Abstract
The etiopathogenesis of the irritable bowel syndrome (IBS), one of the most prevalent gastrointestinal disorders, is not well known. The most accepted hypothesis is that IBS is the result of the disturbance of the 'brain-gut axis.' Although the pathophysiological mechanisms of intestinal dysfunction are complex and not completely understood, stress, infections, gut flora, and altered immune response are thought to play a role in IBS development. The intestinal barrier, composed of a single-cell layer, forms a physical barrier that separates the intestinal lumen from the internal milieu. The loss of integrity of this barrier is related with mucosal immune activation and intestinal dysfunction in IBS. The number of mast cells and T lymphocytes is increased in the intestinal mucosa of certain IBS patients, and the mediators released by these cells could compromise the epithelial barrier function and alter nerve signaling within the enteric nervous system. The association of clinical symptoms to structural and functional abnormalities of the mucosal barrier in IBS patients highlights the importance of understanding the physiological role of the gut barrier in the pathogenesis of this disorder. This review summarizes the clinical and experimental evidences indicating the cellular and molecular mechanisms of IBS symptomatology, and its relevance for future translational research.
Keywords: Intestinal barrier function, Irritable bowel syndrome, Tight junctions, Mast cells
INTRODUCTION
The intestinal epithelial barrier function is a crucial component of gut homeostasis as it represents the first line of defence against many insults, such as bacterial and food antigens and toxins. Loss of molecular and functional integrity leading to disturbed epithelial barrier and subsequent activation of mucosal immune responses is closely related to the pathogenesis of many intestinal disorders,1,2 including irritable bowel syndrome (IBS).
Although reckoned as multifactorial, current understanding of IBS origin and underlying mediators and mechanisms is still fragmentary, but today IBS is thought to be the result of the dysregulation of the interaction between the central and the enteric nervous system, the so-called brain-gut axis.3 The mechanisms behind this disturbance are not clear but a plausible hypothesis postulates that the breakdown of intestinal epithelial barrier's surveillance is a key mechanism in the development of symptoms compatible with IBS. Evidence has evolved from in vivo data showing increased intestinal permeability in IBS patients,4-7 being more pronounced in those diarrhea predominant IBS (IBS-D)4 patients (Table 1). In line with these observations, converging reports have revealed the presence of low-grade inflammation and immune activation8-10 and impaired barrier function11-13 in the intestinal mucosa of certain IBS subgroups as a leading events in the origin and severity of visceral hypersensitivity and bowel dysfunction in IBS. In these patients, the inflammatory infiltrate is dominated by increased populations of mast cells9,10 which have been implicated in intestinal epithelial barrier dysfunction, mainly through modulation of tight junction (TJ) proteins, resulting in increased permeability through the paracellular pathway in the intestinal epithelium.
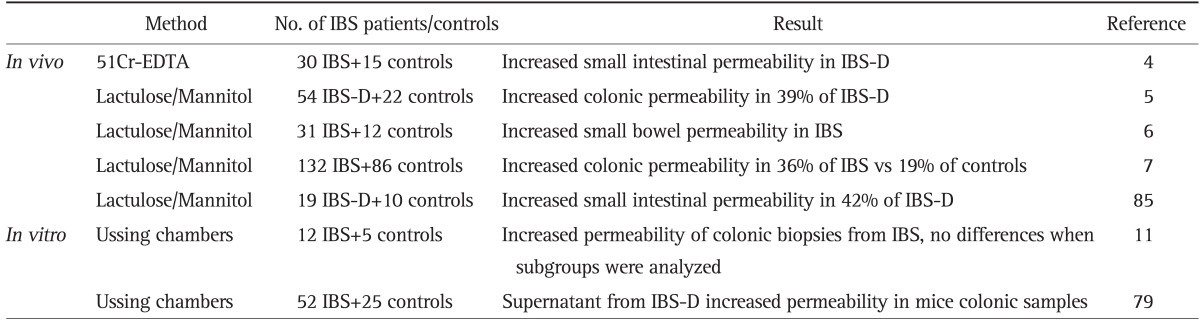
Table 1.
Summary of the in vivo and in vitro Assessments of Intestinal Permeability in Patients with Irritable Bowel Syndrome (IBS)
EDTA, ethylenediaminetetraacetic acid; IBS-D, diarrhea-predominant irritable bowel syndrome.
In this review we summarize clinical and experimental evidence indicating molecular and cellular mechanisms underlying mucosal barrier dysfunction, associated with clinical symptoms in IBS.
INTESTINAL EPITHELIAL BARRIER FUNCTION
The gastrointestinal (GI) tract constitutes one of the largest surface areas that is exposed to and interacts with the external environment. To assure homeostasis, the intestinal epithelium faces a unique challenge to balance the requirements of forming a protective barrier to separate the intestinal lumen from the underlying tissue while simultaneously supporting the absorption of nutrients and water across the epithelial barrier. Several immune and nonimmune defense mechanisms have evolved to fence off the continuous luminal threats. These mechanisms are organized into two coordinated levels of protection and include immune (epithelial and immune cells) as well as nonimmune components (gut motility, mucus layer, and secretion of water). These elements are thoroughly interconnected and defects in any of them can result in barrier dysfunction, altered intestinal permeability, eventually leading to inflammatory processes.
At the same time, small amounts of antigens are allowed to penetrate the epithelial barrier favoring the induction of immune tolerance mechanisms. This transport of molecules from the intestinal lumen to the lamina propria can occur through two main routes: the paracellular pathway, which involves passive movement of small molecules (<600 Da) through the space between adjacent epithelial cells; and the transcellular pathway, which involves both active and passive movement of larger molecules across epithelial cell membranes, usually involving the action of specific transport channels and endocytotic mechanisms mediated or not by specific membrane receptors.
1. Mucosal barrier components
1) Extracellular components of the intestinal barrier
The intestinal mucosal surface is covered with a layer of mucins and lipids that limit the exposure of the monolayer of intestinal epithelial cells to sheer forces and other physical trauma from particles within the lumen, and also prevents direct contact of the epithelium with microorganisms.14 The mucus layer provides the first protection against luminal microorganisms and contributes to the retention of mucosal secretions rich in antibacterial peptides (such as cathelicidins and defensins) and immunoglobulin A which binds, aggregates, or destroys bacteria preventing their adherence to the mucosa and subsequent transepithelial invasion.15,16 Emerging evidence have shown that defensins are not only required to fight invading pathogens, but also participate in modulating microbiota composition and, hence, shaping adaptive immune responses.17
In addition, the high turnover of epithelial cells and intestinal peristalsis together with massive water secretion in response to pathogenic stimulus are also essential factors of the intestinal barrier function.
2) Cellular components of the intestinal barrier
The cellular components of the intestinal barrier consist of a complex array of cell types present within the epithelium: absorptive enterocytes, mucus-producing goblet cells, enteroendocrine cells that produce peptide hormones and Paneth cells involved in secretion of antimicrobial peptides. In addition, both innate and adaptive immune cells, mainly T and B lymphocytes, immunoglobulin A (IgA)-secreting plasma cells, mast cells, dendritic cells, and macrophages are present at steady state and interact together to maintain a balanced immune response against intestinal antigens aimed at preserving gut homeostasis.
