Abstract
Objective
Breast cancer is the second leading cause of cancer death among American women. Risk factors for breast cancer include obesity, alcohol consumption and estrogen therapy. In the present studies we determine the simultaneous effects of these three risk factors on Wnt-1 mammary tumor growth.
Methods
Ovariectomized female mice were fed diets to induce different body weights (calorie restricted, low fat, high fat), provided water or 20% alcohol, implanted with placebo or estrogen pellets and injected with Wnt- mouse mammary cancer cells.
Results
Our results show that obesity promoted the growth of Wnt-1 tumors and induced fatty liver. Tumors tended to be larger in alcohol consuming mice and alcohol exacerbated fatty liver in obese mice. Estrogen treatment promoted weight loss in obese mice which was associated with suppression of tumor growth and fatty liver.
Conclusions
In summary, we show that estrogen protects against obesity which is associated with inhibition of fatty liver and tumor growth.
Keywords: obesity, alchol, estrogen, postmenopausal breast cancer, fatty liver
Introduction
Breast cancer is the second most frequent cancer afflicting American women and accounts for nearly 25% of all diagnosed cancers1. The American Cancer Society predicted approximately 207,000 new cases of invasive breast cancer in 2010; 40,000 of which would succumb to the disease1. Estrogen receptor alpha (ERα)-positive breast cancer is more prevalent among postmenopausal women than premenopausal women2, 3. Modifiable risk factors for postmenopausal breast cancer, such as alcohol consumption, obesity and estrogen plus progestogen therapy, have been shown to increase the risk of ERα-positive breast cancers4–6.
Both obesity and alcohol consumption are established risk factors for breast cancer1, 5, 7–11. Postmenopausal obesity increases the risk of morbidity and mortality from breast cancer7, 8, 10, 11. Excess body weight accounts for nearly 30–50% of all breast cancer deaths11. Adipose tissue can produce a multitude of factors known to influence the carcinogenic process, such as estrogen, leptin, adiponectin, insulin-like growth factor-1 (IGF-1) and cytokines12–15. Obesity increases the risk of developing ERα-positive cancers9. Obesity also increases the risk of developing fatty liver or hepatic steatosis16. Epidemiological studies have reinforced the positive association between alcohol consumption and breast cancer risk17. ERα-positive breast cancers are influenced by alcohol use although the mechanism is unknown5. Alcohol consumption increases breast cancer risk in a dose dependent manner, 10% for every drink consumed per day18. The hormonal status in postmenopausal women is also affected by alcohol; circulating blood estrogen levels increase in women who consume alcohol19–21. Moreover, chronic alcohol consumption increases the risk of developing hepatic steatosis22.
Evidence suggests both obesity and alcohol increase breast cancer risk via the hormone estrogen5, 9, 21, 23–25. In premenopausal women, estrogen is produced largely by the ovaries26. However, after menopause, primary estrogen production shifts to peripheral tissues (e.g. adipose tissue) where aromatase converts androgen to estrogen14. As a result, obese postmenopausal women have higher estrogen levels than lean postmenopausal women24. Elevated estrogen levels are considered a risk factor for breast cancer25, 27, 28. Based on this phenomenon, it is reasonable to understand why obesity tends to increase the risk of estrogen responsive cancers29. Alcohol consumption also increases systemic estrogen levels20, 21, 25, 30. Circulating blood estrogen levels may increase by as much as 20% in women who consume alcohol30. It is possible alcohol increases systemic estrogen levels by increasing the expression level of aromatase31, 32. Studies also show alcohol exposure increases the expression of ERα in breast cancer cells31, 32. Therefore, not only does alcohol increase systemic estrogen levels but also increases the expression of ERα, which may explain why breast cancer cells are more sensitive to estrogen in the presence of alcohol. This suggests alcohol consumption in conjunction with estrogen therapy may increase breast cancer risk to a higher degree than either treatment alone. In fact, Chen et al. show that alcohol in combination with estrogen therapy increases breast cancer risk to a greater extent than estrogen therapy or alcohol only25.
Even though studies show obesity, estrogen and alcohol consumption can increase breast cancer risk, the simultaneous effects of these factors on mammary cancer have not been determined in an experimental setting. To assess the simultaneous effects of these three risk factors on tumor growth, we injected Wnt-1 mammary tumor cells subcutaneously into mice fed a 30% calorie restricted, a low fat diet or a high fat diet, mice consumed either water or alcohol in the drinking water. To determine the combine effects of body weight, alcohol and estrogen on tumor growth, control and obese mice consuming water and alcohol were implanted with pellets delivering estrogen, and then injected with Wnt1 tumor cells.
Methods
Mouse husbandry and diets
All animal procedures were approved by the University of Texas at Austin Institutional Animal Care and Use Committee. Mice were housed according to National Institutes of Health guidelines. 180 ovariectomized, 6 week old female C57Bl/6J mice were purchased from the Jackson Labs (Bar Harbor, MA, USA). The animals were maintained at 24°C on a 12-hour light/dark cycle. Mice were allowed to acclimate for 2 weeks, followed by randomization into treatment groups (9 groups, 20 mice per group). At this point different diets were fed and alcohol treatment commenced for 27 weeks. Mice were fed a 30% calorie restricted (CR), low fat (LF; 10% kcal from fat) or high fat (HF; 60% kcal from fat) diet. The CR diet is a modified version of the LF diet in which the mice receive 70% of the average daily calories and 100% of the vitamins and minerals of the LF diet. All diets have been previously described and used to mimic lean, overweight and obese postmenopausal women33. Water or 20% weight per volume alcohol in the water were given to the animals ad libitum throughout the entire study. Body weight, food consumption and liquid consumption were measured weekly.
Pellet implantation
Estrogen (17 β-estradiol) or placebo pellets were implanted 19 weeks into the study when the mice were 27 weeks old. Implantation occurred under the skin at the dorsal area between the neck and shoulder of the mice. We used estrogen pellets manufactured to deliver 0.72mg of estrogen for 90 days (Innovative Research of America, Sarasota, FL, USA). Therefore, the mice received estrogen/placebo treatment from the time the pellets were implanted until the end of the study when the mice were euthanized; this was approximately 8 weeks.
Wnt-1 transgenic mouse model of breast tumor growth
Mammary tumor cells were generated from mouse mammary tumor virus (MMTV)-Wnt-1 transgenic mice on a C57Bl/6J genetic background. Tumor cells were isolated from transgenic females as previously described34. Wnt-1 mouse tumors are estrogen receptor alpha-positive like the majority of human breast tumors in postmenopausal women35. For implantation, 1 × 105 cells were implanted subcutaneously in 100μl serum-free RPMI 1640 medium into the lower back, halfway between the middle of the back and the base of the tail at 22 weeks into the study when the mice were 30 weeks of age. Once tumors became palpable, tumor volume was determined three times a week with calipers by measuring length, width and depth of the tumor.
