47.Dyce PW, Wen L, Li J. In vitro germline potential of stem cells derived from fetal porcine skin. Nat Cell Biol. 2006;8:384–390. doi: 10.1038/ncb1388. [DOI] [PubMed] [Google Scholar]
- 48.La Salle S, Mertineit C, Taketo T, Moens PB, Bestor TH, et al. Windows for sex-specific methylation marked by DNA methyltransferase expression profiles in mouse germ cells. Dev Biol. 2004;268:403–415. doi: 10.1016/j.ydbio.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 49.Zuccotti M, Monk M. Methylation of the mouse Xist gene in sperm and eggs correlates with imprinted Xist expression and paternal X-inactivation. Nat Genet. 1995;9:316–320. doi: 10.1038/ng0395-316. [DOI] [PubMed] [Google Scholar]
- 50.Lucifero D, Mertineit C, Clarke HJ, Bestor TH, Trasler JM. Methylation dynamics of imprinted genes in mouse germ cells. Genomics. 2002;79:530–538. doi: 10.1006/geno.2002.6732. [DOI] [PubMed] [Google Scholar]
- 51.Song Z, Min L, Pan Q, Shi Q, Shen W. Maternal imprinting during mouse oocyte growth in vivo and in vitro. Biochem Biophys Res Commun. 2009;387:800–805. doi: 10.1016/j.bbrc.2009.07.131. [DOI] [PubMed] [Google Scholar]
- 52.Hiura H, Obata Y, Komiyama J, Shirai M, Kono T. Oocyte growth-dependent progression of maternal imprinting in mice. Genes Cells. 2006;11:353–361. doi: 10.1111/j.1365-2443.2006.00943.x. [DOI] [PubMed] [Google Scholar]
- 53.Zhang P, Chao H, Sun X, Li L, Shi Q, et al. Murine folliculogenesis in vitro is stage-specifically regulated by insulin via the Akt signaling pathway. Histochem Cell Biol. 2010;134:75–82. doi: 10.1007/s00418-010-0708-8. [DOI] [PubMed] [Google Scholar]
- 54.Su YQ, Denegre JM, Wigglesworth K, Pendola FL, O’Brien MJ, et al. Oocyte-dependent activation of mitogen-activated protein kinase (ERK1/2) in cumulus cells is required for the maturation of the mouse oocyte-cumulus cell complex. Dev Biol. 2003;263:126–138. doi: 10.1016/s0012-1606(03)00437-8. [DOI] [PubMed] [Google Scholar]
- 55.Su YQ, Wigglesworth K, Pendola FL, O’Brien MJ, Eppig JJ. Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology. 2002;143:2221–2232. doi: 10.1210/endo.143.6.8845. [DOI] [PubMed] [Google Scholar]
- 56.Abrieu A, Dorée M, Fisher D. The interplay between cyclin-B-Cdc2 kinase (MPF) and MAP kinase during maturation of oocytes. J Cell Sci 114(Pt. 2001;2):257–267. doi: 10.1242/jcs.114.2.257. [DOI] [PubMed] [Google Scholar]
- 57.Fan HY, Li MY, Tong C, Chen DY, Xia GL, et al. Inhibitory effects of cAMP and protein kinase C on meiotic maturation and MAP kinase phosphorylation in porcine oocytes. Mol Reprod Dev. 2002;63(4):480–487. doi: 10.1002/mrd.10194. [DOI] [PubMed] [Google Scholar]
- 58.Tong C, Fan HY, Chen DY, Song XF, Schatten H, et al. Effects of MEK inhibitor U0126 on meiotic progression in mouse oocytes: microtuble organization, asymmetric division and metaphase II arrest. Cell Res. 2003;13(5):375–283. doi: 10.1038/sj.cr.7290183. [DOI] [PubMed] [Google Scholar]
- 59.Odor DL, Blandau RJ. Organ cultures of fetal mouse ovaries. I. Light microscopic structure. Am J Anat. 1971;131(4):387–414. doi: 10.1002/aja.1001310402. [DOI] [PubMed] [Google Scholar]
- 60.De Felici M, Dolci S. Cellular interactions of mouse fetal germ cells in in vitro systems. Curr Top Dev Biol. 1987;23:147–62. doi: 10.1016/s0070-2153(08)60623-7. [DOI] [PubMed] [Google Scholar]
