Abstract
Multiple endocrine neoplasia type 1 (MEN1) is an inherited autosomal dominant disease presenting with pancreatic neuroendocrine tumors (pNETs), parathyroid tumors, or pituitary tumors. Using the PubMed database, we reviewed the literature on information regarding the proper diagnosis and treatment of MEN1-associated pNET. Many cases of MEN1-associated pNET are functioning pNETs. Gastrinomas and insulinomas tend to occur frequently in the duodenum and pancreas, respectively. In addition to diagnostic imaging, the selective arterial secretagogue injection test (SASI test) is useful for localizing functioning pNET. The standard treatment is surgical resection. However, in the case of a functioning pNET, the tumor should first be accurately located using the SASI test before an appropriate surgical method is selected. In cases of a MEN1-associated non-functioning pNET that exceeds 2 cm in diameter, the incidence of distant metastasis is significantly increased, and surgery is recommended. In cases of unresectable pNET, a somatostatin analog has been shown to demonstrate antitumor effects and is considered to be a promising treatment. In addition, molecular-targeted drugs have recently been found to be effective in phase III clinical trials.
Keywords: Multiple endocrine neoplasia type 1, Pancreatic neuroendocrine tumor, Multiple tumors, Selective arterial secretagogue injection test
INTRODUCTION
Multiple endocrine neoplasia (MEN) is an autosomal dominant inherited disease presenting with tumorous lesions, mainly in various endocrine organs. In 1954, Wermer1 first indicated that patients with multiple tumors in the parathyroid gland, pituitary gland, and pancreatic islets of Langerhans were actually suffering from an autosomal dominant inherited syndrome instead of simple concomitant onset of the tumors. In 1961, Sipple2 reported a syndrome complicated with medullary thyroid carcinoma and pheochromocytoma. A syndrome consisting of mainly pancreatic neuroendocrine tumor (pNET), parathyroid tumor, pituitary tumor, etc., that was formally called "multiple endocrine adenomatosis," which was found to be hereditary by Wermer,1 was subsequently classified as MEN type 1 (MEN1). A syndrome consisting of mainly medullary thyroid carcinoma and pheochromocytoma, as reported by Sipple,2 was classified as MEN2.
Patients with MEN1-associated pNET often have multiple tumors or malignant tumors that determine patients' prognosis for survival. Therefore, it is considered important to make a cautious diagnosis and select the most appropriate treatment method for each patient. In light of this, we used the PubMed database to review the literature on MEN1-associated pNET and examine the diagnosis and treatment methods appropriate for MEN1-associated pNET.
ETIOLOGY OF MEN1
MEN1, which was identified by Chandrasekharappa et al.3 in 1997 as the gene that causes MEN1, is located on the long arm of chromosome 11 (11q13). This gene, which is also called menin, consists of about 10 exons dispersed over a region of approximately 7,000 base pairs, and encodes a 610-amino acid protein.3 Menin mRNA has been found in all normal human tissues thus far examined. Menin protein has also been found in pancreatic exocrine cells, where MEN1 tumors do not develop.4 Menin protein is mainly located in the nucleus where it binds with nuclear proteins, including JunD, NFκB, and Smad3, as well as with histone deacetylase and histone methyl-transferase. It is thought to be involved in cell growth, apoptosis, DNA repair, and transcriptional regulation.5,6
In patients with MEN1, the inactivated MEN1 gene has existed heterozygously from the time of ontogeny, while the other normal gene has lost function through somatic mutation, thus causing tumor formation of specific cells.
In familial and non-familial MEN1, the detection rate of MEN1 mutations is 90% and 70%, respectively,7 and the lifetime incidence of MEN1 is nearly 100% in those with the mutations. Therefore, if a patient clinically suspected of having MEN1 cannot be definitely diagnosed, a genetic test is required.
CLINICAL FEATURES OF pNET IN MEN1 PATIENTS
It is reported that the prevalence of MEN1 is estimated to be approximately 1 in 10,000 to 30,000, and that non-familial MEN1 patients account for approximately 15% of total MEN1 patients.8 In people with MEN1 mutations, a few percent of those 10 years of age and under develop MEN1, and while, by 40 years of age, nearly 100% of them develop the disease.9 Hyperparathyroidism is the most frequent initial disease in MEN1 (85%), and in people in their 40s, the incidence of parathyroid adenoma is higher than that of other tumor types.10 Pituitary adenoma and pNET are the second most frequent diseases.