Body composition and uterine weight
Baseline percent body fat was measured on un-anesthetized mice using an Echo MRI (Echo Medical Systems, Houston, TX, USA) when the mice were 8 weeks old. Percent body fat and bone mineral density (BMD) were analyzed by dual energy x-ray absorptiometry (DXA) using a GE Lunar Piximus II densitometer (Madison, WI, USA) on the mouse carcasses at the end of the study when they were 35 weeks old. To evaluate uterine weight mouse uteri were removed from the carcasses and weighed at the end of the study when the mice were 35 weeks of age.
Serum analysis and blood chemistry
Serum was collected from the animals at baseline (24 weeks old) and at the end of the study (35 weeks old). Insulin (pg/ml; Mercodia, Uppsala, Sweden) and 17 β-estradiol (pg/ml; Alpha Diagnostic, San Antonio, TX, USA) ELISA kits were used to measure serum insulin and estrogen, respectively. Similarly, serum adiponectin (ng/ml), vascular endothelial growth factor (VEGF; pg/ml)), leptin (pg/ml) and insulin-like growth factor-1 (IGF-1; ng/ml) were also analyzed using ELISA kits (R&D Systems, Minneapolis, MN, USA). Alanine aminotransferase (ALT) levels were measured in the serum with an ELISA kit (BIOO Scientific, Austin, TX, USA). A nicotinamide adenine dinucleotide (NAD)/reduced form of NAD (NADH) kit (Sigma, St. Louis, MO, USA) was used to quantify blood alcohol levels (BAC; mg/dl). All kits were used according to the manufacturer instructions.
Histology
Tissues were collected at sacrifice, 35 weeks of age, and fixed in 10% neutral buffered formalin for 24 h followed by 70% ethanol until embedded in paraffin. Tissues were cut into 4 μm sections and stained with hematoxylin and eosin by standard procedures at the Histology and Tissue Processing Core at the University of Texas MD Anderson Science Park (Smithville, TX, USA).
Statistical analysis
Statistical comparisons for all analyses (body composition, tumor volume, uterine weight, BMD, serum analysis) were determined using multivariate analysis of variance and post hoc comparison of the means using Tukey's Honestly Significant Difference. SPSS version 15.0 software (SPSS Inc., Chicago, IL, USA) was used to perform statistical evaluations. All results are presented as the mean ± standard error of the mean (SEM) and statistical significance was considered p < 0.05.
Results
Body weight and percent body fat were unaffected by alcohol and drastically modified by exogenous estrogen
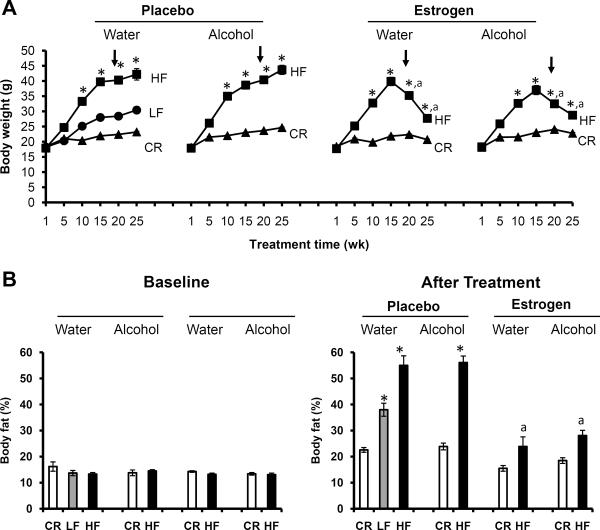
At the start of the experiment, baseline body weights were comparable among all groups (p>0.05), as shown in Fig. 1A. Baseline body weights were taken when the mice were 8 weeks old and immediately before diet and food regimens were implemented (depicted as week 1 on the graph). After administration of the different diet regimens, a nice shift in body weight was observed between CR, LF and HF mice on water. Specifically, CR mice weighed the least, LF mice had an intermediate weight and HF mice were the heaviest. Mice receiving the LF diet and water served as the control group for these experiments. Similar to the water group, mice receiving alcohol displayed the same trend in body weight; alcohol had no affect on body weight (p>0.05). These results were surprising as mice receiving alcohol consumed additional calories from alcohol which provided 1.42 kcal/g; no differences in food consumption (p>0.05) were observed as highlighted in Table 1. The blood alcohol concentration (BAC) of mice consuming 20% alcohol are also depicted in Table 1 which ranged from 57–90 mg/dl (0.057–0.09%), levels equivalent to physiological levels known to increase breast cancer risk in women18, 36, 37. After exogenous estrogen supplementation, the HF group (p<0.05), but not the CR group (p>0.05), underwent drastic weight loss, regardless of water or alcohol consumption. Exogenous estrogen modified body weight but had no affect on alcohol consumption.
Fig. 1.
Exogenous estrogen dramatically affected body weight and percent body fat. 20 mice per group; HF: high fat, LF: low fat, CR: caloric restriction. A. Body weight. Measurements were recorded weekly after diet and liquid regimens were given and throughout the course of the 25 week treatment. Graph depicts averages recorded for every 5 weeks of treatment. Arrow indicates pellet implantation at week 19 when the mice were 27 weeks old, *Significantly different from corresponding CR, aSignificantly different from corresponding placebo. B. Percent body fat. Pre-estrogen treatment body fat levels were recorded at baseline when the mice were 8 weeks old and before pellets were implanted. Post-estrogen treatment body fat levels were evaluated at the end of the study when the mice were 35 weeks of age. *Significantly different from corresponding CR, aSignificantly different from corresponding placebo.
Table I.
Final caloric intake and blood alcohol levels
| Placebo |
Estrogen |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Water |
Alcohol |
Water |
Alcohol |
||||||
| CR | LF | HF | CR | HF | CR | HF | CR | HF | |
| Food (kcal) | 7.6 ± 0.0 | 12.2 ± 0.4 | 15.0 ± 0.6 | 7.6 ± 0.0 | 13.6 ± 0.7 | 7.6 ± 0.0 | 13.9 ± 0.5 | 7.6 ± 0.0 | 11.2 ± 0.3 |
| Alcohol (kcal) | 3.3 ± 0.1 | 3.1 ± 0.3 | 4.6 ± 0.1 | 3.8 ± 0.1 | |||||
| Total (kcal) | 7.6 ± 0.0 | 12.2 ± 0.4 | 15.0 ± 0.6 | 10.9 ± 0.1 | 16.6 ± 0.7 | 7.6 ± 0.0 | 13.9 ± 0.5 | 12.2 ± 0.1 | 14.9 ± 0.3 |
| BAC (mg/dl) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 9 | 90.4 ± 24.6c | 62.4 ± 12.7c | 0.0 ± 0.0 | 0.0 ± 0.0 | 84.2 ± 19.0c | 57.1 ± 5.8c |
Significantly different from corresponding water group
Values are represented as the mean ± SEM (n=10/group)
BAC: blood alcohol concentration, CR: caloric restriction, LF: low fat, HF: high fat
Moreover, much like body weight, no differences in percent body fat were seen between groups at baseline (8 weeks old), as shown in Fig. 1B. However, after treatment (35 weeks old), notable variations were detected between CR and HF groups; CR mice had less percent body fat than HF mice for the placebo groups (p<0.05). Alcohol consumption did not affect body fat levels. However, upon exogenous estrogen supplementation percent body fat drastically decreased, especially for mice in the HF groups, compared to placebo groups.