Intestinal epithelial cells also contribute to the mucosal immune surveillance through the expression of a wide range of pattern recognition receptors, such as toll-like receptor (TLR) and intracellular nucleotide-binding oligomerization domain (NOD)-like receptors.18 Signaling through NODs and TLRs is regulated by intestinal microbiota and its activation in enterocytes and Paneth cells leads to the expression and release of antimicrobial peptides.15 These molecules participate actively in the intestinal host-microbial homeostasis reinforcing the barrier function. In addition, the enterocytes are able to act as nonprofessional antigen presenting cells and communicate to and modulate diverse underlying populations of intestinal immunocytes. Indeed, epithelial cells are able to release several chemokines and cytokines involved in neutrophil and macrophage recruitment (CXC-chemokine ligand [CXCL]-1 and CXCL-2) and in the regulation of the inflammatory potential of dendritic cells (thymic stromal lymphopoietin). Furthermore, recognition of bacteria through TLRs could also account for the production of CD40-independent IgA class switch recombination-inducing signals such as B-cell activating factor of the tumor necrosis factor (TNF) family and proliferation-inducing ligand by epithelial cells. The dysregulation of this immunomodulatory function of the intestinal epithelial cells might contribute to both, the loss of tolerogenic mechanisms and the induction of inflammatory responses, ultimately leading to the development of intestinal inflammation.19
2. The apical juntional complex
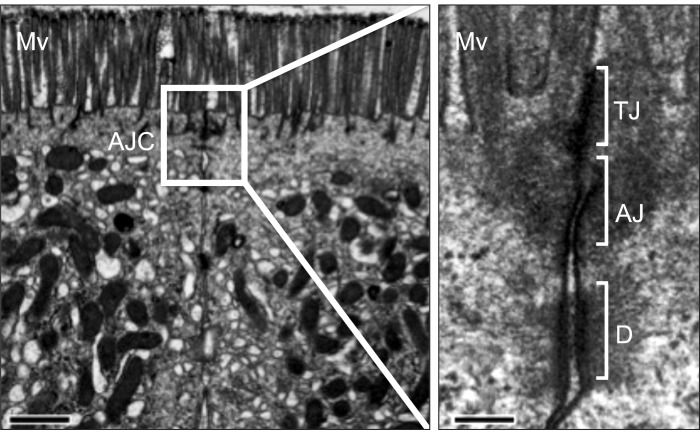
The epithelial cells within the intestinal epithelium form a continuous, polarized monolayer where the individual cell membranes are connected together through the apical junctional complex. This intercellular complex is composed of TJ, adherens junctions, and desmosomes, as well as gap junctions for cell-to-cell communication (Fig. 1).
Fig. 1.
The apical junctional complex. Transmission electron micrograph showing the apical junctional complex (AJC) between two adjacent enterocytes in the human jejunal mucosa. Just below the base of the microvilli (Mv), the plasma membranes of adjacent cells are in intimate contact at the tight junction (TJ), followed by the adherens junction (AJ) and desmosomes (D). Bar, 1 µm; amplified area bar, 150 nm.
1) TJs
Intestinal permeability to small, water-soluble molecules is determined by TJs, which seal the paracellular spaces between the epithelial cells lining the lumen of the gut. TJs are the major constituents of the apical junctional complex, located at the most luminal site of the epithelium. TJs act as both a gate that limits the paracellular transport of ions and the translocation of luminal antigens (microorganisms and their toxins), and a fence that separates the plasma membrane into the apical and basolateral domains.1
TJs are multi-protein complexes composed of transmembrane proteins (occludin, claudins, and junctional adhesion molecule) and peripheral membrane (scaffolding) proteins (zonula occludens [ZO]) (Fig. 2). The structural and functional integrity of the TJ depends on the presence of a peri-junctional ring of actin and myosin, which also contributes to the regulation of paracellular permeability. Indeed, contraction of the peri-junctional cytoskeleton induced after phosphorylation of myosin light chain (MLC) has been shown to lead to enhanced permeability in intestinal epithelia.20 It is believed that the tension resulting from this contraction is transmitted to the lateral membranes of adjacent epithelial cells perturbing, thereby, TJ barrier function.
Fig. 2.
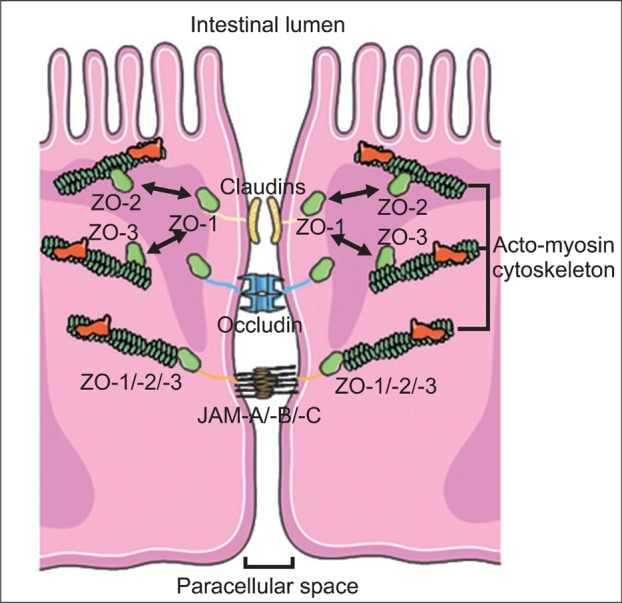
Molecular components of the tight junction. Tight junctions are composed of integral transmembrane proteins (claudins, occludin and junctional adhesion molecules [JAM]-A, -B and -C) that interact in the paracellular space with proteins on adjacent cells. These transmembrane proteins are connected to the actomyosin cytoskeleton filaments through scaffolding proteins (zonula occludens [ZO] [ZO-1, ZO-2, and ZO-3]).
