Abstract
Caspi et al. (2003) found an interaction between the serotonin transporter polymorphism gene (5-HTTLPR) and stressful life events on depression. Subsequent attempts to replicate have been inconsistent. The present research included long allele variants modified by SNP rs25531 and tested the interaction on adolescents’ trajectories of anxious/depressed symptoms, with consideration of possible age effects. Adolescents (N = 574), of whom 436 were genotyped, were followed from ages 12 to 17. Analyses demonstrated a G × E interaction in predicting the development of anxious/depressed symptoms. Specifically, adolescents with lower serotonin transcriptional efficiency (TE) genotypes whose mothers reported more stressful events were reported to show more anxious/depressed symptoms and greater increases in the development of symptoms of anxiety and depression than were higher TE adolescents, particularly at ages 16 and 17. Interactions did not differ by gender. Findings demonstrate that stress may affect adolescents’ likelihood of experiencing anxious/depressed symptoms when they have a low serotonin TE (A/G-modified 5-HTTLPR) genotype and suggest that the vulnerability may be stronger in late than early adolescence.
Keywords: 5-HTTLPR serotonin transporter gene polymorphism, family stress, anxiety and depression, adolescence, longitudinal
Anxiety and depression place an enormous burden on individuals and society (Hoffman, Dukes, & Wittchen, 2008; Simon, 2003). Environmental stress, often indexed by stressful life events (SLEs), has been consistently linked to depression. Nevertheless, the effect of SLEs on depression differs between individuals, and most people who experience SLEs never develop depression (Brown, Bifulco, & Harris, 1987), suggesting that individuals may differ in their susceptibility to stress.
Susceptibility to stress may have biological roots, especially in the serotonergic system. The role of the serotonin transporter has been of focal interest in the study of depression-related phenotypes and other psychiatric disorders because serotonergic neurotransmission appears to be related to psychological functioning (Good-nick & Goldstein, 1998). Consequently, researchers have investigated the serotonin transporter gene (SLC6A4) that encodes the serotonin transporter.
There are several polymorphisms in the serotonin transporter gene, including a functional polymorphism consisting of a 44-basepair insertion/deletion in the 5′ promoter region, known as the serotonin-transporter-linked promoter region (5-HTTLPR). 5-HTTLPR includes a long allele, “L,” and a short allele, “S,” which influence the rate of serotonin transcription. Specifically, the L allele has a higher transcriptional efficiency (TE; i.e., higher serotonin transporter activity and greater reuptake) than the S allele (Lesch et al., 1996). The majority of studies on 5-HTTLPR and depressive phenotypes have only focused on two allele variants, L and S (possible genotypes include LL, LS, SS), with the S allele typically considered the high-risk allele. More recently, studies have investigated 5-HTTLPR with LA and LG variants because an adenine/guanine (A/G) single nucleotide polymorphism (SNP) called rs25531 is located within the repeats of 5-HTTLPR and subdivides the L allele into LA and LG variants. Specifically, the LG variant has lower TE similar to the S allele (Hu et al., 2006). Another study, however, has questioned the functional interpretation of the LG allele (Martin, Cleak, Willis-Owen, Flint, & Shifman, 2007). Nevertheless, reclassification of LG alleles may provide a richer measure of serotonin TE compared with traditional classifications.
Biochemical and behavioral differences observed in individuals with varying 5-HTTLPR genotypes suggest that 5-HTTLPR may be partially responsible for differential biological stress reactivity and that behavioral differences between those carrying the S versus L allele may be most prominent in stressful situations. Given that stress is a consistent predictor of subsequent depression and that individuals differ in their sensitivity to stress, it seems plausible that individuals with 5-HTTLPR-S alleles would be more prone than L-allele carriers to experience anxiety and develop depression following stress.
Caspi et al. (2003) found an interaction between 5-HTTLPR and SLEs on depression, whereby individuals with one or two S alleles (SS or LS) had more depressive symptoms when exposed to SLEs than did individuals with two L alleles (LL). Subsequently, dozens of research teams have attempted to replicate the original findings (for reviews, see Caspi, Hariri, Holmes, Uher, & Moffitt, 2010; Uher & McGuffin, 2008), some successfully (e.g., Kendler, Kuhn, Vittum, Prescott, & Riley, 2005), and some unsuccessfully (e.g., Surtees et al., 2006).
Many reasons have been proposed for the discordant findings, including differences in sample age, how depression is rated (e.g., self-report, parent report, observer), and the use of categorical predictors and outcomes, which may reduce the ability to detect a subthreshold or continuous severity effect. Another methodological concern is that the A/G substitution may affect the TE of the 5-HTTLPR-L allele. Reclassification of LG alleles may reflect a more accurate biological model of TE, and may lead to different findings compared with studies that treat LG alleles the same as LA alleles (Gunthert et al., 2007; Zalsman et al., 2006). Findings so far with LG alleles reclassified as lower TE have not been entirely clear—some studies have found that lower TE individuals have greater depression severity (Zalsman et al., 2006), anxious mood (Gunthert et al., 2007), and suicidal behavior (A. Roy, Hu, Janal, & Goldman, 2007) in response to stress. Other studies, however, have found higher TE individuals at greater risk for depression (Chorbov et al., 2007) and anxiety or depression (Laucht et al., 2009) in response to stress.
Beyond the discordance in findings, prior studies have numerous limitations. Few studies have been prospective with longitudinal measures of SLEs and depressive outcomes. Without longitudinal measures of predictors and outcomes, researchers cannot determine whether the Gene × Environment interaction (G × E) predicts the development of depression over time. Another methodological limitation includes the use of solely self-report measures of depressive symptomatology, which may be biased. In addition, many studies have relied on depression diagnosis rather than continuous measures of depressive symptoms, whereas depression appears to be dimensional rather than categorical, so continuous measures of depression are preferable to binary diagnoses (Hankin, Fraley, Lahey, & Waldman, 2005).
Furthermore, most studies have investigated depression in adults, whereas few studies have focused on adolescence—one of the most important periods for onset of depression. To our knowledge, only seven studies of adolescents have focused on the 5-HTTLPR × Stress interaction predicting depression (Åslund et al., 2009; Benjet, Thompson, & Gotlib, 2010; Chipman et al., 2007; Eley et al., 2004; Uddin et al., 2010) or emotional problems (Kumsta et al., 2010; Nobile et al., 2009). Of the seven studies, only one tested repeated measures of outcomes (Kumsta et al., 2010) and none included multiple informant measures of depressive symptoms or diagnosis. Interestingly, three of the studies on adolescent populations partially replicated the original Caspi et al. (2003) findings, but only in girls (Åslund et al., 2009; Benjet et al., 2010; Eley et al., 2004). This pattern of findings has led some researchers to speculate that 5-HTTLPR may have different effects in males and females (Åslund et al., 2009; Sjöberg et al., 2006; Uddin et al., 2010). In contrast, another adolescent study found that the L allele may confer risk for depression (Chipman et al., 2007). Only one of the adolescent studies reclassified LG alleles (Kumsta et al., 2010) and found that the effect of institutional deprivation (being raised in an orphanage) predicted emotional problems more strongly for adolescents with traditionally classified S alleles (compared with L alleles), but that A/G-modified genotypes did not moderate the effect of institutional deprivation on emotional problems. Given the paucity of studies on adolescent populations, in addition to the limitations in studies of adult populations, research needs to clarify the role that 5-HTTLPR plays in the development of depression over time.
Two recent meta-analyses (Munafò, Durrant, Lewis, & Flint, 2009; Risch et al., 2009) found no consistent interaction between SLEs and 5-HTTLPR genotype on depression and suggested that findings in previous studies may have been due to chance. They observed that, although many studies reported replications of the Caspi et al. (2003) findings, most failed to fully replicate the original findings. Criticisms of these meta-analyses, however, include binary outcomes of depression, selection bias in favor of null findings, and in the case of Munafò et al. (2009), use of a binary predictor for SLEs (Kaufman, Gelernter, Kaffman, Caspi, & Moffitt, 2010). A more recent meta-analysis, however, supported the 5-HTTLPR × SLE interaction effect on depression (Karg, Burmeister, Shedden, & Sen, 2011). Nevertheless, the lack of consistency in the reported replications indicates the need for further research on the 5-HTTLPR Gene × Stress interaction in the development of depression. It can also be noted that most previous studies have used the traditional coding of 5-HTTLPR and not the A/G-modified coding.
If future studies provide support for the 5-HTTLPR Gene × Stress interaction, this could provide a basis for developing more effective assessment tools to identify and target at-risk individuals for treatment. The adolescence era is an ideal period to investigate because many adolescents experience anxiety and depression symptoms. In addition, there are well-known gender differences in the prevalence rates of depression that emerge around adolescence. Females are approximately 2 times more likely than males to develop depression over the lifetime (Lewinsohn, Hops, Roberts, Seeley, & Andrews, 1993), but females do not begin to show higher rates than males until around mid-adolescence (Piccinelli & Wilkinson, 2000).
Much of the G × E literature on depression pertains to anxiety, as well, because depression and anxiety appear to be related phenomena. For instance, they are highly comorbid and share the same genetic risk factors (M.-A. Roy, Neale, Pedersen, Mathé, & Kendler, 1995). The 5-HTTLPR Gene × Stress interaction has been investigated in anxiety. Some cross-sectional studies that did not reclassify LG alleles found no interaction in predicting anxiety (Kendler et al., 2005; Taylor et al., 2006) and anxious/depressed symptoms (Middeldorp & Boomsma, 2009), whereas a longitudinal study found a significant A/G-modified 5-HTTLPR Gene × Stress interaction in predicting anxiety (Gunthert et al., 2007). Research on the 5-HTTLPR × Stress interaction in anxiety is characterized by many of the same problems as in the G × E interaction in depression, and future research should elucidate the role that 5-HTTLPR plays in the presence of stress in the development of both anxiety and depression.