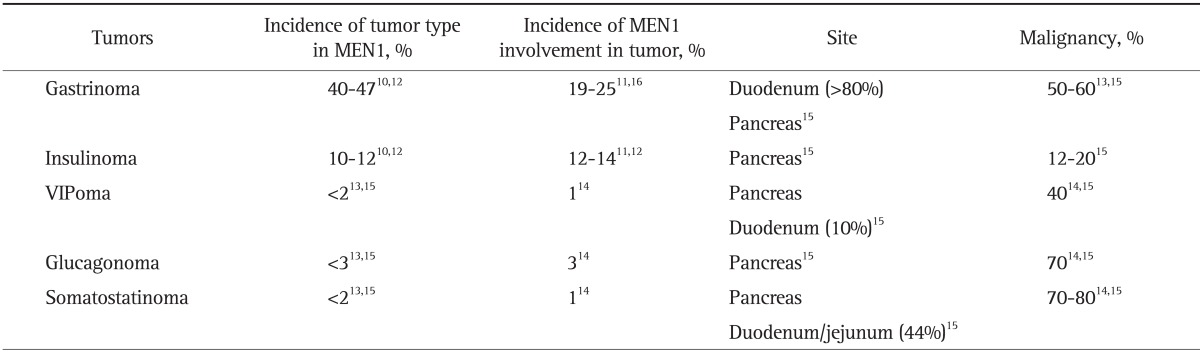
According to a recent nationwide survey on gastroenteropancreatic neuroendocrine tumors in Japan,11 MEN1 was concurrently found in 10% of pNET patients. As for the breakdown of pNET in MEN1 patients, gastrinoma was the most frequent (25%), followed by insulinoma (14%). Interestingly, non-functioning pNET (6.1%) occurred less frequently in Japan than in Western countries.11 VIPoma and glucagonoma were also found, although rarely, as other functioning pNET that present with specific symptoms due to the hormones produced. Table 1 summarizes the characteristics of the MEN1-associated functioning pNET reported in literature.10-16
Table 1.
The Characteristics of Functioning Pancreatic Neuroendocrine Tumors in Patients with MEN1
MEN1, multiple endocrine neoplasia type 1.
In cases of gastrinoma, refractory multiple ulcers and diarrhea, etc., develop due to increased gastrin concentrations in the blood (Table 2).14,17,18 This disease is called Zollinger-Ellison syndrome (ZES). In gastrinoma, the incidence of concurrent MEN1 is as high as 20% to 38%;16,19 and therefore, gastrinoma should be treated with MEN1 in mind. Gastrinoma is often found in the duodenum of MEN1 patients. Since gastrinoma occurs as multiple tumors that are usually smaller than 0.5 mm in size, it is sometimes difficult to detect by imaging.
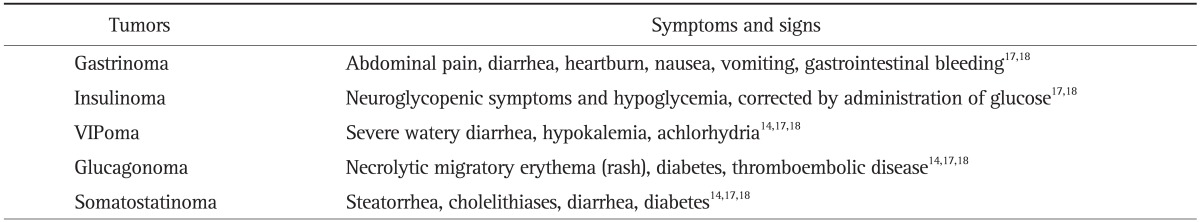
Table 2.
The Symptoms and Signs of Functioning Pancreatic Neuroendocrine Tumors
Insulinoma often presents with symptoms of hypoglycemia caused by an elevation of insulin levels in the blood. Insulinoma usually develops as an isolated disease; however, a patient with multiple pancreatic tumors is likely to have MEN1. Like gastrinoma, it is usually difficult to identify all the pancreatic tumors by imaging studies in MEN1 patients.
