Spine-related disorders are among the most frequently encountered problems in clinical medicine. Low back pain (LBP) alone affects up to 80% of the population at some point in life, and 1% to 2% of the United States adult population is disabled because of LBP.1,2 The substantial need for care of these patients, coupled with our poor understanding of the fundamental basis of LBP in many individuals, has led to an ever-expanding array of treatment options, including medications, manipulative care, percutaneous interventional spine procedures, and an increasing repertoire of surgical approaches.3 The estimated total cost of direct medical expenditures in the United States for spine care in 2006 was more than $85 billion, and the data suggest that the use and costs of spine care have been increasing at an alarming rate in recent years.4,5 Self-reported health status among people with spine problems (including physical functioning, mental health, work, and social limitations) does not seem to be improving, and the overall numbers of people seeking Social Security Disability Insurance for spine-related problems and the percentage of people with disability caused by musculoskeletal pain are increasing.4 Complication rates, including deaths, associated with treatments for spinal pain are also increasing.3,4,6,7 The statistics are concerning and raise a fundamental question: are spine problems worsening over time or are we simply using an increasing number of costly treatments that are not effective?
Valuable insights into this question can be obtained by examining the available epidemiologic data on spinal pain and treatment. This article addresses in particular the epidemiologic data on percutaneous and surgical spinal procedures and examines national trends in the use of noninterventional treatments such as physical therapy and exercise programs, and opioid medications. The costs associated with these treatments, the potential risks, and the overall effect on the quality of care provided to persons with spine problems in the United States are also examined.
PREVALENCE OF LBP
Despite the vast amount of research devoted to LBP, the epidemiology of this condition is not well understood, and the overall prevalence of LBP in the United States is unclear. There are several techniques for estimating the prevalence of spine problems, including survey techniques as well as the use of medical billing claims data. Each method has its strengths and weaknesses. Retrospective surveys obtain information directly from affected individuals but may be subject to recall bias. Claims-based data may avoid this limitation and are not dependent on individual reporting but detect only those subjects whose physicians coded for back pain associated with a given episode of care in the office. Additional challenges arise from heterogeneity in many other aspects of published studies, including the definitions of LBP that are used. The varying methodologies often lead to different patient groups being studied, which can be problematic from clinical and policy perspectives. Based on health care use data (ie, people who present to a health care provider for care for LBP), the prevalence estimates for LBP are as low as 12% to 15%.8 However, estimates from self-report survey data on the prevalence of LBP range from 28% to 40% of the population depending on the methodology used.8,9
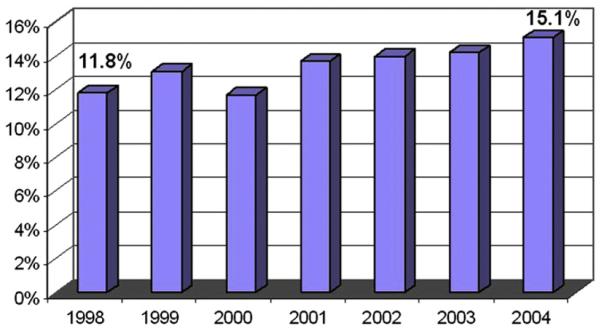
Given the difficulty associated with accurately estimating the prevalence of LBP at any specific time, it seems apparent that there are also difficulties with assessing changes in prevalence over time. A recent study reported telephone survey data collected in 1992 and again in 2006 from a sample in North Carolina indicating that the prevalence of chronic, disabling LBP rose significantly during that time frame, increasing from 3.9% of the population in 1992 to 10.2% in 2006.10 Although this study was not designed to address causation, the investigators suggest that increasing rates of obesity, depression, or other psychosocial factors may explain this increase in LBP prevalence. Claims-based data seem to indicate a smaller increase in prevalence. The percentage of people presenting to physician offices for LBP steadily, but only slightly, increased from 12% in 1998 to 15% in 2004 (Fig. 1). Although both sets of data could suggest that the overall prevalence of LBP is increasing, it is difficult to tell to what degree these changes represents a true increase in the proportion of the population suffering from LBP versus a change in the propensity of people to either report or seek care for low back complaints. If it is the latter, this situation may represent evolving societal beliefs about pain rather than a change in the number of people who suffer from LBP.
Fig. 1.
Percentage of the US population with LBP. (From Katz S, editor. The burden of musculoskeletal diseases in the United States. Rosemont (IL): Bone and Joint Decade, American Academy of Orthopaedic Surgeons; 2008. Copyright © 2008 American Academy of Orthopedic Surgeons; Modified with permission from The Burden of Musculoskeletal Diseases in the United States. Source of data: National Center for Health Statistics, National Ambulatory Medical Care Survey, 1998-2004.)
The uncertainty regarding a potential increase in the prevalence of LBP in the US population becomes relevant when analyzing the array of data indicating escalating rates of use of health care services related to LBP. Is the increase in use due to an increase in the relative number of patients with pain, an increase in the percentage of those with pain seeking and/or receiving care, or, as was suggested in recent data from Martin and colleagues,4 an increase in the per-patient use of care? These questions are important when considering the policy and care implications because the answers speak to the efficacy of, and hence necessity for, specific treatments. Given the available data, the answer is likely that the increasing use rates represent a combination of factors including an increased prevalence of chronic LBP, changes in the treatments used, and changes in societal beliefs regarding pain.
THE USE OF INTERVENTIONAL SPINE PROCEDURES
Interventional spine procedures have been used for many decades for a variety of spinal disorders and range from percutaneous injections to surgery. For a variety of reasons, there has been a recent proliferation in the number of techniques available for use and marked increases in the use rates for many of the procedures. Percutaneous interventions include epidural steroid injections (ESIs) via several different approaches, facet/zygapophysial joint (z-joint) procedures, spinal cord stimulation, several intradiscal procedures, and, more recently, procedures intended to remove disc or other material in the spinal canal or to obtain segmental fusion. Surgical procedures range from well-established approaches for discectomy and/or spinal canal decompression to multiple means of addressing segmental fusion using several different approaches, materials, instruments, and indications. However, the evidence to support the use of many of these procedures is limited.
From the surgical perspective, there are sound data on the efficacy of discectomy for acute radicular pain associated with a disc herniation, decompressive laminectomy for symptomatic spinal stenosis, and fusion for degenerative spondylolisthesis in well-selected patients.11-16 However, selecting appropriate candidates for surgery can be challenging because of variability in diagnosis and characterizing clinical and radiographic characteristics of these conditions. In addition, the clinical benefit of surgery even in well-selected patients can wane, and many patients may do just as well long-term with more conservative options.17,18 The data on the efficacy of surgery for isolated LBP are more suspect, and there are many additional patients in whom surgical procedures are either not indicated or contraindicated because of other issues.17,19 Surgical care also may carry significant risks and costs.11,20,21
Because the role of surgery is limited for the larger population of patients with low back pain, there is a need for less invasive options. As noted earlier, several percutaneous procedures for spinal disorders have been developed and are frequently used clinically, most with limited scientific evidence of efficacy. The most commonly used and studied procedures of this type are ESIs, followed in frequency by z-joint procedures, which include intraarticular injections, medial branch blocks, and radiofrequency neurotomy (RFN). The use of each of these techniques has increased, and the evidence to support their use varies depending on the location in the spine (eg, cervical vs lumbar spine) as well as specific characteristics of the spinal disorder (eg, acute sciatica) and patient (eg, worker’s compensation status). The supportive evidence that is available for these procedures predominantly notes short-term improvements in pain. However, even in the best of circumstances the evidence for long-term benefit from any of these is lacking. To better understand the role of interventional care in the management of spinal pain, this review focuses on ESIs because similar trends and issues exist for the other procedures.
