Abstract
Background/Aims
We aimed to assess the effectiveness of self-expanding metal stent (SEMS) insertion by evaluating the learning curve in relation to the experience of an endoscopist.
Methods
We retrospectively analyzed the outcomes of 120 SEMS insertion procedures performed by one endoscopist in patients with malignant colorectal obstruction. We compared the technical and clinical success rates, complication rates, and duration of the procedures by quartiles.
Results
The mean age of the patients (76 men and 44 women) was 64.6 years. The overall technical success rate was 95.0% (114/120), and the clinical success rate was 90.0% (108/120). The median procedure duration was 16.2 minutes (range, 3.4 to 96.5 minutes). From the first to the last quartile, the technical success rates were 90.0%, 96.7%, 96.7%, and 96.7% (p=0.263), and the clinical success rates were 90.0%, 90.0%, 96.7%, and 83.3% (p=0.588), respectively. Procedure-related complications were observed in 28 patients (23.3%). The complication rates for SEMS insertion when patients were divided by quartiles were 26.7%, 23.3%, 10.0%, and 33.3% (p=0.184), respectively. Moreover, the number of stents per procedure was 1.13, 1.03, 1.00, and 1.00 (p=0.029), respectively. The median duration of SEMS insertion decreased significantly, 20.9 to 14.8 minutes after the first 30 procedures (p=0.005).
Conclusions
An experienced endoscopist was able to perform the SEMS insertion procedure easily and effectively after performing 30 SEMS insertions.
Keywords: Learning curve, Colorectal stents, Self-expanding metallic stents, Malignant colorectal obstruction, Colorectal neoplasms
INTRODUCTION
Colorectal cancer is the third most common cancer in Korea and its incidence is rapidly increasing due to more westernized dietary habits.1,2 Approximately 20% of colorectal cancer patients are not eligible for curative surgery.3 Abdominal pain, nausea, vomiting, constipation, abdominal distension, and the inability to pass gas in patients with colorectal cancer can be signs of malignant colorectal obstruction, which can become a surgical emergency.4 Previously, surgical treatment in these cases was the only way to decompress the bowel. Since the first case of palliative stent insertion in patients and cases of stent insertion as a bridge to surgery were reported, endoscopic stenting with self-expanding metal stents (SEMS) has been widely used.5,6 Most studies on the effectiveness and safety of SEMS show that SEMS provide a safe single-stage surgical procedure that avoids colostomy in patients that received SEMS preoperatively. It also improves clinical outcomes and quality of life for patients undergoing palliative treatment.7,8 According to recent studies, the technical and clinical success rates of SEMS insertion are reported to be above 90%.9,10 Therefore, the placement of SEMS for initial management of obstructive colorectal cancer is universally accepted.
Despite its general acceptance, the adequate level of training necessary for technical competence in SEMS insertion remains unknown. Detailing the learning curve for SEMS insertion could serve as a reference for gastroenterology specialists who would like to perform the procedure. Therefore, the aim of this study was to assess the effectiveness of SEMS insertion by evaluating the learning curve in relation to the experience of an endoscopist.
MATERIALS AND METHODS
1. Patients
In this study, we analyzed the outcomes of 158 SEMS insertion procedures performed by a single endoscopist (Cheon JH) in patients with malignant colorectal obstruction who were treated at Severance Hospital, Yonsei University College of Medicine, Seoul, Korea between March 2006 and December 2009. Our study was performed by a retrospective review of prospectively collected data on patient sex, age, cancer location, type of obstruction, purpose of SEMS insertion, type of stent, stent length, and number of stents. Exclusion criteria were as follows: 1) extrinsic obstruction by other cancerous lesions (n=30); 2) a previous history of stent insertion at other hospitals (n=2); 3) benign stricture after a colon cancer operation (n=5); and 4) recurrence after a colon cancer operation (n=1). After excluding 38 patients, we compared the technical success rates, clinical success rates, complication rates, number of stents needed per procedure and procedure duration by quartiles.
This study was approved by the Institutional Review Board of Severance Hospital.