The most characteristic sign of glucagonoma is necrotic migratory erythema. While glucagonoma is usually considered malignant due to distant metastasis, MEN1-associated cases apparently have tiny tumors with fewer symptoms.20
Most of endocrine tumors with MEN1 are benign, while MEN1-associated pNET is considered to be relatively high risk for malignancy. It is reported that pNET is an important determinant of survival in MEN1 patients and the most important cause of MEN1-related death.21,22 Therefore, the surveillance for MEN1-associated pNET is recommended for patients as follows: 1) asymptomatic individuals with MEN1 mutation, 2) individuals with a clinical diagnosis of MEN1, and 3) symptomatic individuals at risk for MEN1 with an affected parent who have not undergone genetic testing. A diagnostic interval of 1 to 3 years is widely considered to be appropriate for asymptomatic individuals because of the low MEN1-associated tumor proliferation rate.18
DIAGNOSIS
1. Diagnosis of MEN1
MEN1 is diagnosed when at least 2 of the main tumorous diseases (parathyroid tumor, pNET, and pituitary tumor) are found. However, these tumors may occur at different times; therefore, whenever one of these endocrine tumors is detected, the other sites should be monitored over time. As mentioned earlier, genetic testing for MEN1 mutation may be useful, if a definite diagnosis is difficult, or if it is a familial case. Nevertheless, such tests should be implemented cautiously, since it could become a social issue.
2. Imaging diagnosis of MEN1-associated pNET
1) Conventional imaging studies: detection of pNET
As is the case for sporadic pNET that does not involve MEN1, MEN1-associated pNET is also a plethoric tumor involving many tumor vessels. Therefore, imaging diagnosis of MEN1-associated pNET includes an early-phase staining pattern on enhanced computed tomography (CT) (Figs 1A and 2A), and tumor staining image on angiography. CT imaging, in particular, is useful for delineating neuroendocrine tumors in extrapancreatic lesions. Furthermore, while contrast magnetic resonance imaging shows an early-phase staining pattern, it may be useful for differentiating solid pseudopapillary neoplasms or metastatic tumors from pNET, especially in non-functioning pNET.
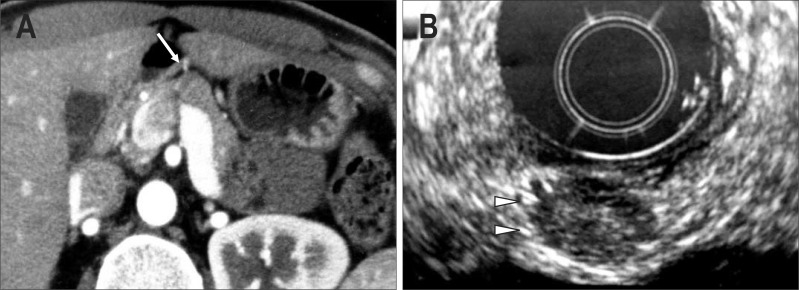
Fig. 1.
A case of MEN1-unassociated insulinoma (34-year-old female). (A) Abdominal imaging computed tomography (CT). A tumor with a slight early phase stain pattern is observed at the pancreatic head and body border (arrow). (B) Endoscopic ultrasonography. A tumor is observed at the site shown using CT (arrowheads). No other tumors are detected in the pancreas.
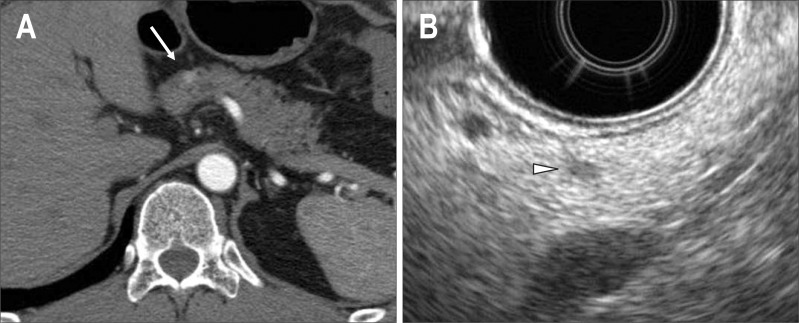
Fig. 2.
A case of MEN1-associated insulinoma (44-year-old male). (A) Abdominal imaging computed tomography (CT). A tumor with an early phase stain pattern is observed at the pancreatic head and body border (arrow). (B) Endoscopic ultrasonography. In addition to the lesion shown using CT, small tumors are found at the pancreatic head, body, and tail. The case is considered to have multiple pancreatic lesions. The arrowhead shows a 3-mm lesion in the pancreatic body.
MEN1-associated pNET is characterized by multiple tumors sporadically distributed across the pancreas. Though multiple intrapancreatic tumors are often tiny, endoscopic ultrasonography (EUS) and intraductal ultrasonography can delineate such minute tumors due to their excellent spatial resolution (Figs 1B and 2B). Since EUS, in particular, can be used for outpatients, it is valuable for monitoring patients over time and the delineation of multiple minute intrapancreatic lesions.