EPIDURAL STEROID INJECTIONS
Although ESIs for the treatment of lumbosacral radicular pain were first introduced in the early 1950s,22 there has been a lot of interest recently in the use of these injections as an alternative to more invasive surgical procedures for treating spinal pain. Since the introduction pf ESIs, many investigators have examined them for the treatment of lumbosacral radiculopathy as well as axial (nonradicular) spinal pain syndromes.23-33 Although most studies have addressed the use of ESIs for isolated lumbosacral radiculopathy resulting from discogenic or other causes, some investigators have advocated their use for more diffuse symptoms associated with lumbar spinal stenosis.34-36 The current literature reports success rates of 18% to 90% for ESIs, depending on methodology, outcome measures, patient selection, and technique.37 Some studies,23,32,38 but not all,30,33,39 have found that ESIs can offer short-term pain reduction to a select group of patients, but there is little evidence of long-term improvement in pain or function.39-42 Given the overall spectrum of treatment approaches available for spinal pain, short-term pain relief can offer significant clinical benefits in appropriate circumstances, and this seems to be the primary beneficial effect of ESIs advocated by many investigators.
Several studies have attempted to examine the influence of ESIs on the subsequent need for lumbar surgery as an outcome measure. The results have been mixed. An initial prospective randomized controlled trial (RCT) by Riew and colleagues27 reported that a significantly higher proportion of patients receiving transforaminal ESIs with anesthetic and corticosteroid opted not to have surgery compared with a control group receiving similar procedures with anesthetic alone. This study followed patients for up to 2 years after the first injection. However, in a separate report on 5-year follow-up of the same patients, there were no differences between the treatment and control groups in terms of lumbar surgery.38 A significant percentage of the treatment group was lost to follow-up for this study, making it difficult to draw any definitive conclusions from these data. A more recent study by Schaufele and colleagues43 in 2006 compared interlaminar versus transforaminal ESIs for persons with chronic LBP who had failed other conservative treatments. In this retrospective case-control study of 40 patients, the investigators found that 2 (10%) of the patients receiving transforaminal ESIs and 5 (25%) of the patients receiving interlaminar ESIs underwent subsequent lumbar surgery within 1 year after the initial injection. Follow-up beyond 1 year was not reported for these patients. Although the sample size in this study is small, this study is representative of the type of evidence available. Overall, the data indicating a significant effect of ESIs on surgical rates are not robust.
Some limited data are available on the cost-effectiveness of ESIs. Price and colleagues40 performed an RCT of ESIs in the United Kingdom and concluded that they did not meet the national guidelines for cost-effectiveness, specifically noting that “ESIs do not provide good value for money.” The investigators believed that further research is needed to compare alternative treatments for LBP and to identify subgroups of patients who might benefit more from ESIs. One drawback of this study is that it was performed in the United Kingdom, where there is a national health service, so it is unclear how applicable their cost analysis is to the US population. To date, there have been no cost-effectiveness studies of ESIs or other interventional pain procedures in the United States.
Regardless of which outcome is considered, one of the biggest challenges in interpreting the literature regarding the efficacy or effectiveness of ESIs is the paucity of high-quality RCTs. A recent survey of the published literature shows that there have been 18 RCTs of ESIs compared with placebo or control treatment for a variety of LBP conditions in the last 25 years. Most of these studies have serious methodological failings that limit their usefulness, including the lack of routine fluoroscopic guidance for injections in any study published before 2000. During this same period, there have been 78 systematic reviews of ESIs, with divergent conclusions based on critical review of the same studies. Although isolated investigators have believed the data support the use of ESIs for specific populations, several more thorough reviews point to the limited or absent data on long-term pain relief or functional improvement associated with these procedures.44,45 The most recent Cochrane review on the subject takes issue with the entire spectrum of percutaneous spine procedures, concluding “there is insufficient evidence to support the use of injection therapy in subacute and chronic low-back pain.”45
Despite the ambiguities in the data supporting ESIs, these procedures have developed widespread acceptance and are used with increasing frequency as a treatment of radiculopathy and other LBP disorders.46 In the United States, the use rates for ESIs of all types are escalating dramatically. In one analysis of Medicare claims from 1994 to 2001, there was a nearly 3-fold increase in ESI rates (Fig. 2) and a 7-fold increase in subsequent reimbursed costs.47 These rates outpace the growth in the Medicare population in that time frame as well as the estimated increase in the prevalence of persons with LBP.8 Similar findings were noted in a study by Carrino and colleagues,46 also using Medicare claims data. The data from these studies do not offer insight into the cause of the increasing rates for ESIs, but this may be related to several possible factors such as expanding clinical usefulness, cultural changes, or socioeconomic issues.
Fig. 2.
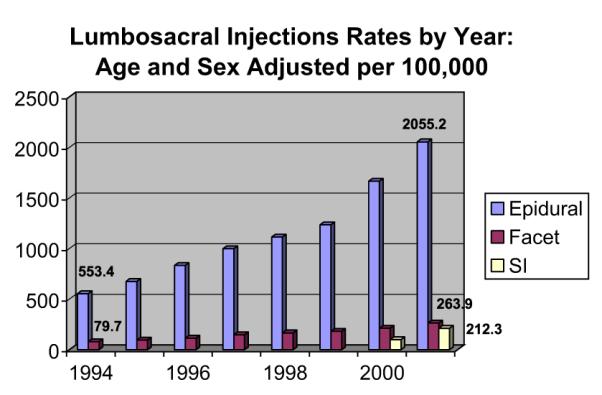
Cumulative number of ESI systematic reviews and RCTs. (From Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine 2007;32(16):1756.)
UNDERSTANDING THE INCREASE IN USE
One of the more optimistic explanations available for the disproportionately escalating rate of ESI use could be improvements in health care delivery, including more effective management of spinal pain in the general population. If these improvements are occurring, improvements in measures of health or disability or a decrease in rates of surgical intervention for specific spine problems might be expected. The available data do not seem to indicate that this is the case. Paradoxically, measures of spine health in the United States show declines in recent years, and surgical rates for the treatment of degenerative spine problems have been dramatically increasing.48,49 When specifically looking at the effect of ESIs on surgical rates, Medicare claims data suggest that the performance of these procedures is positively associated with higher surgical rates rather than falling rates. In addition, epidemiologic studies have shown that the total number of people receiving ESIs who subsequently undergo lumbar surgery is increasing, as is the proportion of people undergoing surgery who have received ESIs.47,50 This particularly seems to be the case in areas of the country that have higher overall rates of injections.50 A study conducted using national Veteran’s Administration (VA) data on more than 13,000 individuals also showed that those who underwent more than 3 ESIs during a 2-year period were at increased risk of undergoing subsequent surgery compared with those who received 3 or fewer.50 Although none of these data indicate that performing ESIs increases surgical rates, they do not support the argument that ESIs (especially greater than 3 in the same patient) are substituting for lumbar surgery on a large-scale basis. Taken as an aggregate, these studies raise serious questions as to our ability to lower the need for surgery through the use of ESIs, which, along with pain reduction, is one of the prime benefits cited by many advocates of these procedures.
