Abstract
Atrial fibrillation (AF) is one of the most common arrhythmias seen in clinical practice. Current evidence suggests that serum uric acid (SUA) could be a marker of oxidative damage a factor reported as part of the mechanisms of AF. The purpose of this study was to evaluate if SUA predicted AF in the Atherosclerosis Risk in Communities study (ARIC). This analysis included 15,382 AF-free black and white men and women, aged 45-64, from the ARIC study, a population-based prospective cohort in the US. SUA was determined using the uricase-peroxidase method at baseline. The primary outcome was the incidence of AF defined as the occurrence of AF detected from hospital discharge codes, scheduled study electrocardiograms (ECG) and /or death certificates during follow-up period (1987-2004). We identified 1085 cases of incident AF. In Cox proportional hazards models adjusted for age, sex, race, center, education, body-mass index, serum glucose, systolic and diastolic blood pressure, LDL cholesterol, alcohol use, prevalent coronary heart disease and heart failure, serum creatinine, use of diuretics, and p wave duration on the ECG (as a measure of left atrial size) at baseline, the hazard ratio (HR) of AF associated with a 1-standard deviation increment in SUA was 1.16; 95% CI 1.06 -1.26. The association of SUA with AF risk differed by race and gender (p for interaction<0.01). In conclusion, elevated SUA is associated with a higher risk of AF, particularly among blacks and women. Further studies should replicate this association and explore potential mechanisms.
Keywords: Atrial fibrillation, Uric acid, Epidemiology
Introduction
Serum uric acid (SUA) is a by-product of purine catabolism, the terminal steps of which are catalyzed by xanthine oxidoreductase (XOR). SUA as an independent predictor of cardiovascular diseases and mortality1, 2. The rationale for this association is that SUA levels rise due to increased purine catabolism resulting from tissue hypoxia and apoptosis and this excess leads to the formation of reactive oxygen species (ROS) capable of oxidative stress damage 3. Therefore, SUA might be an inexpensive marker of the effects of oxidative stress on the heart 4, 5. We hypothesized that in a population-based study of middle aged men and women from four United States communities, those with elevated SUA at baseline are at increased risk for the development of future AF and that this association is independent of cardiovascular risk factors.
Methods
The ARIC Study is a longitudinal community-based cohort of 15,792 men and women adults aged 45 to 64 years at enrollment. The cohort was sampled from 4 US communities: Forsyth County, North Carolina; Jackson, Mississippi; the northwest suburbs of Minneapolis, Minnesota; and Washington County, Maryland. By design, the Jackson site exclusively recruited African Americans, thereby accounting for 90% of African Americans in the study. Most of the remaining African Americans came from Forsyth County. The sampling procedures and methods used in ARIC have been described in detail elsewhere 6. Baseline visits were conducted from 1987 through 1989.
Participants were followed up subsequently by annual telephone interviews and three field center visits every 3 years (last one in 1996-98). For this study, participants were excluded for the following reasons: electrocardiogram (ECG) diagnosed AF at baseline (n=37), missing SUA measurement at baseline (n= 128), and absent ECG examination (n=245). This left 15,382 ARIC participants for analysis.
Participants were asked to fast for at least 12 hours before morning blood collection. After applying a tourniquet, blood was drawn from the antecubital vein while participants were seated. Blood specimens were collected into vacuum tubes containing serum-separator gel (glucose, creatinine and SUA) and EDTA (lipids). Tubes were centrifuged at 3000g for 10 minutes at 4°C. After separation, aliquots were quickly frozen at -70°C until analysis was performed (within a few weeks). Uric acid was measured using the uricase-peroxidase enzymatic method. The reliability coefficient of SUA was 0.91 assessed by repeated measurements taken ≥1 week apart in 40 subjects (10 per ARIC center) and within person variability was 7.2% [13]. SUA was measured at baseline (n= 15,646) and at visit 2 (n= 14,296).
