Abstract
OBJECTIVE
To investigate the frequency of lipid testing in clinical practice and explore the relationship between rheumatoid arthritis (RA), dyslipidemia, and other cardiovascular (CV) risk factors, with RA treatment.
METHODS
Patients in the retrospective database study were ≥18 years old and had ≥2 physician diagnoses for RA or osteoarthritis (OA) [comparator group] between March 2004-March 2008. Outcomes of interest included the percentage of RA and OA patients receiving lipid tests, lipid profiles (total cholesterol [TC], low-density lipoprotein cholesterol [LDL-C], and high-density lipoprotein cholesterol [HDL-C]) of RA vs. OA patients, and lipid profiles of RA patients before and after initiation with a tumor necrosis factor inhibitor (TNFi). We used multivariable regression to control potential confounders between the cohorts.
RESULTS
Over a median 2+ year follow-up, fewer RA patients than OA patients had at least one lipid test (62% [95% CI, 60-64] vs. 68% [95% CI, 65-71]). Mean TC and LDL-C were each 4 mg/dL lower in the RA cohort (P<0.0001); HDL-C was similar between cohorts. Across the RA cohort, 25.2% of patients had suboptimal LDL-C levels (≥130 mg/dL). Among RA patients not using lipid-lowering therapy who initiated TNFi therapy (n=96), mean TC and LDL-C increased by 5.4 and 4.0 mg/dL, respectively.
CONCLUSION
RA patients were less likely to be tested for hyperlipidemia and had more favorable lipid profiles than OA patients. TNFi therapy modestly increased all lipid parameters. Additional studies are needed to determine the effect of traditional CV risk factors, inflammation, and the impact of biologics on CV outcomes in RA patients.
Patients with rheumatoid arthritis (RA) have higher rates of morbidity and mortality than the general population, which is highly attributed to an increased risk of cardiovascular disease (CVD) among RA patients. [1, 2] The increased risk of CVD appears to be linked to coronary atherosclerosis [3, 4] and may be directly caused by chronic inflammation or secondarily caused by physical inactivity and drugs used to treat RA [5]. Not surprisingly, RA treatment guidelines reflect this increased CV risk among RA patients. Evidence-based and expert-opinion based recommendations from the European League Against Rheumatism (EULAR) for the screening and management of RA patients include annual CV risk assessment, management of identified CV risk factors, and aggressive suppression of the inflammatory process to further lower the CV risk [6].
Lipid levels appear to be altered as a result of RA disease activity. Data on total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels in RA patients are conflicting: some studies demonstrate similar [7] or lower [8] levels of TC, while others demonstrate increased levels of TC and LDL-C in patients with early RA [9]. Although reports on lipid profiles in RA patients vary, growing evidence suggests that patients with active untreated RA have reduced total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) levels [8, 10, 11]. Regardless of the TC changes in RA patients, with a decrease in HDL-C, several studies support the notion that RA leads to a more atherogenic lipid profile (TC to HDL-C ratio) which is correlated with disease activity and improves after treatment with antirheumatic medications [7-9, 12].
Inflammation is a common denominator in both RA and atherosclerosis. A growing body of evidence supports the involvement of common proinflammatory cytokines—such as macrophage migration inhibitory factor (MIF), interleukin (IL)-1, IL-6, and tumor necrosis factor-alpha (TNF-α)—in the development and progression of both RA and atherosclerosis [3, 13]. Several studies have demonstrated that the use of disease-modifying anti-rheumatic drugs (DMARDs) and biologic agents that affect these cytokines reduce inflammation in RA patients and may be associated with a reduced risk of CVD [12, 14-20].
Given that inflammation in RA patients alters the lipid profile, it is not surprising that treatment to control inflammation in RA patients may affect also lipid levels. A recent meta-analysis of 24 observational studies evaluating the effect of TNFi therapy on lipids in RA patients showed a small trend in an increase in TC, mostly due to an increase in HDL-C levels [21]. In light of uncertainties regarding the relation between RA, lipid profiles, and potent anti-inflammatory medications such as TNFi therapy, we used a large population-based database to investigate the frequency of lipid testing in clinical practice and to explore the relationship between RA, dyslipidemia, CVD risk factors, and RA treatment. The primary objective of this study was to evaluate the proportion of RA patients receiving lipid testing and the frequency of testing compared to controls (patients with osteoarthritis [OA]). We hypothesized that patients with RA would be tested less frequently than patients with OA. Secondary objectives included 1) comparing lipid levels in RA patients versus controls (OA patients) among patients tested for hyperlipidemia, and 2) describing changes in lipid levels in patients with RA who initiated therapy with a TNFi.
MATERIALS AND METHODS
Patient Identification
A retrospective analysis was conducted using data from the OptumInsight IMPACT database. This database includes medical claims, pharmacy claims, laboratory data, and patient eligibility data for 86.4 million covered lives, of which 63.7 million (74%) have pharmacy benefits and 12.6 million have laboratory results. The database includes patients 65 years of age or older, all of which are covered by standard commercial or managed care plans. The Impact database is derived from 46 health plans located across all census regions in the US (predominantly located in the North, North Central, and Atlantic regions).
Patients were included in the study if they were at least 18 years old and had at least two separate physician diagnoses (≥2 months apart) for RA (ICD-9-CM 714.xx) or for OA (ICD-9-CM 715.xx) between March 2004 and March 2008 (Table 1). Patients were excluded from the study if they had a diagnosis for any other autoimmune inflammatory disease at any time during the study observation period (Appendix Table A). Patients with OA were chosen as a comparator group for RA patients because OA is a chronic condition that affects joints and may limit physical functioning, but which does not have the systemic inflammatory features more common to RA. Furthermore, OA patients were expected to be more comparable to RA patients given a higher expected prevalence of NSAID use (a recognized CV risk factor which might impact CV risk assessment) and more frequent contact with the medical system (which can motivate more use of appropriate screening tests) compared to the general population without chronic medical conditions. Additional study eligibility requirements and study design details which varied by the three study objectives are listed in Table 1.