To our knowledge, no studies have investigated how 5-HTTLPR and SLEs may interact to predict changes in anxiety or depression over time among adolescents. It would be expected that increases in anxious/depressed symptoms over time would be related to both changing levels of stress and the person’s genetic predisposition. It is possible that the 5-HTTLPR × Stress interaction partially mediates the changes in depression across development as well as the gender differences in developing depression. Moreover, no studies have investigated whether the 5-HTTLPR × Stress interaction differs across development, a notable limitation given that researchers have called for examining how G × E effects differ by age (Lenroot & Giedd, 2011). Prospective longitudinal studies can test these important developmental questions.
In the present study, the year-to-year change in growth of anxious/depressed symptoms was analyzed over time as a function of a youth’s A/G-modified 5-HTTLPR genotype and SLEs in the youth’s family during the preceding year. Because of the increasing importance of depression in adolescence, we focused on anxious/depressed symptoms from ages 12 to 17 in the present study, which appears to be a critical time period in the development of depression. We hypothesized that youths with lower TE of 5-HTTLPR with the A/G substitution would experience more anxious/depressed symptoms when exposed to SLEs and would have greater increases in their growth of anxious/depressed symptoms compared with those with higher TE. In addition, in accordance with previous research on adolescents, we predicted that the Gene × Stress interaction would be stronger for females than males. Furthermore, we tested whether the hypothesized effects differed over development in adolescence. To test these hypotheses, we used a community-based sample of individuals from the Child Development Project (CDP; Dodge, Bates, & Pettit, 1990).
Method
Participants
Children (N = 585) were recruited to participate in the CDP from three sites: Nashville, Tennessee; Knoxville, Tennessee; and Bloomington, Indiana. Children’s parents were approached at random during kindergarten preregistration in 1987 and 1988. To represent those participants who did not preregister, parents were also approached on the first day of class, and via phone or mail. About 75% of individuals who were approached agreed to participate. The schools and the composite sample reflected a broad range of socioeconomic status (SES) groups that were representative of the populations at the respective sites. The Hollingshead four-factor index of SES (M = 39.53, SD = 14.01) ranged from 8 to 66 for the original sample, which was 52% male, 81% European American, 17% African American, and 2% of “other” ethnicity. To attenuate concerns of population stratification owing to ancestral heterogeneity among the individuals of “other” ethnicity, they were removed from all analyses, resulting in an N of 574.
Participation ranged from 72% to 81% from ages 12 to 17 (the focus of the present study). Adolescents with maternal ratings of anxious/depressed symptoms at all six time points numbered 297 (52%), 91 (16%) individuals with five time points, 38 (7%) with four time points, 27 (5%) with three time points, 28 (5%) with two time points, 29 (5%) with one time point, and 64 (11%) with no time points. Adolescents with self-reports of anxious/depressed symptoms at all five time points (self-reports were not collected at age 13) numbered 283 (49%), 107 (19%) individuals with four time points, 46 (8%) with three time points, 33 (6%) with two time points, 29 (5%) with one time point, and 76 (13%) with no time points. Overall, the 574 adolescents accounted for 2,555 (74% of the possible 3,444) individual maternal reports and 2,076 (72% of the possible 2,870) individual self-reports of anxious/depressed symptoms, with 387 adolescents having both genotypic data and at least one rating of mother- or self-reported anxious/depressed symptoms. Adolescents with reports of anxious/depressed symptoms were not statistically different from adolescents without ratings in terms of gender, χ2(1, N = 574) = 0.83, p = .363; ethnicity, χ2(1, N = 574) = 2.41, p = .121; and 5-HTTLPR genotype, χ2(2, N = 436) = 3.43, p = .180, but were in SES, t(558) = −2.67, p = .008, with nonparticipating adolescents more likely to be from a lower SES family.
DNA collection took place in 2006–2007 during the annual follow-up and during special visits for those who had moved away from the original three sites. Participants (n = 436) gave saliva for DNA collection via Oragene collection kits under the supervision of a trained interviewer. The kits were mailed to Washington University in St. Louis for DNA extraction and genotyping. Genotyping was conducted using a polymerase chain reaction/restriction enzyme digestion method (Wendland, Martin, Kruse, Lesch, & Murphy, 2006). The genotypic success rates for 5-HTTLPR and rs25531 were 98.7% and 98.5%, respectively. Concordance rates were calculated by comparing genotypes from technical replicates. The concordance rates for 5-HTTLPR and rs25531 were 98.5% and 95.2%, respectively.
Measures
Anxiety/depression
The Anxious/Depressed subscale of the Achenbach Child Behavior Checklist (CBCL; Achenbach, 1991a) and Youth Self-Report (YSR; Achenbach, 1991b) was used as the measure of anxiety and depression symptoms. Although it would be feasible to separate items more specific to anxiety from those more specific to depression, it has been suggested that the Anxious/Depressed subscale be kept together because of its similarity to the items on the Children’s Depression Inventory (Gerhardt, Compas, Connor, & Achenbach, 1999), in addition to findings that the Anxious/Depressed subscale yields a single rather than two separate depression and anxiety factor loadings using principal components analysis (Gerhardt et al., 1999), and evidence failing to demonstrate patterns indicating separate anxiety or depressive disorders from the Anxious/Depressed subscale (Kendler, Gardner, & Lichtenstein, 2008; Wadsworth, Hudziak, Heath, & Achenbach, 2001).
Mother-report anxious/depressed symptoms were measured by the CBCL from ages 12 to 17. Self-reported anxious/depressed symptoms were assessed using the YSR at ages 12 and 14–17. The CBCL and YSR consist of 112 items, asking whether a given behavior is not true, somewhat or sometimes true, or very or often true (scored 0, 1, and 2, respectively). The Anxious/Depressed subscale consists of 16 items, such as “unhappy, sad, depressed,” “complains of loneliness,” “cries a lot,” “feels or complains that no one loves him/her,” “feels worthless or inferior,” “worries,” and the like, with a total possible score of 32.
Descriptive statistics revealed that the overall cross-age mean of mother-reported anxious/depressed symptoms was 3.34 (SD = 3.64), with an average of 3.89 (3.67) at age 12, 3.88 (3.80) at age 13, 3.14 (3.45) at age 14, 2.95 (3.62) at age 15, 3.33 (3.64) at age 16, and 2.82 (3.51) at age 17. As for self-reported anxious/depressed symptoms, the mean score was 4.88 (4.60), with an average of 5.63 (4.34) at age 12, 4.60 (4.41) at age 14, 5.02 (4.79) at age 15, 4.65 (4.61) at age 16, and 4.52 (4.77) at age 17. In addition, Cronbach’s alpha on the Anxious/Depressed subscale of the maternal-report CBCL ranged from .82 to .86, depending on year, and from .83 to .88 for self-report YSR. Cross-age analyses of convergent validity revealed that mother-report measures of anxious/depressed symptoms were correlated across time, ranging from .51 to .72, all significant at the p < .001 level. Cross-age convergences of the self-report ratings of anxious/depressed symptoms ranged from .29 to .69, all significant at the p < .001 level. The concurrent Pearson correlation coefficients between mother- and self-reported ratings of anxious/depressed symptoms were .16 at age 12, .41 at age 14, .36 at age 15, .46 at age 16, .41 at age 17, all significant at the p < .01 level. The moderate associations between mother- and self-reported ratings of anxious/depressed symptoms in adolescence are consistent with prior studies (Achenbach, McConaughy, & Howell, 1987) and provide justification for using both informants in a latent factor.
As a check of the sample’s comparability to epidemiological findings and as validation of the CBCL/YSR index of anxious/depressed symptoms, the National Institute of Mental Health Diagnostic Interview Schedule (DIS; Robins, Cottler, Bucholz, & Compton, 1995) was administered by a specially trained interviewer to participants at age 18. Major depressive disorder diagnoses according to Diagnostic and Statistical Manual of Mental Disorders, fourth edition criteria were found in 72 (17%) of the adolescents, which is consistent with prevalence rates in large epidemiological studies (Bourdon, Rae, Locke, Narrow, & Regier, 1992). The DIS has been shown to have good convergent validity to clinical scales (Fantoni-Salvador & Rogers, 1997) and reliability (Hasin et al., 2006).
Stress
The number of SLEs experienced by the child was assessed by the mother-reported Changes and Adjustments Questionnaire (Dodge, Pettit, & Bates, 1994) regarding the family’s experiences within the past year from ages 12 to 17. The questionnaire consists of 18 SLEs that might have described experiences of the family, such as death of a close relative, divorce of the child’s parents, or financial problems, and the scale has been shown to have high reliability (Dodge, Pettit, & Bates, 1994). Descriptive statistics revealed that the mean of SLEs was 2.64 (SD = 2.30), with an average of 2.50 (2.00) at age 12, 3.03 (2.20) at age 13, 2.27 (2.14) at age 14, 2.29 (2.27) at age 15, 3.22 (2.32) at age 16, and 2.51 (2.66) at age 17.
Genotyping
There are three basic genotypes of the 5-HTTLPR gene: combinations of “short/short” (SS), “long/short” (LS), and “long/long” alleles (LL), coded additively for the number of L alleles (0, 1, or 2, respectively). Those homozygous for the S allele numbered 71 (16%), 199 (46%) were heterozygous, and 166 (38%) were homozygous for the L allele. The 5-HTTLPR alleles were reclassified according to their serotonin TE as a function of SNP rs25531: SS, SLG, LGLG as “low” (0), SLA, LGLA as “medium” (1), and LALA as “high” (2). Despite the strong association between the two forms of coding (r = .86), 57 (13%) participants changed in TE classification following reclassification of the LG alleles. In the reclassification, 101 (24%) participants were coded as low in TE, 196 (46%) as medium, and 133 (31%) as high. The 5-HTTLPR genotype was tested and found to be in Hardy-Weinberg equilibrium, χ2(1, N = 436) = 0.76, p = .383. Because allele frequencies sometimes differ across ethnicities, the genotype frequencies were analyzed by ethnicity and are presented in Table 1. Pearson’s chi-square tests confirmed that allele frequencies were not different by ethnicity for genotypes before, χ2(2, N = 436) = 2.86, p = .239, or after, χ2(2, N = 430) = 0.55, p = .758, reclassification of LG alleles.