- 61.Pesce M, Cerrito MG, Travia G, Russo MA, De Felici M. In vitro development of growing oocytes from fetal and early postnatal mouse ovaries. Int J Dev Biol. 1996. pp. 229S–230S. [PubMed]
- 62.Lees-Murdock DJ, Lau HT, Castrillon DH, De Felici M, Walsh CP. DNA methyltransferase loading, but not de novo methylation, is an oocyte-autonomous process stimulated by SCF signalling. Dev Biol. 2008;321(1):238–50. doi: 10.1016/j.ydbio.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 63.Li ZB, Zhang P, Zhang ZP, Pan B, Chao HH, et al. A co-culture system with preantral follicular granulosa cells in vitro induces meiotic maturation of immature oocytes. Histochem Cell Biol. 2011;135:513–522. doi: 10.1007/s00418-011-0812-4. [DOI] [PubMed] [Google Scholar]
- 64.Luberda Z. The role of glutathione in mammalian gametes. Reprod Biol. 2005;5(1):5–17. [PubMed] [Google Scholar]
- 65.Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- 66.Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. 1997;Nature(385(6616)):525–9. doi: 10.1038/385525a0. [DOI] [PubMed] [Google Scholar]
- 67.Ackert CL, Gittens JE, O’Brien MJ, Eppig JJ, Kidder GM. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol. 2001;233:258–270. doi: 10.1006/dbio.2001.0216. [DOI] [PubMed] [Google Scholar]
- 68.Gittens JE, Mhawi AA, Lidington D, Ouellette Y, Kidder GM. Functional analysis of gap junctions in ovarian granulosa cells: distinct role for connexin43 in early stages of folliculogenesis. Am J Physiol Cell Physiol. 2003;284(4):C880–887. doi: 10.1152/ajpcell.00277.2002. [DOI] [PubMed] [Google Scholar]
- 69.Veitch GI, Gittens JE, Shao Q, Laird DW, Kidder GM. Selective assembly of connexin37 into heterocellular gap junctions at the oocyte/granulosa cell interface. J Cell Sci 117(Pt. 2004;13):2699–2707. doi: 10.1242/jcs.01124. [DOI] [PubMed] [Google Scholar]
- 70.Silva CC, Knight PG. Modulatory actions of activin-A and follistatin on the developmental competence of in vitro-matured bovine oocytes. Biol Reprod. 1998;58(2):558–565. doi: 10.1095/biolreprod58.2.558. [DOI] [PubMed] [Google Scholar]
- 71.Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132(2):191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- 72.Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Cook RW, et al. Postnatal regulation of germ cells by activin: the establishment of the initial follicle pool. Dev Biol. 2006;298:132–148. doi: 10.1016/j.ydbio.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 73.Martins da Silva SJ, Bayne RA, Cambray N, Hartley PS, McNeilly AS, et al. Expression of activin subunits and receptors in the developing human ovary: activin A promotes germ cell survival and proliferation before primordial follicle formation. Dev Biol. 2004;266:334–345. doi: 10.1016/j.ydbio.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 74.Reik W, Walter J. Genomic imprinting: parental influence on the genome. Nat Rev Genet. 2001;2(1):21–32. doi: 10.1038/35047554. [DOI] [PubMed] [Google Scholar]