Table 1. Patient Eligibility Requirements by Objective.
| Objective | Time Perioda |
Continuous Eligibility Requirement |
Lipid Test Requirement |
Index Date Definition |
Variables Controlled for in the Regression Analysis |
|---|---|---|---|---|---|
| 1) Comparison of Frequency of Lipid Testing [RA patients vs. OA patients] |
Apr 2005 – Mar 2008 |
Medical and pharmacy benefits for ≥ 18 months [6 months before and 12 months after RA/OA diagnosis] |
None | Date of 2nd physician RA/OA diagnosis |
Baseline: Demographicsb Insurance typec Comorbidities, comorbidity scoresd RA medication usee Lipid-lowering medication usef Healthcare utilizationg |
| 2) Comparison of Lipid Levels [RA patients vs. OA patients] |
Mar 2004 – Mar 2008 |
Medical and pharmacy benefits for ≥ 12 months |
≥ 1 lipid test result (TC, HDL-C, LDLC, or TG) after 2nd RA/OA diagnosis |
Date of lipid test |
Baseline: Demographicsb Insurance typec Comorbidities, comorbidity scoresd RA medication usee Lipid-lowering medication usef Healthcare utilizationg Lipid tests (HDL-C, LDL-C, TC, TG) Days from diagnosis to lipid test |
| 3) Comparison of Changes in Lipid Levels in RA patients [90 days before / after starting TNFi therapy]h |
Mar 2004 – Mar 2008 |
Medical and pharmacy benefits for ≥ 6 months before TNFi initiation |
≥ 1 lipid test before and another ≥ 6 weeks after TNFi initiation |
Date of TNFi (etanercept, adalimumab, infliximab) initiation |
Baseline: Demographicsb Comorbidities, comorbidity scoresd RA medication usee Lipid-lowering medication usef Time from TNFi initiation to lipid test Lipid levels (HDL-C, LDL-C, TC, TG) |
All objectives had a 6 month baseline period which occurred prior to the index date;
Age, gender, geographic location;
Capitated or not capitated;
individual comorbidities adjusted for include those listed in the clinical characteristics table for each cohort; comorbidity scores include the Charlson Comorbidity Index, Chronic Disease Score;
TNF inhibitors, DMARDs, corticosteroids, NSAIDs;
Bile acid sequestrants, cholesterol absorption inhibitors, fibric acid derivatives, statins, niacin;
Inpatient, physician visits, outpatient visits, ER visits; hSensitivity analyses widened the windows before and after initiating TNFi therapy to ± 120 days and ± 180 days
All measures of a complete lipid panel—TC, HDL-C, LDL-C, and triglycerides (TG)—that occurred at any time during the observation period were evaluated in this study in time windows specific for each objective (Table 1). Classification of lipid levels was based on the Adult Treatment Panel III (ATP-III) cholesterol management guidelines [22]. Use of lipid-lowering medications—including niacin, fibric acid derivatives, bile acid binding resins, cholesterol absorption inhibitors, and HMG-CoA reductase inhibitors—was also evaluated in this study.
Statistical Analyses
All study variables, including baseline and outcome measures, were analyzed descriptively. Dichotomous variables were expressed as numbers and percentages and continuous variables were expressed as means (± standard deviation, SD), medians, or percentiles.
For dichotomous variables, p-values were calculated using the Mann-Whitney U Test, and for continuous variables, p-values were calculated using t-tests. Multivariable regression was used to control for confounders and to increase the efficiency of the estimators. The outcome variables and covariates used to control for baseline differences varied between objectives and are described in Table 1.
RESULTS
Objective 1: Frequency of Lipid Testing, Comparing RA versus OA Patients
The sample of individuals initially meeting eligibility requirements included 30,586 patients with an RA diagnosis, and 107,534 patients with an OA diagnosis (control group). Characteristics of each group at baseline, defined as six months prior to RA or OA diagnosis, are presented in Table 2. The groups were significantly different in most covariates; therefore, regression analysis was performed to control for these differences.
Table 2a. Demographic Characteristics of Patients Included in the Lipid Testing Sample (Objective 1) Measured during the 6-month Baseline Period.
| RA Patients | OA Patients | |
|---|---|---|
| N=30,586 | N=107,534 | |
| Parameter | N (%) or Mean (SD) | N (%) or Mean (SD) |
| Female Gender | 22,658 (74.1%) | 62,689 (58.3%) |
| Age Group | ||
| 18-49 | 13,883 (45.4%) | 27,430 (25.5%) |
| 50-64 | 13,560 (44.3%) | 58,716 (54.6%) |
| 65-74 | 2,124 (6.9%) | 12,289 (11.4%) |
| 75-84 | 1,019 (3.3%) | 9,099 (8.5%) |
| Age (years) | 50.2 (12.0) | 56.1 (10.6) |
| Insurance Type | ||
| Capitated | 7,683 (25.1%) | 40,499 (37.7%) |
| Census Region | ||
| Midwest | 4,389 (14.4%) | 14,208 (13.2%) |
| Northeast | 10,846 (35.5%) | 52,408 (48.7%) |
| South | 11,778 (38.5%) | 30,069 (28.0%) |
| West | 3,569 (11.7%) | 10,815 (10.1%) |
Over a median follow-up of 2.2 years (interquartile range, 1.6-2.8 years) for patients in the RA group and 2.5 years (interquartile range, 1.9-3.1 years) for patients in the OA group, there were somewhat fewer patients in the RA group than in the OA group who had at least one test for TC (59.1% [95% CI, 58.1-60.1] vs. 68.4% [95% CI, 64.4-70.4]), HDL-C (57.2% [95% CI, 56.0-58.4] vs. 66.4% [95% CI, 64.4-68.4]), LDL-C (58.6% [95% CI, 57.3-59.9] vs. 68.4% [95% CI, 67.1-69.7), or TG (59.1% [95% CI, 58.2-60.0] vs. 68.4% [95% CI, 67.3-69.5]). Furthermore, the analysis of the risk-adjusted differences in the number of tests revealed that among patients who received a lipid test, slightly fewer tests were performed for RA than OA patients (TC, 2.0 [95% CI, 1.8-2.2] vs. 2.9 [95% CI, 2.6-3.2]; HDL-C, 1.8 [95% CI, 1.7-1.9] vs. 2.7 [95% CI, 2.5-2.9]; LDL-C, 1.9 [95% CI, 1.4-2.4] vs. 2.9 [95% CI, 2.5-3.3]; TG, 1.0 [95% CI, 0.8-1.2] vs. 2.9 [95% CI, 2.7-3.2]). Because follow-up time was somewhat longer for OA vs. RA patients, a subgroup analysis restricted the cohort to patients with at least 18 months of observation time after the start of follow-up and fixed the ascertainment period to these 18 months. Among this subgroup (71% of RA cohort and 68% of OA cohort), RA patients were less likely compared to OA patients to receive any TC testing (56% [95% CI, 53-59] vs. 67% [95% CI, 63-71]).