Table 1.
Genotype Frequencies by Ethnicity
| 5-HTTLPR genotype | European American |
African American |
|---|---|---|
| 5-HTTLPR genotype | ||
| LL | 138 (37.1%) | 28 (43.8%) |
| LS | 169 (45.4%) | 30 (46.9%) |
| SS | 65 (17.5%) | 6 (9.4%) |
| A/G-modified 5-HTTLPR genotype | ||
| High TE | 116 (31.6%) | 17 (27.0%) |
| Med TE | 166 (45.2%) | 30 (47.6%) |
| Low TE | 85 (23.2%) | 16 (25.4%) |
| A/G-modified 5-HTTLPR alleles | ||
| LA | 402 (54.5%) | 63 (50.0%) |
| LG | 40 (5.4%) | 20 (15.9%) |
| S | 295 (40.0%) | 43 (34.1%) |
Note. Some individuals were missing one or both alleles. LL = long/long allele; LS = long/short allele; SS = short/short allele; TE = transcriptional efficiency; A/G = adenine/guanine.
Statistical Analysis
To test whether the 5-HTTLPR gene interacts with SLEs in the prediction of anxious/depressed symptoms over time, an individual growth model was employed using longitudinal multilevel modeling. The model was a random intercepts model with random slopes for time and the G × E interaction term. SLEs and time were Level 1 predictors, whereas 5-HTTLPR genotype was a Level 2 predictor, as were gender and ethnicity, which were included as covariates. The count variable for SLEs has a meaningful zero point representing absence of SLEs, so it was not mean-centered in the model (although the results remained unaffected when mean centering the predictors). As for time (in years), its deviation from age 12 was computed (i.e., 0–5 for ages 12–17) to set age 12 as the temporal baseline.
The model was tested with the A/G-modified 5-HTTLPR genotype in predicting the average of mother- and self-reported anxious/depressed symptoms (except at age 13, which was only mother report). The individual growth model was tested with maximum likelihood estimation using the lme function of the nlme package (Pinheiro, Bates, DebRoy, Sarkar, & the R Core Team, 2009) in R (R Development Core Team, 2009). The data were examined for normality. Because anxious/depressed symptoms and SLEs were count variables, they were nonnormal. To test the predictions with a normalized distribution, multilevel Poisson regression was performed with a more basic model using the glmer function of the lme4 package (Bates, 2005) in R, but the substantive results of the analyses remained unaffected by Poisson regression, so the analyses presented here describe the standard (i.e., non-Poisson) multilevel modeling.
To test whether the 5-HTTLPR gene interacts with SLEs in the development of anxious/depressed symptoms over time and to examine the timing of the effects, a second-order dual-change score model (CSM; Malone et al., 2004; McArdle & Hamagami, 2001) was used. A CSM is an example of a latent difference score model that estimates intercept, slope, and acceleration parameters. The main advantage of a CSM over traditional growth curve models is that a CSM estimates both changes across time (change scores) and changes in the growth (acceleration scores) of a variable over time. The annual acceleration scores for anxious/depressed symptoms can be predicted in the model to clarify the timing of the effects of genes, stress, and their interaction by estimating the extent to which the effects deflect adolescents away from their expected trajectories of anxiety and depression at various points in time. Thus, a CSM is ideal for estimating the effects of time-varying predictors such as stress and the Gene × Stress interaction on the development of anxiety/depression.
In light of the developmental perspective of the present study, the CSM was probed to determine whether the effect of the interaction was strongest during a particular time frame. Additionally, models tested whether the effects differed by gender or ethnicity. All CSMs were tested with full-information maximum likelihood estimation using the maximum likelihood ratio estimator in Mplus 5 (L. K. Muthén & Muthén, 2007), which uses robust estimation of standard errors when data are nonnormal, as is the case in the present study with anxious/depressed symptoms and SLEs. The genotype and stress variables were not mean-centered in the analyses because both had a meaningful zero point. Nevertheless, the analyses in the CSM were also rerun with mean-centered stress terms (from which the G × E interaction terms were recalculated). The results were substantively the same, so all analyses are presented with variables in their raw metric.
A power analysis was run to determine our power to detect a G × E effect in multiple regression using Quanto 1.2.4 (Gauderman, 2002). Assuming that main effects of 5-HTTLPR and SLEs each account for 1% of the variance in anxious/depressed symptoms, we would have .80 power to detect a G × E interaction that accounts for 1.8% of the variance with a sample of 436 people and an alpha of .05. Thus, the present study has adequate power to detect G × E interactions of fairly small effect in the context of multiple regression. This may partly reflect the use of continuous outcomes, as analyses of continuous outcomes are more powerful than analyses with dichotomous outcomes. The power estimations are most relevant to the multilevel models in the present study. Latent CSMs are likely underpowered relative to the multilevel models because of the additional model complexity.
Results
As an initial step of measurement validation, we computed the correlations of the Anxious/Depressed subscale at each age with the later age 18 DIS major depression diagnosis. The CBCL and YSR anxious/depressed scores from each year significantly predicted later depression diagnosis, with rs ranging from .12 (p = .029) to .37 (p < .001). Although the Achenbach scales are not the same as the diagnostic interview, they show some predictive validity. The depression diagnoses were not used in the G × E models, because, unlike the YSR and CBCL, diagnoses were not assessed longitudinally, nor were they assessed within the time frame of the SLEs measured in the present study. A correlation matrix of model variables is presented in Table 2. Females experienced more anxious/depressed symptoms (ages 15–17 mother report and ages 12, 14, 15, 16, and 17 self-report) on average than did males, as would be expected from previous findings with adolescents. Females also were in families who experienced a greater number of SLEs compared with males at age 17, which was not predicted.
Table 2.
Pearson and Point-Biserial Correlation Matrix of Variables Included in the Multilevel Models
| SLE | CBCL | YSR | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| 1. Female | — | |||||||||||||||||||
| 2. AfrAm | .03 | — | ||||||||||||||||||
| 3. Gene | .02 | .03 | — | |||||||||||||||||
| 4. SLE12 | −.01 | −.01 | .06 | — | ||||||||||||||||
| 5. SLE13 | −.01 | .04 | .08 | .47** | — | |||||||||||||||
| 6. SLE14 | .07 | .10* | −.03 | .39** | .42** | — | ||||||||||||||
| 7. SLE15 | .03 | .07 | .01 | .34** | .34** | .58** | — | |||||||||||||
| 8. SLE16 | .06 | .12* | .04 | .37** | .32** | .44** | .52** | — | ||||||||||||
| 9. SLE17 | .11* | .08 | −.01 | .26** | .24** | .40** | .33** | .43** | — | |||||||||||
| 10. CBCL12 | .06 | −.06 | .01 | .30** | .22** | .21** | .26** | .27** | .15** | — | ||||||||||
| 11. CBCL13 | .06 | −.12* | .04 | .19** | .26** | .20** | .18** | .25** | .19** | .72** | — | |||||||||
| 12. CBCL14 | .09 | −.07 | −.01 | .26** | .26** | .32** | .32** | .34** | .22** | .65** | .70** | — | ||||||||
| 13. CBCL15 | .10* | −.07 | .01 | .20** | .22** | .29** | .33** | .33** | .16** | .55** | .61** | .70** | — | |||||||
| 14. CBCL16 | .16** | −.05 | .11* | .18** | .20** | .22** | .18** | .36** | .18** | .56** | .65** | .59** | .64** | — | ||||||
| 15. CBCL17 | .14** | −.04 | .01 | .25** | .23** | .31** | .23** | .29** | .32** | .51** | .56** | .62** | .60** | .67** | — | |||||
| 16. YSR12 | .11* | −.02 | −.05 | .07 | .04 | .12* | .03 | .06 | .02 | .16** | .22** | .21** | .23** | .19** | .13* | — | ||||
| 17. YSR14 | .20** | −.14** | .01 | .13* | .16** | .17** | .14** | .11* | .10* | .28** | .34** | .41** | .38** | .30** | .29** | .47** | — | |||
| 18. YSR15 | .22** | −.12* | .01 | .10 | .12* | .13* | .10 | .15** | .08 | .30** | .30** | .34** | .36** | .39** | .35** | .37** | .64** | — | ||
| 19. YSR16 | .22** | −.13** | .03 | .10 | .11* | .13* | .12* | .19** | .12* | .31** | .35** | .40** | .42** | .46** | .37** | .34** | .55** | .69** | — | |
| 20. YSR17 | .20** | −.16** | .01 | .22** | .16** | .13* | .15** | .15* | .17** | .32** | .30** | .37** | .35** | .37** | .41** | .29** | .56** | .61** | .67** | — |
Note. AfrAm = African American ethnicity (dummy coded); SLE = Stressful Life Events; CBCL = Child Behavior Checklist anxious/depressed symptoms; YSR = Youth Self-Report anxious/depressed symptoms.
p < .05.
p < .01.
Is There a G × E Effect on Anxious/Depressed Symptoms in Adolescence?