An additional surrogate measure to consider as an indicator of the broader health effect of ESIs is the use of opioid pain medications for those undergoing spinal procedures. As is the case with spinal injections and surgery, epidemiologic data indicate that opioid use in the United States is increasing at an alarming rate and that complications/deaths associated with opioid use (both prescribed and recreational/illicit use) are increasing.6,7,51,52 Although there are no data to indicate a causal relationship between the increase in interventional procedures for back pain and the use of opioid medications, one might hope that there would be evidence of a reduction in opiate use following ESIs. The data available are not supportive of this idea, either. An epidemiologic study performed within the VA system over a 2-year period showed no reduction in the use of opioids after ESI.50 Most people in this study were using opioids both before and after the intervention (64% vs 67%).50 This study did not determine the indication for the use of opioid medications in these patients and was not an RCT, both of which pose limitations on interpretation of the data. However, that there was no evidence of a reduction in opioid use raises the concern that in clinical practice the use of injections may not be associated with a significant decrease in opioid use as would be expected. When considered with the information on health status and surgical rates, the data make it difficult to argue that the expanding use of ESIs is accounting for major improvements in health outcomes.
Other explanations for the changes seen in use rates for ESIs may be found in more socioeconomic factors, such as alterations in the distribution and numbers of the providers who are performing the procedures. In the last 10 years, interventional pain management has blossomed, including a substantial increase in the number of fellowships offered (usually either anesthesiology or physical medicine and rehabilitation [PM&R]). The American Board of Pain Management began board certification in 2000. As of March 2009, more than 2200 physicians had been board certified by the American Board of Pain Medicine (http://www.abpm.org/). This figure represents approximately 16% of the total number of providers board certified in PM&R and anesthesiology in the same period according to statistics from the American Board of Medical Specialties. In one study on geographic variations of ESI use, ESI rates strongly correlated with the number of providers performing these procedures in a given area (r 5 0.79, P<.001), suggesting that the supply of physicians who perform injections may be a significant factor in the increase in ESI use.53 The influx of specialists board certified in interventional pain care and the increasing role of PM&R in interventional spine care could thus be major contributors to the increase in interventional pain procedures being performed. Some have argued that board certification programs and improved training in pain management have led to improvements in the quality of care provided for persons suffering from chronic pain and that the increase in the number of procedures being performed is partially in response to a previously unmet need. However, given the foregoing discussion it has yet to be shown that the increase in supply of board-certified physicians and the treatments provided has made a substantial difference in the functioning, quality of life, or pain levels of people with chronic LBP.
Another socioeconomic issue potentially affecting the use of ESIs is the growth of physician-owned ambulatory surgical centers (ASCs) in the United States. ASCs are freestanding facilities designed specifically for outpatient surgical procedures that do not require an overnight or inpatient hospital admission. The number of Medicare-certified ASCs increased by more than 60% from 2000 to 2007, totaling nearly 5000 across the United States. During this time, Medicare payments for services provided at ASCs more than doubled, from $1.4 billion to $2.9 billion.54 Ninety-five percent of Medicare-certified ASCs are privately owned, for-profit businesses, most of which are owned by local physician investors. The Stark self-referral law, established in 1989 to ensure that economic conflicts of interest do not drive up use, does not apply to ASCs, making it possible for physicians to refer their own patients to the ASCs with which they may have financial relationships. By delivering care in these centers, physician investors can increase practice revenues by receiving facility fees that often are larger than the professional fees they receive for the procedures. This type of arrangement may affect physician behavior by creating financial conflicts of interest for physicians practicing in ASCs and potentially increase use rates for various forms of interventional care. In support of this concept, there are data on imaging and spine surgery rates to suggest that financial incentives associated with physician-owned facilities may alter physician practice patterns.55,56
In addition to financial interests, there are several legitimate potential reasons why a variety of procedures may be performed in ASCs as opposed to other locations such as hospitals and physician offices. Included in these reasons are the possibility of improved outcomes related to specialized staffing, more efficient protocols, dedicated equipment, better customer service, and the presence of more convenient locations with shorter wait times than hospital settings.57 Although the Center for Medicare and Medicaid Services (CMS) examines cost and use trends to determine the appropriate reimbursement for procedures performed at ASCs, there have been few published data comparing outcomes for procedures performed in these settings with those in more traditional hospital settings57,58 and none examining interventional pain procedures for back pain.
These issues are of concern because, although interventional spine procedures can be performed in physician offices, ASCs, or outpatient hospital settings, there are some data to suggest that an increasing percentage are being performed at ASCs.59 ESIs are one of the most frequently performed procedures at ASCs, outpaced only by colonoscopies/endoscopies and cataract surgeries.60 This situation may be related to increased physician reimbursement for procedures performed at these sites compared with offices or outpatient hospitals, particularly when accounting for facility fees captured by physician ASC owners as dividends.61,62 These data leave open the idea that financial incentives for providers performing ESIs in physician-owned facilities may be a significant driver of use rates.
When looking at the totality of the published data on ESIs, a concern is that the increase in their use is more related to economic factors than to clinical ones. Although the rates of ESIs and other interventional spine procedures are expanding rapidly, there is no evidence of broad societal or clinical benefits. There is some literature indicating short-term improvements in pain after ESIs, but the literature base is replete with numerous systematic reviews built on a paucity of well-designed RCTs. More data are needed on indications, patient selection, frequency, route of administration, and cost-effectiveness to establish the true clinical usefulness of these procedures. Considering the state of the literature alongside the societal need and desire to use nonsurgical treatment options and the economic factors related to physician supply and reimbursement, it becomes difficult to argue that the primary impetus behind the recent increased use of ESIs and other spinal procedures is clinical usefulness.
ADDITIONAL PERCUTANEOUS INTERVENTIONAL SPINE PROCEDURES
Z-joint Injections and RFN
There are fewer epidemiologic data examining the use of z-joint intraarticular steroid injections and RFN (ie, radiofrequency ablation of the z-joint medial branch nerve) than there are for ESIs. There is good evidence to indicate that z-joints are potential sources of back pain.63-66 Injection of these joints with corticosteroids, as is performed for many peripheral joints, is believed to have the potential to transiently relieve pain. In addition, these joints are innervated by the medial branches of the lumbar dorsal rami, and these nerves are targets for nerve blocks using anesthetics, steroid medications, or RFN, the latter of which may result in destruction of the nerves believed to convey pain from the joints.
Similar to the situation for ESIs, the literature on z-joint procedures is underwhelming. Most studies on intraarticular z-joint injections have been unable to convincingly show significant benefit for LBP, although optimizing patient selection is challenging and the overall quality of these studies is limited. There is some evidence to suggest that RFN of the medial branches may provide pain relief in highly selected patients.67-70 Although there are ardent advocates for these procedures, recent reviews are not supportive, with Chou and colleagues17 noting that “insufficient evidence exists to reliably validate” RFN. Despite the equivocal evidence to support their use, z-joint procedures are being used with increasing frequency.46,47 For example, Medicare payments for z-joint injections increased from $141 million in 2003 to $307 million in 2006 (Office of Inspector General report, September 2008) and the number of claims during that period increased by 78%. Because of the lack of evidence to support the use of lumbar z-joint injections as well as concerns regarding the percentage of injections billed inappropriately (including overbilling for bilateral procedures because of coding and documentation errors), there has recently been intensive review of coverage policies for z-joint injections and RFN. There are currently no published epidemiologic data on the use of radiofrequency ablation in the lumbar spine, and this needs to be further examined. The reactive stance of the payers to the data indicates the larger concern over the effectiveness and use of interventional spine procedures.
