Abstract
Presenilins (PSs) are catalytic components of the γ-secretase proteolytic complexes that produce Aβ and cell signaling peptides. γ-Secretase substrates are mostly membrane-bound peptides derived following proteolytic cleavage of the extracellular domain of typeI transmembrane proteins. Recent work reveals that γ-secretase substrate processing is regulated by proteins termed γ-Secretase Substrate Recruiting Factors (γSSRFs) that bridge substrates to γ-secretase complexes. These factors constitute novel targets for pharmacological control of specific γ-secretase products such as Aβ and signaling peptides. PS familial Alzheimer’s disease (FAD) mutants cause a loss of γ-secretase cleavage function at epsilon sites of substrates thus inhibiting production of cell signaling peptides while promoting accumulation of uncleaved toxic substrates. Importantly, γ-secretase inhibitors may cause toxicity in vivo by similar mechanisms. Here we review novel mechanisms that control γ-secretase substrate selection and cleavage and examine their relevance to AD.
Keywords: Alzheimer’s disease, Presenilin, Familial AD mutations, γ-Secretase Substrate Recruiting Factors (γSSRFs), metalloproteinases, ADAMs, toxic substrates, γ-Secretase-produced signaling peptides
1. Introduction
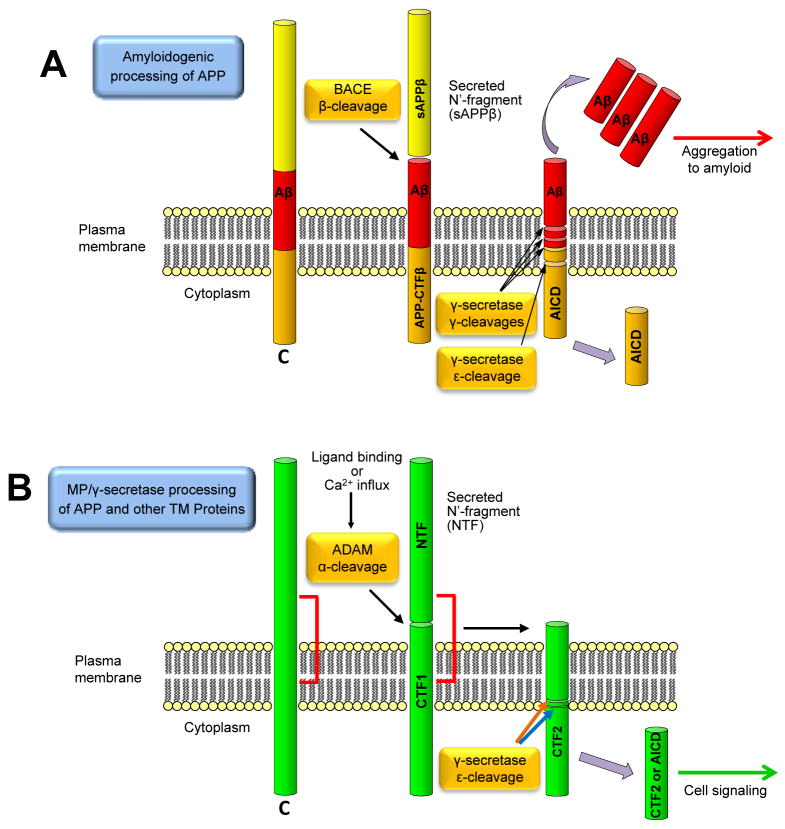
γ-Secretase, a proteolytic complex that contains presenilin (PS) at its catalytic core, processes the Amyloid Precursor Protein (APP) producing Aβ peptides, the structural components of the amyloid fibers found in amyloid plaques (AP) and cerebrovascular amyloidoisis (CVA). Substrates of γ-secretase are mostly membrane-bound polypeptides derived from the cleavage of the extracellular domain of transmembrane proteins usually by members of the ADAM (A Disintegrin And Metalloproteinase) family of metalloproteinases (MPs). γ-Secretase participates in two distinct processing pathways of APP termed non-amyloidogenic and amyloidogenic to denote production of Aβ through the later pathway. Amyloidogenic processing involves cleavage of extracellular APP by β-secretase (Luo et al., 2001) resulting in the production of membrane bound peptide APP-CTFβ, the immediate precursor of Aβ. It is now generally accepted that APP-CTFβ is cleaved by γ-secretase at several γ sites producing soluble Aβ peptides with different C-terminal ends (Fig 1A). Recent data however, raise the intriguing possibility that these peptides are derived from cleavage at the epsilon (ε) site of substrates (see below). Secreted Aβ peptides may then aggregate to form the amyloid depositions found in Alzheimer’s disease (AD), Down’s syndrome and to a fraction of aged non-demented individuals. In the non-amyloidogenic processing, extracellular APP is cleaved by ADAMs within the Aβ sequence, also called α-secretase processing (Anderson et al., 1991; Buxbaum et al., 1998), thus inhibiting production of Aβ (Fig. 1B). Recent work revealed a large number of cell surface proteins and receptors, including Notch1, cadherins, and Erb4 that are processed similar to the non-amyloidogenic processing of APP. Proteins are cleaved by ADAMs to yield membrane-bound peptides termed CTF1s (c-terminal fragments) that are then cleaved by γ-secretase at ε sites close to membrane/cytoplasm interface to produce cytosolic peptides termed CTF2s or APP intracellular domain (AICD) for the APP CTF2 (Fig 1B). AICD can also be produced via the amyloidogenic cleavage of APP (Fig 1A). CTF2 peptides have been shown to function in signal transduction and gene expression indicating that processing of cell surface proteins along the MP/γ-secretase pathways result in the production of peptides with important cellular functions (Robakis, 2003).
Figure 1. Amyloidogenic and ADAM/γ-secretase signaling processing of APP and other substrates.
(A) Extracellular APP is cleaved by β-secretase (BACE) producing soluble N-terminal fragments (sAPPβ) shed to the extracellular space. It is currently believed that the remaining membrane-bound C-terminal fragment (APP-CTFβ) is then cleaved by γ-secretase at several γ sites to produce Aβ peptides with variable c-terminal ends, Aβ 40 and 42 being the most abundant. Cleavage of APP-CTFβ by γ-secretase at the ε site produces AICD released to the cytosol. The temporal relationship of the γ-secretase cleavages however is unclear and available data are also consistent with the suggestion that γ-secretase cleaves only at the ε site of APP-CTFβ releasing AICD (see text). The carboxy terminus of the remaining membrane peptide is then “trimmed” by carboxypeptidases producing Aβ peptides of variable lengths (not shown here). Produced soluble Aβ peptides may then aggregate to form amyloid depositions. (B) Cleavage of APP and other transmembrane proteins by ADAMs near the extracellular face of the plasma membrane, produces a shed ectodomain (N-terminal fragment; NTF) and a membrane bound peptide termed CTF1 (C-Terminal Fragment 1) that is then processed by γ-secretase at the ε site to produce CTF2 peptides (C-terminal Fragment 2) released to the cytosol. CTF2 peptides have been shown to function in cellular signaling (green arrow, see also text). This processing pathway may be stimulated by calcium influx through NMDA receptor and/or by ligand binding to receptors (Fig. 6). Brown arrow indicates an APP-like ε-cleavage that occurs two or three residues inside the membrane while the blue arrow indicates a cadherin-like cleavage that occurs on the membrane/cytoplasm interface (see also text). The red bracket indicates the position of Aβ sequence of APP cleaved in the non-amyloidogenic (α-secretase) processing pathway
Molecular mechanisms of the extracellular and γ-secretase cleavages of proteins are now emerging, indicating that activity and substrate selectivity of these proteolytic events are regulated at multiple levels including ligand-receptor binding, calcium influx, NMDA receptor activation, substrate recruitment, and enzyme trafficking. Recent efforts to reduce Aβ involve inhibition of γ-secretase by pharmacological agents such as γ-secretase inhibitors (GSI). In addition to decreasing Aβ however, GSIs inhibit the ε-cleavage of many proteins including Notch1, cadherins and EphB2 thus inhibiting production of functionally important CTF2 peptides, an outcome with potentially toxic consequences. Here we review mechanisms that regulate γ-secretase substrate selectivity and cleavage and examine how these mechanisms may be used to control production of Aβ and other products of γ-secretase.