AF diagnoses were obtained from 3 sources: ECGs at the 4 scheduled study exams through 1998, hospital discharge records through 2004, and death certificates through 2004. Study participants underwent standard 12-lead ECG recordings at baseline and at each of the three follow-up exams. All ECG recordings were done with MAC PC Personal Cardiographs (Marquette Electronics, Inc, Milwaukee, WI). All ECG recordings automatically coded as AF were visually rechecked to confirm the diagnosis 7.
As part of standard follow-up procedures in ARIC, a trained abstractor obtained and recorded all International Classification of Diseases, Ninth Revision (ICD-9) hospital discharge diagnoses codes from each participant’s hospitalizations reported in the annual follow-up interview or via hospital surveillance. AF was defined as the presence of ICD-9 code 427.31 in the discharge codes. Participants with a diagnosis of atrial flutter (ICD-9 code 427.32) not developing AF in subsequent follow-up were not considered as having AF (n = 53) and their follow-up time was continued until censored. Transient AF can occur during cardiac operative procedures. Therefore, we excluded transient AF occurring simultaneously with heart revascularization surgery (ICD-9 code 36.X) or other cardiac surgery involving heart valves or septa (ICD-9 code 35.X), without evidence of AF in subsequent hospitalizations or study exams (n = 86 cases) and their follow-up time was continued until censored. Participants were also labeled as AF cases if the underlying cause of death was AF (ICD-10 code I48 or ICD-9 code 427.3). A validation study of the AF ICD code in a sample of ARIC hospitalizations reported a sensitivity and positive predictive values close to 90% 8.
The incidence date of AF was defined as the date of the first ECG showing AF, the first hospital discharge with an AF or atrial flutter diagnosis (the latter only if AF was identified later in the same patient), or death certificate with AF, whichever occurred earlier.
Additional variables were collected at visit 1 (baseline). Information on age, sex, race, educational attainment, alcohol and cigarette use, and physical activity were based on self-report. Other variables like blood pressure and body mass index were measured. Serum creatinine, glucose and triglycerides were measured. Use of diuretics within the two weeks before the baseline interview were identified from medication containers and used as a categorical variable. Metabolic syndrome was defined according to the National Cholesterol Education Program 9.
Prevalent heart failure was defined as current use of medications for heart failure at the baseline exam or evidence of manifest heart failure, stage 3, according to the Gothenburg criteria 10. Prevalent MI was defined as self-reported history of physician-diagnosed MI, silent MI by electrocardiography, or hospitalized MI at baseline.
Incidence of cardiovascular events was collected during the follow-up. Incident heart failure (HF) cases were identified through review of death certificates and local hospital discharge lists and defined according to the International Classification of Diseases Codes (ICD-9 or ICD-10). Incident HF was defined as first HF hospitalization (428, ICD-9) or any death where the death certificate included an HF code (428, ICD-9 and I50, ICD-10). Non-hospitalized, nonfatal HF was not captured. Hospitalized MI was defined according to ARIC standardized criteria 11.
Follow-up started the date of first examination and ended at the development of AF, death, lost to follow-up, or December 31, 2004 (whatever occurred earlier). Incidence rates were calculated for each quartile of SUA dividing the number of incident AF cases over the person-years of follow-up. We calculated the hazard ratio (HR) of developing AF per 1 standard deviation (SD) of SUA using Cox proportional hazards models, adjusting for potential confounding factors. We also evaluated the association of quartiles of SUA with AF incidence creating dummy variables and using the lowest quartile of SUA as the reference in Cox proportional hazards models. We conducted a chi-square goodness of fit test and adjusted our analysis adjusted for age, gender, education, race, center, body mass index, alcohol use, serum glucose, systolic and diastolic blood pressure, low density lipoprotein (LDL) cholesterol, prevalent coronary heart disease and heart failure, serum creatinine, use of diuretics and p wave duration on ECG at baseline. To assess the robustness of our analysis we performed two subsidiary analyses. First, we calculated the mean SUA level using the first (baseline) and second (visit 2) measurements and determined event rates and hazard ratios. Second, SUA was used as a time varying covariate in the multivariate Cox proportional hazards models. Finally, we included interaction terms to evaluate if the association between AF and SUA differed by race, gender, or the metabolic syndrome.