2. Definitions
The location of the cancer was categorized into two groups: right colon and left colon. The ascending colon and the transverse colon were included as the right colon and the other areas were classified as the left colon. Technical failure was defined as failure to deploy a stent across the entire length of a colon stricture. Clinical failure was defined as the absence of the resolution of obstructive symptoms (abdominal distension, vomiting, and abdominal pain) and the absence of gas and stool passage despite achieving technical success.11 Problems such as immediate migration, bleeding, and perforation were considered stent-induced complications.12 Subtotal obstruction was defined as a state with narrow stool caliber or the ability to pass only small amounts of liquid stool or gas. Total obstruction was defined as decreased or absent bowel sounds or the inability to pass any stool or gas.7 Procedure time was calculated only after the colonoscope was advanced to the site of obstruction, then the time required to insert a guidewire and stent and to confirm correct positioning and expansion using fluoroscopy was included.
3. Endoscopic technique
Before placing colonic stents, all patients underwent a computed tomography (CT) scan and bowel preparation by glycerin and warm saline enema. We evaluated the CT scans to assess the extent of the tumor and the location, degree, and length of the obstruction. Stents were placed by one experienced colonoscopist (Cheon JH) from our hospital as previously described.1 Before initiating SEMS insertion, the endoscopist had performed more than 1,000 colonoscopy procedures per year for several years but had no experience with endoscopic retrograde cholangiopancreatography.
A flexible colonoscope (CF-H260AI; Olympus, Tokyo, Japan) was advanced to the site of the obstruction. A biliary guidewire (Jagwire; Boston Scientific, Natick, MA, USA) was inserted into the lumen of a catheter (ERCP-Catheter; MTW Endoskopie, Wesel, Germany), and the guidewire and the catheter were then advanced together beyond the obstruction. The distal and proximal ends of the stricture were confirmed under fluoroscopic guidance by injecting a water-soluble contrast agent (Gastrograffin; Bayer Schering Pharm., Seoul, Korea) through the catheter after removal of the guidewire. The compressed SEMS delivery system was then introduced through the working channel of the endoscope over the guidewire and passed beyond the stricture. Stent release and expansion progressed from the proximal to the distal portion under fluoroscopic and endoscopic control. Abdominal X-rays were obtained on the same day of the procedure, as well as the next day, to confirm correct positioning and expansion.
Stent type was selected according to the preference of the patient and the experience of the endoscopist. Stent length was selected by allowing for at least an additional 2 cm to be exposed distal and proximal to the obstructing lesion. Four types of stents were used in our study: 1) covered Niti-s colonic covered stents (Taewoong Medical, Seoul, Korea); 2) newly developed, covered Comvi stents (Taewoong Medical); 3) uncovered Wall-Flex colonic stents (Boston Scientific, Denver, CO, USA); and 4) uncovered Niti-s colonic D type stents (Taewoong Medical).13
4. Statistical analysis
Data were analyzed to identify the baseline patient characteristics, sites of obstructing lesions, reasons for stenting, outcomes, procedure times, and complications. The data were expressed as the mean±SD, median (range), or no. (%) as appropriate. We compared categorical variables with the chi-square test and one-way ANOVA. Correlations between success rates, procedure times, and level of experience were assessed using Tukey's multiple comparison test and Dunn procedure. A p<0.05 on a two-tailed test was considered statistically significant. Statistical analysis was performed using IBM SPSS version 18.0 (IBM, New York, NY, USA).