Objective 2: Comparison of Lipid Levels in RA versus OA Patients
From the initial sample size of 136,996 RA patients, and based upon the availability of lab data in the database, 12,319 (9.0%) were eligible for the lipid level objective analysis compared with the 29,621 patients from the 194,192 OA controls (15.3%). Similar to the study population for Objective 1, there were significant differences among eligible RA and OA patients at baselinel (Table 3).
Table 3a. Demographic Characteristics of Patients Included in the Lipid Level Sample (Objective 2) Measured during the 6-month Baseline Period.
| RA Patients | OA Patients | |
|---|---|---|
| N=12,319 | N=29,621 | |
| Parameter | N (%) or Mean (SD) | N (%) or Mean (SD) |
| Gender | ||
| Female | 9,357 (76.0%) | 18,410 (62.8%) |
| Age Group | ||
| 18-49 | 3,895 (31.6%) | 5,216 (17.8%) |
| 50-64 | 6,565 (53.3%) | 16,429 (56.0%) |
| 65-74 | 1,287 (10.5%) | 4,291 (14.63%) |
| 75-84 | 572 (4.6%) | 3,400 (11.6%) |
| Age (years) | 54.2 (11.0) | 58.7 (10.3) |
| Insurance Type | ||
| Capitated | 4,208 (34.2%) | 11,953 (40.8%) |
| Census Region | ||
| Midwest | 1,165 (9.5%) | 3,100 (10.6%) |
| Northeast | 4,872 (39.6%) | 10,809 (36.9%) |
| South | 5,595 (45.4%) | 13,981 (47.7%) |
| West | 685 (5.6%) | 1,439 (4.9%) |
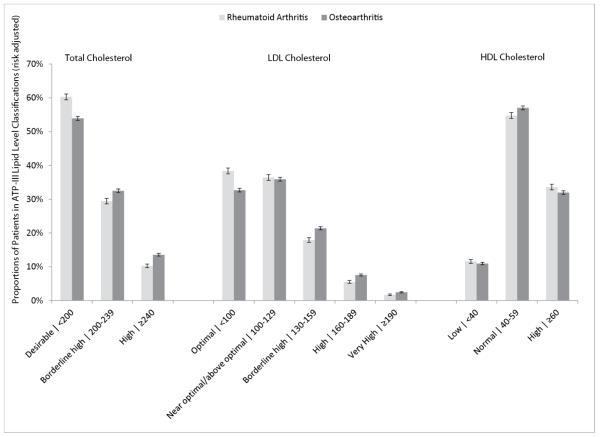
Multivariable-adjusted regression analysis was performed to assess risk-adjusted lipid levels and CVD-related comorbidity prevalence. Mean TC, LDL-C, and TG levels were significantly lower in the RA than in the OA cohort (TC, 195 mg/dL [95% CI, 191-199] vs. 199 mg/dL [95% CI, 192-205]; LDL-C, 112 mg/dL [95% CI, 111-113] vs. 116 mg/dL [95% CI, 114-118]; TG, 132 mg/dL [95% CI, 129-135] vs. 138 mg/dL [95% CI, 136-140]; P<0.0001 for all comparisons), while HDL-C levels were marginally higher for the RA cohort relative to the OA cohort (56.8 mg/dL vs. 56.1 mg/dL; P=0.02). Furthermore, significantly fewer RA patients than OA patients had borderline high or high TC and LDL-C levels, based on ATP-III lipid classification levels (P≤0.0001; Figure 1). RA patients were significantly less likely than OA patients to have a recorded diagnosis of hyperlipidemia (33% [95% CI, 28-35] vs. 45% [95% CI, 41-48]) and hypertension (45% [95% CI, 41-49] vs. 54% [95% CI, 49-56]; P<0.0001 for both).
Figure 1. Proportions of RA and OA Patients in Various Lipid Categories Based On ATP-III Classifications.
RA and OA cohorts were adjusted using multivariable regression based on factors listed in Table 1. Error bars indicate the 95% confidence interval of the mean.
Objective 3: Comparison of Lipid Levels Before and After TNFi Therapy in RA Patients
A total of 1,393 RA patients were eligible for the analysis of changes in lipid levels after TNFi therapy initiation. Of these patients, 289 had a lipid test within 90 days preceding and following TNFi initiation, 477 patients had a lipid test within 120 days, and 766 had a test within 180 days of TNFi initiation. Lipid test results were further stratified by use or non-use of lipid-lowering medications before and after TNFi initiation. Patients were classified as consistent users (lipid-lowering medication use before and after TNFi therapy), non-users (no lipid-lowering medication use before or after TNFi therapy), or mixed users (lipid-lowering medication therapy either before or after TNFi therapy, but not both). Mixed lipid-lowering medication users’ results were not reported due to an inadequate sample size to characterize the various patterns of use with these agents. Furthermore, we did not observe any patients who started TNFi therapy, then was tested for hyperlipidemia but who did not have a result available in the data, initiated a lipid-lowering medication, and then was re-tested and had lipid results available.