Emerging Techniques
There are several emerging interventional spine procedures. The role that many of these play in spine care is unclear. Perhaps the best way to address the future is to examine the past. A cautionary note on the broader spectrum of spinal procedures may be seen in the example of intradiscal electrothermal therapy (IDET). This procedure was developed and introduced in the 1990s as a less invasive alternative to fusion for discogenic back pain. The intent was to introduce a thermal coil into the annulus fibrosis of a painful lumbar disc and, through heating of the thermal coil, achieve pain relief via either collagenous remodeling or anular denervation (the exact mechanism of action was still theoretic at the time of clinical release).71 The device used in this procedure (SpineCath produced by ORATEC, Inc, Menlo Park, CA) was approved as a 510-K device with only limited safety data to show its equivalence to a cautery probe previously approved by the US Food and Drug Administration (FDA). Once FDA approval was obtained for the device and preliminary data from small nonrandomized cohort studies funded by the manufacturer were published, IDET quickly gained popularity as a percutaneous spine intervention for discogenic pain. There was a great deal of enthusiasm for this procedure based on early studies, and its use increased dramatically in the 1990s and early 2000s. After these initial positive reports were published, the company that developed IDET and the SpineCath used for the procedure was sold to Smith & Nephew for considerable profit, and Smith & Nephew began a marketing campaign to advance the sales of the SpineCath used in IDET procedures. However, subsequently published RCTs on IDET were not so encouraging and failed to show anything other than perhaps a narrow benefit from the procedure.72,73 Despite the increasing RCT evidence that IDET was not effective, 2 subsequent industry-sponsored systematic reviews were published that concluded that IDET was safe and effective for discogenic pain.74,75 However, a nonindustry-sponsored systematic review did not identify any significant benefit of IDET. After a review of the available data and input from multiple medical specialty societies, CMS issued a noncoverage decision regarding IDET (http://www.cms.gov/). Consequently, the use of IDET has decreased and insurance coverage and reimbursement for this procedure have been limited dramatically. Despite the lack of evidence to show effectiveness and the resultant limitations on coverage for IDET, some providers still strongly advocate for the use of this procedure.74,76
The penetration of IDET into the clinical marketplace and its continued use despite lack of substantial evidence of benefit and lack of coverage by many insurers speaks both to the perceived need for effective and less invasive alternatives to surgical care for LBP and to some of the potential problems with patient expectations, financial incentives, and evidence dissemination or interpretation that may exist in the medical arena. Although some of the issues with IDET are particularly problematic, its story is emblematic of problems present throughout interventional spine care. The widespread clinical application of new techniques before the establishment of a sound physiologic rationale for their use, an appropriate safety profile, and evidence of efficacy in a clinically valid group of patients may be detrimental to patients, providers, and the financial stability of our health system.
The IDET story is not unique in interventional pain. There are several other spine interventions introduced into clinical practice with limited data whose effectiveness has subsequently been called into question by rigorous RCTs. Vertebroplasty is the most recent example. Vertebroplasty is a widely used spine intervention now under substantial scrutiny after the publication of 2 large RCTs that show no additional benefit to the procedure over sham or placebo intervention.77,78
Surgical Interventions
Given the epidemiology of LBP within the United States and the extraordinary health care costs associated with its treatment,79,80 it is not surprising to find that rates of lumbar spine surgery, like those for spinal injections, are increasing dramatically.48,49 The rates of spinal fusion, in particular, have been increasing rapidly. The Agency for Healthcare Research and Quality’s Healthcare Cost and Usefulness Project reported up to a 40% increase in spinal fusions in the 7-year period from 1998 to 2004,8 and Deyo and colleagues81 recently reported that the rate of complex fusion procedures being performed for lumbar spinal stenosis increased 15-fold from 2002 to 2007. Data on discectomies and other spine surgeries also reflect an increase over time, although not to the same degree as for spinal fusions.49 It is possible that discectomies and other less invasive spine procedures may be underreported in current data because many are being performed in outpatient settings and are thus not captured in analyses of the inpatient databases typically used to estimate spine surgery rates.49 The advancing rates for lumbar fusion surgeries are troublesome because these procedures generally have less well-defined indications and less well-documented success rates than some of the procedures mentioned previously such as microdiscectomy. In addition, fusion procedures are associated with increased costs, complications, and reoperation rates when compared with less complex surgical approaches. As with ESIs, there is no clear answer in the medical literature as to why the rates of lumbar fusion are escalating in such a disproportionate manner to other surgical approaches to the spine, but some of this is likely related to the availability of numerous new technologies and approaches to attaining fusion, many of which, like IDET, have entered the clinical arena without substantial data on clinical superiority over existing techniques.
Physical Therapy and Exercise Programs
The use of physical therapy and other specific exercise programs for back pain has also been controversial. Although there is strong evidence to suggest that staying active and engaging in physical activity can reduce disability associated with back pain,82 there are conflicting data regarding the clinical effectiveness as well as the cost-effectiveness of physical therapy in chronic LBP.83 One recent study suggested that adherence to an early physical therapy program in people with acute LBP was associated with lower subsequent health care costs.84 Another study using the Medicare claims database determined that early physical therapy after a first visit to a doctor for LBP is associated with decreased subsequent health care use (emergency department visits, ESIs, surgery).85 However, a recent systematic review of the cost-usefulness of physical therapy found that only 54% of the studies showed a net benefit in terms of cost, whereas the remainder found no benefit to physical therapy.83
Several difficulties are associated with studying the efficacy of physical therapy and exercise programs for LBP, including the variability in treatment protocols and patient characteristics within and between studies. Defining clinical subgroups for study has also been challenging, and there is no widely accepted method of distinguishing between many individuals with LBP in any meaningful way for research purposes. Numerous studies on exercise and LBP apply multifaceted treatment programs to what are likely widely heterogeneous groups of patients, frequently showing unimpressive results. Given the complexities associated with this topic, these studies are also frequently methodologically challenged and underpowered. Although positive benefits of physical therapy interventions for LBP have been reported, one systematic review found that only one-third of studies on this topic reporting positive outcomes showed clinically significant changes in addition to statistically significant changes.86 In general, it has been difficult to determine if any one physical therapy protocol is more effective than others, and there is still controversy about the overall usefulness of physical therapy treatments for LBP.
There are scant data on the changes in use of physical therapy. A large cross-sectional survey study in 2006 reported that use of physical therapy to treat LBP is common, with 30% of people with LBP reporting being seen by a physical therapist, with an average of 21 visits per person per year.87 In this sample population, use of passive modalities such as transcutaneous electrical nerve stimulation units, corsets, traction, electrostimulation, and ultrasound, all of which have limited data on efficacy, were more common than better-studied structured rehabilitation or exercise-based programs.87 Limited data suggest that the use of physical therapy is increasing, but at a slower rate than other available treatments.5 This is a topic that will likely come under increasing scrutiny as the drive toward evidence-based medicine and cost-effectiveness analysis continues.
Opioid Use
The safety and efficacy of opioids for treatment of nonmalignant pain, particularly chronic spinal pain, is another controversial topic. Some clinicians advocate the use of opioids in this setting as a pain relief strategy,88 whereas others strongly oppose their use. Opioid use among persons with chronic LBP is common. Survey data from the late 1990s on more than 20,000 individuals revealed that about 12% of those with back pain had received prescriptions for opioids.80 Data from the Medicaid system in the United States showed that overall opiate use increased by 309% from 1992 to 2002.89
Concerns regarding opioid use for chronic LBP arise from issues related to the potential for abuse, a possible lack of effectiveness, and the high rates of concurrent mental illness in those with chronic LBP. A recent systematic review of opioid use in the setting of chronic pain suggested a lifetime prevalence of substance abuse of 36% to 56% among those using prescription opiates, with up to 24% of patients inappropriately taking their opioid medications.90 In addition, a meta-analysis of the available study data showed a nonsignificant difference in pain relief comparing those taking opioids and those not taking opioids.90 The quality of the available studies is suboptimal and there are few data on the long-term efficacy of opioids for chronic pain. Despite the limitations of the data on the direct clinical benefit of opiates for LBP, a large survey has shown that patients who were prescribed opioids at their last office visit for back pain were more satisfied with their care and less likely to seek care from additional providers than those not prescribed opioids.91
Further complicating the picture of opiate use are recent data indicating that people taking opioids for back pain were more likely to have underlying depression, anxiety, and other comorbid medical conditions.92 These findings suggest that there may be factors associated with opioid use that are unrelated to the severity or specific cause of back pain but more related to broader physical and mental health issues. Given the strong relationship between chronic LBP and psychosocial factors such as depression, anxiety, and somatization,93-98 effective treatment of this problem often necessitates a multimodal approach that encompasses the full scope of comorbidities associated with chronic spinal pain. The concept that addressing the psychological state of the individual with LBP is an important component of care is supported by a large literature base on operative and nonoperative therapies.99,100
Reliance on the use of opiates to manage chronic LBP raises many of the same issues seen in the previous discussions on interventional spine care. There is increasing concern that the shift in training of providers has led to an increasing number of interventionalists who perform only interventional procedures without addressing underlying mental health issues or other psychosocial factors associated with chronic back pain that may influence outcomes and health care use. Successful administration of opioid medications in those with spinal pain may depend on using these medications in a comprehensive framework that addresses the biopsychosocial nature of the problem.