Occludin was the first transmembrane protein to be discovered at the TJ; however, its function is not yet fully delineated. Despite occludin being an important constituent of TJs, in occludin knockout animals, TJ assembly and paracellular permeability remained unchanged.21,22 However, a detailed characterization suggested that occludin play a role in the regulation of TJ integrity rather than in the de novo assembly of the TJ.21 Furthermore, in vitro data suggests that TJ assembly, transepithelial resistance, and localization of occludin to the TJ are regulated by phosphorylation.23,24 In homeostasis, occludin is highly phosphorylated on Ser and Thr residues, while phosphorylation of Tyr residues is kept at minimum. This hyperphosphorylated occludin is selectively located at the TJ. However, during the disruption of TJs, in response to various stimuli, occludin undergoes dephosphorylation at Ser/Thr residues, which results in redistribution of the protein from the membrane to the cytoplasm.24 The precise role of Tyr-phosphorylation in TJ disruption is not clear at present, but it has been suggested that it may attenuate the interaction between occludin and ZO proteins, leading to the disruption of the TJ.25
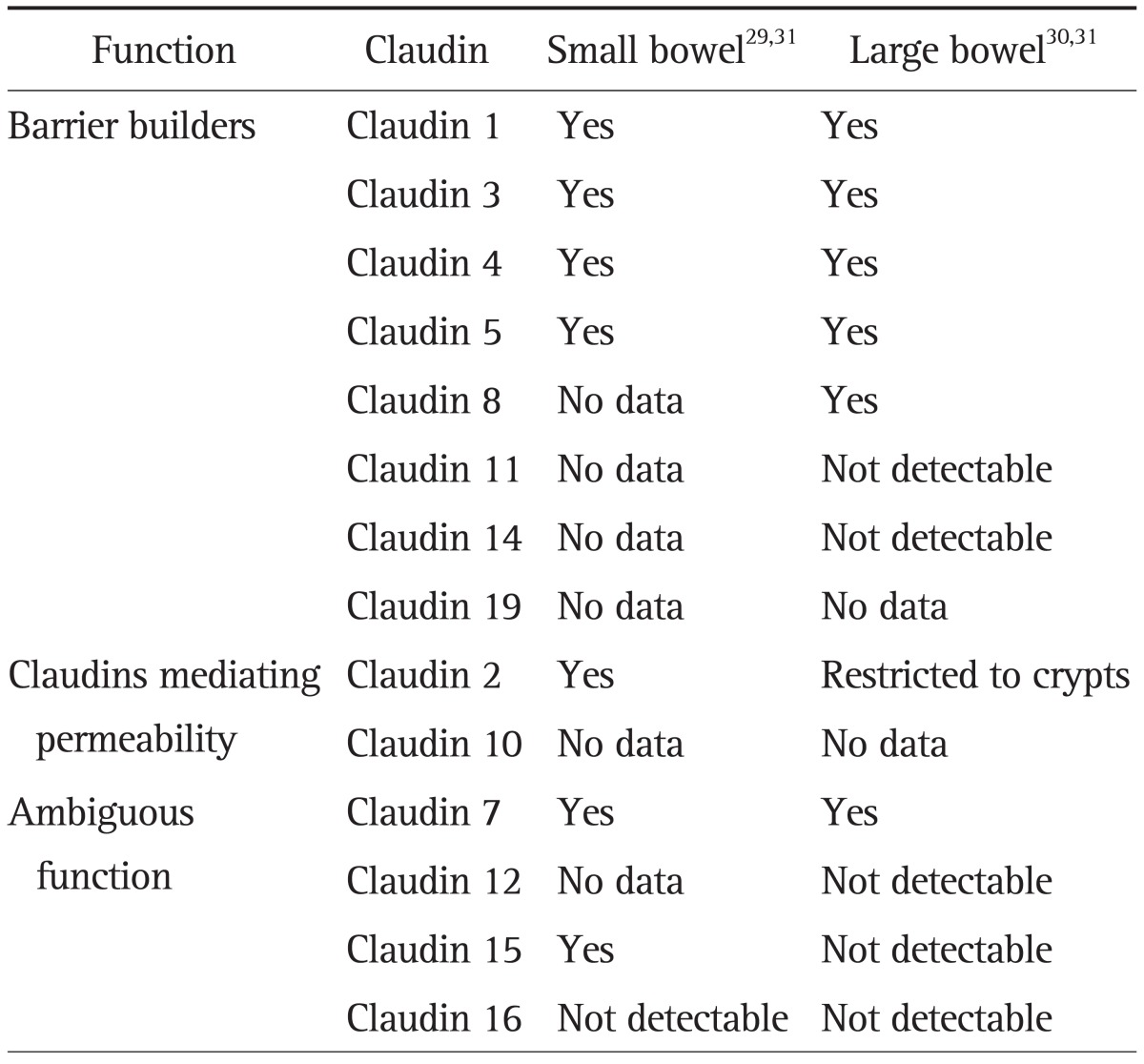
The observation that barriers can develop in the absence of occludin22 prompted a continued search of essential barrier-forming components of the TJ leading to the identification of the claudin family of transmembrane proteins, which consists of 24 members between 20 and 27 kDa in size. Claudins play a central role in the coordination of barrier function and their differential expression and properties appear to determine the tissue-specific variations in electrical resistance and paracellular ionic selectivity.26 The functional characterization of single claudins has identified specific contributions to the barrier properties in the different epithelia of the organism. Some claudins form pores that allow preferential passage of specific ions, while other claudins contribute to strengthening the barrier by preventing the passage of specific ions (Table 2). Within the intestine, the ion-selective properties of the paracellular barrier vary considerably between the individual segments due to their specific physiological function showing an overall decrease in permeability towards the distal ends of the GI tract.27 Similarly, the ability of intestinal epithelial TJ to discriminate and restrict passage of solutes based on size varies along the crypt-villus axis.28 At the molecular level, this is achieved thanks to the segment-specific expression of different claudins (Table 2). Thus, barrier properties along the intestinal epithelium, and along the crypt-villus axis, are in accordance with the localization of claudins. Studies in the human intestine have revealed the expression of tightening claudin-1, -3, -4, -5, and -7 in the ileum and colonic mucosa,29,30 with barely detectable expression of the permeability mediator claudin-2 restricted to the colonic crypts.30,31 However, claudin-2 expression has been detected in both, the crypt and the villus of the small bowel.31 This change in the expression and distribution of claudins may respond to the specific physiological function carried out by the intestinal epithelium along the intestine, as it has to guarantee the absorption of fluid and nutrients in the small bowel while protecting against a vast amount of luminal antigens from the resident microbiota in the colon.
Table 2.
Functional Classification of the Claudin Family of Proteins and Their Specific Expression Pattern in the Human Gut
The TJ transmembrane proteins, claudins and occludin, are structurally associated to the cytoskeleton, and are composed of actin, myosin II and other proteins, through the scaffolding proteins ZO.32 This association to the peri-junctional actomyosin ring, which encircles the cell at the apical surface, is crucial for the dynamic regulation of paracellular permeability. Interestingly, simultaneous elimination of ZO-1 and ZO-2 expression prevents claudin recruitment, TJ formation and development of epithelial barrier function.33
Although a large number of other TJ-associated proteins have been also identified, the manner by which they interact to form the selectively permeable barrier is not fully defined. Alterations in these structural and regulatory TJ proteins have been linked to barrier dysfunction and increased intestinal permeability in response to a broad range of inflammatory and infectious stimuli.1 However, the molecular mechanisms by which TJ-related proteins alter paracellular permeability in specific intestinal diseases require further investigation.