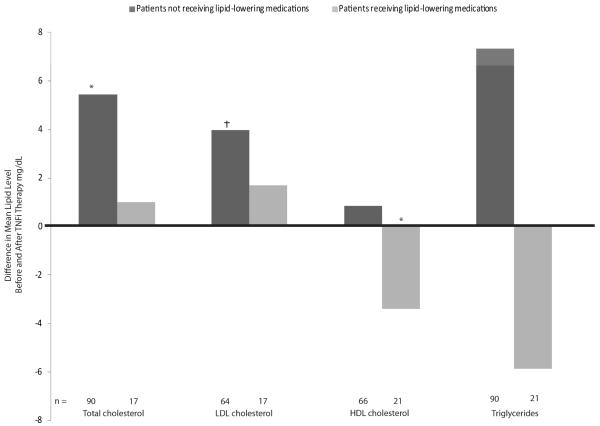
We identified 289 patients with paired lipid levels within ± 90 days preceding and following the start of TNFi treatment (mean 65 days between the paired lipid tests). Among non-users of lipid-lowering medications starting therapy with a TNFi (n=96), mean HDL-C, TC, and LDL-C levels increased modestly: HDL-C, 0.9 mg/dL [95% CI, 0.1-1.6]; TC, 5.4 mg/dL [95% CI, 2.6-18.3]; LDL-C, 4.0 mg/dL [95% CI, 0.3-7.7] (Figure 2). Mean TG levels also increased in these patients (mean change, 7.3 mg/dL, P=0.08). Among consistent lipid-lowering medication users (n=21), HDL-C levels decreased by a mean of 3.4 mg/dL [95% CI, 2.2-10.0]; no significant changes were observed in TC, LDL-C, and TG levels (Figure 2). The atherogenic index (ratio of TC to HDL) decreased in both patients consistently using and not using lipid-lowering medications (3.8 to 3.6 and 3.7 to 3.6, respectively; P=0.08)
Figure 2. Changes in Lipid Levels after TNFi Initiation in Patients with RA.
Includes patients who underwent lipid testing within 90 days before and after TNFi initiation. Not all patients underwent the complete set of lipid tests (e.g., patients might have undergone only TC testing but not HDL or LDL testing). A linear regression model was used to control for the factors listed in Table 1. *p ≤ 0.0001; †p ≤ 0.01 for the null hypothesis that there was no change in lipid values after TNFi initiation
The within-person analysis described above was repeated for the groups who had lipid testing within ±120 and ±180 days of TNFi initiation. Study results were consistent, with adjusted LDL levels consistently higher after TNFi initiation in patients not taking lipid-lowering medications, regardless of the timing of the test (data not shown).
DISCUSSION
This real-world analysis demonstrated that patients with RA had mean TC, LDL-C, and TG levels that were lower than OA patients. Although RA patients were slightly more likely to be in a favorable ATP-III category, approximately 25% of RA patients had suboptimal lipid levels based on current ATP-III guidelines [22]. Among RA patients initiating TNFi therapy and who had their TC and LDL-C re-tested within three months, mean TC and LDL-C increased 5 and 4 mg/dL after TNFi therapy was initiated. Finally, we observed that while RA patients were only slightly less likely to receive any lipid testing than OA patients, approximately one-third of RA patients did not receive any testing during the observation period of more than 2 years.
Among RA patients not receiving lipid-lowering medications, we observed that treatment with TNFi was associated with modest increases in TC and LDL-C levels. This is consistent with results from other studies that observed increases in lipid levels after treatment with biological agents. In a recent meta-analysis of 24 observational studies evaluating the effect of TNFi therapy on lipids in RA patients, a small trend of an increase in TC was observed [21]. Of the four controlled studies which measured the atherogenic index, one study found a significant increase of 8.9% in the TNFi therapy group and a significant decrease of 10.4% in the control group [23], two studies reported non-significant decreases in the TNFi group with no changes in the control groups [24, 25], and one study reported a significant decrease in cases compared to controls, but data were not provided [26]. In our study, we found minimal changes in lipid profiles among RA patients who were treated with lipid-lowering medication prior to and during TNFi therapy. However, our results could not be compared with the meta-analysis since no similar sub-group analysis of lipid level changes among patients using lipid-lowering medication treatments was performed [21].
Aside from TNFi, other biologic agents have been shown to affect lipid profiles. Tocilizumab (TCZ), which inhibits the proinflammatory cytokine interleukin-6 (IL-6) binding to its receptors,[27] is associated with decreases in inflammatory markers [28]. TCZ is associated with increased lipid levels in RA patients (e.g. an increase in LDL of 20 mg/dL among TCZ+MTX users), but has not been associated with an increase in CV events during short-term follow-up.[29-32] In a recently completed long-term follow-up study of TCZ in RA patients (mean treatment duration of 2.4 years), TC, HDL-C, LDL-C, and TG levels increased after 6 weeks of treatment and remained relatively stable at the elevated level thereafter [31, 32].
The clinical importance of lipid levels on CVD risk in RA is not completely understood. Recent evidence suggests that there may be a paradoxical effect of lipids on the risk of CVD in RA, where lower and not higher TC and LDL-C levels are associated with increased cardiovascular risk [11]. Furthermore, although HDL-C is generally considered to be cardioprotective—both through its ability to promote cholesterol efflux from artery cell walls and anti-inflammatory properties which project LDL-C from oxidation—a growing body of evidence suggests that in inflammatory conditions such as RA and systemic lupus erythematosus, patients have non-protective “pro-inflammatory HDL” (piHDL) which promotes accumulation of oxidixed phospholipids in LDL-C [33, 34].
Based upon what appears to be more favorable TC and LDL-C distributions in RA patients compared to OA patients, the results of this analysis suggest that lipid profiles in RA patients only partially explain the previously-observed excess CVD risk associated with the systemic inflammation of RA [35]. Other inflammation-induced factors, such as increased oxidative stress, insulin resistance, endothelial dysfunction, pro-thrombotic state, and elevated homocysteine levels,[36] as well as non-inflammatory mechanisms, such as genetic polymorphism and CV toxicity associated with certain anti-rheumatic drugs (e.g., glucocorticoids) may also contribute to the increased CVD risk in RA [36].
A major strength of this study is that it is based on real-world clinical practice. The dataset closely represents the United States in terms of population age, gender, and geographic region [37]. Comparing the United States versus the Impact dataset, the age distributions are as follows: 0-20 (27% US vs. 25% Impact), 21-39 (27% vs. 24%), 40-64 (31% vs. 40%), and 65+ (13% vs. 11%). In terms of regions, the distributions are as follows: northeast (18% US vs. 29% Impact), Midwest (22% vs. 26%), South (37% vs. 37%), and West (23% vs. 11%). Similar proportions of men are in each (49% in the United States versus 51% in the Impact dataset).
