Abstract
CC chemokine ligand 20 (CCL20) attracts CC chemokine receptor 6 (CCR6)-expressing cells. Using endoscopic biopsies taken from the gastric antrum of 42 subjects infected with H. pylori and 42 uninfected subjects, mucosal CCL20 mRNA and protein levels were measured by real-time polymerase chain reaction and enzyme-linked immunosorbent assay, respectively. CCL19 mRNA and protein levels, as well as CCL21 mRNA levels, were also measured. The CCL20 mRNA and protein levels were significantly elevated in H. pylori-positive patients and substantially decreased after successful eradication. CCL19 and CCL21 expression levels were comparable in the H. pylori-infected and the uninfected groups. The CCL20 concentrations correlated with the degree of chronic gastritis. Immunohistochemistry and the in vitro infection assay showed that CCL20 was principally produced by the gastric epithelium. CCR6-expressing cells, including CD45RO+ memory T lymphocytes and fascin+-CD1a+ immature dendritic cells, infiltrated close to the CCL20-expressing epithelial cells. The CCL20/CCR6 interaction may be involved in the development of H. pylori-associated gastritis.
Keywords: CC chemokine ligand 20, CC chemokine receptor 6, H. pylori, Dendritic cells, cag pathogenicity island
Introduction
Helicobacter pylori is one of the most common infections in humans [1,2]. This noninvasive organism colonizes the gastric epithelium and causes chronic gastritis that is characterized by dense infiltration of neutrophils and mononuclear cells into the lamina propria [3,4]. H. pylori elicits specific antibodies against various immunogenic proteins derived from the bacterium [5]. Despite the cellular and humoral immune responses, H. pylori infection shows lifelong persistence in the majority of cases [1,2]. The complex interplay between the bacterium and the host immune reaction is not fully understood.
Chemokines are a family of cytokines that are characterized by their biological ability to attract different cell types through their action on specific receptors, and they play a pivotal role in various immune and inflammatory conditions, including H. pylori-associated gastritis (HAG) [6–12]. We have previously demonstrated enhancement of mucosal production of interleukin 8 (IL-8), a representative chemoattractant for neutrophils, monocyte chemoattractant protein 1 (MCP-1), and regulated on activation normal T-cell expressed and presumably secreted (RANTES), both of which induce an influx of mononuclear cells, such as macrophages and lymphocytes, in patients with HAG [8]. Mucosal IL-8 concentrations have been associated with the degree of neutrophil infiltration, whereas the MCP-1 and RANTES levels have been shown to be correlated with the grades of mononuclear cell infiltration in H. pylori-infected gastric mucosa [8]. Accumulating evidence has shown that a variety of chemokines are involved in the development of HAG.
Dendritic cells (DCs) are dedicated antigen-presenting cells and function as central mediators between the innate and cognate immune systems [13]. DC trafficking is tightly controlled by differential expression of chemokine ligands and specific receptors; immature DCs that express CC-chemokine receptor 6 (CCR6) migrate towards its exclusive chemokine ligand, CC-chemokine ligand 20 (CCL20, also known as macrophage inflammatory protein 3α, liver and activation-regulated chemokines, or exodus-1) at the inflammatory site, where they capture and process antigens [14,15]. Nishi et al. have reported that H. pylori infection upregulates CCL20 expression in gastric epithelial cells and induces an influx of DCs into the infected gastric mucosa of mice [10]. Recent studies have shown CCL20 upregulation and recruitment of CCR6-expressing CD3+ cells in gastric mucosa during H. pylori infection in humans [11], suggesting that CCL20 may play a role in HAG through interaction with the corresponding receptor.
The aim of the present study was to determine the role of CCL20 expression in the development of HAG in a relatively large number of subjects. The study was also designed to examine the expression of other lymphoid chemokines, CCL19 and CCL21, which are exclusive ligands for CCR7 expressed on mature type DCs [16,17]. To determine whether H. pylori infection leads to CCL20 upregulation in vitro CCL20 expression by gastric epithelial cells infected with diverse H. pylori strains was investigated.
Materials and methods
Subjects and tissue samples
Consecutive outpatients who underwent upper gastrointestinal endoscopy for dyspepsia between June 2006 and May 2007 were recruited. The study was approved by the Nagasaki University Ethics Committee. All samples were obtained with the written informed consent of the patients prior to their inclusion, in accordance with the Helsinki Declaration. Exclusion criteria were: age <18 or >80 years, pregnancy, body mass index (BMI) >30 kg/m2, diabetes mellitus, cachectic state (including cancer), systemic infection, liver disease, renal impairment, use of medications effective against H. pylori during the preceding 3 months, alcohol abuse, drug addiction, and chronic corticosteroid or nonsteroidal anti-inflammatory drug use. None of the subjects had undergone gastrointestinal surgery. During endoscopy, five biopsy specimens were obtained from the gastric antrum along the lesser curvature. Two of these samples were snap-frozen for measurement of mucosal protein levels of CCL20 and CCL19, and two were also snap-frozen for relative mRNA quantification of CCL20, CCL19, and CCL21. The other specimen was fixed in 10% formalin and embedded in paraffin for histopathological and immunohistological assessment.
Of the H. pylori-positive patients, eight were treated with eradication therapy consisting of: lansoprazole, 30 mg twice daily; amoxicillin, 750 mg twice daily; and clarithromycin, 400 mg twice daily, for 7 days. Four weeks after cessation of the treatment, the patients were again examined by endoscopy, and biopsy specimens were obtained in a similar fashion to that before treatment.