INTESTINAL PERMEABILITY REGULATION
Far from being a static element, the epithelial barrier function is incredibly dynamic and functionally responsive to several physiologic, pathologic, and pharmacologic stimuli. Thus, the intestinal TJ are constantly being restructured by dietary factors, neuro-humoral signaling, inflammatory mediators and a variety of cellular signaling pathways which, in turn, are susceptible of being usurped by pathogenic bacteria to gain access to the intestinal mucosa.22
Regulation of intestinal permeability involves different functional pathways. Fast changes in permeability occur via MLC kinase (MLCK)-mediated cytoskeleton contraction and by endocytosis of TJ proteins,34,35 whereas more lasting permeability disturbances involve the transcriptional modulation of TJ proteins, epithelial cell apoptosis and structural alterations in the epithelium.36
MLCK is a Ca2+-calmodulin-dependent Ser-Thr kinase that specifically phosphorylates MLC in response to both physiological and pathological stimuli.37,38 Phosphorylation of MLC results in a conformational change that triggers the contraction of the actomyosin cytoskeleton leading to epithelial barrier dysfunction. In addition, MLCK activity is also involved in the endocytosis of occludin in response to the pro-inflammatory mediator TNF. This TNF-induced occludin internalization has been reported to be specifically mediated by caveolae endocytosis highlighting the role of vesicular traffic pathways in intestinal TJ regulation in response to cytokine-mediated barrier dysfunction.39
In vivo functional assessment of the intestinal epithelial barrier can be achieved by measuring differential permeability of the intestinal mucosa to nonabsorbable molecular markers, including monosaccharides and disaccharides, along the crypt-villus axis (Table 1).40 Moreover, the physiological properties of the paracellular gap can also be accurately assessed in vitro by measuring the transepithelial electrical resistance and ionic diffusion potentials, and by quantifying the flux of either labeled or electron-dense tracer molecules of various sizes.41 Using both approaches, several reports have shown that abnormal intestinal permeability characterizes several chronic inflammatory disorders in which this phenomenon has been connected to the disappearance of key structural proteins of the intestinal epithelial barrier.42 Although altered intestinal permeability has also been reported in a subset of patients with IBS4-7 and the grade of barrier defect related to the onset of symptoms,43 the mechanisms responsible and the mediators remain mostly ignored.
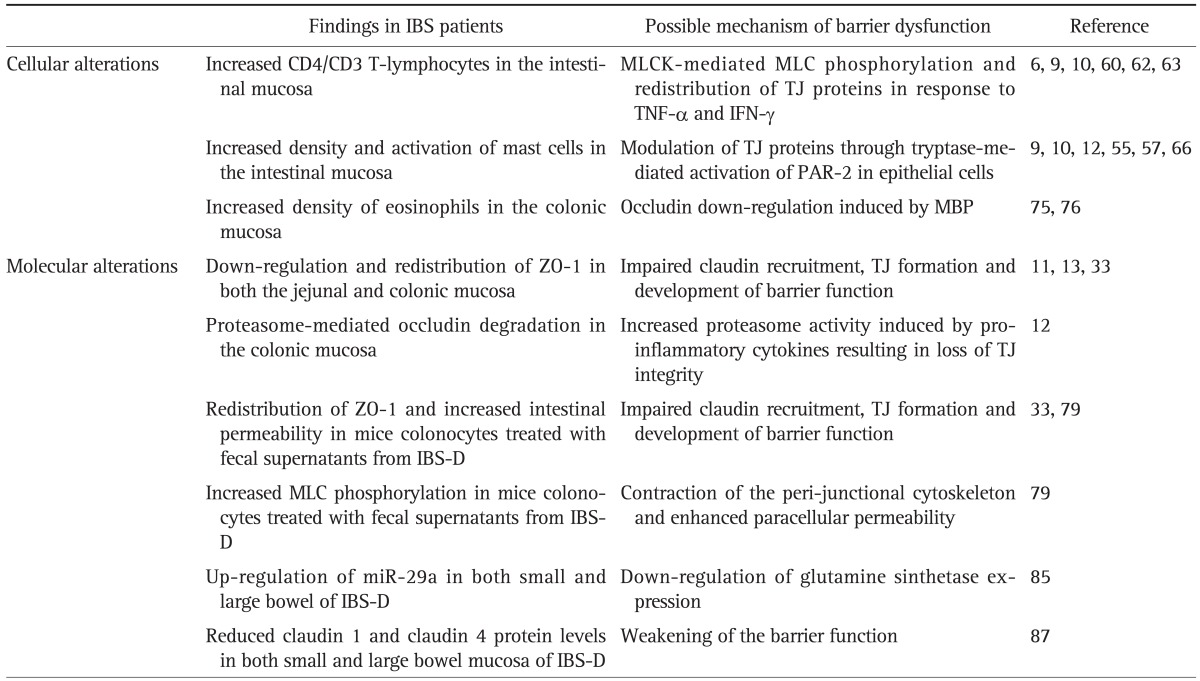
A complete discussion of all the mechanisms involved in the regulation of intestinal permeability is beyond the scope of this article but a few deserve closer attention as they are important in our current understanding of IBS pathogenesis (Table 3).
Table 3.
Summary of the Cellular and Molecular Alterations That Affect the Intestinal Barrier Function in IBS
IBS, irritable bowel syndrome; MLCK, myosin light chain kinase; MLC, myosin light chain; TJ, tight junction; TNF-α, tumor necrosis factor alpha; IFN-γ, interferon gamma; ZO, zonula occludens; PAR-2, protease-activated receptor 2; MBP, major basic protein; IBS-D, diarrhea-predominant irritable bowel syndrome.
REGULATION OF INTESTINAL BARRIER FUNCTION IN IBS
1. Stress-induced intestinal barrier dysfunction
Stress, either physical or psychological, represents a threat to the internal homeostasis. In response to stress, a coordinated autonomic, endocrine, and immune response is generated to maintain stability. However, excessive stress exposure, in susceptible individuals, impairs this adaptive response, eventually predisposing those subjects to the development of new diseases or to the exacerbation of previously existing ones.44 This may be the case of IBS as epidemiological, empirical, and clinical observations have related stressful or traumatic life events with symptom onset, severity, and persistence in certain IBS subtypes.45 In fact, around 50% of IBS patients present comorbidity with psychiatric disorders, commonly anxiety and depression,46 and suffer more chronic stress than the healthy population.10
The core system of the stress response involves the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic branch of the autonomic nervous system. The latter mediates brain-gut communication through the enteric nervous system, modulating and coordinating GI motility, secretion, and immune function. The release of corticotropin releasing factor (CRF) from the hypothalamus in response to activation of the HPA promotes the synthesis of corticotropin in the pituitary gland, which stimulates the adrenal cortex to release cortisol that finally circulate in the blood reaching every tissue and facilitating the coordination of brain and peripheral functions. The effects of stress on gut function are universal; however, IBS patients appear to have enhanced reactivity to stress compared to healthy subjects regarding to intestinal motility, sensitivity and permeability, and HPA axis hormone levels.47
In the GI tract, the stress-induced mucosal barrier impairment is critical for the development of mucosal inflammation. Indeed, both acute and chronic stress, have been shown to increase ion and water secretion and intestinal permeability in the jejunum and the colon of laboratory animals.48-50 These changes were paralleled by a marked increase in epithelial macromolecular permeability48 and were mimicked by the intraperitoneal injection of CRF through mast cells and neural pathways.48,51 In addition, studies using segmental perfusion techniques in the human jejunum have revealed that acute stress, either psychological or physical, reduced net water absorption52 or increased secretion.53 Moreover, in healthy volunteers, an excessive exposure to stressful life events determines an abnormal jejunal epithelial response to incoming acute stressful stimuli.54 This abnormal response indicates the loss of regulatory mechanisms and may represent an initial step in the development of prolonged mucosal dysfunction, a finding that could be linked to IBS etiopathology.