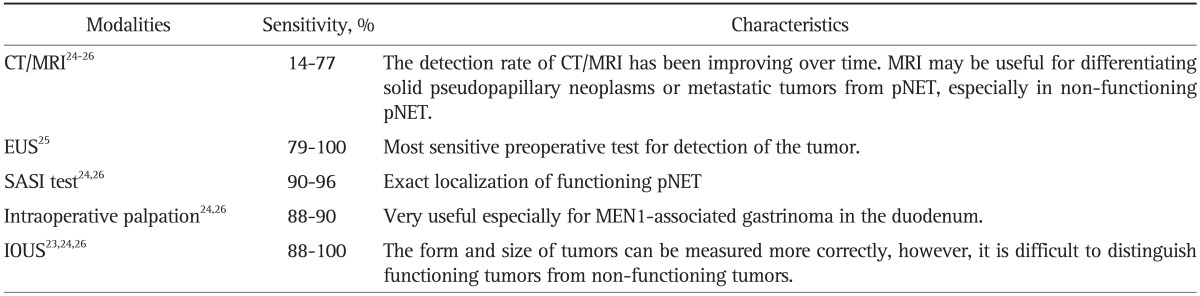
Intraoperative ultrasonography (IOUS) is useful for estimating the character of a tumor and to measure the distance between a tumor and the main pancreatic duct which is very important information to determine whether enucleation can be undergone safely or not. In addition, the form and size of pNET can be measured more correctly with IOUS than any other preoperative imaging techniques (Table 3).23-26
Table 3.
The Characteristics and Sensitivities of the Diagnostic Modalities for pNET
pNET, pancreatic neuroendocrine tumor; CT, computed tomography; MRI, magnetic resonance imaging; EUS, endoscopic ultrasonography; SASI test, selective arterial secretagogue injection test; MEN1, multiple endocrine neoplasia type 1; IOUS, intraoperative ultrasonography.
2) Functional imaging studies: exact localization of functioning pNET
Nevertheless, even the combination of these diagnostic imaging techniques cannot necessarily delineate all of the multiple lesions. In the case of functioning pNET, selective arterial secretagogue injection test (SASI test) can be useful for the localizing tumors, since hormone is secreted by the tumor cells in response to secretin or calcium stimulant under arteriography. SASI test was developed by Imamura et al.27 to locate gastrinoma using secretin as stimulant. Subsequently, Doppman et al.28 applied this method for localizing insulinoma by changing the stimulant from secretin to calcium. In this test, a catheter is placed inside a hepatic vein under abdominal angiography in order to measure gastrin or insulin in the hepatic venous blood, and then secretin or calcium is injected into the artery responsible for each pancreatic region. The tumor is located in the region where gastrin or insulin has increased. The injection is usually made into the splenic artery, the gastroduodenal artery, or the superior mesenteric artery.28,29 Furthermore, according to another report, an additional injection into the dorsal pancreatic artery may improve diagnostic performance.30 These tests can locate the nutrient vessel for the tumor, and are therefore useful in determining which surgical method to use in order to remove the entire tumor.29 In addition, in some cases of MEN1-associated pNET, functioning tumors and non-functioning tumors exist at the same time. Therefore, in the case of MEN1-associated gastrinoma and insulinoma, SASI test should be performed before operation to localize functioning tumors exactly.