DISCUSSION
Individuals with LBP are receiving an increasing number of interventional treatments for pain without any available evidence to support a substantial overall improvement in functional status. This situation presents a challenging dilemma for health care providers. There is a vast and ever-expanding array of potential treatment options for LBP, many of which have some evidence of efficacy in select patients but none of which has offered long-term benefit for most patients with LBP. There are many difficulties with patient selection, and it is often unclear which patients respond to which treatments. Nonetheless, it seems that patient demand for services to address LBP is increasing, and there may be shifts in societal belief systems that create unrealistic expectations of improvement from focused interventions. The conflict between the level of patient demand and the level of evidence for various care options for LBP often leaves physicians obligated to provide treatment recommendations based on an insufficient database. Entering this care environment is an increasing supply of physicians trained in the delivery of interventional spine care, and several economic factors seem to provide incentives for them to perform these procedures with greater frequency. The result is a system that increasingly emphasizes the performance of narrowly focused and insufficiently studied procedures to address what are likely complex biopsychosocial pain problems.
The outcome of this is not good from the perspective of the individual or the society as a whole. Given the increasing health care costs associated with interventions for LBP, and the subsequent scrutiny of third-party payers, we are facing an extremely important crossroads in the care of people with chronic LBP. If we are going to identify the optimal treatment modalities for patients with LBP and show the value in our care, we must invest in high-quality outcomes research and clinical trials to determine which treatments are effective for which subsets of patients. It is imperative that we carefully examine each patient individually and determine a rational, evidence-based approach to treat their pain.
There is a need for high-quality RCTs comparing the efficacy and effectiveness of many of the interventions commonly used to treat spinal pain, including ESIs. However, there are several significant barriers to performing research of this type. There are few physicians performing these interventions with research training and interest; funding for this research is often limited; there is no consensus as to the appropriate methodology; and recruitment of patients into placebo-controlled studies for pain is difficult. There has also been significant discussion and debate about the various outcome tools used in spine research, particularly in trials of treatments for pain. What are the most important outcome measures to assess and how do we define success? Are self-reported pain, satisfaction with treatment, function, and global assessment of improvement adequate outcome measures? How do you interpret studies that document improvement in some measures of success, but not others (ie, improvement in pain scores, but no differences in opioid use, subsequent surgery rates, or return to work)? From a societal perspective, are return to work and health care use more important than self-reported pain relief or patient satisfaction? Recent recommendations from the IMMPACT (Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials) group include the use of a composite outcome measure that includes at least 2 individual outcome measures encompassing self-reported pain, physical function, emotional well-being, and global assessment of improvement in clinical trials of pain interventions.101 Many of the available studies report improvements in only one outcome measure and fail to meet the recommended criteria for a successful outcome.
Future study designs will also have to identify and address the most important predictors of success for each of the available treatments. There are subsets of patients who benefit from many of the available treatments, but there are no consistent ways to predict the success of any given treatment of LBP. The evidence suggests that the outcomes for people with nonspecific LBP are better predicted by psychosocial factors such as current job satisfaction, fear avoidance beliefs/behaviors, and gender than they are by biomedical factors.102 The type of provider seen, insurance coverage, age, and ethnicity also may contribute to a patient’s perceived improvement in pain as well as satisfaction with care.91 Although these psychosocial yellow flags are important predictors of the development of chronic pain,98 it is less clear how they may be used to predict response to individual interventions for people with chronic back pain. However, they are clearly indicators of the complexity of the situation.
When considering the role of interventional care for chronic LBP, it is important to take a step back and make sure that we are addressing the associated medical and psychosocial factors that are likely contributing to the ongoing pain and disability. Factors such as sleep disturbance, fear avoidance, cata-strophizing, obesity,103 depression, anxiety, and socioeconomic issues need to be addressed in any treatment plan for chronic LBP, or the intervention is doomed to fail. Perhaps it is time to revisit old concepts with respect to the treatment of chronic LBP: interdisciplinary, comprehensive programs that encourage physical activation despite pain, improving coping strategies and self-efficacy, addressing underlying mental health issues, and fostering lifestyle behavior modification.100,104-106 As Deyo and colleagues3 have recently stated, “chronic back pain may benefit from sustained commitment from health care providers, involvement of patients as partners in their care, education in self-care strategies, coordination of care, and involvement of community resources to promote exercise, provide social support and facilitate a return to work.” The conflicting evidence regarding the effectiveness of a myriad of treatments for LBP is likely reflective of the multifactorial and complex nature of the problem. In most circumstances, it seems that LBP, particularly in the chronic state, cannot be successfully treated with individual interventions of any kind.