RESULTS
1. Baseline characteristics
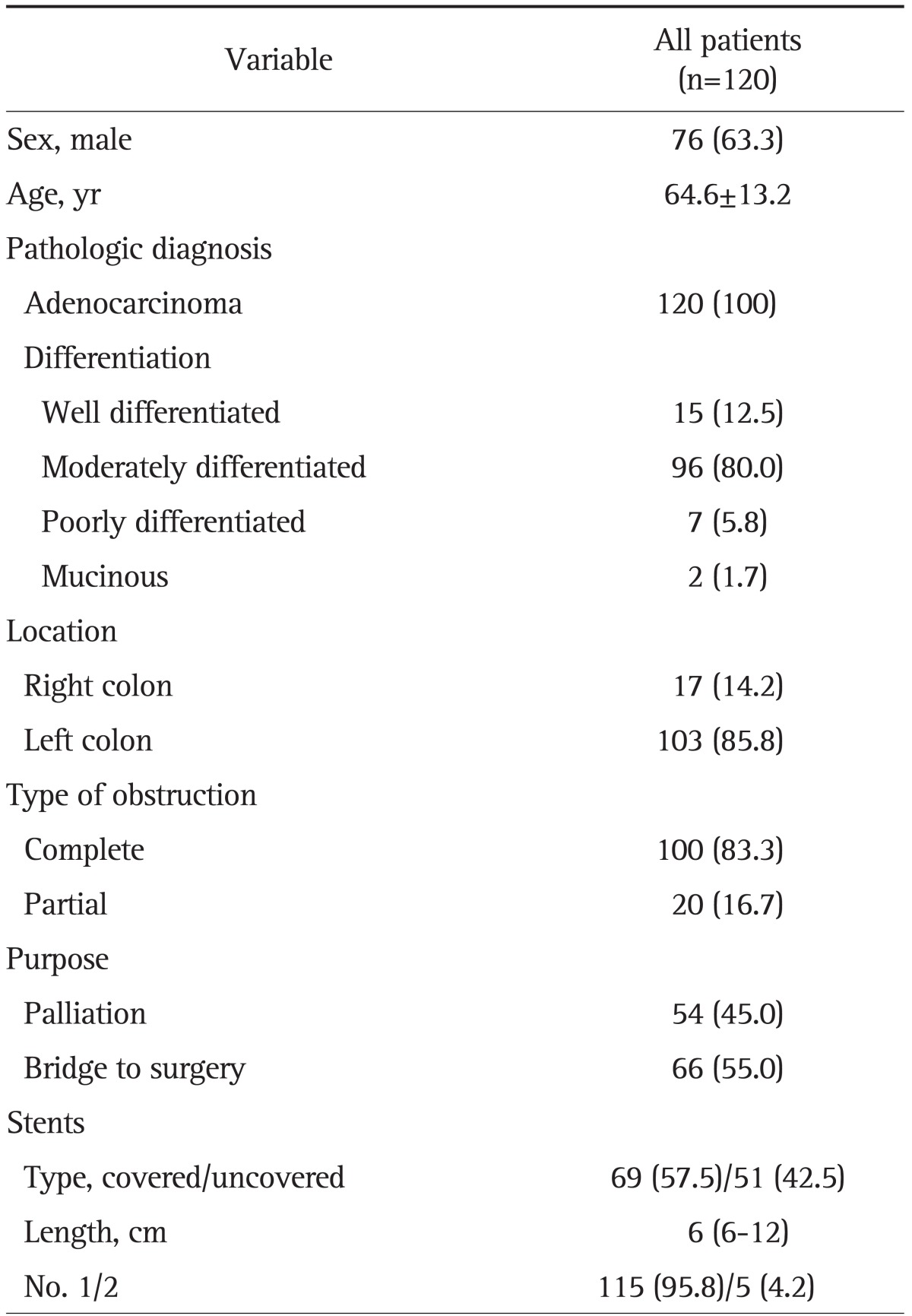
The baseline characteristics of the 120 patients are summarized in Table 1. The mean age of the patients (76 men and 44 women) was 64.6 years. The pathological diagnosis of all patients was adenocarcinoma. There were 103 (85.8%) patients with left-sided colonic obstruction, complete obstruction was present in 100 patients (83.3%) and partial obstruction was noted in 20 (16.7%). In terms of SEMS insertions, 45% (n=54) were performed for palliation and 55% (n=66) were performed as a bridge to surgery. The type of stent was classified into covered (n=69, 57.5%) and uncovered stents (n=51, 42.5%). The median length of SEMS was 6 cm (range, 6 to 12 cm). Most patients (95%) underwent SEMS insertion when first diagnosed with colorectal cancer, whereas six patients underwent SEMS insertion during the follow-up period. The median period from the diagnosis of colorectal cancer to stent insertion in these six patients was 13.6 months (range, 3.1 to 33.0 months).
Table 1.
Characteristics of the Patients and Lesions
Data are presented as mean±SD or number (%).