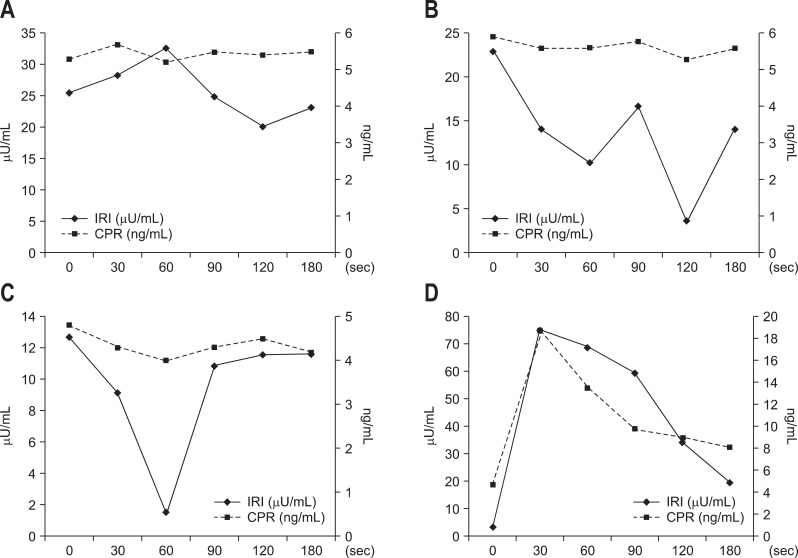
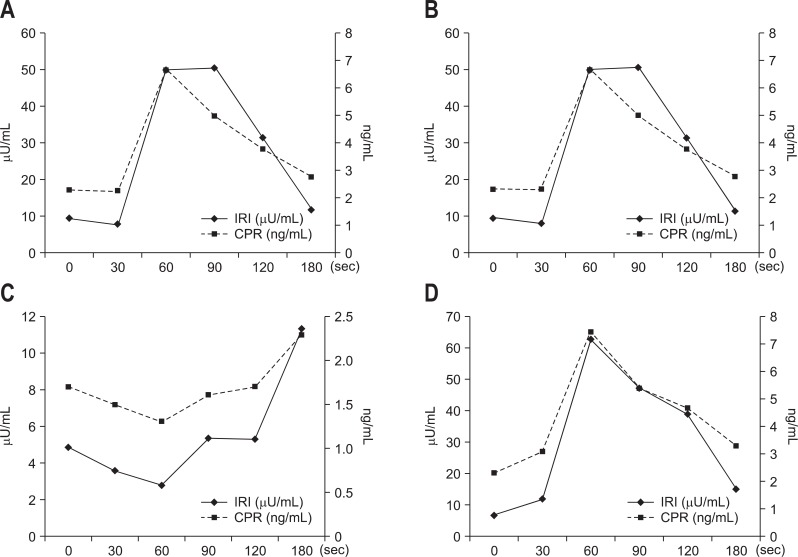
Our department conducts the SASI test using calcium. After the blood is collected, 0.025 meq/kg calcium gluconate is administered. Immunoreactive insulin or immunoreactive gastrin is measured before calcium administration and at 30, 60, 90, 120, and 180 seconds after calcium administration. For insulinoma, C-peptide immunoreactivity is also measured. Figs 3 and 4 show our SASI test data for MEN1-unassociated insulinoma and MEN1-associated insulinoma, respectively. As demonstrated by the results of CT and EUS, MEN1-unassociated insulinoma was fed by a single vessel; and therefore, it was a solitary tumor. In contrast, in the case of MEN1-associated insulinoma, CT showed only 1 tumor; however, the SASI test revealed that the insulinoma was fed by multiple vessels and that multiple minute tumors existed in the pancreas.
Fig. 3.
The selective arterial secretagogue injection test data from the case in Fig. 1. (A) Gastroduodenal artery. (B) Splenic artery. (C) Superior mesenteric artery, and (D) dorsal pancreatic artery. Step-up is found only in (D).
IRI, immunoreactive insulin; CPR, C-peptide immunoreactivity.
Fig. 4.
The selective arterial secretagogue injection test data from the case in Fig. 2. (A) Gastroduodenal artery. (B) Splenic artery. (C) Superior mesenteric artery, and (D) dorsal pancreatic artery. Step-up is found in (A), (B), and (D). In this case, a parathyroid tumor is found in addition to the pancreatic endocrine tumor.
IRI, immunoreactive insulin; CPR, C-peptide immunoreactivity.
Somatostatin receptor (receptor subtype 2 in particular) is frequently expressed in pNET. Based on this observation, somatostatin receptor scanning is useful for localizating both the primary tumor and metastatic lesions.17
TREATMENT OF pNET IN MEN1 PATIENTS
Differing reports have been published regarding resection of MEN1-associated gastrinoma with ZES for the following reasons: the symptoms can be controlled with H2 blocker or proton pump inhibitor, it is likely to be malignant, and complete cure is difficult considering frequent postoperative recurrence. At present, there are many authorities who do not recommend subjecting patients with MEN1-associated gastrinoma to aggressive surgery such as a Whipple resection because it is extensive, the long-term consequences are unclear, postoperative morbidity can be significant.17 Some groups recommend that surgical exploration should be reserved for patients with an imageable tumor 2 to 3 cm.31 In addition, there is a report that patients with MEN1-associated gastrinoma have excellent long-term survival without surgery and, even with metastatic disease, they have a 15-year survival of 52%.32 On the other hand, some reports have suggested that the tumor should be proactively removed except in cases with hepatic metastasis.29 A previous report regarding multivariate analysis of metastatic gastroenteropancreatic neuroendocrine tumors revealed that age, the number of liver metastases, tumor slope and initial surgery were predictive of survival.33 Therefore, one of the purpose of surgery for pNET is preventing hepatic metastasis. Various surgical procedures have been attempted. Akerström et al.34 recommended Thompson's procedure for MEN1-associated gastrinoma. In this procedure, a duodenotomy is performed since gastrinoma is often found in the duodenum. The tumor is then located by palpation and IOUS and removed. The mass at the pancreatic head is enucleated, and then 80% of the pancreatic tail is resected and lymph node is removed.34,35 Bartsch et al.36 recommended pylorus-preserving partial duodenectomy, in order to prevent duodenal recurrence and to maintain postoperative quality of life. Imamura et al.29,37 recommended pancreaticoduodenectomy or pancreas-preserving total duodenectomy after fully identifying the location of tumor with SASI test, in order to improve the prognosis for complete cure and to prevent hepatic metastasis, provided that the tumor is located at the pancreatic head or duodenum; a high biochemical cure (87.5%) was obtained for gastrinoma.37 In this way, surgical treatment for MEN1-associated gastrinoma remains controversial. It is also true that a controlled study has not been done to determine the exact timing and role of surgery. Additional studies are needed to clearly define whether a more aggressive approach is indicated.