2. Cellular mechanisms of intestinal barrier function in IBS
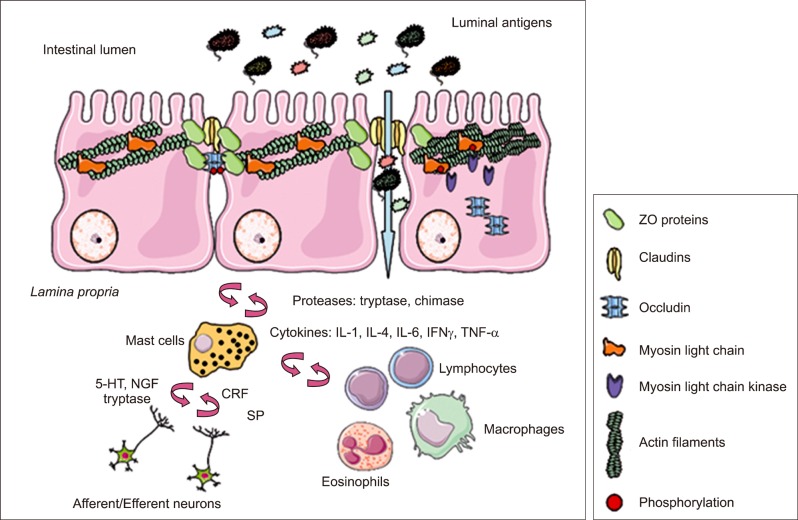
Although epithelial cells represent the primary cellular determinant of the epithelial barrier function, in the GI tract the properties of the intestinal barrier are not intrinsic to epithelial cells but also induced by interactions with the immune tissue (Fig. 3). Immune cells are associated with the physical barrier to provide immunity at entry sites. In the GI tract, immune cells reside both within the epithelium and in the lamina propria and their movement into the gut lumen is regulated to preserve homeostasis and to avoid inflammation in response to microbial-associated inflammatory stimuli and to induce tolerance toward food antigens.55
Fig. 3.
Neuro-immune regulation of intestinal barrier function. In the intestinal mucosa, mast cells are strategically located in close proximity to epithelial cells and nerve endings, establishing a bidirectional communication through the release of specific mediators. In addition, mast cells can recruit and activate other immune cells. The interaction of all these elements is crucial to assure an appropriate intestinal barrier homeostatic balance. However, chronic stimulation and the consequent release of inflammatory mediators may lead to tight junction disruption and intestinal barrier dysfunction through myosin light chain kinase-mediated phosphorylation of myosin light chain and subsequent changes in the expression and localization of tight junction proteins.
IL, interleukin; IFNγ, interferon gamma; TNF-α, tumor necrosis factor alpha; NGF, nerve growth factor; CRF, corticotropin releasing factor; SP, substance P; ZO, zonula occludens.
A growing number of independent studies have revealed a mucosal pathobiological substrate, a process of low-grade inflammation and immune activation, common to the intestine of certain IBS subgroups.8,9 Notably, the inflammatory infiltrate is dominated by mast cells and T lymphocytes,10,56 cells that have been largely implicated in stress and infectious intestinal barrier dysfunction, and in the generation of intestinal motor abnormalities and visceral pain in IBS.57-59
1) T lymphocytes
T cells are involved in adaptive immunity and display many functions, including the activation of other cell types, such as B lymphocytes and macrophages and the killing of infected host cells. Increased density of CD4+ T-cells and CD3+ or CD8+ intraepithelial T-cells have been reported along the intestinal mucosa of IBS patients.6,9,10,60
T cell-derived pro-inflammatory interleukins (ILs) and cytokines have been implicated in the disruption of intestinal epithelial barrier. TNF-α and interferon gamma (IFNγ) have been shown to be key effector molecules in the dysregulation of both the transcellular and the paracellular pathways through several mechanisms including impairment of Na+ absorption and Na+-glucose co-transport and TJ disruption.61 Both mediators have been shown to increase the expression and activity of MLCK leading to increased MLC phosphorylation and cytoskeleton contraction.62 Moreover, IFNγ has been also implicated in the redistribution of transmembrane TJ proteins occludin, claudin 1, and claudin 4.63 Notably, IL-4 and IL-13 have been shown to increase intestinal permeability by inducing apoptosis of epithelial cells and regulating claudin 2 expression.64,65
2) Mast cells
Mast cells are common residents of the intestine, which are part of the innate immune system and contribute to the modulation of a wide variety of GI pathophysiological processes.66 Mast cells have been widely studied in IBS and shown to be increased in number9,10,55 and activation10 and directly related to the generation of visceral hypersensitivity and abdominal pain in IBS.57,58 Moreover, proximity of mast cells to enteric nerves has been identified in the colonic mucosa of IBS patients.55 This anatomical relationship provides a physical substrate for bidirectional communication between the central nervous system and the gut, by which stress, luminal bacteria and other regulatory factors may influence GI function and inflammation.65 In fact, mast cells respond to neuropeptides and neurotransmitters (among other molecules) and synthesize and secrete molecules that modulate neural responses as well (CRF, substance P, serotonin, nerve growth factor, etc.).65
Mast cells have been largely implicated in the modulation of paracellular permeability. Upon activation they release potent inflammatory mediators, including prostaglandins, leukotriens, platelet-activating factor, cytokines (TNF-α, IL-3, IL-4, IL-5, IL-6, and the granulocyte and monocyte colony-stimulating factor) and specific proteases (tryptase, chymase, carboxypeptidase-A).67,68 Among all these mediators, tryptase seems particularly relevant as it can activate protease-activated receptor 2 on epithelial cells, resulting in modulation of TJ proteins and increase in permeability through paracellular pathways in the intestinal epithelium.57,66 Noteworthy, increased tryptase mucosal expression13,57 and release to the intestinal lumen10 has been recently described in IBS patients.
In the GI tract, mast cells play a major role in stress-induced regulation of epithelial, motor, and visceral responses.65 In addition to pro-inflammatory mediators, human mucosal mast cells produce and respond to key molecules mediating the stress response such as CRF and related peptides (urocortin).69,70 Activation of these receptors on the cell surface leads to mast cell degranulation and selective release of cytokines and other pro-inflammatory mediators resulting in altered intestinal barrier function.68 Pharmacologic inhibition of mast cells and treatment with CRF antagonists have been shown to inhibit the effect of mast cell-mediated changes in colonic epithelial physiology in animal models.47,71
3) Monocytes/Macrophages
Monocytes and macrophages trigger an acute inflammatory response to infectious agents. Activated macrophages release pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6 which are involved in the recruitment of other inflammatory cells and in intestinal barrier dysfunction.61,72,73 The number of resident macrophages in the intestinal mucosa of IBS patients has been assessed with discrepant results. One study has reported unchanged infiltration of macrophages in the colonic mucosa of IBS as compared to healthy individuals,74 while other showed reduced number in the mucosa of the rectum of patients that developed IBS after a bout of gastroenteritis compared to asymptomatic controls.6 This reduction in the macrophage population in IBS patients is further supported by decreased expression of the macrophage-recruiting chemokines CC-chemokine ligand 2 and CXCL10.6
4) Eosinophils
Eosinophils are multifunctional leukocytes implicated in the pathogenesis of numerous inflammatory processes, including infection, asthma and GI disorders. Eosinophils are also resident cells throughout the GI tract (excluding the squamous mucosa of the esophagus) and can synthesize and store potent immunologic mediators. Among these mediators, major basic protein has been described to induce epithelial barrier dysfunction associated with the down-regulation of occluding.75 It has recently been identify increased number of eosinophils along with mucosal hyperplasia and lymphocyte aggregation in the lamina propria of the colon in IBS patients;76 however, this finding has not yet been linked to the pathophysiology of IBS. Interestingly, eosinophilic cationic protein and eotaxin-1 have been shown to be released in the intestinal lumen of IBS patients after CRF injection,77 uncovering a putative role of stress-mediated eosinophil activation in IBS physiopathology.