Conclusions from this study need to be weighed within the confines of some limitations of this data source. Clinical data related to risk factors such as smoking, CVD history, family history of CVD, and blood pressure, as well as RA disease severity and activity, were not available in the database. Studies have shown that while some traditional risk factors (dyslipidemia, family cardiac history, hypertension, diabetes mellitus, and obesity) impart similar risk for a CV event among RA and non-RA patients, other traditional CV risk factors (male gender, smoking, and personal cardiac history) impart significantly less relative CV risk in RA versus non-RA patients [38]. Beyond traditional CV risk factors, several disease severity and disease activity markers in RA—such as extra-articular manifestations, elevated erythrocyte sedimentation rate (ESR), rheumatoid factor (RF) seropositivity, higher joint count, and functional status—correlate with the rate of CVD and major CV events, including myocardial infarction (MI), congestive heart failure (CHF), and death [4, 39-41].
Another limitation of this study is that this analysis did not investigate longitudinal changes in lipid levels associated with TNFi therapy beyond 180 days. A study by Popa et al. (2007) showed that although short-term effects of TNFi therapy on lipids seemed beneficial and anti-atherogenic, the atherogenic index increased after six months from the start of therapy. Furthermore, changes in disease activity and inflammatory status were inversely correlated with changes in TC and HDL-C levels and positively correlated with the variation of atherogenic index [42]. When evaluated over a longer period, such as six months or beyond, infliximab treatment has been associated with significantly increased levels of TC and TG, with no change in HDL-C and LDL-C or atherogenic indices at 6 months [43]. In other studies, RA patients treated with infliximab had increased lipid levels (TC, HDL-C, LDL-C) initially, which returned to baseline by six months to one year of treatment (except for TC levels, which remained increased in one study) [26, 44]. The effect of time was partially addressed in this study by sensitivity analysis in which similar results were obtained when lipid tests were carried out before or after 120 and 180 days. However, further investigations into the long-term effect of TNFi therapy on lipid levels are needed. Finally, we excluded patients who initiated lipid-lowering therapy after initiation of TNFi therapy and before follow-up lipid testing was performed (i.e. described as ‘mixed’ lipid lowering medication users). While this may have excluded some individuals with elevated lipids, these patients initiated a lipid lowering medication prior to follow-up lipid testing, avoiding concern for a selection bias related to the effect of TNFi on lipids.
CONCLUSION
Patients with RA have a higher mortality rate than the general population. Much of this risk is due to CVD. This study showed that in clinical practice RA patients were tested for dyslipidemia less frequently than their OA counterparts. Furthermore, although RA patients tended to have relatively lower lipid levels, more than 25% of patients had suboptimal lipid levels based on current ATP-III guidelines. Analysis of lipid levels in RA patients before and after TNFi therapy initiation showed modest increases in TC, LDL-C, and HDL-C levels among patients not using lipid-lowering medications. Due to the increased risk of CVD and mortality among RA patients, more aggressive and early lipid management, including greater use of statin therapy may be appropriate to reduce CVD among RA patients who have elevated lipid and CRP levels. Additional prospective, long-term studies are needed to comprehensively determine the role of inflammation and the impact of biologics on lipid levels and CV outcomes in patients with RA.
SIGNIFICANCE AND INNOVATION.
Our retrospective analysis of a representative US database demonstrated RA patients are significantly less likely to be tested for hyperlipidemia than controls (patients with osteoarthritis).
Approximately 25% of patients with RA have suboptimal LDL-C levels according to the ATP-III cholesterol management guidelines.
Initiation of anti-TNF therapy increased total cholesterol levels by approximately 5mg/dL among RA patients not on treatment with lipid lowering agents
Table 2b. Clinical Characteristics of Patients Included in the Lipid Testing Sample (Objective 1) Measured during the 6-month Baseline Period.
| RA Patients | OA Patients | |
|---|---|---|
| N=30,586 | N=107,534 | |
| Parameter | N (%) or Mean (SD) | N (%) or Mean (SD) |
| Comorbidities and Scores | ||
| CCI Score [48] | 1.1 (0.9) | 0.4 (0.9) |
| Chronic Disease Score [49] | 2.5 (3.0) | 2.8 (2.9) |
| Fibromyalgia | 2,593 (8.5%) | 5,336 (5.0%) |
| Respiratory Infections | 7,844 (25.7%) | 32,472 (30.2%) |
| Diabetes | 1,880 (6.2%) | 10,385 (9.7%) |
| Obesity | 664 (2.2%) | 4,445 (4.1%) |
| Hypertension | 7,077 (23.1%) | 41,259 (38.4%) |
| Healthcare Utilization | ||
| Inpatient | 0.1 (0.3) | 0.1 (0.3) |
| Physician visits | 4.4 (6.2) | 5.9 (6.6) |
| Outpatient visits | 1.2 (2.8) | 1.7 (3.4) |
| ER visits | 0.2 (1.3) | 0.3 (1.3) |
| RA-related Medications | ||
| Non-cytotoxic DMARDsa | 3,498 (11.4%) | 399 (0.4%) |
| Cytotoxic DMARDsb | 795 (2.6%) | 680 (0.6%) |
| Methotrexate | 3,764 (12.3%) | 118 (0.1%) |
| Corticosteroids | 7,421 (24.3%) | 16,772 (15.6%) |
| NSAIDs | 13,047 (42.7%) | 50,215 (46.7%) |
| Lipid-lowering Medications | 3,570 (11.7%) | 26,896 (25.0%) |
CCI, Charlson Comorbidity Index; DMARD, disease-modifying antirheumatic drug; NSAID, non-steroidal antiinflammatory drug
gold compounds, penicillamine, chloroquine, hydroxychloroquine, sulfasalazine;
azathioprine, cyclosporine, cyclophosphamide, leflunomide
Table 3b. Clinical Characteristics of Patients Included in the Lipid Level Sample (Objective 2) Measured during the 6-month Baseline Period.