2. Core components and binding partners of the γ-secretase enzymatic complex
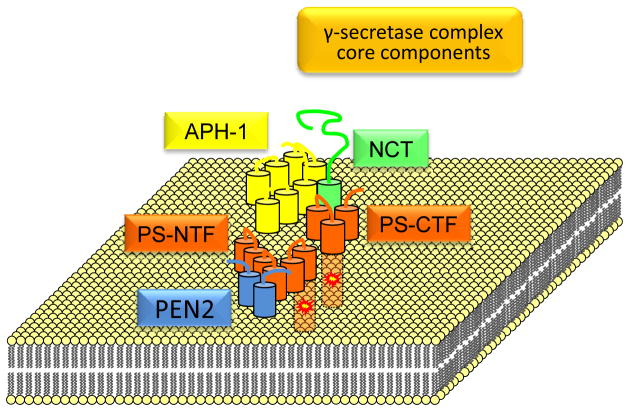
γ-Secretase is a multi-subunit proteolytic enzyme that contains a functional core of at least four proteins including presenilin (PS), anterior pharynx-defective 1 (APH-1), nicastrin (NCT) and presenilin enhancer 2 (PEN-2) (Fig. 2). Together, the four subunits of γ-secretase comprise 19 transmembrane domains (TMs) predicting a complicated tertiary protein complex. PS, the catalytic component of the complex has two homologs, PS1 and PS2. Both PSs are cleaved during maturation producing heterodimeric complexes consisting of an N-terminal and a C-terminal fragment termed PS-NTF and PS-CTF respectively (Fig. 2). APH-1 and NCT form a sub-complex that binds the PS-CTF fragment of the PS heterodimer while PEN-2 binds the PS-NTF fragment. This arrangement places PS-CTF at the center of the proteolytic complex (Barthet et al., 2011; LaVoie et al., 2003; Shirotani et al., 2004b; Steiner et al., 2008). γ-Secretase is an aspartyl protease (Wolfe et al., 1999) with its catalytic site forming at the interface of TM domain 6 of PS-NTF and TM domain 7 of PS-CTF each contributing one catalytic aspartate (Fig. 2). Of the remaining three partners, PEN-2 is crucial for the endoproteolysis of full length PS into PS-NTF and PS-CTF (Ahn et al., 2010; Prokop et al., 2004), while NCT and APH-1 are thought to be important for PS stabilization and trafficking (LaVoie et al., 2003; Zhang et al., 2005). In addition, it has been proposed that NCT participates in the recruitment of Notch1 and APP substrates to γ-secretase (Chen et al., 2001; Dries et al., 2009) but this function has been challenged (Chavez-Gutierrez et al., 2008; Futai et al., 2009; Shirotani et al., 2004a; Zhao et al., 2010) and recent reports indicate that APH-1 may play that role (Chen et al., 2010). APH-1 has also been proposed to participate in the catalytic functions of γ-secretase via its conserved histidine residues in TM sequences 5 and 6 (Pardossi-Piquard et al., 2009b; Pei et al., 2011; Serneels et al., 2009).
Figure 2. Structure of core the γ-secretase proteolytic complex.
The core γ-secretase complex is composed of PS (either PS1 or PS2), anterior pharynx-defective 1 (APH-1), nicastrin (NCT) and presenilin enhancer 2 (PEN-2). The latter protein has been reported to stimulate the endoproteolysis of full-length PS zymogen into catalytically active fragments PS-NTF and PS-CTF. The two catalytic aspartates on TMs VI and VII of PS are shown as yellow-red sparks.
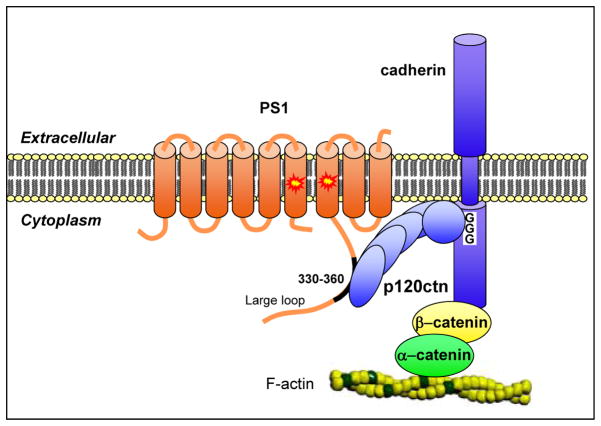
In addition to the four subunits that make up the catalytic core of γ-secretase, more than 50 other proteins have been shown to associate with the γ-secretase complex, consistent with the detection of PS-containing high molecular weight protein complexes (Chen and Schubert, 2002; Georgakopoulos et al., 1999; Kiss et al., 2008; McCarthy et al., 2009; Verdile et al., 2007). Many of these proteins bind to core enzyme components and may function to regulate substrate recruitment or subcellular localization of γ-secretase complex components. For example, catenin protein p120 (p120ctn) that binds the juxtamembrane sequence of cytoplasmic cadherins also binds PS1-CTF (Kouchi et al., 2009) bridging γ-secretase with the cadherin/catenin adherens junctions and forming supercomplexes of more than 1 mega Dalton (Kiss et al., 2008) (Fig. 3). Below we discuss recent work that illuminates mechanisms by which γ-secretase partners regulate substrate selection and processing.
Figure 3. γSSRF p120ctn recruits cadherins to γ-secretase by binding PS1-CTF.
Catenin protein p120 (p120ctn) known to bind the juxtamembrane region of cadherins (glycine repeat GGG) also binds to the 330–360 amino acid sequence of γ-secretase component PS1-CTF thus recruiting cadherins to γ-secretase complexes for processing (Kouchi et al., 2009). For better clarity, the other core components of γ-secretase are not shown. The cadherin/catenin junction complex binds the actin cytoskeleton via α-catenin. Thus the PS/γ-secretase complex may affect functions of the cellular cytoskeleton (see also Marambaud et al., 2002).
3. γ-secretase substrate recruiting factors (γSSRF)
Efforts to treat AD by inhibiting amyloid formation with GSIs have been associated with cellular toxicity (Cummings, 2010), probably because in addition to inhibiting Aβ, GSIs affect the γ-secretase processing of many substrates, reducing production of peptides with useful biological functions and promoting accumulation of toxic precursors (see also below). Thus, new strategies aim at developing agents that will selectively inhibit γ-secretase cleavage of APP-CTFβ, the precursor of Aβ, without affecting other substrates. These efforts received a boost by recent discoveries that cellular factors control the γ-secretase cleavage of specific substrates by binding and recruiting them to γ-secretase for processing.
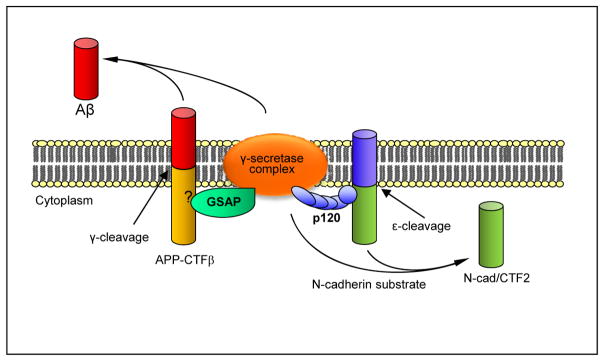
Protein p120ctn is a first example of a factor that binds both, cadherins and γ-secretase core component PS1-CTF thus recruiting cadherin substrates to γ-secretase for processing (Kouchi et al., 2009). This observation suggested the existence of cellular mechanisms that select and recruit substrates to γ-secretase. A key role in these mechanisms is played by specific proteins termed γ-secretase-substrate recruiting factors (γSSRFs) that, similar to p120ctn, bind both, a substrate and a γ-secretase core component thus linking substrates to the γ-secretase proteolytic complex (Fig. 3). These factors constitute new targets for the regulation of γ-secretase processing of substrates and may be used therapeutically to inhibit specific products of γ-secretase as inhibition of binding between a γSSRF and either γ-secretase or its substrate will decrease substrate processing. GSAP (γ-secretase activating protein) is a recent example of a γSSRF that binds APP-CTFs recruiting them to γ-secretase for processing. As a result, GSAP has been proposed as a target for the specific inhibition of Aβ (He et al., 2010). Presently, the core component of γ-secretase that binds GSAP is not known and it is thus unclear whether GSAP binds directly or indirectly to γ-secretase. Similarly, the APP sequence that binds to GSAP has not been determined. In vitro studies however, show that although GSAP stimulates production of Aβ, it reduces AICD, the product of γ-secretase cleavage of APP at the ε site (He et al., 2010). The source of this discrepancy is unclear but suggests the unexpected possibility that GSAP may have differential effects on the production of Aβ peptides and AICD. Protein X11/Mint has also been shown to bind both APP and γ-secretase core component PS1-CTF (Lau et al., 2000) but its role as γSSRF is unclear as there is no evidence that X11/Mint recruits APP to γ-secretase and the role of X11/Mint in Aβ production is in dispute (see also below) (Borg et al., 1998; Sano et al., 2006).