Because SUA might increase the risk of AF through an increased risk of heart failure or myocardial infarction, we conducted an additional analysis censoring individuals when they developed heart failure or myocardial infarction before they developed AF.
Proportional hazard assumptions and assumptions of linearity of the association were examined using scaled Schoenfeld residuals. Analyses were performed using STATA version 10 (College Station, TX) and all tests were two-tailed with p value of <0.05 considered as statistically significant.
Results
Selected baseline characteristics of the cohort of 15,382 adults free of AF are shown by quartiles of baseline SUA in Table 1. Participants in the highest quartile of SUA were more likely to be older, black, males and had a higher prevalence of cardiovascular risk factors, incident heart failure and myocardial infarction when compared to participants in the lowest quartile of SUA.
Table 1.
Baseline characteristics of 15,382 adults free of atrial fibrillation by quartile of serum uric acid
| Characteristic * | Baseline serum uric acid | ||||
|---|---|---|---|---|---|
| SUA cut-points(mg/dl) | Quartile 1 < 5.0 | Quartile 2 5.0 - 5.9 | Quartile 3 6.0 – 7.0 | Quartile 4 >7.0 | |
| N | 3880 | 3919 | 3927 | 3656 | p for trend |
| Age (years) | 53.0 (5.6) | 54.1 (5.6) | 54.3 (5.6) | 54.6 (5.8) | <0.01 |
| Female | 87% | 63% | 39% | 26% | <0.01 |
| Black | 19% | 23% | 22% | 30% | <0.01 |
| Body-mass index (kg/m2) | 24.8 (4.1) | 26.8(4.8) | 28.0 (5.0) | 29.4 (5.1) | <0.01 |
| Serum creatinine (mg/dl) | 0.98 (0.20) | 1.06 (0.26) | 1.14 (0.35) | 1.25 (0.67) | <0.01 |
| Diuretic use | 9% | 12% | 18% | 32% | <0.01 |
| Systolic BP (mm Hg) | 136.4 (17.1) | 138.8 (17.0) | 141.1 (16.7) | 144.8 (17.8) | <0.01 |
| Serum glucose (mg/dl) | 107.7 (44.5) | 109.8 (41.2) | 111.0 (38.1) | 114.9 (39.9) | <0.01 |
| Prevalent heart failure | 2% | 3% | 5% | 9% | <0.01 |
| Prevalent coronary artery disease | 1% | 3% | 5% | 8% | <0.01 |
| Incident heart failure | 7% | 10% | 12% | 17% | <0.01 |
| Incident myocardial infarction | 6% | 7% | 9% | 13% | <0.01 |
Results shown as mean (standard deviation) or percentage.
We identified 1085 participants with incident AF over a median follow-up of 16.8 years. Incident AF was positively associated with SUA levels, with an AF rate of 3 per 1000 person-years in the lowest quartile of SUA vs. 8 per 1000 person-years in the highest quartile (p<0.01) (table 2). This nearly three-fold risk increment persisted when we used the mean SUA of visits 1 and 2 as the main exposure (p<0.01).
Table 2.