In the case of MEN1-associated insulinoma, surgery is usually performed due to the intense symptoms caused by hyperinsulinemia, even if the tumor cannot be identified by imaging.34 MEN1-associated insulinoma occurring mostly in the pancreas is multiple and minute. Therefore, an SASI test should be performed preoperatively to determine the extent of resection. When only the preoperatively identified tumor is removed, recurrence rate would be high; and therefore, distal pancreatectomy is often conducted in addition to tumor enucleation.18
The treatment method for MEN1-associated non-functioning pNET has been debated. The above-mentioned nationwide survey on gastroenteropancreatic neuroendocrine tumors in Japan reported that the rate of distant metastasis is significantly high when a non-functioning pNET exceeds 2 cm in size.11 According to the guidelines of European Neuroendocrine Tumor Society, surgery is recommended for MEN1-associated non-functioning pNET exceeding 2 cm in diameter, in order to prevent further progression.38 However, more data is needed.
In contrast, unresectable malignant pancreatic endocrine tumors are mainly treated by controlling the symptoms of hormone excess, managing hepatic metastasis, and with systemic chemotherapy, as is the case for MEN1-unassociated pNET. A somatostatin analog that can inhibit secretion of various hormones has demonstrated anti-tumor effects (PROMID study),39 and is considered a promising treatment. According to various reports, other systemic chemotherapy agents include streptozotocin, dacarbazine, and temozolomide.40-42 A report on the latest molecular targeted drugs for pNET showed that the mTOR inhibitor everolimus extended the median progression-free survival from 4.6 to 11.0 months and reduced the progression risk by 65% (hazard ratio, 0.35; p<0.0001) in a worldwide Phase III study (RADIANT-3).43 It is a very promising drug for unresectable pNET and will likely become the first choice of treatment. Additionally, the median progression-free survival of the group treated with the tyrosine kinase inhibitor sunitinib was 11.4 months, which was significantly longer than the 5.5 months of the placebo group in a Phase III study.44
CONCLUSION
MEN1-associated pNET is mostly functioning pNET such as gastrinoma and insulinoma, which frequently manifest as multiple minute tumors. For a functioning pNET, surgery and other therapeutic strategies should be determined after SASI test is conducted to identify the exact location of the tumors. In contrast, for MEN1-associated non-functioning pNET, surgery is recommended when the tumor exceeds 2 cm in diameter, since the likelihood of distant metastasis is high.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Wermer P. Genetic aspects of adenomatosis of endocrine glands. Am J Med. 1954;16:363–371. doi: 10.1016/0002-9343(54)90353-8. [DOI] [PubMed] [Google Scholar]
- 2.Sipple JH. The association of pheochromocytoma with carcinoma of the thyroid gland. Am J Med. 1961;31:163–166. [Google Scholar]
- 3.Chandrasekharappa SC, Guru SC, Manickam P, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404–407. doi: 10.1126/science.276.5311.404. [DOI] [PubMed] [Google Scholar]
- 4.Cavallari I, D'Agostino DM, Ferro T, et al. In situ analysis of human menin in normal and neoplastic pancreatic tissues: evidence for differential expression in exocrine and endocrine cells. J Clin Endocrinol Metab. 2003;88:3893–3901. doi: 10.1210/jc.2002-021840. [DOI] [PubMed] [Google Scholar]
- 5.Marx SJ. Molecular genetics of multiple endocrine neoplasia types 1 and 2. Nat Rev Cancer. 2005;5:367–375. doi: 10.1038/nrc1610. [DOI] [PubMed] [Google Scholar]
- 6.Poisson A, Zablewska B, Gaudray P. Menin interacting proteins as clues toward the understanding of multiple endocrine neoplasia type 1. Cancer Lett. 2003;189:1–10. doi: 10.1016/s0304-3835(02)00509-8. [DOI] [PubMed] [Google Scholar]