REFERENCES
- 1.Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344(5):363–70. doi: 10.1056/NEJM200102013440508. [DOI] [PubMed] [Google Scholar]
- 2.Anderson G. The epidemiology of spinal disorders. In: Frymoyer JW, editor. The adult spine: principles and practice. 2nd edition Lippincott-Raven; Philadelphia: 1997. [Google Scholar]
- 3.Deyo RA, Mirza SK, Turner JA, et al. Overtreating chronic back pain: time to back off? J Am Board Fam Med. 2009;22(1):62–8. doi: 10.3122/jabfm.2009.01.080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299(6):656–64. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 5.Weiner DK, Kim YS, Bonino P, et al. Low back pain in older adults: are we utilizing healthcare resources wisely? Pain Med. 2006;7(2):143–50. doi: 10.1111/j.1526-4637.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- 6.Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15(9):618–27. doi: 10.1002/pds.1276. [DOI] [PubMed] [Google Scholar]
- 7.Paulozzi LJ, Ryan GW. Opioid analgesics and rates of fatal drug poisoning in the United States. Am J Prev Med. 2006;31(6):506–11. doi: 10.1016/j.amepre.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Katz S, editor. The burden of musculoskeletal diseases in the United States. Bone and Joint Decade, American Academy of Orthopaedic Surgeons; Rosemont (IL): 2008. [Google Scholar]
- 9.Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from U.S. national surveys, 2002. Spine. 2006;31(23):2724–7. doi: 10.1097/01.brs.0000244618.06877.cd. [DOI] [PubMed] [Google Scholar]
- 10.Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169(3):251–8. doi: 10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA. 2006;296(20):2451–9. doi: 10.1001/jama.296.20.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am. 2009;91(6):1295–304. doi: 10.2106/JBJS.H.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watters WC, 3rd, Bono CM, Gilbert TJ, et al. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. Spine J. 2009;9(7):609–14. doi: 10.1016/j.spinee.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Martin CR, Gruszczynski AT, Braunsfurth HA, et al. The surgical management of degenerative lumbar spondylolisthesis: a systematic review. Spine (Phila Pa 1976) 2007;32(16):1791–8. doi: 10.1097/BRS.0b013e3180bc219e. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356(22):2257–70. doi: 10.1056/NEJMoa070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atlas SJ, Keller RB, Wu YA, et al. Long-term outcomes of surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: 10 year results from the Maine lumbar spine study. Spine (Phila Pa 1976) 2005;30(8):927–35. doi: 10.1097/01.brs.0000158954.68522.2a. [DOI] [PubMed] [Google Scholar]
- 17.Chou R, Baisden J, Carragee EJ, et al. Surgery for low back pain: a review of the evidence for an American Pain Society Clinical Practice Guideline. Spine (Phila Pa 1976) 2009;34(10):1094–109. doi: 10.1097/BRS.0b013e3181a105fc. [DOI] [PubMed] [Google Scholar]
- 18.Deyo RA. Back surgery-who needs it? N Engl J Med. 2007;356(22):2239–43. doi: 10.1056/NEJMp078052. [DOI] [PubMed] [Google Scholar]
- 19.Birkmeyer NJ, Weinstein JN. Medical versus surgical treatment for low back pain: evidence and clinical practice. Eff Clin Pract. 1999;2(5):218–27. [PubMed] [Google Scholar]
- 20.Mirza SK, Deyo RA. Systematic review of randomized trials comparing lumbar fusion surgery to nonoperative care for treatment of chronic back pain. Spine (Phila Pa 1976) 2007;32(7):816–23. doi: 10.1097/01.brs.0000259225.37454.38. [DOI] [PubMed] [Google Scholar]
- 21.Wang MC, Chan L, Maiman DJ, et al. Complications and mortality associated with cervical spine surgery for degenerative disease in the United States. Spine (Phila Pa 1976) 2007;32(3):342–7. doi: 10.1097/01.brs.0000254120.25411.ae. [DOI] [PubMed] [Google Scholar]
- 22.Lievre JA, Block-Michel H. L’injection transsacree. [Etude clinique and radiologigue] Bull Soc Med Paris. 1957;73:1110–8. in French. [PubMed] [Google Scholar]
- 23.Carrette S, Leclaire R, Marcoux S, et al. Epidural corticosteroid injections for sciatica due to herniated nucleus pulposus. N Engl J Med. 1997;336:1634–40. doi: 10.1056/NEJM199706053362303. [DOI] [PubMed] [Google Scholar]
- 24.Nelemans PJ, deBie RA, deVet HC, et al. Injection therapy for subacute and chronic benign low back pain. Spine. 2001;26(5):501–15. doi: 10.1097/00007632-200103010-00014. [DOI] [PubMed] [Google Scholar]
- 25.Fukusaki M, Kobayashi I, Hara T, et al. Symptoms of spinal stenosis do not improve after epidural steroid injection. Clin J Pain. 1998;14(2):148–51. doi: 10.1097/00002508-199806000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Butterman GR. Treatment of lumbar disc herniation: epidural steroid injection compared with discectomy. A prospective, randomized study. J Bone Joint Surg Am. 2004;86(4):670–9. [PubMed] [Google Scholar]
- 27.Riew KD, Yin Y, Gilula L, et al. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain. J Bone Joint Surg Am. 2000;82(11):1589–93. doi: 10.2106/00004623-200011000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Weinstein SM, Herring SA. Lumbar epidural steroid injections. Spine J. 2003;3(3 Suppl):37S–44S. doi: 10.1016/s1529-9430(02)00560-0. [DOI] [PubMed] [Google Scholar]
- 29.Rivest C, Katz JN, Ferrante FM, et al. Effects of epidural steroid injection on pain due to lumbar spinal stenosis or herniated disks: a prospective study. Arthritis Care Res. 1998;11(4):291–7. doi: 10.1002/art.1790110410. [DOI] [PubMed] [Google Scholar]
- 30.Valat JP, Giraudeau B, Rozenberg S. Epidural corticosteroid injections for sciatica: a randomized, double blind, controlled clinical trial. Ann Rheum Dis. 2003;62(7):639–43. doi: 10.1136/ard.62.7.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delport EG, Cucuzzella AR, Marley JK, et al. Treatment of lumbar spinal stenosis with epidural steroid injections: a retrospective outcome study. Arch Phys Med Rehabil. 2004;85(3):479–84. doi: 10.1016/s0003-9993(03)00472-6. [DOI] [PubMed] [Google Scholar]
- 32.Vad VB, Chat A. Transforaminal epidural steroid injections in lumbosacral radiculopathy: a prospective randomized study. Spine. 2002;27(1):11–5. doi: 10.1097/00007632-200201010-00005. [DOI] [PubMed] [Google Scholar]
- 33.Arden NK, Price C, Reading I, et al. A multicentre randomized controlled trial of epidural corticosteroid injections for sciatica: the WEST study. Rheumatology (Oxford) 2005;44(11):1399–406. doi: 10.1093/rheumatology/kei028. [DOI] [PubMed] [Google Scholar]
- 34.Botwin KP, Gruber RD, Bouchlas CG, et al. Fluoroscopically guided lumbar transforaminal epidural steroid injections in degenerative lumbar stenosis: an outcome study. Am J Phys Med Rehabil. 2002;81(12):898–905. doi: 10.1097/00002060-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Rydevik BL, Cohen DB, Kostuik JP. Spine epidural steroids for patients with lumbar spinal stenosis. Spine. 1997;22(19):2313–7. doi: 10.1097/00007632-199710010-00024. [DOI] [PubMed] [Google Scholar]
- 36.Banaszkiwicz PA, Kader D, Wardlaw D. The role of caudal epidural injections in the management of low back pain. Bull Hosp Jt Dis. 2003;61(3-4):127–31. [PubMed] [Google Scholar]
- 37.Cluff R, Mehio AK, Cohen SP, et al. The technical aspects of epidural steroid injections: a national survey. Anesth Analg. 2003;95(2):403–8. doi: 10.1097/00000539-200208000-00031. [DOI] [PubMed] [Google Scholar]
- 38.Riew KD, Park JB, Cho YS, et al. Nerve root blocks in the treatment of lumbar radicular pain. A minimum five-year follow-up. J Bone Joint Surg Am. 2006;88(8):1722–5. doi: 10.2106/JBJS.E.00278. [DOI] [PubMed] [Google Scholar]