3. Molecular mechanisms of intestinal barrier function in IBS
Although clinical studies have shown that increased intestinal permeability occur along the whole intestine, studies aimed at unraveling the molecular basis of this phenomenon in IBS are mainly focused on the large bowel. In this sense, application of high throughput technologies such as microarray gene profiling of mucosal samples from IBS patients are providing meaningful insights on the complex network of molecular interactions regulating the intestinal barrier function and its relation to IBS clinical manifestations. Indeed, colonic transcriptomic signatures of IBS patients have reported significant changes in the expression of a wide variety of genes involved in the impairment of mucosal immune response to microbial pathogens.78 Moreover, increased expression of genes involved in production of mucins (MUC20) have been found in the colon of IBS suggesting altered mucus composition;76 however, the potential implication of this finding in IBS pathophysiology remains unclear. Recently, differential gene expression patterns of jejunal biopsies from IBS-D patients have been linked to pathways involved in mast cell activation, intestinal permeability and TJ signaling.13
Barrier dysfunction in IBS has been linked to the down-regulation of the TJ scaffolding protein ZO-111 and proteasome-mediated occludin degradation in the colonic mucosa of IBS patients.12 Down-regulation and redistribution of ZO-1 linked to mast cell activation and disturbed bowel habits has been also described in the jejunal epithelium of IBS-D patients.13 Interestingly, the same effect on ZO-1 localization together with enhanced phosphorylation of MLC resulting in increased intestinal permeability has been found in mouse colonocytes added with fecal supernatants from IBS-D.79 Given that tryptase, the most abundant serine proteases in the mast cells, alters ZO-1 distribution, associated with increased paracellular permeability in colonocytes,66 it can be speculated that the clinical manifestations of IBS-D may rely on mast cell-related impairment of jejunal TJ function.
Besides protein down-regulation, structural and functional modulation of barrier function can be also achieved by endocytosis of TJ proteins by a mechanism that depends on the stimulus involved.34 Pro-inflammatory factors such as TNF and bacterial products have been shown to induce the internalization of ZO-1 and occludin by caveolar-mediated endocytosis38 together with a concomitant loss of barrier function. Notably, caveolar-mediated endocytosis has been identified as the most significant signaling pathway associated with the transcriptional signature of IBS-D in the jejunum.13 Taken together, these results suggest that ZO-1 down-regulation and redistribution may help to explain the increased intestinal permeability observed in IBS-D, although further studies are needed to define the mechanisms and functional consequences of this alteration.
The major focus of attention in above efforts has been the characterization of expression of protein-coding genes and their use for determining clinical outcomes. However, the majority of the human genome consists of non-protein-coding RNA (ncRNA).80 Increasing evidence points to an important functional or regulatory role of ncRNA in cellular processes as well as a contribution of aberrant ncRNA expression to disease phenotypes.81 These ncRNAs appear to function by the targeting of different epigenetic regulatory complexes to their intended targets.82 Among the different species of ncRNAs, microRNAs (miRNAs) have been identified as playing a fundamental role in differentiation, survival and function of immune cells, cytokine responses as well as intracellular signaling pathways.83,84 It has recently been shown shown that IBS-D patients harbour a distinctive miRNA profiling,85 which reflects an elevated transcription regulation activity that may account for some of the pathological hallmarks observed in this disorder, i.e., distorted barrier function, low-grade inflammation and immune activation. Small bowel and colon tissues of IBS-D patients with increased intestinal permeability showed a consistent molecular profile defined by up-regulation of miR-29a and down-regulation of glutamine synthetase expression, when compared to healthy and IBS-D with normal permeability. Noteworthy, the expression of miR-29a has been found increased in the colon of ulcerative colitis patients, a disorder also associated with increased barrier permeability, though, on the contrary, its expression was nonsignificantly altered in a small control population of IBS patients.86 It should be equally noted that miR-29a has over 1,100 estimated targets, whose contribution to the effect of miR-29a on intestinal permeability cannot be excluded. One of such putative targets is the TJ core protein claudin-1. Intriguingly, the intestine of IBS-D patients show decreased claudin-1 and claudin-4 protein levels in both the mucosa of the small bowel and the colon, whereas elevated claudin-1 and claudin-3 levels are found in constipated IBS patients.87 These facts support a role for miR-29a in controlling gut inflammation and permeability, but also bring into question its specific utility as a diagnostic marker or therapeutic target for just only IBS-D patients with increased permeability.
CONCLUSIONS
Recent studies conducted in IBS patients provide strong evidence for the presence of low-grade inflammation together with altered intestinal permeability; however, whether one phenomena precedes the other remains to be established. Stress or bacterial-mediated disruption of epithelial barrier function in IBS may result in adaptive neuro-immunological responses that, together with the malfunctioning of inflammation tuning-down mechanisms may lead to longstanding increase of gut permeability and hypersensitivity. The TJ is a critical determinant of mucosal barrier function. In the absence of mucin deficiency or gross epithelial damage, the TJ is the primary determinant of paracellular permeability. Defining how TJ-associated molecules are regulated and how they signal to modulate changes in epithelial cells in IBS would be essential to understand their contribution to the pathogenesis of this disorder.