Effect of exogenous estrogen on physiology
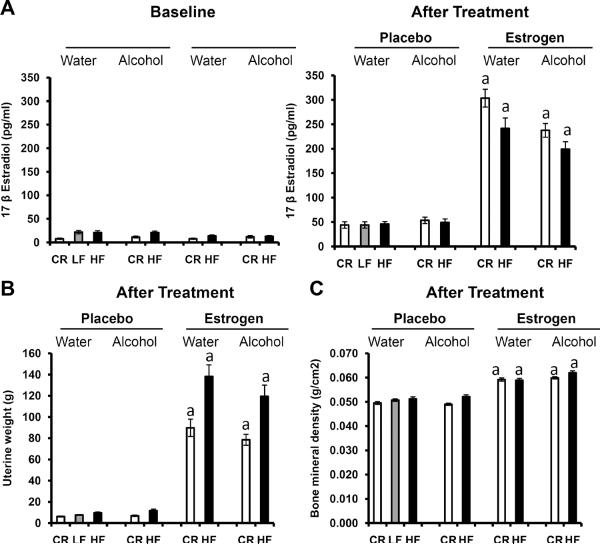
Estrogen has been shown to increase uterine weight and increase bone mineral density38. Because the animals used for the current study have been ovariectomized and lack endogenous estrogen, we decided to verify the exogenous estrogen treatment was effective by analyzing the aforementioned physiological parameters in our mice. Fig. 2A depicts serum estrogen levels at baseline (24 weeks of age) and after treatment (end of study, 35 weeks old) with estrogen and placebo pellets. No statistically significant difference in serum estrogen was noted among groups before estrogen supplementation at baseline (p>0.05). However, after estrogen treatment, serum estrogen levels greatly increased compared to the placebo groups (p<0.01). After estrogen treatment, uterine weight (35 weeks old, Fig. 2B) significantly increased for both CR and HF mice on water and alcohol compared to the placebo group (p<0.01). BMD also increased in the estrogen group compared to the placebo (Fig. 2C, 35 weeks of age, p<0.01). Collectively, the increased circulating blood estrogen levels, uterine weight and BMD, along with the decreased percent body fat suggest the estrogen implantation was successful.
Fig. 2.
Physiological effects of exogenous estrogen treatment. HF: high fat, LF: low fat, CR: caloric restriction, 17 β estradiol: estrogen. A. Levels of circulating serum estrogen. Estrogen levels were recorded at baseline when the mice were 24 weeks old and before pellets were implanted. Post-estrogen treatment levels were evaluated at the end of the study when the mice were 35 weeks of age and after pellets were implanted. 10 mice per group, aSignificantly different from corresponding placebo. B. Uterine weight was analyzed at the end of the study when the mice were 35 weeks old. 12 mice per group, aSignificantly different from corresponding placebo. C. Bone mineral density was evaluated when the mice were 35 weeks of age. 20 mice per group, aSignificantly different from corresponding placebo.
Obesity and alcohol tended to promote while exogenous estrogen suppressed tumor growth
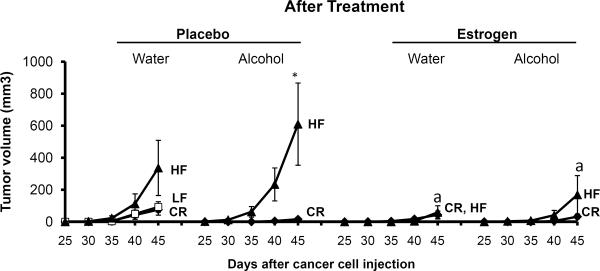
As portrayed in Fig. 3, diet tended to promote mammary tumor growth. HF mice developed larger tumors than CR mice; though the difference was not significant for the water group (p=0.153) but was significant for the alcohol group before estrogen treatment (p<0.032). Alcohol treatment had a tendency to increase tumor volume in obese mice compared to mice on water for both the placebo and estrogen groups even though the difference was not significant (p>0.05). In contrast, alcohol did not impact the growth of CR tumors compared to water animals (p>0.05). Fascinatingly, compared to placebo, tumor growth was considerably suppressed in HF mice (p<0.05) after estrogen pellet implantation. No distinctions were noticed for CR mice in either the estrogen or placebo group (p>0.05). Estrogen treatment inhibited the effects of both obesity (HF) on tumor growth.
Fig. 3.
Tumor volume. Estrogen or placebo treatments began when the mice were 27 weeks of age. Cancer cells were injected when the mice were 30 weeks old. Following cancer cell injection, tumor volume was measured 2–3 times per week until the mice were euthanized at the end of the study. The graph depicts the average tumor volume recorded every 5 days once tumors became palpable around 25 days after cancer cell injection. 20 mice per group; HF: high fat, LF: low fat, CR: caloric restriction; aSignificantly different from corresponding placebo, *Significantly different from corresponding CR.
