Abstract
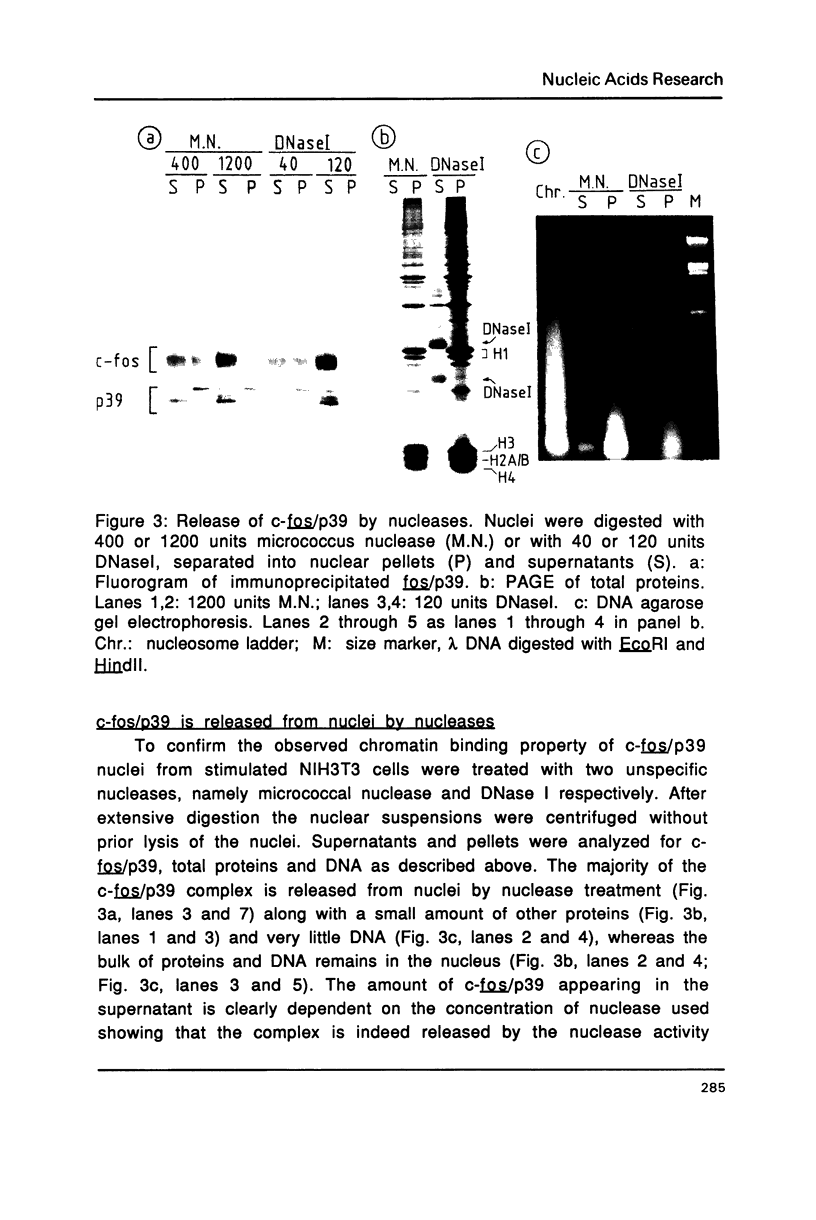
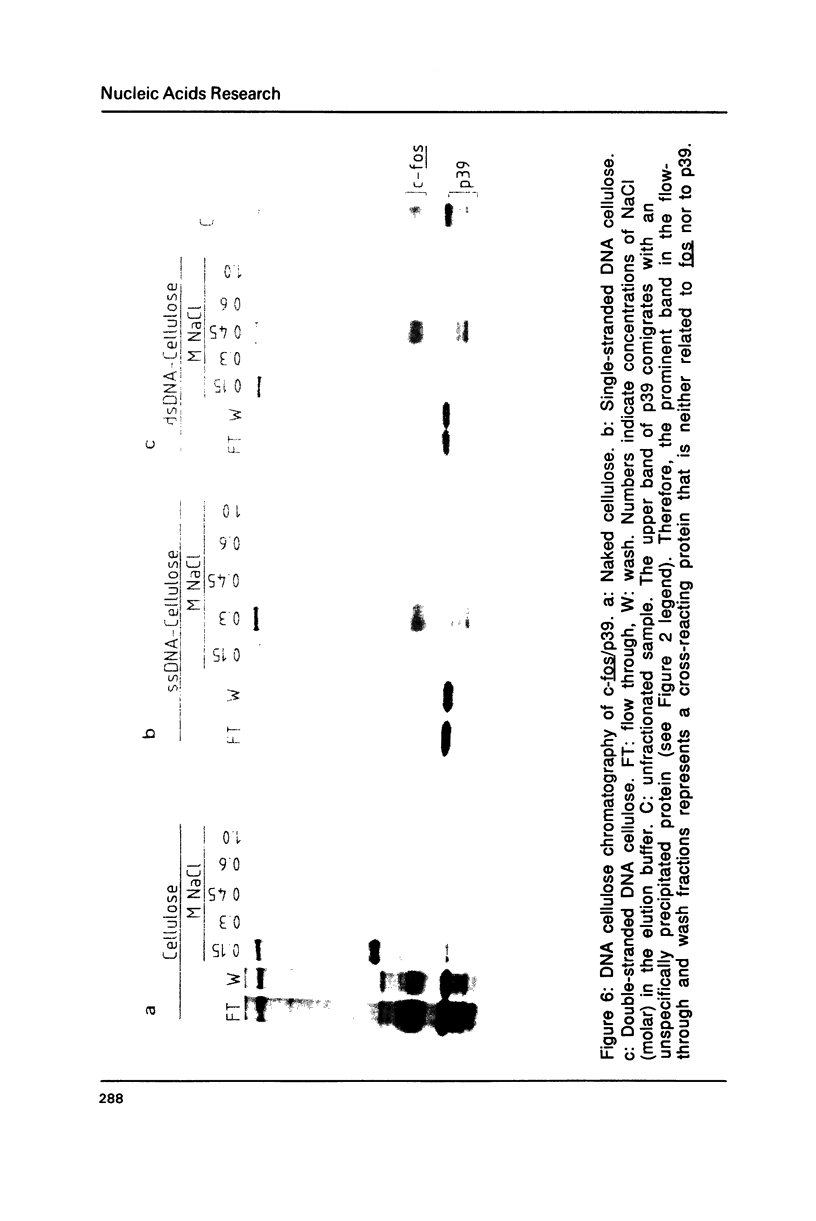
As a first step in the analysis of the molecular function of the nuclear c-fos proto-oncogene product we have studied its subnuclear localization in serum-stimulated mouse fibroblasts where it forms a non-covalent, apparently monodisperse complex with another nuclear protein, p39. The c-fos/p39 complex is almost quantitatively released from intact nuclei by DNasel or micrococcus nuclease treatment under conditions where only a minor fraction of DNA and nuclear proteins is released. In gel filtration experiments, c-fos/p39 comigrates with chromatin and seems to be associated with regions of increased DNasel accessibility. c-fos/p39 is bound to chromatin by electrostatic forces of moderate strength since greater than 90% of the complex can be eluted from nuclei at 0.4 M NaCl. In vitro, the c-fos/p39 complex in nuclear extracts binds to double- and single-stranded calf thymus DNA, suggesting that the association of c-fos/p39 with chromatin is at least in part due to its interaction with DNA. In agreement with this conclusion, c-fos/p39 is released from nuclei by incubation with tRNA, presumably due to competition for binding sites. Our observations are compatible with the hypothesis that c-fos may play a role in the regulation of gene expression.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Bunte T., Greiser-Wilke I., Donner P., Moelling K. Association of gag-myc proteins from avian myelocytomatosis virus wild-type and mutants with chromatin. EMBO J. 1982;1(8):919–927. doi: 10.1002/j.1460-2075.1982.tb01272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Miller A. D., Zokas L., Verma I. M. Viral and cellular fos proteins: a comparative analysis. Cell. 1984 Feb;36(2):259–268. doi: 10.1016/0092-8674(84)90219-8. [DOI] [PubMed] [Google Scholar]
- Curran T., Teich N. M. Identification of a 39,000-dalton protein in cells transformed by the FBJ murine osteosarcoma virus. Virology. 1982 Jan 15;116(1):221–235. doi: 10.1016/0042-6822(82)90415-9. [DOI] [PubMed] [Google Scholar]