Formation of stable complexes between enzyme and substrate is unfavorable for processing and catalysis is more efficient under conditions of transient interactions between enzyme and substrate. Paradoxically however, cadherins are both excellent γ-secretase substrates and stable partners of PS1 as indicated by the detection of abundant cadherin/PS1 complexes (Georgakopoulos et al., 1999; Serban et al., 2005). The discovery that p120ctn bridges cadherins to PS1-CTF (Kouchi et al., 2009) resolves this paradox as it suggests a model according to which cadherins are linked to PS/γ-secretase via p120ctn. During catalysis, cadherins may move to the catalytic site of the PS/γ-secretase enzyme where they undergo proteolysis. Presently, it is unclear what stimulates cadherin movement to the catalytic site of γ-secretase. It is possible this movement is triggered by the ADAM 10 cleavage of extracellular cadherins although this model needs experimental testing. Thus, formation of a tripartite complex between γ-secretase, p120ctn and cadherins (Fig. 3) at the plasma membrane (Georgakopoulos et al., 1999) may increase the efficiency of the coupled processing of cadherins first by an ADAM at the ectodomain followed by γ-secretase cleavage at the ε site of fragment N-Cad/CTF1 produced from the ADAM cleavage. Importantly, δ-catenin, another member of the p120ctn family of proteins, is present at the synapse where it binds PS1 tethering it to post synaptic densities (PSD) and positioning γ-secretase closer to synaptic cadherin substrates (Restituito et al., 2011; Zhou et al., 1997). It is an interesting possibility that the γSSRF GSAP may play analogous roles in the processing of synaptic APP. Other studies however, reported that substrates bind directly to γ-secretase core components although reports have been contradictory. Accordingly, Shah et al. (Shah et al., 2005), concluded that NCT functions as a γ-secretase substrate receptor recruiting APP and Notch1 to the enzyme. In contrast, Kornilova et al. (Kornilova et al., 2005), reported that APP substrates bind initially to presenilin and Zhao et al. (Zhao et al., 2010), reported that γ-secretase complexes lacking NCT are able to cleave both APP and Notch. The contradictory literature may reflect the heretofore unknown role of γ-SSRFs in recruiting substrates to γ-secretase complexes. Thus, the model that cellular γ-SSRFs are used to recruit substrates to γ-secretase predicts that a specific γ-SSRF recruits Notch1 to γ-secretase for processing. Identification of this putative factor will facilitate efforts to inhibit production of Aβ without affecting processing of Notch1.
4. Distinct γ-secretase complexes process specific substrates
Although PS1 and its homolog PS2 have similar catalytic activities, no PS1-PS2 hybrids have been found indicating that cells contain distinct γ-secretase complexes incorporating either PS1- or PS2 fragments. It is unclear however, what determines whether a specific substrate is processed by PS1- or PS2-containing γ-secretase. Thus, by interacting with specific substrates and either PS1 or PS2, γSSRFs may determine not only which protein will be processed by γ-secretase but also whether it will be processed by a PS1- or PS2-containing enzyme or both. For example, p120ctn binds amino acids 330 to 360 of PS1-CTF, a sequence not present in PS2 (Kouchi et al., 2009). As a result, cadherins are processed only by PS1-containing γ-secretase complexes. In contrast, others substrates like Notch1 (Steiner et al., 1999) and APP are processed by both, PS1- and PS2-containing γ-secretase complexes. This observation predicts GSAP that recruits APP binds either to a sequence common to both PS1 and PS2 or to another core component common to all γ-secretase complexes. Answer to this important question will also indicate additional methodologies to specifically decrease the γ-secretase processing of APP and production of Aβ.
The above observations suggest that γSSRFs rather than the substrates themselves compete for specific populations of γ-secretase complexes. Indeed, overexpression of p120ctn inhibits APP processing and Aβ production (Kouchi et al., 2009) presumably by competing with GSAP for PS1-containing γ-secretase complexes that process both cadherins and APP. This model also predicts that increased expression of GSAP will inhibit cadherin processing. On the other hand, under physiological conditions where there is no p120ctn overexpression, N-cadherin and APP do not compete for γ-secretase complexes as processing of N-cadherin is not increased in the absence of APP (Barthet et al., 2011). This outcome is consistent with a mechanism where substrates do not compete directly for catalysis but instead the rate limiting step of these cleavage events is the binding of γSSRF to both enzyme and substrate (Fig. 4). Interestingly, different cell types may use predominantly PS1- or PS2- containing γ-secretase complexes. Accordingly, in microglia most γ-secretase complexes contain PS2 (Jayadev et al., 2010) while in fibroblasts only a minor fraction of complexes contain PS2 (Franberg et al., 2011). Whether any pathological conditions, such as AD, are associated with modifications or expression abnormalities of γSSRFs remains to be investigated. Finally, it is worth mentioning that protein adaptors are widespread among proteolytic systems. Thus, in addition to γ-secretase, several other complex proteolytic systems, including the AAA+ proteases, use enzyme-substrate binding proteins for substrate recruitment (Sauer and Baker, 2011).
Figure 4. γSSRFs compete for γ-secretase complexes.
γSSRFs p120ctn and GSAP bound to cadherins or APP respectively, compete for limited amounts of γ-secretase complexes promoting processing of their respective substrates and production of Cad-CTF2 or Aβ. Thus, overexpression of p120ctn inhibits γ-secretase processing of APP substrates and production of Aβ.
5. Effects of protein trafficking on γ-secretase substrate processing
Enzymatic reactions occur when substrates and enzymes co-localize in subcellular compartments. Thus, factors that regulate trafficking of APP and its processing enzymes are intensely investigated as their manipulation may reveal ways to control production of Aβ. A fraction of APP is cleaved intracellularly in the trans-Golgi netwok and post-Golgi vesicles as it travels to the cell surface (Sambamurti et al., 1992). It is currently thought that a portion of APP that reaches the cell surface is then internalized and migrates to late endosomes that contain high amounts of β-secretase, a protease that promotes the amyloidogenic processing of APP and production of Aβ. Thus, increased APP internalization may lead to increased production of intracellular Aβ peptides at sites where they may cause their deleterious effects. Factors like SorLA and SNX17 may regulate this pathway (Andersen et al., 2005; Lee et al., 2008). In contrast, it has been reported that X11/Mint suppresses Aβ by promoting cell surface expression of APP, a localization rich in α-secretase that promotes non-amyloidogenic processing (Rogelj et al., 2006). This role of X11/Mint however, needs further examination as others reported that this protein increases Aβ in a transgenic mouse model that overexpresses APP (Ho et al., 2008). Substrate cleavage is also controlled by the subcellular localization of γ-secretase components, a process regulated at multiple levels. Thus, glycosylated NCT may promote cell surface localization of PS (Yang et al., 2002) and APP has also been reported to regulate trafficking of PS1 to the cell surface, a process regulated by protein trafficking factor phospholipase D1 (PLD1) (Liu et al., 2009). Calsenilin, a Ca2+-binding protein, has been reported to stimulate APP processing and Aβ (Lilliehook et al., 2003). In contrast to its effects on APP however, calsenilin inhibits the ε-cleavage of N-cadherins. The opposite effects of calsenilin on the processing of these two substrates may be related to the inhibitory effects of calsenilin on the trafficking of PS1-CTF and APH-1 to cell surface (Jang et al., 2011). By retaining γ-secretase components in internal compartments such as endosomes, calsenilin may promote the amyloidogenic processing of APP and limit cadherin processing that occurs mainly at the cell surface.
Recently, G-protein-coupled receptors (GPCRs) emerged as key regulators of γ-secretase cleavages. Evidence suggests these receptors promote formation of complexes specific to the amyloidogenic or non-amyloidogenic processing of APP (Teng et al., 2010). Thus, β2-adrenergic (AR) and δ-opioid receptors increase Aβ in vitro by forming complexes with β- and γ-secretases. This process involves the association of β2-AR with PS and requires agonist-induced endocytosis and trafficking of the β2-AR/PS complex to late endosomes/lysosomes that promote amyloidogenic processing of APP. Similar effects are observed with δ-opioid receptor (Ni et al., 2006; Teng et al., 2010). Overexpression of the constitutively active GPR3 receptor however, increases Aβ and AICD via unknown mechanisms (Thathiah et al., 2009). Involvement of this receptor in APP processing is also supported by a robust reduction in Aβ upon inhibition of GPR3 expression suggesting that inhibition of GPR3 by inverse agonists may reduce Aβ. Finally, it is important to note that some factors regulate γ-secretase activity by interacting with enzymatic complex components in specific compartments. For example, TMP21, a member of the p24 protein family, is expressed in intracellular compartments where it binds PS and decreases Aβ by blocking the amyloidogenic processing of APP-CTFβ which takes place in these compartments. Since TMP21 is not expressed at cell surface, it does not affect cleavage of substrates that are mainly processed at the plasma membrane such as Notch and cadherins (Chen et al., 2006; Pardossi-Piquard et al., 2009a). For a more extensive review of factors affecting intracellular trafficking of APP and γ-secretase components please see (Thinakaran and Koo, 2008).