2. Stent insertion outcomes
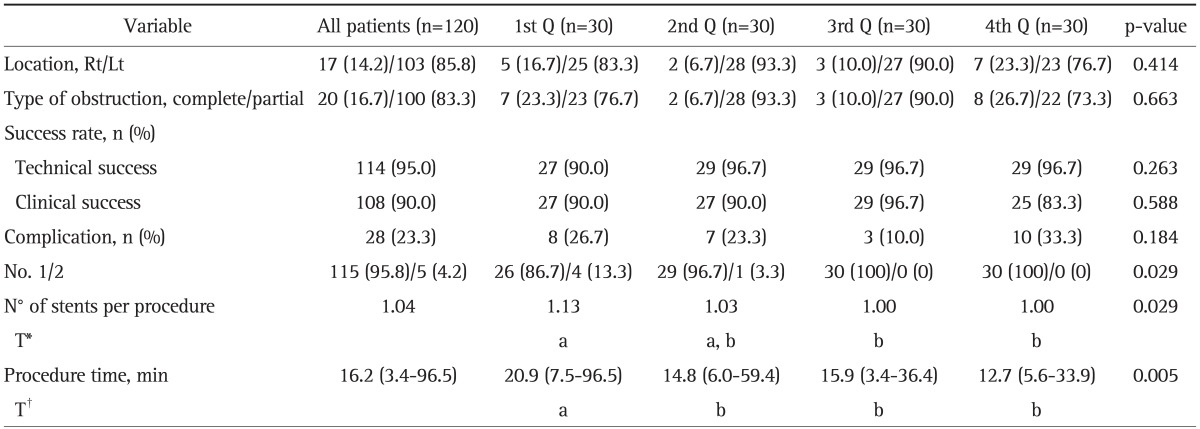
The outcomes of stent insertion are shown in Table 2. Most patients (n=115, 95.8%) underwent the SEMS procedure with a single stent. Only five patients required two stents because of inadequate measurement of the length of the stricture (n=2) or technical failure (n=3). The overall technical success rate was 95.0% (114/120), and the clinical success rate was 90.0% (108/120). The median procedure duration was 16.2 minutes (range, 3.4 to 96.5 minutes). Re-intervention for SEMS insertion was required in 25 patients (20.8%). The median interval between the initial stenting and the second attempt was 89 days (range, 1 to 947 days).
Table 2.
Outcomes of Stent Insertion
Data are presented as median (range) or number (%).
Q, quartile; Rt, right; Lt, left.
*The same letters indicate a nonsignificant difference between groups based on Tukey's multiple comparison test; †The same letters indicate a nonsignificant difference between groups based on the multiple comparison test (Dunn procedure).
Procedure-related complications were observed in 28 patients (23.3%): obstruction in 11, hemorrhage in 4, stent migration in 11, and both hemorrhage and stent migration in 2 patients. There was no perforation, erosion/ulcer, or mortality. Among the patients with hemorrhage, there was no major bleeding necessitating blood transfusion. Stent migration occurred in 13 patients, and one of these patients underwent emergent surgery, another was treated with re-positioning of a stent that had already been inserted, and the remaining patients had a new stent inserted. The median number of days between the insertion and migration was 47 days (range, 1 to 567 days). Stent obstruction was noted in 11 patients. One of them was transferred to another hospital, another underwent emergent surgery, and three of the remaining patients underwent new stent insertion due to tumor ingrowth. The others recovered after removal of impacted stool. The median duration of obstruction was 3 days (range, 1 to 947 days). Bleeding was noted in six patients a median of 2.7 days (range, 1 to 4 days) after the procedure.