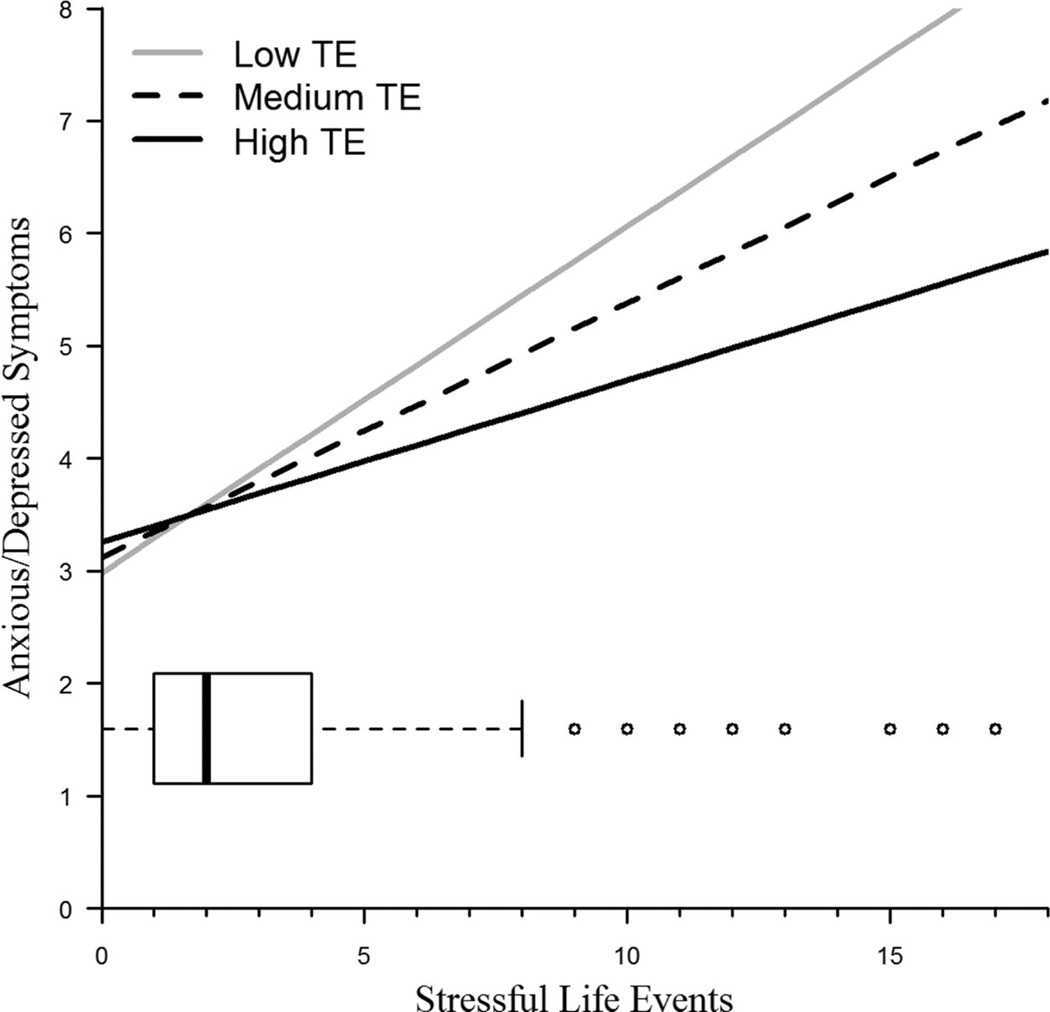
An individual growth model tested the interactive effects of the 5-HTTLPR genotype and SLEs in predicting anxious/depressed symptoms over time. The results are presented in Table 3. When considering the 5-HTTLPR genotype with the A/G substitution in predicting anxious/depressed symptoms, SLEs and the interaction between genotype and SLEs were significant as predicted. Specifically, those with lower TE who experienced more SLEs had more anxious/depressed symptoms than those with higher TE and those who experienced fewer SLEs, as illustrated in Figure 1.
Table 3.
Fixed Effects Predicting Anxious/Depressed Symptoms Over Time
| Predictor | Value | SE | df | t | p |
|---|---|---|---|---|---|
| Intercept | 3.40 | 0.32 | 1653 | 10.71 | <.001 |
| Female | 0.80 | 0.27 | 403 | 2.92 | .004 |
| African American | −1.11 | 0.40 | 403 | −2.76 | .006 |
| Time | −0.10 | 0.04 | 1653 | −2.80 | .005 |
| Gene | 0.14 | 0.20 | 403 | 0.68 | .500 |
| SLEs | 0.31 | 0.05 | 1653 | 6.25 | <.001 |
| Gene × SLEs | −0.08 | 0.04 | 1653 | −2.12 | .035 |
Note. SLEs = stressful life events.
Figure 1.
Interaction between transcriptional efficiency (TE; defined by 5-HTTLPR + adenine/guanine substitution) and stressful life events (SLEs) in predicting anxious/depressed symptoms. Includes overlaid box and whisker plot of SLEs.
Does the G × E Effect Predict the Development of Anxious/Depressed Symptoms, and Does the G × E Effect Differ Across Development?
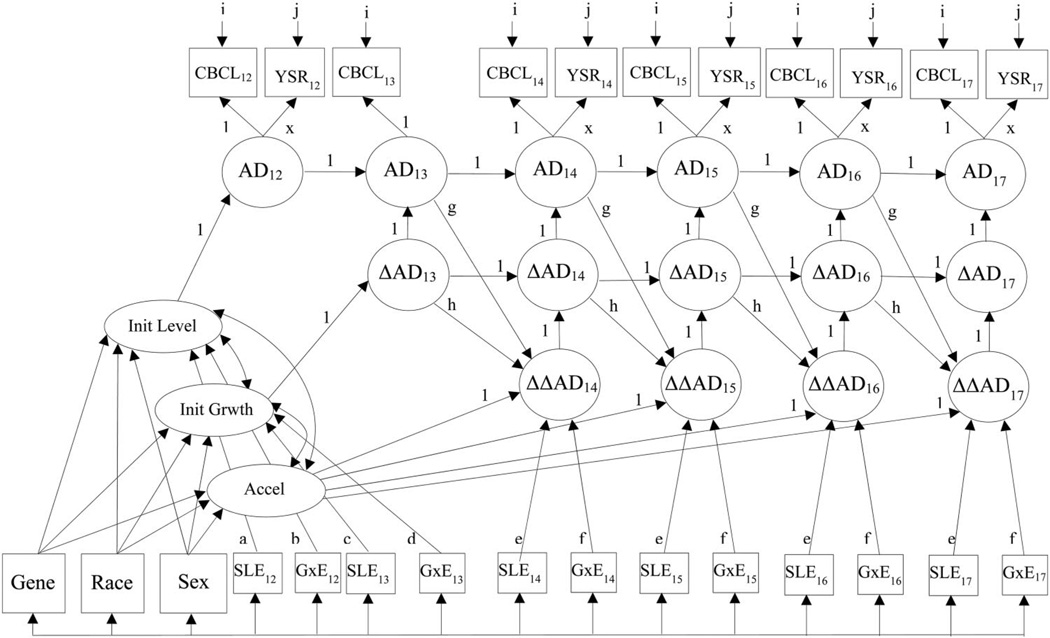
Because of the significant G × E interaction in the omnibus individual growth model, we wanted to examine the developmental timing of the effect. To determine whether the G × E effect predicted the development of anxious/depressed symptoms and to determine the timing of the effect, we fit a second-order dual CSM, as depicted in Figure 2, examining whether there was an A/G-modified 5-HTTLPR gene–environment interaction effect on the intercept (initial level at age 12), slope (initial growth at age 13), and acceleration terms (annual changes in growth from ages 14 to 17). Main effects of SLEs and genotype were also included as predictors of the initial level, initial growth, and each acceleration term in the model in order to rule out the possibility that the G × E interaction effects owed to the main effects (unlike the depiction in Figure 2, which, for the sake of simplicity, shows genotype as only predicting the initial level, initial growth, and the latent acceleration factor). Loadings on the four acceleration parameters were constrained to be equal, but they were allowed to vary from the intercept, and both the acceleration and intercept parameters were allowed to vary from the slope because the intercept, slope, and acceleration represent qualitatively different phenomena.
Figure 2.
Second-order dual change score model. Loadings with matching labels are constrained to be equal; unlabeled paths are freely estimated. For the sake of pictorial simplicity, the depicted model shows genotype predicting the latent acceleration factor, but in the actual analysis, genotype predicted each acceleration term separately. Parameters a and b only predict the initial level; parameter c only predicts the initial growth. CBCL = Child Behavior Checklist Anxious/Depressed symptoms; YSR = Youth Self-Report Anxious/Depressed symptoms; AD = Anxious/Depressed symptoms; Init Level = Initial Level (intercept); Init Growth = Initial Growth (slope); Accel = Acceleration; SLE = Stressful Life Events; G×E = Gene × Environment interaction.
To test whether the interaction differed by age, the CSM was modified in a two-step model process, with a piecewise shifting of the time frame. Three sets of acceleration timeframes were considered: (a) Time Frame A = age 14; Time Frame B = ages 15–17, (b) A = 14–15; B = 16–17, and (c) A = 14–16; B = 17. The main effects of genotype and SLEs on the acceleration terms in Time Frame B were allowed to freely vary from their corresponding effects in Time Frame A (Model 1). Then, the interaction terms in Time Frame B were allowed to freely vary from their corresponding effects in Time Frame A (Model 2). The chi-square change test evaluated whether freeing the interaction terms in Time Frame B led to a significantly better fitting model (i.e., whether the effect of the interaction on the acceleration scores differed by age from Timeframe A to Timeframe B). Of the three sets of acceleration time frames, the only significantly better fitting model allowed the interaction effects to differ at ages 16–17 from ages 14–15 (Satorra-Bentler scaled chi-square difference [1] = 4.76, p = .029), which we refer to as the ages 16–17 CSM, and which serves as the main model on the question of whether the G × E varies with development.