6. Inhibitors and modulators of γ-secretase
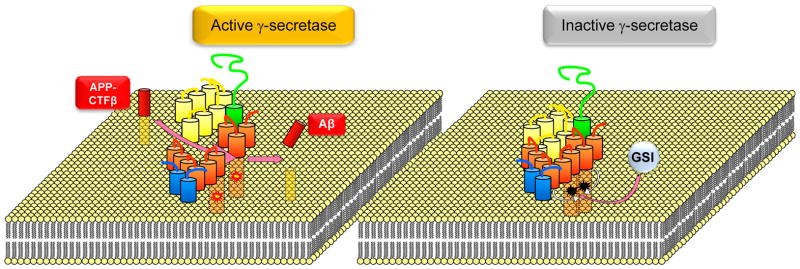
Inhibitors of γ-secretase activity have been used to block production of Aβ peptides. The first potent GSI was a peptide aldehyde (Higaki et al., 1995) followed by construction of difluoro-ketone peptidomimetic inhibitors based on the sequence of the APP transmembrane domain (Wolfe et al., 1998). These early attempts were followed by a second generation of γ-secretase inhibitors including the non-transition and transition state analogs DAPT and L-685,458 respectively. Both are widely used in research as they inhibit processing of almost all known substrates. Their use in vivo however has been associated with toxic effects, probably due to the inhibition of the ε-cleavage which is important to cell signaling (see below). Interestingly, all known GSIs, including transition state analogs that bind the catalytic site, are non-competitive inhibitors suggesting that substrates bind at sites independent of the catalytic cleft (Esler et al., 2002; Tian et al., 2003; Tian et al., 2002). It has been reported that instead of preventing substrate binding, these inhibitors strengthen interactions between PS-CTF, the central component of the γ-secretase complex, and its partners the APH-1/NCT and PS-NTF/PEN-2 sub-complexes thus stabilizing the γ-secretase complex and inhibiting its proteolytic activity (Fig. 5). Indeed, this stabilization effect correlates with inhibition of substrate processing including inhibition of N-cadherin cleavage (Barthet et al., 2011). Unfortunately, these GSIs block APP γ-secretase processing at concentrations higher than those required for the inhibition of other substrates such as ephrinB and cadherin (Barthet et al., 2011), indicating that at useful concentrations, GSIs will inhibit processing of important cellular substrates before Aβ is inhibited. This observation may explain reported clinical side effects in patients treated with GSIs (Cummings, 2010) and indicates that different pharmacological strategies may be required to decrease cellular Aβ without causing toxicity.
Figure 5. Mechanism of γ-secretase inhibition.
Left: γ-secretase catalyzes proteolysis of transmembrane substrates including APP-CTFβ shown here. Processing of this substrate at the active site of the enzyme which includes two aspartate residues shown as yellow-red sparks (one on PS-NTF and the other on PS-CTF) generates AICD when substrate is cleaved at the ε site and Aβ when cleaved at γ sites. Right: γ-secretase is inhibited by GSIs that bind the active site and enhance interactions between γ-secretase subunits thus locking the enzyme in a closed conformation, characteristic of inhibited enzymes (inactivated aspartates are shown in black) (Barthet et al., 2011).
More recent therapeutic efforts have been concentrating on the development of selective allosteric γ-secretase modulators (GSMs) that modify Aβ peptides without affecting the ε-cleavage of other substrates. One strategy aims at increasing the fraction of less amyloidogenic peptides Aβ40 and Aβ38 at the expense of the more amyloidogenic peptide Aβ42. To this end, efforts concentrate on the development of pharmacological agents that stimulate a “trimming” exopeptidase activity able to remove carboxyl-terminal amino acid residues from Aβ42 without affecting γ-secretase cleavage at the ε-site (Kounnas et al., 2010). Interestingly, such agents tend to bind PS-NTF (Ebke et al., 2011; Ohki et al., 2011) or PEN-2 instead of PS-CTF (Kounnas et al., 2010) where most GSI act (Morohashi et al., 2006). These observations implicate the PS-NTF/PEN-2 heterodimer in the Aβ carboxyl-terminal trimming activity associated with γ-secretase, a theory that needs further experimental testing. Additional evidence suggests that Aβ peptides may be derived from a unique γ-secretase cleavage at the ε site of APP-CTFβ. This cleavage produces a “long” Aβ peptide comprising the 49 amino-terminal residues of APP-CTFβ. This long Aβ is then “trimmed” by a γ-secretase-associated carboxypeptidase activity (see above) that removes carboxyl-terminal amino acids producing shorter Aβ peptides including those of 38, 40, and 42 residues commonly found in brain amyloid depositions (Funamoto et al., 2004). This mechanism of Aβ production is further supported by the detection in human samples and cell lines of almost all Aβ peptides between 38 and 49 residues long predicted by this hypothesis (Funamoto et al., 2004; Miller et al., 1993; Qi-Takahara et al., 2005). These data support the hypothesis that γ-secretase may cleave its APP-CTF substrates only at the biologically relevant ε site, a suggestion supported by absence of any evidence for the production of an AICD (APP-CTF2) peptide expected to be derived from the predicted γ-secretase cleavage at γ sites.
Another approach for inhibiting Aβ without affecting processing of other substrates is to develop agents that target proteins acting as γSSRFs for APP. An example is Gleevec which binds GSAP and prevents recruitment of APP-CTFs to γ-secretase thus decreasing production of Aβ (He et al., 2010). This approach may be used to specifically limit γ-secretase processing of APP and production of Aβ peptides in both familial and sporadic AD. Finally, APH-1 represents another potential target for Aβ inhibition as evidence suggests that this protein regulates the γ-secretase activity through histidine residues located at APH-1 TM domains 5 and 6 (Pardossi-Piquard et al., 2009b; Pei et al., 2011). Interestingly, γ-secretase complexes containing APH-1β produce longer, more amyloidogenic Aβ than complexes containing APH-1α (Serneels et al., 2009) suggesting that agents that target APH-1β may reduce production of amyloidogenic peptides.
7. Stimulation of MP/γ-secretase receptor processing by ligands, calcium influx and NMDA receptor agonists
γ-Secretase substrates are mostly membrane-bound peptides produced by the MP processing of the extracellular domain of full length substrates. An important exception is APP which is also cleaved at the extracellular domain by the non-MP β-secretase. The extracellular cleavages of these substrates take place close to the plasma membrane releasing most of the extracellular sequence to the intercellular space. The remaining membrane-bound C-terminal fragments (CTF1s) are then cleaved by γ-secretase at ε sites producing CTF2 peptides which may function in signal transduction and gene expression (Fig. 6). The list includes CTF2 peptides derived from the processing of Notch1 (De Strooper et al., 1999), ErbB4 (Ni et al., 2001), N-cadherin (Marambaud et al., 2003), EphB2 (Xu et al., 2009) and the AICD of APP (Weidemann et al., 2002).
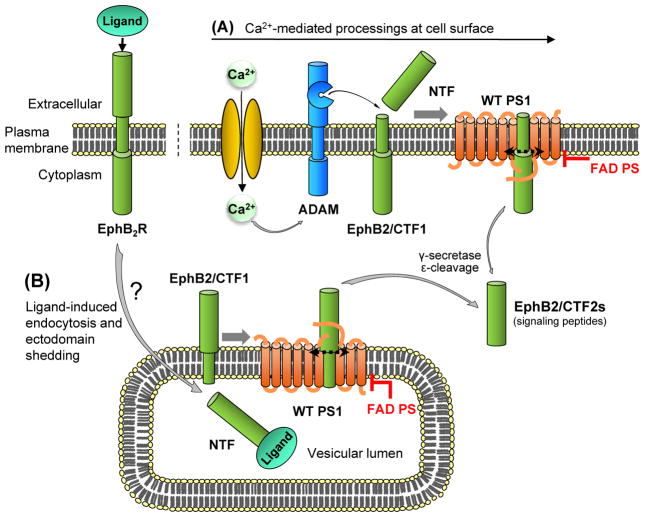
Figure 6. MP/γ-secretase processing of EphB2 receptor can occur at distinct subcellular localizations and is inhibited by FAD mutations.
Calcium influx and NMDA receptor agonists promote cell surface (plasma membrane) processing of EphB2 receptor (A) while binding of ephrinB ligands promote a processing pathway that requires endocytosis and takes place in intracellular compartments (B). Both pathways include γ-secretase cleavage at the ε site of substrates producing biologically active CTF2 peptides that contain the cytoplasmic sequence of substrates (Litterst et al., 2007). Importantly, the ε-cleavage of substrates has been shown to be inhibited by PS FAD mutants reducing the levels of biologically active CTF2 products and increasing the amounts of potentially toxic CTF1 precursors. Questionmark indicates the unknown enzyme that cleaves extracellular EphB2 in the endocytic pathway.