Histopathological examination
The paraffin-embedded biopsy specimens were cut into 5-µm-thick sections and stained with hematoxylin and eosin. Intestinal metaplasia was defined by the presence of goblet cells in the glandular mucosa with Alcian blue (pH 2.5)/periodic acid-Schiff staining. The histological degree of infiltration of neutrophils (activity) and mononuclear cells (chronic inflammation), glandular atrophy, and intestinal metaplasia was scored 0, 1, 2, or 3, corresponding to none, mild, moderate, or marked, in accordance with the updated Sydney system. Furthermore, the density of H. pylori colonization on the epithelia was assessed by histology with Giemsa staining and scored as 0, 1, 2 or 3, based on the above classification system. Two independent observers who were blinded to the endoscopic findings and experimental results examined the tissue sections, and their agreement was sought.
Measurement of gastric mucosa levels of chemokines
Measurements of local tissue concentrations of CCL19 and CCL20 were performed as described previously [8]. Briefly, the biopsy samples were immediately placed in 1.0 mL phosphate-buffered saline (PBS), frozen on dry ice, and stored at −80 °C until use. The samples were homogenized, and aliquots of homogenate supernatants, obtained by centrifugation (10,000 ×g for 10 min), were assayed for total protein by a modified Lowry method. The supernatants, diluted to 0.50 mg/mL total protein concentration, were frozen at −80 °C until assayed. The concentrations of cytokines in the biopsy homogenate supernatants were measured using commercially available assay kits (Research and Diagnostics, Minneapolis, MN, USA), which use the quantitative immunometric sandwich enzyme immunoassay technique. These assays were performed in duplicate according to the manufacturer's instructions. Cytokine concentrations were expressed in pg/mg protein. Inter-and intra-assay variabilities were <10%.
Real-time polymerase chain reaction
Total RNA from the biopsy samples was extracted using a commercial kit according to the supplier's instructions (Isogen, Nippon Gene Co., Toyama, Japan). One microgram of total RNA was reverse transcribed into complementary deoxyribonucleic acid (cDNA) in a volume of 25 µL with MuLV reverse transcriptase and random hexamers (both from PE Applied Biosystems, Foster City, CA, USA). Using the TaqMan® RT-PCR system, amplification of CCL20, CCl19, CCL21, and β-actin cDNA was performed in a MicroAmp® optical 96-well reaction plate (PE Applied Biosystems). The primer and probe sequences were designed to be cDNA-specific and to work under equivalent reaction conditions using primer express software (PE Applied Biosystems), and they were labeled with FAM (6-carboxyfluorescein) at the 5′-end and with TAMRA (6-carboxytetramethylrhodamine) at the 3′-end, as previously described [18]. A negative PCR control without template and a positive PCR control with a template of known amplification were included in each assay. The reactions were performed in an ABI PRISM 7700 Sequence Detection System (PE Applied Biosystems). During PCR, the Ct values (the cycle number at which the detected fluorescence exceeds the threshold) for each amplification product were determined using a threshold value of 0.03. The specific signals were normalized by constitutively expressed β-actin signals using the formula 2−DCt=2−(Ct, β-actin − Ct,Specific) as described in the ABI PRISM 7700 SDS relative quantification of gene expression protocol by PE Applied Biosystems. Thermal cycling was initiated with a first denaturation step at 95 °C for 10 min and followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min.
Immunohistochemistry
Immunohistochemical staining was performed with the streptavidin–biotin–peroxidase-complex method (Histofine SAB-PO® kits, Nichirei Co., Tokyo, Japan), as described previously [8,18]. The following steps were performed at room temperature unless otherwise specified. Paraffin-embedded biopsy specimens were cut into 5-µm-thick sections, deparaffinized, and rehydrated. After inhibition of endogenous peroxidase activity for 30 min with methanol containing 0.3% H2O2, the sections were reacted for 20 min with 5% albumin to prevent nonspecific binding. They were then incubated overnight at 4 °C with the primary antibodies, including CCL20 (Research and Diagnostics, diluted at 1:100), CCR6 (Research and Diagnostics, diluted at 1:500), fascin (Dako, Grostrup, Denmark, diluted at 1:25), CD1a (LabVision, Fremont, CA, USA, diluted at 1:25), CD45RO (Dako, diluted at 1:200), CD20 (Dako, dilution, 1:100), and CD68 (Dako, diluted at 1:100). On the next day, the sections were washed in 0.01 M phosphate-buffered saline (PBS) and incubated for 20 min with 10 mg/mL biotinylated antiserum corresponding to each primary antibody. After washing in PBS, the sections were reincubated for 20 min with 100 µg/mL horseradish peroxidase-conjugated streptavidin and stained with 0.02% 3,3′-diaminobenzidine tetrahydrochloride in 0.05 M tris–HCl buffer containing 0.03% H2O2. Finally, the sections were washed in PBS and counterstained with hematoxylin.
Detection of H. pylori infection
H. pylori status was assessed by the rapid urease test (Helicocheck, Otsuka Pharmaceutical Co., Tokushima, Japan) and histology with Giemsa staining using each biopsy sample taken from the antrum within 2 cm of the pyloric ring and in the middle portion of the corpus along the greater curvature. Patients were considered positive for H. pylori infection when at least one of these examinations yielded positive results. On the other hand, patients were defined as H. pylori-negative if all test results were negative. Eradication of H. pylori was considered successful when the 13C-urea breath test was negative.
In vitro H. pylori infection assay
A human gastric cancer cell line, AZ-521 (Culture Collection of Health Science Resource Bank, Japan Health Science Foundation, Tokyo, Japan) was used for the in vitro H. pylori infection assay, as described previously [19]. Briefly, the gastric epithelial cells (106/mL) were grown in Earle's minimal essential medium (Sigma-Aldrich, St. Louis, MO, USA) containing 10% fetal calf serum (Invitrogen, Carlsbad, CA, USA) under a 5% CO2 atmosphere at 37 °C, and they were infected with H. pylori ATCC 49503 (wild, American Type Culture Collection, Rockville, MD, USA) grown in Brucella broth at a ratio of 100 bacteria per cell. The ATCC 49503 stain possesses both the entire cag pathogenicity island (cagPAI) and vacA. Isogenic mutants lacking the whole cagPAI (ΔcagPAI), cagA (ΔcagA), or vacA (ΔvacA) were also inoculated to the gastric epithelial cells in a similar fashion. Three hours after incubation, total RNA was extracted from the gastric epithelial cells as described above and reverse transcribed to cDNA, which was subjected to quantification of CCL20 mRNA expression by real-time PCR.