- 7.Tsukada T, Yamaguchi K, Kameya T. The MEN1 gene and associated diseases: an update. Endocr Pathol. 2001;12:259–273. doi: 10.1385/ep:12:3:259. [DOI] [PubMed] [Google Scholar]
- 8.Trump D, Farren B, Wooding C, et al. Clinical studies of multiple endocrine neoplasia type 1 (MEN1) QJM. 1996;89:653–669. doi: 10.1093/qjmed/89.9.653. [DOI] [PubMed] [Google Scholar]
- 9.Bassett JH, Forbes SA, Pannett AA, et al. Characterization of mutations in patients with multiple endocrine neoplasia type 1. Am J Hum Genet. 1998;62:232–244. doi: 10.1086/301729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandi ML, Gagel RF, Angeli A, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658–5671. doi: 10.1210/jcem.86.12.8070. [DOI] [PubMed] [Google Scholar]
- 11.Ito T, Sasano H, Tanaka M, et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010;45:234–243. doi: 10.1007/s00535-009-0194-8. [DOI] [PubMed] [Google Scholar]
- 12.Marx S, Spiegel AM, Skarulis MC, et al. Multiple endocrine neoplasia type 1: clinical and genetic topics. Ann Intern Med. 1998;129:484–494. doi: 10.7326/0003-4819-129-6-199809150-00011. [DOI] [PubMed] [Google Scholar]
- 13.Marini F, Falchetti A, Del Monte F, et al. Multiple endocrine neoplasia type 1. Orphanet J Rare Dis. 2006;1:38. doi: 10.1186/1750-1172-1-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty GM. Rare endocrine tumours of the GI tract. Best Pract Res Clin Gastroenterol. 2005;19:807–817. doi: 10.1016/j.bpg.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Tonelli F, Giudici F, Fratini G, Brandi ML. Pancreatic endocrine tumors in multiple endocrine neoplasia type 1 syndrome: review of literature. Endocr Pract. 2011;17(Suppl 3):33–40. doi: 10.4158/EP10376.RA. [DOI] [PubMed] [Google Scholar]
- 16.Jensen RT. Management of the Zollinger-Ellison syndrome in patients with multiple endocrine neoplasia type 1. J Intern Med. 1998;243:477–488. doi: 10.1046/j.1365-2796.1998.00281.x. [DOI] [PubMed] [Google Scholar]
- 17.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–1492. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pieterman CR, Vriens MR, Dreijerink KM, van der Luijt RB, Valk GD. Care for patients with multiple endocrine neoplasia type 1: the current evidence base. Fam Cancer. 2011;10:157–171. doi: 10.1007/s10689-010-9398-6. [DOI] [PubMed] [Google Scholar]
- 19.Akerström G, Hessman O, Skogseid B. Timing and extent of surgery in symptomatic and asymptomatic neuroendocrine tumors of the pancreas in MEN 1. Langenbecks Arch Surg. 2002;386:558–569. doi: 10.1007/s00423-001-0274-6. [DOI] [PubMed] [Google Scholar]
- 20.Frankton S, Bloom SR. Gastrointestinal endocrine tumours. Glucagonomas. Baillieres Clin Gastroenterol. 1996;10:697–705. doi: 10.1016/s0950-3528(96)90019-6. [DOI] [PubMed] [Google Scholar]
- 21.Dean PG, van Heerden JA, Farley DR, et al. Are patients with multiple endocrine neoplasia type I prone to premature death? World J Surg. 2000;24:1437–1441. doi: 10.1007/s002680010237. [DOI] [PubMed] [Google Scholar]
- 22.Doherty GM, Olson JA, Frisella MM, et al. Lethality of multiple endocrine neoplasia type I. World J Surg. 1998;22:581–586. doi: 10.1007/s002689900438. [DOI] [PubMed] [Google Scholar]
- 23.Grant CS, van Heerden J, Charboneau JW, James EM, Reading CC. Insulinoma. The value of intraoperative ultrasonography. Arch Surg. 1988;123:843–848. doi: 10.1001/archsurg.1988.01400310057009. [DOI] [PubMed] [Google Scholar]
- 24.Kuzin NM, Egorov AV, Kondrashin SA, et al. Preoperative and intraoperative topographic diagnosis of insulinomas. World J Surg. 1998;22:593–597. doi: 10.1007/s002689900440. [DOI] [PubMed] [Google Scholar]