Recent evidence shows that the MP/γ-secretase processing of cell surface receptors is regulated by ligand binding and calcium influx (Georgakopoulos et al., 1999; Litterst et al., 2007; Mumm et al., 2000). In addition, it has been shown that endocytosis is required for ligand-induced MP/γ-secretase processing of EphB2 receptor (Litterst et al., 2007). Endocytosis is followed by cleavage of the extracellular (lumenal) domain of EphB2 while the remaining membrane-bound peptide EphB2-CTF1 is cleaved at the ε-site by γ-secretase releasing fragment EphB2-CTF2 to the cytosol (Fig. 6). In contrast to the ligand-induced processing however, the Ca2+ influx-stimulated processing of EphB2 takes place at the plasma membrane and is independent of endocytosis (Litterst et al., 2007). In addition, there is evidence that the enzyme that cleaves the extracellular sequence of EphB2 in response to calcium influx differs from the enzyme that cleaves the extracellular receptor in response to ligand binding. Thus, depending on stimulation conditions, cleavage of the extracellular domain of a receptor can take place in different subcellular compartments by distinct enzymes. It seems however, that in both pathways, γ-secretase processes the products of the extracellular cleavage (Litterst et al., 2007). Interestingly, EphB2/CTF2, the end product of the MP/γ-secretase processing of EphB2 receptor, promotes phosphorylation and surface expression of NMDAR (Xu et al., 2009), a process that may contribute to the effects of EphB2 on the functions of NMDA receptor (Nolt et al., 2011; Takasu et al., 2002).
Similar to EphB2, processing of E- and N-cadherins along the MP/γ-secretase pathway is also stimulated by calcium influx and NMDAR agonists (Marambaud et al., 2002; Marambaud et al., 2003). Cadherins are first cleaved by ADAM10, a step stimulated by calcium (Reiss et al., 2005), to produce membrane-bound Cad-CTF1 that is then cleaved by γ-secretase. These cleavages dissociate adherens junctions from the cytoskeleton and release α- and β-catenins to the cytosol. Since soluble β-catenin is an important regulator of the Wnt pathway, its release via the MP/γ-secretase processing of cadherins may affect cell signaling (Marambaud et al., 2002). Interestingly, the membrane topology of the ε-cleavage site seems to depend on the exact amino sequence of each substrate. Accordingly, some substrates are cleaved close to the carboxy-terminal end of the transmembrane sequence a few residues upstream from the membrane/cytoplasm interface, while cadherins are cleaved by γ-secretase at the membrane-cytoplasm interface rather than within the membrane (Marambaud et al., 2002).
8. Familial AD (FAD) mutations inhibit the ε-cleavage reducing production of signaling peptides while increasing potentially toxic precursors
Recent work reveals that many PS1 FAD mutants inhibit production of CTF2 peptides indicating these mutants cause a loss of γ-secretase cleavage function at the ε site of substrates such as Notch1, N-cadherin, efnB2, and EphB2 (Georgakopoulos et al., 2006; Litterst et al., 2007; Marambaud et al., 2003; Song et al., 1999). These data support the hypothesis that PS FAD mutations contribute to neurotoxicity by inhibiting production of peptides with useful cellular functions (Fortini, 2003; Marambaud et al., 2003; Robakis, 2011). Furthermore, by reducing the γ-secretase-catalyzed ε-cleavage, PS FAD mutations promote accumulation of membrane-bound CTF1 fragments, the precursors of CTF2 peptides (Litterst et al., 2007; Marambaud et al., 2003). Recent evidence suggests that increased levels of CTF1s, including those derived from APP and netrin, cause cellular toxicity (Bai et al., 2011; Jiang et al., 2010; Lu et al., 2000) and their accumulation may contribute to the neurodegeneration of FAD. Presently, it is unclear why CTF1s are toxic. It is possible that accumulation of high levels of these transmembrane peptides in the membrane (Fig. 6) interferes with the movements of receptors and other factors on the plane of the membrane with toxic consequences. Thus, PS FAD mutations may promote neurotoxicity by both, reducing production of signaling CTF2 peptides and increasing accumulation of toxic CTF1s. Both effects can result from the reduction of γ-secretase cleavage activity at the ε site of substrates suggesting that increasing γ-secretase cleavage activity at ε sites may be of therapeutic interest in PS FAD cases (Marambaud et al., 2003).
Importantly, in addition to reducing Aβ, GSIs inhibit the ε-cleavage of substrates, an effect shown to result in reduced production of CTF2 peptides with a concomitant increase of their precursors CTF1 peptides (Barthet et al., 2011; Georgakopoulos et al., 2006; Litterst et al., 2007; Marambaud et al., 2003). These effects of GSI on the ε-cleavage may explain the toxicity observed in AD patients participating in clinical trials of GSI (Barthet et al., 2011; Cummings, 2010; Sambamurti et al., 2011). Thus, GSIs may act in vivo similar to PS FAD mutations. Both may cause toxicities by a double hit, reducing production of functionally important CTF2 peptides and increasing the levels of toxic CTF1 peptides.
9. Conclusions
γ-Secretase is a proteolytic complex whose activity is regulated by different mechanisms including a) subunit composition b) interaction with modulator proteins c) substrate recruitment by γSSRFs d) subcellular localization of enzyme and its substrates and e) drugs that modulate its activity. A common theory posits that AD is caused by Aβ and its amyloid derivatives. Treatment of patients with GSIs that reduce Aβ however, failed to show therapeutic benefits and patients displayed toxic side effects, thought to result from inhibition of biologically important ε-cleavage of substrates. Thus, recent efforts concentrate on specific modulators of γ-secretase able to inhibit Aβ without affecting other substrates. In this respect, targeting γSSRFs such as GSAP is a promising approach. On the other hand, it has been argued that Aβ and derivatives may not be the main causative agents of AD (Neve and Robakis, 1998; Pimplikar et al., 2010; Robakis, 2003; Shen and Kelleher, 2007). Recent data shows that PS FAD mutants inhibit γ-secretase cleavage at the ε site of substrates supporting the hypothesis that these mutants promote neurodegeneration by reducing functionally important CTF2 peptides while at the same time promoting accumulation of toxic CTF1 precursor peptides. Importantly, GSIs may cause toxicity in vivo by similar mechanisms. Thus, stimulators of the ε-cleavage of substrates may be of therapeutic interest in FAD.
Highlights.
Review explores the concept that γ-secretase cleavage is regulated by γ-Secretase Substrate Recruiting Factors (γSSRFs).