Statistical analysis
Statistical analyses were performed using Fisher's exact test, the χ2 test, Student's t-test, the Mann–Whitney U test, and the Kruskal–Wallis test. A p value <0.05 was accepted as statistically significant. Data were expressed as mean± standard deviation (SD).
Results
Baseline characteristics
A total of 84 patients (mean age: 64 years; range: 49–80 years), consisting of 58 men and 46 women, was studied. There were 32 current smokers and 37 alcohol drinkers. Based on the rapid urease test and histopathology, 42 subjects each were designated as either positive or negative for H. pylori infection. Baseline characteristics, including age, gender, alcohol intake, smoking habits, and body mass index, were not statistically different between the H. pylori-infected group and the uninfected group.
Mucosal CCL20 and CCL19 levels in gastric mucosa tissues
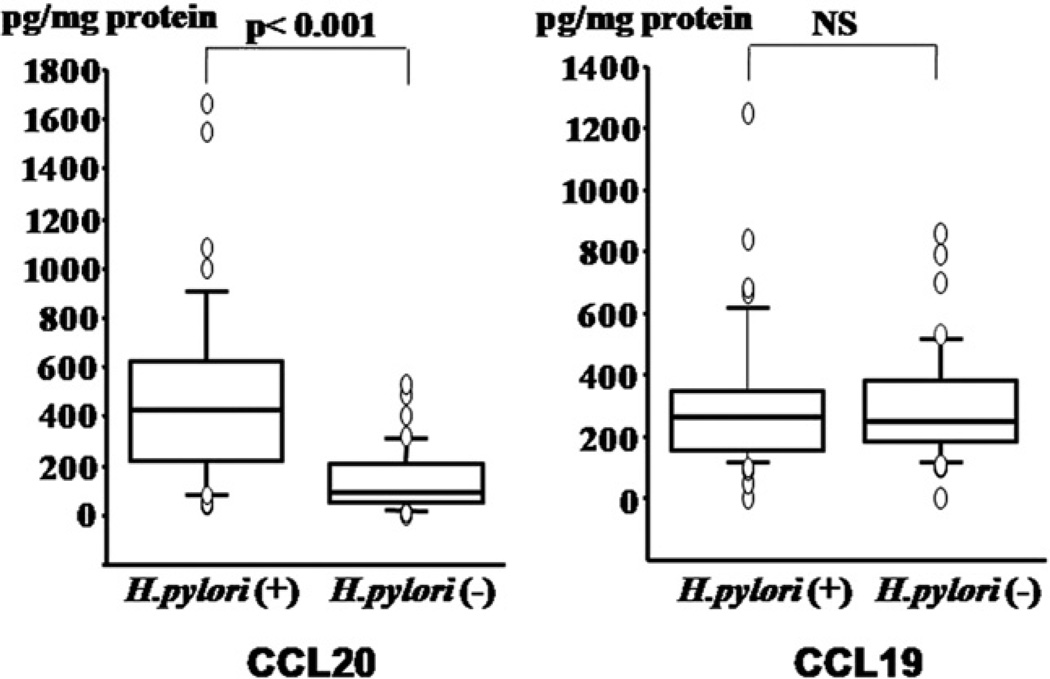
Fig. 1 shows the concentrations of CCL20 and CCL19 in the gastric mucosa. Mucosal CCL20 levels were significantly elevated in H. pylori-positive patients compared with H. pylori-negative patients (p<0.001), whereas there was no difference in the mucosal CCL19 concentrations between the H. pylori-infected group and the uninfected group.
Figure 1.
Concentrations of CC chemokine ligand 20 (CCL20) and CCL19 in gastric mucosa are shown by H. pylori status.
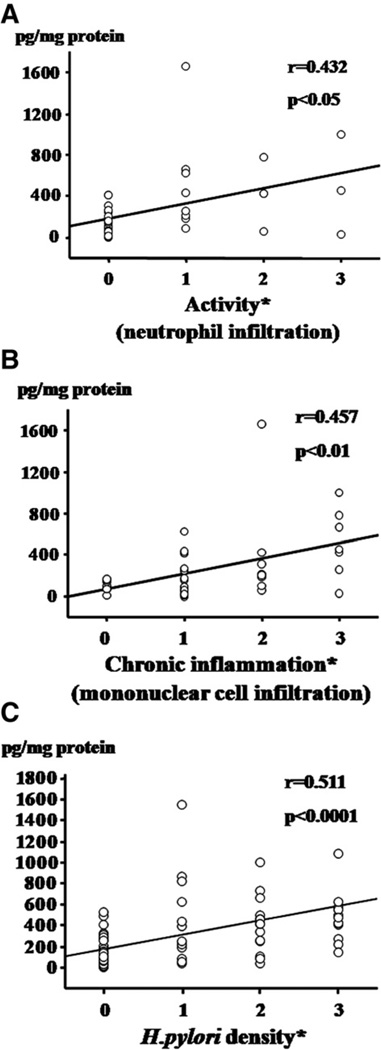
Fig. 2 shows the relationship between the mucosal CCL20 concentrations and the activity, chronic inflammation, and H. pylori density scores. There was a significant correlation between CCL20 concentrations and the activity (correlation coefficient, r=0.457, p<0.01, Fig. 2A) and chronic inflammation (r=432, p<0.05, Fig. 2B) scores. The CCL20 levels were significantly increased in parallel with the H. pylori density score (r=0.511, p<0.0001 Fig. 2C). On the other hand, no associations were observed between the mucosal CCL20 concentrations and atrophy (r=0.006) or the intestinal metaplasia score (r=0.046). No correlations were seen between the CCL19 levels and any gastritis scores or the H. pylori density score.