Effect of alcohol, obesity and exogenous estrogen on growth factors
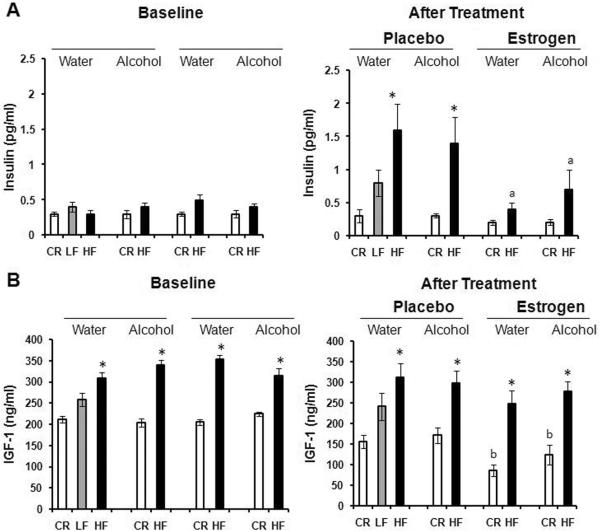
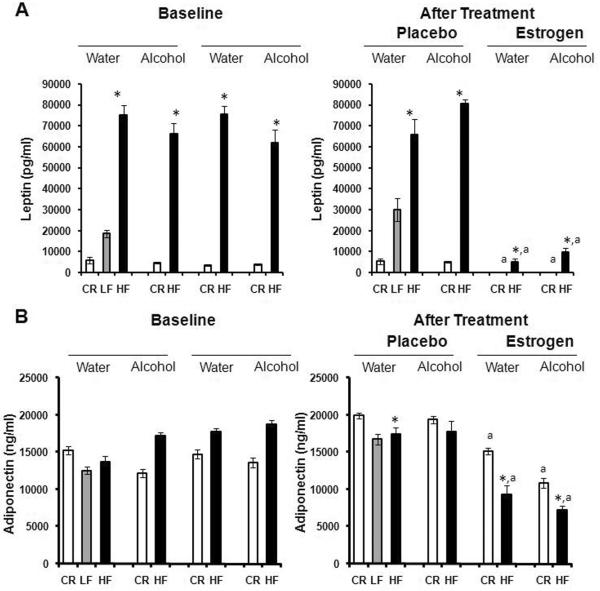
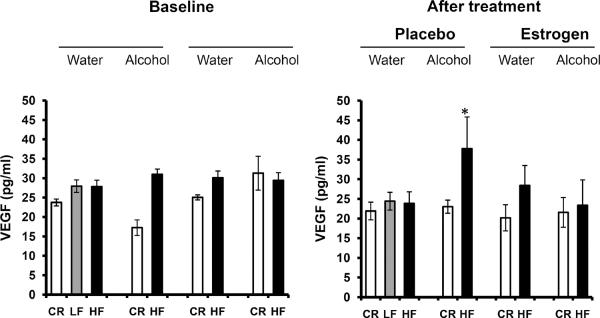
A multitude of growth factors, including but not limited to insulin, IGF-1, leptin and VEGF, have been implicated in cancer39–47. Therefore, we decided to analyze the serum levels of these hormones to determine the role they may play in tumor growth in the present study. Serum insulin and IGF-1 levels increased in Fig. 4A, B as body fat levels increased; CR mice had significantly lower levels of IGF-1 compared to HF mice, after estrogen treatment (p<0.01). Alcohol had no influence on insulin or IGF-1 compared to mice receiving water. However, supplementation of estrogen tended to significantly decrease the circulation of insulin in the blood of HF mice compared to placebo (p<0.01). IGF-1 levels were also greatly diminished in CR mice post-estrogen treatment (p<0.05). Much like insulin and IGF-1, serum leptin levels increased with body adiposity as portrayed in Fig. 5A, p<0.05. Alcohol did not modify the effect of obesity on leptin expression. Nevertheless, serum leptin levels were dramatically reduced after estrogen treatment for both CR (p<0.05) and HF (p<0.01) mice in contrast to placebo. Adiponectin levels, on the other hand, have been shown to inversely correlate with body fat48. Adiponectin levels in Fig. 5B support this idea after treatment (35 weeks old) where CR mice have higher serum adiponectin than HF mice in all groups but the alcohol/placebo group (p<0.05). This was not the case at baseline (24 weeks old). In addition, estrogen treatment caused a down shift in adiponectin expression for both CR and HF animals versus placebo (p<0.01). Finally, we determined blood levels of the pro-angiogenic growth factor VEGF. Fig. 6 shows diet had no affect on VEGF levels after treatment. Among alcohol fed mice, HF diet compared to CR diet stimulated VEGF levels after placebo treatment (p<0.05). The addition of estrogen tended to block the effects of alcohol on VEGF levels in the HF mice post treatment, although the difference was not significant (p>0.05).
Fig. 4.
Modulation of hormones by alcohol, obesity and exogenous estrogen. Hormones were measured at the end of the study when the mice were 35 weeks old. 10 mice per group; HF: high fat, LF: low fat, CR: caloric restriction. A. Levels of circulating Insulin. *Significantly different from corresponding CR, aSignificantly different from corresponding placebo. B. Levels of circulating IGF-1. *Significantly different from corresponding CR, bSignificantly different from corresponding baseline.
Fig. 5.
Modulation of adipokines by alcohol, obesity and exogenous estrogen. Hormone levels were analyzed at the end of the study when the mice were 35 weeks of age. 10 mice per group; HF: high fat, LF: low fat, CR: caloric restriction. A. Levels of circulating Leptin. *Significantly different from corresponding CR, aSignificantly different from corresponding placebo. B. Levels of circulating Adiponectin. *Significantly different from corresponding CR, aSignificantly different from corresponding placebo.
Fig. 6.
Levels of circulating VEGF. VEGF levels were measured at the end of the study when the mice were 35 weeks old. 10 mice per group; HF: high fat, LF: low fat, CR: caloric restriction. *Significantly different from corresponding CR.
Alcohol and obesity negatively affected liver histopathology which was reversed by exogenous estrogen
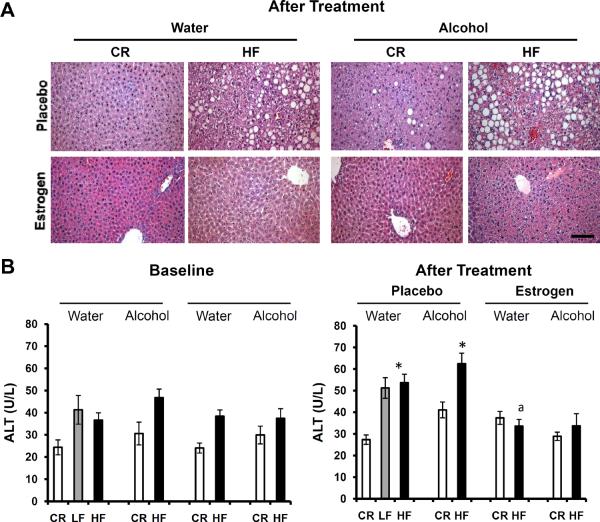
Fatty liver disease, or hepatic steatosis, is commonly associated with alcohol consumption and obesity49–52. It is unclear how exogenous estrogen affects the disease. We qualitatively evaluated liver histology in the current study to determine if estrogen has a deleterious or protective affect on hepatic steatosis. Fig. 7A shows the induction of fatty liver with increasing adiposity as characterized by fat droplets in the hepatocytes. HF displayed more fatty liver than CR as shown in the literature53. Moreover, the condition seemed to worsen after alcohol consumption in HF animals compared to HF mice on water. Alcohol even had a negative effect on CR livers compared to the water group; however, not near the extent of the HF animals. Importantly, estrogen treatment alleviated the fatty liver phenotype for all body weights and liquid groups and provided a protective effect. ALT is used as a marker of liver damage and overall liver health54. High levels of ALT are suggestive of fatty liver, as well as a myriad of other liver pathologies. To confirm the histology results, serum ALT levels were analyzed as depicted in Fig. 7B. Serum ALT levels were consistent with the microscopic findings of the liver; ALT levels were higher in obese mice (p<0.01), even higher in obese mice consuming alcohol (not significant) and ultimately suppressed to baseline levels (24 weeks of age, p>0.05) after estrogen treatment, especially in HF mice.