ACKNOWLEDGEMENTS
Supported in part by the Fondo de Investigación Sanitaria and CIBERehd, Instituto Carlos III, Subdirección General de Investigación Sanitaria, Ministerio de Ciencia e Innovación (CP10/00502, MV; EC07/90148 & PI/080940 JS; CB06/04/0021, JS), the International Foundation for Functional Gastrointestinal Disorders (2008 IFFGD) and the 2010 Rome Foundation Award (JS). CIBERehd is funded by the Instituto de Salud Carlos III.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 2.Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010;140:859–870. doi: 10.1016/j.cell.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med. 2011;62:381–396. doi: 10.1146/annurev-med-012309-103958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunlop SP, Hebden J, Campbell E, et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288–1294. doi: 10.1111/j.1572-0241.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146:41–46. doi: 10.1016/j.pain.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall JK, Thabane M, Garg AX, et al. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther. 2004;20:1317–1322. doi: 10.1111/j.1365-2036.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- 8.Gwee KA, Collins SM, Read NW, et al. Increased rectal mucosal expression of interleukin 1beta in recently acquired post-infectious irritable bowel syndrome. Gut. 2003;52:523–526. doi: 10.1136/gut.52.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–1783. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- 10.Guilarte M, Santos J, de Torres I, et al. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut. 2007;56:203–209. doi: 10.1136/gut.2006.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piche T, Barbara G, Aubert P, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58:196–201. doi: 10.1136/gut.2007.140806. [DOI] [PubMed] [Google Scholar]
- 12.Coeffier M, Gloro R, Boukhettala N, et al. Increased proteasome-mediated degradation of occludin in irritable bowel syndrome. Am J Gastroenterol. 2010;105:1181–1188. doi: 10.1038/ajg.2009.700. [DOI] [PubMed] [Google Scholar]
- 13.Martínez C, Vicario M, Ramos L, et al. The jejunum of diarrhea-predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations. Am J Gastroenterol. 2012;107:736–746. doi: 10.1038/ajg.2011.472. [DOI] [PubMed] [Google Scholar]
- 14.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 16.Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 17.Salzman NH, Hung K, Haribhai D, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marques R, Boneca IG. Expression and functional importance of innate immune receptors by intestinal epithelial cells. Cell Mol Life Sci. 2011;68:3661–3673. doi: 10.1007/s00018-011-0829-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011;32:256–264. doi: 10.1016/j.it.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell. 2005;16:3919–3936. doi: 10.1091/mbc.E04-12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saitou M, Furuse M, Sasaki H, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saitou M, Fujimoto K, Doi Y, et al. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao R. Occludin phosphorylation in regulation of epithelial tight junctions. Ann N Y Acad Sci. 2009;1165:62–68. doi: 10.1111/j.1749-6632.2009.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simonovic I, Rosenberg J, Koutsouris A, Hecht G. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell Microbiol. 2000;2:305–315. doi: 10.1046/j.1462-5822.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- 25.Kale G, Naren AP, Sheth P, Rao RK. Tyrosine phosphorylation of occludin attenuates its interactions with ZO-1, ZO-2, and ZO-3. Biochem Biophys Res Commun. 2003;302:324–329. doi: 10.1016/s0006-291x(03)00167-0. [DOI] [PubMed] [Google Scholar]
- 26.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 27.Cereijido M, Contreras RG, Shoshani L, Flores-Benitez D, Larre I. Tight junction and polarity interaction in the transporting epithelial phenotype. Biochim Biophys Acta. 2008;1778:770–793. doi: 10.1016/j.bbamem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Fihn BM, Sjöqvist A, Jodal M. Permeability of the rat small intestinal epithelium along the villus-crypt axis: effects of glucose transport. Gastroenterology. 2000;119:1029–1036. doi: 10.1053/gast.2000.18148. [DOI] [PubMed] [Google Scholar]
- 29.Schumann M, Günzel D, Buergel N, et al. Cell polarity-determining proteins Par-3 and PP-1 are involved in epithelial tight junction defects in coeliac disease. Gut. 2012;61:220–228. doi: 10.1136/gutjnl-2011-300123. [DOI] [PubMed] [Google Scholar]
- 30.Zeissig S, Bürgel N, Günzel D, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Escaffit F, Boudreau F, Beaulieu JF. Differential expression of claudin-2 along the human intestine: implication of GATA-4 in the maintenance of claudin-2 in differentiating cells. J Cell Physiol. 2005;203:15–26. doi: 10.1002/jcp.20189. [DOI] [PubMed] [Google Scholar]
- 32.Tsukita S, Katsuno T, Yamazaki Y, Umeda K, Tamura A. Roles of ZO-1 and ZO-2 in establishment of the belt-like adherens and tight junctions with paracellular permselective barrier function. Ann N Y Acad Sci. 2009;1165:44–52. doi: 10.1111/j.1749-6632.2009.04056.x. [DOI] [PubMed] [Google Scholar]
- 33.Umeda K, Ikenouchi J, Katahira-Tayama S, et al. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 34.Shen L, Turner JR. Role of epithelial cells in initiation and propagation of intestinal inflammation. Eliminating the static: tight junction dynamics exposed. Am J Physiol Gastrointest Liver Physiol. 2006;290:G577–G582. doi: 10.1152/ajpgi.00439.2005. [DOI] [PubMed] [Google Scholar]
- 35.Utech M, Mennigen R, Bruewer M. Endocytosis and recycling of tight junction proteins in inflammation. J Biomed Biotechnol. 2010;2010:484987. doi: 10.1155/2010/484987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulzke JD, Bojarski C, Zeissig S, Heller F, Gitter AH, Fromm M. Disrupted barrier function through epithelial cell apoptosis. Ann N Y Acad Sci. 2006;1072:288–299. doi: 10.1196/annals.1326.027. [DOI] [PubMed] [Google Scholar]
- 37.Turner JR, Rill BK, Carlson SL, et al. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997;273:C1378–C1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- 38.Yuhan R, Koutsouris A, Savkovic SD, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;113:1873–1882. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]
- 39.Marchiando AM, Shen L, Graham WV, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teshima CW, Meddings JB. The measurement and clinical significance of intestinal permeability. Curr Gastroenterol Rep. 2008;10:443–449. doi: 10.1007/s11894-008-0083-y. [DOI] [PubMed] [Google Scholar]
- 41.Albin DM, Tappenden KA. Advances in methods to evaluate gastrointestinal transport function. Curr Opin Clin Nutr Metab Care. 2001;4:351–354. doi: 10.1097/00075197-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55:1512–1520. doi: 10.1136/gut.2005.085373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camilleri M, Gorman H. Intestinal permeability and irritable bowel syndrome. Neurogastroenterol Motil. 2007;19:545–552. doi: 10.1111/j.1365-2982.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- 44.Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- 45.Chang L. The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology. 2011;140:761–765. doi: 10.1053/j.gastro.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lydiard RB. Irritable bowel syndrome, anxiety, and depression: what are the links? J Clin Psychiatry. 2001;62(Suppl 8):38–45. [PubMed] [Google Scholar]
- 47.Dinan TG, Quigley EM, Ahmed SM, et al. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 48.Santos J, Saunders PR, Hanssen NP, et al. Corticotropin-releasing hormone mimics stress-induced colonic epithelial pathophysiology in the rat. Am J Physiol. 1999;277(2 Pt 1):G391–G399. doi: 10.1152/ajpgi.1999.277.2.G391. [DOI] [PubMed] [Google Scholar]