- 39.Cuckler JM, Bernini PA, Wiesel SW, et al. The use of epidural steroids in the treatment of lumbar radicular pain. A prospective, randomized, double-blind study. J Bone Joint Surg Am. 1985;67(1):63–6. [PubMed] [Google Scholar]
- 40.Price C, Arden N, Coglan L, et al. Cost-effectiveness and safety of epidural steroids in the management of sciatica. Health Technol Assess. 2005;9(33):1–58. iii. doi: 10.3310/hta9330. [DOI] [PubMed] [Google Scholar]
- 41.Wilson-MacDonald J, Burt G, Griffin D, et al. Epidural steroid injection for nerve root compression. A randomised, controlled trial. J Bone Joint Surg Br. 2005;87(3):352–5. doi: 10.1302/0301-620x.87b3.15338. [DOI] [PubMed] [Google Scholar]
- 42.Bush K, Hillier S. A controlled study of caudal epidural injections of triamcinalone plus procaine for the management of intractable sciatica. Spine. 1991;16:572–5. doi: 10.1097/00007632-199105000-00015. [DOI] [PubMed] [Google Scholar]
- 43.Schaufele MK, Hatch L, Jones W. Interlaminar versus transforaminal epidural injections for the treatment of symptomatic lumbar intervertebral disc herniations. Pain Physician. 2006;9(4):361–6. [PubMed] [Google Scholar]
- 44.DePalma MJ, Slipman CW. Evidence-informed management of chronic low back pain with epidural steroid injections. Spine J. 2008;8(1):45–55. doi: 10.1016/j.spinee.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 45.Staal JB, de Bie RA, de Vet HC, et al. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine (Phila Pa 1976) 2009;34(1):49–59. doi: 10.1097/BRS.0b013e3181909558. [DOI] [PubMed] [Google Scholar]
- 46.Carrino JA, Morrison WB, Parker L, et al. Spinal injection procedures: volume, distribution, and reimbursement in the U.S. Medicare populations from 1993 to 1999. Radiology. 2002;225(3):723–9. doi: 10.1148/radiol.2253011401. [DOI] [PubMed] [Google Scholar]
- 47.Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007;32(16):1754–60. doi: 10.1097/BRS.0b013e3180b9f96e. [DOI] [PubMed] [Google Scholar]
- 48.Weinstein JN, Bronner KK, Morgan TS, et al. Trends and geographic variations in major surgery for degenerative diseases of the hip, knee, and spine. Health Aff (Millwood) 2004;(Suppl Web Exclusive):VAR81–9. doi: 10.1377/hlthaff.var.81. [DOI] [PubMed] [Google Scholar]
- 49.Deyo RA, Mirza SK. Trends and variations in the use of spine surgery. Clin Orthop Relat Res. 2006;443:139–46. doi: 10.1097/01.blo.0000198726.62514.75. [DOI] [PubMed] [Google Scholar]
- 50.Friedly J, Nishio I, Maynard C, et al. The relationship between repeated epidural steroid injections on subsequent opioid use and lumbar surgery. Arch Phys Med Rehabil. 2008;89(6):1011–5. doi: 10.1016/j.apmr.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 51.Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103–7. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 52.Vogt MT, Starz TW, Kwoh CK, et al. Opioid usage among older adults with low back pain: comment on the article by Solomon. Arthritis Rheum. 2006;55(3):513. doi: 10.1002/art.21997. author reply: 513-4. [DOI] [PubMed] [Google Scholar]
- 53.Friedly J, Chan L, Deyo R. Geographic variation in epidural steroid injection use in Medicare patients. J Bone Joint Surg Am. 2008;90(8):1730–7. doi: 10.2106/JBJS.G.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menachemi N, Chukmaitov A, Brown LS, et al. Quality of care in accredited and nonaccredited ambulatory surgical centers. Jt Comm J Qual Patient Saf. 2008;34(9):546–51. doi: 10.1016/s1553-7250(08)34069-0. [DOI] [PubMed] [Google Scholar]
- 55.Mitchell JM. The prevalence of physician self-referral arrangements after Stark II: evidence from advanced diagnostic imaging. Health Aff (Millwood) 2007;26(3):w415–24. doi: 10.1377/hlthaff.26.3.w415. [DOI] [PubMed] [Google Scholar]
- 56.Mitchell JM. Utilization changes following market entry by physician-owned specialty hospitals. Med Care Res Rev. 2007;64(4):395–415. doi: 10.1177/1077558707301953. [DOI] [PubMed] [Google Scholar]
- 57.MedPac report to congress: Medicare payment policy. Chapter. 2004;3F:185–204. [Google Scholar]
- 58.Chukmaitov AS, Menachemi N, Brown S, et al. A comparative study of quality outcomes in freestanding ambulatory surgery centers and hospital-based outpatient departments: 1997-2004. Health Services Research. 2008;43(5):1485–504. doi: 10.1111/j.1475-6773.2007.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manchikanti L, Boswell MV. Interventional techniques in ambulatory surgical centers: a look at the new payment system. Pain Physician. 2007;10(5):627–50. [PubMed] [Google Scholar]
- 60.Centers for Medicare & Medicaid Services (CMS), HHS Medicare program; revised payment system policies for services furnished in ambulatory surgical centers (ASCs) beginning in CY 2008. Final rule. Fed Regist. 2007;72(148):42469–626. [PubMed] [Google Scholar]
- 61.Strope S, Sarma A, Ye Z, et al. Disparities in the use of ambulatory surgical centers: a cross sectional study. BMC Health Serv Res. 2009;9:121. doi: 10.1186/1472-6963-9-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strope SA, Daignault S, Hollingsworth JM, et al. Medicare reimbursement changes for ambulatory surgery centers and remuneration to urological physician-owners. J Urol. 2008;180(3):1070–4. doi: 10.1016/j.juro.2008.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Binder DS, Nampiaparampil DE. The provocative lumbar facet joint. Curr Rev Musculoskelet Med. 2009;2(1):15–24. doi: 10.1007/s12178-008-9039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilde VE, Ford JJ, McMeeken JM. Indicators of lumbar zygapophyseal joint pain: survey of an expert panel with the Delphi technique. Phys Ther. 2007;87(10):1348–61. doi: 10.2522/ptj.20060329. [DOI] [PubMed] [Google Scholar]
- 65.Kalichman L, Hunter DJ. Lumbar facet joint osteoarthritis: a review. Semin Arthritis Rheum. 2007;37(2):69–80. doi: 10.1016/j.semarthrit.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 66.Cavanaugh JM, Lu Y, Chen C, et al. Pain generation in lumbar and cervical facet joints. J Bone Joint Surg Am. 2006;88(Suppl 2):63–7. doi: 10.2106/JBJS.E.01411. [DOI] [PubMed] [Google Scholar]
- 67.Dreyfuss PH, Dreyer SJ, Vaccaro A. Lumbar zygapophysial (facet) joint injections. Spine J. 2003;3(3 Suppl 1):50–9. doi: 10.1016/s1529-9430(02)00450-3. [DOI] [PubMed] [Google Scholar]
- 68.Nath S, Nath CA, Pettersson K. Percutaneous lumbar zygapophysial (Facet) joint neurotomy using radiofrequency current, in the management of chronic low back pain: a randomized double-blind trial. Spine. 2008;33(12):1291–7. doi: 10.1097/BRS.0b013e31817329f0. discussion: 1298. [DOI] [PubMed] [Google Scholar]
- 69.Bogduk N. Evidence-informed management of chronic low back pain with facet injections and radiofrequency neurotomy. Spine J. 2008;8(1):56–64. doi: 10.1016/j.spinee.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 70.Slipman CW, Bhat AL, Gilchrist RV, et al. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J. 2003;3(4):310–6. doi: 10.1016/s1529-9430(03)00025-1. [DOI] [PubMed] [Google Scholar]
- 71.Saal JS, Saal JA. Management of chronic discogenic low back pain with a thermal intradiscal catheter. A preliminary report. Spine (Phila Pa 1976) 2000;25(3):382–8. doi: 10.1097/00007632-200002010-00021. [DOI] [PubMed] [Google Scholar]
- 72.Pauza KJ, Howell S, Dreyfuss P, et al. A randomized, placebo-controlled trial of intradiscal electrothermal therapy for the treatment of discogenic low back pain. Spine J. 2004;4(1):27–35. doi: 10.1016/j.spinee.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 73.Freeman BJ, Fraser RD, Cain CM, et al. A randomized, double-blind, controlled trial: intradiscal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain. Spine (Phila Pa 1976) 2005;30(21):2369–77. doi: 10.1097/01.brs.0000186587.43373.f2. discussion: 2378. [DOI] [PubMed] [Google Scholar]