The parameters for the ages 16–17 CSM are presented in Table 4. In the model, the parameters predicting the acceleration terms at ages 16 and 17 were constrained to be equal and allowed to vary from the estimates at ages 14 and 15. Fit indices suggested that the model fit the data adequately, χ2(N = 574) = 500.14, comparative fit index [CFI] = 0.87, root-mean-square error of approximation [RMSEA] = 0.049. The G × E interaction terms were not significant in predicting the initial level at age 12 or the initial growth at age 13. In addition, the G × E interaction was not significant in predicting the acceleration terms from ages 14 to 15. There was, however, a significant Gene × Environment interaction in the prediction of the acceleration terms from ages 16 to 17. Specifically, those with lower serotonin TE genotypes showed greater acceleration (i.e., change in the growth) of anxious/depressed symptoms than higher TE individuals when they had experienced more SLEs measured at ages 16 and 17. In other words, high risk levels of the genotype and stress predicted an increased rate of growth in anxious/depressed symptoms in later adolescence. The p value of the interaction effect (.009) is less than a Bonferroni-corrected alpha criterion for multiple tests (.013), based on four tests: (a) the intercept at age 12, (b) the initial growth at age 13, (c) the acceleration at ages 14–15, and (d) the acceleration at ages 16–17. The R2 change in the latent acceleration term with the addition of the interaction term was .022, suggesting that the Gene × Stress interaction accounted for modest amounts (less than 3%) of variance in anxious/depressed symptoms.
Table 4.
Parameter Estimates From the CSM Allowing Effects Predicting Acceleration Terms From Ages 16–17 to Vary From Those Predicting Acceleration at Ages 14–15
| Predictor | B | β | SE | z | p |
|---|---|---|---|---|---|
| Intercepts | |||||
| Initial level | 3.23 | 1.62 | 0.38 | 8.60 | <.001 |
| Initial growth | −0.39 | −0.80 | 0.36 | −1.08 | .280 |
| Annual acceleration | 0.08 | 0.13 | 0.54 | 0.15 | .878 |
| Proportional change | |||||
| ADt-1 on ΔΔADt (g) | −0.31 | −0.82a | 0.17 | −1.84 | .066 |
| ΔADt-1 on ΔΔADt (h) | −1.53 | −0.90b | 0.18 | −8.75 | <.001 |
| Effects of race | |||||
| African American on initial level | −0.46 | −0.09 | 0.34 | −1.36 | .173 |
| African American on initial growth | −0.70 | −0.55 | 0.34 | −2.09 | .037 |
| African American on acceleration | −0.47 | −0.27 | 0.20 | −2.37 | .018 |
| Effects of sex | |||||
| Female on initial level | 0.56 | 0.14 | 0.24 | 2.38 | .017 |
| Female on initial growth | 0.16 | 0.17 | 0.22 | 0.74 | .462 |
| Female on acceleration | 0.44 | 0.33 | 0.16 | 2.71 | .007 |
| Effects of stress | |||||
| SLE12 on initial level | 0.18 | 0.18 | 0.12 | 1.48 | .139 |
| SLE13 on initial growth | 0.22 | 1.00 | 0.12 | 1.78 | .076 |
| SLE14–15 on acceleration (14–15) | 0.14 | 0.39c | 0.09 | 1.59 | .111 |
| SLE16–17 on acceleration (16–17) | 0.30 | 0.95d | 0.06 | 5.45 | <.001 |
| Effects of genotype | |||||
| Gene on initial level | −0.15 | −0.06 | 0.28 | −0.53 | .594 |
| Gene on initial growth | −0.03 | −0.04 | 0.26 | −0.11 | .910 |
| Gene on acceleration (14–15) | −0.05 | −0.05e | 0.12 | −0.40 | .688 |
| Gene on acceleration (16–17) | 0.12 | 0.11f | 0.11 | 1.07 | .286 |
| Effects of Gene × Stress interaction | |||||
| G×E12 on initial level | 0.10 | 0.17 | 0.09 | 1.12 | .265 |
| G×E13 on initial growth | −0.04 | −0.30 | 0.09 | −0.47 | .642 |
| G×E14–15 on acceleration (14–15) | 0.03 | 0.12g | 0.05 | 0.54 | .592 |
| G×E16–17 on acceleration (16–17) | −0.10 | −0.54h | 0.04 | −2.61 | .009 |
Note. Values with superscript letters represent mean standardized estimates (from multiple standardized estimates of multiple paths: e.g., the values listed below for superscript a represent the four standardized estimates from the four g paths in Figure 2). CSM = change score model; AD = anxious/depressed symptoms; SLE = stressful life events.
(−0.89, −0.84, −0.79, −0.75).
(−1.01, −0.91, −0.85, −0.84).
(0.35, 0.42).
(0.95, 0.95).
(−0.04, −0.05).
(0.11, 0.12).
(0.11, 0.14).
(−0.55, −0.53).
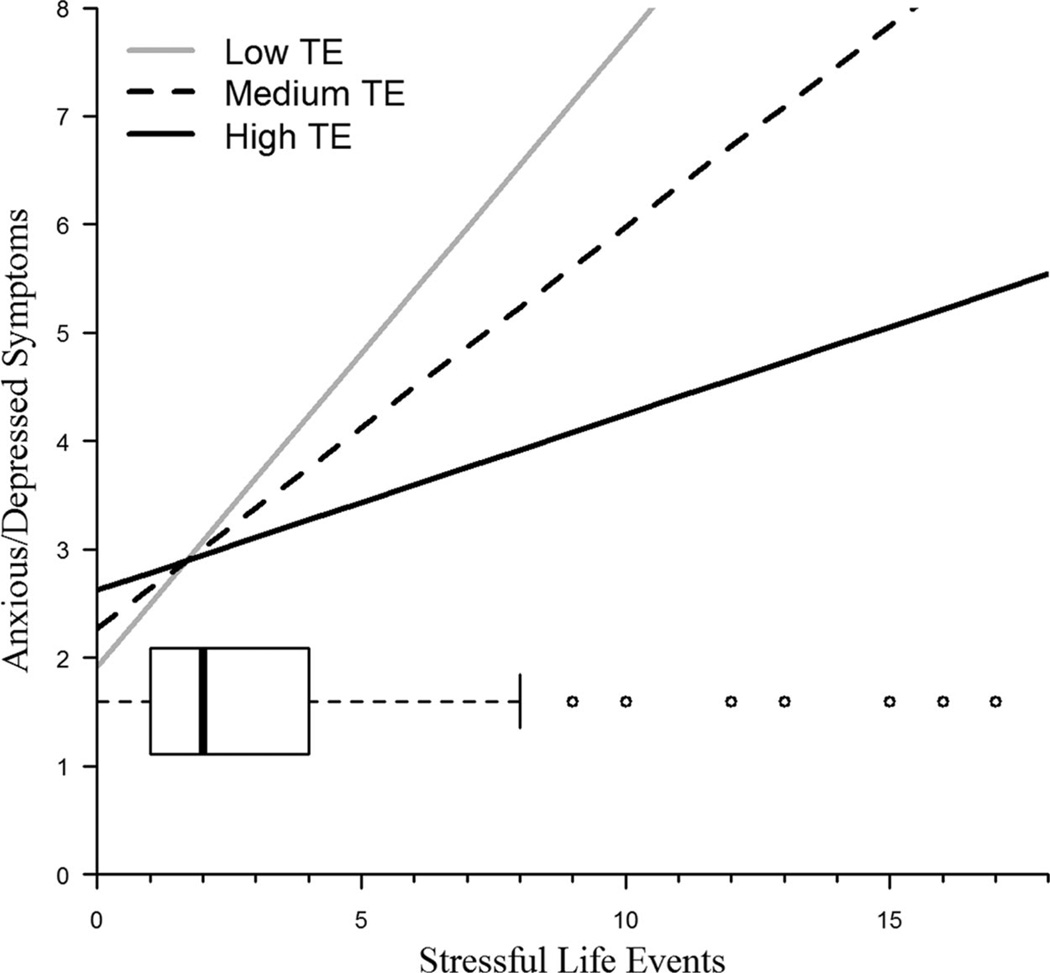
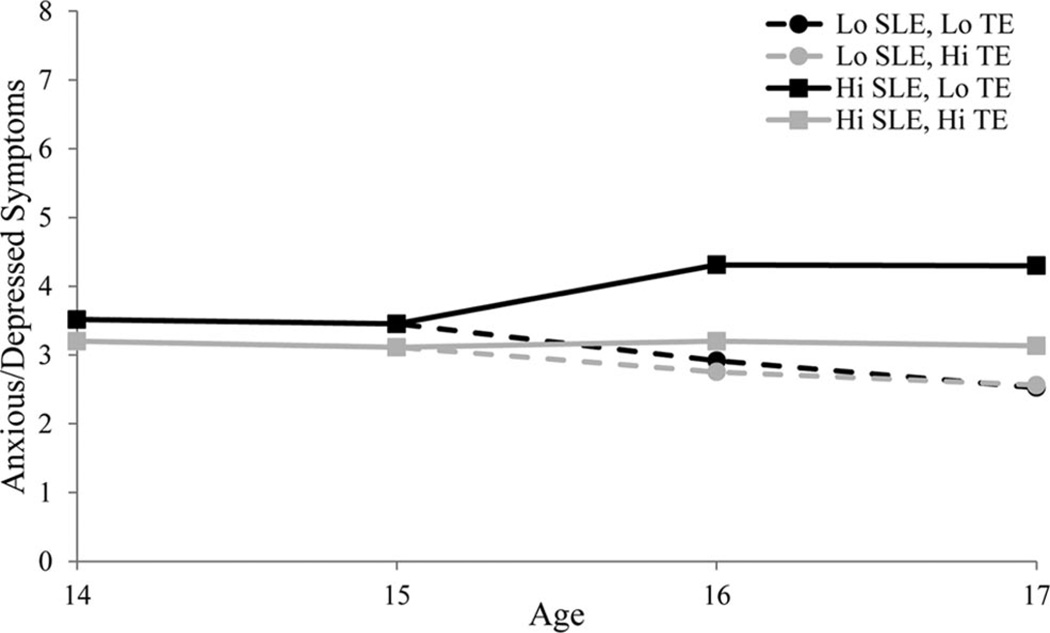
A latent estimate (i.e., combined mother- and self-report ratings) of anxious/depressed symptoms at age 17 is plotted in Figure 3 as a function of genotype and number of SLEs experienced at age 17. The model-implied trajectories of mother-reported anxious/depressed symptoms over time as a function of genotype and SLEs are depicted in Figure 4, showing the heightened levels in the trajectories of anxious/depressed symptoms in late adolescence among those with the risk genotype who experienced high levels of stress at ages 16–17.