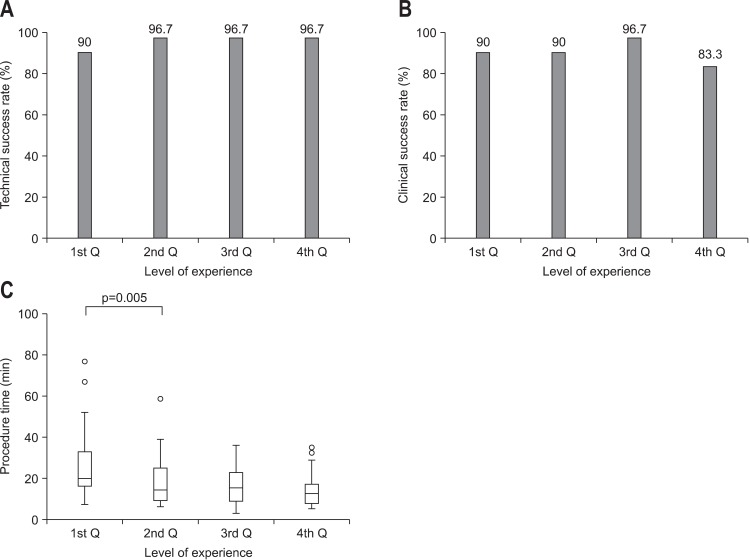
The technical and clinical success rates, complications, the number of stents used, and procedure time for each quartile are shown in Table 2 and Fig. 1. The location of obstruction site and the type of obstruction by quartile was not statistically different (p=0.414, p=0.663), respectively. From the first to the last quartile, the technical success rates were 90.0%, 96.7%, 96.7%, and 96.7% (p=0.263), and the clinical success rates were 90.0%, 90.0%, 96.7%, and 83.3% (p=0.588), respectively. Complications occurred in 26.7%, 23.3%, 10.0%, and 33.3% (p=0.184) of the patients, and the number of stents per procedure was 1.13, 1.03, 1.00, and 1.00 (p=0.029) by quartiles, respectively. The median procedure duration for SEMS insertion significantly decreased, 20.9-14.8 minutes, after the first 30 procedures (p=0.005).
Fig. 1.
(A) Technical success rate. (B) Clinical success rate. (C) Procedure duration. (A) and (B) illustrate the learning curves for colorectal self-expanding metal stents insertion, which reveal correlations between technical success rates (A), clinical success rates (B), and the endoscopist's level of experience (p>0.05). (C) It demonstrates the correlation between procedure duration and level of experience. The median procedure duration decreased significantly after the first 30 procedures (p<0.05 with the multiple comparison test [Dunn procedure]).
The technical success rate of the patients with right-sided colon obstruction and that of the patients with left-sided colon obstruction were similar (94.1% vs 95.2%, p=0.858), whereas the clinical success rate in the patients with right-sided colon obstruction was higher than that in the patients with left-sided colon obstruction (100% vs 88.4%, p<0.001). There was no significant difference in procedure duration between patients with right-sided colon obstruction and patients with left-sided colon obstruction (24.5 minutes vs 19.1 minutes, p=0.118).
DISCUSSION
Malignant colorectal obstruction, partial colonic obstruction, and complete obstruction are observed in approximately 20%, 10% to 20%, and 8% to 29% of patients with colorectal cancer, respectively.14-16 Complete colonic obstruction is a surgical emergency because intestinal perforation, sepsis, and death can occur.4 Nowadays, SEMS are effectively, safely, and widely used. In recent studies, both technical and clinical success rates of SEMS insertion were reported to be above 90%.9,10 Although SEMS insertion is widely used to relieve malignant colorectal obstruction, the optimal learning requirements for SEMS insertion remain unknown. Therefore, we aimed to assess the effectiveness of SEMS insertion by evaluating the learning curve in relation to the experience of an endoscopist.
According to recent studies, the technical and clinical success rates of SEMS insertion are reported to be above 90%.9,10 In this study, the overall technical success rate (95%) and the clinical success rate (90%) were similar to those of previous studies. Furthermore, the technical and clinical success rates of the first quartile were both 90%, which indicates that stent insertion is not a difficult technique for experienced colonoscopists. Therefore, the learning curve based on the success rates of stent insertion per se might be less meaningful. However, the technical success rates had a tendency to be lower, although not significantly different, in the first quartile than in the other periods (90% vs 96.7%). Further large-scale studies concerning the success rate of SEMS insertion are needed to explain this tendency more precisely. The clinical success rate seems to be lower in the fourth quartile, though the difference was not statistically significant. The causes of failures were mostly (n=3/5, 60%) obstruction by stool impaction that resolved easily after removal of the stool. Sebastian et al.9 and Cho et al.17 reported that the technical success rate was lower in the group with proximal colon obstruction than in the group with distal colon obstruction. However, we observed similar results between right-sided and left-sided colon obstructions (94.1% vs 95.2%, p=0.858). The procedure duration was similar between the patients with right-sided colon obstruction and those with left-sided colon obstruction (24.5 minutes vs 19.1 minutes, p=0.118). In this study, the procedure duration was calculated after the colonoscope was advanced to the obstruction site. The time required to reach the obstruction site was not included.