Figure 2.
Relationship between the CCL20 concentrations in gastric mucosa and the activity (A), chronic inflammation (B), and H. pylori density (C) scores. The each histological degree was scored 0, 1, 2, or 3, corresponding to none, mild, moderate, or marked, in accordance with the updated Sydney system.
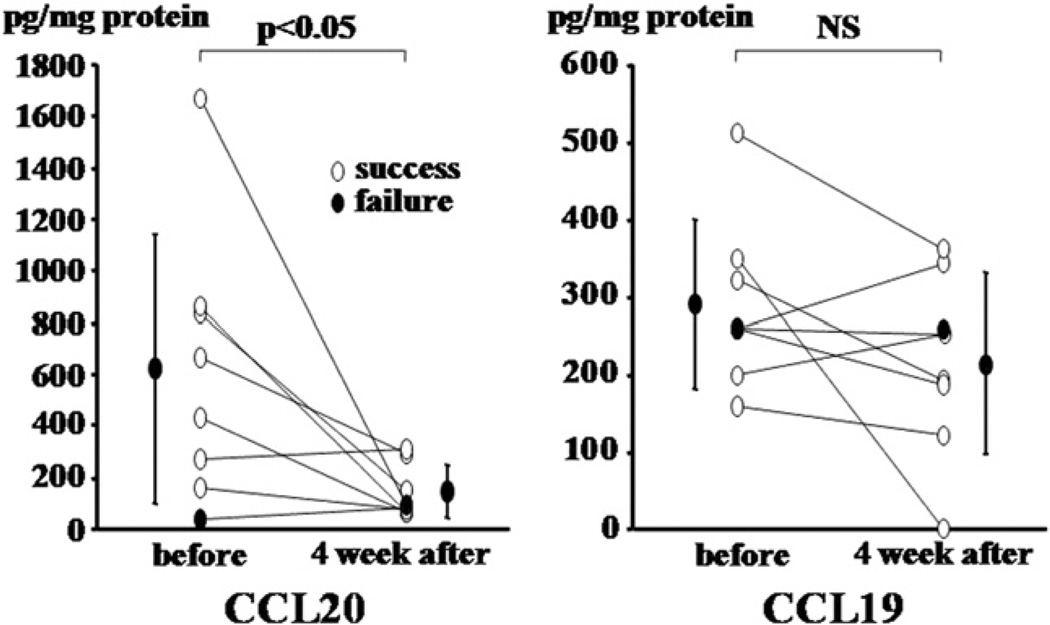
Successful eradication of H. pylori was achieved in seven (88%) of eight patients with triple therapy. Mucosal CCL20 concentrations significantly decreased 4 weeks after successful eradication (p<0.05), whereas no significant changes were observed following cure of the infection in the CCL19 concentrations (Fig. 3). In a patient who did not show eradication of H. pylori the CCL20 concentrations remained unchanged (270 and 305 pg/mg protein for pre- and post-treatment, respectively).
Figure 3.
CCL20 and CCL19 concentrations before and after eradication therapy.
Relative expression levels of CCL20, CCL19, and CCL21 mRNA and H. pylori infection
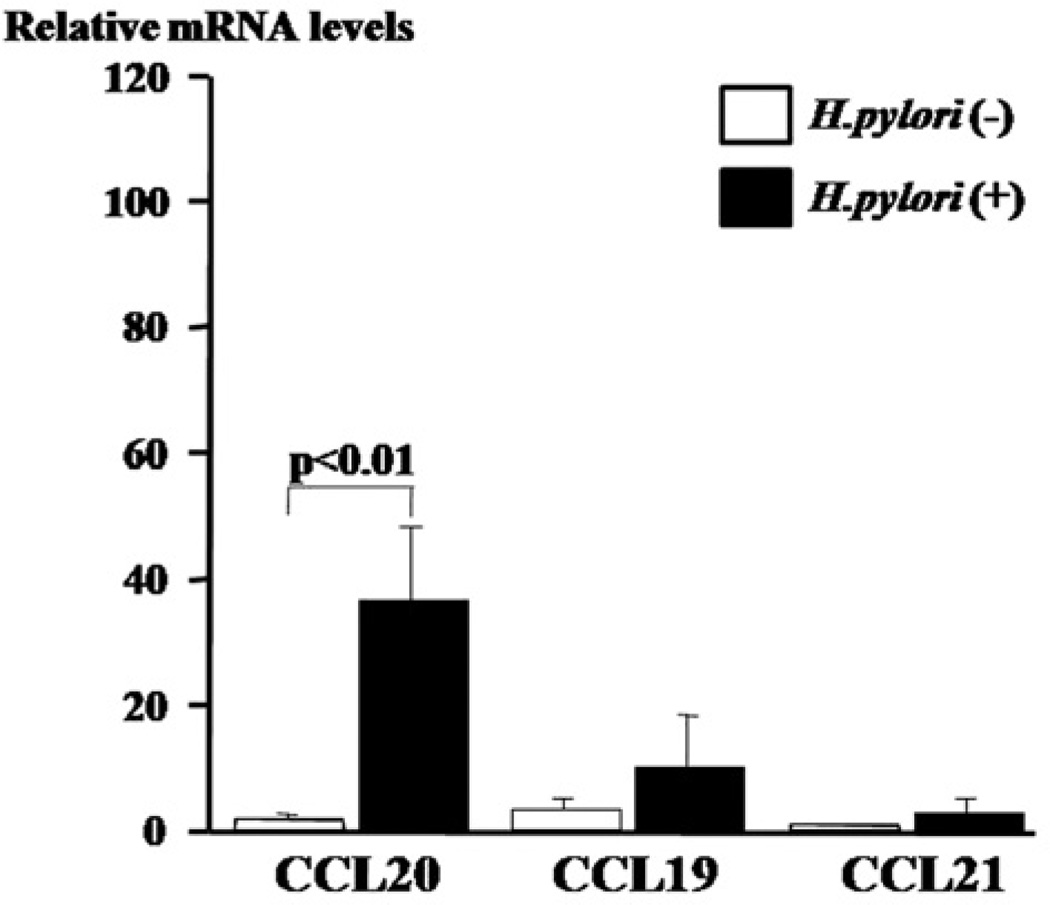
The relative expression levels of CCL20 mRNA in the gastric mucosa specimens of H. pylori-positive patients were significantly higher than those of H. pylori negative patients (Fig. 4). Similarly, the relative CCL20 mRNA expression levels were significantly correlated with the H. pylori density score (r=0.661, p<0.001). There was a significantly positive correlation between the relative CCL20 mRNA and protein levels (r=0.516, p<0.01).