- 49.Santos J, Benjamin M, Yang PC, Prior T, Perdue MH. Chronic stress impairs rat growth and jejunal epithelial barrier function: role of mast cells. Am J Physiol Gastrointest Liver Physiol. 2000;278:G847–G854. doi: 10.1152/ajpgi.2000.278.6.G847. [DOI] [PubMed] [Google Scholar]
- 50.Vicario M, Alonso C, Guilarte M, et al. Chronic psychosocial stress induces reversible mitochondrial damage and corticotropin-releasing factor receptor type-1 upregulation in the rat intestine and IBS-like gut dysfunction. Psychoneuroendocrinology. 2012;37:65–77. doi: 10.1016/j.psyneuen.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Yates DA, Santos J, Söderholm JD, Perdue MH. Adaptation of stress-induced mucosal pathophysiology in rat colon involves opioid pathways. Am J Physiol Gastrointest Liver Physiol. 2001;281:G124–G128. doi: 10.1152/ajpgi.2001.281.1.G124. [DOI] [PubMed] [Google Scholar]
- 52.Barclay GR, Turnberg LA. Effect of psychological stress on salt and water transport in the human jejunum. Gastroenterology. 1987;93:91–97. doi: 10.1016/0016-5085(87)90319-2. [DOI] [PubMed] [Google Scholar]
- 53.Santos J, Saperas E, Nogueiras C, et al. Release of mast cell mediators into the jejunum by cold pain stress in humans. Gastroenterology. 1998;114:640–648. doi: 10.1016/s0016-5085(98)70577-3. [DOI] [PubMed] [Google Scholar]
- 54.Alonso C, Guilarte M, Vicario M, et al. Maladaptive intestinal epithelial responses to life stress may predispose healthy women to gut mucosal inflammation. Gastroenterology. 2008;135:163–172. doi: 10.1053/j.gastro.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 55.Iweala OI, Nagler CR. Immune privilege in the gut: the establishment and maintenance of non-responsiveness to dietary antigens and commensal flora. Immunol Rev. 2006;213:82–100. doi: 10.1111/j.1600-065X.2006.00431.x. [DOI] [PubMed] [Google Scholar]
- 56.Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 57.Cenac N, Andrews CN, Holzhausen M, et al. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636–647. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 59.Buhner S, Li Q, Vignali S, et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology. 2009;137:1425–1434. doi: 10.1053/j.gastro.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 60.Cremon C, Gargano L, Morselli-Labate AM, et al. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104:392–400. doi: 10.1038/ajg.2008.94. [DOI] [PubMed] [Google Scholar]
- 61.Musch MW, Clarke LL, Mamah D, et al. T cell activation causes diarrhea by increasing intestinal permeability and inhibiting epithelial Na+/K+-ATPase. J Clin Invest. 2002;110:1739–1747. doi: 10.1172/JCI15695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005;19:923–933. doi: 10.1096/fj.04-3260com. [DOI] [PubMed] [Google Scholar]
- 64.Weber CR, Raleigh DR, Su L, et al. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem. 2010;285:12037–12046. doi: 10.1074/jbc.M109.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wisner DM, Harris LR, 3rd, Green CL, Poritz LS. Opposing regulation of the tight junction protein claudin-2 by interferon-gamma and interleukin-4. J Surg Res. 2008;144:1–7. doi: 10.1016/j.jss.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 66.Santos J, Guilarte M, Alonso C, Malagelada JR. Pathogenesis of irritable bowel syndrome: the mast cell connection. Scand J Gastroenterol. 2005;40:129–140. doi: 10.1080/00365520410009410. [DOI] [PubMed] [Google Scholar]
- 67.Jacob C, Yang PC, Darmoul D, et al. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. J Biol Chem. 2005;280:31936–31948. doi: 10.1074/jbc.M506338200. [DOI] [PubMed] [Google Scholar]
- 68.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wallon C, Yang PC, Keita AV, et al. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut. 2008;57:50–58. doi: 10.1136/gut.2006.117549. [DOI] [PubMed] [Google Scholar]
- 70.Kempuraj D, Papadopoulou NG, Lytinas M, et al. Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology. 2004;145:43–48. doi: 10.1210/en.2003-0805. [DOI] [PubMed] [Google Scholar]
- 71.Barreau F, Cartier C, Leveque M, et al. Pathways involved in gut mucosal barrier dysfunction induced in adult rats by maternal deprivation: corticotrophin-releasing factor and nerve growth factor interplay. J Physiol. 2007;580(Pt 1):347–356. doi: 10.1113/jphysiol.2006.120907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Al-Sadi R, Ye D, Said HM, Ma TY. Cellular and molecular mechanism of interleukin-1beta modulation of Caco-2 intestinal epithelial tight junction barrier. J Cell Mol Med. 2011;15:970–982. doi: 10.1111/j.1582-4934.2010.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem. 2011;286:31263–31271. doi: 10.1074/jbc.M111.238147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O'Sullivan M, Clayton N, Breslin NP, et al. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12:449–457. doi: 10.1046/j.1365-2982.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 75.Furuta GT, Nieuwenhuis EE, Karhausen J, et al. Eosinophils alter colonic epithelial barrier function: role for major basic protein. Am J Physiol Gastrointest Liver Physiol. 2005;289:G890–G897. doi: 10.1152/ajpgi.00015.2005. [DOI] [PubMed] [Google Scholar]
- 76.Park KS, Ahn SH, Hwang JS, et al. A survey about irritable bowel syndrome in South Korea: prevalence and observable organic abnormalities in IBS patients. Dig Dis Sci. 2008;53:704–711. doi: 10.1007/s10620-007-9930-1. [DOI] [PubMed] [Google Scholar]
- 77.Guilarte M, Santos J, Alonso C, et al. Corticotropin-releasing hormone (CRH) triggers jejunal mast cell and eosinophil activation in IBS patients. Gastroenterology. 2004;126(Suppl 2):A38. [Google Scholar]
- 78.Aerssens J, Camilleri M, Talloen W, et al. Alterations in mucosal immunity identified in the colon of patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:194–205. doi: 10.1016/j.cgh.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gecse K, Róka R, Ferrier L, et al. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut. 2008;57:591–599. doi: 10.1136/gut.2007.140210. [DOI] [PubMed] [Google Scholar]
- 80.Morris KV. The emerging role of RNA in the regulation of gene transcription in human cells. Semin Cell Dev Biol. 2011;22:351–358. doi: 10.1016/j.semcdb.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khalil AM, Rinn JL. RNA-protein interactions in human health and disease. Semin Cell Dev Biol. 2011;22:359–365. doi: 10.1016/j.semcdb.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Costa FF. Non-coding RNAs, epigenetics and complexity. Gene. 2008;410:9–17. doi: 10.1016/j.gene.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 83.Bi Y, Liu G, Yang R. MicroRNAs: novel regulators during the immune response. J Cell Physiol. 2009;218:467–472. doi: 10.1002/jcp.21639. [DOI] [PubMed] [Google Scholar]
- 84.Sheedy FJ, O'Neill LA. Adding fuel to fire: microRNAs as a new class of mediators of inflammation. Ann Rheum Dis. 2008;67(Suppl 3):iii50–iii55. doi: 10.1136/ard.2008.100289. [DOI] [PubMed] [Google Scholar]
- 85.Zhou Q, Souba WW, Croce CM, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut. 2010;59:775–784. doi: 10.1136/gut.2009.181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu F, Zikusoka M, Trindade A, et al. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology. 2008;135:1624–1635. doi: 10.1053/j.gastro.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 87.Kong WM, Gong J, Dong L, Xu JR. Changes of tight junction claudin-1,-3,-4 protein expression in the intestinal mucosa in patients with irritable bowel syndrome. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27:1345–1347. [PubMed] [Google Scholar]