γSSRFs are targets for drugs to inhibit specific γ-secretase products
Review clarifies roles of gamma (γ) and epsilon (ε) γ-secretase cleavages in the production of Aβ and signaling peptides
Roles of epsilon (ε) γ-secretase cleavage in familial Alzheimer disease (FAD) and neurotoxicity of γ-secretase inhibitors
Reviews evidence that presenilin FAD mutations cause loss of γ-secretase cleavage activity and inhibit signaling peptides
Acknowledgments
This work was supported by NIH grants AG-17926, AG-08200, NS 047229 and P50AG05138
Abbreviations
- Aβ
amyloid β peptide
- AD
Alzheimer’s disease
- ADAM
a disintegrin and metalloproteinase
- AICD
APP intracellular domain
- AP
amyloid plaque
- APH-1
anterior pharynx-defective 1
- APP
amyloid precursor protein
- BACE
β-site APP cleaving enzyme
- CTF
C-terminal fragment
- CVA
cerebrovascular amyloidoisis
- DS
Down’s syndrome
- ER
endoplasmic reticulum
- FAD
familial Alzheimer’s disease
- GSI
γ-secretase inhibitor
- GSAP
γ-secretase activating protein
- GSM
γ-secretase modulator
- γSSRF
γ-secretase substrate recruiting factors
- MP
metalloproteinase
- NCT
nicastrin
- NFT
neurofibrillary tangle
- NMDA
N-Methyl-D-aspartate
- NTF
N-terminal fragment
- PEN-2
presenilin enhancer 2
- PS
presenilin
- PSD
post synaptic densities
- sAPP
secreted APP
- TGN
trans-Golgi network
- TM
transmembrane domain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn K, Shelton CC, Tian Y, Zhang X, Gilchrist ML, Sisodia SS, Li YM. Activation and intrinsic {gamma}-secretase activity of presenilin 1. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1013246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, Behlke J, von Arnim CA, Breiderhoff T, Jansen P, Wu X, Bales KR, Cappai R, Masters CL, Gliemann J, Mufson EJ, Hyman BT, Paul SM, Nykjaer A, Willnow TE. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci U S A. 2005;102:13461–13466. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JP, Esch FS, Keim PS, Sambamurti K, Lieberburg I, Robakis NK. Exact cleavage site of Alzheimer amyloid precursor in neuronal PC-12 cells. Neurosci Lett. 1991;128:126–128. doi: 10.1016/0304-3940(91)90775-o. [DOI] [PubMed] [Google Scholar]
- Bai G, Chivatakarn O, Bonanomi D, Lettieri K, Franco L, Xia C, Stein E, Ma L, Lewcock JW, Pfaff SL. Presenilin-dependent receptor processing is required for axon guidance. Cell. 2011;144:106–118. doi: 10.1016/j.cell.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthet G, Shioi J, Shao Z, Ren Y, Georgakopoulos A, Robakis NK. Inhibitors of gamma-secretase stabilize the complex and differentially affect processing of amyloid precursor protein and other substrates. Faseb J. 2011;25:2937–2946. doi: 10.1096/fj.11-183806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg JP, Yang Y, De Taddeo-Borg M, Margolis B, Turner RS. The X11alpha protein slows cellular amyloid precursor protein processing and reduces Abeta40 and Abeta42 secretion. J Biol Chem. 1998;273:14761–14766. doi: 10.1074/jbc.273.24.14761. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Liu KN, Luo Y, Slack JL, Stocking KL, Peschon JJ, Johnson RS, Castner BJ, Cerretti DP, Black RA. Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated alpha-secretase cleavage of the Alzheimer amyloid protein precursor. J Biol Chem. 1998;273:27765–27767. doi: 10.1074/jbc.273.43.27765. [DOI] [PubMed] [Google Scholar]
- Chavez-Gutierrez L, Tolia A, Maes E, Li T, Wong PC, de Strooper B. Glu(332) in the Nicastrin ectodomain is essential for gamma-secretase complex maturation but not for its activity. J Biol Chem. 2008;283:20096–20105. doi: 10.1074/jbc.M803040200. [DOI] [PubMed] [Google Scholar]
- Chen AC, Guo LY, Ostaszewski BL, Selkoe DJ, LaVoie MJ. Aph-1 associates directly with full-length and C-terminal fragments of gamma-secretase substrates. J Biol Chem. 2010;285:11378–11391. doi: 10.1074/jbc.M109.088815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Hasegawa H, Schmitt-Ulms G, Kawarai T, Bohm C, Katayama T, Gu Y, Sanjo N, Glista M, Rogaeva E, Wakutani Y, Pardossi-Piquard R, Ruan X, Tandon A, Checler F, Marambaud P, Hansen K, Westaway D, St George-Hyslop P, Fraser P. TMP21 is a presenilin complex component that modulates gamma-secretase but not epsilon-secretase activity. Nature. 2006;440:1208–1212. doi: 10.1038/nature04667. [DOI] [PubMed] [Google Scholar]
- Chen F, Yu G, Arawaka S, Nishimura M, Kawarai T, Yu H, Tandon A, Supala A, Song YQ, Rogaeva E, Milman P, Sato C, Yu C, Janus C, Lee J, Song L, Zhang L, Fraser PE, St George-Hyslop PH. Nicastrin binds to membrane-tethered Notch. Nat Cell Biol. 2001;3:751–754. doi: 10.1038/35087069. [DOI] [PubMed] [Google Scholar]
- Chen Q, Schubert D. Presenilin-interacting proteins. Expert Rev Mol Med. 2002;4:1–18. doi: 10.1017/S1462399402005008. [DOI] [PubMed] [Google Scholar]
- Cummings J. What can be inferred from the interruption of the semagacestat trial for treatment of Alzheimer’s disease? Biol Psychiatry. 2010;68:876–878. doi: 10.1016/j.biopsych.2010.09.020. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- Dries DR, Shah S, Han YH, Yu C, Yu S, Shearman MS, Yu G. Glu-333 of nicastrin directly participates in gamma-secretase activity. J Biol Chem. 2009;284:29714–29724. doi: 10.1074/jbc.M109.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebke A, Luebbers T, Fukumori A, Shirotani K, Haass C, Baumann K, Steiner H. Novel {gamma}-secretase modulators directly target presenilin. J Biol Chem. 2011 doi: 10.1074/jbc.C111.276972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler WP, Kimberly WT, Ostaszewski BL, Ye W, Diehl TS, Selkoe DJ, Wolfe MS. Activity-dependent isolation of the presenilin- gamma -secretase complex reveals nicastrin and a gamma substrate. Proc Natl Acad Sci U S A. 2002;99:2720–2725. doi: 10.1073/pnas.052436599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortini ME. Neurobiology: double trouble for neurons. Nature. 2003;425:565–566. doi: 10.1038/425565a. [DOI] [PubMed] [Google Scholar]
- Franberg J, Svensson AI, Winblad B, Karlstrom H, Frykman S. Minor contribution of presenilin 2 for gamma-secretase activity in mouse embryonic fibroblasts and adult mouse brain. Biochem Biophys Res Commun. 2011;404:564–568. doi: 10.1016/j.bbrc.2010.12.029. [DOI] [PubMed] [Google Scholar]
- Funamoto S, Morishima-Kawashima M, Tanimura Y, Hirotani N, Saido TC, Ihara Y. Truncated carboxyl-terminal fragments of beta-amyloid precursor protein are processed to amyloid beta-proteins 40 and 42. Biochemistry. 2004;43:13532–13540. doi: 10.1021/bi049399k. [DOI] [PubMed] [Google Scholar]
- Futai E, Yagishita S, Ishiura S. Nicastrin is dispensable for gamma-secretase protease activity in the presence of specific presenilin mutations. J Biol Chem. 2009;284:13013–13022. doi: 10.1074/jbc.M807653200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakopoulos A, Litterst C, Ghersi E, Baki L, Xu C, Serban G, Robakis NK. Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. Embo J. 2006;25:1242–1252. doi: 10.1038/sj.emboj.7601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakopoulos A, Marambaud P, Efthimiopoulos S, Shioi J, Cui W, Li HC, Schutte M, Gordon R, Holstein GR, Martinelli G, Mehta P, Friedrich VL, Jr, Robakis NK. Presenilin-1 forms complexes with the cadherin/catenin cell-cell adhesion system and is recruited to intercellular and synaptic contacts. Mol Cell. 1999;4:893–902. doi: 10.1016/s1097-2765(00)80219-1. [DOI] [PubMed] [Google Scholar]
- He G, Luo W, Li P, Remmers C, Netzer WJ, Hendrick J, Bettayeb K, Flajolet M, Gorelick F, Wennogle LP, Greengard P. Gamma-secretase activating protein is a therapeutic target for Alzheimer’s disease. Nature. 2010;467:95–98. doi: 10.1038/nature09325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki J, Quon D, Zhong Z, Cordell B. Inhibition of beta-amyloid formation identifies proteolytic precursors and subcellular site of catabolism. Neuron. 1995;14:651–659. doi: 10.1016/0896-6273(95)90322-4. [DOI] [PubMed] [Google Scholar]
- Ho A, Liu X, Sudhof TC. Deletion of Mint proteins decreases amyloid production in transgenic mouse models of Alzheimer’s disease. J Neurosci. 2008;28:14392–14400. doi: 10.1523/JNEUROSCI.2481-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang C, Choi JK, Na YJ, Jang B, Wasco W, Buxbaum JD, Kim YS, Choi EK. Faseb J. 2011. Calsenilin regulates presenilin 1/{gamma}-secretase-mediated N-cadherin {varepsilon}-cleavage and {beta}-catenin signaling. [DOI] [PubMed] [Google Scholar]