| RA Patients | OA Patients | |
|---|---|---|
| N=12,319 | N=29,621 | |
| Parameter | N (%) or Mean (SD) | N (%) or Mean (SD) |
| Comorbidities and Scores | ||
| CCI Score [48] | 1.2 (1.1) | 0.5 (1.0) |
| Chronic Disease Score [49] | 4.5 (3.5) | 3.1 (3.0) |
| Fibromyalgia | 912 (7.4%) | 1,262 (4.3%) |
| Respiratory Infections | 4,367 (35.5%) | 9,944 (33.9%) |
| Diabetes | 1,599 (13.0%) | 4,242 (14.5%) |
| Obesity | 330 (2.7%) | 1,295 (4.4%) |
| Hypertension | 5,379 (43.7%) | 15,603 (53.2%) |
| Polycystic Ovarian Disease | 18 (0.2%) | 12 (0.0%) |
| Coronary Artery Disease | 943 (7.7%) | 2,538 (8.7%) |
| Heart Failure | 236 (1.9%) | 632 (2.2%) |
| Hyperlipidemia | 6,073 (49.3%) | 16,635 (56.7%) |
| Lipid Levels | ||
| Total Cholesterol | 196.2 (36.4) | 198.7 (37.8) |
| HDL Cholesterol | 56.6 (15.9) | 55.2 (16.1) |
| LDL Cholesterol | 113.0 (31.7) | 115.9 (33.3) |
| Triglycerides | 133.1 (66.8) | 138.5 (68.8%) |
| Healthcare Utilization | ||
| Inpatient | 0.0 (0.2) | 0.1 (0.3) |
| Physician visits | 4.2 (6.0) | 5.8 (6.5) |
| Outpatient visits | 1.3 (2.2) | 1.8 (3.5) |
| ER visits | 0.2 (1.2) | 0.3 (1.2) |
| RA-related Medications | ||
| DMARD only | 4,439 (36.0%) | 0 (0.0%) |
| Non-TNFi only | 3 (0.0%) | 1 (0.0%) |
| Non-TNFi and DMARDs | 13 (0.1%) | 0 (0.0%) |
| TNFi only | 819 (6.7%) | 0 (0.0%) |
| TNFi and DMARDs | 1,231 (10.0%) | 0 (0.0%) |
| Corticosteroids | 4,166 (33.8%) | 4,587 (15.6%) |
| NSAIDs | 6,639 (53.9%) | 12,849 (43.8%) |
| Lipid-lowering Medications | 3,294 (26.7%) | 8,678 (29.6%) |
DMARD, disease-modifying antirheumatic drug; ER, emergency room; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NSAID, non-steroidal anti-inflammatory drug; TNFi, tumor necrosis factor inhibitor
ACKNOWLEDGEMENT
The authors thank Kristin A. Hanson, PharmD, MS and Jyoti S. Nandi, MD, PhD who provided medical writing services on behalf of United BioSource Corporation, Bethesda, Maryland, USA.
Grant support and competing interests:
JRC is supported by the NIH (AR 053351) and AHRQ (R01HS018517); during the past five years, he has received consulting fees, honoraria, and research funding from Roche/Genentech, UCB, Centocor, CORRONA, Amgen, Pfizer, BMS, Crescendo, and Abbott. AJ is an employee of Genentech. OB is a consultant for Genentech. Funding for this study, analysis, and manuscript preparation was provided by Genentech, Inc. No authors received funding for preparation of the manuscript.
APPENDIX
Table A. Autoimmune Inflammatory Diseases for which Patients were Excluded.
| Condition | ICD-9-CM Code(s) |
|---|---|
| Acute disseminated encephylomyelitis (ADEM) | 323.61 |
| Addison’s disease | 255.4 |
| Ankylosing spondylitis | 720 |
| Antiphospholipid antibody syndrome (APS) | 279.8 |
| Autoimmune hemolytic anemia | 283 |
| Autoimmune hepatitis | 571.42 |
| Bullous pemphigoid | 694.5 |
| Celiac disease | 579 |
| Cushing’s disease | 255 |
| Dermatomyositis | 710.3 |
| Diabetes mellitus type 1 | 250.01, 250.03 |
| Goodpasture’s syndrome | 446.21 |
| Graves’ disease | 242 |
| Guillain-barre syndrome | 357 |
| Hashimoto’s disease | 245.2 |
| Idiopathic thrombycytopenia purpura (ITP) | 287.31 |
| Inflammatory bowel disease | 555, 556 |
| Inflammatory myopathy | 359 |
| Lupus erythematosus | 695.4 |
| Multiple sclerosis | 340 |
| Myasthenia gravis | 358, 258.01 |
| Pemphigus | 694.4, 694.5, 694.6 |
| Pernicious anemia | 281 |
| Polymyalgia | 725 |
| Ploymyositis | 710.4 |
| Primary biliary cirrhosis | 571.6 |
| Psoriasis | 696.1 |
| Psoriatic arthritis | 696 |
| Reactive arthritis | 99.3 |
| Sjögren’s syndrome | 410.2 |
| Systemic lupus erythematosus | 710 |
| Temporal arteritis | 446.5 |
| Vasculitis | 362.18 |
| Wegener’s granulomatosis | 446.4 |
Patients with the above ICD-9-CM codes recorded in the database at any time during the study observation period were excluded from the study.
Footnotes
AUTHORS’ CONTRIBUTIONS
JRC participated in the design of the study and provided critical review of the study results and manuscript. AJ conceived the study, participated in its design and coordination, and provided interpretation of the study results. OB participated in the design of the study, performed the statistical analysis, and provided interpretation of the results. All authors read and approved the final manuscript.