Incidence rates and hazard ratios of atrial fibrillation by quartile of serum uric acid
| Quartile of SUA | Number at risk | Number developing AF | Incidence rate of AF per 1000 person-years | Age, gender and race-adjusted HR (95% C.I) |
|---|---|---|---|---|
| Baseline quartiles | ||||
| Quartile 1 (<5.0 mg/dl) | 3880 | 186 | 3 | 1 (Reference) |
| Quartile 2 (5.0-5.9 mg/dl) | 3919 | 243 | 4 | 1.04(0.83-1.30) |
| Quartile 3 (6.0-7.0 mg/dl) | 3927 | 297 | 5 | 1.30(1.04-1.62) |
| Quartile 4 (>7.0 mg/dl) | 3656 | 359 | 8 | 1.74(1.39-2.17) |
| p for trend | <0.01 | <0.01 | ||
| Mean quartiles * | ||||
| Quartile 1 (<5.0 mg/dl) | 3584 | 158 | 3 | 1 (Reference) |
| Quartile 2 (5.0-5.9 mg/dl) | 3461 | 213 | 4 | 1.29(1.02-1.63) |
| Quartile 3 (6.0-7.0 mg/dl) | 3390 | 250 | 5 | 1.40(1.11-1.78) |
| Quartile 4 (>7.0 mg/dl) | 3448 | 319 | 7 | 2.00(1.58-2.52) |
| p for trend | <0.01 | <0.01 |
Excludes cases of AF between visits 1 and 2 and those who did not attend visit 2.
HR: Hazard ratio, CI: confidence interval
Table 3 shows the adjusted relationship between baseline SUA and incident AF. After simultaneous adjustment for age, sex, race, education, field center, body-mass index, alcohol use, serum glucose, systolic and diastolic blood pressure, LDL cholesterol, prevalent heart failure, prevalent coronary heart disease, serum creatinine, p wave duration on ECG (as a measure of left atrial size) at baseline and use of diuretics, SUA was significantly associated with the subsequent risk of incident AF (HR of 1.16; 95% CI 1.06 -1.26 per 1 SD increase). Similar risk associations were seen using the mean value of SUA between visit 1 and visit 2 and in analysis including SUA as a time varying exposure. When stratified by ARIC recruitment center only the Jackson site and the Minneapolis site had a significant association between SUA and AF.
Table 3.
Adjusted relative hazards of incident AF related to serum uric acid.
| SUA measurement | Model A | Model B | Model C | Model D | |
|---|---|---|---|---|---|
| Baseline (visit 1) | HR† | 1.30 | 1.25 | 1.16 | 1.07 |
| 95% CI | 1.21, 1.40 | 1.15, 1.36 | 1.06,1.26 | 0.97, 1.17 | |
| p-value | <0.01 | <0.01 | <0.01 | 0.12 | |
|
| |||||
| Mean value | HR | 1.24 | 1.20 | 1.14 | 1.07 |
| 95% CI | 1.18,1.31 | 1.13,1.27 | 1.08,1.21 | 1.01,1.14 | |
| p-value | <0.01 | <0.01 | <0.01 | <0.01 | |
|
| |||||
| Time varying | HR | 1.29 | 1.27 | 1.24 | 1.18 |
| 95% CI | 1.02,1.63 | 1.00,1.63 | 0.97,1.57 | 0.95,1.48 | |
| p-value | 0.03 | 0.04 | 0.07 | 0.12 | |
HR: hazard ratio indicates adjusted relative hazards per standard deviation (SD) of serum uric acid difference at visit 1 (SD= 1.56 mg/dl).
Model A adjusts simultaneously for age, sex, race, education and field center.
Model B adjusts simultaneously for all variables in model A as well as, body mass index, alcohol use, serum glucose, systolic and diastolic blood pressure, and LDL-cholesterol.
Model C adjusts simultaneously for all variables in model B as well as, prevalent coronary heart disease and heart failure, serum creatinine, use of diuretics and p wave duration on ECG at baseline.
Model D adjusts for Model C, and censors individuals when they develop incident myocardial infarction or heart failure.
When censoring individuals at the time of developing incident heart failure and myocardial infarction the relation between SUA and AF was attenuated, with a HR of 1.07 (95% CI 0.97-1.17) per 1 SD increase. Similar risk gradients were seen for mean value of SUA between visit 1 and visit 2 and the time varying analysis (table 3).
Figure 1 shows the incidence of AF by quartile over time. Using the baseline measurement of SUA those in the highest quartile (>7.1 mg/dl) had a HR of AF of 1.41 (95% CI 1.11 -1.80) when compared to those in the lowest quartile (<5.0 mg/dl).