Fig. 7.
Effects of alcohol, obesity and exogenous estrogen on liver histopathology. HF: high fat, LF: low fat, CR: caloric restriction. A. Hematoxylin and Eosin stained sections of Fatty Liver. Livers were fixed and analyzed when the mice were 35 weeks old. 3 mice per group, 20× magnification, 100μm bar. B. Levels of circulating ALT. 10 mice per group; *Significantly different from corresponding CR, aSignificantly different from corresponding placebo. Pre-estrogen treatment ALT levels were recorded at baseline when the mice were 24 weeks old. Post-estrogen treatment levels were evaluated at the end of the study when the mice were 35 weeks of age.
Discussion
Postmenopausal women come in all sizes, lean, overweight and obese, many of which drink alcohol and even take estrogen to prevent the side effects of menopause. It is unclear if body weight or exogenous estrogen modulates the effects of alcohol on breast cancer growth. To address this in an experimental setting, we determined the simultaneous effects of alcohol, obesity and exogenous estrogen on tumor growth and fatty liver using mouse models in this study. Mice of different body weights (calorie restricted, low fat, high fat) consuming water or alcohol were injected subcutaneously with Wnt-1 cancer cells and implanted with placebo or estrogen pellets. Our results show that obesity promoted Wnt-1 tumor growth, which was ameliorated by estrogen supplementation. We also show that exogenous estrogen treatment resulted in significant weight loss in obese mice. Others have shown similar effects of exogenous estrogen on body weight55. We correlate the dramatic loss in body weight to Wnt-1 tumor growth suppression. Obese mice consuming alcohol tended to have elevated tumor growth compared to obese mice consuming water. However, the difference was not significant and therefore suggests alcohol does not modulate the effects of body weight on tumor growth. Moreover, our results show that both obesity and alcohol consumption promoted fat accumulation in the liver and exogenous estrogen completely blocked the accumulation of fat in the livers of obese and alcohol consuming mice.
Here, alcohol consumption did not significantly modulate the effect of obesity on tumor growth. This does not rule out alcohol alone as a player in tumor growth. Evidence shows that alcohol accelerates tumor growth in mice56 and in cell culture studies alcohol increases the metastatic potential of breast cancer cells in a dose dependent manner57, 58. Moreover, alcohol and obesity are associated with high levels of hormones, including leptin and VEGF, which have been linked to breast cancer10, 15, 18, 43, 45, 59–63. In cell culture studies, leptin promotes the proliferation and increases the invasiveness of breast cancer cells43, 44. Leptin also up-regulates aromatase activity, suggesting leptin may affect breast cancer via estrogen59. Gonzalez et al. demonstrated that blocking the effects of leptin, with a pegylated leptin peptide receptor antagonist, inhibited tumor growth in mice, suggesting leptin plays an important role in breast cancer growth60. We reasoned that alcohol and obesity may influence mammary tumor growth via systemic hormones, such as leptin and estrogen. VEGF could also play a role. VEGF is a major player in angiogenesis, a process essential for tumor growth and metastasis45, 64, 65. We originally assumed factors, like VEGF, produced by adipose tissue were associated with tumor growth; however, we cannot rule out the involvement of tumor-derived VEGF. VEGF levels were the highest in the HF placebo group receiving alcohol. This was interesting since alcohol had no effect on body weight nor body fat suggesting it must come from the tumor itself, rather than the adipose tissue. Moreover, estrogen treatment decreased VEGF expression in these mice. This data is in contrast to the literature which shows estrogen increases serum levels of VEGF66. In our study, body weight and body fat significantly decreased after estrogen treatment which was associated with a decrease in tumor growth. This suggests that the decreased VEGF expression could correspond to the tumor growth inhibition67.
Much like alcohol consumption, obesity increases the risk of developing and dying from breast cancer in post-menopausal women7, 8, 10, 68, 69. Obesity can lead to multiple physiological alterations that can influence breast cancer susceptibility. Obesity is also associated with a plethora of co-morbidities, such as hepatic steatosis16. Our results show that obesity promoted tumor growth. Moreover, obesity promoted liver steatosis. Serum insulin, IGF-1, leptin and VEGF levels increased with increasing body weight. This suggests obesity may affect tumor growth via these growth factors.
Estrogen is a steroid hormone produced predominantly by the ovaries in premenopausal women 26. However, production shifts to peripheral tissues (e.g. adipose tissue) in postmenopausal women where aromatase converts androgen to estrogen26. Estrogen-producing cells, such as adipocytes, contain aromatase 26. Obese postmenopausal women have higher estrogen levels than lean postmenopausal women because they have more adipose tissue and aromatase24. Elevated estrogen levels are considered a risk factor for breast cancer25, 27, 28. Surprisingly, estrogen treatment suppressed tumor growth in both CR and HF mice. This was unexpected since estrogen is a growth factor for breast cancer cells. The present data challenge the central dogma regarding estrogen and breast cancer; however, these effects of estrogen on tumor growth may be dependent on the presence of obesity. This notion is supported by findings from Nkhata et al. who showed that estrogen treatment inhibited T47-D human breast cancer cell progression in obese mice55. Thus, it is feasible that if estrogen treatment leads to a loss of body weight it may protect against breast cancer. On the other hand the timing of estrogen supplementation may be the key to whether estrogen is a risk factor or a protector against breast cancer. For example, one study from the Woman's Health Initiative highlighted the benefit of initiating estrogen therapy five or more years after menopause and emphasized the resultant decrease in breast cancer70. Most animal studies do not consider this factor when designing experiments and supplement estrogen immediately before ovariectomy71–73. In doing this they ignore physiological changes that occur after long term estrogen deprivation, as seen in postmenopausal women. Others have shown under cell culture conditions that estrogen inhibited cell growth and induced apoptosis (cell death) of MCF-7 breast cancer cells, after long-term estrogen deprivation 74. All of these studies highlight the delicate balance between hormone therapy timing and breast cancer susceptibility. Alternatively, it is possible that estrogen inhibits tumor growth, but promotes breast cancer metastasis72. In our studies we only evaluated primary tumor growth, thus it is feasible that estrogen may increased the metastatic ability of breast cancer cells, while it inhibits tumor growth.
Previous research has shown that estrogen treatment results in weight loss55. Therefore, the inhibition of tumor growth may be due to weight loss after estrogen treatment and not the estrogen treatment itself. In our studies, estrogen supplementation triggered a drastic decrease in body weight and percent body fat in obese mice. What is more, circulating insulin and leptin levels were reduced after estrogen treatment. Thus, the decrease in systemic growth factors, as a result of weight loss, may be responsible for the inhibition of tumor growth by estrogen treatment. The key factor for estrogen to inhibit tumor growth may be dependent in whether or not it promotes weight loss, or more specifically loss of body fat, leading to a drastic decrease in systemic hormone levels (e.g., leptin, insulin and IGF-1).