REFERENCES
- 1.Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, Gabriel SE. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52(2):402–411. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 2.Nicola PJ, Maradit-Kremers H, Roger VL, Jacobsen SJ, Crowson CS, Ballman KV, Gabriel SE. The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Arthritis Rheum. 2005;52(2):412–420. doi: 10.1002/art.20855. [DOI] [PubMed] [Google Scholar]
- 3.Full LE, Ruisanchez C, Monaco C. The inextricable link between atherosclerosis and prototypical inflammatory diseases rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res Ther. 2009;11(2):217. doi: 10.1186/ar2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabriel SE. Heart disease and rheumatoid arthritis: understanding the risks. Ann Rheum Dis. 2010;69(Suppl 1):i61–64. doi: 10.1136/ard.2009.119404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turesson C, Jacobsson LT, Matteson EL. Cardiovascular co-morbidity in rheumatic diseases. Vasc Health Risk Manag. 2008;4(3):605–614. doi: 10.2147/vhrm.s2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters MJ, Symmons DP, McCarey D, Dijkmans BA, Nicola P, Kvien TK, McInnes IB, Haentzschel H, Gonzalez-Gay MA, Provan S, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69(2):325–331. doi: 10.1136/ard.2009.113696. [DOI] [PubMed] [Google Scholar]
- 7.Park YB, Lee SK, Lee WK, Suh CH, Lee CW, Lee CH, Song CH, Lee J. Lipid profiles in untreated patients with rheumatoid arthritis. J Rheumatol. 1999;26(8):1701–1704. [PubMed] [Google Scholar]
- 8.Boers M, Nurmohamed MT, Doelman CJ, Lard LR, Verhoeven AC, Voskuyl AE, Huizinga TW, van de Stadt RJ, Dijkmans BA, van der Linden S. Influence of glucocorticoids and disease activity on total and high density lipoprotein cholesterol in patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62(9):842–845. doi: 10.1136/ard.62.9.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgiadis AN, Papavasiliou EC, Lourida ES, Alamanos Y, Kostara C, Tselepis AD, Drosos AA. Atherogenic lipid profile is a feature characteristic of patients with early rheumatoid arthritis: effect of early treatment--a prospective, controlled study. Arthritis Res Ther. 2006;8(3):R82. doi: 10.1186/ar1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choy E, Sattar N. Interpreting lipid levels in the context of high-grade inflammatory states with a focus on rheumatoid arthritis: a challenge to conventional cardiovascular risk actions. Ann Rheum Dis. 2009;68(4):460–469. doi: 10.1136/ard.2008.101964. [DOI] [PubMed] [Google Scholar]
- 11.Myasoedova E, Crowson CS, Kremers HM, Roger VL, Fitz-Gibbon PD, Therneau TM, Gabriel SE. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70(3):482–487. doi: 10.1136/ard.2010.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Halm VP, Nielen MM, Nurmohamed MT, van Schaardenburg D, Reesink HW, Voskuyl AE, Twisk JW, van de Stadt RJ, de Koning MH, Habibuw MR, et al. Lipids and inflammation: serial measurements of the lipid profile of blood donors who later developed rheumatoid arthritis. Ann Rheum Dis. 2007;66(2):184–188. doi: 10.1136/ard.2006.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Micco P, Ferrazzi P, Libre L, Mendolicchio L, Quaglia I, De Marco M, Colombo A, Bacci M, Rota LL, Lodigiani C. Intima-media thickness evolution after treatment with infliximab in patients with rheumatoid arthritis. Int J Gen Med. 2009;2:141–144. doi: 10.2147/ijgm.s5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi HK, Hernan MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359(9313):1173–1177. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- 15.Del Porto F, Lagana B, Lai S, Nofroni I, Tinti F, Vitale M, Podesta E, Mitterhofer AP, D’ Amelio R. Response to anti-tumour necrosis factor alpha blockade is associated with reduction of carotid intima-media thickness in patients with active rheumatoid arthritis. Rheumatology (Oxford) 2007;46(7):1111–1115. doi: 10.1093/rheumatology/kem089. [DOI] [PubMed] [Google Scholar]
- 16.Naranjo A, Sokka T, Descalzo MA, Calvo-Alen J, Horslev-Petersen K, Luukkainen RK, Combe B, Burmester GR, Devlin J, Ferraccioli G, et al. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther. 2008;10(2):R30. doi: 10.1186/ar2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wijbrandts CA, van Leuven SI, Boom HD, Gerlag DM, Stroes EG, Kastelein JJ, Tak PP. Sustained changes in lipid profile and macrophage migration inhibitory factor levels after anti-tumour necrosis factor therapy in rheumatoid arthritis. Ann Rheum Dis. 2009;68(8):1316–1321. doi: 10.1136/ard.2007.086728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dixon WG, Watson KD, Lunt M, Hyrich KL, Silman AJ, Symmons DP. Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti-tumor necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2007;56(9):2905–2912. doi: 10.1002/art.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Listing J, Strangfeld A, Kekow J, Schneider M, Kapelle A, Wassenberg S, Zink A. Does tumor necrosis factor alpha inhibition promote or prevent heart failure in patients with rheumatoid arthritis? Arthritis Rheum. 2008;58(3):667–677. doi: 10.1002/art.23281. [DOI] [PubMed] [Google Scholar]
- 20.Reiss AB, Carsons SE, Anwar K, Rao S, Edelman SD, Zhang H, Fernandez P, Cronstein BN, Chan ES. Atheroprotective effects of methotrexate on reverse cholesterol transport proteins and foam cell transformation in human THP-1 monocyte/macrophages. Arthritis Rheum. 2008;58(12):3675–3683. doi: 10.1002/art.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollono EN, Lopez-Olivo MA, Lopez JA, Suarez-Almazor ME. A systematic review of the effect of TNF-alpha antagonists on lipid profiles in patients with rheumatoid arthritis. Clin Rheumatol. 2010;29(9):947–955. doi: 10.1007/s10067-010-1405-7. [DOI] [PubMed] [Google Scholar]