- Jayadev S, Case A, Eastman AJ, Nguyen H, Pollak J, Wiley JC, Moller T, Morrison RS, Garden GA. Presenilin 2 is the predominant gamma-secretase in microglia and modulates cytokine release. PLoS One. 2010;5:e15743. doi: 10.1371/journal.pone.0015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Mullaney KA, Peterhoff CM, Che S, Schmidt SD, Boyer-Boiteau A, Ginsberg SD, Cataldo AM, Mathews PM, Nixon RA. Alzheimer’s-related endosome dysfunction in Down syndrome is Abeta-independent but requires APP and is reversed by BACE-1 inhibition. Proc Natl Acad Sci U S A. 2010;107:1630–1635. doi: 10.1073/pnas.0908953107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A, Troyanovsky RB, Troyanovsky SM. p120-catenin is a key component of the cadherin-gamma-secretase supercomplex. Mol Biol Cell. 2008;19:4042–4050. doi: 10.1091/mbc.E08-04-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornilova AY, Bihel F, Das C, Wolfe MS. The initial substrate-binding site of gamma-secretase is located on presenilin near the active site. Proc Natl Acad Sci U S A. 2005;102:3230–3235. doi: 10.1073/pnas.0407640102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchi Z, Barthet G, Serban G, Georgakopoulos A, Shioi J, Robakis NK. p120 catenin recruits cadherins to gamma-secretase and inhibits production of Abeta peptide. J Biol Chem. 2009;284:1954–1961. doi: 10.1074/jbc.M806250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounnas MZ, Danks AM, Cheng S, Tyree C, Ackerman E, Zhang X, Ahn K, Nguyen P, Comer D, Mao L, Yu C, Pleynet D, Digregorio PJ, Velicelebi G, Stauderman KA, Comer WT, Mobley WC, Li YM, Sisodia SS, Tanzi RE, Wagner SL. Modulation of gamma-secretase reduces beta-amyloid deposition in a transgenic mouse model of Alzheimer’s disease. Neuron. 2010;67:769–780. doi: 10.1016/j.neuron.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau KF, McLoughlin DM, Standen C, Miller CC. X11 alpha and x11 beta interact with presenilin-1 via their PDZ domains. Mol Cell Neurosci. 2000;16:557–565. doi: 10.1006/mcne.2000.0898. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Fraering PC, Ostaszewski BL, Ye W, Kimberly WT, Wolfe MS, Selkoe DJ. Assembly of the gamma-secretase complex involves early formation of an intermediate subcomplex of Aph-1 and nicastrin. J Biol Chem. 2003;278:37213–37222. doi: 10.1074/jbc.M303941200. [DOI] [PubMed] [Google Scholar]
- Lee J, Retamal C, Cuitino L, Caruano-Yzermans A, Shin JE, van Kerkhof P, Marzolo MP, Bu G. Adaptor protein sorting nexin 17 regulates amyloid precursor protein trafficking and processing in the early endosomes. J Biol Chem. 2008;283:11501–11508. doi: 10.1074/jbc.M800642200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilliehook C, Bozdagi O, Yao J, Gomez-Ramirez M, Zaidi NF, Wasco W, Gandy S, Santucci AC, Haroutunian V, Huntley GW, Buxbaum JD. Altered Abeta formation and long-term potentiation in a calsenilin knock-out. J Neurosci. 2003;23:9097–9106. doi: 10.1523/JNEUROSCI.23-27-09097.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litterst C, Georgakopoulos A, Shioi J, Ghersi E, Wisniewski T, Wang R, Ludwig A, Robakis NK. Ligand binding and calcium influx induce distinct ectodomain/gamma-secretase-processing pathways of EphB2 receptor. J Biol Chem. 2007;282:16155–16163. doi: 10.1074/jbc.M611449200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang YW, Wang X, Zhang H, You X, Liao FF, Xu H. Intracellular trafficking of presenilin 1 is regulated by beta-amyloid precursor protein and phospholipase D1. J Biol Chem. 2009;284:12145–12152. doi: 10.1074/jbc.M808497200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DC, Rabizadeh S, Chandra S, Shayya RF, Ellerby LM, Ye X, Salvesen GS, Koo EH, Bredesen DE. A second cytotoxic proteolytic peptide derived from amyloid beta-protein precursor. Nat Med. 2000;6:397–404. doi: 10.1038/74656. [DOI] [PubMed] [Google Scholar]
- Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, Fan W, Kha H, Zhang J, Gong Y, Martin L, Louis JC, Yan Q, Richards WG, Citron M, Vassar R. Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat Neurosci. 2001;4:231–232. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, Wisniewski T, Robakis NK. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. Embo J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, Robakis NK. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003;114:635–645. doi: 10.1016/j.cell.2003.08.008. [DOI] [PubMed] [Google Scholar]
- McCarthy JV, Twomey C, Wujek P. Presenilin-dependent regulated intramembrane proteolysis and gamma-secretase activity. Cell Mol Life Sci. 2009;66:1534–1555. doi: 10.1007/s00018-009-8435-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DL, Papayannopoulos IA, Styles J, Bobin SA, Lin YY, Biemann K, Iqbal K. Peptide compositions of the cerebrovascular and senile plaque core amyloid deposits of Alzheimer’s disease. Arch Biochem Biophys. 1993;301:41–52. doi: 10.1006/abbi.1993.1112. [DOI] [PubMed] [Google Scholar]
- Morohashi Y, Kan T, Tominari Y, Fuwa H, Okamura Y, Watanabe N, Sato C, Natsugari H, Fukuyama T, Iwatsubo T, Tomita T. C-terminal fragment of presenilin is the molecular target of a dipeptidic gamma-secretase-specific inhibitor DAPT (N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester) J Biol Chem. 2006;281:14670–14676. doi: 10.1074/jbc.M513012200. [DOI] [PubMed] [Google Scholar]
- Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ, Ray WJ, Kopan R. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol Cell. 2000;5:197–206. doi: 10.1016/s1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- Neve RL, Robakis NK. Alzheimer’s disease: a re-examination of the amyloid hypothesis. Trends Neurosci. 1998;21:15–19. doi: 10.1016/s0166-2236(97)01168-5. [DOI] [PubMed] [Google Scholar]
- Ni CY, Murphy MP, Golde TE, Carpenter G. gamma -Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- Ni Y, Zhao X, Bao G, Zou L, Teng L, Wang Z, Song M, Xiong J, Bai Y, Pei G. Activation of beta2-adrenergic receptor stimulates gamma-secretase activity and accelerates amyloid plaque formation. Nat Med. 2006;12:1390–1396. doi: 10.1038/nm1485. [DOI] [PubMed] [Google Scholar]
- Nolt MJ, Lin Y, Hruska M, Murphy J, Sheffler-Colins SI, Kayser MS, Passer J, Bennett MV, Zukin RS, Dalva MB. EphB controls NMDA receptor function and synaptic targeting in a subunit-specific manner. J Neurosci. 2011;31:5353–5364. doi: 10.1523/JNEUROSCI.0282-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki Y, Higo T, Uemura K, Shimada N, Osawa S, Berezovska O, Yokoshima S, Fukuyama T, Tomita T, Iwatsubo T. Phenylpiperidine-type gamma-secretase modulators target the transmembrane domain 1 of presenilin 1. Embo J. 2011;30:4815–4824. doi: 10.1038/emboj.2011.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardossi-Piquard R, Bohm C, Chen F, Kanemoto S, Checler F, Schmitt-Ulms G, St George-Hyslop P, Fraser PE. TMP21 transmembrane domain regulates gamma-secretase cleavage. J Biol Chem. 2009a;284:28634–28641. doi: 10.1074/jbc.M109.059345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardossi-Piquard R, Yang SP, Kanemoto S, Gu Y, Chen F, Bohm C, Sevalle J, Li T, Wong PC, Checler F, Schmitt-Ulms G, St George-Hyslop P, Fraser PE. APH1 polar transmembrane residues regulate the assembly and activity of presenilin complexes. J Biol Chem. 2009b;284:16298–16307. doi: 10.1074/jbc.M109.000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei J, Mitchell DA, Dixon JE, Grishin NV. Expansion of Type II CAAX Proteases Reveals Evolutionary Origin of gamma-Secretase Subunit APH-1. J Mol Biol. 2011;410:18–26. doi: 10.1016/j.jmb.2011.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimplikar SW, Nixon RA, Robakis NK, Shen J, Tsai LH. Amyloid-independent mechanisms in Alzheimer’s disease pathogenesis. J Neurosci. 2010;30:14946–14954. doi: 10.1523/JNEUROSCI.4305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop S, Shirotani K, Edbauer D, Haass C, Steiner H. Requirement of PEN-2 for stabilization of the presenilin N-/C-terminal fragment heterodimer within the gamma-secretase complex. J Biol Chem. 2004;279:23255–23261. doi: 10.1074/jbc.M401789200. [DOI] [PubMed] [Google Scholar]
- Qi-Takahara Y, Morishima-Kawashima M, Tanimura Y, Dolios G, Hirotani N, Horikoshi Y, Kametani F, Maeda M, Saido TC, Wang R, Ihara Y. Longer forms of amyloid beta protein: implications for the mechanism of intramembrane cleavage by gamma-secretase. J Neurosci. 2005;25:436–445. doi: 10.1523/JNEUROSCI.1575-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss K, Maretzky T, Ludwig A, Tousseyn T, de Strooper B, Hartmann D, Saftig P. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. Embo J. 2005;24:742–752. doi: 10.1038/sj.emboj.7600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restituito S, Khatri L, Ninan I, Mathews PM, Liu X, Weinberg RJ, Ziff EB. Synaptic autoregulation by metalloproteases and gamma-secretase. J Neurosci. 2011;31:12083–12093. doi: 10.1523/JNEUROSCI.2513-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robakis NK. An Alzheimer’s disease hypothesis based on transcriptional dysregulation. Amyloid. 2003;10:80–85. doi: 10.3109/13506120309041729. [DOI] [PubMed] [Google Scholar]