Figure 3.
Interaction between transcriptional efficiency (TE; defined by 5-HTTLPR + adenine/guanine substitution) and stressful life events (SLEs) in predicting the latent factor of anxious/depressed symptoms at age 17 (AD17) from the ages 16–17 change score model. Includes overlaid box and whisker plot of SLEs at age 17.
Figure 4.
Mother-reported anxious/depressive symptoms over time grouped by adenine/guanine-modified 5-HTTLPR genotype transcriptional efficiency (Hi TE vs. Lo TE) and stressful life events (Hi SLEs vs. Lo SLEs). Depicts the model-implied trajectories from the ages 16–17 change score model. SLEs were plotted at ± 1 SD of the mean. For pictorial simplicity, the figure included only High TE (2) and Low TE (0) and excluded Medium TE (1). The Medium TE group was not, however, excluded from any analyses. SLE = stressful life events; TE = transcriptional efficiency.
Does the G × E Effect Differ by Gender?
To test the differential effect of gender on the interaction between 5-HTTLPR and SLEs in predicting anxious/depressed symptoms, we conducted interaction analyses in a multigroup model by gender. The models were the same as the ages 16–17 CSM with the exception of the removal of gender as a covariate because the models were tested on male and female subsets. In the baseline gender model, the structural models were constrained to be equal for both genders. Tests of model fit suggested that the baseline gender model fit the data moderately well, χ2(N = 574) = 902.59, CFI = 0.80, RMSEA = 0.062. In the comparison gender model, the G × E interaction terms at ages 16–17 were allowed to vary by gender. There was no significant improvement, however, in model fit after freeing the interaction terms at ages 16 and 17 to be different by gender (Satorra-Bentler scaled chi-square difference [1] = 1.34, p = .248), suggesting that the interaction effect did not differ by gender.1 Specifically, the G × E effect was significant in predicting the acceleration of anxious/depressed symptoms at ages 16–17 among males (B = −0.14, z = −3.05, p = .002) and females (B = −0.10, z = −2.30, p = .022). In order to determine our power to detect an interaction by gender, we ran a simulation of the multigroup CSM. The model had .80 power to detect a difference of parameter estimates between males and females when the difference in their estimates was 0.087.
Does the G × E Effect Differ by Ethnicity?
Due to possible differences in allelic frequencies and linkage disequilibrium patterns across different racial groups (although no allele frequency differences were found in the present sample), the ages 16–17 CSM (with the exclusion of the ethnicity dummy variable) was tested separately among European Americans to evaluate the interaction between 5-HTTLPR genotype and SLEs. There was no significant interaction predicting the initial level (B = 0.13, z = 1.24, p = .217), initial growth (B = −0.01, z = 0.05, p = .964), or acceleration (B = −0.01, z = −0.15, p = .884) from ages 14 to 15. There was, however, an interaction predicting the acceleration of anxious/depressed symptoms at ages 16–17 (B = −0.09, z = −2.25, p = .024) in the same direction as the effect in the model with the European American and African American sample combined. Unfortunately, we did not have a sufficiently large sample of African American and other racial groups to properly test the interactive effects of 5-HTTLPR genotype and stress in predicting anxious/depressed symptoms in other populations. The results, however, were substantively the same when comparing findings from the European American-only sample with the European American and African American samples combined, suggesting that they could be combined for purposes of the present analyses (see Footnote 1).
Does the G × E Effect Hold for the Traditional Coding of 5-HTTLPR?
Although the focus of the present study was on the A/G-modified 5-HTTLPR Gene × Stress interaction, models using the traditional coding of 5-HTTLPR were considered for the sake of comparison to previous studies. Similar to A/G-modified models, the 5-HTTLPR Genotype × SLEs interaction was not significant in predicting the initial level at age 12 (B = 0.12, z = 1.30, p = .195) or initial growth at age 13 (B = −0.08, z = −0.88, p = .377), but came closer to significance in predicting the acceleration at ages 14–17 (B = −0.06, z = −1.62, p = .105) of anxious/depressed symptoms in the baseline model. Contrary to A/G-modified models, however, the interaction effect in the 5-HTTLPR model was not stronger at ages 16–17 compared with the effect at ages 14–15 (Satorra-Bentler scaled chi-square difference [1] = 1.42, p = .115). Nevertheless, examination of the parameters in the nonsignificantly better fitting model that allowed the effects at ages 16–17 to differ from ages 14–15 demonstrated that the traditional coding of 5-HTTLPR yielded a G × E interaction with effects similar to the A/G-modified model. Specifically, the G × E interaction was not significant in predicting the initial level at age 12 (B = 0.14, z = 1.51, p = .131), initial growth at age 13 (B = −0.08, z = −0.83, p = .404), or acceleration at ages 14–15 (B = 0.01, z = 0.016, p = .871), yet was significant in predicting the acceleration at ages 16–17 (B = −0.10, z = −2.18, p = .030) in the same direction as the A/G-modified G × E effect at ages 16–17.
Discussion
It was hypothesized that the 5-HTTLPR genotype and SLEs would interact in predicting the development of anxious/depressed symptoms and that this interaction would be stronger for females. Because of findings that an SNP modifies the TE of the 5-HTTLPR-L allele, the hypotheses were tested with the A/G-modified classification of 5-HTTLPR. In an individual growth model, we detected a significant G × E interaction in the direction hypothesized. Specifically, those with lower serotonin TE genotypes were reported to experience more anxious/depressed symptoms in response to SLEs. We tested a CSM to examine the developmental timing of the omnibus G × E effect and to determine whether the G × E interaction predicts the development of anxious/depressed symptoms.
After improving model fit by modifying the model to allow effects at ages 16–17 to differ from those at ages 14–15, analyses revealed that there was an interaction between A/G-modified 5-HTTLPR and SLEs in predicting acceleration of anxious/depressed symptoms at ages 16 and 17. Individuals with low TE showed a stronger effect of SLEs on rate of acceleration in anxious/depressed symptoms at ages 16 and 17 than did individuals with high TE. There was no interaction, however, in the prediction of the intercept at age 12, growth at age 13, or acceleration at ages 14 and 15. Follow-up analyses of gender interactions and tests of ethnicity were secondary, exploratory tests that substantiated the findings by providing support for the interaction with or without African Americans and finding no differences across gender in both the multilevel model and CSM. Together, the findings suggest that the interaction effect in the model collapsing across age was driven by the effect at ages 16 and 17 and that the interaction effect was strongest in predicting changes in the growth of anxious/depressed symptoms during late adolescence, which may account for previous null findings with younger populations (Araya et al., 2009; Eley et al., 2004, among males).
The finding that the interaction effect was stronger in later adolescence, particularly at ages 16 and 17, may pertain to previous findings that rates of depression increase dramatically from middle to late adolescence (Hankin & Abela, 2005). The interaction found in this study may support the late-maturing prefrontal cortex (PFC) theory of adolescent depression (Andersen & Teicher, 2008). According to the late-maturing PFC theory, the complexity and continual development of the PFC may render adolescents especially vulnerable to the effects of stress (Andersen et al., 2008). Because one of the roles of the PFC is to modulate activity of limbic areas, including the amygdala, which is involved in processing emotion and fear, researchers have suggested that adolescent depression may result from the developmental lag between the PFC and the earlier developing limbic areas (Davey, Yücel, & Allen, 2008). Furthermore, low (compared with high) 5-HTTLPR TE is associated with increased amygdala activity and decreased connectivity between the PFC and the amygdala among those with depression (Friedel et al., 2009). Thus, low serotonin TE may render adolescents particularly vulnerable to stress by the serotonin transporter gene’s effect of increasing the reactivity of the amygdala to stress while reducing the PFC’s capacity to modulate the effects of stress experienced in the amygdala. In other words, adolescents with lower serotonin TE may be more likely to experience anxiety or depression following stress because the stresses during adolescence may be experienced more acutely as a result of the developed amygdala, but the regulatory functions of the PFC are lagging, especially for those with lower TE.
Increased stress responsivity in later relative to earlier adolescence may arise from dynamic changes in genetic effects across development. For example, studies have shown developmental changes in genetic factors from early to late adolescence in terms of anxious/depressed symptoms (Kendler et al., 2008) and anxiety sensitivity (Zavos, Gregory, & Eley, 2012). Specifically, Zavos et al. (2012) observed that new genetic effects on anxiety sensitivity emerge in late adolescence and that “the most substantial period of genetic flux was from 15 to 17 years” (p. 211). Thus, it is possible that environmental effects such as stress in interaction with the emerging effects of 5-HTTLPR may play a greater role in late compared with early adolescence.
Before the discovery of the A/G substitution affecting serotonin TE, 5-HTTLPR was coded with only L and S alleles. As a point of comparison to previous studies, we tested the CSM with the traditional coding of 5-HTTLPR (i.e., LL, LS, and SS, which may misclassify LG alleles), and the G × E effect was significant at ages 16–17 but not at other ages. Thus, although the A/G-modified coding of 5-HTTLPR sought to refine the biological measurement of serotonin TE, the coding did not moderate the results of the present study. Future studies should examine which coding is a more robust predictor of anxiety and depression in the presence of stress.