- 22.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 23.Dahlqvist SR, Engstrand S, Berglin E, Johnson O. Conversion towards an atherogenic lipid profile in rheumatoid arthritis patients during long-term infliximab therapy. Scand J Rheumatol. 2006;35(2):107–111. doi: 10.1080/03009740500474578. [DOI] [PubMed] [Google Scholar]
- 24.Seriolo B, Paolino S, Ferrone C, Cutolo M. Effects of etanercept or infliximab treatment on lipid profile and insulin resistance in patients with refractory rheumatoid arthritis. Clin Rheumatol. 2007;26(10):1799–1800. doi: 10.1007/s10067-007-0702-2. [DOI] [PubMed] [Google Scholar]
- 25.Seriolo B, Paoliono S, Ferrone C, Cutolo M, Comments on the original article by Soubrier et al. Effects of anti-tumor necrosis factor therapy on lipid profile in patients with rheumatoid arthritis. Joint Bone Spine. 2009;76(1):117–118. doi: 10.1016/j.jbspin.2008.09.004. author reply 118. [DOI] [PubMed] [Google Scholar]
- 26.Popa C, Netea MG, Radstake T, Van der Meer JW, Stalenhoef AF, van Riel PL, Barerra P. Influence of anti-tumour necrosis factor therapy on cardiovascular risk factors in patients with active rheumatoid arthritis. Ann Rheum Dis. 2005;64(2):303–305. doi: 10.1136/ard.2004.023119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mihara M, Kasutani K, Okazaki M, Nakamura A, Kawai S, Sugimoto M, Matsumoto Y, Ohsugi Y. Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family. Int Immunopharmacol. 2005;5(12):1731–1740. doi: 10.1016/j.intimp.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Genovese M, Smolen J, Emery P, Jones G, Lee J, Alecock E, Kremer J. Lipid and inflammatory biomarker profiles in patients receiving tocilizumab for rheumatoid arthritis: analysis of five phase 3 clinical trials. Arthritis & Rheumatism. 2008;58(9):S531–S532. [Google Scholar]
- 29.Maini RN, Taylor PC, Szechinski J, Pavelka K, Broll J, Balint G, Emery P, Raemen F, Petersen J, Smolen J, et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006;54(9):2817–2829. doi: 10.1002/art.22033. [DOI] [PubMed] [Google Scholar]
- 30.Nishimoto N, Hashimoto J, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Murata N, van der Heijde D, Kishimoto T. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007;66(9):1162–1167. doi: 10.1136/ard.2006.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Vollenhoven R, Siri D, Furie R, Krasnow J, Alecock E, Alten R. Long term safety and tolerability of tocilizumab treatment in patients with rheumatoid arthritis and a mean treatment duration of 2.4 year. Arthritis Rheum. 2009;60(Suppl 10):1955. [Google Scholar]
- 32.van Vollenhoven R, Scali J, Curtis J, Krasnow J, Vernon E, Alten R. Safety of tocilizumab in patients with rheumatoid arthritis: anyalysis of median of 2.6 years of treatment in long-tern extension studies. Ann Rheum Dis. 2010;69(Suppl3):544. [Google Scholar]
- 33.Charles-Schoeman C, Watanabe J, Lee YY, Furst DE, Amjadi S, Elashoff D, Park G, McMahon M, Paulus HE, Fogelman AM, et al. Abnormal function of high-density lipoprotein is associated with poor disease control and an altered protein cargo in rheumatoid arthritis. Arthritis Rheum. 2009;60(10):2870–2879. doi: 10.1002/art.24802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hahn BH, Grossman J, Ansell BJ, Skaggs BJ, McMahon M. Altered lipoprotein metabolism in chronic inflammatory states: proinflammatory high-density lipoprotein and accelerated atherosclerosis in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res Ther. 2008;10(4):213. doi: 10.1186/ar2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dessein PH, Stanwix AE, Joffe BI. Cardiovascular risk in rheumatoid arthritis versus osteoarthritis: acute phase response related decreased insulin sensitivity and high-density lipoprotein cholesterol as well as clustering of metabolic syndrome features in rheumatoid arthritis. Arthritis Res. 2002;4(5):R5. doi: 10.1186/ar428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ku IA, Imboden JB, Hsue PY, Ganz P. Rheumatoid arthritis: model of systemic inflammation driving atherosclerosis. Circ J. 2009;73(6):977–985. doi: 10.1253/circj.cj-09-0274. [DOI] [PubMed] [Google Scholar]
- 37.Statistical Abstract of the United States: 2000. 120th ed US Bureau of the Census; Washington, DC: 2000. [Google Scholar]
- 38.Gonzalez A, Maradit Kremers H, Crowson CS, Ballman KV, Roger VL, Jacobsen SJ, O’ Fallon WM, Gabriel SE. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis. 2008;67(1):64–69. doi: 10.1136/ard.2006.059980. [DOI] [PubMed] [Google Scholar]
- 39.Avouac J, Allanore Y. Cardiovascular risk in rheumatoid arthritis: effects of anti-TNF drugs. Expert Opin Pharmacother. 2008;9(7):1121–1128. doi: 10.1517/14656566.9.7.1121. [DOI] [PubMed] [Google Scholar]
- 40.Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008;26(5 Suppl 51):S35–61. [PubMed] [Google Scholar]
- 41.Solomon DH, Kremer J, Curtis JR, Hochberg MC, Reed G, Tsao P, Farkouh ME, Setoguchi S, Greenberg JD. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis. 2010;69(11):1920–1925. doi: 10.1136/ard.2009.122226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Popa C, van den Hoogen FH, Radstake TR, Netea MG, Eijsbouts AE, den Heijer M, van der Meer JW, van Riel PL, Stalenhoef AF, Barrera P. Modulation of lipoprotein plasma concentrations during long-term anti-TNF therapy in patients with active rheumatoid arthritis. Ann Rheum Dis. 2007;66(11):1503–1507. doi: 10.1136/ard.2006.066191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiortsis DN, Mavridis AK, Filippatos TD, Vasakos S, Nikas SN, Drosos AA. Effects of infliximab treatment on lipoprotein profile in patients with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 2006;33(5):921–923. [PubMed] [Google Scholar]
- 44.Peters MJ, Vis M, van Halm VP, Wolbink GJ, Voskuyl AE, Lems WF, Dijkmans BA, Twisk JW, de Koning MH, van de Stadt RJ, et al. Changes in lipid profile during infliximab and corticosteroid treatment in rheumatoid arthritis. Ann Rheum Dis. 2007;66(7):958–961. doi: 10.1136/ard.2006.059691. [DOI] [PMC free article] [PubMed] [Google Scholar]