Figure 1.
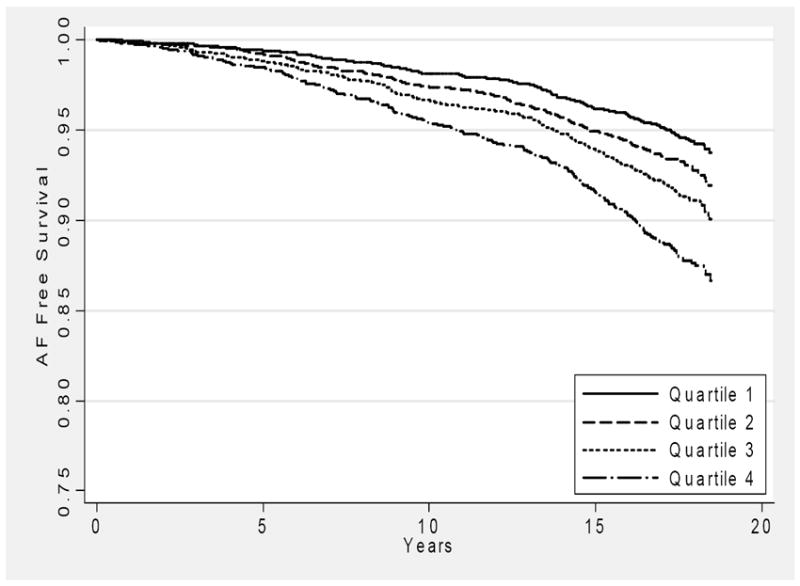
Survival free of atrial fibrillation by quartile of SUA*, The Atherosclerosis Risk in Communities study, 1987-2004.
* Quartile 1 <5.0 mg/dl, Quartile 2 5.0-5.9 mg/dl, Quartile 3 6.0-7.0 mg/dl, Quartile 4 >7.0 mg/dl
Baseline SUA was associated with incident AF in blacks (HR per 1 SD difference 1.56; 95% CI 1.28-1.90) but not in whites (HR 1.05 per 1 SD difference; 95% CI 0.95-1.11) (p for interaction<0.01), in women (HR 1.25 per 1 SD difference; 95% CI 1.08-1.43) but not in men (HR 1.05 per 1 SD difference; 95% CI 0.94-1.18) (p for interaction=0.01). The association of SUA with incident AF was similar in participants with metabolic syndrome (HR per 1 SD 1.18; 95% CI 1.03-1.33) and those without metabolic syndrome (HR per 1 SD difference 1.10; 95% CI 0.97-1.23) (p for interaction=0.24) after adjustment for other covariates.
Discussion
We report that SUA is independently and associated with incident AF in the ARIC cohort. This relationship was graded and independent of a wide range of established AF risk factors and differed by race and sex. Strengths of the study that lend weight to these conclusions are its prospective design, large community-based sample, and carefully standardized assessments.
Several population based studies have found SUA to be an independent marker of CVD disease 1, 12, however, other studies have found that the association is potentially explained by the cardiovascular risk factor load 13. A prior case-control study reported an association between permanent AF and SUA 14. A potential explanation for this association is the link between SUA and oxidative stress 4, 5. SUA is a by-product of purine catabolism, the terminal steps of which are catalyzed by xanthine oxidoreductase (XOR). XOR is a major source of ROS production which has been found to be elevated in both animal and human left atria with AF 15. Animal studies have shown that oxidative stress leads to the development of atrial electrical remodeling 16, which in turn results in reentry and a decrease in nitric oxide production leading to S-nitrosylation of ion channels that can shorten the plateau phase of the action potential causing accelerated repolarization and AF 17, 18.
In humans, cross-sectional and case-control studies have consistently reported associations between markers of oxidative stress and valvular and post-CABG related AF. In patients with AF after radiofrequency ablation, ROS markers had a strong, graded relationship to the recurrence of paroxysmal AF after 4 years of follow-up 19. Also, a recent study found an association between NADPH and incident AF in consecutive elective patients undergoing coronary artery bypass grafting 20.