Figure 4.
Relative expression levels of CCL20, CCL19, and CCL21 mRNA in gastric mucosa are shown by H. pylori status.
The relative expression levels of CCL19 and CCL21 mRNA were comparable in the H. pylori-infected and the uninfected groups.
Immunohistochemical analyses
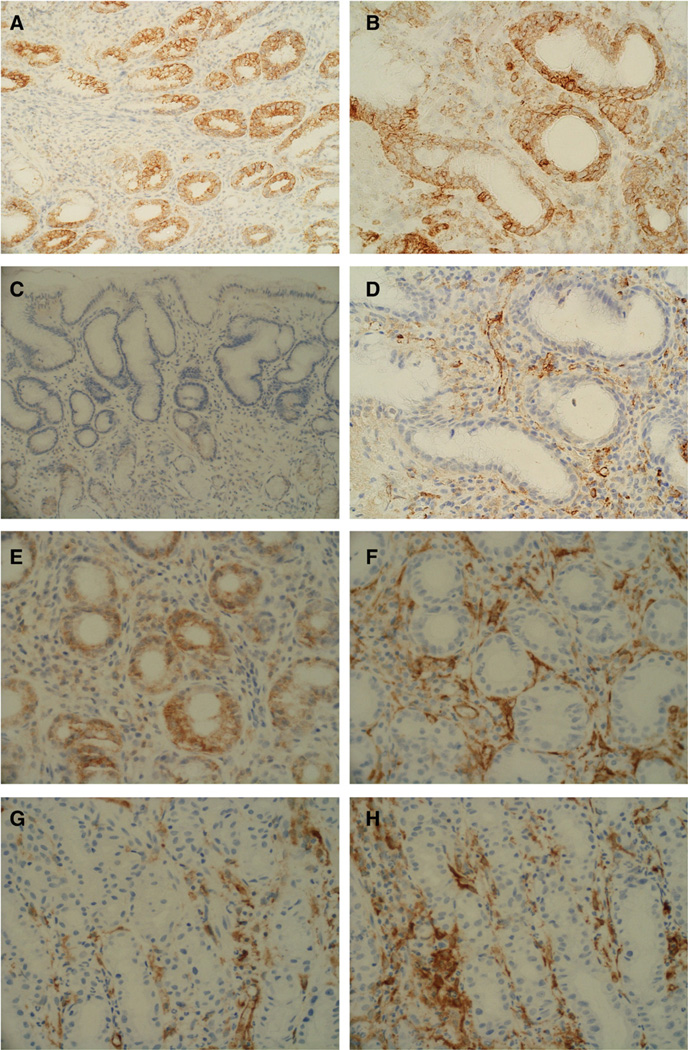
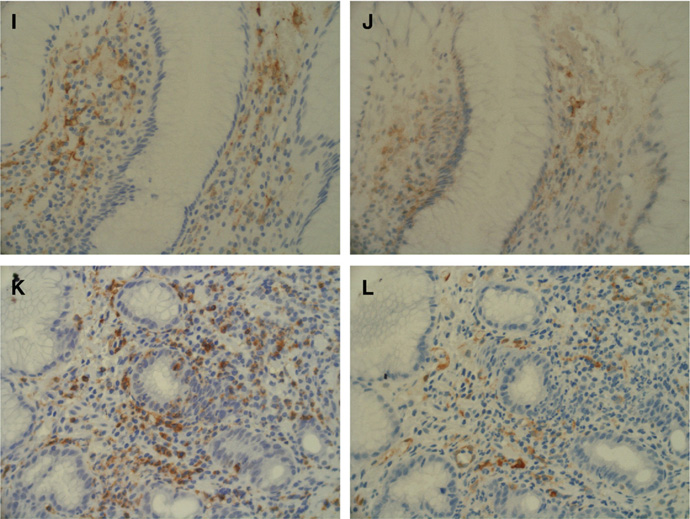
As shown in Figs. 5A, B, CCL20 immunoreactivity was seen exclusively in the gastric epithelial cells of patients with HAG. In contrast, CCL20 staining was faint or undetectable in the uninfected normal mucosa (Fig. 5C). The CCR6-expressing cells infiltrated in proximity to the CCL20-expressing cells (Figs. 5E, F). Of note, DCs positive for fascin, a pan-DC marker [20], were predominantly infiltrated in proximity to the CCL20-expressing epithelia (Fig. 5D). These DCs were positive for CCR6 (Fig. 5F), as were CD45RO-positive memory T cells (Figs. 5K, L). CD20-positive (B cell lineage) but not CD68-positive (macrophage) cells sometimes showed immunoreactivity with anti-CCR6 antibody (data not shown). Again, fascin-expressing DCs showed immunoreactivity for CD1a, indicating the immature DC subset [20] (Figs. 5I, J).