Additional analyses tested findings in previous studies among adolescents that the Gene × Stress interaction was stronger among females than males (Åslund et al., 2009; Benjet et al., 2010; Eley et al., 2004). In contrast with previous studies, the Genotype × SLEs interaction did not differ by gender, and the interaction effect was significant for both males and females. Our finding may be supported by findings in a twin study that the genetic influences on anxious/depressed symptoms are similar for males and females in childhood and adolescence (Kendler et al., 2008). It is possible, however, that there was insufficient power to detect a three-way interaction, so we recommend that future studies test the interaction longitudinally with larger samples.
A significant interaction between A/G-modified 5-HTTLPR and SLEs in predicting anxious/depressed symptoms is consistent with some findings (Gunthert et al., 2007; Zalsman et al., 2006) and inconsistent with others (Chorbov et al., 2007; Laucht et al., 2009). Regarding previous findings specific to anxiety and anxiety/depression (but not depression by itself), Gunthert et al. (2007) found an A/G-modified 5-HTTLPR × Stress interaction in the development of anxiety, similar to the findings in the present study.
The present study has several strengths. A fairly large sample of individuals was followed prospectively for 6 years over an important time period in the development of depression. Developmental perspectives are essential to understanding how factors contribute to individual growth in psychopathology. Latent CSMs evaluated the timing of the effects of genes and stress on anxiety/depression in adolescence, and the same pattern of effects held across gender and with or without African Americans in the analysis. Other researchers have noted the importance of using longitudinal studies to examine age-varying genetic effects (Lasky-Su et al., 2008). To our knowledge, no other study has used latent CSMs to evaluate the interaction between 5-HTTLPR and SLEs on the development of anxiety or depression. With few exceptions (e.g., Gunthert et al., 2007), most studies have been cross-sectional and cannot predict intraindividual change (trajectories) of depression. Other strengths of the present study include the use of multiple informant measures of anxious/depressed symptoms and continuous outcome measures. Continuous measures of symptoms facilitate better analysis of change in subsyndromal anxiety/depression, accounting for the dimensionality of depression and anxiety. The vast majority of previous findings have been presented using single informant report or diagnosis of depressive symptoms, which may be biased. To our knowledge, only one other study has included a multiple informant report of depressive symptoms (Caspi et al., 2003).
There are several limitations of the present study. One limitation is the use of a combined Anxious/Depressed subscale of the YSR and CBCL, limiting comparisons of the present findings with those of previous studies using a pure depression index. It may be noted, however, that the CBCL and YSR subscales at each age were significantly associated with later depressive disorder diagnoses in the present study. A second limitation of the present study deals with the sample size. The study is likely underpowered to detect interaction effects, particularly the three-way interaction with gender. Consequently, we present interaction analyses cautiously, particularly the gender interaction analyses, with the hope that future research will clarify the role of the proposed Gene × Stress interaction among males and females. It may be noted, however, that there is an increase in statistical power associated with repeated measures designs (B. O. Muthén & Curran, 1997), which increases our ability to detect an interaction and, therefore, our confidence in the present findings.
Although the analyses examined change in anxious/depressed symptoms, the correlational nature of the study limits our ability to draw causal inferences. Associations could owe to a third variable (e.g., a gene–environment correlation) or to the opposite direction of effect (i.e., anxious depressed symptoms lead to greater family stress). The findings and interpretations are supported, however, by experimental evidence on the effects of manipulation of the 5-HTT gene in mice on stress reactivity (Carroll et al., 2007) as well as the theoretical construct validity of the 5-HTTLPR stress-sensitivity hypothesis (Caspi et al., 2010).
Although we probed the CSM to examine the developmental timing of the G × E effect after finding a significant omnibus interaction effect when collapsing across age, there remains a possibility that the interaction effect at ages 16–17 or the lack thereof at earlier ages may owe to chance. We attempted to minimize the possibility of chance findings by correcting for multiple testing and validating our findings with different subsets in our sample and different models. Our findings suggest the possibility of a developmental shift in the G × E effect. Nevertheless, the finding requires replication in independent samples. If the finding of a later adolescence timing effect for the 5-HTTLPR × Stress interaction holds in future research, this could result in a more developmentally appropriate targeting of at-risk individuals. The finding might also encourage more developmentally informed work at the molecular level on the role of the serotonergic system in response to stress and in modulating mood, especially in relation to structural and functional studies of neural systems, particularly in late adolescence.
The present study shows the value in taking a developmental perspective when investigating gene–environment interactions. Findings demonstrate that the 5-HTTLPR genotype affects likelihood of experiencing anxious/depressed symptoms in situations of stress, particularly during late adolescence.
Acknowledgments
The Child Development Project has been funded by National Institute of Mental Health Grants MH42498, MH56961, MH57024, and MH57095; Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant HD30572; and National Institute on Drug Abuse Grant DA016903. Isaac T. Petersen is supported by the National Institute of Health and National Research Service Award HD007475-17. Danielle M. Dick is supported by K02AA018755. The authors acknowledge the role of John Budde and Alison Goate in producing the genotypic data used in this article.
Footnotes
Results from the follow-up tests of gender and ethnicity were the same in the multilevel model as the findings from the CSM, suggesting that the G × E effect did not differ by gender or ethnicity.
Contributor Information
Isaac T. Petersen, Department of Psychological and Brain Sciences, Indiana University
John E. Bates, Department of Psychological and Brain Sciences, Indiana University
Jackson A. Goodnight, Department of Psychology, University of Dayton
Kenneth A. Dodge, Center for Child and Family Policy, Duke University
Jennifer E. Lansford, Center for Child and Family Policy, Duke University
Gregory S. Pettit, Department of Human Development and Family Studies, Auburn University
Shawn J. Latendresse, Department of Psychiatry, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University
Danielle M. Dick, Department of Psychiatry, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University
References
- Achenbach TM. Manual for the Child Behavior Checklist and 1991 profile. Burlington: University of Vermont, Department of Psychiatry; 1991a. [Google Scholar]
- Achenbach TM. Manual for the Youth Self-Report and 1991 profile. Burlington: University of Vermont, Department of Psychiatry; 1991b. [Google Scholar]
- Achenbach TM, McConaughy SH, Howell CT. Child/adolescent behavioral and emotional problems: Implications of cross-informant correlations for situational specificity. Psychological Bulletin. 1987;101:213–232. [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends in Neurosciences. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. Journal of Neuropsychiatry and Clinical Neurosciences. 2008;20:292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya R, Hu X-Z, Heron J, Enoch M-A, Evans J, Lewis G, Goldman D. Effects of stressful life events, maternal depression and 5-HTTLPR genotype on emotional symptoms in pre-adolescent children. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2009;150B:670–682. doi: 10.1002/ajmg.b.30888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åslund C, Leppert J, Comasco E, Nordquist N, Oreland L, Nilsson KW. Impact of the interaction between the 5HTTLPR polymorphism and maltreatment on adolescent depression. A population-based study. Behavior Genetics. 2009;39:524–531. doi: 10.1007/s10519-009-9285-9. [DOI] [PubMed] [Google Scholar]
- Bates D. Fitting linear mixed models in R. R News. 2005;5:27–30. Retrieved from http://www.r-project.org/doc/Rnews/Rnews_2005-1.pdf. [Google Scholar]
- Benjet C, Thompson RJ, Gotlib IH. 5-HTTLPR moderates the effect of relational peer victimization on depressive symptoms in adolescent girls. Journal of Child Psychology and Psychiatry. 2010;51:173–179. doi: 10.1111/j.1469-7610.2009.02149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon KH, Rae DS, Locke BZ, Narrow WE, Regier DA. Estimating the prevalence of mental disorders in U.S. adults from the Epidemiologic Catchment Area Survey. Public Health Reports. 1992;107:663–668. [PMC free article] [PubMed] [Google Scholar]
- Brown GW, Bifulco A, Harris TO. Life events, vulnerability and onset of depression: Some refinements. British Journal of Psychiatry. 1987;150:30–42. doi: 10.1192/bjp.150.1.30. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Boyce-Rustay JM, Millstein R, Yang R, Wiedholz LM, Murphy DL, Holmes A. Effects of mild early life stress on abnormal emotion-related behaviors in 5-HTT knockout mice. Behavior Genetics. 2007;37:214–222. doi: 10.1007/s10519-006-9129-9. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003 Jul 18;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chipman P, Jorm AF, Prior M, Sanson A, Smart D, Tan X, Eastel S. No interaction between the serotonin transporter polymorphism (5-HTTLPR) and childhood adversity or recent stressful life events on symptoms of depression: Results from two community surveys. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2007;144B:561–565. doi: 10.1002/ajmg.b.30480. [DOI] [PubMed] [Google Scholar]
- Chorbov VM, Lobos EA, Todorov AA, Heath AC, Botteron KN, Todd RD. Relationship of 5-HTTLPR genotypes and depression risk in the presence of trauma in a female twin sample. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2007;144B:830–833. doi: 10.1002/ajmg.b.30534. [DOI] [PubMed] [Google Scholar]