- 25.Khashab MA, Yong E, Lennon AM, et al. EUS is still superior to multidetector computerized tomography for detection of pancreatic neuroendocrine tumors. Gastrointest Endosc. 2011;73:691–696. doi: 10.1016/j.gie.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 26.Wiesli P, Brändle M, Schmid C, et al. Selective arterial calcium stimulation and hepatic venous sampling in the evaluation of hyperinsulinemic hypoglycemia: potential and limitations. J Vasc Interv Radiol. 2004;15:1251–1256. doi: 10.1097/01.RVI.0000140638.55375.1E. [DOI] [PubMed] [Google Scholar]
- 27.Imamura M, Takahashi K, Adachi H, et al. Usefulness of selective arterial secretin injection test for localization of gastrinoma in the Zollinger-Ellison syndrome. Ann Surg. 1987;205:230–239. doi: 10.1097/00000658-198703000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doppman JL, Miller DL, Chang R, et al. Insulinomas: localization with selective intraarterial injection of calcium. Radiology. 1991;178:237–241. doi: 10.1148/radiology.178.1.1984311. [DOI] [PubMed] [Google Scholar]
- 29.Imamura M. Recent standardization of treatment strategy for pancreatic neuroendocrine tumors. World J Gastroenterol. 2010;16:4519–4525. doi: 10.3748/wjg.v16.i36.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baba Y, Miyazono N, Nakajo M, et al. Localization of insulinomas. Comparison of conventional arterial stimulation with venous sampling (ASVS) and superselective ASVS. Acta Radiol. 2000;41:172–177. doi: 10.1080/028418500127345000. [DOI] [PubMed] [Google Scholar]
- 31.Norton JA, Fraker DL, Alexander HR, et al. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999;341:635–644. doi: 10.1056/NEJM199908263410902. [DOI] [PubMed] [Google Scholar]
- 32.Norton JA, Alexander HR, Fraker DL, et al. Comparison of surgical results in patients with advanced and limited disease with multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. Ann Surg. 2001;234:495–505. doi: 10.1097/00000658-200110000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durante C, Boukheris H, Dromain C, et al. Prognostic factors influencing survival from metastatic (stage IV) gastroenteropancreatic well-differentiated endocrine carcinoma. Endocr Relat Cancer. 2009;16:585–597. doi: 10.1677/ERC-08-0301. [DOI] [PubMed] [Google Scholar]
- 34.Akerström G, Hessman O, Hellman P, Skogseid B. Pancreatic tumours as part of the MEN-1 syndrome. Best Pract Res Clin Gastroenterol. 2005;19:819–830. doi: 10.1016/j.bpg.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Thompson NW. Surgical treatment of the endocrine pancreas and Zollinger-Ellison syndrome in the MEN 1 syndrome. Henry Ford Hosp Med J. 1992;40:195–198. [PubMed] [Google Scholar]
- 36.Bartsch DK, Langer P, Wild A, et al. Pancreaticoduodenal endocrine tumors in multiple endocrine neoplasia type 1: surgery or surveillance? Surgery. 2000;128:958–966. doi: 10.1067/msy.2000.109727. [DOI] [PubMed] [Google Scholar]
- 37.Imamura M, Komoto I, Ota S, et al. Biochemically curative surgery for gastrinoma in multiple endocrine neoplasia type 1 patients. World J Gastroenterol. 2011;17:1343–1353. doi: 10.3748/wjg.v17.i10.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plöckinger U, Rindi G, Arnold R, et al. Guidelines for the diagnosis and treatment of neuroendocrine gastrointestinal tumours. A consensus statement on behalf of the European Neuroendocrine Tumour Society (ENETS) Neuroendocrinology. 2004;80:394–424. doi: 10.1159/000085237. [DOI] [PubMed] [Google Scholar]
- 39.Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 40.Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519–523. doi: 10.1056/NEJM199202203260804. [DOI] [PubMed] [Google Scholar]
- 41.Arnold R, Rinke A, Schmidt Ch, Hofbauer L. Endocrine tumours of the gastrointestinal tract: chemotherapy. Best Pract Res Clin Gastroenterol. 2005;19:649–656. doi: 10.1016/j.bpg.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Yalcin S. Advances in the systemic treatment of pancreatic neuroendocrine tumors. Cancer Treat Rev. 2011;37:127–132. doi: 10.1016/j.ctrv.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]