- Curran T., Van Beveren C., Verma I. M. Viral and cellular fos proteins are complexed with a 39,000-dalton cellular protein. Mol Cell Biol. 1985 Jan;5(1):167–172. doi: 10.1128/mcb.5.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplan C., Theis J., O'Farrell P. H. The Drosophila developmental gene, engrailed, encodes a sequence-specific DNA binding activity. Nature. 1985 Dec 19;318(6047):630–635. doi: 10.1038/318630a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R. N., Tachibana C. Y., Abrams H. D., Hann S. R. V-myc- and c-myc-encoded proteins are associated with the nuclear matrix. Mol Cell Biol. 1985 Jan;5(1):114–126. doi: 10.1128/mcb.5.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgin S. C. Anatomy of hypersensitive sites. Nature. 1984 May 17;309(5965):213–214. doi: 10.1038/309213a0. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Hancock D. C. Studies on the interaction of the human c-myc protein with cell nuclei: p62c-myc as a member of a discrete subset of nuclear proteins. Cell. 1985 Nov;43(1):253–261. doi: 10.1016/0092-8674(85)90030-3. [DOI] [PubMed] [Google Scholar]
- Ilyin Y. V., Varshavsky A. Y., Mickelsaar U. N., Georgiev G. P. Studies on deoxyribonucleoprotein structure. Redistribution of proteins in mixtures of deoxyribonucleoproteins, DNA and RNA. Eur J Biochem. 1971 Sep 24;22(2):235–245. doi: 10.1111/j.1432-1033.1971.tb01537.x. [DOI] [PubMed] [Google Scholar]
- Jensen R. H., Chalkley R. The physical state of nucleohistone under physiological ionic strength. The effect of interaction with free nucleic acids. Biochemistry. 1968 Dec;7(12):4388–4395. doi: 10.1021/bi00852a035. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Sippel A. E. Subnuclear localization of proteins encoded by the oncogene v-myb and its cellular homolog c-myb. Mol Cell Biol. 1986 Jan;6(1):62–69. doi: 10.1128/mcb.6.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Simanis V., Bartsch R., Yewdell J., Gannon J., Mole S. Cellular targets for SV40 large T-antigen. Proc R Soc Lond B Biol Sci. 1985 Oct 22;226(1242):25–42. doi: 10.1098/rspb.1985.0077. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Curran T., Verma I. M. c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell. 1984 Jan;36(1):51–60. doi: 10.1016/0092-8674(84)90073-4. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Müller R. Cellular and viral fos genes: structure, regulation of expression and biological properties of their encoded products. Biochim Biophys Acta. 1986;823(3):207–225. doi: 10.1016/0304-419x(86)90003-x. [DOI] [PubMed] [Google Scholar]
- Müller R., Müller D., Verrier B., Bravo R., Herbst H. Evidence that expression of c-fos protein in amnion cells is regulated by external signals. EMBO J. 1986 Feb;5(2):311–316. doi: 10.1002/j.1460-2075.1986.tb04214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Verma I. M., Adamson E. D. Expression of c-onc genes: c-fos transcripts accumulate to high levels during development of mouse placenta, yolk sac and amnion. EMBO J. 1983;2(5):679–684. doi: 10.1002/j.1460-2075.1983.tb01484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Wagner E. F. Differentiation of F9 teratocarcinoma stem cells after transfer of c-fos proto-oncogenes. Nature. 1984 Oct 4;311(5985):438–442. doi: 10.1038/311438a0. [DOI] [PubMed] [Google Scholar]
- Rüther U., Wagner E. F., Müller R. Analysis of the differentiation-promoting potential of inducible c-fos genes introduced into embryonal carcinoma cells. EMBO J. 1985 Jul;4(7):1775–1781. doi: 10.1002/j.1460-2075.1985.tb03850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staufenbiel M., Deppert W. Different structural systems of the nucleus are targets for SV40 large T antigen. Cell. 1983 May;33(1):173–181. doi: 10.1016/0092-8674(83)90346-x. [DOI] [PubMed] [Google Scholar]
- Verma S. S., Gupta R. K., Kishore N., Sen Gupta J. A simple relationship between maximal aerobic power and body weight in Indian adolescent boys. Indian J Med Sci. 1986 Apr;40(4):93–96. [PubMed] [Google Scholar]
- Verrier B., Müller D., Bravo R., Müller R. Wounding a fibroblast monolayer results in the rapid induction of the c-fos proto-oncogene. EMBO J. 1986 May;5(5):913–917. doi: 10.1002/j.1460-2075.1986.tb04303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]