Figure 5.
CCL20 immunoreactivity was exclusively in the gastric epithelial cells of patients with H. pylori-associated gastritis (A, lower power view; B, higher power view). In contrast, CCL20 staining was almost absent in the uninfected normal mucosa (C). The CC chemokine receptor 6 (CCR6)-expressing cells (E) were found in close proximity to CCL20 expressing cells (F). Dendritic cells (DCs) positive for fascin, a pan-DC marker (D), can be seen to be predominantly infiltrated in the vicinity of the CCL20-expressing epithelia (B). Surface CCR6 primarily co-localized with DCs (G, H). The fascin-expressing DCs (I) show immunoreactivity for CD1a (J), indicating the immature phenotype. CD45RO-expressing memory T lymphocytes (K) are positive for CCR6 (L).
CCL20 mRNA expression in gastric epithelial cells infected with diverse H. pylori strains in vitro
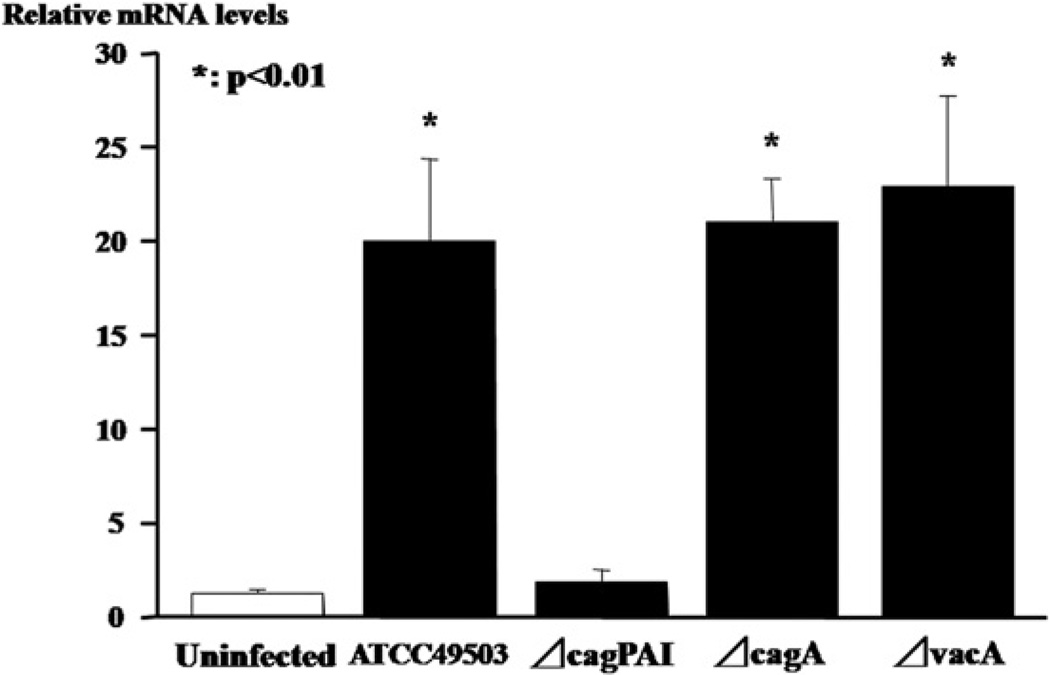
Infection with wild type H. pylori strain (ATCC49503) led to significantly increased CCL20 mRNA levels in AZ-521 cells compared to uninfected controls (Fig. 6). Similarly, infection with the ΔcagA or the ΔvacA strain significantly induced CCL20 mRNA expression. However, the relative CCL20 mRNA expression levels in the AZ-521 cells infected with the ΔcagPAI H. pylori strain were extremely low compared to those in the uninfected controls.
Figure 6.
Relative CCL20 mRNA levels in a gastric epithelial cell line (AZ-521) infected with diverse H. pylori strains including wild H. pylori strain (ATCC49503), the isogenic mutant strain lacking the entire cag pathogenecity island PAI (ΔcagPAI), the isogenic cagA (ΔcagA) mutant strain and the isogenic vacA (ΔvacA) mutant strain in vitro.
Discussion
The present results showed increased local production of CCL20, a representative lymphoid chemokine, in the gastric mucosa of patients infected with H. pylori. Nishi et al. were the first to report the association of H. pylori infection with CCL20 overexpression in murine gastric tissue [10]. Recent studies using RT-PCR showed that CCL20 mRNA expression was upregulated in H. pylori-infected gastric mucosa in humans, though only a small number of patients was examined [11,12]. In the present study involving a larger number of subjects, we confirmed significantly increased CCL20 protein and mRNA levels in gastric mucosa infected with H. pylori. The mucosal CCL20 mRNA and protein levels were positively correlated with the H. pylori density of the colonization. In addition, the CCL20 concentrations were significantly decreased after successful eradication therapy. Thus, it is evident that H. pylori infection induces CCL20 biosynthesis and secretion in the gastric mucosa of humans.