Consistent with the literature, our studies show both alcohol consumption and obesity promote liver steatosis (fatty liver)49–52. Fatty liver is a reversible condition where lipids accumulate in hepatocytes75. Fatty liver is strongly associated with obesity16, 76, 77. In our studies, consumption of alcohol by obese mice exacerbated liver steatosis to a greater extent than either alcohol or obesity alone. Liver steatosis was alleviated by estrogen treatment. Interestingly, fatty liver disease is more prevalent in men and postmenopausal women than in premenopausal women, suggesting that indeed estrogen is a protective factor against the development of liver steatosis78, 79. Additionally, Ogawa et al. reported a dramatic induction of fatty liver in breast cancer patients treated with tamoxifen, an estrogen receptor antagonist80. Thus, these studies along with our findings support the notion that indeed estrogen may protect against the development of fatty liver. Although interesting, the data presented here is limited to the Wnt-1 allograft mouse model of breast cancer. The results reflect the effects of exogenous estrogen on this model and were restricted to 20% alcohol.
Conclusions
We show that both alcohol and obesity promoted tumor growth and fatty liver. Surprisingly, estrogen treatment inhibited the effects of obesity and alcohol consumption on tumor growth and fatty liver. This is the first study to show that estrogen is a protective factor to the effects of alcohol and obesity on mammary tumor growth and fatty liver development. It is interesting that estrogen can behave in such a dichotomous fashion as both a risk factor for breast cancer and suppressor of tumor growth in obese mice. Studies are warranted to determine if indeed certain estrogen therapies can be used in postmenopausal women without leading to an increased risk of breast cancer.
Acknowledgements
This work was supported by grants ACS RSG CNE-113703 (NPN), NCI 1K22CA127519-01A1 (NPN), NIEHS National Institutes of Health ES09145, and National Institute of Environmental Health Sciences Center ES007784.
Financial Support: ACS RSG CNE-113703 (NPN), NCI 1K22CA127519-01A1 (NPN), NIEHS National Institutes of Health ES09145, National Institute of Environmental Health Sciences Center ES007784.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society . Breast Cancer Facts & Figures 2009–2010. American Cancer Society, Inc.; Atlanta: [Google Scholar]
- 2.Li CI, Daling JR, Malone KE. Age-specific incidence rates of in situ breast carcinomas by histologic type, 1980 to 2001. Cancer Epidemiol Biomarkers Prev. 2005;14(4):1008–1011. doi: 10.1158/1055-9965.EPI-04-0849. [DOI] [PubMed] [Google Scholar]
- 3.Murillo-Ortiz B, Perez-Luque E, Malacara JM, Daza-Benitez L, Hernandez-Gonzalez M, Benitez-Bribiesca L. Expression of estrogen receptor alpha and beta in breast cancers of pre- and post-menopausal women. Pathol Oncol Res. 2008;14(4):435–442. doi: 10.1007/s12253-008-9088-y. [DOI] [PubMed] [Google Scholar]
- 4.Sweeney C, Blair CK, Anderson KE, Lazovich D, Folsom AR. Risk factors for breast cancer in elderly women. Am J Epidemiol. 2004;160(9):868–875. doi: 10.1093/aje/kwh276. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki R, Ye W, Rylander-Rudqvist T, Saji S, Colditz GA, Wolk A. Alcohol and postmenopausal breast cancer risk defined by estrogen and progesterone receptor status: a prospective cohort study. J Natl Cancer Inst. 2005;97(21):1601–1608. doi: 10.1093/jnci/dji341. [DOI] [PubMed] [Google Scholar]
- 6.Lee SA, Ross RK, Pike MC. An overview of menopausal oestrogen-progestin hormone therapy and breast cancer risk. Br J Cancer. 2005;92(11):2049–2058. doi: 10.1038/sj.bjc.6602617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 8.McTiernan A. Behavioral risk factors in breast cancer: can risk be modified? Oncologist. 2003;8(4):326–334. doi: 10.1634/theoncologist.8-4-326. [DOI] [PubMed] [Google Scholar]
- 9.Setiawan VW, Monroe KR, Wilkens LR, Kolonel LN, Pike MC, Henderson BE. Breast cancer risk factors defined by estrogen and progesterone receptor status: the multiethnic cohort study. Am J Epidemiol. 2009;169(10):1251–1259. doi: 10.1093/aje/kwp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3(9):565–574. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 11.Petrelli JM, Calle EE, Rodriguez C, Thun MJ. Body mass index, height, and postmenopausal breast cancer mortality in a prospective cohort of US women. Cancer Causes Control. 2002;13(4):325–332. doi: 10.1023/a:1015288615472. [DOI] [PubMed] [Google Scholar]
- 12.Pischon T, Nothlings U, Boeing H. Obesity and cancer. Proc Nutr Soc. 2008;67(2):128–145. doi: 10.1017/S0029665108006976. [DOI] [PubMed] [Google Scholar]
- 13.Grossmann ME, Ray A, Nkhata KJ, Malakhov DA, Rogozina OP, Dogan S, et al. Obesity and breast cancer: status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 29(4):641–653. doi: 10.1007/s10555-010-9252-1. [DOI] [PubMed] [Google Scholar]
- 14.Siiteri PK. Adipose tissue as a source of hormones. Am J Clin Nutr. 1987;45(1 Suppl):277–282. doi: 10.1093/ajcn/45.1.277. [DOI] [PubMed] [Google Scholar]
- 15.Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351(9113):1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 16.Dixon JB, Bhathal PS, O'Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121(1):91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 17.Williams RR, Horm JW. Association of cancer sites with tobacco and alcohol consumption and socioeconomic status of patients: interview study from the Third National Cancer Survey. J Natl Cancer Inst. 1977;58(3):525–547. doi: 10.1093/jnci/58.3.525. [DOI] [PubMed] [Google Scholar]
- 18.Smith-Warner SA, Spiegelman D, Yaun SS, van den Brandt PA, Folsom AR, Goldbohm RA, et al. Alcohol and breast cancer in women: a pooled analysis of cohort studies. JAMA. 1998;279(7):535–540. doi: 10.1001/jama.279.7.535. [DOI] [PubMed] [Google Scholar]
- 19.Ginsburg ES, Walsh BW, Gao X, Gleason RE, Feltmate C, Barbieri RL. The effect of acute ethanol ingestion on estrogen levels in postmenopausal women using transdermal estradiol. J Soc Gynecol Investig. 1995;2(1):26–29. [PubMed] [Google Scholar]
- 20.Chung KW. Effects of chronic ethanol intake on aromatization of androgens and concentration of estrogen and androgen receptors in rat liver. Toxicology. 1990;62(3):285–295. doi: 10.1016/0300-483x(90)90052-i. [DOI] [PubMed] [Google Scholar]