- Davey CG, Yücel M, Allen NB. The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neuroscience & Biobehavioral Reviews. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Bates JE, Pettit GS. Mechanisms in the cycle of violence. Science. 1990 Dec 21;250:1678–1683. doi: 10.1126/science.2270481. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Pettit GS, Bates JE. Socialization mediators of the relation between socioeconomic status and child conduct problems. Child Development. 1994;65:649–665. [PubMed] [Google Scholar]
- Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, Craig IW. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Molecular Psychiatry. 2004;9:908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- Fantoni-Salvador P, Rogers R. Spanish versions of the MMPI-2 and PAI: An investigation of concurrent validity with Hispanic patients. Assessment. 1997;4:29–39. [Google Scholar]
- Friedel E, Schlagenhauf F, Sterzer P, Park SQ, Bermpohl F, Ströhle A, Wrase J. 5-HTT genotype effect on prefrontal–amygdala coupling differs between major depression and controls. Psychopharmacology. 2009;205:261–271. doi: 10.1007/s00213-009-1536-1. [DOI] [PubMed] [Google Scholar]
- Gauderman WJ. Sample size requirements for matched case-control studies of gene–environment interaction. Statistics in Medicine. 2002;21:35–50. doi: 10.1002/sim.973. [DOI] [PubMed] [Google Scholar]
- Gerhardt CA, Compas BE, Connor JK, Achenbach TM. Association of a mixed anxiety-depression syndrome and symptoms of major depressive disorder during adolescence. Journal of Youth and Adolescence. 1999;28:305–323. [Google Scholar]
- Goodnick PJ, Goldstein BJ. Selective serotonin reuptake inhibitors in affective disorders—I. Basic pharmacology. Journal of Psychopharmacology. 1998;12:S5–S20. doi: 10.1177/0269881198012003021. [DOI] [PubMed] [Google Scholar]
- Gunthert KC, Conner TS, Armeli S, Tennen H, Covault J, Kranzler HR. Serotonin transporter gene polymorphism (5-HTTLPR) and anxiety reactivity in daily life: A daily process approach to gene-environment interaction. Psychosomatic Medicine. 2007;69:762–768. doi: 10.1097/PSY.0b013e318157ad42. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abela JRZ. Depression from childhood through adolescence and adulthood. In: Hankin BL, Abela JRZ, editors. Development of psychopathology: A vulnerability-stress perspective. Thousand Oaks, CA: Sage Publications; 2005. pp. 245–288. [Google Scholar]
- Hankin BL, Fraley RC, Lahey BB, Waldman ID. Is depression best viewed as a continuum or discrete category? A taxo-metric analysis of childhood and adolescent depression in a population-based sample. Journal of Abnormal Psychology. 2005;114:96–110. doi: 10.1037/0021-843X.114.1.96. [DOI] [PubMed] [Google Scholar]
- Hasin D, Samet S, Nunes E, Meydan J, Matseoane K, Waxman R. Diagnosis of comorbid psychiatric disorders in substance users assessed with the Psychiatric Research Interview for Substance and Mental Disorders for DSM-IV. The American Journal of Psychiatry. 2006;163:689–696. doi: 10.1176/ajp.2006.163.4.689. [DOI] [PubMed] [Google Scholar]
- Hoffman DL, Dukes EM, Wittchen H-U. Human and economic burden of generalized anxiety disorder. Depression and Anxiety. 2008;25:72–90. doi: 10.1002/da.20257. [DOI] [PubMed] [Google Scholar]
- Hu X-Z, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. The American Journal of Human Genetics. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress and depression meta-analysis revisited. Archives of General Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Gelernter J, Kaffman A, Caspi A, Moffitt T. Arguable assumptions, debatable conclusions. Biological Psychiatry. 2010;67:e19–e20. doi: 10.1016/j.biopsych.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Lichtenstein P. A developmental twin study of symptoms of anxiety and depression: Evidence for genetic innovation and attenuation. Psychological Medicine. 2008;38:1567–1575. doi: 10.1017/S003329170800384X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression. Archives of General Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Kumsta R, Stevens S, Brookes K, Schlotz W, Castle J, Beckett C, Sonuga-Barke E. 5HTT genotype moderates the influence of early institutional deprivation on emotional problems in adolescence: Evidence from the English and Romanian Adoptee (ERA) study. Journal of Child Psychology & Psychiatry. 2010;51:755–762. doi: 10.1111/j.1469-7610.2010.02249.x. [DOI] [PubMed] [Google Scholar]
- Lasky-Su J, Lyon HN, Emilsson V, Heid IM, Molony C, Raby BA, Lange C. On the replication of genetic associations: Timing can be everything! The American Journal of Human Genetics. 2008;82:849–858. doi: 10.1016/j.ajhg.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laucht M, Treutlein J, Blomeyer D, Buchmann AF, Schmid B, Becker K, Banaschewski T. Interaction between the 5-HTTLPR serotonin transporter polymorphism and environmental adversity for mood and anxiety psychopathology: Evidence from a high-risk community sample of young adults. International Journal of Neuropsychopharmacology. 2009;12:737–747. doi: 10.1017/S1461145708009875. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Annual research review: Developmental considerations of gene by environment interactions. Journal of Child Psychology and Psychiatry. 2011;52:429–441. doi: 10.1111/j.1469-7610.2011.02381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch K-P, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996 Nov 29;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Hops H, Roberts RE, Seeley JR, Andrews JA. Adolescent psychopathology: I. Prevalence and incidence of depression and other DSM-III—R disorders in high school students. Journal of Abnormal Psychology. 1993;102:133–144. doi: 10.1037//0021-843x.102.1.133. [DOI] [PubMed] [Google Scholar]
- Malone PS, Lansford JE, Castellino DR, Berlin LJ, Dodge KA, Bates JE, Pettit GS. Divorce and child behavior problems: Applying latent change score models to life event data. Structural Equation Modeling. 2004;11:401–423. doi: 10.1207/s15328007sem1103_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Cleak J, Willis-Owen SAG, Flint J, Shifman S. Mapping regulatory variants for the serotonin transporter gene based on allelic expression imbalance. Molecular Psychiatry. 2007;12:421–422. doi: 10.1038/sj.mp.4001952. [DOI] [PubMed] [Google Scholar]
- McArdle J, Hamagami F. Latent difference score structural models for linear dynamic analyses with incomplete longitudinal data. In: Collins LM, Sayer AG, editors. New methods for the analysis of change, decade of behavior. Washington, DC: American Psychological Association; 2001. pp. 139–175. [Google Scholar]
- Middeldorp CM, Boomsma DI. Genetics and psychopathology. In: Berntson GG, Cacioppo JT, editors. Handbook of neuroscience for behavioral sciences. New York, NY: Wiley; 2009. pp. 1180–1202. [Google Scholar]
- Munafò MR, Durrant C, Lewis G, Flint J. Gene × environment interactions at the serotonin transporter locus. Biological Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Muthén BO, Curran PJ. General longitudinal modeling of individual differences in experimental designs: A latent variable framework for analysis and power estimation. Psychological Methods. 1997;2:371–402. [Google Scholar]
- Muthén LK, Muthén BO. Mplus (version 5) Los Angeles, CA: Author; 2007. [Google Scholar]
- Nobile M, Rusconi M, Bellina M, Marino C, Giorda R, Carlet O, Battaglia M. The influence of family structure, the TPH2 G-703T and the 5-HTTLPR serotonergic genes upon affective problems in children aged 10–14 years. Journal of Child Psychology and Psychiatry. 2009;50:317–325. doi: 10.1111/j.1469-7610.2008.01958.x. [DOI] [PubMed] [Google Scholar]
- Piccinelli M, Wilkinson G. Gender differences in depression. Critical review. British Journal of Psychiatry. 2000;177:486–492. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D the R Core Team. nlme: Linear and nonlinear mixed effects models. R package version 3.1–93. 2009 Retrieved from http://cran.r-project.org/web/packages/nlme/index.html. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. Retrieved from http://www.R-project.org. [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K-Y, Eaves L, Hoh J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. Journal of the American Medical Association. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM. Diagnostic Interview Schedule for DSM-IV (DIS-IV) St. Louis, MO: Washington University School of Medicine; 1995. [Google Scholar]
- Roy A, Hu X-Z, Janal MN, Goldman D. Interaction between childhood trauma and serotonin transporter gene variation in suicide. Neuropsychopharmacology. 2007;32:2046–2052. doi: 10.1038/sj.npp.1301331. [DOI] [PubMed] [Google Scholar]
- Roy M-A, Neale MC, Pedersen NL, Mathé AA, Kendler KS. A twin study of generalized anxiety disorder and major depression. Psychological Medicine. 1995;25:1037–1049. doi: 10.1017/s0033291700037533. [DOI] [PubMed] [Google Scholar]
- Simon GE. Social and economic burden of mood disorders. Biological Psychiatry. 2003;54:208–215. doi: 10.1016/s0006-3223(03)00420-7. [DOI] [PubMed] [Google Scholar]
- Sjöberg RL, Nilsson KW, Nordquist N, Öhrvik J, Leppert J, Lindström L, Oreland L. Development of depression: Sex and the interaction between environment and a promoter polymorphism of the serotonin transporter gene. International Journal of Neuropsychopharmacology. 2006;9:443–449. doi: 10.1017/S1461145705005936. [DOI] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NWJ, Willis-Owen SAG, Luben R, Day NE, Flint J. Social adversity, the serotonin transporter (5-HTTLPR) polymorphism and major depressive disorder. Biological Psychiatry. 2006;59:224–229. doi: 10.1016/j.biopsych.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biological Psychiatry. 2006;60:671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Uddin M, Koenen KC, de los Santos R, Bakshis E, Aiello AE, Galea S. Gender differences in the genetic and environmental determinants of adolescent depression. Depression and Anxiety. 2010;27:658–666. doi: 10.1002/da.20692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: Review and methodological analysis. Molecular Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Wadsworth ME, Hudziak JJ, Heath AC, Achenbach TM. Latent class analysis of Child Behavior Checklist anxiety/depression in children and adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40:106–114. doi: 10.1097/00004583-200101000-00023. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Molecular Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Zalsman G, Huang Y-Y, Oquendo MA, Burke AK, Hu X-z, Brent DA, Mann JJ. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. The American Journal of Psychiatry. 2006;163:1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]
- Zavos HMS, Gregory AM, Eley TC. Longitudinal genetic analysis of anxiety sensitivity. Developmental Psychology. 2012;48:204–212. doi: 10.1037/a0024996. [DOI] [PubMed] [Google Scholar]