Besides oxidative stress, there are other potential epidemiological explanations for an association between SUA and AF. First, SUA could potentially be a marker of cardiovascular risk factor burden 21, and even though we found that the relationship with AF was independent of most cardiovascular risk factors, the potential for residual confounding by not accounting for factors like inflammatory markers could explain our results. Also, SUA is a marker of endothelial dysfunction, metabolic syndrome and diabetes 21, all of which are important predictors of AF. Finally, the important attenuation of HRs after censoring for the occurrence of myocardial infarction or heart failure, indicates that the association between SUA and AF could be partially explained by increased risk of other cardiovascular diseases. Because of the long follow-up and likely progression of heart disease over time our intent with this analysis was to account for the effect that incident heart failure and myocardial infarction could have as mediators on incident atrial fibrillation.
We found that SUA was associated with AF in Blacks but not Whites. This racial heterogeneity has been reported in other ARIC studies for other diseases and its correlates 22, 23. Higher uric acid levels have been found in Blacks because of higher prevalence of SLC2A9, a gene, related to the re-absorption of uric acid in the proximal tubule 24. The higher SUA levels, could potentially lead to endothelial dysfunction and the activation of the renin-angiotensin 25 system which could potentially end in AF. However, a clear mechanism for the race and gender differences is yet to be determined.
Nonetheless, several limitations deserve mention. First, the current analysis is based on one or two measurements of SUA and the impact of the change in SUA levels on incidence of AF over time is unknown. However, our analysis included a time varying evaluation of SUA over 3 years and the results were similar to the single or multiple averaged measurements. Second, potential unmeasured confounders not adjusted for, such as kidney function, could explain our results. Though we were able to adjust for blood creatinine levels, we lacked data on albuminuria and hematuria, and hence ARIC participants with normal glomerular filtration rate and kidney damage evidenced by these signs could have been misclassified as having normal kidney function. However, this number is expected to be small. Third, the method of AF ascertainment could lead to lower identification rates of outpatient AF cases and our hospital-based AF ascertainment could lead to some false positives. In the ARIC cohort we were able to confirm only 89% of AF events in a sample of 125 discharge summaries. However, the AF incidence and lifetime risk in the white ARIC participants were similar to those from other populations, such as the Mayo Clinic study in Olmsted County, Minnesota, Cardiovascular Health Study or the Framingham Heart Study 8, 26-28.
In summary, we have found that SUA might be an independent risk factor for AF, suggesting that oxidative stress could play an important role in the development of AF. Whether modification of SUA might influence AF risk awaits further study.
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. This study was additionally funded by grants RC1HL099452-01 and RC1HL101056-01 from NHLBI and 09SDG2280087 from the American Heart Association. The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Financial disclosure: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fang J, Alderman MH. Serum Uric Acid and Cardiovascular Mortality. JAMA: The Journal of the American Medical Association. 2000;283:2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 2.Liese AD, Hense HW, Lowel H, Doring A, Tietze M, Keil U. Association of serum uric acid with all-cause and cardiovascular disease mortality and incident myocardial infarction in the MONICA Augsburg cohort. World Health Organization Monitoring Trends and Determinants in Cardiovascular Diseases. Epidemiology. 1999;10:391–397. doi: 10.1097/00001648-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Zimmet JM, Hare JM. Nitroso-redox interactions in the cardiovascular system. Circulation. 2006;114:1531–1544. doi: 10.1161/CIRCULATIONAHA.105.605519. [DOI] [PubMed] [Google Scholar]