- 21.Gapstur SM, Potter JD, Sellers TA, Folsom AR. Increased risk of breast cancer with alcohol consumption in postmenopausal women. Am J Epidemiol. 1992;136(10):1221–1231. doi: 10.1093/oxfordjournals.aje.a116430. [DOI] [PubMed] [Google Scholar]
- 22.Sozio M, Crabb DW. Alcohol and lipid metabolism. Am J Physiol Endocrinol Metab. 2008;295(1):E10–16. doi: 10.1152/ajpendo.00011.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li CI, Chlebowski RT, Freiberg M, Johnson KC, Kuller L, Lane D, et al. Alcohol consumption and risk of postmenopausal breast cancer by subtype: the women's health initiative observational study. J Natl Cancer Inst. 102(18):1422–1431. doi: 10.1093/jnci/djq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modugno F, Kip KE, Cochrane B, Kuller L, Klug TL, Rohan TE, et al. Obesity, hormone therapy, estrogen metabolism and risk of postmenopausal breast cancer. Int J Cancer. 2006;118(5):1292–1301. doi: 10.1002/ijc.21487. [DOI] [PubMed] [Google Scholar]
- 25.Chen WY, Colditz GA, Rosner B, Hankinson SE, Hunter DJ, Manson JE, et al. Use of postmenopausal hormones, alcohol, and risk for invasive breast cancer. Ann Intern Med. 2002;137(10):798–804. doi: 10.7326/0003-4819-137-10-200211190-00008. [DOI] [PubMed] [Google Scholar]
- 26.Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of aromatase: saga of an important biological mediator and therapeutic target. Endocr Rev. 2009;30(4):343–375. doi: 10.1210/er.2008-0016. [DOI] [PubMed] [Google Scholar]
- 27.Colditz GA. Relationship between estrogen levels, use of hormone replacement therapy, and breast cancer. J Natl Cancer Inst. 1998;90(11):814–823. doi: 10.1093/jnci/90.11.814. [DOI] [PubMed] [Google Scholar]
- 28.Hankinson SE, Colditz GA, Willett WC. Towards an integrated model for breast cancer etiology: the lifelong interplay of genes, lifestyle, and hormones. Breast Cancer Res. 2004;6(5):213–218. doi: 10.1186/bcr921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9(9):631–643. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 30.Maskarinec G, Morimoto Y, Takata Y, Murphy SP, Stanczyk FZ. Alcohol and dietary fibre intakes affect circulating sex hormones among premenopausal women. Public Health Nutr. 2006;9(7):875–881. doi: 10.1017/phn2005923. [DOI] [PubMed] [Google Scholar]
- 31.Etique N, Chardard D, Chesnel A, Merlin JL, Flament S, Grillier-Vuissoz I. Ethanol stimulates proliferation, ERalpha and aromatase expression in MCF-7 human breast cancer cells. Int J Mol Med. 2004;13(1):149–155. [PubMed] [Google Scholar]
- 32.Fan S, Meng Q, Gao B, Grossman J, Yadegari M, Goldberg ID, et al. Alcohol stimulates estrogen receptor signaling in human breast cancer cell lines. Cancer Res. 2000;60(20):5635–5639. [PubMed] [Google Scholar]
- 33.Hong J, Smith RR, Harvey AE, Nunez NP. Alcohol consumption promotes insulin sensitivity without affecting body fat levels. Int J Obes (Lond) 2009;33(2):197–203. doi: 10.1038/ijo.2008.266. [DOI] [PubMed] [Google Scholar]
- 34.Nunez NP, Perkins SN, Smith NC, Berrigan D, Berendes DM, Varticovski L, et al. Obesity accelerates mouse mammary tumor growth in the absence of ovarian hormones. Nutr Cancer. 2008;60(4):534–541. doi: 10.1080/01635580801966195. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Hively WP, Varmus HE. Use of MMTV-Wnt-1 transgenic mice for studying the genetic basis of breast cancer. Oncogene. 2000;19(8):1002–1009. doi: 10.1038/sj.onc.1203273. [DOI] [PubMed] [Google Scholar]
- 36.Boffetta P, Hashibe M, La Vecchia C, Zatonski W, Rehm J. The burden of cancer attributable to alcohol drinking. Int J Cancer. 2006;119(4):884–887. doi: 10.1002/ijc.21903. [DOI] [PubMed] [Google Scholar]
- 37.Chemical Test Section, Wisconsin Department of Transportation, Division of State Patrol. http://www.dot.wisconsin.gov/safety/docs/08law.pdf.
- 38.Modder UI, Riggs BL, Spelsberg TC, Fraser DG, Atkinson EJ, Arnold R, et al. Dose-response of estrogen on bone versus the uterus in ovariectomized mice. Eur J Endocrinol. 2004;151(4):503–510. doi: 10.1530/eje.0.1510503. [DOI] [PubMed] [Google Scholar]
- 39.Gu JW, Young E, Patterson SG, Makey KL, Wells J, Huang M, et al. Postmenopausal obesity promotes tumor angiogenesis and breast cancer progression in mice. Cancer Biol Ther. 11(10):910–917. doi: 10.4161/cbt.11.10.15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101(1):48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong J, Holcomb VB, Tekle SA, Fan B, Nunez NP. Alcohol consumption promotes mammary tumor growth and insulin sensitivity. Cancer Lett. 294(2):229–235. doi: 10.1016/j.canlet.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin--a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. 2002;94(22):1704–1711. doi: 10.1093/jnci/94.22.1704. [DOI] [PubMed] [Google Scholar]
- 43.Sulkowska M, Golaszewska J, Wincewicz A, Koda M, Baltaziak M, Sulkowski S. Leptin--from regulation of fat metabolism to stimulation of breast cancer growth. Pathol Oncol Res. 2006;12(2):69–72. doi: 10.1007/BF02893446. [DOI] [PubMed] [Google Scholar]
- 44.Caldefie-Chezet F, Damez M, de Latour M, Konska G, Mishellani F, Fusillier C, et al. Leptin: a proliferative factor for breast cancer? Study on human ductal carcinoma. Biochem Biophys Res Commun. 2005;334(3):737–741. doi: 10.1016/j.bbrc.2005.06.077. [DOI] [PubMed] [Google Scholar]
- 45.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 46.Brakenhielm E, Veitonmaki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, et al. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2004;101(8):2476–2481. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goodwin PJ, Ennis M, Bahl M, Fantus IG, Pritchard KI, Trudeau ME, et al. High insulin levels in newly diagnosed breast cancer patients reflect underlying insulin resistance and are associated with components of the insulin resistance syndrome. Breast Cancer Res Treat. 2009;114(3):517–525. doi: 10.1007/s10549-008-0019-0. [DOI] [PubMed] [Google Scholar]
- 48.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 49.O'Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 51(1):307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 50.Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 5:145–171. doi: 10.1146/annurev-pathol-121808-102132. [DOI] [PubMed] [Google Scholar]