Several immunohistochemical analyses have shown CCL20-positive epithelial cells in infected gastric mucosa in humans and mice [10,12]. Consistent with this, in the present study, CCL20 immunoreactivity was primarily associated with the gastric epithelia of specimens from HAG patients. In the in vitro H. pylori infection assay, the ATCC 49503 wild strain containing the entire cag PAI can induce >20-fold increase in CCL20 mRNA levels in gastric epithelium-derived cells, further supporting the notion that the epithelium is the main source of CCL20 production in H. pylori-infected gastric tissue. In contrast, an isogenic cagPAI mutant (ΔcagPAI), but not an isogenic ΔcagA or ΔvacA mutant, failed to induce CCL20 mRNA expression, suggesting that an intact cagPAI is involved in sufficient CCL20 induction. It was also found that the ability of H. pylori to induce CCL20 expression was independent of the vacA locus. Tomimori et al. have shown that H. pylori containing the entire cagPAI genes activates nuclear factor-κB (NF-κB), a pivotal transcription factor regulating many immune and inflammation-related genes, through an intracellular signaling pathway that involves I-κB kinase and NF-κB-inducing kinase, leading to CCL20 gene transcription in gastric cancer cell lines [12]. Moreover, they have shown that the NF-κB binding site present in the CCL20 gene promoter region is required for CCL20 induction [12]. Again, CCL20 is known to be upregulated in the presence of IL-1 and tumor necrosis factor α (TNF-α), which activate NF-κB, in gastric and colonic epithelial cells [11,21]. Wu et al. have demonstrated that CCL20 production is substantially augmented in response to H. pylori when there is stimulation by IL-1β and TNF-α [11]. Thus, CCL20 overexpression by gastric epithelial cells upon interaction with H. pylori may be mediated in part through induction of the proinflammatory cytokines produced locally in the inflamed stomach. In a previous study, we demonstrated nuclear localization of activated NF-κB not only in infiltrating inflammatory cells but also in epithelial cells in the gastric mucosa of patients with HAG [8]. Thus, infection with H. pylori, in particular the virulent cagPAI-carrying strain, may cause NF-κB activation in the gastric epithelium directly or via mucosal inflammatory responses, leading to CCL20 overexpression [12,19].
In the present study, there was a positive correlation between the mucosal CCL20 protein levels and the grading scores of mononuclear cell infiltration of HAG. A similar result was reported in a previous study, where the CCL20 mRNA levels were correlated with the degree of chronic gastritis, [12]. CCL20 is chemotactic for memory T cells and immature DCs through interaction with CCR6 [14,15]. In fact, the present study confirmed gastric mucosal infiltration of CD45RO+ memory T lymphocytes and fascin+-CD1a+ DCs, both of which were positive for CCR6. The CCR6-expressing cells were found in close proximity to CCL20-expressing gastric epithelial cells in patients with HAG. CCR6 was also expressed on B lymphocytes [22], as shown in the present study, and is known to be present on activated neutrophils [23], possibly explaining the association of the CCL20 mucosal levels with the degree of gastritis activity. Enhanced CCL20 production from gastric epithelium may be involved in the attraction of CCR6-bearing immune cells into the gastric mucosa in response to H pylori infection, thus perpetuating the mucosal inflammation.
CCL19 and CCL20 are structurally related chemokines that share the common receptor CCR7 [16,17]. DCs pick up antigen in the mucosa and travel to the T-cell areas of secondary lymphoid organs, where antigen presentation takes place. CCL19 and CCL21 are expressed in the T-cell zone of secondary lymphoid organs, where they exert potential chemotactic action for mature DCs through the interaction with CCR7, promoting immune and inflammatory processes [16, 17,24]. In fact, DCs with the mature phenotype have been identified in gastric mucosa in humans and mice with autoimmune gastritis [25], suggesting that they might contribute to gastric inflammation. A recent in vitro study has shown that DCs express CCR7 and migrate in response to CCL19 after exposure to H. pylori [24]. However, there were no differences in the relative CCL19 mRNA and protein levels, as well as in the CCL21 mRNA levels, in the gastric mucosa in the H. pylori-infected and the uninfected groups. On the contrary, the CCL19 and CCL20 gene expression levels were significantly increased in colonic tissue obtained from patients with Crohn's disease who underwent bowel resection compared to the unaffected tissue obtained from diverticular disease or colon cancer [20]. The reason for this discrepancy is not clear, but the differences in inflammatory sites, severity of inflammation, and specimen size may be responsible; biopsies usually sample the mucosal tissue only, which might result in underestimation of the CCL20 and CCL19 expression in the deeper layers in the present study. Nevertheless, the numbers of DCs with immature phenotype were not increased in the mucosa of patients with Crohn's disease compared to controls [20], suggesting that DC subsets have diverse roles in the pathogenesis of these diseases. As for HAG, immature DC accumulation via the CCL20/CCR6 interaction would favor priming, whereas low expression of CCL19 and CCL21 indicates DC immaturity, which may lead to immune tolerance, possibly explaining why the immune system fails to eliminate H. pylori infection.
In conclusion, the present study demonstrated that H. pylori infection induces local CCL20 production by the gastric epithelium of patients with HAG. CCL20 upregulation may perpetuate mucosal trafficking of immature DCs, memory T lymphocytes, and other immune cells through surface CCR6 expression, and thereby favor to the development of HAG.
Acknowledgment
J Moss was supported by the intramural research program, NIH, NHLBI.