- 74.Andersson GB, Mekhail NA, Block JE. Intradiscal electrothermal therapy (IDET) Spine (Phila Pa 1976) 2006;31(12):1402. doi: 10.1097/01.brs.0000219362.82997.97. author reply: 1402-3. [DOI] [PubMed] [Google Scholar]
- 75.Appleby D, Andersson G, Totta M. Meta-analysis of the efficacy and safety of intradiscal electrothermal therapy (IDET) Pain Med. 2006;7(4):308–16. doi: 10.1111/j.1526-4637.2006.00172.x. [DOI] [PubMed] [Google Scholar]
- 76.Manchikanti L, Boswell MV, Singh V, et al. Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician. 2009;12(4):699–802. [PubMed] [Google Scholar]
- 77.Buchbinder R, Osborne RH, Kallmes D. Vertebroplasty appears no better than placebo for painful osteoporotic spinal fractures, and has potential to cause harm. Med J Aust. 2009;191(9):476–7. doi: 10.5694/j.1326-5377.2010.tb03467.x. [DOI] [PubMed] [Google Scholar]
- 78.Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361(6):569–79. doi: 10.1056/NEJMoa0900563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pai S, Sundaram LJ. Low back pain: an economic assessment in the United States. Orthop Clin North Am. 2004;35(1):1–6. doi: 10.1016/S0030-5898(03)00101-9. [DOI] [PubMed] [Google Scholar]
- 80.Luo X, Pietrobon R, Sun SX, et al. Estimates and patterns of direct health care expenditures among individuals with low back pain in the United States. Spine. 2004;29:79–86. doi: 10.1097/01.BRS.0000105527.13866.0F. [DOI] [PubMed] [Google Scholar]
- 81.Deyo RA, Mirza SK, Martin BI, et al. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303(13):1259–65. doi: 10.1001/jama.2010.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weiner SS, Nordin M. Prevention and management of chronic back pain. Best Pract Res Clin Rheumatol. 2010;24(2):267–79. doi: 10.1016/j.berh.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 83.Roine E, Roine RP, Räsänen P, et al. Cost-effectiveness of interventions based on physical exercise in the treatment of various diseases: a systematic literature review. Int J Technol Assess Health Care. 2009;25(4):427–54. doi: 10.1017/S0266462309990353. [DOI] [PubMed] [Google Scholar]
- 84.Fritz JM, Cleland JA, Speckman M, et al. Physical therapy for acute low back pain: associations with subsequent healthcare costs. Spine (Phila Pa 1976) 2008;33(16):1800–5. doi: 10.1097/BRS.0b013e31817bd853. [DOI] [PubMed] [Google Scholar]
- 85.Gellhorn A, Martin B, Chan L, et al. Management patterns in acute low back pain: the role of physical therapy. J Spine. doi: 10.1097/BRS.0b013e3181d79a09. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Tulder M, Malmivaara A, Hayden J, et al. Statistical significance versus clinical importance: trials on exercise therapy for chronic low back pain as example. Spine (Phila Pa 1976) 2007;32(16):1785–90. doi: 10.1097/BRS.0b013e3180b9ef49. [DOI] [PubMed] [Google Scholar]
- 87.Carey TS, Freburger JK, Holmes GM, et al. A long way to go: practice patterns and evidence in chronic low back pain care. Spine (Phila Pa 1976) 2009;34(7):718–24. doi: 10.1097/BRS.0b013e31819792b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mahowald ML, Singh JA, Majeski P. Opioid use by patients in an orthopedics spine clinic. Arthritis Rheum. 2005;52(1):312–21. doi: 10.1002/art.20784. [DOI] [PubMed] [Google Scholar]
- 89.Zerzan JT, Morden NE, Soumerai S, et al. Trends and geographic variation of opiate medication use in state Medicaid fee-for-service programs, 1996 to 2002. Med Care. 2006;44(11):1005–10. doi: 10.1097/01.mlr.0000228025.04535.25. [DOI] [PubMed] [Google Scholar]
- 90.Martell BA, O’Connor PG, Kerns RD, et al. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146(2):116–27. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- 91.Wallace AS, Freburger JK, Darter JD, et al. Comfortably numb? Exploring satisfaction with chronic back pain visits. Spine J. 2009;9(9):721–8. doi: 10.1016/j.spinee.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 92.Rhee Y, Taitel MS, Walker DR, et al. Narcotic drug use among patients with lower back pain in employer health plans: a retrospective analysis of risk factors and health care services. Clin Ther. 2007;29(Suppl):2603–12. doi: 10.1016/j.clinthera.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Campello MA, Weiser SR, Nordin M, et al. Work retention and nonspecific low back pain. Spine (Phila Pa 1976) 2006;31(16):1850–7. doi: 10.1097/01.brs.0000227288.00378.d5. [DOI] [PubMed] [Google Scholar]
- 94.Pincus T, Burton AK, Vogel S, et al. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine (Phila Pa 1976) 2002;27(5):E109–20. doi: 10.1097/00007632-200203010-00017. [DOI] [PubMed] [Google Scholar]
- 95.Nickel R, Egle UT, Eysel P, et al. Health-related quality of life and somatization in patients with long-term low back pain: a prospective study with 109 patients. Spine (Phila Pa 1976) 2001;26(20):2271–7. doi: 10.1097/00007632-200110150-00020. [DOI] [PubMed] [Google Scholar]
- 96.Bacon NM, Bacon SF, Atkinson JH, et al. Somatization symptoms in chronic low back pain patients. Psychosom Med. 1994;56(2):118–27. doi: 10.1097/00006842-199403000-00007. [DOI] [PubMed] [Google Scholar]
- 97.Cleland JA, Fritz JM, Brennan GP. Predictive validity of initial fear avoidance beliefs in patients with low back pain receiving physical therapy: is the FABQ a useful screening tool for identifying patients at risk for a poor recovery? Eur Spine J. 2008;17(1):70–9. doi: 10.1007/s00586-007-0511-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Deyo RA, Diehl AK. Psychosocial predictors of disability in patients with low back pain. J Rheumatol. 1988;15(10):1557–64. [PubMed] [Google Scholar]
- 99.Palmer KT, Calnan M, Wainwright D, et al. Disabling musculoskeletal pain and its relation to somatization: a community-based postal survey. Occup Med (Lond) 2005;55(8):612–7. doi: 10.1093/occmed/kqi142. [DOI] [PubMed] [Google Scholar]
- 100.Newman RI, Seres JL, Yospe LP, et al. Multidisciplinary treatment of chronic pain: long-term follow-up of low-back pain patients. Pain. 1978;4(3):283–92. doi: 10.1016/0304-3959(77)90140-3. [DOI] [PubMed] [Google Scholar]
- 101.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–21. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 102.Ritzwoller DP, Crounse L, Shetterly S, et al. The association of comorbidities, utilization and costs for patients identified with low back pain. BMC Musculoskelet Disord. 2006;7:72. doi: 10.1186/1471-2474-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shiri R, Karppinen J, Leino-Arjas P, et al. The association between obesity and low back pain: a meta-analysis. Am J Epidemiol. 2010;171(2):135–54. doi: 10.1093/aje/kwp356. [DOI] [PubMed] [Google Scholar]
- 104.Ahern DK, Bishop D, Follick MJ, et al. Interdisciplinary perspectives on mechanisms and management of low back pain. R I Med J. 1990;73(1):21–31. [PubMed] [Google Scholar]
- 105.Beekman CE, Axtell L. Ambulation, activity level, and pain. Outcomes of a program for spinal pain. Phys Ther. 1985;65(11):1649–57. doi: 10.1093/ptj/65.11.1649. [DOI] [PubMed] [Google Scholar]
- 106.Branthaver B, Stein GF, Mehran A. Impact of a medical back care program on utilization of services and primary care physician satisfaction in a large, multispecialty group practice health maintenance organization. Spine (Phila Pa 1976) 1995;20(10):1165–9. doi: 10.1097/00007632-199505150-00011. [DOI] [PubMed] [Google Scholar]