- 51.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134(5):1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Musso G, Gambino R, Bo S, Uberti B, Biroli G, Pagano G, et al. Should nonalcoholic fatty liver disease be included in the definition of metabolic syndrome? A cross-sectional comparison with Adult Treatment Panel III criteria in nonobese nondiabetic subjects. Diabetes Care. 2008;31(3):562–568. doi: 10.2337/dc07-1526. [DOI] [PubMed] [Google Scholar]
- 53.Bowman TA, Ramakrishnan SK, Kaw M, Lee SJ, Patel PR, Golla VK, et al. Caloric restriction reverses hepatic insulin resistance and steatosis in rats with low aerobic capacity. Endocrinology. 151(11):5157–5164. doi: 10.1210/en.2010-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245(3):194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 55.Nkhata KJ, Ray A, Dogan S, Grande JP, Cleary MP. Mammary tumor development from T47-D human breast cancer cells in obese ovariectomized mice with and without estradiol supplements. Breast Cancer Res Treat. 2008 doi: 10.1007/s10549-008-9991-7. [DOI] [PubMed] [Google Scholar]
- 56.Watabiki T, Okii Y, Tokiyasu T, Yoshimura S, Yoshida M, Akane A, et al. Long-term ethanol consumption in ICR mice causes mammary tumor in females and liver fibrosis in males. Alcohol Clin Exp Res. 2000;24(4 Suppl):117S–122S. [PubMed] [Google Scholar]
- 57.Singletary K. Ethanol and experimental breast cancer: a review. Alcohol Clin Exp Res. 1997;21(2):334–339. [PubMed] [Google Scholar]
- 58.Meng Q, Gao B, Goldberg ID, Rosen EM, Fan S. Stimulation of cell invasion and migration by alcohol in breast cancer cells. Biochem Biophys Res Commun. 2000;273(2):448–453. doi: 10.1006/bbrc.2000.2942. [DOI] [PubMed] [Google Scholar]
- 59.Roth MJ, Baer DJ, Albert PS, Castonguay TW, Dorgan JF, Dawsey SM, et al. Relationship between serum leptin levels and alcohol consumption in a controlled feeding and alcohol ingestion study. J Natl Cancer Inst. 2003;95(22):1722–1725. doi: 10.1093/jnci/djg090. [DOI] [PubMed] [Google Scholar]
- 60.Rene Gonzalez R, Watters A, Xu Y, Singh UP, Mann DR, Rueda BR, et al. Leptin-signaling inhibition results in efficient anti-tumor activity in estrogen receptor positive or negative breast cancer. Breast Cancer Res. 2009;11(3):R36. doi: 10.1186/bcr2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gu JW, Elam J, Sartin A, Li W, Roach R, Adair TH. Moderate levels of ethanol induce expression of vascular endothelial growth factor and stimulate angiogenesis. Am J Physiol Regul Integr Comp Physiol. 2001;281(1):R365–372. doi: 10.1152/ajpregu.2001.281.1.R365. [DOI] [PubMed] [Google Scholar]
- 62.Hong J, Holcomb VB, Dang F, Porampornpilas K, Nunez NP. Alcohol consumption, obesity, estrogen treatment and breast cancer. Anticancer Res. 30(1):1–8. [PubMed] [Google Scholar]
- 63.Delli Carpini J, Karam AK, Montgomery L. Vascular endothelial growth factor and its relationship to the prognosis and treatment of breast, ovarian, and cervical cancer. Angiogenesis. 13(1):43–58. doi: 10.1007/s10456-010-9163-3. [DOI] [PubMed] [Google Scholar]
- 64.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13(1):9–22. [PubMed] [Google Scholar]
- 65.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20(21):4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 66.Dabrosin C, Margetts PJ, Gauldie J. Estradiol increases extracellular levels of vascular endothelial growth factor in vivo in murine mammary cancer. Int J Cancer. 2003;107(4):535–540. doi: 10.1002/ijc.11398. [DOI] [PubMed] [Google Scholar]
- 67.Poon RT, Fan ST, Wong J. Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol. 2001;19(4):1207–1225. doi: 10.1200/JCO.2001.19.4.1207. [DOI] [PubMed] [Google Scholar]
- 68.Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. Am J Clin Nutr. 2007;86(3):s823–835. doi: 10.1093/ajcn/86.3.823S. [DOI] [PubMed] [Google Scholar]
- 69.Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev. 2007;8(5):395–408. doi: 10.1111/j.1467-789X.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 70.Prentice RL, Chlebowski RT, Stefanick ML, Manson JE, Langer RD, Pettinger M, et al. Conjugated equine estrogens and breast cancer risk in the Women's Health Initiative clinical trial and observational study. Am J Epidemiol. 2008;167(12):1407–1415. doi: 10.1093/aje/kwn090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yue W, Wang JP, Li Y, Fan P, Liu G, Zhang N, et al. Effects of estrogen on breast cancer development: Role of estrogen receptor independent mechanisms. Int J Cancer. 127(8):1748–1757. doi: 10.1002/ijc.25207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liang Y, Benakanakere I, Besch-Williford C, Hyder RS, Ellersieck MR, Hyder SM. Synthetic progestins induce growth and metastasis of BT-474 human breast cancer xenografts in nude mice. Menopause. 17(5):1040–1047. doi: 10.1097/gme.0b013e3181d3dd0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kang JS, Kang MR, Han SB, Yoon WK, Kim JH, Lee TC, et al. Low dose estrogen supplementation reduces mortality of mice in estrogen-dependent human tumor xenograft model. Biol Pharm Bull. 2009;32(1):150–152. doi: 10.1248/bpb.32.150. [DOI] [PubMed] [Google Scholar]
- 74.Lewis JS, Meeke K, Osipo C, Ross EA, Kidawi N, Li T, et al. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Natl Cancer Inst. 2005;97(23):1746–1759. doi: 10.1093/jnci/dji400. [DOI] [PubMed] [Google Scholar]
- 75.Everhart JE, Bambha KM. Fatty liver: think globally. Hepatology. 51(5):1491–1493. doi: 10.1002/hep.23659. [DOI] [PubMed] [Google Scholar]
- 76.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 77.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55(7):434–438. [PubMed] [Google Scholar]
- 78.Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41(2):372–379. doi: 10.1002/hep.20554. [DOI] [PubMed] [Google Scholar]
- 79.Yatsuji S, Hashimoto E, Tobari M, Tokushige K, Shiratori K. Influence of age and gender in Japanese patients with non-alcoholic steatohepatitis. Hepatol Res. 2007;37(12):1034–1043. doi: 10.1111/j.1872-034X.2007.00156.x. [DOI] [PubMed] [Google Scholar]
- 80.Ogawa Y, Murata Y, Nishioka A, Inomata T, Yoshida S. Tamoxifen-induced fatty liver in patients with breast cancer. Lancet. 1998;351(9104):725. doi: 10.1016/S0140-6736(05)78493-2. [DOI] [PubMed] [Google Scholar]