- Robakis NK. Mechanisms of AD neurodegeneration may be independent of Abeta and its derivatives. Neurobiol Aging. 2011;32:372–379. doi: 10.1016/j.neurobiolaging.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogelj B, Mitchell JC, Miller CC, McLoughlin DM. The X11/Mint family of adaptor proteins. Brain Res Rev. 2006;52:305–315. doi: 10.1016/j.brainresrev.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Sambamurti K, Greig NH, Utsuki T, Barnwell EL, Sharma E, Mazell C, Bhat NR, Kindy MS, Lahiri DK, Pappolla MA. Targets for AD treatment: conflicting messages from gamma-secretase inhibitors. J Neurochem. 2011;117:359–374. doi: 10.1111/j.1471-4159.2011.07213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambamurti K, Refolo LM, Shioi J, Pappolla MA, Robakis NK. The Alzheimer’s amyloid precursor is cleaved intracellularly in the trans-Golgi network or in a post-Golgi compartment. Ann N Y Acad Sci. 1992;674:118–128. doi: 10.1111/j.1749-6632.1992.tb27481.x. [DOI] [PubMed] [Google Scholar]
- Sano Y, Syuzo-Takabatake A, Nakaya T, Saito Y, Tomita S, Itohara S, Suzuki T. Enhanced amyloidogenic metabolism of the amyloid beta-protein precursor in the X11L-deficient mouse brain. J Biol Chem. 2006;281:37853–37860. doi: 10.1074/jbc.M609312200. [DOI] [PubMed] [Google Scholar]
- Sauer RT, Baker TA. AAA+ proteases: ATP-fueled machines of protein destruction. Annu Rev Biochem. 2011;80:587–612. doi: 10.1146/annurev-biochem-060408-172623. [DOI] [PubMed] [Google Scholar]
- Serban G, Kouchi Z, Baki L, Georgakopoulos A, Litterst CM, Shioi J, Robakis NK. Cadherins mediate both the association between PS1 and beta-catenin and the effects of PS1 on beta-catenin stability. J Biol Chem. 2005;280:36007–36012. doi: 10.1074/jbc.M507503200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serneels L, Van Biervliet J, Craessaerts K, Dejaegere T, Horre K, Van Houtvin T, Esselmann H, Paul S, Schafer MK, Berezovska O, Hyman BT, Sprangers B, Sciot R, Moons L, Jucker M, Yang Z, May PC, Karran E, Wiltfang J, D’Hooge R, De Strooper B. gamma-Secretase heterogeneity in the Aph1 subunit: relevance for Alzheimer’s disease. Science. 2009;324:639–642. doi: 10.1126/science.1171176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Lee SF, Tabuchi K, Hao YH, Yu C, LaPlant Q, Ball H, Dann CE, 3rd, Sudhof T, Yu G. Nicastrin functions as a gamma-secretase-substrate receptor. Cell. 2005;122:435–447. doi: 10.1016/j.cell.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Shen J, Kelleher RJ., 3rd The presenilin hypothesis of Alzheimer’s disease: evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci U S A. 2007;104:403–409. doi: 10.1073/pnas.0608332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirotani K, Edbauer D, Kostka M, Steiner H, Haass C. Immature nicastrin stabilizes APH-1 independent of PEN-2 and presenilin: identification of nicastrin mutants that selectively interact with APH-1. J Neurochem. 2004a;89:1520–1527. doi: 10.1111/j.1471-4159.2004.02447.x. [DOI] [PubMed] [Google Scholar]
- Shirotani K, Edbauer D, Prokop S, Haass C, Steiner H. Identification of distinct gamma-secretase complexes with different APH-1 variants. J Biol Chem. 2004b;279:41340–41345. doi: 10.1074/jbc.M405768200. [DOI] [PubMed] [Google Scholar]
- Song W, Nadeau P, Yuan M, Yang X, Shen J, Yankner BA. Proteolytic release and nuclear translocation of Notch-1 are induced by presenilin-1 and impaired by pathogenic presenilin-1 mutations. Proc Natl Acad Sci U S A. 1999;96:6959–6963. doi: 10.1073/pnas.96.12.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Duff K, Capell A, Romig H, Grim MG, Lincoln S, Hardy J, Yu X, Picciano M, Fechteler K, Citron M, Kopan R, Pesold B, Keck S, Baader M, Tomita T, Iwatsubo T, Baumeister R, Haass C. A loss of function mutation of presenilin-2 interferes with amyloid beta-peptide production and notch signaling. J Biol Chem. 1999;274:28669–28673. doi: 10.1074/jbc.274.40.28669. [DOI] [PubMed] [Google Scholar]
- Steiner H, Winkler E, Haass C. Chemical cross-linking provides a model of the gamma-secretase complex subunit architecture and evidence for close proximity of the C-terminal fragment of presenilin with APH-1. J Biol Chem. 2008;283:34677–34686. doi: 10.1074/jbc.M709067200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasu MA, Dalva MB, Zigmond RE, Greenberg ME. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science. 2002;295:491–495. doi: 10.1126/science.1065983. [DOI] [PubMed] [Google Scholar]
- Teng L, Zhao J, Wang F, Ma L, Pei G. A GPCR/secretase complex regulates beta- and gamma-secretase specificity for Abeta production and contributes to AD pathogenesis. Cell Res. 2010;20:138–153. doi: 10.1038/cr.2010.3. [DOI] [PubMed] [Google Scholar]
- Thathiah A, Spittaels K, Hoffmann M, Staes M, Cohen A, Horre K, Vanbrabant M, Coun F, Baekelandt V, Delacourte A, Fischer DF, Pollet D, De Strooper B, Merchiers P. The orphan G protein-coupled receptor 3 modulates amyloid-beta peptide generation in neurons. Science. 2009;323:946–951. doi: 10.1126/science.1160649. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283:29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian G, Ghanekar SV, Aharony D, Shenvi AB, Jacobs RT, Liu X, Greenberg BD. The mechanism of gamma-secretase: multiple inhibitor binding sites for transition state analogs and small molecule inhibitors. J Biol Chem. 2003;278:28968–28975. doi: 10.1074/jbc.M300905200. [DOI] [PubMed] [Google Scholar]
- Tian G, Sobotka-Briner CD, Zysk J, Liu X, Birr C, Sylvester MA, Edwards PD, Scott CD, Greenberg BD. Linear non-competitive inhibition of solubilized human gamma-secretase by pepstatin A methylester, L685458, sulfonamides, and benzodiazepines. J Biol Chem. 2002;277:31499–31505. doi: 10.1074/jbc.M112328200. [DOI] [PubMed] [Google Scholar]
- Verdile G, Gandy SE, Martins RN. The role of presenilin and its interacting proteins in the biogenesis of Alzheimer’s beta amyloid. Neurochem Res. 2007;32:609–623. doi: 10.1007/s11064-006-9131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A, Eggert S, Reinhard FB, Vogel M, Paliga K, Baier G, Masters CL, Beyreuther K, Evin G. A novel epsilon-cleavage within the transmembrane domain of the Alzheimer amyloid precursor protein demonstrates homology with Notch processing. Biochemistry. 2002;41:2825–2835. doi: 10.1021/bi015794o. [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Citron M, Diehl TS, Xia W, Donkor IO, Selkoe DJ. A substrate-based difluoro ketone selectively inhibits Alzheimer’s gamma-secretase activity. J Med Chem. 1998;41:6–9. doi: 10.1021/jm970621b. [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- Xu J, Litterst C, Georgakopoulos A, Zaganas I, Robakis NK. Peptide EphB2/CTF2 generated by the gamma-secretase processing of EphB2 receptor promotes tyrosine phosphorylation and cell surface localization of N-methyl-D-aspartate receptors. J Biol Chem. 2009;284:27220–27228. doi: 10.1074/jbc.M109.048728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DS, Tandon A, Chen F, Yu G, Yu H, Arawaka S, Hasegawa H, Duthie M, Schmidt SD, Ramabhadran TV, Nixon RA, Mathews PM, Gandy SE, Mount HT, St George-Hyslop P, Fraser PE. Mature glycosylation and trafficking of nicastrin modulate its binding to presenilins. J Biol Chem. 2002;277:28135–28142. doi: 10.1074/jbc.M110871200. [DOI] [PubMed] [Google Scholar]
- Zhang YW, Luo WJ, Wang H, Lin P, Vetrivel KS, Liao F, Li F, Wong PC, Farquhar MG, Thinakaran G, Xu H. Nicastrin is critical for stability and trafficking but not association of other presenilin/gamma-secretase components. J Biol Chem. 2005;280:17020–17026. doi: 10.1074/jbc.M409467200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Liu Z, Ilagan MX, Kopan R. Gamma-secretase composed of PS1/Pen2/Aph1a can cleave notch and amyloid precursor protein in the absence of nicastrin. J Neurosci. 2010;30:1648–1656. doi: 10.1523/JNEUROSCI.3826-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Liyanage U, Medina M, Ho C, Simmons AD, Lovett M, Kosik KS. Presenilin 1 interaction in the brain with a novel member of the Armadillo family. Neuroreport. 1997;8:2085–2090. doi: 10.1097/00001756-199705260-00054. [DOI] [PubMed] [Google Scholar]