References
- 1.Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J. Clin. Invest. 2004;113:321–333. doi: 10.1172/JCI20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ernst PB, Gold BD. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu. Rev. Microbiol. 2000;54:615–640. doi: 10.1146/annurev.micro.54.1.615. [DOI] [PubMed] [Google Scholar]
- 3.Allen LAH. The role of the neutrophils and phagocytosis in infection caused by Helicobacter pylori. Curr. Opin. Infect. Dis. 2001;14:273–277. doi: 10.1097/00001432-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Shimoyama T, Crabtree JE. Bacterial factors and immune pathogenesis in Helicobacter pylori infection. Gut. 1998;43:S2–S5. [PMC free article] [PubMed] [Google Scholar]
- 5.Zevering Y, Jacob L, Meyer TF. Naturally acquired human immune responses against Helicobacter pylori and implications for vaccine development. Gut. 1999;45:465–474. doi: 10.1136/gut.45.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 7.Luster AD. Chemokines—chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 8.Isomoto H, Mizuta Y, Miyazaki M, Takeshima F, Omagari K, Murase K, Nishiyama T, Inoue K, Murata I, Kohno S. Implication of NF-kappaB in Helicobacter pylori-associated gastritis. Am. J. Gastroenterol. 2000;95:2768–2776. doi: 10.1111/j.1572-0241.2000.02304.x. [DOI] [PubMed] [Google Scholar]
- 9.Hansson M, Hermansson M, Svensson H, Elfvin A, Hansson LE, Johnsson E, Sjöling A, Quiding-Järbrink M. CCL28 is increased in human Helicobacter pylori-induced gastritis and mediates recruitment of gastric immunoglobulin A-secreting cells. Infect. Immun. 2008;76:3304–3311. doi: 10.1128/IAI.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishi T, Okazaki K, Kawasaki K, Fukui T, Tamaki H, Matsuura M, Asada M, Watanabe T, Uchida K, Watanabe N, Nakase H, Ohana M, Hiai H, Chiba T. Involvement of myeloid dendritic cells in the development of gastric secondary lymphoid follicles in Helicobacter pylori-infected neonatally thymectomized BALB/c mice. Infect. Immun. 2003;71:2153–2162. doi: 10.1128/IAI.71.4.2153-2162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu YY, Tsai HF, Lin WC, Hsu PI, Shun CT, Wu MS, Hsu PN. Upregulation of CCL20 and recruitment of CCR6+ gastric infiltrating lymphocytes in Helicobacter pylori gastritis. Infect. Immun. 2007;75:4357–4363. doi: 10.1128/IAI.01660-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomimori K, Uema E, Teruya H, Ishikawa C, Okudaira T, Senba M, Yamamoto K, Matsuyama T, Kinjo F, Fujita J, Mori N. Helicobacter pylori induces CCL20 expression. Infect. Immun. 2007;75:5223–5232. doi: 10.1128/IAI.00731-07. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 14.Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Aït-Yahia S, Brière F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaser A, Ludwiczek O, Holzmann S, Moschen AR, Weiss G, Enrich B, Graziadei I, Dunzendorfer S, Wiedermann CJ, Mürzl E, Grasl E, Jasarevic Z, Romani N, Offner FA, Tilg H. Increased expression of CCL20 in human inflammatory bowel disease. J. Clin. Immunol. 2004;24:74–85. doi: 10.1023/B:JOCI.0000018066.46279.6b. [DOI] [PubMed] [Google Scholar]
- 16.Sallusto F, Palermo B, Lenig D, Miettinen M, Matikainen S, Julkunen I, Forster R, Burgstahler R, Lipp M, Lanzavecchia A. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur. J. Immunol. 1999;29:1617–1625. doi: 10.1002/(SICI)1521-4141(199905)29:05<1617::AID-IMMU1617>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Dieu-Nosjean MC, Vicari A, Lebecque S, Caux C. Regulation of dendritic cell trafficking: a process that involves the participation of selective chemokines. J. Leukoc. Biol. 1999;66:252–262. doi: 10.1002/jlb.66.2.252. [DOI] [PubMed] [Google Scholar]
- 18.Isomoto H, Ueno H, Saenko VA, Mondal MS, Nishi Y, Kawano N, Ohnita K, Mizuta Y, Ohtsuru A, Yamashita S, Nakazato M, Kohno S. Impact of Helicobacter pylori infection on gastric and plasma ghrelin dynamics in humans. Am. J. Gastroenterol. 2005;100:1711–1720. doi: 10.1111/j.1572-0241.2005.41492.x. [DOI] [PubMed] [Google Scholar]
- 19.Lu H, Wu JY, Kudo T, Ohno T, Graham DY, Yamaoka Y. Regulation of Interleukin-6 promoter activation in gastric epithelial cells infected with Helicobacter pylori. Mol. Biol. Cell. 2005;16:4954–4966. doi: 10.1091/mbc.E05-05-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Middel P, Raddatz D, Gunawan B, Haller F, Radzun HJ. Increased number of mature dendritic cells in Crohn's disease: evidence for a chemokine mediated retention mechanism. Gut. 2006;55:220–227. doi: 10.1136/gut.2004.063008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon JH, Keates S, Bassani L, Mayer LF, Keates AC. Colonic epithelial cells are a major site of macrophage inflammatory protein 3alpha (MIP-3alpha) production in normal colon and inflammatory bowel disease. Gut. 2002;51:818–826. doi: 10.1136/gut.51.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao F, Shirakawa AK, Foley JF, Rabin RL, Farber JM. Human B cells become highly responsive to macrophage-inflammatory protein-3 alpha/CC chemokine ligand-20 after cellular activation without changes in CCR6 expression or ligand binding. J. Immunol. 2002;168:4871–4880. doi: 10.4049/jimmunol.168.10.4871. [DOI] [PubMed] [Google Scholar]
- 23.Akahoshi T, Sasahara T, Namai R, Matsui T, Watabe H, Kitasato H, Inoue M, Kondo H. Production of macrophage inflammatory protein 3alpha (MIP-3alpha) (CCL20) and MIP-3beta (CCL19) by human peripheral blood neutrophils in response to microbial pathogens. Infect. Immun. 2003;71:524–526. doi: 10.1128/IAI.71.1.524-526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansson M, Lundgren A, Elgbratt K, Quiding-Järbrink M, Svennerholm AM, Johansson EL. Dendritic cells express CCR7 and migrate in response to CCL19 (MIP-3beta) after exposure to Helicobacter pylori. Microbes Infect. 2006;8:841–850. doi: 10.1016/j.micinf.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Ninomiya T, Matsui H, Akbar SM, Murakami H, Onji M. Localization and characterization of antigen-presenting dendritic cells in the gastric mucosa of murine and human autoimmune gastritis. Eur. J. Clin. Invest. 2000;30:350–358. doi: 10.1046/j.1365-2362.2000.00629.x. [DOI] [PubMed] [Google Scholar]