- 75.Hashiyama K, Hayashi Y, Kobayashi S. Drosophila Sex lethal gene initiates female development in germline progenitors. Science. 2011;333(6044):885–8. doi: 10.1126/science.1208146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Development of immature oocytes in vitro. (A) Representative pictures of the oocytes and PAGCs of the four experimental groups. Arrows indicate degenerating oocytes. Green cells are EGFP transgenic ones, and gray cell are normal cells. Scale bars: 100 µm. (B) Growth of the immature oocytes from 12–14-day-old mice in the four experimental groups (see, Fig. 1). Oocytes cultured onto PAGCs (DOs+PAGCs) or onto PAGCs in the presence ActA show a significant increase of diameter. (C) Intracellular GSH levels in the oocytes of the four experimental groups as above. Oocytes of the DOs+PAGCs+ActA show a significant increase of GSH levels. For comparison the GSH levels of 14 and 21 dpp oocytes are also shown. (D) Evaluation of apoptosis in the oocytes of the four experimental groups. The culture onto PAGCs results in a significant reduction of the number of apoptotic oocytes. (E) Real-time quantitative PCR analysis of Bax and Bcl-2 transcripts in the oocytes of the four experimental groups. ActA causes a significant reduction of the Bax mRNA both in DOs and in DOs+PAGCs. (F) Real-time quantitative PCR analysis of Cx37 transcripts in the oocytes of the four experimental groups. Oocytes cultured onto PAGCs show a significant higher level of Cx37 mRNA; ActA causes a further increase of the transcript levels.
(TIF)
Effects of ActA on PAGCs in vitro . (A) Incubation for two days in the presence of ActA increases the number of BrdU positive PAGCs in vitro. (B) ActA decreases the number of apoptotic PAGCs cultured in vitro for 7 days as evaluated by the TUNEL staining.
(TIF)
The histology of 10 dpp mouse ovary injected with 60 µg/kg/day ActA for 6 days. (A, B) The quantity of the primordial follicles in 10 dpp mouse injected with 60 µg/kg/day, and the 10 and 18 dpp mice as the controls. (C) The “primitive follicle-rich region” in 10 dpp mouse injected with ActA or physiological saline for 4 days. *P<0.05; **P<0.01.
(TIF)
Morphologies of oocytes and follicles within the ovarian explants in the presence of ActA. (A, B) Primary and preantral secondary follicle-like structures around growing oocytes at different culture times (arrows). (C, D) Many oocytes grew together as ‘siamese twins’ and oocytes shared the zona pellucida (white arrows). The quantity of oocytes in the ovaries cultured in vitro in the presence of ActA revealed a reasonable density (E), not to be overmuch (F).
(TIF)
In vitro maturation of oocytes co-cultured with PAGCs. Examples of oocytes isolated from ovaries cultured for 28 days +ActA and transferred onto PAGCs + or – ActA in IVC at the beginning (Co-IVC 0d) and after 7 day of culture (Co-IVC 7d); oocytes onto PAGCs + or – ActA in IVC for 7 days matured in IVM at the beginning (IVM 0 h) and after 16–18 h (IVM 16–18 h).
(TIF)
Characterization of the oocytes generated in vitro from 12.5 dpc embryonic ovaries. (A) Percent of apoptotic oocytes (TUNEL positivity) isolated from the ovary explants after 28 days in the presence or absence of ActA. (B,D) Real-time quantitative PCR analysis of Bax, Bcl-2 and Cx37. Oocytes generated in vitro from explants of ovaries of 12.5 dpc embryos after 28 days of culture in the presence or absence of ActA. (C) GSH levels in the oocytes as above.
(TIF)
Assay of intracellular glutathione (GSH) in oocytes.
(DOC)
Proliferation and apoptosis assays of preantral granulosa cells.
(DOC)
Establishment of in vitro maturation culture system for immature oocytes from prepubertal ovaries.
(DOC)
Details of primers used for Real-time PCR.
(DOCX)
The primers used for amplification of imprint gene by nested PCR.
(DOCX)