The complications of stent placement are perforation, stent migration, stent obstruction, and bleeding.18 Perforation is one of the most serious complications and occurred in 3.7% of patients in a pooled analysis.9 In our study, the overall complication rate was 23.3%, which is similar to that of another study.17 The complication rates by quartile were not statistically different (p=0.184), which suggests that the effects of the learning curve on complications might be less significant in this study. Stent migration rates of 11% to 40% are reported, particularly with covered stents.9,19 We found no differences in the total complication rates between the covered and uncovered stent groups (17/69 [24.6%] vs 11/51 [21.6%]) (p=0.478), but the migration rate was higher in the covered stent group than in the uncovered stent group (11/69 [15.9%] vs 2/51 [3.9%]) (p=0.041).
Interestingly, the first 30 cases of SEMS insertion required an average of 1.13 stents per procedure. However, in the third and fourth quartiles, all patients needed only one SEMS insertion. Moreover, the insertion time decreased significantly, 20.9 to 14.8 minutes, after 30 procedures (p=0.005). This indicates that endoscopists who are not familiar with the SEMS insertion procedure can perform the procedure effectively and safely after performing 30 SEMS insertions.
In this study, we collected the data in a prospective manner, and the learning curve was calculated using the results of SEMS insertion performed by one endoscopist. Bowel obstruction can also be caused by extrinsic compression or intraluminal invasion from other abdominal malignancies, such as stomach or ovarian cancer.18 Other studies of the SEMS procedure involve both extrinsic and intrinsic causes of colon obstruction. However, this study only investigated malignant colorectal obstruction caused by colorectal cancer. In this study, we enrolled a larger number of patients compared to another study.20 We also analyzed complication rates and procedure durations by quartiles in addition to technical and clinical success rates. Based on our results, the duration of the SEMS insertion procedure was dramatically reduced after the endoscopist had performed 30 procedures. Similar to our results, Williams et al.20 reported the learning curve of colorectal stenting in 40 malignant colorectal obstruction patients, which revealed that at least 20 cases are required for an operator to be considered experienced based on the success rate and number of stents per procedure.
Our study had several limitations. The data were collected prospectively but were analyzed retrospectively. The long-term follow-up of stent patency was not included in this study, so the comparison of stent patency of SEMS by quartiles needs further investigation. In conclusion, our study demonstrated that an experienced endoscopist can perform the SEMS insertion procedure effectively and safely after performing 30 SEMS insertions.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kim TI. Effect of stenting for obstructing colorectal cancers, according to the location of obstructing lesion. Korean J Gastroenterol. 2009;54:413–414. doi: 10.4166/kjg.2009.54.6.413. [DOI] [PubMed] [Google Scholar]
- 2.Kim JS, Oh SY, Seo KU, et al. Comparison of effects of preoperative stenting for obstructing colorectal cancers according to the location of the obstructing lesion. Korean J Gastroenterol. 2009;54:384–389. doi: 10.4166/kjg.2009.54.6.384. [DOI] [PubMed] [Google Scholar]
- 3.Súarez J, Jiménez J, Vera R, et al. Stent or surgery for incurable obstructive colorectal cancer: an individualized decision. Int J Colorectal Dis. 2010;25:91–96. doi: 10.1007/s00384-009-0814-z. [DOI] [PubMed] [Google Scholar]