- 4.Cappola TP, Kass DA, Nelson GS, Berger RD, Rosas GO, Kobeissi ZA, Marban E, Hare JM. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation. 2001;104:2407–2411. doi: 10.1161/hc4501.098928. [DOI] [PubMed] [Google Scholar]
- 5.Tsukimori K, Yoshitomi T, Morokuma S, Fukushima K, Wake N. Serum uric acid levels correlate with plasma hydrogen peroxide and protein carbonyl levels in preeclampsia. Am J Hypertens. 2008;21:1343–1346. doi: 10.1038/ajh.2008.289. [DOI] [PubMed] [Google Scholar]
- 6.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 7.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC., Jr Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40:1204–1211. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson H, Caidahl K, Larsson B, Ohlson LO, Welin L, Wilhelmsen L, Svardsudd K. Cardiac and pulmonary causes of dyspnoea--validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987;8:1007–1014. doi: 10.1093/oxfordjournals.eurheartj.a062365. [DOI] [PubMed] [Google Scholar]
- 11.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 12.Niskanen LK, Laaksonen DE, Nyyssonen K, Alfthan G, Lakka HM, Lakka TA, Salonen JT. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch Intern Med. 2004;164:1546–1551. doi: 10.1001/archinte.164.14.1546. [DOI] [PubMed] [Google Scholar]
- 13.Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131:7–13. doi: 10.7326/0003-4819-131-1-199907060-00003. [DOI] [PubMed] [Google Scholar]
- 14.Letsas KP, Korantzopoulos P, Filippatos GS, Mihas CC, Markou V, Gavrielatos G, Efremidis M, Sideris A, Kardaras F. Uric acid elevation in atrial fibrillation. Hellenic J Cardiol. 2010;51:209–213. [PubMed] [Google Scholar]
- 15.Korantzopoulos P, Kolettis TM, Galaris D, Goudevenos JA. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int J Cardiol. 2007;115:135–143. doi: 10.1016/j.ijcard.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, Kanderian A, Pavia S, Hamlin RL, McCarthy PM, Bauer JA, Van Wagoner DR. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–8. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez DR, Treuer A, Sun QA, Stamler JS, Hare JM. S-Nitrosylation of cardiac ion channels. J Cardiovasc Pharmacol. 2009;54:188–195. doi: 10.1097/FJC.0b013e3181b72c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill JS, McKenna WJ, Camm AJ. Free radicals irreversibly decrease Ca2+ currents in isolated guinea-pig ventricular myocytes. Eur J Pharmacol. 1995;292:337–340. doi: 10.1016/0926-6917(95)90042-x. [DOI] [PubMed] [Google Scholar]
- 19.Shimano M, Shibata R, Inden Y, Yoshida N, Uchikawa T, Tsuji Y, Murohara T. Reactive oxidative metabolites are associated with atrial conduction disturbance in patients with atrial fibrillation. Heart Rhythm. 2009;6:935–940. doi: 10.1016/j.hrthm.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Kim YM, Kattach H, Ratnatunga C, Pillai R, Channon KM, Casadei B. Association of atrial nicotinamide adenine dinucleotide phosphate oxidase activity with the development of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51:68–74. doi: 10.1016/j.jacc.2007.07.085. [DOI] [PubMed] [Google Scholar]
- 21.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283:2253–2259. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt MI, Duncan BB, Watson RL, Sharrett AR, Brancati FL, Heiss G. A metabolic syndrome in whites and African-Americans. The Atherosclerosis Risk in Communities baseline study. Diabetes Care. 1996;19:414–418. doi: 10.2337/diacare.19.5.414. [DOI] [PubMed] [Google Scholar]
- 24.Charles BA, Shriner D, Doumatey A, Chen G, Zhou J, Huang H, Herbert A, Gerry NP, Christman MF, Adeyemo A, Rotimi CN. A genome-wide association study of serum uric acid in African Americans. BMC Med Genomics. 2011;4:17. doi: 10.1186/1755-8794-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson RJ, Rodriguez-Iturbe B, Kang DH, Feig DI, Herrera-Acosta J. A unifying pathway for essential hypertension. Am J Hypertens. 2005;18:431–440. doi: 10.1016/j.amjhyper.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 26.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 27.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 28.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]