- 4.Nagula S, Ishill N, Nash C, et al. Quality of life and symptom control after stent placement or surgical palliation of malignant colorectal obstruction. J Am Coll Surg. 2010;210:45–53. doi: 10.1016/j.jamcollsurg.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 5.Wong KS, Cheong DM, Wong D. Treatment of acute malignant colorectal obstruction with self-expandable metallic stents. ANZ J Surg. 2002;72:385–388. doi: 10.1046/j.1445-2197.2002.02431.x. [DOI] [PubMed] [Google Scholar]
- 6.Tejero E, Mainar A, Fernández L, Tobío R, De Gregorio MA. New procedure for the treatment of colorectal neoplastic obstructions. Dis Colon Rectum. 1994;37:1158–1159. doi: 10.1007/BF02049822. [DOI] [PubMed] [Google Scholar]
- 7.Small AJ, Coelho-Prabhu N, Baron TH. Endoscopic placement of self-expandable metal stents for malignant colonic obstruction: long-term outcomes and complication factors. Gastrointest Endosc. 2010;71:560–572. doi: 10.1016/j.gie.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Tilney HS, Lovegrove RE, Purkayastha S, et al. Comparison of colonic stenting and open surgery for malignant large bowel obstruction. Surg Endosc. 2007;21:225–233. doi: 10.1007/s00464-005-0644-1. [DOI] [PubMed] [Google Scholar]
- 9.Sebastian S, Johnston S, Geoghegan T, Torreggiani W, Buckley M. Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol. 2004;99:2051–2057. doi: 10.1111/j.1572-0241.2004.40017.x. [DOI] [PubMed] [Google Scholar]
- 10.Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg. 2002;89:1096–1102. doi: 10.1046/j.1365-2168.2002.02148.x. [DOI] [PubMed] [Google Scholar]
- 11.Dronamraju SS, Ramamurthy S, Kelly SB, Hayat M. Role of self-expanding metallic stents in the management of malignant obstruction of the proximal colon. Dis Colon Rectum. 2009;52:1657–1661. doi: 10.1007/DCR.0b013e3181a8f4af. [DOI] [PubMed] [Google Scholar]
- 12.Donnellan F, Cullen G, Cagney D, et al. Efficacy and safety of colonic stenting for malignant disease in the elderly. Int J Colorectal Dis. 2010;25:747–750. doi: 10.1007/s00384-010-0917-6. [DOI] [PubMed] [Google Scholar]
- 13.Park S, Cheon JH, Park JJ, et al. Comparison of efficacies between stents for malignant colorectal obstruction: a randomized, prospective study. Gastrointest Endosc. 2010;72:304–310. doi: 10.1016/j.gie.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 14.Tekkis PP, Kinsman R, Thompson MR, Stamatakis JD Association of Coloproctology of Great Britain, Ireland. The Association of Coloproctology of Great Britain and Ireland study of large bowel obstruction caused by colorectal cancer. Ann Surg. 2004;240:76–81. doi: 10.1097/01.sla.0000130723.81866.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goligher JC, Smiddy FG. The treatment of acute obstruction or perforation with carcinoma of the colon and rectum. Br J Surg. 1957;45:270–274. doi: 10.1002/bjs.18004519109. [DOI] [PubMed] [Google Scholar]
- 16.Ragland JJ, Londe AM, Spratt JS., Jr Correlation of the prognosis of obstructing colorectal carcinoma with clinical and pathologic variables. Am J Surg. 1971;121:552–556. doi: 10.1016/0002-9610(71)90137-1. [DOI] [PubMed] [Google Scholar]
- 17.Cho YK, Kim SW, Lee BI, et al. Clinical outcome of self-expandable metal stent placement in the management of malignant proximal colon obstruction. Gut Liver. 2011;5:165–170. doi: 10.5009/gnl.2011.5.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suh JP, Kim SW, Cho YK, et al. Effectiveness of stent placement for palliative treatment in malignant colorectal obstruction and predictive factors for stent occlusion. Surg Endosc. 2010;24:400–406. doi: 10.1007/s00464-009-0589-x. [DOI] [PubMed] [Google Scholar]
- 19.Ely CA, Arregui ME. The use of enteral stents in colonic and gastric outlet obstruction. Surg Endosc. 2003;17:89–94. doi: 10.1007/s00464-002-8809-7. [DOI] [PubMed] [Google Scholar]
- 20.Williams D, Law R, Pullyblank AM. Colorectal stenting in malignant large bowel obstruction: the learning curve. Int J Surg Oncol. 2011;2011:917848. doi: 10.1155/2011/917848. [DOI] [PMC free article] [PubMed] [Google Scholar]